Abstract
Objectives
To evaluate the recent developments in robotic urological surgery, as the introduction of robotic technology has overcome many of the difficulties of pure laparoscopic surgery enabling surgeons to perform complex minimally invasive procedures with a shorter learning curve. Robot-assisted surgery (RAS) is now offered as the standard for various surgical procedures across multiple specialities.
Methods
A systematic search of MEDLINE, PubMed and EMBASE databases was performed to identify studies evaluating robot-assisted simple prostatectomy, salvage radical prostatectomy, surgery for urolithiasis, distal ureteric reconstruction, retroperitoneal lymph node dissection, augmentation ileocystoplasty, and artificial urinary sphincter insertion. Article titles, abstracts, and full text manuscripts were screened to identify relevant studies, which then underwent data extraction and analysis.
Results
In all, 72 studies evaluating the above techniques were identified. Almost all studies were retrospective single-arm case series. RAS appears to be associated with reduced morbidity, less blood loss, reduced length of stay, and comparable clinical outcomes in comparison to the corresponding open procedures, whilst having a shorter operative duration and learning curve compared to the equivalent laparoscopic techniques.
Conclusion
Emerging data demonstrate that the breadth and complexity of urological procedures performed using the da Vinci® platform (Intuitive Surgical Inc., Sunnyvale, CA, USA) is continually expanding. There is a gaining consensus that RAS is producing promising surgical results in a wide range of procedures. A major limitation of the current literature is the sparsity of comparative trials evaluating these procedures.
Abbreviations: AUS, artificial urinary sphincter; ICUD, intracorporeal urinary diversion; HoLEP, holmium laser enucleation of the prostate; LOS, length of hospital stay; MIS, minimally invasive surgery; PCNL, percutaneous nephrolithotomy; (L-)(O-)(R-) RPLND, (laparoscopic)(open)(robot-assisted) retroperitoneal lymph node dissection; (L)(RA) PN, (laparoscopic)(robot-assisted) partial nephrectomy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RAI, robot-assisted augmentation ileocystoplasty; (s)RALP, (salvage)robot-assisted laparoscopic prostatectomy; RAS, robot-assisted surgery; (RA)RC, (robot-assisted) radical cystectomy; RCT, randomised controlled trial; (L)(R)RP, (laparoscopic)(retropubic)radical prostatectomy; sRRP, salvage RRP; RNL, robot-assisted nephrolithotomy; RPL, robot-assisted pyelolithotomy; (O)(L)(RA)SP, (open)(laparoscopic)(robot-assisted)simple prostatectomy; (S)UI, (stress) urinary incontinence
Keywords: Robot-assisted surgery, Robotic surgery, Urology
Introduction
The introduction of the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) has dramatically transformed the landscape of minimally invasive surgery (MIS). This surgical platform, whilst maintaining the benefits of standard laparoscopy, provides the surgeon with additional advantages of greater dexterity, a wider range of movement, tremor filtration, three-dimensional vision, and primary surgeon camera control. These benefits are useful, especially when there is a deep and narrow field and when intracorporeal suturing and fine tissue dissection are required, as is the case for pelvic and retroperitoneal surgery [1], [2]. This technology has therefore enabled surgeons to replicate complex open procedures using MIS with a much faster learning curve than standard laparoscopy and the potential to supersede the results of open surgery.
Robot-assisted surgery (RAS) has now become the contemporary ‘gold standard’ treatment modality for many urological conditions. Perhaps the most established procedure being robot-assisted laparoscopic prostatectomy (RALP) [3]. After first being described by Menon et al. [4], RALP has now replaced open retropubic radical prostatectomy (RRP) and laparoscopic RP (LRP) in most modern healthcare systems [3]. Despite the lack of high-quality randomised controlled trials (RCTs) showing a benefit over open RRP [5], there is an abundance of non-randomised data that have shown clear advantages for intraoperative blood loss, transfusion rates, duration of catheterisation, length of hospital stay (LOS), positive margins, potency, continence, and readmission rates [6], [7].
Since its first report, again by Menon et al. [8] in 2003, robot-assisted radical cystectomy (RARC) has likewise been adopted by several large institutions. A recent systematic review comparing RARC (with mainly extracorporeal urinary diversion) with open RC showed that RARC benefited from fewer perioperative complications, greater lymph node yield, lower blood loss, and a shorter LOS [9]. With many units now routinely performing intracorporeal urinary diversion (ICUD), further benefits can be derived by a reduction in incision size, postoperative pain, and bowel-related complications [10]. A recent study demonstrated that introduction of RARC and ICUD represented the principal factor leading to the benefits of a RC enhanced-recovery programme [11], and furthermore cost-efficiency analyses have shown promising results even when factoring in purchase, consumable and maintenance expenses [12], [13].
A robot-assisted approach for partial nephrectomy (RAPN) has also yielded benefits including reduced blood loss, postoperative pain and LOS [14]. A systematic review comparing RAPN and laparoscopic PN (LPN) showed RAPN to have shorter ischaemia times and a lower overall complication rate than the LPN [15]. RAPN also has a shorter learning curve than LPN and enables more complex cases (>4 cm, multifocal, central location, solitary kidney) to be carried out [16], [17]. These benefits are mirrored in other upper tract procedures including robot-assisted pyeloplasty, where authors present the case for RAS becoming the standard of care [18], [19].
With the widespread uptake of RAS in urology, the aim of the present review was to evaluate its expanding role within the speciality.
Methods
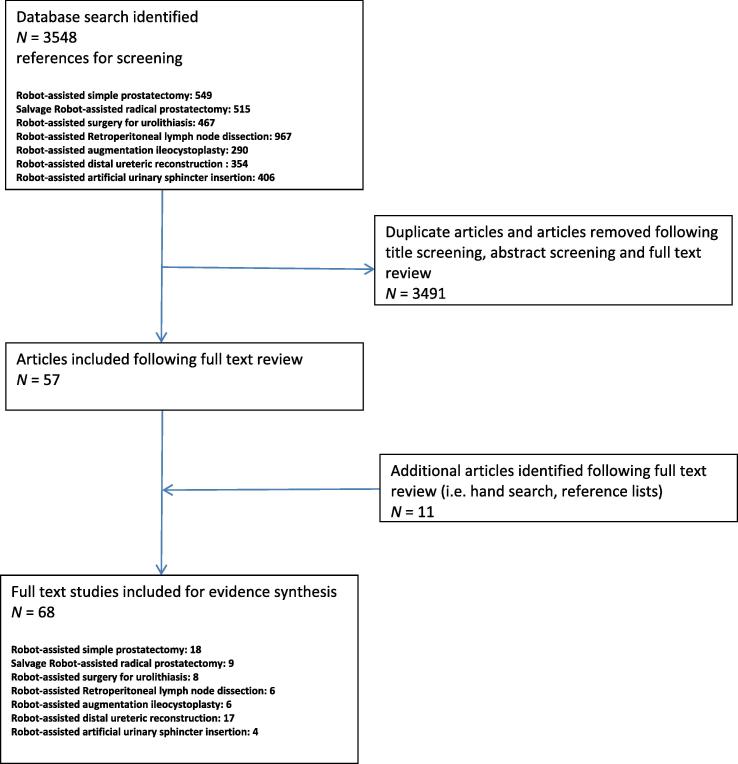
We identified several RAS procedures, which have recently gained attention in the contemporary literature. A systematic review was performed for several urological procedures, with an endeavour to adhere to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. For each procedure, we performed a structured, comprehensive literature review searching MEDLINE, PubMed and EMBASE databases. We retrieved citations using the described search combinations (Table 1) for the relevant procedure. Article titles, abstracts and full text manuscripts were then screened to identify relevant studies (Fig. 1). We excluded single case reports and case series with less than three patients. As the majority of relevant articles were case series, risk-of-bias assessment was not performed.
Table 1.
List of terms used for relevant database searches.
| Procedure | Search terms |
|---|---|
| Robot-assisted simple prostatectomy (RASP) | ‘robotic’ or ‘robot’ or ‘robot-assisted’ AND ‘prostatectomy’ AND ‘benign’ or ‘simple’ |
| Salvage robot-assisted radical prostatectomy (sRALP) | ‘salvage’ or ‘salvage therapy’ AND ‘prostatectomy’ or ‘surgery’ AND ‘robotic’ or ‘robot’ or ‘robot-assisted’ |
| Robot-assisted surgery (RAS) for urolithiasis | ‘robotic’ or ‘robot’ or ‘robot-assisted’ AND ‘urolithiasis’ or ‘pyelolithotomy’ or ‘nephrolithotomy’ or ‘stones’ or ureterolithotomy’ |
| Robot-assisted distal ureteric reconstruction | ‘robotic’ or ‘robot’ or ‘robot-assisted’ AND ‘Boari’ or ‘Psoas’ or ‘reimplant’ or ‘uretero-ureterostomy’ or ‘ureteroureterostomy’ or ‘ureteroneocystostomy’ or ‘uretero-neocystostomy’ |
| Robot-assisted retroperitoneal lymph node dissection (R-RPLND) | ‘robotic’ or ‘robot’ or ‘robot-assisted’ AND ‘testicular’ or ‘retroperitoneal’ or ‘RPLND’ |
| Robot-assisted augmentation ileocystoplasty (RAI) | ‘robotic’ or ‘robot’ or ‘robot-assisted’ AND ‘ileocystoplasty’ or ‘enetrocystoplasty’ or ‘augmentation’ |
| Robot-assisted artificial urinary sphincter (AUS) insertion | ‘robotic’ or ‘robot’ or ‘robot-assisted’ AND ‘sphincter’ or ‘AUS’ or ‘artificial urinary’ |
Fig. 1.
PRISMA diagram showing study acquisition.
Robot-assisted simple prostatectomy (RASP)
Surgical treatment options for benign prostatic obstruction have expanded considerably over the last 20 years. Whereas TURP for <80 mL glands, and open SP (OSP) for larger prostates was considered the ‘gold standard’ [20], the development of laser technologies and MIS has provided treatment alternatives with many potential benefits over traditional modalities [21], [22].
With the intent of replicating the equivalent open procedure, whilst negating the technical challenges of laparoscopic surgery, RASP was first described in 2008 [23]. Subsequently, 18 case series have been reported, of which, there are three non-randomised comparative studies [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. Table 2 summarises data from studies evaluating RASP [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41].
Table 2.
Clinico-demographic data and surgical outcomes from case series/studies evaluating RASP.
| Mean/median: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Number of patients | Age, years | Preoperative TRUS volume, mL | Operative time, min | Blood loss, mL | Resection weight, g | LOS, days | Duration of catheterisation, days | Surgical technique | Transfusion, n |
| Sotelo et al. [23] | 7 | 65 | 77 | 195 | 382 | 52 | 1.3 | 7.5 | TP TV |
1 |
| Yuh et al. [24] | 3 | 77 | 323 | 211 | 558 | 301 | 1.3 | NR | TP | 1 |
| John et al. [25] | 13 | 70 | NR | 210 | 500 | 82 | 6 | 6 | EP | 0 |
| Uffort et al. [26] | 15 | 66 | 71 | 128 | 140 | 46 | 2.5 | 4.6 | TP TV |
0 |
| Matei et al. [27] | 15 | 66 | 98 | 180 | 50 | 103 | 2.7 | 7 | TP TV |
0 |
| Sutherland et al. [28] | 9 | 68 | 166 | 183 | 206 | 112 | 1.3 | 13 | TP TC |
0 |
| Vora et al. [29] | 13 | 67 | NR | 179 | 219 | 163 | 2.7 | 8.8 | TP TV |
0 |
| Matei et al. [30] | 35 | 65 | 107 | 186 | 121 | 87 | 3.2 | 7.4 | TP TV |
0 |
| Coelho et al. [31] | 6 | 69 | 157 | 90 | 208 | 145 | 1 | 4.8 | TP TV |
0 |
| Clavijo et al. [32] | 10 | 71 | 81 | 106 | 375 | 81 | 1 | 9 | TP TV |
1 |
| Banapour et al. [33] | 16 | 68 | 142 | 228 | 197 | 94 | 1.3 | 8 | TP TC or TV |
0 |
| Leslie et al. [34] | 25 | 73 | 149 | 214 | 143 | NR | 4 | 9 | TP TV |
1 |
| Pokorny et al. [35] | 67 | 69 | 129 | 97 | 200 | 84 | 4 | 3 | TP TV |
1 |
| Autorino et al. [36] | 487 | 67 | 110 | 145 | 200 | 75 | 2 | 7 | TP TC or TV |
5 |
| Patel et al. [37] | 20 | 70.8 | NR | NR | NR | 134 | NR | 3–5 | TP TV |
0 |
| Garzon et al. [38] | 79 | 69 | 80 | 152 | 390 | 68 | NR | 9 | TP TC |
5 |
| Umari et al. [39] | 81 | 69 | 130 | 105 | NR | 89 | 2 | 2 | TP TV |
1 |
| Stolzenburg et al. [40] | 10 | 63 | 143 | 122 | 228 | 102 | 8 | 7 | EP TV |
0 |
| Sorokin et al. [41] | 63 | 69 | 136 | 160 | 327 | 81 | 1.5 | 5.7 | TP TV |
2 |
TP, transperitoneal; EP, extraperitoneal; TV, transvesical; TC, transcapsular; NR, not reported.
Several surgical techniques for RASP are described. Patients are initially positioned as for RALP and in all but two studies a transperitoneal approach is described. Authors perform adenoma excision either through an anterior transcapsular incision (as for a Millen’s OSP), an anterior horizontal cystotomy in proximity to the prostate–vesical junction (as for a Freyer’s OSP), or through a vertical incision at the dome of the bladder. For the two former approaches the space of Retzius is initially developed, whereas for the latter the bladder remains attached to the abdominal wall. For both transvesical approaches, once the bladder neck is exposed, an incision over the mucosa overlying the adenoma is made, and the circumferential plane between the adenoma and the capsule is developed towards the apex, at which point the adenoma is divided from the urethra.
Despite an abundance of RASP cases being described, the literature is limited by the lack of any randomised trial evaluating RASP. In comparison to the contemporary literature on OSP [42], a systematic review of case series evaluating RASP tend to report a longer operative time but benefits include reduced blood loss, rate of transfusion, LOS, need for irrigation, catheterisation time, and incision size (Table 2) [20], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. Overall complication rates (Clavien–Dindo Grade >II) appear to be similar for both OSP and RASP, as are functional outcomes [20], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]. All studies report on significant improvement in IPSS and uroflowmetry parameters after RASP, similar to that obtained for OSP. These findings were also confirmed by the one non-randomised comparative study of RASP vs OSP [41].
Further non-randomised comparative studies between laparoscopic SP (LSP) and holmium laser enucleation of the prostate (HoLEP) have been performed. In a single comparative study, outcomes following RASP and LSP were similar but one could conclude that the qualities and widespread availability of robotic surgical systems are likely to favour RASP if minimally-invasive SP is to become widely adopted [36], [38]. A recent retrospective non-randomised study evaluated RASP vs HoLEP and reported that operative times, blood loss, functional outcomes, and complications rates were similar in both groups, despite patients undergoing RASP having greater co-morbidity and a higher IPSS [39]. However, the LOS and catheterisation time were less in HoLEP, due to the avoidance of a cystotomy incision.
Cost-effective analyses of RASP have shown a favourable economic profile against OSP due to reduced LOS, irrigation, and transfusion requirements [30]. Additionally, the cost of RASP has been shown to be equivalent to bipolar-TURP, but this was based on bipolar-TURP having a longer hospitalisation period than RASP [30].
Further limitations of the existing literature are present. Feasibility of RASP on very large prostate glands has only been truly demonstrated in select studies, as only three (18 patients) have evaluated mean preoperative prostate volumes of >150 mL [24], [28], [31]. The majority of studies had mean prostate volumes of <150 mL and five studies were in glands of <100 mL [24], [26], [27], [32], [38]. Studies evaluating RASP in smaller prostate glands are of questionable value given the minimal morbidity and good functional outcomes of transurethral surgery in this cohort.
RASP may achieve better adenoma clearance than standard endoscopic procedures potentially having superior durable effects. Furthermore, in established robotic surgical units were laser technology for benign prostatic obstruction are not available, RASP may provide an excellent treatment alternative for larger glands. However, further randomised studies with long-term follow-up and cost-efficiency assessment are required to determine if RASP has a role in routine clinical practice.
Salvage RALP (sRALP)
Although many consider radiotherapy after surgery for prostate cancer to be a routinely offered second-line treatment modality in appropriate patients, the converse cannot be said. Salvage RRP (sRRP) for radio-recurrent prostate cancer, in addition to having high biochemical relapse rates, has traditionally been associated with significant morbidity. In a systematic review by Chade et al. [43], rectal injury was reported in up to 19% of patients, urinary incontinence (UI) in up to 80%, and stricture disease in as many as 40%. Therefore, the view that open surgery carried a low chance of cure with significant associated morbidity has deterred many clinicians from offering sRRP routinely.
The first report of a sRALP was published in 2008 [44], and subsequently nine further case series have followed reporting on a total of 197 patients (Table 3) [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]. As for sRRP, oncological outcomes after sRALP are worse than in men undergoing primary RALP, this being indicative of the increased risk of micrometastatic disease in the men with radio-recurrent prostate cancer [54]. In stage-matched cohorts, although positive surgical margin rates are equivalent between primary and sRALP, relapse rates are higher after sRALP despite shorter reported follow-up periods [54]. A recent systematic review showed that with a mean of 18 months follow-up, 56/193 of the reported cases of sRALP had biochemical recurrence [54].
Table 3.
Clinico-demographic data and surgical outcomes from case series/studies evaluating sRALP.
| Reference |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Jamal et al. [44] | Kaouk et al. [45] | Boris et al. [46] | Strope et al. [47] | Eandi et al. [48] | Zugor et al. [49] | Yuh et al. [50] | Kaffenberger et al. [51] | Bates et al. [52] | Vora et al. [53] |
| Number of patients | 1 | 4 | 11 | 6 | 18 | 13 | 51 | 34 | 53 | 6 |
| Age, years, mean/median | 50 | – | 65 | – | 67 | 63 | 68 | 61 | 65 | 65 |
| Blood loss, mL, mean/median | 100 | 117 | 113 | 280 | 150 | 130 | 175 | 192 | 120 | – |
| Operative time, min, mean/median | 150 | 125 | 183 | 356 | 156 | 154 | 179 | 176 | 130 | – |
| Duration of catheterisation, days, mean/median | 14 | 15 | 10 | – | 14 | 6 | – | – | 11 | – |
| LOS, days, mean/median | 1 | 3 | 1 | 2 | 2 | 6 | 2 | 1 | 2 | – |
| Clavien–Dindo Grade > II complications, n | 0 | 0 | 1 | 1 | 3 | 0 | 8 | 3 | 0 | 0 |
| Rectal injury, n | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Follow-up, months, mean/median | 3 | 5 | 21 | 12 | 18 | 23 | 36 | 16 | 27 | 7 |
| Time to salvage treatment, months, mean/median | NR | NR | NR | NR | NR | 48 | 68 | 48 | NR | NR |
| PSM, n | 0 | 2 | 3 | 1 | 5 | 0 | 16 | 9 | 10 | NR |
| Incontinent, n | 0 | 1 | 3 | 6 | 12 | 3 | 28 | 21 | 12 | 1 |
| BCR, n | 0 | NR | 3 | 2 | 6 | 8 | 22 | 6 | 8 | 1 |
| Stricture, n | NR | 0 | 1 | 1 | 3 | 0 | 8 | 3 | 0 | NR |
BCR, biochemical recurrence; NR not reported.
Additionally, functional outcomes after sRALP remain worse than for primary RALP [52]. However, the morbidity after sRALP seems less and functional outcomes better than sRRP [43], [54]. Almost 40% of patients undergoing sRALP will experience significant stress UI (SUI), up to 15% will require surgery for this [54], [50]. Comparing continence rates between studies is difficult due to differing definitions used, but at first glance, these rates of UI appear to be better following sRALP in comparison to data on sRRP, where the majority of series report UI rates of >40% [43], [54]. Furthermore, the reported stricture rate of 8% after sRALP appears far less than that reported for open surgery (7–41%). Blood loss is less in sRALP than in sRRP, as is the incidence of rectal injury (0.5% sRALP vs 2–19% for sRRP) [43], [54]. Additionally, the incidence of Calvien–Dindo Grade >II complications appears to be lower for sRALP [43], [54].
Whether the seemingly reduced morbidity and complication rate after sRALP in comparison to sRRP is due to advantages of robotic surgery or technical innovations in contemporary radiation delivery, is not clear. Although UI rates remain high after sRALP, and longer term outcome data are required to establish its oncological value, the technical feasibility and safety of sRALP shown in these studies renders this treatment a viable oncological option for well-selected patients.
RAS for urolithiasis
Although endoscopic surgery has largely displaced open surgery in the management of urolithiasis, MIS has the potential to be a useful modality for treatment in selected cases. Whilst avoiding the morbidity of open surgery, MIS could be used in the management of complex stone disease not amenable to endoscopic or standard minimally-invasive management strategies. In a meta-analysis comparing laparoscopic pyelolithotomy with percutaneous nephrolithotomy (PCNL), laparoscopy had significantly lower rates of bleeding and sepsis, whilst displaying a trend towards higher stone-free rates [55]. More recently, robot-assisted pyelolithotomy (RPL) and robot-assisted nephrolithotomy (RNL) have been reported as possible management options in the treatment of renal stones.
To date seven case series have reported on 78 patients undergoing either RNL or RPL (Table 4) [56], [57], [58], [59], [60], [61], [62]. Although anatomical and stone characteristics play a crucial role in case selection, RPL appears to be the technique most widely used, conceivably as parenchymal bleeding and potential nephron loss are avoided. Therefore, renal pelvic rather than calyceal stones may be more amenable to RAS.
Table 4.
Clinico-demographic data and surgical outcomes from case series/studies evaluating RAS for urolithiasis.
| Reference |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Atug et al. [56] | Badani et al. [57] | Lee et al. [58] | Mufarrji et al. [59] | Ghani et al. [60] | King et al. [61] | Swaringen et al. [62] |
| Number of patients | 8 | 13 | 5 | 13 | 4 | 7 | 28 |
| Technique | RPL | RPL | RPL | RPL | RPL/RNL | RNL | RPL/RNL |
| Stone size, cm, mean/median | 10.8 | 4.2 | 3.8 | NR | NR | NR | 2.74 |
| Operative time, min, mean/median | 275 | 158 | 315 | 235 | 216 | 222 | 182 |
| Blood loss, mL, mean/median | 48 | 100 | 19 | 60 | 37 | 121 | 38 |
| LOS, days, mean/median | 1 | NR | 3.8 | 2 | 2 | 3 | 1.7 |
| Clavien–Dindo Grade ≥ III complications, n | 0 | 0 | NR | 0 | 0 | 0 | 2 |
| Stone-free after surgery, n/N (%) | 8/8 | 12/13 | 3/5 | 13/13 | 4/4 | 2/7 | 27/28 (96) |
| Follow-up, months, mean/median | 13 | 1 | 18 | 28 | NR | 5 | 9 |
NR, not reported.
RPL and RNL are performed using either transperitoneal or retroperitoneal approaches. Surgical positioning and initial renal mobilisation are similar to RAPN. For RPL, once the stone within the collecting system has been identified, a vertical pyelotomy is made in the pelvis, exposing the stone, which is removed with ProGrasp forceps. The pyelotomy is closed using a continuous absorbable suture. The technique described for RNL replicates that for open surgery. After identification of the stone with intraoperative ultrasonography, the renal parenchyma is cleared from renal fat. A nephrotomy is made, if possible, where the parenchyma is thinnest, often without the need for renal ischaemia. En bloc stone removal is performed, and the incised parenchyma is closed using sliding clip renorrhaphy technique as for RAPN.
Characteristics of these case series evaluating RPL and RNL are shown in Table 4. The mean stone size treated varies significantly between studies, from 2.7 to 10.8 cm [56], [57], [58], [59], [60], [61], [62]. All studies report low blood loss, a short LOS (usually ≤3 days), and only three Clavien–Dindo Grade ≥III complications have been reported in 78 patients. The majority of studies report high stone-free rates, largely with the avoidance of stone fragmentation. However, no comparative studies evaluating robot-assisted stone surgery to standard contemporary treatments exist [56], [57], [58], [59], [60], [61], [62]. A key advantage of RPL or RNL over PCNL is complete stone removal avoiding longer-term morbidity and the need for further surgical treatment.
Interestingly, the perception that RPL/RNL is more expensive than standard stone treatments may be erroneous. Studies in the USA have reported that a PCNL costs $19 845. Data on the expense of neither RPL nor RNL have been presented, but if using RAPN ($11 962 per case) as a surrogate for the cost of RPL/RNL, the anticipated cost of robotic-assisted stone surgery may justify its future routine use [63], [64].
The feasibility and outcomes of robot-assisted laparoscopic ureterolithotomy for large, impacted, lower ureteric stones have also been explored. Dogra et al. [65] presented data from a series of 16 cases undergoing robot-assisted laparoscopic ureterolithotomy for stones not amenable to endoscopic management. The mean console time was only 20 min and stone clearance rates were 100%, due to en bloc removal. LOS, interval to drain removal, and complication rates, were significantly shorter than for comparative laparoscopic series. At 20 months follow-up no ureteric strictures were evident [65].
Although many authors acknowledge that endourology remains the standard of care, with falling anticipated costs and rising experience, RAS has the potential to displace procedures such as PCNL in selected cases. However, prospective RCTs evaluating its short- and long-term safety and efficacy against standard treatment modalities are required prior to implementation.
Robot-assisted distal ureteric reconstruction
The surgical management of both benign and malignant ureteric pathology often requires simultaneous reconstruction to restore normal renal drainage. Increasingly, authors are demonstrating that this is becoming technically feasible with RAS.
On review of the literature we identified 17 studies (Table 5 [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82]) evaluating distal ureteric reconstructive surgery, with institutions presenting their experience using a number of differing techniques including uretero-ureterostomy and uretero-neocystostomy (often with adjunct procedures such as Boari flap formation or Psoas hitch).
Table 5.
Clinico-demographic data and surgical outcomes from case series/studies evaluating robot-assisted distal ureteric reconstruction.
| Reference | Number of patients | Age, years, mean/median | Aetiology, n | Procedure, n | Operative time, min, mean/median | Blood loss, mL, mean/median | LOS, days, mean/median | Follow-up, months, mean/median | Clavian–Dindo Grade ≥ III complication, n | Type of study | Stenosis during follow-up, n |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Williams et al. [66] | 9 | 44 | Benign 9 | URI 9 | 247 | 110 | 2 | 18 | 1 | Case series | 1 |
| Schimpf et al. [67] | 11 | 66 | Benign 3 Cancer 8 |
PH 3 BF 2 URI 6 |
189 | 82 | 2.4 | 12 | 1 | Case series | NR |
| Glinianski et al. [68] | 9 | 78 | Cancer 9 | PH 6 URI 1 UU 1 |
252 | 44 | 1.5 | 23 | 0 | Case series | 1 |
| Hemal et al. [69] | 30 | NR | Benign 24 Cancer 6 |
UU 12 PH 5 URI 13 |
137 | 98 | 2.4 | 13.5 | 1 | Case series | 0 |
| Patil et al. [70] | 12 | 43 | Benign 12 | PH 12 | 208 | 48 | 4.3 | 15.5 | 0 | Case series | 2 |
| McClain et al. [71] | 6 | 68 | Cancer 6 | UU 2 PH 2 URI 2 |
268 | 72.5 | 1.8 | 33 | 0 | Case series | 0 |
| Kozinn et al. [72] | 10 | 53 | Benign 10 | URI 4 PH 4 BF 2 |
306 | 30 | 2.4 | 30 | NR | Comparative non-RCT vs open surgery | 0 |
| Baldie et al. [73] | 16 | 44 | Benign 16 | PH 8 URI 4 UU 3 BF 1 |
258 | 171 | 2.5 | 4.4 | 1 | Comparative non-RCT vs laparoscopic surgery | 0 |
| Isac et al. [74] | 25 | 49 | Benign 25 | URI 11 PH 4 BF 10 |
279 | 100 | 7.5 | NR | 2 | Comparative non-RCT vs open surgery | 2 |
| Musch et al. [75] | 16 | 63 | Benign 12 Cancer 4 |
URI 7 PH 4 BF 5 |
260 | NR | 2.4 | 10 | 2 | Case series | 1 |
| Gellhaus et al. [76] | 37 | 52 | Benign 37 | UU 15 PH 22 |
219 | 89 | 2.3 | 14 | 2 | Case series | 2 |
| Fifer et al. [77] | 55 | 52 | Benign 45 Cancer 10 |
UU 5 URI/PH 45 BF 9 UL 5 Other 3 |
233 | 50 | 1.6 | 6 | 2 | Case series | 3 |
| Slater et al. [78] | 14 | 39 | Benign 9 Congen. 4 |
UU 1 URI 10 BF 3 |
286 | 40 | 2.3 | 20.7 | 0 | Case series | 0 |
| Wason et al. [79] | 13 | 46 | Benign 9 | URI 8 PH 6 |
282 | 123 | 2.5 | 9.8 | 2 | Case series | 0 |
| Stolzenberg et al. [80] | 11 | 39 | Benign 6 Cancer 5 |
BF 11 | 166 | 155 | NR | 15.2 | 0 | Case series | 0 |
| Franklin et al. [81] | 9 | 44 | Benign 9 | PH 4 BF 1 URI 4 |
295 | 77 | 2.7 | 8.3 | 0 | Case series | 1 |
| Schiavina et al. [82] | 26 | 44 | Benign 26 | UU 7 URI12 PH 7 |
185 | 47 | 7 | 26 | 1 | Comparative non-RCT vs laparoscopic surgery | 1 |
BF, uretero-neocystotomy with Boari flap reconstruction; Congen., congenital; NR, not reported; PH, uretero-neocystotomy with Psoas hitch repair; UL, ureterolysis; URI, direct uretero-neosytotomy / ureteric re-implantation; UU, ureteroureterostomy.
Standardising data from these studies is challenging due to the heterogeneity of the type of pathology, location and length of the diseased ureteric segment, patient comorbidity, preoperative evaluation, and surgical procedure described.
Most authors describe use of a dorsal lithotomy position (particularly for distal lesions). Adhesiolysis and colonic mobilisation are performed to allow longitudinal mobilisation of the ureter. RAS techniques follow the same principles of ureteric reconstruction as for open surgery; mobilisation of the ureter to healthy tissue avoiding direct ureteric handling and devascularisation, spatulation of the ureter, a water-tight tension-free anastomosis with absorbable sutures over a ureteric stent, placement of a bladder catheter and tube drain overlying the anastomosis, and if feasible an omental wrap is performed.
Comparisons with laparoscopic and open ureteric reconstruction have been made. Baldie et al. [73] and Schiavina et al. [82], both performed non-randomised comparisons of pure laparoscopic against robot-assisted ureteric reconstruction. Using similar reconstructive techniques, short- and long-term complications were similar between both groups, although RAS was associated with a lower operative time, blood loss, and LOS [73], [82]. In similar retrospective non-randomised comparisons of RAS vs open surgery, RAS was associated with less blood loss and LOS, whilst operative durations, and short- and long-term complication rates were similar in both groups [72], [74]. Additionally stricture rates after robot-assisted ureteric re-implantation appear low. Therefore, the current evidence shows that robot-assisted ureteric excision and reconstruction can be safely performed.
Robot-assisted retroperitoneal lymph node dissection (R-RPLND)
Laparoscopic RPLND (L-RPLND) is associated with improved cosmesis, reduced complication rate, lower morbidity, and shorter LOS compared to open RPLND (O-RPLND) [83]. However, L-RPLND has a steep learning curve and may have inferior oncological outcomes to O-RPLND due to the associated difficulties of retroaortic and retrocaval dissection, often necessitating patients to have routine adjuvant chemotherapy [84].
R-RPLND was first described in 2006 [85], and subsequent to this there have been a further eight case series of R-RPLND. Two of these case series were in less than three patients. Data summarised from the remaining six larger case series are presented in Table 6 [86], [87], [88], [89], [90], [91].
Table 6.
Clinico-demographic data and surgical outcomes from case series/studies evaluating R-RPLND.
| Reference |
||||||
|---|---|---|---|---|---|---|
| Variable | Cheney et al. [86] | Harris et al. [87] | Kamel et al. [88] | Pearce et al. [89] | Stepanien et al. [90] | Singh et al. [91] |
| Number of patients | 18 | 16 | 12 | 47 | 20 | 13 |
| Article type | Case series | Non-randomised comparative study vs L-RPLND | Case series | Case series | Case series | Case series |
| Age, years, mean/median | 32 | 29.8 (median) | 37.8 | 30 | 31 | 26 |
| TNM stage, n | ||||||
| I | 10 | 14 | 0 | 42 | 11 | 0 |
| II | 8 | 2 | 5 | 5 | 6 | 3 |
| III | 0 | 0 | 6 | 0 | 3 | 10 |
| Prior chemotherapy, n | ||||||
| Yes | 8 | 0 | 12 | 0 | 4 | 13 |
| No | 10 | 16 | 0 | 47 | 16 | 0 |
| Histology, n | ||||||
| NSGCT | 18 | 16 | 9 | 47 | 20 | 13 |
| Seminoma | 0 | 0 | 3 | 0 | 0 | 0 |
| Operative time, min, mean/median | 343 | 271 | 312 | 235 | 293 | 200 |
| Blood loss, mL, mean/median | 172 | 75 | 475 | 50 | 50 | 120 |
| Clavien–Dindo Grade ≥ III complications, n | 0 | 1 | 1 | 0 | 1 | 2 |
| LOS, days, mean/median | 2.4 | – | 3.6 | 1 | 1 | 4 |
| Conversion, n | 3 | 1 | 2 | 1 | 0 | 0 |
| Node yield, mean/median | 20 | 30 | 12 | 26 | 19.5 | 20 |
| Follow up, months, mean/median | – | 13.5 | 31 | 22 | 49 | 23 |
| Retroperitoneal recurrence, n | 0 | 0 | 0 | 0 | 0 | 0 |
NSGCT, non-seminomatous germ cell tumour.
R-RPLND is most commonly performed using a transperitoneal technique, with most patients in a left lateral decubitus position [86], [87], [88], although a supine dorsal lithotomy technique with a Trendelenburg tilt is also described [90]. Unilateral templates have been preferred in chemotherapy naïve patients, whereas bilateral templates have been described in surgery for post-chemotherapy residual masses. Boundaries of dissection are analogous with open surgery, with sparing of the hypogastric plexus similarly feasible.
The data show acceptable operative durations and all studies report a mean blood loss of <500 mL with most studies describing a LOS of ≤3 days (Table 6). Of the 126 cases described, there were nine open conversions and only five Clavien–Dindo Grade ≥III complications. The node yield following R-RPLND appears equivalent to open surgery, and oncological outcomes, although limited by the immature follow-up periods, appear acceptable [92]. Morbidity appears to be less in comparison to O-RPLND, with a lower reported incidence of postoperative ileus, respiratory and wound complications, and anejaculation [92]. The one study performing a comparative analysis of R-RPLND vs L-RPLND, showed no clear benefit in favour of R-RPLND [87]. Whereas three-dimensional vision, improved dexterity, reduced hand tremor and surgeon fatigue would potentially favour R-RPLND over L-RPLND, the lack of tactile feedback and the time consuming de-docking process should open conversion be required due to vascular injury make L-RPLND a more attractive option.
One limitation of the existing literature is the bias in studies including mainly chemotherapy naïve Stage I patients. In the largest series of 47 patients (all chemotherapy naïve), Pearce et al. [89] performed R-RPLND in 42 patients with clinical Stage I disease. Although R-RPLND in post-chemotherapy patients has been described, this is only in 37 of the 126 reported cases [86], [87], [88], [89], [90], [91]. The preference to perform R-RPLND in chemo-naïve clinical Stage I patients is not consistent with contemporary practice [93]. The majority of Stage I patients managed conservatively will not relapse, chemotherapy in this stage of the disease has improved disease-free survival in comparison to surgery, and contemporary practice would suggest that most O-RPLND are performed in post-chemotherapy patients with clinical Stage II disease [93], [94]. In those studies that do compare outcomes in pre- and post-chemotherapy, post-chemotherapy surgery appears to be associated with an increased operative duration, higher conversion rates, and increased blood loss [86], [90]. Proponents argue that if R-RPLND can establish durable long-term response in Stage I patients, it may offer an equitable compromise between the side-effects of chemotherapy, morbidity of an O-RPLND, and the recurrence rates of observation, but this opinion deviates from recommendations by international authorities [93] and therefore future studies need to focus on selecting post-chemotherapy patients.
Robot-assisted augmentation ileocystoplasty (RAI)
Augmentation enterocystoplasty is typically reserved for treatment-refractory cases of detrusor overactivity and neuropathic bladder dysfunction. The surgical complexity of the procedure carries significant surgical morbidity, particularly in the neuropathic population in whom it is frequently performed. The increasing use of RARC with ICUD has reinforced the feasibility of performing intracorporeal bowel division and re-anastomosis with associated reduced morbidity and bowel-related complications [10], and has opened the door for other such procedures such as augmentation enterocystoplasty to be performed by RAS.
Following the first case report of RAI by Gundeti et al. [95] in 2008, 64 cases have subsequently been reported in the literature in 13 publications. Seven of these were single case reports and of the remaining six articles, five were published from the same group encompassing similar time periods [96], [97], [98], [99], [100], [101].
The solitary adult case series evaluating RAI reported by Madec et al. [96] comprised 19 patients undergoing supra-trigonal RC followed by RAI. In this series, indications for surgery included treatment‐refractory detrusor overactivity, low bladder compliance, and painful bladder syndrome. Although robot-assisted intracorporeal supra-trigonal RC was performed in all cases, bowel segment isolation, anastomosis and de-tubularisation were all performed extracorporeally, with a subsequent robot-assisted intracorporeal ileal graft anastomosis performed onto the bladder in the sagittal plane. There were no Clavien–Dindo Grade >II complications and the authors report on a rapid learning curve, even for surgeons less experienced in robotic surgery, and long‐term functional results were quoted as excellent. However, a limitation of this study was the need for extracorporeal bowel division and re-anastomosis.
Murthy et al. [97] reported outcomes from a cohort of 15 paediatric patients undergoing a RAI, performing a non-randomised retrospective comparison against open augmentation enterocystoplasty. Indications for surgery included neuropathic bladder dysfunction (poor compliance, detrusor overactivity refractory to medical treatment, high detrusor leak point pressure) or bladder dysfunction secondary to posterior urethral valves. Most cases also had a concomitant robotic procedure (Mitrofanoff appendicovesicostomy, antegrade colonic enema channel, bladder neck closure). The RAI technique involved intracorporeal isolation bowel division and end-to-end anastomosis, and then bi-valving the bladder with a coronal cystotomy and a subsequent ileal graft continuous anastomosis (all performed intracorporeally). Although the authors report a shorter median LOS with RAI (6 vs 8 days; P = 0.01), there were no differences in median blood loss, opiate requirement, and return to full diet, although epidural usage was less in the robotic cohort [97]. Functional outcomes and complications were similar in both groups. The operative duration for RAI in this study was 623 min (vs 265 min for open) indicating this procedure remains in its infancy [97].
Therefore, based on the limited data available, although being a safe and technically feasible procedure the benefits of RAI over open surgery remain to be confirmed. Further studies expanding on the case numbers currently described are required before transition into routine clinical practice occurs.
Robot-assisted artificial urinary sphincter insertion (AUS)
The most common indication of AUS insertion in contemporary practice is for post-prostatectomy UI, where the AUS cuff is placed around the bulbar urethra. However, bladder neck cuff placement is the preferred site in men with neurogenic SUI (in order to minimise device erosion) and women with SUI with prior unsuccessful conservative and surgical treatments. Traditionally bladder neck AUS insertion has been performed via an open incision, but recent studies have shown that a RAS approach is feasible.
Six publications were identified reporting on a RAS approach for AUS implantation. Two case series in men have been reported [102], [106], a further three publications were case series in women with SUI (103–105), and one paediatric case report, have been published.
Yates et al. [102] reported on six men with neurogenic (spinal cord injury) sphincter incompetence causing refractory SUI. Patients underwent bladder neck AUS (AMS 800, American Medical Systems) placement using a robot-assisted transperitoneal approach. Posterior bladder neck dissection was initially performed via a posterior peritoneal incision overlying the seminal vesicles/vas deferens. Subsequently, the space of Retzius was developed, and lateral prostate dissection was performed to identify the precise location of the bladder neck circumferentially. Tape measurement of the bladder neck preceded intracorporeal placement of the AUS at this site. Device tubing, pump and reservoir were all inserted via a small right iliac fossa incision, with the reservoir placed intra-abdominally in the lateral vesicular space, and pump placed in the scrotum, before the device was connected and pressurised. The authors report good functional outcomes, with no Clavien–Dindo Grade ≥II complications and blood loss of >150 mL in only one patient. However, in a series of four male patients with neurogenic SUI undergoing bladder neck AUS cuff placement, Hervé et al. [106] reported ongoing UI in two patients due to insufficient traction of the cuff around the bladder neck.
Three studies have reported on 25 female patients with intrinsic sphincter deficiency causing SUI treated with robot-assisted AUS insertion [103], [104], [105]. In these studies all patients had multiple failed previous procedures for SUI. All authors used a similar surgical approach. The space of Retzius was developed down to the bladder neck, endopelvic fascia incised bilaterally, and the bladder neck dissected from the vagina below the periurethral fascia. Subsequently, tape measurement and subsequent bladder neck cuff placement was performed. The reservoir was placed in the pre-vesical or lateral para-vesical space and the pump placed in the labia majora. All authors describe use of a planned cystotomy in selected patients to aid dissection around the bladder neck in an attempt to avoid bladder or vaginal injury.
In one study a non-randomised comparison against open AUS insertion was performed demonstrating a reduced operative duration, blood loss, LOS, and overall complication rate with RAS, but with no significant difference in functional outcomes in comparison to open surgery [105].
However, complication rates following robot-assisted AUS insertion appear high. Peyronnet et al. [105] reported that two of eight patients in the RAS group had intraoperative vaginal injuries, although this was equivalent to a comparative cohort undergoing open surgery. However, the incidence of bladder injury was lower in the RAS group [one of eight patients vs seven of 16 (44%)]. Biardeau et al. [104] also reported a high incidence (four of 11 patients) of visceral injury (two vaginal and two bladder) with robot-assisted AUS insertion. Although the authors’ state patient factors heavily contributed to visceral injuries, with such high intraoperative complications [104], [105], clearly other prospective studies comparing RAS vs open/laparoscopic insertion are required to assess its safety and benefit over open surgery.
Conclusions
The role of RAS continues to develop within the field of urological surgery. Here we present the growing evidence demonstrating that the breadth and complexity of surgical procedures performed using the da Vinci platform is continually expanding. There is a gaining consensus that RAS is producing promising surgical results in wide range of procedures and with accumulating expertise will continue to do so. However, a major limitation of the current literature evaluating urological RAS is the sparsity of comparative trials, in particular those randomising patients to RAS and conventional approaches. This is not only for emerging RAS techniques, but also for more established oncological RAS procedures, which are now routinely used.
The foremost controversy concerning the use of RAS lies in the associated capital and running costs of this technology. Therefore it is essential that future studies should not only assess the potential clinical advantages of RAS, but also the cost-effectiveness.
Conflict of interest
None.
Source of funding
None.
Setting the Scene
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Honda M., Morizane S., Hikita K., Takenaka A. Current status of robotic surgery in urology. Asian J Endosc Surg. 2017;10:372–381. doi: 10.1111/ases.12381. [DOI] [PubMed] [Google Scholar]
- 2.Iannetti A., Gnech M., Rossanese M., Abbinante M., De Giorgi G., Mottrie A. Robot-assisted renal surgery: current indications and results. Minerva Urol Nefrol. 2014;66:15–24. [PubMed] [Google Scholar]
- 3.Lowrance W.T., Eastham J.A., Savage C., Maschino A.C., Laudone V.P., Dechet C.B. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–2092. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon M., Shrivastava A., Tewari A., Sarle R., Hemal A., Peabody J.O. Laparoscopic and robot assisted radical prostatec-tomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168:945–949. doi: 10.1016/S0022-5347(05)64548-X. [DOI] [PubMed] [Google Scholar]
- 5.Yaxley J.W., Coughlin G.D., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 6.Tang K., Jiang K., Chen H., Chen Z., Xu H., Ye Z. Robotic vs. Retropubic radical prostatectomy in prostate cancer: a systematic review and an meta-analysis update. Oncotarget. 2017;8:32237–32257. doi: 10.18632/oncotarget.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsay C., Pickard R., Robertson C., Close A., Vale L., Armstrong N. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16:1–313. doi: 10.3310/hta16410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon M., Hemal A.K., Tewari A., Shrivastava A., Shoma A.M., El-Tabey N.A. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232–236. doi: 10.1046/j.1464-410x.2003.04329.x. [DOI] [PubMed] [Google Scholar]
- 9.Li K., Lin T., Fan X., Xu K., Bi L., Duan Y. Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy. Cancer Treat Rev. 2013;39:551–560. doi: 10.1016/j.ctrv.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed K., Khan S.A., Hayn M.H., Agarwal P.K., Badani K.K., Balbay M.D. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2014;65:340–347. doi: 10.1016/j.eururo.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Koupparis A., Villeda-Sandoval C., Weale N., El-Mahdy M., Gillatt D., Rowe E. Robot-assisted radical cystectomy with intracorporeal urinary diversion: impact on an established enhanced recovery protocol. BJU Int. 2015;116:924–931. doi: 10.1111/bju.13171. [DOI] [PubMed] [Google Scholar]
- 12.Lee R., Chughtai B., Herman M., Shariat S.F., Scherr D.S. Cost-analysis comparison of robot-assisted laparoscopic radical cystectomy (RC) vs open RC. BJU Int. 2011;108:976–983. doi: 10.1111/j.1464-410X.2011.10468.x. [DOI] [PubMed] [Google Scholar]
- 13.Bansal S.S., Dogra T., Smith P.W., Amran M., Auluck I., Bhambra M. Cost analysis of open radical cystectomy versus robot-assisted radical cystectomy. BJU Int. 2018;121:437–444. doi: 10.1111/bju.14044. [DOI] [PubMed] [Google Scholar]
- 14.Ghani K.R., Sukumar S., Sammon J.D., Rogers C.G., Trinh Q.D., Menon M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the Nationwide inpatient sample. J Urol. 2014;191:907–912. doi: 10.1016/j.juro.2013.10.099. [DOI] [PubMed] [Google Scholar]
- 15.Aboumarzouk O.M., Stein R.J., Eyraud R., Haber G.P., Chlosta P.L., Somani B.K. Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2012;62:1023–1033. doi: 10.1016/j.eururo.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Eyraud R., Long J.A., Snow-Lisy D., Autorino R., Hillyer S., Klink J. Robot-assisted partial nephrectomy for hilar tumors: perioperative outcomes. Urology. 2013;81:1246–1252. doi: 10.1016/j.urology.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 17.Shuch B., Singer E.A., Bratslavsky G. The surgical approach to multifocal renal cancers: hereditary syndromes, ipsilateral multifocality, and bilateral tumors. Urol Clin North Am. 2012;39:133–148. doi: 10.1016/j.ucl.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Autorino R., Eden C., El-Ghoneimi A., Guazzoni G., Buffi N., Peters C.A. Robot-assisted and laparoscopic repair of ureteropelvic junction obstruction: a systematic review and meta-analysis. Eur Urol. 2014;65:430–452. doi: 10.1016/j.eururo.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravas S, Bach T, Drake M, Gacci M, Gratzke C, Herrmann TR et al. EAU Guidelines Managment of Non-neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). European Association of Urology, 2017. Available at: https://uroweb.org/wp-content/uploads/13-Non-Neurogenic-Male-LUTS_2017_web.pdf. Accessed June 2018.
- 21.Humphreys M.R., Miller N.L., Handa S.E., Terry C. Holium laser enucleation of the prostate—outcomes independent of prostate size? J Urol. 2008;180:2431–2435. doi: 10.1016/j.juro.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Asimakopoulos A.D., Mugnier C., Hoepffner J.L., Spera E., Vespasiani G., Gaston R. The surgical treatment of a large prostatic adenoma: the laparoscopic approach—a systematic review. J Endourol. 2012;26:960–967. doi: 10.1089/end.2012.0055. [DOI] [PubMed] [Google Scholar]
- 23.Sotelo R., Clavijo R., Carmona O., Garcia A., Banda E., Miranda M. Robotic simple prostatectomy. J Urol. 2008;179:513–515. doi: 10.1016/j.juro.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 24.Yuh B., Laungani R., Perlmutter A., Eun D., Peabody J.O., Mohler J.L. Robot-assisted Millin’s retropubic prostatectomy: case series. Can J Urol. 2008;15:4101–4105. [PubMed] [Google Scholar]
- 25.John H., Bucher C., Engel N., Fischer B., Fehr J.L. Preperitoneal robotic prostate adenomectomy. Urology. 2009;73:811–815. doi: 10.1016/j.urology.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Uffort E.E., Jensen J.C. Robotic-assisted laparoscopic simple prostatectomy: an alternative minimal invasive approach for prostate adenoma. J Robot Surg. 2010;4:7–10. doi: 10.1007/s11701-010-0180-4. [DOI] [PubMed] [Google Scholar]
- 27.Matei D.V., Spinelli M.G., Nordio A., Brescia A., Crisan N., Coman I. Robotic simple prostatectomy. Eur Urol Suppl. 2008;9:337. [Google Scholar]
- 28.Sutherland D.E., Perez D.S., Weeks D.C. Robot-assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol. 2011;25:641–644. doi: 10.1089/end.2010.0528. [DOI] [PubMed] [Google Scholar]
- 29.Vora A., Mittal S., Hwang J., Bandi G. Robot-assisted simple prostatectomy: multi-institutional outcomes for glands larger than 100 grams. J Endourol. 2012;26:499–502. doi: 10.1089/end.2011.0562. [DOI] [PubMed] [Google Scholar]
- 30.Matei D.V., Brescia A., Mazzoleni F., Spinelli M., Musi G., Melegari S. Robot-assisted simple prostatectomy (RASP): does it make sense? BJU Int. 2012;110:972–979. doi: 10.1111/j.1464-410X.2012.11192.x. [DOI] [PubMed] [Google Scholar]
- 31.Coelho R.F., Chauhan S., Sivaraman A. Modified technique of robotic-assisted simple prostatectomy: advantages of a vesico-urethral anastomosis. BJU Int. 2012;109:426–433. doi: 10.1111/j.1464-410X.2011.010401.x. [DOI] [PubMed] [Google Scholar]
- 32.Clavijo R., Carmona O., Palmer K.J., Orvieto M.A., Rocco B. Robot-assisted simple prostatectomy: novel technique. J Endourol. 2013;27:328–332. doi: 10.1089/end.2012.0212. [DOI] [PubMed] [Google Scholar]
- 33.Banapour P., Patel N., Kane C.J., Cohen S.A., Parsons J.K. Robotic-assisted simple prostatectomy: a systematic review and report of a single institution case series. Prostate Cancer Prostatic Dis. 2014;17:1–5. doi: 10.1038/pcan.2013.52. [DOI] [PubMed] [Google Scholar]
- 34.Leslie S., de Castro Abreu A.L., Chopra S., Ramos P., Park D., Berger A.K. Transvesical robotic simple prostatectomy: initial clinical experience. Eur Urol. 2014;66:321–329. doi: 10.1016/j.eururo.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Pokorny M., Novara G., Geurts N., Dovey Z., De Groote R., Ploumidis A. Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic centre. Euro Urol. 2015;68:451–457. doi: 10.1016/j.eururo.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Autorino R., Zargar H., Mariano M.B., Sanchez-Salas R., Sotelo R.J., Chlosta P.L. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European-American multi-institutional analysis. Eur Urol. 2015;68:86–94. doi: 10.1016/j.eururo.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 37.Patel N.D., Parsons J.K. Robotic-assisted simple prostatectomy: is there evidence to go beyond the experimental stage? Curr Urol Rep. 2014;15:443. doi: 10.1007/s11934-014-0443-0. [DOI] [PubMed] [Google Scholar]
- 38.Martín Garzón O.D., Azhar R.A., Brunacci L., Ramirez-Troche N.E., Medina Navarro L., Hernández L.C. One-year outcome comparison of laparoscopic, robotic, and robotic intrafascial simple prostatectomy for benign prostatic hyperplasia. J Endourol. 2016;30:312–318. doi: 10.1089/end.2015.0218. [DOI] [PubMed] [Google Scholar]
- 39.Umari P., Fossati N., Gandaglia G., Pokorny M., De Groote R., Geurts N. Robotic assisted simple prostatectomy (RASP) versus holmium laser enucleation of the prostate (HoLEP) for lower urinary tract symptoms in patients with large volume prostates (>100ml): a comparative analysis from a high volume center. J Urol. 2017;197:1108–1114. doi: 10.1016/j.juro.2016.08.114. [DOI] [PubMed] [Google Scholar]
- 40.Stolzenburg J.U., Kallidonis P., Qazi H., Ho Thi P., Dietel A., Liatsikos E.N. Extraperitoneal approach for robotic-assisted simple prostatectomy. Urology. 2014;84:1099–1105. doi: 10.1016/j.urology.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 41.Sorokin I., Sundaram V., Singla N., Walker J., Margulis V., Roehrborn C. Robot-assisted versus open simple prostatectomy for benign prostatic hyperplasia in large glands: a propensity score-matched comparison of perioperative and short-term outcomes. J Endourol. 2017;31:1164–1169. doi: 10.1089/end.2017.0489. [DOI] [PubMed] [Google Scholar]
- 42.Li M., Qiu J., Hou Q., Wang D., Huang W., Hu C. Endoscopic enucleation versus open prostatectomy for treating large benign prostatic hyperplasia: a meta-analysis of randomized controlled trials. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chade D.C., Eastham J., Graefen M., Hu J.C., Karnes R.J., Klotz L. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol. 2012;61:961–971. doi: 10.1016/j.eururo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Jamal K., Challacombe B., Elhage O., Popert R., Kirby R., Dasgupta P. Successful salvage robotic-assisted radical prostatectomy after external beam radiotherapy failure. Urology. 2008;72:1356–1358. doi: 10.1016/j.urology.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Kaouk J.H., Hafron J., Goel R., Haber G.P., Jones J.S. Robotic salvage retropubic prostatectomy after radiation/brachy-therapy: initial results. BJU Int. 2008;102:93–96. doi: 10.1111/j.1464-410X.2008.07570.x. [DOI] [PubMed] [Google Scholar]
- 46.Boris R.S., Bhandari A., Krane L.S., Eun D., Kaul S., Peabody J. Salvage robotic-assisted radical prostatectomy: initial results and early report of outcomes. BJU Int. 2009;103:952–956. doi: 10.1111/j.1464-410X.2008.08245.x. [DOI] [PubMed] [Google Scholar]
- 47.Strope S.A., Coelho M., Wood D.P., Hollenbeck B.K. Robot-assisted salvage prostatectomy: evaluation of initial patient-reported outcomes. J Endourol. 2010;24:425–427. doi: 10.1089/end.2009.0143. [DOI] [PubMed] [Google Scholar]
- 48.Eandi J.A., Ba Link, Nelson R.A., Josephson D.Y., Lau C., Kawachi M.H. Robotic assisted laparoscopic sal-vage prostatectomy for radiation resistant prostate cancer. J Urol. 2010;183:133–137. doi: 10.1016/j.juro.2009.08.134. [DOI] [PubMed] [Google Scholar]
- 49.Zugor V., Labanarisa P., Porres D., Heidenreich A., Witt J.H. Robot-assisted radical prostatectomy for the treatment of radiation-resistant prostate cancer: surgical, oncological and short-term functional outcomes. Urol Int. 2014;92:20–26. doi: 10.1159/000351948. [DOI] [PubMed] [Google Scholar]
- 50.Yuh B., Ruel N., Muldrew S., Mejia R., Novara G., Kawachi M. Complications and outcomes of salvage robot-assisted radical prostatectomy: a single-institution experience. BJU Int. 2014;113:769–776. doi: 10.1111/bju.12595. [DOI] [PubMed] [Google Scholar]
- 51.Kaffenberger S.D., Keegan K.A., Bansal N.K., Morgan T.M., Tang D.H., Barocas D.A. Salvage robotic assisted laparoscopic radical prostatectomy: a single institution, 5-year experience. J Urol. 2013;189:507–513. doi: 10.1016/j.juro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bates A.S., Samavedi S., Kumar A., Mouraviev V., Rocco B., Coelho R. Salvage robot assisted radical prostatectomy: a propensity matched study of perioperative, oncological and functional outcomes. Eur J Surg Oncol. 2015;41:1540–1546. doi: 10.1016/j.ejso.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Vora A., Agarwal V., Singh P., Patel R., Rivas R., Nething J. Single-institution comparative study on the outcomes of salvage cryotherapy versus salvage robotic prostatectomy for radio-resistant prostate cancer. Prostate Int. 2016;4:7–10. doi: 10.1016/j.prnil.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zargar H., Lamb A.D., Rocco B., Porpiglia F., Liatsikos E., Davis J. Salvage robotic prostatectomy for radio recurrent prostate cancer: technical challenges and outcome analysis. Minerva Urol Nefrol. 2017;69:26–37. doi: 10.23736/S0393-2249.16.02797-1. [DOI] [PubMed] [Google Scholar]
- 55.Wang X., Li S., Liu T., Guo Y., Yang Z. Laparoscopic pyelolithotomy compared to percutaneous nephrolithotomy as surgical management for large renal pelvic calculi: a meta-analysis. J Urol. 2013;190:888–893. doi: 10.1016/j.juro.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 56.Atug F., Castle E.P., Burgess S.V., Thomas R. Concomitant management of renal calculi and pelvi-ureteric junction obstruction with robotic laparoscopic surgery. BJU Int. 2005;96:1365–1368. doi: 10.1111/j.1464-410X.2005.05819.x. [DOI] [PubMed] [Google Scholar]
- 57.Badani K.K., Hemal A.K., Fumo M., Kaul S., Shrivastava A., Rajendram A.K. Robotic extended pyelolithotomy for treatment of renal calculi: a feasibility study. World J Urol. 2006;24:198–201. doi: 10.1007/s00345-006-0099-6. [DOI] [PubMed] [Google Scholar]
- 58.Lee R.S., Passerotti C.C., Cendron M., Estrada C.R., Borer J.G., Peters C.A. Early results of robot assisted laparoscopic lithotomy in adolescents. J Urol. 2007;177:2306–2309. doi: 10.1016/j.juro.2007.01.178. [DOI] [PubMed] [Google Scholar]
- 59.Mufarrij P.W., Woods M., Shah O.D., Palese M.A., Berger A.D., Thomas R. Robotic dismembered pyeloplasty: a 6-year, multi-institutional experience. J Urol. 2008;180:1391–1396. doi: 10.1016/j.juro.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Ghani K.R., Rogers C.G., Sood A., Kumar R., Ehlert M., Jeong W. Robot-assisted anatrophic nephrolithotomy with renal hypothermia for managing staghorn calculi. J Endourol. 2013;27:1393–1398. doi: 10.1089/end.2013.0266. [DOI] [PubMed] [Google Scholar]
- 61.King S.A., Klaassen Z., Madi R. Robot-assisted anatrophic nephrolithotomy: description of technique and early results. J Endourol. 2014;28:325–329. doi: 10.1089/end.2013.0597. [DOI] [PubMed] [Google Scholar]
- 62.Swearingen R., Sood A., Madi R., Klaassen Z., Badani K., Elder J.S. Zero-fragment nephrolithotomy: a multi-center evaluation of robotic pyelolithotomy and nephrolithotomy for treating renal stones. Eur Urol. 2017;72:1014–1021. doi: 10.1016/j.eururo.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Mir S.A., Cadeddu J.A., Sleeper J.P., Lotan Y. Cost comparison of robotic, laparoscopic, and open partial nephrectomy. J Endourol. 2011;25:447–453. doi: 10.1089/end.2010.0510. [DOI] [PubMed] [Google Scholar]
- 64.Hyams E.S., Shah O. Percutaneous nephrostolithotomy versus flexible ureteroscopy/holmium laser lithotripsy: cost and outcome analysis. J Urol. 2009;182:1012–1017. doi: 10.1016/j.juro.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 65.Dogra P.N., Regmi S.K., Singh P., Saini A.K., Nayak B. Lower ureteral stones revisited: expanding the horizons of robot. Urology. 2013;82:95–99. doi: 10.1016/j.urology.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 66.Williams S.K., Leveillee R.J. Expanding the horizons: robot-assisted reconstructive surgery of the distal ureter. J Endourol. 2009;23:457–461. doi: 10.1089/end.2008.0269. [DOI] [PubMed] [Google Scholar]
- 67.Schimpf M.O., Wagner J.R. Robot-assisted laparoscopic distal ureteral surgery. JSLS. 2009;13:44–49. [PMC free article] [PubMed] [Google Scholar]
- 68.Glinianski M., Guru K.A., Zimmerman G., Mohler J., Kim H.L. Robot-assisted ureterectomy and ureteral reconstruction for urothelial carcinoma. J Endourol. 2009;23:97–100. doi: 10.1089/end.2007.0279. [DOI] [PubMed] [Google Scholar]
- 69.Hemal A.K., Nayyar R., Gupta N.P., Dorairajan L.N. Experience with robot assisted laparoscopic surgery for upper and lower benign and malignant ureteral pathologies. Urology. 2010;76:1387–1393. doi: 10.1016/j.urology.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 70.Patil N.N., Mottrie A., Sundaram B., Patel V.R. Robotic-assisted laparoscopic ureteral reimplantation with psoas hitch: a multi-institutional, multinational evaluation. Urology. 2008;72:47–50. doi: 10.1016/j.urology.2007.12.097. [DOI] [PubMed] [Google Scholar]
- 71.McClain P.D., Mufarrij P.W., Hemal A.K. Robot-assisted reconstructive surgery for ureteral malignancy: analysis of efficacy and oncologic outcomes. J Endourol. 2012;26:1614–1617. doi: 10.1089/end.2012.0219. [DOI] [PubMed] [Google Scholar]
- 72.Kozinn S.I., Canes D., Sorcini A., Moinzadeh A. Robotic versus open distal ureteral reconstruction and reimplantation for benign stricture disease. J Endourol. 2012;26:147–151. doi: 10.1089/end.2011.0234. [DOI] [PubMed] [Google Scholar]
- 73.Baldie K., Angell J., Ogan K., Hood N., Pattaras J.G. Robotic management of benign mid and distal ureteral strictures and comparison with laparoscopic approaches at a single institution. Urology. 2012;80:596–601. doi: 10.1016/j.urology.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Isac W., Kaouk J., Altunrende F., Rizkala E., Autorino R., Hillyer S.P. Robot-assisted ureteroneocystostomy: technique and comparative outcomes. J Endourol. 2013;27:318–323. doi: 10.1089/end.2012.0196. [DOI] [PubMed] [Google Scholar]
- 75.Musch M., Hohenhorst L., Pailliart A., Loewen H., Davoudi Y., Kroepfl D. Robot-assisted reconstructive surgery of the distal ureter: single institution experience in 16 patients. BJU Int. 2013;111:773–783. doi: 10.1111/j.1464-410X.2012.11673.x. [DOI] [PubMed] [Google Scholar]
- 76.Gellhaus P.T., Bhandari A., Monn M.F., Gardner T.A., Kanagarajah P., Reilly C.E. Robotic management of genitourinary injuries from obstetric and gynaecological operations: a multi-institutional report of outcomes. BJU Int. 2015;115:430–436. doi: 10.1111/bju.12785. [DOI] [PubMed] [Google Scholar]
- 77.Fifer G.L., Raynor M.C., Selph P., Woods M.E., Wallen E.M., Viprakasit D.P. Robotic ureteral reconstruction distal to the ureteropelvic junction: a large single institution clinical series with short-term follow up. J Endourol. 2014;28:1424–1428. doi: 10.1089/end.2014.0227. [DOI] [PubMed] [Google Scholar]
- 78.Slater R.C., Farber N.J., Riley J.M., Shilo Y., Ost M.C. Contemporary series of robotic-assisted distal ureteral reconstruction utilizing side docking position. Int Braz J Urol. 2015;41:1154–1159. doi: 10.1590/S1677-5538.IBJU.2014.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wason S.E., Lance R.S., Given R.W., Malcolm J.B. Robotic-assisted ureteral re-implantation: a case series. J Laparoendosc Adv Surg Tech A. 2015;25:503–507. doi: 10.1089/lap.2014.0051. [DOI] [PubMed] [Google Scholar]
- 80.Stolzenburg J.U., Rai B.P., Do M., Dietel A., Liatsikos E., Ganzer R. Robot-assisted technique for Boari flap ureteric reimplantation: replicating the techniques of open surgery in robotics. BJU Int. 2016;118:482–484. doi: 10.1111/bju.13502. [DOI] [PubMed] [Google Scholar]
- 81.Franklin A., Pokala N., Jones C., Johans C., Strom K., Cummings J. Is the robotic approach feasible for repair of iatrogenic injuries of the lower ureter? World J Urol. 2016;34:1323–1328. doi: 10.1007/s00345-016-1768-8. [DOI] [PubMed] [Google Scholar]
- 82.Schiavina R., Zaramella S., Chessa F., Pultrone C.V., Borghesi M., Minervini A. Laparoscopic and robotic ureteral stenosis repair: a multi-institutional experience with a long-term follow-up. J Robot Surg. 2016;10:323–330. doi: 10.1007/s11701-016-0601-0. [DOI] [PubMed] [Google Scholar]
- 83.Rassweiler J.J., Scheitlin W., Heidenreich A., Laguna M.P., Janetschek G. Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol. 2008;54:1004–1015. doi: 10.1016/j.eururo.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Steiner H., Leonhartsberger N., Stoehr B., Peschel R., Pichler R. Postchemotherapy laparoscopic retroperitoneal lymph node dissection for low-volume, stage II, nonseminomatous germ cell tumor: first 100 patients. Eur Urol. 2013;63:1013–1017. doi: 10.1016/j.eururo.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 85.Davol P., Sumfest J., Rukstalis D. Robotic-assisted laparoscopic retroperitoneal lymph node dissection. Urology. 2006;67(199):e7–e8. doi: 10.1016/j.urology.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 86.Cheney S.M., Andrews P.E., Leibovich B.C., Castle E.P. Robot-assisted retroperitoneal lymph node dissection: technique and initial case series of 18 patients. BJU Int. 2015;115:114–120. doi: 10.1111/bju.12804. [DOI] [PubMed] [Google Scholar]
- 87.Harris K.T., Gorin M.A., Ball M.W., Pierorazio P.M., Allaf M.E. A comparative analysis of robotic vs laparoscopic retroperitoneal lymph node dissection for testicular cancer. BJU Int. 2015;116:920–923. doi: 10.1111/bju.13121. [DOI] [PubMed] [Google Scholar]
- 88.Kamel M.H., Littlejohn N., Cox M., Eltahawy E.A., Davis R. Post-chemotherapy robotic retroperitoneal lymph node dissection: institutional experience. J Endourol. 2016;30:510–519. doi: 10.1089/end.2015.0673. [DOI] [PubMed] [Google Scholar]
- 89.Pearce S.M., Golan S., Gorin M.A., Luckenbaugh A.N., Williams S.B., Ward J.F. Safety and early oncologic effectiveness of primary robotic retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer. Eur Urol. 2017;71:476–482. doi: 10.1016/j.eururo.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Stepanian S., Patel M., Porter J. Robot-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer: evolution of the technique. Eur Urol. 2016;70:661–667. doi: 10.1016/j.eururo.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 91.Singh A., Chatterjee S., Bansal P., Bansal A., Rawal S. Robot-assisted retroperitoneal lymph node dissection: feasibility and outcome in postchemotherapy residual mass in testicular cancer. Indian J Urol. 2017;33:304–309. doi: 10.4103/iju.IJU_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heidenreich A., Pfister D., Witthuhn R., Thüer D., Albers P. Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol. 2009;55:217–224. doi: 10.1016/j.eururo.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 93.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizaziet K et al. EAU Guidelines on. Testicular cancer. European Association of Urology 2017. Available at: https://uroweb.org/wp-content/uploads/11-Testicular-Cancer_2017_web.pdf. Accessed June 2018.
- 94.Albers P., Siener R., Krege S., Schmelz H.U., Dieckmann K.P., Heidenreich A. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I Nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966–2972. doi: 10.1200/JCO.2007.12.0899. [DOI] [PubMed] [Google Scholar]
- 95.Gundeti M.S., Eng M.K., Reynolds W.S., Zagaja G.P. Pediatric robotic-assisted laparoscopic augmentation ileocys-toplasty and Mitrofanoff appendicovesicostomy: complete intracorporeal – initial case report. Urology. 2008;72:1144–1147. doi: 10.1016/j.urology.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 96.Madec F.X., Hedhli O., Perrouin-Verbe M.A., Levesque A., Le Normand L., Rigaud J. Feasibility, morbidity, and functional results of supratrigonal cystectomy with augmentation ileocystoplasty by combined robot-assisted laparoscopy and mini-laparotomy approach. J Endourol. 2017;31:655–660. doi: 10.1089/end.2017.0107. [DOI] [PubMed] [Google Scholar]
- 97.Murthy P., Cohn J.A., Selig R.B., Gundeti M.S. Robot-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy in children: updated interim results. Eur Urol. 2015;68:1069–1075. doi: 10.1016/j.eururo.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 98.Cohen A.J., Brodie K., Murthy P., Wilcox D.T., Gundeti M.S. Comparative outcomes and perioperative complications of robotic vs open cystoplasty and complex reconstructions. Urology. 2016;97:172–178. doi: 10.1016/j.urology.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 99.Gundeti M.S., Acharya S.S., Zagaja G.P., Shalhav A.L. Paediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy (RALIMA): feasibility of and initial experience with the University of Chicago technique. BJU Int. 2011;107:962–969. doi: 10.1111/j.1464-410X.2010.09706.x. [DOI] [PubMed] [Google Scholar]
- 100.Gundeti M.S., Acharya S.S., Zagaja G.P. The University of Chicago technique of complete intracorporeal pediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy. J Robot Surg. 2009;3:89–93. doi: 10.1007/s11701-009-0137-7. [DOI] [PubMed] [Google Scholar]
- 101.Razmaria A.A., Marchetti P.E., Prasad S.M., Shalhav A.L., Gundeti M.S. Does robot-assisted laparoscopic ileocystoplasty (RALI) reduce peritoneal adhesions compared with open surgery? BJU Int. 2014;113:468–475. doi: 10.1111/bju.12284. [DOI] [PubMed] [Google Scholar]
- 102.Yates D.R., Phé V., Rouprêt M., Vaessen C., Parra J., Mozer P. Robot-assisted laparoscopic artificial urinary sphincter insertion in men with neurogenic stress urinary incontinence. BJU Int. 2013;111:1175–1179. doi: 10.1111/bju.12072. [DOI] [PubMed] [Google Scholar]
- 103.Fournier G., Callerot P., Thoulouzan M., Valeri A., Perrouin-Verbe M.A. Robotic-assisted laparoscopic implantation of artificial urinary sphincter in women with intrinsic sphincter deficiency incontinence: initial results. Urology. 2014;84:1094–1098. doi: 10.1016/j.urology.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Biardeau X., Rizk J., Marcelli F., Flamand V. Robot-assisted laparoscopic approach for artificial urinary sphincter implantation in 11 women with urinary stress incontinence: surgical technique and initial experience. Eur Urol. 2015;67:937–942. doi: 10.1016/j.eururo.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 105.Peyronnet B., Vincendeau S., Tondut L., Bensalah K., Damphousse M., Manunta A. Artificial urinary sphincter implantation in women with stress urinary incontinence: preliminary comparison of robot-assisted and open approaches. Int Urogynecol J. 2016;27:475–481. doi: 10.1007/s00192-015-2858-7. [DOI] [PubMed] [Google Scholar]
- 106.Hervé F., Lumen N., Goessaert A.S., Everaert K. Persistent urinary incontinence after a robot-assisted artificial urinary sphincter procedure: lessons learnt from two cases. BMJ Case Rep. 2016 doi: 10.1136/bcr-2016-216971. pii: bcr2016216971. [DOI] [PMC free article] [PubMed] [Google Scholar]