Abstract
Tissue engineered vascular grafts (TEVGs) are beginning to achieve clinical success and hold promise as a source of grafting material when donor grafts are unsuitable or unavailable. Significant technological advances have generated small-diameter TEVGs that are mechanically stable and promote functional remodeling by regenerating host cells. However, developing a biocompatible blood-contacting surface remains a major challenge. The TEVG luminal surface must avoid negative inflammatory responses and thrombogenesis immediately upon implantation and promote endothelialization. The surface has therefore become a primary focus for research and development efforts. The current state of TEVGs is herein reviewed with an emphasis on the blood-contacting surface. General vascular physiology and developmental challenges and strategies are briefly described, followed by an overview of the materials currently employed in TEVGs. The use of biodegradable materials and stem cells requires careful control of graft composition, degradation behavior, and cell recruitment ability to ensure that a physiologically relevant vessel structure is ultimately achieved. The establishment of a stable monolayer of endothelial cells (ECs) and the quiescence of smooth muscle cells (SMCs) are critical to the maintenance of patency. Several strategies to modify blood-contacting surfaces to resist thrombosis and control cellular recruitment are reviewed, including coatings of biomimetic peptides and heparin.
Keywords: Vascular Engineering, Vascular Graft, Blood-Contacting Surface, Endothelialization, Surface Modification
1. INTRODUCTION
Cardiovascular disease (CVD) is currently the leading cause of death in the United States and the world. Approximately 800,000 CVD deaths occur annually in the US as reported in 2017 by the American Heart Association [1]. Additionally, the World Health Organization estimated that 17.5 million people died worldwide from cardiovascular related diseases in the year 2013, and this number is expected to grow to 23.6 million by 2030 [2]. CVD can cause stenosis or occlusion of blood vessels and is commonly associated with coronary artery disease, cerebrovascular disease, deep vein thrombosis, atherosclerosis, and other serious complications [3].
Treatment methods of CVD include lifestyle modification, pharmaceuticals, or surgery. For situations requiring surgery, autologous grafts are the current gold standard treatment method. However, although autologous grafts are preferred over synthetic grafts, acceptable donor sites are not always available. Synthetic grafts are a useful alternative for large diameter vessels but not ideal for small-diameter vessels (diameters less than 6 mm) due to poor patency rates. Tissue engineered vascular grafts (TEVGs) hold promise to overcome the limitations seen with synthetic grafts and serve as alternative replacement vessels for clinical applications. However, engineering materials to mimic the properties of native tissues is challenging due to the complex structure and composition. In order to develop a functional small-diameter vascular graft, it is essential to understand the blood-contacting surface and antithrombogenic mechanisms. The engineering strategies for TEVGs have been extensively reviewed by Pashneh-Tala et al. [3]. This review paper focuses on the blood-contracting surface of TEVGs.
1.1. Blood-Contacting Surface and Antithrombogenicity
The blood-contacting surface of a vessel’s lumen is covered with a confluent monolayer of endothelial cells (ECs). A healthy endothelium plays a critical role in hemostasis as it directly contacts and interacts with substances within circulating blood. The ECs are anchored to an underlying basement membrane, which consists of a thin mat of laminin, collagen, and assorted extracellular matrix (ECM) biomacromolecules. This matrix often contains basic fibroblast growth factor (bFGF) and transforming growth factor (TGF)-β1, which activates SMCs to synthesize elastin and other components of ECM [3, 4]. The morphology and function of ECs are critical for maintaining vascular health. Endothelial abnormalities are well known for their significant role in cardiovascular diseases, especially atherosclerosis [5, 6].
Hemostasis and thrombus formation are regulated by several interconnected systems and biological factors, including the coagulation and complement cascades, biomechanical forces, and circulating cells and blood borne molecules. ECs synthesize and control many key factors such as heparans, thrombomodulin, tissue plasminogen activator (tPA), plasminogen activator inhibitor-1 (PAI-1), adhesion proteins, and platelet activating factor (PAF) to regulate thrombus formation through interactions with secondary molecules. These EC-secreted molecules include thrombin, antithrombin III (AT III), protein C, endothelial protein coupled receptor (EPCR), platelet activating factor receptor (PAFR), plasmin, and plasminogen. Figure 1 depicts the ECs’ key role in regulating the antithrombogenic properties of blood vessels.
Figure 1. The role of endothelial cells (ECs) in the regulation of thrombogenesis.
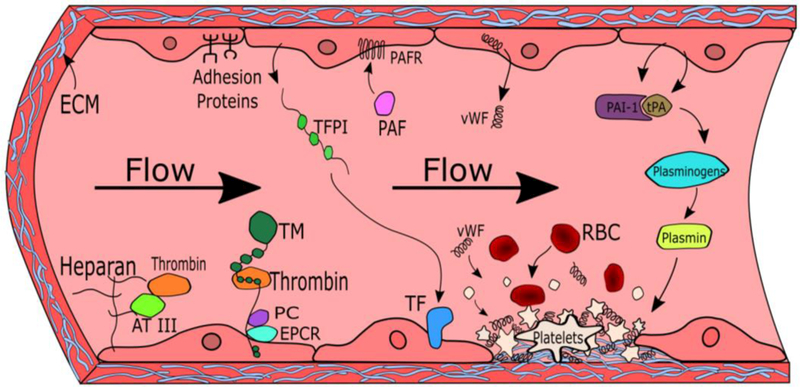
Heparans, thrombomodulin, tissue plasminogen activator (tPA), plasminogen activator inhibitor-1 (PAI-1), adhesion proteins, and platelet activating factor (PAF) play key roles in regulating coagulation. These important factors interact with other molecules such as thrombin, antithrombin III (AT III), protein C, endothelial protein coupled receptor (EPCR), platelet activating factor receptor (PAFR), plasmin, and plasminogen to promote or prevent thrombus formation. Thrombi are formed when damaged endothelium expose extracellular matrix (ECM) proteins such as collagen, which then recruit platelets, von Willebrand factor (vWF), and red blood cells (RBCs).
1.1.1. Platelet adhesion, coagulation cascade and regulation of thrombus formation
The coagulation cascade is divided into an intrinsic and extrinsic pathway. The endothelium plays a central role in coagulation cascades due to its unique anti-thrombogenic, fibrinolytic, and anticoagulant properties. Because collagen and other ECM proteins are highly thrombogenic, trauma or disease that damages the endothelium and exposes underlying thrombogenic materials can trigger thrombosis. Platelets initiate the coagulation cascade by immediately binding to exposed ECM components such as collagen, fibronectin, laminin, and thrombospondin at the site of vascular damage. Binding to these molecules activates platelets, which release prothrombotic molecules, including adenosine diphosphate (ADP) and thromboxane, leading to further activation and aggregation of platelets [7, 8]. Platelet activation leads toward synthesis of additional adhesive molecules, which further increase the platelet adhesion rate. For example, the de novo expression of vascular cell adhesion molecule (VCAM-1), also known as CD106, facilitates the adhesion of leukocytes to ECs [6, 9]. von Willebrand factor (vWF) is another platelet adhesive protein that is synthesized by ECs and links platelets to collagen. vWF is present in all ECs but is not secreted unless activated by the coagulation cascade [7, 10]. CD31 (PECAM1), CD54, (ICAM-1), P-selectin, and E-selectin are cellular adhesion proteins present on ECs that can bind with platelets and leukocytes [6]. In order to stabilize the blood clot formed by platelet aggregation, components of the intrinsic pathway activate thrombin, which converts fibrinogen into fibrin polymers to facilitate thrombus cross-linking. The presence of a fibrin clot further increases platelet incorporation [11]. Avoiding activation of these adhesive proteins can reduce blood coagulation and thrombus formation after TEVG implantation.
Prothrombin, fibrinogen, tissue factor (TF), and kallikrein are key pro-coagulation factors. These thrombus-forming components are controlled by several anticoagulation factors, including tissue factor pathway inhibitor (TFPI), thrombomodulin, protein C, and tissue plasminogen activator. Thrombomodulin and EPCR stimulate protein C, which contributes to anticoagulation [6, 8, 10]. TF initiates the extrinsic pathway of the coagulation cascade and is released from damaged sub-endothelial tissues. TF is regulated by TFPI, which is a serine protease inhibitor secreted by healthy vascular ECs. Undamaged ECs also express thrombomodulin, an integral membrane protein that binds thrombin and stimulates the activation of protein C, another anticoagulant. In addition, it is crucial to disintegrate and clear the thrombus after damage is repaired in order to maintain homeostasis. Tissue plasminogen activator (tPA) promotes fibrinolysis by catalyzing the breakdown of plasminogen to plasmin and is inhibited by plasminogen activator inhibitor (PAI-1). tPA and PAI-1 are secreted by ECs to regulate fibrinolysis of thrombi [5, 6, 8–10].
The natural anticoagulation mechanism involves several factors that can be used or mimicked to improve patency of TEVGs. AT III and TFPI are upregulated by the presence of an intact endothelial layer, and they prevent activation of thrombus promoting factors in the coagulation cascade. Other anticoagulation factors present on ECs include heparans, thrombomodulin, and ADPase. ECs synthesize heparans (or heparin-like glycosaminoglycans), which bind to AT III and greatly decrease thrombogenesis by deactivating coagulation factors [5, 7, 12]. Several studies have demonstrated the anti-thrombogenic properties of heparin-coated vascular grafts [13].
1.1.2. Inflammation and complement cascade activation
The complement cascade is an important system to terminate foreign cells. There are more than 20 types of plasma proteins involved in the complement system, which function as either binding proteins or catalysts in both the classical and alternative pathway [14]. Several components of the complement cascade are controlled by ECs. Complement factors, including C1s, C3, factor B, and the terminal complex components are secreted from ECs upon activation and initiate the cascade. Several other EC-secreted factors inhibit the complement cascade, including C1 inhibitor, factor H, and factor I. The complement inhibitors CD46, CD55, and CD59 are bound to the surface of ECs [6, 15].
Endothelial inflammation plays a key role in atherosclerosis and vascular occlusion. Endothelial inflammation is a complex response triggered by either an acute endothelial response or a phenotypic alteration, which can stimulate a prolonged reaction. Monocyte chemoattractant protein (MCP)-1, secreted by actively inflamed ECs, contributes to the inflammatory and immune response by recruiting monocytes (macrophages) to an injury site. Agonists (such as endotoxin, interleukin-1, and tumor necrosis factor (TNF)), oxidized lipoproteins, advanced glycation end products, and mechanical stimulation mediate endothelial activation and secretion of MCP-1. These factors regulate gene expression within ECs by activating the pleiotropic transcription factor, nuclear factor-κB. Prevention of endothelial activation can help prevent the accumulation of atherosclerotic plaque, which could otherwise lead to vascular occlusion [9, 16].
1.1.3. Influence of mechanical force on vascular tone maintenance
Pulsatile flow and mechanical stresses in the lumen of blood vessels also influence vascular patency. Blood flow produces shear stress on ECs and causes hyperpolarization of the cell membrane via potassium channels. This activation stimulates the release of nitric oxide (NO) by transcriptional upregulation of endothelial nitric oxide synthase (eNOS) and generates prostacyclin (PGI2), leading to vasodilation. The lipid molecule PGI2 acts as an antagonist to thromboxane A2, inhibits platelet aggregation, and promotes vasodilation [6, 17].
Relaxing factors are important for regulating vascular tone and contribute to the anti-thrombotic properties of the endothelium [5]. Endothelium-derived relaxing factor (EDRF) relaxes smooth muscle cells (SMCs) and inhibits thrombus formation. EDRF was identified as NO by Luis Ignarro and his colleagues, for which they won a Nobel Prize in 1998 [18]. PGI2 and NO inhibit platelet aggregation by stimulating increased levels of cAMP and cGMP. Endothelium-derived hyperpolarizing factor (EDHF) also contributes to vasodilation by hyperpolarization of the cell membrane through K+ channels, which causes the vascular smooth muscle layer to relax [5, 19]. In recent literature, NO release was found to decrease thrombus formation and improve vascular patency, demonstrating that eNOS and NO are important factors in regulating the anti-thrombogenic properties of ECs [20].
The endothelium acts as a mechanoreceptor, sensing changes in blood flow and pressure, which triggers the release of vasodilation or vasoconstriction signaling molecules to the vascular smooth muscle cells. To counter vasodilation, ECs produce endothelin-1 if activated by shear stress in the presence of thrombin or under hypoxic conditions, which stimulates SMCs to contract, leading to vasoconstriction [6, 21]. Studies investigating the effects of biomechanical stimulation on ECs have shown that laminar shear stress plays an important role in maintaining natural endothelial morphology. Cultured ECs under laminar shear stress upregulated transcription factors such as KLF-2 (Kruppel-like factor), KLF-4, and nuclear factor erythroid 2-related factor-2 (NRF-2). This biomechanical stimulation promotes an anti-thromobogenic phenotype and normal cell shape, alignment, and organization. It also promotes glycocalyx formation, a glycosaminoglycan-rich layer on the surface of ECs, which triggers the expression of eNOS and subsequent release of NO [9, 22]. In contrast, ECs under disturbed flow (ie non-directed, as found in branched or curved arteries) express the pleiotropic transcription factor nuclear factor-κB and mimic the abnormal phenotype of ECs found in patients with atherosclerotic cardiovascular disease [9, 23].
1.1.4. The influence of endothelial cell activation on thrombus formation
Activation of ECs can stimulate phenotypic changes that influence the anti-thrombogenic properties of blood vessels. ECs can be activated by vascular endothelial growth factor (VEGF), cellular adhesion, several cytokines, mechanical stimulation, or endotoxin. An understanding of endothelial activation and thrombotic endothelial diseases can help design TEVGs with improved anti-thrombogenic properties [5, 9].
Surgical implantation of a TEVG damages neighboring tissues and can disrupt endothelial continuity. It is particularly important to avoid surgical trauma induced intimal hyperplasia at the interface between the implant and native vasculature. This disease promoting tissue has mismatching mechanical properties and can protrude into the vascular lumen, which can disrupt laminar blood flow and predispose towards thrombus formation. Vascular trauma induces platelet adhesion, migration of pro-inflammatory cells especially monocytes/macrophages, and promotes excessive cellular proliferation and activation, which can result in atherosclerotic plaque development [5, 9, 24]. Activation of ECs or damage to the endothelial layer compromises the anti-thrombogenic properties of blood vessels. Therefore, maintaining a healthy and undamaged endothelial layer is the first and most important consideration to enhance the patency of cellularized TEVGs. Various strategies have been developed to address this issue that are reviewed in the present contribution. These strategies include novel in vitro anti-thrombogenic cell seeding, EC maturation by provision of physiological pulsatile flow, as well as promoting efficient in vitro and/or in situ endothelialization by modifying the blood-contacting surface via attachment of biomimetic peptides, antibodies and growth factors.
When developing vascular grafts for CVD patients, a broad range of disease characteristics that affect the confluence and functionality of the endothelium need to be considered. Symptoms of vascular dysfunction include generalized or localized vascular spasms, abnormal thrombosis, atherosclerosis, and restenosis [5]. Understanding mechanisms of endothelial dysfunction and treatment methods are critical in TEVG development to prevent coagulation and improve patency results.
2. BLOOD INTERFACE OF VASCULAR GRAFTS
Vascular graft implantation triggers protein absorption, complement activation, platelet adhesion and foreign reaction. Recapitulating a hemocompatible blood-contacting interface on vascular grafts is a major goal of bioengineering replacement vascular tissues. The character of the blood-material interface is determined by material properties, blood flow effects and the biological environment [25]. Material selection and understanding their affect on blood and blood borne cells is critical for TEVG engineering.
2.1. Naturally Derived Materials
A major benefit of naturally derived materials is that their chemical structure and biological effects are similar to their human counterparts. Most of the natural materials are biodegradable and can be remodeled and replaced by native cells and ECM. Consequently, grafts fabricated from naturally derived materials can be integrated and attain a close approximation to a native vessel [26].
2.1.1. Elastin
Elastin, composed of tropoelastin and fibrillin, is an important ECM constituent in vertebrate tissues that require elastic recoil [27]. As an essential constituent of blood vessels, it plays a vital role in vessel elasticity and SMC phenotype regulation [28]. Tropoelastin can be secreted by SMCs, ECs and fibroblasts. Elastin has become an important component for constructing TEVGs, as it exhibits anti-thrombogenic and anti-inflammatory properties in addition to imparting material properties of elasticity. Its degradation products, bioactive elastin-derived peptides (EDPs), retain the property of decreasing platelet aggregation and thrombosis [29]. Elastin directly isolated from vasculature was found to cause only minor platelet adhesion [30]. A study by Simionescu et al. obtained pure elastin from porcine aortas and tested its potential as a TEVG scaffold. In vitro results revealed reduced platelet adhesion and aggregation compared to collagen scaffolds derived from porcine aortas. The research demonstrated that elastin scaffolds promote EC proliferation, viability, and endothelial layer formation [31]. Another study applied purified porcine arterial elastin combined with fibrin-bonded layers from acellular small intestinal mucosa to improve the mechanical properties of elastin [32]. The researchers tested acute thrombogenicity of the construct using a porcine carotid artery interposition model. The elastin-containing construct remained patent significantly longer than commercially available PTFE. While thrombus in the elastin construct was restricted to the suture sites, thrombosis was elicited along the entire length of the PTFE graft. Incorporating elastin into TEVGs is therefore a promising approach to achieve a stable blood-contacting surface based on its antithrombogenic and natural properties.
2.1.2. Silk fibroin
Fibroin is the main component of silk fibers, composed of antiparallel beta sheets. Silk fibroin is a biodegradable and biocompatible scaffold material with high mechanical strength and a low degradation rate. It has been shown to be non-thrombogenic, as it avoided activation of platelets [33]. The anti-thrombotic property of fibroin was also observed by Enomoto et al, in a long term implantation of a vascular graft fabricated with silk fibroin thread by plaiting [34]. The fibroin graft maintained high patency after a one-year implantation. Implantation of grafts by Soffer that were fabricated into tubular silk fibroin scaffolds by electrospinning demonstrated sufficient structural integrity to allow handling and to maintain open conduits when hydrated [35]. The scaffolds possess a promising elastic modulus (average 2.45MPa) and ultimate tensile strength (average 2.42MPa) for potential use as vascular grafts, including the ability to withstand arterial pressures and to behave mechanically similar to native vessels. These systems also support EC and SMC proliferation [35, 36], suggesting their future potential in TEVG applications.
2.1.3. Collagen
Collagen is a main protein component of arteries and veins, made from amino acid-packed triple helices, providing mechanical support along the protein longitudinal axis. Although present in each layer of vasculature, larger bundles of collagen are concentrated in the vessel adventitia. TEVG scaffolds with high collagen content exhibit high mechanical strength, biocompatibility, degradability, and accessibility to native cells. The collagen fibrils contain integrin binding sites, which allow cells to attach and migrate [37]. However, at the same time, collagen can bind vWF and blood coagulation proteins, which promote platelet adhesion on the TEVG surface [38]. Similarly, activated platelets also express adhesion proteins including Gp1b, GpIIb-IIIa, CD40L, P-selectin, GPIbα46 and ICAM-2, which induce platelet aggregation and vessel wall invasion of activated leucocytes [39]. Therefore, when constructing TEVGs from collagen-containing matrices, antithromgenic materials or anticoagulation reagents should be incorporated into the scaffold, especially on the blood-contacting surface, to reduce the thrombogenic effects of collagen [40]. Huynh et al. developed an acellular collagen-based TEVG by depositing bovine type I collagen on the submucosa of swine small intestines, which is itself a highly collagenous material [41]. After treatment with an anti-thrombosis reagent and crosslinking, the graft was implanted into a rabbit model. Histological analysis identified SMCs infiltrating within the graft and an endothelial layer at 90 days. The grafts were responsive to various vasoactive agents, indicating successful functional remodeling.
2.1.4. Decellularized ECM
ECM is the collection of biomacromolecules secreted and organized by cells, which contains complex components including collagen, laminin, hyaluronic acid and fibronectin. The ECM provides an ideal environment for cells to proliferate and carry out their functions. It can mechanically support cells to form an integrated and functional tissue, as well as provide biomolecules to maintain cell motility and viability. Decellularized tubular tissues which contain a relatively organized ECM structure, such as human greater saphenous vein and intestine, have been investigated for use as vascular scaffolds [42]. Following decellularization processing, decellularized tissues have been found to maintain a high compositional similarity to native vasculature with respect to collagen and elastin content. Furthermore, the mechanical strength of decellularizeds scaffold, including suture retention and compliance, are comparable to native vasculature [43]. In vitro testing revealed that the decellularized human greater saphenous vein exhibits similar burst strength and suture-holding strength as fresh veins (fresh: 2480 ± 460 mm Hg, 185 ± 30 gm, decellularized: 2380 ± 620 mm Hg, 178 ± 66 gm). However, tissue engineering approaches that rely upon xenograft ECM must surmount pathogenic concerns, while allograft and homograft ECM is in limited supply. To solve this problem, one unique strategy developed tubular ECM by body foreign reaction over 3 weeks of subcutaneous implantation of polymer rods [44]. The tubular fibrocellular tissue capsule can be applied as a TEVG material. The graft can also be decellularized and repopulated with specialized cell types for improved performance as a TEVG. Widespread experience demonstrates that the ECM supports cell attachment, proliferation and infiltration, which permits their use as potential TEVG candidates [45]. Nevertheless, based on the complex composition of ECM, a bulk ECM implanted into the vascular system will promote thrombosis [46]. Antithrobogenic strategies need to be incorporated into these ECM constructs to improve the TEVG patency.
Besides decellularization of natural tissues, cell-derived ECM has been developed to circumvent pathogenic concerns associated with xenografts. [47, 48]. Our group has derived highly aligned ECM nanofibers by decellularizing aligned human dermal fibroblasts sheets. The nanofibers are around 80 nm in diameter, similar to the physiological size of collagen nanofibers [49]. Recently, we assembled a completely biological and anisotropic vascular graft by combining the aligned ECM nanofibrous sheet and hMSCs in a rotating wall bioreactor system [47]. The TEVG demonstrated increased tensile strength and anisotropy compared with its static culture counterpart. Dr. Tranquillo’s group has created a TEVG by growing ovine dermal fibroblasts in a sacrificial tubular fibrin gel mold and then decellularizing the resulting tubular cellular assembly. The 4 weeks implantation of this TEVG in sheep femoral arteries demonstrated graft patency and mechanical anisotropy along with extensive recellularization [50]. A similar approach was applied to create an “off-the-shelf” decellularized vascular graft, which was implanted into young lambs [51]. After 50 weeks, explanted grafts displayed physiological stiffness and complete lumen endothelialization without any signs of calcification, aneurysm or restenosis [51]. Most recently this group has developed decellularized arteriovenous grafts (AVGs) by seeding neonatal human dermal fibroblasts in the sacrificial fibrin mold [52]. These decellularized constructs were implanted into baboons and tested as hemodialysis access points. AVGs were extensively recellularized, showed an 83% and 60% patency rate at 3- and 5-months, respectively, without calcifications, loss of burst strength, or outflow stenosis [52]. Although significant progress has been achieved using this strategy, anticoagulation drugs were required for the entire duration of the study, and thus the study focused on long term graft function and biointegration without considering the blood contacting interface.
2.2. Synthetic Materials
Synthetic materials provide a reproducible, controllable and inexpensive source for TEVG engineering. Synthetic materials usually have higher mechanical strength than natural derived materials, which make them promising in blood pressure-bearing applications. One major obstacle of constructing TEVGs from synthetic materials is their lack of cell communication signals and integrin binding sites, which might hinder cell attachment and infiltration into the graft. Moreover, a broad mechanical compliance mismatch further undermines the long-term patency of these grafts. To improve the properties of synthetic materials, innovative modifications have been developed.
2.2.1. Non-biodegradable synthetic materials
Biostable polymers, such as polyethylene terephthalate (aka PET, Dacron) and expanded polytetrafluoroethylene (ePTFE), have been utilized as prosthetic large vascular grafts (ID > 6mm) [53]. When applied as large diameter vessels, the higher rate of blood flow and larger flow area together with the relatively inert property of PET and ePTFE decreases the possibility of TEVG occlusion. They also provide strong mechanical support to tolerate blood pressure and pulsatile blood flow [54]. However, when applied in small diameter vessels (ID < 6mm) that have a much lower blood flow rate, the vascular grafts tend to fail by occlusive thrombus. Moreover, biostable materials are prone to chronic foreign body responses that form fibrotic capsules around the graft. The mechanical mismatch between material and host tissue may also compromise vessel functionality, promoting cell hyperplasia near the graft-tissue interface. In order to improve the blood-contacting surface of non-biodegradable polymeric TEVGs, ECs have been seeded into the vascular lumen. Nevertheless, the generated endothelium fails to completely cover the neointima, which contributes to thrombus formation [55]. Thus, the biostable polymers require surface modification and bioactive molecular incorporation to improve EC confluence on the material surface [56].
2.2.2. Biodegradable synthetic materials
Linear aliphatic polyesters such as poly (caprolactone) (PCL), poly (lactic acid) (PLA), poly (glycolic acid) (PGA), and their copolymers poly (lactic acid-co-glycolic acid) (PLGA) are biodegradable and biocompatible polymers that have achieved approval from the US Food and Drug Administration (FDA) for clinical use [57]. The first preliminary study evaluating a PGA tube as a vascular graft was conducted in a rat model by Lauritzen in 1983 utilizing grafts with an internal diameter of 1mm, [58]. This initial experiment discovered that half the venous PGA grafts were completely replaced by regenerated vascular tissue with complete endothelium and developed sub endothelial layers over the duration of the study, suggesting that PGA tubes are promising constructs for vascular graft engineering. It should be noted that the failure mode for the other half of the PGA grafts was due to damage caused to the intima during surgery. PCL is a versatile biomaterial that has been extensively investigated. It degrades by the hydrolysis of ester linkages, and giant cells and macrophages can digest the fragments. Unlike PGA or PLA, the degradation of PCL is significantly slower (longer than 24 month), making it more compatible with the slow rate of tissue healing [59]. One study compared small-diameter PCL grafts with ePTFE grafts at 24 weeks in rats in terms of endothelial coverage, intima formation, and cell infiltration after implantation [60]. The authors found faster endothelialization and intima formation in the PCL grafts relative to ePTFE, with better cell infiltration and ECM deposition, indicating that PCL is a more appropriate candidate than ePTFE for TEVGs. Varied combinations of polyesters have been used to synthesize biodegradable materials with different mechanical properties and degradation rates. A PGA non-woven fabric sheet was combined with P(CL/LA) (50:50) by pouring P(CL/LA) solution onto the sheet followed by freeze-drying. Tubular grafts were constructed with the material to a diameter of 8 mm. The grafts were implanted in the inferior vena cava of canines for a long term performance test at low pressure [61]. The goal was to achieve a biodegradable scaffold that was capable of promoting regeneration through optimized mechanics and degradation time without the need for cell seeding. After two years of implantation the graft exhibited well-formed vasculature with high similarity to native vessels without observations of serious thrombus in the inner layer of the graft. However, due to the hydrolysis of the graft, the loss of mechanical strength at early stages of implantation resulted in stenosis. Reinforcement to increase mechanical strength and endothelialization of the graft is needed to improve the TEVG performance. Polydioxanone ((C4H6O3)n, PDO) is a biocompatible and biodegradable polymer that has been widely used as wound closure sutures. Similar to Dacron and ePTFE, PDO provides strong mechanical retention when applied as a TEVG scaffold [62]. Its degradation rate is lower than PLGA and PGA [63], but faster than PCL. Sell et al. co-spun PDO with elastin at a 1:1 ratio, using PDO as a backbone to provide mechanical support while elastin provided bioactive signals to prevent platelet adhesion [64]. The mechanical strength of the construct was similar to that of native vessels and the incorporation of elastin promoted cell infiltration. A similar study by Smith et al employed an electrospun PDO-elastin tube reinforced by crosslinking to withstand suturing. The burst strength of the tube was significantly elevated by the processing [65].
Overall, natural materials are more biocompatible since they are remodelable by host cells and their degradation products can be assimilated or metabolized and excreted by the body. However, using natural or naturally derived materials often requires complicated and time-consuming purification or isolation processes, and reproducibility may be challenged due to natural variations. The low mechanical strength of natural materials often necessitates additional processing for reinforcement. Synthetic materials are more reproducible and easily processed but require incorporation of bioactive molecules and careful design to obtain suitable degradation rates and mechanical strength. More importantly, a functional TEVG design must be antithrombotic. Incorporation of antithrombotic materials such as elastin and designing synthetic materials with non-thromobogenic surfaces provide new directions for engineering TEVGs.
2.3. Influence of Surface Roughness, Topography and Mechanical Properties on TEVG Hemocompatibility
In addition to considerations related to TEVG material selection, the architecture and topography of the blood-contacting surface are also important factors affecting thrombus formation. The aortic endothelium is rough at the submicron scale, with ridges and grooves that are generally aligned in the blood flow direction [66, 67]. These ridges have a ~500 nm width and ~100 nm height [66, 68]. These natural topographical features may provide increased cellular attachement as well as alignment in the direction of blood flow. In recent years, various studies have focused on elucidating the impact of biophysical stimulation on the behavior of platelets and ECs. These studies can help in designing appropariate blood-contacting surfaces for TEVGs that can provide better hemocompatibility.
Platelet adhesion and activation are significantly affected by material surface nano-topography gradients. A rough surface has been created by coating gold nano particles (36 nm and 56 nm of diameter) onto a smooth gold substrate, which was further coated with fibrinogen cell adhesion molecules [69]. Results showed that platelets were more adherent and readily activated on a smooth surface relative to a rough one. Others have also found that increased surface roughness at a micrometer level increases thrombogenicity, possibly due to the increased surface area available for platelet adhesion and activation [70]. Surfaces with roughness larger than a platelet dimension (~ 2 μm) have higher thrombogenic potential. Interestingly, roughness from a 36 nm nanoparticle coating does not affect platelet adhesion and activation, suggesting platelets may have a minimum roughness detection limit of ~36 nm for stimulating thrombogenic effects [71]. Variations in surface topography also affect platelet activity. In contrast to roughness, surface topography with structured ridges or grooves (50 nm - 2 μm) significantly reduce platelet adhesion area, as platelet attachment is generally restricted to the groove top. Thus, structured surfaces can be highly thrombo-resistant. Similarly, electrospun fibers having diameter less than 1 μm effectively avoid platelet adhesion and blood coagulation, while a significantly increased platelet adhesion has been observed on fibers with diameters of 2–3 μm [72]. Therefore, a well-designed TEVG surface with appropriate roughness and topography could significantly improve the hemocompatibility of the TEVG blood-contacting surface [69].
The native endothelial vascular basement membrane has nano to micro scale topographical features, which guide EC orientation [67]. These surface topographical features can be used to modulate EC behavior in order to enhance TEVG hemocompatibility. For example, a study conducted to explore rat aortic endothelial cell (RAEC) behavior on a patterned titanium surface having periodic arrays of grooves with width and pitch ranging from 750 nm to 100 μm demonstrated that a smaller feature size elicits enhanced cellular effects [73]. The patterned surface with a feature size less than 10 μm promoted increased cell density, elongation and proliferation compared to smooth or randomly pattered surfaces [73]. A similar study was conducted on patterns with ridge and groove sizes ranging from 200 nm to 2,000 nm and at a fixed 300 nm groove depth. Four different vascular cell types including human umbilical vein ECs, human dermal microvascular ECs, human aortic ECs and human saphenous vein ECs exhibited significantly increased cell orientation and alignment on aligned patterns ridges larger than 800 nm [74]. Moreover, all of the cell types excluding human aortic ECs showed a significantly increased rate of cell migration on aligned patterns having ridges larger than 1,200 nm [74].
The mechanical properties of the blood-contacting surface are also important regulators of platelet adhesion, spreading and activation [75–77]. A fibrinogen-immobilized polyacrylamide gel with varied stiffness (0.25 kPa - 100 kPa) revealed that stiffer surfaces (5 to 50 kPa) significantly enhance platelet adhesion and spreading through activation of integrin αIIbβ2, which binds fibrinogen [76]. They found that platelets respond to surface stiffness via Rac1 and actomyosin activity by secreting more alpha granules on stiffer substrates [76]. Another study with a collagen-conjugated polyacrylamide gel with varying stiffness from 0.25 kPa to 5 kPa confirmed this result, as the platelet spreading area increased (50–60 μm2) on gels stiffer than 5 kPa, compared to a spreading area of 30–40 of μm2 on softer gels with a stiffness between 0.25 to 2.5 kPa [75]. This study found that collagen mediated the stiffness effects by regulating extracellular Ca+2 levels and actomyosin pathways rather than through Rho-associated protein kinase pathways [75]. Although the influence of a TEVG’s blood-contacting surface topography and mechanical properties on platelet activation/adhesion is a relatively new research direction, this needs to be carefully considered for successful TEVG design.
3. STRATEGIES TO IMPROVE THE BLOOD INTERFACE OF VASCULAR GRAFTS
Whether off-the-shelf grafts made from biomaterials or cellular grafts fabricated by allogeneic or autologous cells, TEVGs all require a functional blood-contacting surface to reduce thrombogenicity and maintain patency. Different strategies, such as including anti-thrombotic cells in the graft lumen, stimulating in vivo endothelialization, utilizing thrombosis-resistant graft biomaterials, and chemical modification of the graft surface, have been employed to produce successful blood-contacting surfaces in TEVGs (Figure 2).
Figure 2. Modifying blood-contacting surface of TEVG.
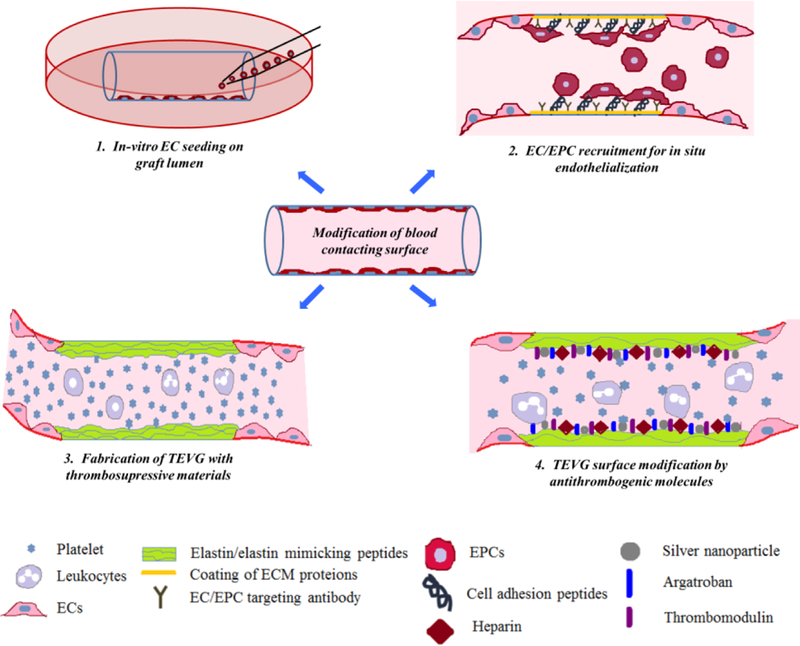
(1). In vitro endothelial cell seeding on the graft lumen provides a biomimetic environment and prevents direct contact of blood with the graft surface. (2). In-situ endothelialization can be achieved by coating the graft lumen with ECM proteins, growth factors, EC/EPC specific cell binding peptides or antibodies. (3). Fabrication of TEVG with anti-thrombogenic natural or synthetic materials maintains graft patency by preventing platelet adhesion and activation. (4). Coating of various anti-thrombogenic molecules such as heparin, silver nano-particles, argatroban and thrombomodulin actively maintains graft patency by preventing platelet adhesion and activation.
3.1. Incorporating Anti-Thrombotic Luminal Cells to Improve TEBV Blood Interface
In most TEVGs, thrombosis occurs due to the lack of an EC layer or the failure to properly re-endothelialize after implantation. Pre-implant luminal cell seeding to provide a functional endothelium or anti-thrombotic cell layer would provide an effective blood-contacting surface. Seeding cells before implantation, if they are accepted by the host immune system, shortens the duration of TEVG regeneration post-implantation. An ideal cell source is one that is easy to obtain and expands quickly to large quantities in vitro, retains native function, and is non-immunogenic in the intended recipient.
3.1.1. Cell types
An ideal TEVG should have biomimetic components and structure. It should be able to withstand the natural mechanical forces from blood flow without rupture and should be functionally anti-thrombogenic, a property in which the vascular cells play a vital role. Including vascular cells in the design of TEVGs would help to reconstruct functional vascular tissue. Currently, ECs and SMCs are widely applied in TEVGs. Compared to traditional vascular cell types, stem cells expand the range of options when designing TEVGs, since the process to obtain vascular cells is invasive and the vitality and proliferative capability of adult cells are limited. Furthermore, the multi-differentiation capability of stem cells may decrease the number of required cell types involved in the whole procedure, which could shorten the time required to fabricate TEVGs. Table 1 provides examples of typical cell types that have been incorporated in TEVG fabrication to improve the TEVG-blood interface.
Table 1.
TEVG with Incorporated Cells
| ECs | Nondegradable scaffolds: Dacron [78,
79], Dacron-collagen [80], PTFE [81]; Degradable scaffolds: PCL [82], P(LLA/CL) [83], P(CS/CL) [84], collagen [85], decellularized vessels [86, 87], fibroblast cell sheets [88] |
Human vein [78], Human artery [79, 81, 86, 88], Canine artery [83, 84], Porcine artery [87] |
ECs | EC seeding significantly improved TEVG patency. Dynamic seeding increased endothelium confluence after implantation. |
ECs participated in re- endothelialization. Detached ECs circulated in peripheral blood. EC attachment depended upon EC source, seeding strategy and scaffold material. |
| ECFCs | Degradable scaffolds: decellularized vessels [89–91], decellularized small intestine submucosa [92],collagen [93] |
Sheep artery [89, 91], Canine artery [90, 93] |
ECFCs, ECFCs- derived ECs |
ECFCs promoted and directly integrated into the regenerating endothelium. Cell implantation improved TEVG patency. |
ECFCs differentiated into ECs and integrated into the reformed endothelium. ECFCs recruited circulating blood progenitor cells to promote re-endothelialization. |
| MSCs | Degradable scaffolds: P(CL/LA) [94], PLLA [95], PCL-gelatin [96], decellularized vessels [97, 98], PLGA- PU [99] |
Rat artery [95, 98], Ovine artery [97], Canine artery [99] |
MSCs, MSCs- derived ECs |
MSCs possess anti- thromobogenic properties, increasing TEVG patency. MSC-derived ECs involved in re-endothelialization. |
MSCs recruited endothelial lineage cells and stabilized EC colonization through paracrine factors. MSC-derived ECs directly participated in re- endothelialization. |
|
BM- MNCs |
Degradable scaffolds: P(CL/LA) [100- 102], decellularized vessels [103, 104], PGLA-P(CL/LA) [105] |
Canine artery [100], Lamb vein [101], Canine artery [103], Human vein [104, 105], Mice artery [102] |
BM-MNCs, BM-MNC derived ECs |
BM-MNCs enabled TEVG to regenerate SMCs and endothelium. BM-MNCs reduced platelet activation and inflammation, improving TEVG patency. |
BM-MNCs differentiated into endothelial lineage cells, lining the endothelium. The MSC sub- population within the BM-MNCs further stabilized the endothelium. |
Abbreviations: EC: endothelial cell; ECFC: endothelial colony forming cell; MSC: mesenchymal stem cell; BM-MNC: bone marrow mononuclear cell; PCL: poly(ɛ-caprolactone); CS: chitosan; PLLA: poly(L-lactide); PTFE: Polytetrafluoroethylene; PLGA: poly(lactic-glycolic acid); PU: polyurethane
3.1.1.1. Vascular cells
A monolayer of ECs line the vasculature, which plays a vital role in avoiding thrombus formation. The permeability of the vessel, fibrinolysis, and inflammation are also associated with a healthy EC layer that secretes an assortment of molecules to regulate the function of SMCs and blood borne cells. The expression level of these molecules is controlled by interactions between circulating blood, ECs and other vascular cells [6]. Early stage studies recognized the importance of ECs in the TEVG blood-contacting layer to avoid thrombosis post implantation. An endothelialized Dacron vascular graft was implanted in a patient for 9 months before requiring a resection, and upon explant the inner surface of the graft revealed an absence of thrombus due to the presence of a uniform endothelial layer similar to native vasculature [78]. Including ECs in the inner layer of TEVGs or applying growth factors to stimulate EC recruitment onto TEVGs contributes to the formation of a stable endothelium and reduces thrombosis after implantation. SMCs are distributed in the vascular media layer, sandwiched between layers of elastin. Although these cells do not directly contact the blood, they indirectly regulate the function of ECs through secretion, maturation, and organization of ECM [106], in addition to their mechanical role. SMC and EC interactions through paracrine factors influence vessel wall assembly and vessel maturation [107]. The presence of SMCs in EC culture decreases EC apoptosis and stimulates EC quiescence via Tie2 signaling, which increases vascular stability and EC survival [108]. Research by Neff et al. compared TEVG functionality in which ECs were seeded on the inner surface of decellularized porcine arteries either with or without SMCs on the outer layer [91]. The group with SMCs not only exhibited higher tensile strength and more rapid medial healing, but also displayed a greater EC physiologic response to receptor-mediated agonists compared with TEVGs seeded without SMCs. Unfortunately, although an engineered endothelial layer is the most straightforward and biomimetic analogue of the natural endothelium, ECs have limited proliferative and regenerative ability. Moreover, the sudden exposure to blood flow and physiological conditions could reduce the attachment of EC on TEVGs [109]. In order to improve endothelialization as well as reduce processing complexity, different cell sources and strategies have been explored.
3.1.1.2. Endothelial progenitor cells (EPCs)
Endothelial progenitor cells (EPCs) are defined as cells that can differentiate into ECs and contribute to new blood vessel formation [110]. By using cell culture technology, two phenotypes of EPCs have been produced: myeloid angiogenic cells (MACs) and endothelial colony forming cells (ECFCs). MACs do not have the ability to differentiate into ECs but can secrete paracrine factors to promote angiogenesis. ECFCs give rise to ECs and directly participate in vasculature formation [111]. ECFCs are positive for CD31, CD146, VEGFR-2, and vWF, but negative for CD45 and CD14, reflecting their normal morphology and function for EC differentiation [24]. ECFCs are recruited on-site where there is injured endothelium and differentiate into ECs to repair vascular damage. Compared to ECs, ECFCs have a higher proliferation ability and are therefore easier to expand in vitro [112], which provides an alternative source of ECs. ECFCs from dogs were isolated and expanded in vitro, and then prelined on a collagen mesh that was wrapped with segmented polyurethane. The graft exhibited high patency post implantation at 3 months, as 11 of 12 of the grafts were patent and covered confluently with cells expressing factor VIIIb-related antigen, indicating that the prelined ECFCs had formed into a functional vascular graft with a confluent EC layer [93]. ECFCs derived from sheep peripheral blood were seeded on decellularized porcine iliac vessels and then implanted in a sheep model [113]. The graft exhibited vascular function similar to native arteries, including contraction and relaxation responses. The results suggest that ECFCs could be used as an alternative source of ECs in TEVG biofabrication.
3.1.1.3. Mesenchymal stem cells (MSCs)
MSCs are a promising cell type for vascular engineering. They are widely available in the body and can be derived from various tissue sources, including adipose tissue, bone marrow, and umbilical vein blood. They can be easily expanded through at least 40 population doublings in vitro [114]. MSCs are immunoregulatory and non-autologous sources do not cause severe immune responses when implanted into foreign recipients [115]. More importantly, they are anti-thromobogenic because of heparan sulfate proteoglycan expression on their surface [95]. They can also recruit ECFCs and ECs on site [95], and be transdifferentiated into vascular cell types, including ECs and SMCs [116]. These properties make MSCs especially attractive as a cell source for TEVG engineering, as they could increase graft patency [117]. A completely biological TEVG was synthesized by MSC sheets by Zhao et al [118]. The TEVG integrated with native vasculature and formed an endothelial inner layer after 4 weeks implantation, which maintained the patency of the TEVG. Zhou et al developed a completely biological TEVG by combining MSC derived ECs and SMCs from transdifferentiation [119]. MSC derived SMCs were seeded on a PCL-gelatin mesh to help SMCs maintain a tubular shape and then the scaffold was sacrificed, leaving behind an SMC filled layer. MSC derived ECs were then seeded in the lumen under pulsatile stimulation. This method produced a TEVG with similar structure as native vessels and comparable mechanical properties including elastic modulus (12.7±1.19MPa), suture retention (1.62±0.1N) and burst pressure (1.72±0.14MPa) to the human saphenous vein. The study demonstrated that the multi-differentiation property of MSC makes it a viable cell source for TEVGs.
3.1.1.4. Induced pluripotent stem cells (iPSCs)
iPSCs are pluripotent stem cells that are derived by reprogramming adult cells [120]. iPSCs can avoid the ethical issues involved in the use of embryonic stem cells (ESCs) while retaining the pluripotent ability to differentiate into all cell types. In use, iPSCs are always induced to differentiate into specific lineages before seeding since their pluripotent property might otherwise produce a teratoma. iPSCs provide a novel source for obtaining vascular cells. The SMCs derived from iPSCs have been shown to maintain phenotype stability and perform better than vascular derived SMCs in terms of SMC specific proteins and gene expression [121]. Since iPSCs can be induced into an EC type with phenotypic plasticity [122, 123], their use as blood contacting cells in a TEVG may reduce platelet activation and thrombogenesis post implantation. Studies utilizing iPSC-derived ECs in engineering vasculature in 3D tissues [122, 124] demonstrated the pro-angiogenic capability of the ECs. Research by Nakayama et al. engineered a bilayered vascular graft by incorporating iPSC-derived SMCs and ECs into aligned fibrillary collagen. The aligned EC layer significantly reduced the inflammatory response of monocytes as compared to randomly-oriented ECs [125]. The study demonstrated the feasibility of applying iPSC derived ECs in building TEVGs. The iPSCs provide a promising cell source for engineering TEVGs, overcoming the limitations of ESCs while possessing pluripotent differentiation capability.
3.1.1.5. Bone marrow mononuclear cells (BM-MNCs)
BM-MNCs include EPCs, ECs, MSCs, immune-related cells and hematopoietic stem cells. By combining their features, BM-MNCs may provide an anti-thrombogenic property when incorporated into TEVGs. Harvesting BM-MNCs from bone marrow is also less invasive than collecting vascular cells from blood vessels [126]. The BM-MNCs can be differentiated into ECs and SMCs, prior to seeding them in a scaffold [127], or seeded while undifferentiated [128]. In a study by Cho et al, BM-MNCs were differentiated to SMC and EC phenotypes and seeded on decellularized canine carotid arteries [129]. The resulting TEVGs were evaluated in a canine model. They found a significantly improved patency of the BM-MNC graft compared with unseeded groups after 8 weeks. The structure of the TEVG was similar to native tissue and the BM-MN derived ECs and SMCs were detected in the construct, demonstrating the long-term self-renewal capacity of the BM-MNCs. Fukunishi et al combined BM-MNCs with a nanofibrous scaffold employed as an infrarenal inferior vena cava graft. The patency of the conduits with BM-MNCs was significantly increased (from 1/10 to 9/10) relative to the non-seeded group after a six month implantation [130]. Histological results depicted concentric laminated SMCs and a confluent EC layer, demonstrating the differentiation ability of BM-MNCs and their applicability in TEVGs. A clinical study was also conducted employing BM-MNCs in vascular tissue engineering. BM-MNCs were seeded on a polymer tube composed of PLA and PCL and reinforced by PGLA [131]. The conduits were implanted into patients with a median age of 5.5 years. In a follow-up with a median duration of 16.7 months post operation there were no complications due to thrombosis or obstruction. The maximum follow-up time of 32 months demonstrated the long-term feasibility of TEVGs constructed from BM-MNCs with an effective blood-contacting luminal surface.
The establishment of an EC lining or a complete layer of non-EC anti-thrombotic cells on the lumen of vascular grafts offers the most effective method to avoid thrombus formation and maintain patency after implantation. The cell seeding strategy is as important as the cell type selection to ensure a confluent and functional blood-contacting surface in the TEVG lumen.
3.1.2. Strategies for preventing immune rejection
Vascular grafts seeded with autologous ECs normally do not evoke host immune responses. However, obtaining autologous cells requires a biopsy, which might be distressing for patients. Moreover, this approach suffers from various limitations including a long culture period for host cell expansion and decreased cellular health depending upon the patient’s age and disease status. Allogenic ECs are readily available from donors but increase the risk of immune rejection. In addition to humeral responses, host T cells can detect allogenic antigens by allorestricted (direct) recognition and/or self-restricted (indirect) recognition, which might result in massive cell death and graft rejection [132]. Monoclonal antibody treatment is an efficient way to modulate host immune responses. Antibodies targeted to immune cell receptors can block chronic graft rejection. Treatment with an anti VCAM-1 monoclonal antibody after allogenic vascular graft implantation resulted in prolonged graft survival [133]. This treatment reduced macrophage and T cell infiltration at the implant site. Moreover, infiltrated T cells appeared to be quiescent after antibody treatment [133]. Very late antigen-4 (VLA-4) can bind with VCAM-1 and induce a costimulatory immune reaction. Monoclonal antibody treatment against VLA-4 and VCAM-1 prevents active graft rejection and provides immosupression to cardiac allografts [134]. In another study, a rat anti-CD2 monoclonal antibody was tested in a pre-clinical model of pig-to-baboon xenograft transplantation [135]. This treatment prevented direct cytotoxicity of baboon immune cells toward pig aortic ECs by depleting all peripheral CD2 positive cells [135]. A similar study was performed to determine the influence of an anti-CD4 monoclonal antibody and gallium nitrate for murine cardiac allograft acceptance. During this treatment, immune processes similar to the acute graft rejection were observed. Moreover, transplanted allografts did not undergo long-term tissue remodeling [136].
Although current immunosuppression regimens are adequate for preventing acute allograft rejection, these treatments pose a risk of malignancy, infection, hypertension, diabetes and hypercholesterolemia [137]. Interestingly, ECFC express all EC markers but are negative for CD14, CD115 and CD45 [138], which might reduce the risk of immune rejection. ECFCs can be cultured in endothelial differentiation medium to induce their differentiated into ECs. In order to compare immunogenicity of allogenic ECFC derived ECs with allogenic aortic ECs (not differentiated from ECFC), J. Ladhoff, et al., seeded both of these cell types into an acellular aortic graft, which was implanted into an allogeneic mismatch rat model [139]. Their results indicated that ECFC derived ECs moderately upregulate MHC-II and are protected against allogenic humoral as well as cytotoxic T cell mediated immune responses. In addition, allogenic ECFC derived ECs showed excellent endothelialization and very mild inflammatory responses without any signs of graft rejection compared to allogenic aortic ECs [139]. Besides ECFCs, MSCs can also be used to modulate host immune responses. Allogenic MSCs can alter the cytokine secretion profile of major immune cells, including dendritic cells (DCs), natural killer (NK) cells and effector T cells [140]. It has been shown that allogenic hMSCs can reduce secretion of TNF-α, Interferon-γ and increase secretion of IL-4, IL-10 from DCs, T helper cells and macrophages [140]. These immune-modulatory properties of MSCs make them an appropriate candidate for development of vascular grafts. Another study demonstrated that IL-1β activated macrophages can induce MSC differentiation into α-actin positive SMC like cells, which can provide contractility to vascular grafts [141]. Most recently, a novel approach was developed to reduce immunogenicity of ECs by silencing the human leukocyte antigen class I (HLA I) using lentiviral vector mediated RNA interfearence (RNAi), which could silence HLA-I expression up to 67 percent [142]. Importantly, HLA I silenced ECs were positive for CD31 and vascular endothelial (VE)-cadherin, which are critical for successful tight junction formation. In addition, HLA I silenced ECs were able to synthesize vWF and NO [142]. This approach can possibly reduce immunogenicity of autologous and allogenic ECs for successful TEVG construction. It is crucial to design efficient approaches to modulate host immune responses in order to prevent TEVG rejection. These approaches might include (but are not limited to) the use of autologous cell types, systemic immune suppression treatment, inclusion of immunosuppressive antibodies into graft design and use of immunomodulatory stem cells for allogenic cellular TEVGs.
3.1.2. Cell seeding strategies
Various strategies have been developed for cell seeding in small diameter vascular grafts. In vitro cell seeding techniques include static/gravitational cell seeding, dynamic cell seeding, magnetic cell seeding and electrostatic cell seeding [143]. These techniques are briefly summarized in Figure 3.
Figure 3: Cell-seeding strategies for TEVG Endothelialization.
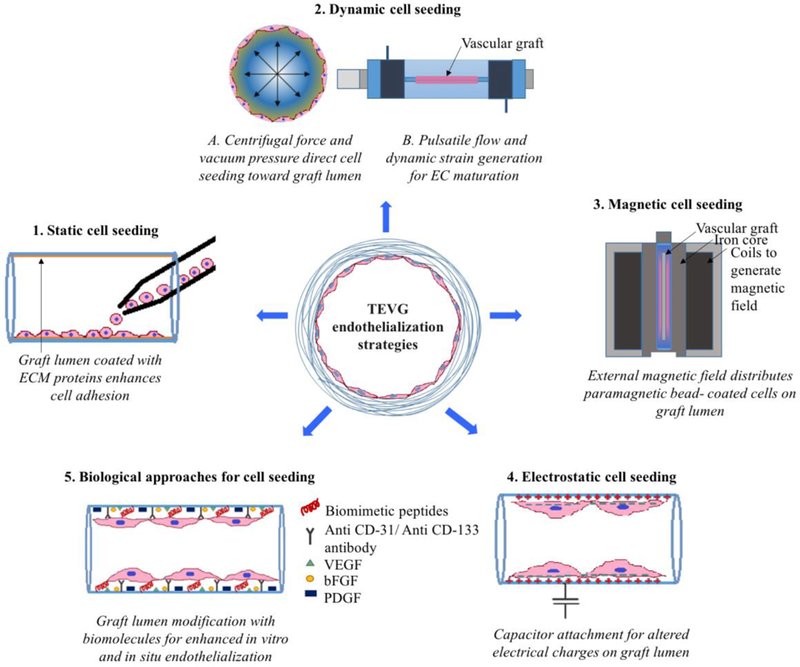
(1). Static cell seeding: ECs/ EPCs can be directly seeded on the vascular graft lumen. A graft lumen can be coated with individual or combinations of various ECM proteins for enhanced cell attachment and retention [144, 145]. (2). Dynamic cell seeding: (A) Vacuum pressure and centrifugal force applied toward graft lumen increases cell seeding efficiency and reduces cell culture period [146, 147]. (B) Bioreactors can provide a physiological pulsatile flow environment, which can be used to promote a mature, non-activated EC phenotype [148–150]. (3). Magnetic cell seeding: An external magnetic field can efficiently guide and regulate seeding of paramagnetic/superparamagnetic particle coated EC/EPCs on a graft lumen [151–153]. (4). Electrostatic cell seeding: attachment of e-PTFE graft with capacitor generates temporary positive charge on graft lumen. Cell surface is usually negatively charged; thus, this temporary positive charge on graft lumen can be used for successful adhesion of ECs/EPCs [154]. (5). Biological approaches for cell seeding: A graft lumen can be coated with biomimetic cell adhesion peptides [155–164], specific antibodies to capture ECs/EPCs [165–168] and various growth factors to promote in vitro/in situ migration and maturation of ECs/EPCs during cell culture and/or after implantation [169–173].
3.1.2.1. Static cell seeding
Static or gravitational cell seeding is the simplest technique, in which cells are seeded directly on the lumen of vascular scaffolds. Static cell seeding usually requires coating the graft lumen with cell adhesion molecules, such as ECM proteins. The cell adhesion molecule fibronectin has been used to coat small-caliber PTFE and Dacron vascular grafts in order to promote EC attachment and growth [145]. Similarly, other ECM proteins such as collagen, gelatin, fibrin and laminin, alone or in various combinations, promotes in vitro EC adhesion and proliferation on the lumen of PTFE and Dacron grafts [144]. However, static cell seeding is associated with several notable problems including non-uniform seeding, poor seeding efficiency, longer culture periods (typically 5 to 9 days), and the risk of contamination [145, 174]. In addition, coating the lumen with adhesion molecules increases the risk of platelet adhesion [175]. If the EC layer is damaged during implantation, the underlying thrombogenic adhesion molecules can promote serious complications including graft occlusion. Thus, with cellularized grafts, it is crucial to protect the surface with a confluent layer of anti-thrombotic cells.
3.1.2.2. Dynamic cell seeding
Dynamic cell seeding uses centrifugal force, vacuum pressure, and fluid sheer stress to enhance seeding efficiency. Graft porosity plays a crucial role for successful cell seeding under dynamic conditions, especially in vacuum seeding [147]. However, synthetic grafts such as PTFE and Dacron need to be pre-clotted after cell seeding in order to close pores present in the constructs. There are several challenges associated with pre-clotting procedures, as review by Burkel, W.E. [176]. For example, the pre-clotted surface is rough and can promote platelet adhesion or activation, which eventually leads to the induction of neointimal hyperplasia. Thus, patients are required to take systemic heparin treatments to prevent platelet activation after implantation [177].
Tissue engineered scaffolds can be used to mitigate these problems. It is possible to create vascular scaffolds with desired pore size and mechanical properties similar to native arteries [178]. Tissue-engineering approaches eliminate the need for pre-clotting the scaffolds with a patient’s blood. Park, I.S., et al., used a dynamic perfusion reactor for seeding cells into elastic scaffolds to form small diameter vascular grafts [146]. They seeded a collagen-SMC mixture into poly (L-lactide-co-ϵ-caprolactone) (PLCL) scaffolds under vacuum and cultured the constructs under dynamic strain in a perfusion bioreactor to promote ECM synthesis and uniform SMC distribution. ECs were seeded on the lumen of the graft under the same dynamic culture conditions to complete the TEVG. The engineered grafts showed a confluent EC layer on the lumen along with secreted elastin content similar to native arteries. [146]. Various types of bioreactors are being used to generate negative pressure for enhanced cell seeding along with dynamic fluid flow for uniform cell distribution and matrix deposition.
With advances in bioreactor design and technology, various physiological conditions can be generated during cell culture. For example, physiological pulse dynamics can be mimicked using perfusion bioreactors to improve endothelialization in small diameter TEVGs. Decellularized umbilical vein scaffolds promote efficient cell seeding and development of uniform EC monolayers on the graft lumen under axial rotation [150]. Moreover, use of perfusion circuits (flow-ramping paradigm) to generate physiological fluid sheer stress and pressure of 1.45 pascals (Pa) with pulse frequencies at 80 pulses/min significantly improves expression of a non-activated EC phenotype, which inhibits the induction of neointimal hyperplasia [150].
The physiological pulsatile flow generated by a perfusion bioreactor creates temporal and spatial variations in shear stress experienced at the graft lumen, which directly affects the function and phenotype of luminally seeded ECs. ECs respond to differences in fluid flow conditions, including laminar, sinusoidal, and physiological pulsatile flow. These different flow conditions affect EC morphology, retention rate, alignment, and apoptosis [148]. Among these various flow conditions, physiological pulsatile fluid flow with a pulse frequency of 1.25 Hz and a time-average pressure of 100 mmHg produces excellent cell retention with unidirectional EC alignment and actin bundles oriented in the same direction. In addition, physiological pulsatile fluid flow significantly reduces the rate of apoptosis compared to laminar flow and sinusoidal flow conditions [148]. Novel advances in pulsatile perfusion bioreactors are being made by optimizing culture conditions for continuous maintenance of O2 and CO2 concentrations along with constant delivery of nutrients and removal of waste products [149]. This approach eliminates the conventional need to replace culture medium.
3.1.2.3. Magnetic cell seeding
One of the major obstacles in the development of TEVGs is long cell culture periods and the maintenance of complicated dynamic physiological conditions for proper cell seeding into vascular scaffolds. A novel strategy for faster and more efficient seeding of vascular cells involves the use of magnetic beads to label desired cells and application of an external magnetic field to regulate cellular distribution. Carboxydextran coated superparamagnetic nanoparticles (Resovist®, Schering, Berlin, Germany) with a diameter of 50nm can be used to label human umbilical vein endothelial cells (HUVECs), which is a typical and widely used EC model [151]. Concentrations of superparamagnetic nanoparticles of 10–200 μg/ml do not affect cellular viability or eNOS expression. An external magnetic field can be used to seed labeled HUVECs on the lumen of a PTFE graft. In addition, cell seeding can be observed non-invasively using magnetic resonance imaging (MRI). This method produces a complete and homogeneous monolayer of HUVECs on the graft lumen. However, long-term cell retention and potential side effects from the beads needs to be evaluated to validate the usefulness of this technology.
Internalization of magnetic nanoparticles may negatively affect cellular physiology and function. Thus, non-invasive cell coating with magnetic beads may provide a better solution. Superparamagnetic polymer particles known as Dynabeads® (DynaBiotec, Oslo, Norway), have been used to coat the cell surface. Similarly, an external magnetic field is used to uniformly seed cells on graft lumens. However, the concentration of beads on a single cell surface must be optimized. Tiwari, A., et al., used CD31 coated Dynabeads to attach them on the surface of HUVECs (4, 10 and 50 beads per cell) and seeded these cells on the lumen of PTFE TEVGs using an external magnetic field [153]. Their results indicate that a higher number of beads per cell produced detrimental effects on cell proliferation and metabolism over time. Although this is an effective method for cell seeding, the internalization of the nanoparticles and their side effects were not discussed.
In addition to distributing cells on synthetic grafts, such as PTFE and Dacron, Dynabeads can be used to cover tissue-engineered scaffolds with different vascular cell types. For example, Perea, H., et al., labeled SMCs and HUVECs with CD44 and CD31 coated Dynabeads, respectively [152]. They seeded SMCs into the lumen of a collagen membrane under a magnetic field for 40 minutes, followed by HUVEC seeding for another 20 minutes under an external magnetic force of 2 pN on each magnetic bead, with 90% seeding efficiency. Magnetic cell seeding can create uniform and monolayer cell coatings on the lumen of both synthetic and natural scaffolds, accurately and within short culture times. However, the long-term cell retention, phenotypic changes, and other side effects after in vivo implantation need to be addressed.
3.1.2.4. Electrostatic cell seeding
The material properties of vascular grafts also play a crucial role in successful endothelialization processing. For example, synthetic grafts such as e-PTFE are highly negatively charged [179]. The surfaces of ECs and platelets are also negatively charged [180]. Thus, ECs must overcome the repulsive force present between the material and cell interface for successful cell attachment. To overcome this challenge, various research groups have evaluated the effects of a range of ECM protein coatings on the graft lumen for successful cell adhesion [144, 163]. However, these coatings create a risk of platelet adhesion and activation if the EC layer becomes damaged during or after implantation.
Electrostatic cell seeding temporarily alters the electrical charge of the graft lumen to enhance cell attachment. For example, e-PTFE is dielectric. When the graft is attached to a capacitor, negatively charged nuclei (electrons) of dielectric (graft) material are attracted toward the surface of the capacitor, temporarily imposing a positively charged lumen surface [154]. This temporary positive charge facilitates EC attachment. It is important to remember that the graft is made up of insulating material, so there is no electric current passing from graft to capacitor. When the graft is removed from the capacitor, the negative charge returns to the e-PTFE surface. After implantation, this negatively charged surface prevents platelet adhesion and contributes to graft patency if the EC layer becomes damaged.
In summary, small diameter vascular grafts are especially vulnerable to occlusion. They must be engineered with favorable surface properties to avoid thrombogenesis [181]. Endothelialization and chemical stability have been shown to be the most important factors controlling the thrombosis property [182]. As ECs form the interface between blood and tissues, the presence of a confluent EC monolayer in a vascular graft can inhibit the bioactive substances responsible for SMC migration and proliferation and their production of ECM, and thus improve thromboresistance and reduce intimal hyperplasia. However, innovative approaches are needed to improve the limited capacity of ECs to re-endothelialize. In the absence of a confluent endothelium, surface treatments have the potential to improve thrombosis resistance of small diameter grafts [183]. For instance, elastic laminae and modified elastin coatings, or heparin conjugates can control platelet distribution and decrease the degree of platelet activation [184].
3.2. Biological Approaches for Enhancing Cell Recruitment and Retention on TEVGs
Various biological approaches are being developed for successful in vitro and/or in situ recruitment of EPCs and ECs. These include attachment of EPC/EC specific adhesion peptides, growth factors, and EPC/EC capturing antibodies for enhanced endothelialization of the graft lumen. This section will explore the advantages and limitations associated with various biological cell adhesion approaches that have been developed for TEVGs.
3.2.1. Biomimetic peptides
Coating TEVGs with the Arg–Gly–Asp (RGD) peptide is an effective approach to promote EC adhesion and migration [185]. Several research groups have used variants of RGD peptides to enhance endothelialization, including Gly–Arg–Gly–Asp–Ser–Pro (GRGDSP) [162], RGD combined with the Pro-His-Ser-Arg-Asn (PHSRN) peptide (a fibronectin associated binding sequence), and RGD combined with the Tyr-Ile-Gly-Ser-Arg (YIGSR) peptide (part of the laminin β1 chain) [156]. However, coating the graft lumen with chemically synthesized low molecular weight RGD peptides has some limitations. For instance, instead of increasing EC adhesion, the RGD peptide can detach from the graft substrate after becoming attached to a cell’s surface via integrin receptors. In order to increase the molecular weight of RGD peptides and their retention capability on the graft lumen, a novel recombinant RGD-containing fusion protein was created by connecting the cellulose-binding domain (CBD) to RGD peptides. This fusion protein was coated on an inner gelatin layer of polyurethane grafts [158] and significantly increased EC retention and reduced platelet adhesion/activation .
Other biomimetic substances, such as the mussel inspired adhesive polydopamine, are being investigated to determine their potential for enhancing endothelialization. A study conducted by Lee et al., found that a polydopamine coating in combination with RGD and YIGSR on decellularized vein matrix promotes EPC differentiation into ECs with enhanced focal adhesions [161]. However, platelet adhesion/activation was not investigated in this study. In order to promote in situ recruitment of EPCs and ECs, a microporous PCL graft coated with fusion proteins containing the mussel adhesive protein (MAP) and RGD peptide was evaluated over four weeks in the rabbit carotid artery [159]. Results showed that the MAP-RGD promoted in situ EPC/EC recruitment with a 66% patency rate. For unknown reasons, the graft endothelialized prior to implantation was less effective at maintaining patency than the graft that was endothelialized in situ [159].
Various studies suggest that stromal cell–derived factor-1α (SDF-1α) plays a major role in the transportation of hematopoietic stem cells from bone marrow to the peripheral blood. It also promotes EPC recruitment into ischemic microenvironments and regulates neoangiogenesis [155, 163]. Thus, SDF-1α can be used to promote TEVG endothelialization. Nanofibrous vascular scaffolds prepared from a PLLA and PCL polymer blend, with the luminal surface coated with heparin and SDF-1α, reduces platelet adhesion and activation. Moreover, the combination of heparin and SDF-1α improved long-term graft patency as well as in situ recruitment of EPCs and smooth muscle progenitor cells (SMPCs). The SMPCs differentiated into SMCs, which significantly improved the elastic modulus of the grafts [164].
Currently, peptides/proteins that bind selectively to ECs or EPCs are being developed to allow highly specific cell recruitment for endothelialization with minimal risk of platelet adhesion and activation side-effects. The basement membrane that separates ECs and SMCs in arteries contains a high collagen type IV content. Trimeric peptides found with high redundancy in human collagen type IV were screened using peptide array based cell adhesion effects [186]. By comparing results between EC and SMC adhesion using cysteine-alanine-glycine (CAG) peptides, it was found that CAG has a high affinity for ECs with minimal cross-reactivity for SMCs [160]. Small caliber PCL grafts containing the CAG peptide sequence, incorporated by blending the CAG peptide with the polymer during electrospinning, experience a significantly increased endothelialization and eNOS expression after implantation compared to grafts without the CAG peptide. [160]. Hao, D., et al., studied the cell recruitment potential for the LXW7 ligand, which specifically binds to the αvβ3 integrin present on the EC/EPC surface. LXW7 is a disulfide cyclic octa-peptide with unnatural amino acids flanking both sides of the main functional motif. Due to the presence of unnatural amino acids, it is more resistant to proteolysis in vivo compared to linear and natural amino acid containing peptides. [157]. They found that the LXW7 ligand exhibits very strong binding affinity toward ECs/EPCs and weaker binding to platelets compared to the conventional GRGD ligand for αvβ3 integrin. Moreover, it promotes cellular proliferation, possibly due to activation of VEGFR2 and the MAPK pathway [157]. These EPC/EC specific proteins/peptide sequences can significantly improve the rate of endothelialization in vitro and/or after graft implantation.
3.2.2. Growth factors
VEGF and bFGF are known regulators of angiogenesis, vascular growth, and blood vessel maturation. VEGF in particular is a potent mitogen and chemoattractant for ECs [187]. Thus, it is a good promoter for TEVG endothelialization [188]. Decellularized carotid arteries can be coated with VEGF and heparin [173]. This modified graft can sustainably release VEGF up to 20 days and promotes complete endothelialization within 6 months of implantation. Moreover, they showed significantly reduced neointimal hyperplasia and increased patency compared to unmodified grafts following in vivo implantation [173]. In addition to decellularized scaffolds, nanofiberous scaffolds coated with VEGF and heparin exhibited anticoagulation properties and enhanced EPC growth in vitro [169].
PDGF plays a critical role in the recruitment of pericytes and smooth muscle cells, which stabilizes blood vessels [172]. Multilayer electrospun nanofibrous scaffolds with an inner layer of poly(ethylene glycol)-b-poly(Llactide-co-ε-caprolactone) (PELCL) along with gelatin and VEGF, a middle layer containing poly(L-lactide-co-glycolide) (PLGA) along with gelatin and PDGF and an outer layer containing poly(ε-caprolactone) (PCL) enhances recruitment of vascular ECs and SMCs. Release of VEGF and PDGF increases endothelialization and inhibits SMC hyperproliferation, with enhanced patency over 8 weeks [170]. The crosslinking process to conjugate various growth factors depends on the type of scaffold (i.e. synthetic grafts, decellularized scaffolds, and electrospun scaffolds), and details can be found in articles cited for each graft type.
In addition to growth factor coatings, plasmids encoding genes of specific growth factors can be incorporated into the graft lumen. Lahtinen, M., et al., developed this novel approach for vascular graft endothelialization via incorporation of plasmids encoding the human VEGF165 and FGF-2 genes into the lumen of ePTFE and pre-clotted polyester grafts [171]. They found that the VEGF165 plasmid improves graft endothelialization while combined FGF-2 and VEGF165 plasmids reduce graft endothelialization. They also found that VEGF165 plasmid incorporation into pre-clotted polyester grafts increases endothelialization and patency relative to ePTFE grafts [171].
3.2.3. Antibody coating
Antibodies can specifically target and recruit ECs / EPCs on the graft lumen by engaging with specific surface antigens or receptors. For example, several studies have reported that coating cardiovascular stents with anti-CD34 antibodies enhances in situ recruitment, adhesion, and proliferation of ECs and circulating EPCs [189]. Similarly, ePTFE graft lumens can be coated with anti-CD34 antibodies using peptide linkages for faster endothelialization and enhanced patency [168]. After 28 days of implantation into pig models, 85% of the graft surface was covered with an EC layer compared to 32% EC coverage in non-coated grafts. However, despite a promising endothelialization, anti-CD34 antibody coated grafts evoke an excessive intimal hyperplasia at the anastomoses [168]. Similarly, another study showed that anti-CD34-coating significantly increases platelet adhesion along with protein adsorption, compared to non-coated grafts [167].
Although an anti-CD34 coating improves endothelialization, this approach risks platelet adhesion and induction of neointimal hyperplasia and thus alternative solutions need to be investigated. In situ recruitment of EPCs can be achieved by coating antibodies targeted toward EPC specific cell receptors. CD133 is an EPC specific surface receptor that can be used to enhance EPC recruitment on the graft lumen using an anti-CD133 coating [166]. ePTFE grafts coated with multilayers of anti-CD133 along with heparin and collagen showed accelerated endothelialization and patency at 7 days after implantation in a pig model [165]. However, long-term patency has yet to be investigated. Although antibody coatings accelerate cell recruitment with high cell type specificity, increased manufacturing costs compromise their commercial application for TEVG production.
3.3. Modification of Blood-Contacting Surface
Synthetic, naturally derived, and biodegradable TEVGs require blood-contacting surface processing to reduce thrombogenicity and maintain patency. Systemic anticoagulatant drug regimens are commonly used to maintain graft patency in clinical applications, particularly when working with synthetic materials. Modification of the blood-contacting surface can reduce or eliminate the need for such treatments, decreasing the corresponding risk of bleeding by restricting anticoagulation effects locally to the TEVG. This section will examine methods currently employed to produce successful blood-contacting TEVG surfaces.
3.3.1. Elastic lamina and modified elastin as blood-contacting surface
Elastin is a key regulator within the vasculature. In addition to providing the physical elasticity and resilience necessary for the conduction of pulsatile blood flow, elastin regulates the migration and proliferation of SMCs by inducing a quiescent, contractile phenotype via a non-integrin dependent G-protein coupled signaling pathway [190]. Elastin exhibits antithrombic and inflammation-resistant properties in vivo, resisting the adhesion and activation of leukocytes and platelets, suggesting a lack of integrin binding sites on these cell types [191].
These properties make elastin an attractive choice as a blood-contacting material. Elastin has been successfully employed as a blood contacting surface in a small diameter vascular graft by McCarthy et al [192]. The elastin-rich elastic laminae of donor vessels was decellularized and mechanically reinforced with biodegradable poly(ether urethane) (PEU). This study utilized naturally sourced elastin. However, elastin can also be used to coat synthetic materials through use of its precursor, tropoelastin, and peptide mimics, as will be discussed later [193].
3.3.1.1. Elastin mimicry
Synthetic materials can be fabricated with elastin-mimicking surfaces or coated with elastin-mimicking peptides to activate the receptor-mediated signaling mechanisms that inhibit platelet and inflammation activation [194]. These mechanisms are incompletely understood, but are thought to be due to the lack of integrin binding sites for elastin. It has been shown that the elastin-rich arterial elastic laminae is able to suppress inflammatory responses, in part by inhibiting leukocyte adhesion via activation of Src homology 2 domain-containing protein-tyrosine phosphatase (SHP)-1 through signal regulatory protein (SIRP) α [195]. Elastin contains ~75% hydrophobic amino acids and is therefore highly insoluble [196]. Novel approaches to synthetically mimic elastin have produced materials with a similar inhibition of platelet adhesion and activation [194, 197–199]. Polyurethane surfaces modified with peptides modeled from the cross linking domains of elastin have been used to crosslink elastin-like peptides to their surfaces, resulting in decreased platelet adhesion and activation, increased endothelialization, and increased eNOS expression relative to uncoated controls in vitro [194, 197]. Inspired by the ability of elastin to self-aggregate and self-organize [199], researchers were able to genetically engineer elastin mimics capable of both chemical and physical crosslinking [198]. The elastin mimic LysB10, a triblock amphiphilic copolymer with hydrophobic endblocks and a hydrophilic midblock, was able to maintain complete patency for a two week in vivo study when used as the blood contacting surface for an acellular vascular graft [200]. Histological evaluation identified a stable neointima positive for vWF, indicating the acellular graft had successfully re-endothelialized.
3.3.1.2. Tropoelastin
The elastin precursor, tropoelastin, rapidly and spontaneously self-aggregates under physiological conditions to be cross-linked into elastin fibers by the recruitment of the enzyme lysyl oxidase [201]. This property made isolation and extraction difficult and inefficient prior to the development of a synthetic elastin gene in E. Coli [202]. Tropoelastin has since been covalently coated on metal stents [203] and polymer vascular grafts [204] where it has successfully imparted anti-thrombogencity, reduced SMC proliferation, and enhanced the development of a stable endothelium to improve biocompatibility. Another study was able to determine that the first 10 N-terminal domains (N10) and first 18 N-terminal domains (N18) of tropoelastin facilitate EC attachment and proliferation [205]. These domains retained the anti-thrombogenicity of full-length tropoelastin when plasma coated onto stainless steel and evaluated in vitro.
Elastin provides a promising biocompatible material for the construction of vascular grafts, able to closely recreate the natural structure and mechanics of native tissue while providing a hemocompatible surface for immediate biocompatibility and being able to regulate EC and SMC proliferation for long term stability [206]. A close recreation of native tissue mechanics may be more important than previously thought. While many researchers attempt to approximate native compliance and elasticity as closely as is necessary to facilitate undisturbed flow without risk of aneurysm or burst, recent work has implicated alterations in the mechanical properties of blood vessels as risk factors for atherosclerosis [207].
3.4. Development of Scaffolds Incorporating Anticoagulation Molecules
A variety of methods have been developed to actively maintain TEVG patency. Figure 4 illustrates the mechanisms of some of these methods. Many of these methods, such as heparin, inhibit coagulation by targeting either thrombin or platelets. Heparin is perhaps the most widely utilized antithrombic agent for coating blood-contacting TEVG surfaces and vascular devices. This well characterized molecule has been reproduced synthetically and is therefore inexpensive and readily available. But alternatives, such as argatroban are available if necessary [208].
Figure 4. Inclusion of anticoagulation molecules in TEVGs.

(1) Thrombomodulin (TM) is a transmembrane protein present on ECs that binds thrombin. The thrombin-TM complex activates protein C (PC, APC), which then forms a membrane-bound complex with protein S (S) that inactivates factors Va and VIIIa. Recombinant TM can be covalently bound to synthetic materials [209]. (2) Argatroban locally and directly inhibits thrombin by non-covalently interacting with Asp-189 in the S1 binding pocket of thrombin to block its enzymatic activity. Truncated forms were developed which retained the guanidyl group for ionic interaction and featured a spacer arm on the piperazinyl amide moiety for surface grafting [210]. (3) Nitric oxide (NO) activates soluble guanylate cyclase (sGC) to increase cyclic guanosine monophosphate (cGMP) production by binding to the sixth position of the heme ring, breaking the bond to His-105. cGMP then decreases expression of thromboxane A2 and the platelet surface adhesion protein P-selectin. (4) Silver nanoparticles (SNP) inhibit platelet aggregation by blocking biochemical pathways associated with membrane restructuring, preventing the expression of surface adhesion proteins.
3.4.1. Thrombin regulation
The enzyme thrombin plays a pivotal role in coagulation. It is responsible for the activation of platelets, the conversion of fibrinogen into a fibrin network during clotting, and provideing feedback amplification for coagulation. However, thrombin is not exclusively a procoagulant. Thrombomodulin, a membrane protein present on ECs, has the ability to bind thrombin, which activates protein C and exerts an inhibitory effect on the coagulation system [211]. Thus, a variety of approaches aimed at imparting anticoagulation properties to biomaterials target thrombin.
3.4.1.1. Thrombomodulin
Since thrombomodulin not only prevents thrombin from serving as a procoagulation factor but also repurposes it for anticoagulation, engineering active thrombomodulin onto blood contacting surfaces would locally generate protein C to inhibit thrombin activation. One study aimed at optimizing surface activity by controlling the random orientation associated with traditional covalent linkages utilized a truncated form of thrombomodulin containing the binding sites for thrombin and protein C. The truncated thrombomodulin was selectively bound to the surface of ePTFE grafts through Staudinger ligation [209]. For evaluation purposes, an ex vivo femoral arteriovenous shunt model was used in which the treated ePTFE grafts were placed between a collagen coated ePTFE graft, as a thrombogenic source, and an expansion chamber. The authors found a near 50% reduction in platelet deposition in the expansion chamber compared to ePTFE grafts without the truncated thrombomodulin [209].
3.4.1.2. Argatroban
Argatroban interacts with Asp-189 of thrombin, also known as the specificity pocket S1 binding site, to directly and locally inhibit thrombin. Argatroban is one of only two synthetic thrombin inhibitors currently approved for medical use by the FDA [210, 212]. Argatroban loaded small diameter (1–3mm) vascular grafts fabricated by subcutaneous implantation and maturation of silicone rods exhibited patency for up to 12 weeks in 3/4 samples implanted into rabbit carotid arteries, as compared to 0/4 patency by 2 weeks for untreated grafts [213]. These grafts were composed primarily of collagen and fibroblasts, known to trigger severe thrombosis [214]. Argatroban has also been used as an antithrombic coating for balloon catheters and stents [215]. Nakayama et al. developed water-soluble argatroban for improved adsorption on biological materials by utilizing ionization of the carboxyl group to dissolve it in an alkaline solution, followed by freeze-drying to produce a water-soluble argatroban sodium salt [216]. Small diameter vascular grafts (1.5mm) and heart tri-leaflet valves formed via subcutaneous implantation of silicone molds treated with the water-soluble argatroban avoided any signs of thrombus or neointimal hyperplasia for up to 12 weeks in a canine model, although they were covered by native tissue [216].
3.4.2. Platelet regulation
The activation of a platelet negatively charges its surface by activating scramblase, which transports negatively charged phospholipids from its inner to outer membrane and creates an optimal surface for binding the tenase and prothrombic complex [217]. Regulating the activation or aggregation of platelets thus offers another target for controlling coagulation.
3.4.2.1. Silver nanoparticles
While investigating the potential for spherical silver nanoparticles (SNP) (10–15 nm in diameter) as cytotoxic antimicrobials, it was found that they inhibited platelet aggregation in a concentration dependent manner [218]. Further characterization of this phenomenon determined that platelets incubated with SNP retained significantly reduced aggregation potential after removal of the nanoparticles. Thrombin stimulation increased release of ATP/ADP from platelets. Pretreatment with SNP significantly reduced ATP/ADP release in aggregated platelets, indicating the SNP interfered with an integrin-mediated mechanism since platelet aggregation involves ligation of surface integrins [218]. More recent studies have suggested that SNPs reversibly inhibit platelet aggregation by blocking biochemical pathways associated with platelet membrane restructuring during activation [219]. This activity is critically dependent upon their size, as SNPs larger than 24 nm have been found to promote platelet activation and the coagulation cascade [220]. SNPs have been synthesized and immobilize onto a porous PCL scaffold through the reaction of PEG and silver nitrate to inhibit platelet adhesion and aggregation in vitro while retaining antimicrobial properties [219, 221]. The aggregation of SNP in the medium indicates the protective PEG coating is lost as SNP leach out of the scaffold, but will not cause any cytotoxic effects as long as the initial concentration is set optimally low.
3.4.2.2. Nitric oxide (NO)
NO primarily serves as a vasodilator in the regulation of blood pressure and as a cytotoxic agent for nonspecific immune responses. NO also inhibits platelet adhesion and aggregation via the activation of soluble guanylate cyclase and consequent increase in the concentration of cyclic guanosine monophosphate (GMP) [222, 223]. Platelets themselves produce NO as a negative feedback mechanism against aggregation, and its short half-life makes NO an ideal agent to achieve local, biocompatible inhibition of thrombosis [223, 224]. Recently, Gao et al. developed a small diameter (2mm) vascular graft from electrospun PCL with a surface treatment of organoselenium modified polyethyleneimine (SePEI) to catalyze the production of NO in situ [225]. The grafts displayed a steady generation of NO in vitro that could be controlled by adjusting the number of SePEI layers, allowing it to promote rapid adhesion and proliferation of ECs while inhibiting the adhesion and activation of SMCs and macrophages. The use of an NO generating catalyst rather than NO storage avoids the limitations of NO depletion and cytotoxic complications from a burst release profile.
3.4.2.3. Heparin
Heparin is a glycosaminoglycan (GAG) composed of alternating chains of D-glucosamine and uronic acid residues [226, 227]. It functions as an anticoagulant by binding with AT III, enacting a conformational change that enhances the ability of AT III to inactivate thrombin by forming a ternary complex with heparin and thrombin [228]. However, the unique pentasaccharide responsible for facilitating this binding is only present in about a third of heparin molecules, making its effectiveness variable [229]. Heparin is heterogenous in molecular size, with molecular weight ranging from 5,000 to 30,000 with a mean molecular weight of 15,000, or approximately 50 monosaccharide chains [226]. Heparin molecules shorter than 18 chains are unable to simultaneously bind thrombin and AT III, but retain anticoagulation properties by their ability to catalyze AT III to inhibit factor Xa [230]. Heparin is also able to catalyze the inhibition of thrombin through heparin cofactor II. This cofactor does not require the AT III-binding pentasaccharide, is specific for thrombin, but requires much higher doses of heparin for comparative effectiveness to AT III catalysis [231]. Investigation into this alternative mechanism led to the development of low molecular weight (LMW) heparin, however a detailed distinction will not be drawn here due to their similar clinical effectiveness. Barrowcliffe’s History of Heparin provides a comprehensive review [232]. More recently, heparin has found application in the biomedical field as a means to improve hemocompatibility of biomaterials by reducing platelet adhesion, inhibiting coagulation kinetics, and supporting re-endothelialization even on synthetic materials [233]. Clinical trials have determined that heparin immobilized to the surface of ePTFE vascular grafts via covalent bonding remain bioactive for up to 12 weeks after implantation, producing results comparable to autologous grafting and preferable when the saphenous vein is absent or unsuitable for transplantation, as may be the case in systemic CVD [234]. Materials may also be engineered to release heparin by utilizing responsive polymers to break the conjugate bonds [235].
Of particular interest is the application of heparin to vascular scaffolds, where thrombosis poses a serious challenge to the use of synthetic materials and the ideal vascular replacement is one that promotes native tissue regrowth. Several long-term clinical studies showed that heparin significantly improves the patency of synthetic vascular grafts, concurrent with the results of animal studies, but host integration has remained elusive [236, 237, 238]. Stable binding of heparin to the luminal surface of ePTFE grafts significantly improved patency compared to untreated controls in the canine model and remained active for up to 12 weeks [238]. A 1-year clinical trial comparing heparin-bonded PTFE to untreated PTFE grafts in femoral artery bypass found patency rates favoring the heparin-bonded grafts (86.4% to 79.9% primary patency, 88% to 81% secondary patency) with an overall 37% reduction in risk of graft failure [239]. Another 5-year clinical study comparing heparin-bonded Dacron to untreated PTFE in femoral artery bypass found similar results (46% to 35% primary patency, 47% to 36% secondary patency) [237].
Heparin holds potential to further enhance vascular engineering through its ability to promote endothelialization and regulate SMCs [240]. Heparin contributes to the hemocompatability of synthetic scaffolds, especially in the case of acellular biodegradable scaffolds where they endow an immediate, off-the-shelf potential [241]. Novel approaches are being developed to exploit heparin-mediated tissue regrowth activity. Smith et al. demonstrated that a heparin-bound poly-L-lysine (PLL) surface could be used to immobilize VEGF for the capture of ECs under shear with high selectivity (37 +/− 2 cells/mm2 at shear of 15 dyne/cm2 compared to 10 +/− 4 cells/mm2 for heparin controls without immobilized VEGF) [242]. This highlights the potential use of heparin’s growth factor binding properties to accelerate endothelialization and graft integration under in vivo flow. A similar method was employed to recruit a confluent and functional endothelium on an acellular TEVG fabricated from small intestinal submucosa (SIS), within one month in a sheep model [243]. The graft possessed functional host SMCs at one month, and both endothelium and SMCs were aligned with flow direction and vessel circumference, respectively, after three months. In another study, the sequential surface modification with thrombomodulin followed by heparin imparted a strong local anticoagulation effect via generation of activated protein C (APC). This effect was demonstrated in an arteriovenous (AV) shunt constructed from decellularized porcine SIS, using a baboon model [244]. Covalent attachment of heparin to a decellularized rat pancreas scaffold significantly improved the proliferation and growth of seeded HUVECs [245]. These successes demonstrate that heparinized TEVG surfaces can effectively inhibit thrombogenesis and stimulate endothelialization.
4. Conclusion Remarks and TRANSLATIONAL CHALLENGES
Although considerable effort has been invested to develop small-diameter TEVGs, presently there is no commercialized vascular graft for small diameter vessels, including coronary arteries. The failures of small diameter vascular grafts are generally associated with thrombosis, intimal hyperplasia, or inflammation, all linked to the blood-contacting TEVG surface. The blood interface has thus become an important focus for translational applications. Some clinical results are encouraging and present attractive directions for future development.
The early clinical trials for small vascular grafts produced by cell self-assembly were undertaken for hemodialysis access. The L’Heureux’s group clinically tested the durability of a 4.8 mm diameter TEVG with autologous fibroblasts and ECs obtained from small skin and vein biopsies. The grafts were grown in culture supplemented with bovine serum and implanted as arteriovenous shunts in ten patients receiving hemodialysis through an access graft that had at least one previous failure. Three grafts failed within the first three months, one patient was withdrawn because of severe gastrointestinal bleeding, one died of unrelated cause, and five grafts functioned for six to twenty months. Although an endothelium was included in the lumen, the graft failure at mid- to long-term was associated with the formation of thrombosis and aneurysm. A major limitation of this approach is the long time required to build the completely autologous vascular graft [246]. To address this problem, the group reported in 2011 the first implantation of a frozen, devitalized, completely autologous Lifeline™ 4.8 mm vascular graft derived entirely from a fibroblast layer. Living autologous ECs were seeded in the lumen five days before implantation. The graft functioned effectively within eight weeks in a patient who required a semi-permanent dialysis catheter [247]. Grafts produced by this method can be stored “off-the-shelf”. The grafts were implanted into three hemodialysis patients and no degradation or dilation were observed up to eleven months [248]. However, the time required to prepare the grafts and the lack of mature elastin may limit the application of the technology. While vascular grafts produced by self-assembly approaches have proceeded to more clinical trials, they cannot be conveniently commercialized. Although self-assembly grafts can utilize devitalization to achieve long-term storage similar to scaffold-based designs, doing so still requires a preemptive harvest to collect autologous cells.
To solve the problems associated with “off-the-shelf” grafts, Dahl and coworkers from Humacyte Inc. constructed a completely biological tubular small-diameter vascular graft (3 to 6 mm in diameter) using cadaver SMC secreted ECM [249]. These decellularized grafts can be stored for at least 12 months and provide “off-the-shelf” grafts without using anti-thrombotic drugs for clinical use [250]. The clinical trials for the 6 mm acellular grafts have been registered both in Poland and the USA. The grafts were implanted as arteriovenous (AV) conduits in 40 patients with end-stage renal disease, in Poland starting from December 2012 (ClinicalTrials.gov, NCT01744418), followed by a U.S. clinical trial in 20 patients with end-stage renal disease, beginning in May 2013 (ClinicalTrials.gov, NCT01840956). The study reported adequate blood flow through the vessels, no dilation and no post-cannulation bleeding after 16 months [251]. The clinical trials are still ongoing. Clinical trials of completely biological “off-the-shelf” small-diameter vascular grafts have achieved promising initial results, but their long-term efficacy still needs to be assessed.
An ideal small-diameter vascular graft should be “off-the-shelf”, anti-thrombotic, immune-compatible, compliant and eventually completely integrated into native vessels. Biodegradable materials hold the greatest promise for engineering “off-the-shelf” vascular grafts. However, their mechanical properties, composition, degradation behavior, and cell recruitment ability need to be precisely controlled to realize a physiologically relevant vessel structure with a stable endothelium to maintain long-term graft patency. Most recently, a 3D printing technology has been developed to print vascular grafts [252]. Although the technology itself is encouraging for biomanufacturing and scaling-up biological tissues, developmental challenges for vascular grafts are still related to materials, cells and the blood-contracting surface. Improved strategies are in high demand for engineering functional small-diameter TEVGs.
ACKNOWLEDGEMENTS
This study was supported by the National Institutes of Health (1R15CA202656 and 1R15HL115521–01A1) and the National Science Foundation (1703570).
Contributor Information
Daniel Radke, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
Wenkai Jia, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
Dhavan Sharma, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
Kemin Fena, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
Guifang Wang, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
Jeremy Goldman, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
Feng Zhao, Department of Biomedical Engineering, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, U.S..
REFERENCES
- [1].Xu J, Murphy SL, Kochanek KD, Bastian BA, National Vital Statistics Reports 2016, 64, 39. [PubMed] [Google Scholar]
- [2].Mendis S, et al. , The World Health Organization, Geneva, Switzerland: 2014. [Google Scholar]
- [3].Pashneh-Tala S, MacNeil S, Claeyssens F, Tissue Engineering. Part B, Reviews 2016, 22, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lin S, Sandig M, Mequanint K, Tissue engineering. Part A 2011, 17, 1561. [DOI] [PubMed] [Google Scholar]
- [5].Rubanyi GM, J Cardiovasc Pharmacol 1993, 22 Suppl 4, S1. [DOI] [PubMed] [Google Scholar]
- [6].McGuigan AP, Sefton MV, Biomaterials 2007, 28, 2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sarkar S, Sales KM, Hamilton G, Seifalian AM, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2007, 82B, 100. [DOI] [PubMed] [Google Scholar]
- [8].Dee KC, Puleo DA, Bizios R, An Introduction to Tissue-Biomaterial Interfaces, John WIley & Sons, Hoboken, New Jersey: 2002. [Google Scholar]
- [9].Gimbrone MA, García-Cardeña G, Circulation Research 2016, 118, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davie EW, Fujikawa K, Kisiel W, Biochemistry 1991, 30, 10363. [DOI] [PubMed] [Google Scholar]
- [11].Beumer S, IJsseldijk M, de Groot PG, Sixma JJ, Blood 1994, 84, 3724. [PubMed] [Google Scholar]
- [12].Li W, Johnson DJD, Esmon CT, Huntington JA, Nat Struct Mol Biol 2004, 11, 857. [DOI] [PubMed] [Google Scholar]
- [13].Wang Z, Sun B, Zhang M, Ou L, Che Y, Zhang J, Kong D, Journal of Bioactive and Compatible Polymers 2012, 28, 154 [Google Scholar]
- Melchiorri AJ, Hibino N, Yi T, Lee YU, Sugiura T, Tara S, Shinoka T, Breuer C, Fisher JP, Biomacromolecules 2015, 16, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER, Proceedings of the National Academy of Sciences 2000, 97, 6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PH, Chen C, Bush RL, Yao Q, Lumsden AB, Hanson SR, Journal of Vascular Surgery 2004, 39, 1322. [DOI] [PubMed] [Google Scholar]
- Lord MS, Yu W, Cheng B, Simmons A, Poole-Warren L, Whitelock JM, Biomaterials 2009, 30, 4898. [DOI] [PubMed] [Google Scholar]
- [14].Gorbet MB, Sefton MV, Biomaterials 2004, 25, 5681. [DOI] [PubMed] [Google Scholar]
- [15].Bevilacqua M, GIMBRONE M JR, “of Endothelial-Leukocyte Adhesion”, presented at Leukocyte Adhesion Molecules: Proceedings of the First International Conference on :” Structure, Function and Regulation of Molecules Involved in Leukocyte Adhesion”, Held in Titisee, West Germany, September 28-October 2, 1988, 2012. [Google Scholar]
- [16].Csiszar A, Wang M, Lakatta EG, Ungvari Z, Journal of Applied Physiology 2008, 105, 1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Frölich J, Journal of hypertension. Supplement: official journal of the International Society of Hypertension 1990, 8, S73. [PubMed] [Google Scholar]
- MONCADA S, British journal of pharmacology 1982, 76, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Yamaki F, Koike K, Toro L, Current Medicinal Chemistry-Cardiovascular & Hematological Agents 2004, 2, 257. [DOI] [PubMed] [Google Scholar]
- [18].Loscalzo J, Circulation research 2013, 113, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olesen S-P, Claphamt D, Davies P, Nature 1988, 331, 168. [DOI] [PubMed] [Google Scholar]
- [20].Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM, Merz SI, Meyerhoff ME, Bendick PJ, Zelenock GB, Shanley CJ, Journal of Vascular Surgery 2004, 40, 803. [DOI] [PubMed] [Google Scholar]
- Chen S, An J, Weng L, Li Y, Xu H, Wang Y, Ding D, Kong D, Wang S, Materials Science and Engineering: C 2014, 45, 49125491855 [Google Scholar]
- Lim SH, Cho S-W, Park J-C, Jeon O, Lim JM, Kim S-S, Kim B-S, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2008, 85B, 537. [DOI] [PubMed] [Google Scholar]
- Ponnuswamy P, Schröttle A, Ostermeier E, Grüner S, Huang PL, Ertl G, Hoffmann U, Nieswandt B, Kuhlencordt PJ, PLOS ONE 2012, 7, e30193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stow LR, Jacobs ME, Wingo CS, Cain BD, The FASEB Journal 2011, 25, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koo A, Dewey CF, García-Cardeña G, American Journal of Physiology - Cell Physiology 2013, 304, C137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, Nat Clin Pract Cardiovasc Med 2009, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM, Nature 1999, 399, 601. [DOI] [PubMed] [Google Scholar]
- Nam D, Ni C-W, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H, American Journal of Physiology - Heart and Circulatory Physiology 2009, 297, H1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt A-M, Stern DM, Blood 1998, 91, 3527. [PubMed] [Google Scholar]
- [25].Hoffman AS, ACS Publications, 1982. [Google Scholar]
- [26].Naito Y, Shinoka T, Duncan D, Hibino N, Solomon D, Cleary M, Rathore A, Fein C, Church S, Breuer C, Advanced drug delivery reviews 2011, 63, 312. [DOI] [PubMed] [Google Scholar]
- [27].Woodhouse KA, Klement P, Chen V, Gorbet MB, Keeley FW, Stahl R, Fromstein JD, Bellingham CM, Biomaterials 2004, 25, 4543. [DOI] [PubMed] [Google Scholar]
- [28].Patel A, Fine B, Sandig M, Mequanint K, Cardiovascular research 2006, 71, 40. [DOI] [PubMed] [Google Scholar]
- [29].Waterhouse A, Wise SG, Ng MK, Weiss AS, Tissue Engineering Part B: Reviews 2011, 17, 93. [DOI] [PubMed] [Google Scholar]
- Scandolera A, Odoul L, Salesse S, Guillot A, Blaise S, Kawecki C, Maurice P, El Btaouri H, Romier-Crouzet B, Martiny L, Frontiers in pharmacology 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki C, Hézard N, Bocquet O, Poitevin G, Rabenoelina F, Kauskot A, Duca L, Blaise S, Romier B, Martiny L, Arteriosclerosis, thrombosis, and vascular biology 2014, ATVBAHA. 114.304432. [DOI] [PubMed] [Google Scholar]
- [30].Barnes M, Maclntyre D, Pathophysiology of Haemostasis and Thrombosis 1979, 8, 158. [Google Scholar]
- [31].Simionescu DT, Lu Q, Song Y, Lee J, Rosenbalm TN, Kelley C, Vyavahare NR, Biomaterials 2006, 27, 702. [DOI] [PubMed] [Google Scholar]
- [32].Hinds MT, Rowe RC, Ren Z, Teach J, Wu PC, Kirkpatrick SJ, Breneman KD, Gregory KW, Courtman DW, Journal of Biomedical Materials Research Part A 2006, 77, 458. [DOI] [PubMed] [Google Scholar]
- [33].Lovett M, Eng G, Kluge J, Cannizzaro C, Vunjak-Novakovic G, Kaplan DL, Organogenesis 2010, 6, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, Asakura T, Sata M, Journal of vascular surgery 2010, 51, 155. [DOI] [PubMed] [Google Scholar]
- [35].Soffer L, Wang X, Zhang X, Kluge J, Dorfmann L, Kaplan DL, Leisk G, Journal of Biomaterials Science, Polymer Edition 2008, 19, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu H, Li X, Zhou G, Fan H, Fan Y, Biomaterials 2011, 32, 3784. [DOI] [PubMed] [Google Scholar]
- [37].Heino J, Matrix Biology 2000, 19, 319. [DOI] [PubMed] [Google Scholar]
- [38].Badimon L, Badimon JJ, Turitto VT, Vallabhajosula S, Fuster V, Circulation 1988, 78, 1431. [DOI] [PubMed] [Google Scholar]
- Wu D, Vanhoorelbeke K, Cauwenberghs N, Meiring M, Depraetere H, Kotze HF, Deckmyn H, Blood 2002, 99, 3623. [DOI] [PubMed] [Google Scholar]
- [39].Davies MG, Hagen PO, The British journal of surgery 1994, 81, 1254. [DOI] [PubMed] [Google Scholar]
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA, Nature 1998, 391, 591. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA, Blood 1996, 88, 146. [PubMed] [Google Scholar]
- Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA, The Journal of experimental medicine 2000, 192, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Furie B, Furie BC, Cell 1988, 53, 505. [DOI] [PubMed] [Google Scholar]
- [41].Huynh T, Abraham G, Murray J, Brockbank K, Hagen P-O, Sullivan S, Nature biotechnology 1999, 17, 1083. [DOI] [PubMed] [Google Scholar]
- [42].Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, DiMuzio PJ, Journal of vascular surgery 2004, 40, 146. [DOI] [PubMed] [Google Scholar]
- [43].Conklin B, Richter E, Kreutziger K, Zhong D-S, Chen C, Medical engineering & physics 2002, 24, 173. [DOI] [PubMed] [Google Scholar]
- [44].Rothuizen TC, Damanik FF, Lavrijsen T, Visser MJ, Hamming JF, Lalai RA, Duijs JM, van Zonneveld AJ, Hoefer IE, van Blitterswijk CA, Biomaterials 2016, 75, 82. [DOI] [PubMed] [Google Scholar]
- Rothuizen TC, Damanik FF, Anderson JM, Lavrijsen T, Cox MA, Rabelink TJ, Moroni L, Rotmans JI, Tissue Engineering Part C: Methods 2014, 21, 436. [DOI] [PubMed] [Google Scholar]
- [45].Gui L, Muto A, Chan SA, Breuer CK, Niklason LE, Tissue Engineering Part A 2009, 15, 2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang Y, Gallant RC, Ni H, Current opinion in hematology 2016, 23, 280. [DOI] [PubMed] [Google Scholar]
- [47].Xing Q, Qian Z, Tahtinen M, Yap AH, Yates K, Zhao F, Advanced Healthcare Materials 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Quint C, Arief M, Muto A, Dardik A, Niklason LE, Journal of vascular surgery 2012, 55, 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xing Q, Yates K, Tahtinen M, Shearier E, Qian Z, Zhao F, Tissue engineering. Part C, Methods 2015, 21, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Vogt C, Leong KW, Zhao F, Advanced functional materials 2014, 24, 3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Syedain ZH, Meier LA, Lahti MT, Johnson SL, Tranquillo RT, Tissue engineering. Part A 2014, 20, 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Bianco R, Tranquillo RT, Nature Communications 2016, 7, 12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Syedain ZH, Graham ML, Dunn TB, O’Brien T, Johnson SL, Schumacher RJ, Tranquillo RT, Science translational medicine 2017, 9. [DOI] [PubMed] [Google Scholar]
- [53].Walpoth BH, Bowlin GL, Expert Review of Medical Devices 2005, 2, 647. [DOI] [PubMed] [Google Scholar]
- [54].Damme HV, Deprez M, Creemers E, Limet R, Acta chirurgica belgica 2005, 105, 249. [DOI] [PubMed] [Google Scholar]
- [55].Clowes AW, Gown A, Hanson S, Reidy M, The American journal of pathology 1985, 118, 43. [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Kotze HF, Savage B, Harker LA, Arteriosclerosis, Thrombosis, and Vascular Biology 1985, 5, 595. [DOI] [PubMed] [Google Scholar]
- [56].Gabriel M, Weiler H, Mehlhorn U, Vahl C-F, The Thoracic and Cardiovascular Surgeon 2014, 62, OP53 [Google Scholar]
- Gabriel M, Niederer K, Becker M, Raynaud CM, Vahl C-F, Frey H, Bioconjugate chemistry 2016, 27, 1216. [DOI] [PubMed] [Google Scholar]
- [57].Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM, Polymer Degradation and Stability 2010, 95, 2126. [Google Scholar]
- [58].Lauritzen C, Scandinavian journal of plastic and reconstructive surgery 1983, 17, 133. [DOI] [PubMed] [Google Scholar]
- [59].Pitt CG, Marks T, Schindler A, Biodegradable drug delivery systems based on aliphatic polyesters: application to contraceptives and narcotic antagonists, Academic Press: New York, 1980 [PubMed] [Google Scholar]
- Van de Velde K, Kiekens P, Polymer testing 2002, 21, 433. [Google Scholar]
- [60].Pektok E, Nottelet B, Tille J-C, Gurny R, Kalangos A, Moeller M, Walpoth BH, Circulation 2008, 118, 2563. [DOI] [PubMed] [Google Scholar]
- [61].Matsumura G, Nitta N, Matsuda S, Sakamoto Y, Isayama N, Yamazaki K, Ikada Y, PLoS One 2012, 7, e35760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL, Acta Biomaterialia 2005, 1, 115. [DOI] [PubMed] [Google Scholar]
- [63].Goonoo N, Jeetah R, Bhaw-Luximon A, Jhurry D, European Journal of Pharmaceutics and Biopharmaceutics 2015, 97, 371. [DOI] [PubMed] [Google Scholar]
- [64].Sell S, McClure MJ, Barnes CP, Knapp DC, Walpoth BH, Simpson DG, Bowlin GL, Biomedical Materials 2006, 1, 72. [DOI] [PubMed] [Google Scholar]
- [65].Smith MJ, McClure MJ, Sell SA, Barnes CP, Walpoth BH, Simpson DG, Bowlin GL, Acta Biomaterialia 2008, 4, 58. [DOI] [PubMed] [Google Scholar]
- [66].Mao Y, Sun Q, Wang X, Ouyang Q, Han L, Jiang L, Han D, Applied Physics Letters 2009, 95, 013704. [Google Scholar]
- [67].Klee D, Hocker H, Biomedical Applications: Polymer Blends 1999, 149, 1. [Google Scholar]
- [68].Fan H, Chen P, Qi R, Zhai J, Wang J, Chen L, Chen L, Sun Q, Song Y, Han D, Jiang L, Small (Weinheim an der Bergstrasse, Germany) 2009, 5, 2144. [DOI] [PubMed] [Google Scholar]
- [69].Hulander M, Lundgren A, Faxalv L, Lindahl TL, Palmquist A, Berglin M, Elwing H, Colloids and surfaces. B, Biointerfaces 2013, 110, 261. [DOI] [PubMed] [Google Scholar]
- [70].Ikada Y, in Polymers in Medicine, Springer Berlin Heidelberg, Berlin, Heidelberg: 1984, 103 [Google Scholar]
- Hecker JF, Edwards RO, Journal of biomedical materials research 1981, 15, 1. [DOI] [PubMed] [Google Scholar]
- [71].Sun T, Tan H, Han D, Fu Q, Jiang L, Small (Weinheim an der Bergstrasse, Germany) 2005, 1, 959. [DOI] [PubMed] [Google Scholar]
- Chen L, Han D, Jiang L, Colloids and Surfaces B: Biointerfaces 2011, 85, 2. [DOI] [PubMed] [Google Scholar]
- [72].Milleret V, Hefti T, Hall H, Vogel V, Eberli D, Acta biomaterialia 2012, 8, 4349. [DOI] [PubMed] [Google Scholar]
- [73].Lu J, Rao MP, MacDonald NC, Khang D, Webster TJ, Acta biomaterialia 2008, 4, 192. [DOI] [PubMed] [Google Scholar]
- [74].Liliensiek SJ, Wood JA, Yong J, Auerbach R, Nealey PF, Murphy CJ, Biomaterials 2010, 31, 5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kee MF, Myers DR, Sakurai Y, Lam WA, Qiu Y, PloS one 2015, 10, e0126624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA, Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Qiu Y, Ciciliano J, Myers DR, Tran R, Lam WA, Blood reviews 2015, 29, 377. [DOI] [PubMed] [Google Scholar]
- [78].Park PK, Jarrell BE, Williams SK, Carter TL, Rose DG, Martinez-Hernandez A, Carabasi RA, Journal of vascular surgery 1990, 11, 468. [PubMed] [Google Scholar]
- [79].Herring M, Gardner A, Glover J, Journal of vascular surgery 1984, 1, 279. [PubMed] [Google Scholar]
- Örtenwall P, Wadenvik H, Kutti J, Risberg B, Journal of vascular surgery 1990, 11, 403. [PubMed] [Google Scholar]
- [80].Weinberg CB, Bell E, Science 1986, 231, 397. [DOI] [PubMed] [Google Scholar]
- [81].Örtenwall P, Wadenvik H, Risberg B, Journal of vascular surgery 1989, 10, 374. [PubMed] [Google Scholar]
- [82].Ku SH, Park CB, Biomaterials 2010, 31, 9431. [DOI] [PubMed] [Google Scholar]
- [83].Zhai W, Qiu L. j, Mo X. m., Wang S, Xu Y. f., Peng B, Liu M, Huang J. h., Wang G. c., Zheng J. h., Journal of Biomedical Materials Research Part B: Applied Biomaterials 2013 [Google Scholar]
- Wang S, Mo XM, Jiang BJ, Gao CJ, Wang HS, Zhuang YG, Qiu LJ, International journal of nanomedicine 2013, 8, 2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhou M, Qiao W, Liu Z, Shang T, Qiao T, Mao C, Liu C, Tissue Engineering Part A 2013, 20, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Carnagey J, Hern‐Anderson D, Ranieri J, Schmidt CE, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2003, 65, 171. [DOI] [PubMed] [Google Scholar]
- [86].Lamm P, Juchem G, Milz S, Schuffenhauer M, Reichart B, Circulation 2001, 104, I. [PubMed] [Google Scholar]
- [87].Dahan N, Sarig U, Bronshtein T, Baruch L, Karram T, Hoffman A, Machluf M, Tissue Engineering Part A 2017, 23, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].L’heureux N, McAllister TN, de la Fuente LM, New England Journal of Medicine 2007, 357, 1451. [DOI] [PubMed] [Google Scholar]
- [89].Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Nature medicine 2001, 7, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhou M, Liu Z, Liu C, Jiang X, Wei Z, Qiao W, Ran F, Wang W, Qiao T, Liu C, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2012, 100, 111. [DOI] [PubMed] [Google Scholar]
- [91].Neff LP, Tillman BW, Yazdani SK, Machingal MA, Yoo JJ, Soker S, Bernish BW, Geary RL, Christ GJ, Journal of vascular surgery 2011, 53, 426. [DOI] [PubMed] [Google Scholar]
- [92].Rong J-J, Sang H-F, Qian A-M, Meng Q-Y, Zhao T-J, Li X-Q, International journal of clinical and experimental pathology 2015, 8, 1282. [PMC free article] [PubMed] [Google Scholar]
- [93].He H, Shirota T, Yasui H, Matsuda T, The Journal of thoracic and cardiovascular surgery 2003, 126, 455. [DOI] [PubMed] [Google Scholar]
- [94].Gu Y.-q., Li C.-m., Wang C.-r., Feng Z.-g., Qiu R.-x., Chen B, Li J.-x., Zhang S.-w., Wang Z.-g., Zhang J, Acta pharmacologica sinica 2009, 30, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hashi CK, Zhu Y, Yang G-Y, Young WL, Hsiao BS, Wang K, Chu B, Li S, Proceedings of the National Academy of Sciences 2007, 104, 11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhou R, Zhu L, Fu S, Qian Y, Wang D, Wang C, Scientific reports 2016, 6, 35422. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [97].Zhao Y, Zhang S, Zhou J, Wang J, Zhen M, Liu Y, Chen J, Qi Z, Biomaterials 2010, 31, 296. [DOI] [PubMed] [Google Scholar]
- [98].Mirza A, Hyvelin J-M, Rochefort GY, Lermusiaux P, Antier D, Awede B, Bonnet P, Domenech J, Eder V, Journal of vascular surgery 2008, 47, 1313. [DOI] [PubMed] [Google Scholar]
- [99].Zhang L, Zhou J, Lu Q, Wei Y, Hu S, Biotechnology and bioengineering 2008, 99, 1007. [DOI] [PubMed] [Google Scholar]
- [100].Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H, Circulation 2003, 108, 1729. [DOI] [PubMed] [Google Scholar]
- [101].Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X, Shinoka T, Breuer CK, Annals of surgery 2008, 248, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fukunishi T, Best CA, Ong CS, Groehl T, Reinhardt J, Yi T, Miyachi H, Zhang H, Shinoka T, Breuer CK, Tissue Engineering Part A 2018, 24, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cho S-W, Lim SH, Kim I-K, Hong YS, Kim S-S, Yoo KJ, Park H-Y, Jang Y, Chang BC, Choi CY, Annals of surgery 2005, 241, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, Kullberg-Lindh C, Borg H, Ejnell H, Sumitran-Holgersson S, The Lancet 2012, 380, 230. [DOI] [PubMed] [Google Scholar]
- [105].Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T, The Journal of thoracic and cardiovascular surgery 2010, 139, 431. [DOI] [PubMed] [Google Scholar]
- [106].Lee K-W, Stolz DB, Wang Y, Proceedings of the National Academy of Sciences 2011, 108, 2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Korff T, Kimmina S, Martiny-baron G, Augustin HG, The FASEB Journal 2001, 15, 447. [DOI] [PubMed] [Google Scholar]
- [108].Lilly B, Physiology 2014, 29, 234. [DOI] [PubMed] [Google Scholar]
- [109].Baiguera S, Ribatti D, Angiogenesis 2013, 16, 1. [DOI] [PubMed] [Google Scholar]
- [110].Basile DP, Yoder MC, Journal of cellular physiology 2014, 229, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zampetaki A, Kirton JP, Xu Q, Cardiovascular research 2008, 78, 413. [DOI] [PubMed] [Google Scholar]
- Medina RJ, Barber CL, Sabatier F, Dignat‐George F, Melero‐Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JK, Yamaguchi T, Stem Cells Translational Medicine 2017, 6, 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lin Y, Weisdorf DJ, Solovey A, Hebbel RP, Journal of clinical Investigation 2000, 105, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Nature medicine 2001, 7, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nardi NB, da Silva Meirelles L, in Stem cells, Springer, 2008, 249. [DOI] [PubMed] [Google Scholar]
- [115].Aggarwal S, Pittenger MF, Blood 2005, 105, 1815. [DOI] [PubMed] [Google Scholar]
- [116].Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C, Stem cells 2004, 22, 377. [DOI] [PubMed] [Google Scholar]
- Wang T, Xu Z, Jiang W, Ma A, International journal of cardiology 2006, 109, 74. [DOI] [PubMed] [Google Scholar]
- [117].Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I, Pharmacology & therapeutics 2014, 143, 181. [DOI] [PubMed] [Google Scholar]
- [118].Zhao J, Liu L, Wei J, Ma D, Geng W, Yan X, Zhu J, Du H, Liu Y, Li L, Artificial organs 2012, 36, 93. [DOI] [PubMed] [Google Scholar]
- [119].Zhou R, Zhu L, Fu S, Qian Y, Wang D, Wang C, Scientific reports 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [120].Zhang D, Jiang W, BioScience 2015, biv016. [Google Scholar]
- [121].Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang L-J, Chen YE, Ma PX, Yang B, Biomaterials 2014, 35, 8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Qin L, Kural MH, Schwan J, Li X, Bartulos O, Cong X.-q., Ren Y, Gui L, Li G, Biomaterials 2017, 147, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Siewert J, Teodosescu S, Zhao L, Dimitrievska S, Qian H, Huang AH, Niklason L, Stem cells translational medicine 2014, 3, 1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, Lewis R, Daigh C, Hansen TD, Mann DA, Stem Cell Reviews and Reports 2015, 11, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Adams WJ, Zhang Y, Cloutier J, Kuchimanchi P, Newton G, Sehrawat S, Aird WC, Mayadas TN, Luscinskas FW, García-Cardeña G, Stem cell reports 2013, 1, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Abaci HE, Guo Z, Coffman A, Gillette B, Lee W. h., Sia SK, Christiano AM, Advanced healthcare materials 2016, 5, 1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Nakayama KH, Joshi PA, Lai ES, Gujar P, Joubert L-M, Chen B, Huang NF, Regenerative medicine 2015, 10, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang B, Borazjani A, Tahai M, de Jongh Curry AL, Simionescu DT, Guan J, To F, Elder SH, Liao J, Journal of biomedical materials research Part A 2010, 94, 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K.-i., Shimada T, Oike Y, Imaizumi T, Circulation 2001, 103, 2776. [DOI] [PubMed] [Google Scholar]
- [128].Krawiec JT, Vorp DA, Biomaterials 2012, 33, 3388. [DOI] [PubMed] [Google Scholar]
- [129].Cho S-W, Lim SH, Kim I-K, Hong YS, Kim S-S, Yoo KJ, Park H-Y, Jang Y, Chang BC, Choi CY, Annals of surgery 2005, 241, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Fukunishi T, Best CA, Ong CS, Groehl T, Reinhardt J, Yi T, Miyachi H, Zhang H, Shinoka T, Breuer CK, Tissue Engineering Part A 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H, The Journal of thoracic and cardiovascular surgery 2005, 129, 1330. [DOI] [PubMed] [Google Scholar]
- [132].Sayegh MH, Watschinger B, Carpenter CB, Transplantation 1994, 57, 1295. [DOI] [PubMed] [Google Scholar]
- [133].Orosz CG, Ohye RG, Pelletier RP, Van Buskirk AM, Huang E, Morgan C, Kincade PW, Ferguson RM, Transplantation 1993, 56, 453. [DOI] [PubMed] [Google Scholar]
- [134].Isobe M, Suzuki J, Yagita H, Okumura K, Yamazaki S, Nagai R, Yazaki Y, Sekiguchi M, The Journal of Immunology 1994, 153, 5810. [PubMed] [Google Scholar]
- [135].Dehoux JP, de la Parra B, Latinne D, Bazin H, Gianello P, Xenotransplantation 2001, 8, 193. [DOI] [PubMed] [Google Scholar]
- [136].Orosz CG, Wakely E, Sedmak DD, Bergese SD, VanBuskirk AM, Transplantation 1997, 63, 1109. [DOI] [PubMed] [Google Scholar]
- [137].Valantine H, The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2004, 23, S187. [DOI] [PubMed] [Google Scholar]
- [138].Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA, Blood 2007, 109, 1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ladhoff J, Fleischer B, Hara Y, Volk HD, Seifert M, Cardiovascular research 2010, 88, 121. [DOI] [PubMed] [Google Scholar]
- [140].Aggarwal S, Pittenger MF, Blood 2005, 105, 1815. [DOI] [PubMed] [Google Scholar]
- [141].Lee MJ, Kim MY, Heo SC, Kwon YW, Kim YM, Do EK, Park JH, Lee JS, Han J, Kim JH, Arteriosclerosis, thrombosis, and vascular biology 2012, 32, 2733. [DOI] [PubMed] [Google Scholar]
- [142].Lau S, Eicke D, Carvalho Oliveira M, Wiegmann B, Schrimpf C, Haverich A, Blasczyk R, Wilhelmi M, Figueiredo C, Boer U, Tissue engineering. Part A 2017. [DOI] [PubMed] [Google Scholar]
- [143].Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N, Painter C, Mirensky T, Erickson B, Shinoka T, Breuer CK, Tissue engineering. Part B, Reviews 2010, 16, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski KJ, Rittgers SE, Schmidt SP, Bowlin GL, Frontiers in bioscience : a journal and virtual library 2004, 9, 1412. [DOI] [PubMed] [Google Scholar]
- [144].Anderson JS, Price TM, Hanson SR, Harker LA, Surgery 1987, 101, 577. [PubMed] [Google Scholar]
- [145].Foxall TL, Auger KR, Callow AD, Libby P, The Journal of surgical research 1986, 41, 158. [DOI] [PubMed] [Google Scholar]
- [146].Park IS, Kim YH, Jung Y, Kim SH, Kim SH, Journal of biomaterials science. Polymer edition 2012, 23, 1807. [DOI] [PubMed] [Google Scholar]
- [147].van Wachem PB, Stronck JW, Koers-Zuideveld R, Dijk F, Wildevuur CR, Biomaterials 1990, 11, 602. [DOI] [PubMed] [Google Scholar]
- [148].Gong X, Liu H, Ding X, Liu M, Li X, Zheng L, Jia X, Zhou G, Zou Y, Li J, Huang X, Fan Y, Biomaterials 2014, 35, 4782. [DOI] [PubMed] [Google Scholar]
- [149].Song L, Zhou Q, Duan P, Guo P, Li D, Xu Y, Li S, Luo F, Zhang Z, PloS one 2012, 7, e42569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Uzarski JS, Cores J, McFetridge PS, Tissue engineering. Part C, Methods 2015, 21, 1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Perea H, Aigner J, Heverhagen JT, Hopfner U, Wintermantel E, Journal of tissue engineering and regenerative medicine 2007, 1, 318. [DOI] [PubMed] [Google Scholar]
- [152].Perea H, Aigner J, Hopfner U, Wintermantel E, Cells, tissues, organs 2006, 183, 156. [DOI] [PubMed] [Google Scholar]
- [153].Tiwari A, Punshon G, Kidane A, Hamilton G, Seifalian AM, Cell biology and toxicology 2003, 19, 265. [DOI] [PubMed] [Google Scholar]
- [154].Bowlin GL, Rittgers SE, Cell transplantation 1997, 6, 631. [DOI] [PubMed] [Google Scholar]
- [155].De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M, Blood 2004, 104, 3472. [DOI] [PubMed] [Google Scholar]
- [156].Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, Davies N, Biomaterials 2005, 26, 167. [DOI] [PubMed] [Google Scholar]
- [157].Hao D, Xiao W, Liu R, Kumar P, Li Y, Zhou P, Guo F, Farmer DL, Lam KS, Wang F, Wang A, ACS chemical biology 2017, 12, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hsu SH, Sun SH, Chen DC, Artificial organs 2003, 27, 1068. [DOI] [PubMed] [Google Scholar]
- [159].Kang TY, Lee JH, Kim BJ, Kang JA, Hong JM, Kim BS, Cha HJ, Rhie JW, Cho DW, Biofabrication 2015, 7, 015007. [DOI] [PubMed] [Google Scholar]
- [160].Kuwabara F, Narita Y, Yamawaki-Ogata A, Kanie K, Kato R, Satake M, Kaneko H, Oshima H, Usui A, Ueda Y, The Annals of thoracic surgery 2012, 93, 156. [DOI] [PubMed] [Google Scholar]
- [161].Lee JS, Lee K, Moon SH, Chung HM, Lee JH, Um SH, Kim DI, Cho SW, Macromolecular bioscience 2014, 14, 1181. [DOI] [PubMed] [Google Scholar]
- [162].Li J, Ding M, Fu Q, Tan H, Xie X, Zhong Y, Journal of materials science. Materials in medicine 2008, 19, 2595. [DOI] [PubMed] [Google Scholar]
- [163].Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T, Circulation 2003, 107, 1322. [DOI] [PubMed] [Google Scholar]
- [164].Yu J, Wang A, Tang Z, Henry J, Lee B, Zhu Y, Yuan F, Huang F, Li S, Biomaterials 2012, 33, 8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lu S, Zhang P, Sun X, Gong F, Yang S, Shen L, Huang Z, Wang C, ACS Appl Mater Interfaces 2013, 5, 7360. [DOI] [PubMed] [Google Scholar]
- [166].Miller-Kasprzak E, Jagodzinski PP, Archivum immunologiae et therapiae experimentalis 2007, 55, 247. [DOI] [PubMed] [Google Scholar]
- [167].Mrówczyński W, Rungatscher A, Buchegger F, Tille J-C, Namy S, Ratib O, Kutryk M, Walpoth BH, Kardiochirurgia i Torakochirurgia Polska = Polish Journal of Cardio-Thoracic Surgery 2014, 11, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Rotmans JI, Heyligers JM, Verhagen HJ, Velema E, Nagtegaal MM, de Kleijn DP, de Groot FG, Stroes ES, Pasterkamp G, Circulation 2005, 112, 12. [DOI] [PubMed] [Google Scholar]
- [169].Chen X, Wang J, An Q, Li D, Liu P, Zhu W, Mo X, Colloids and surfaces. B, Biointerfaces 2015, 128, 106. [DOI] [PubMed] [Google Scholar]
- [170].Han F, Jia X, Dai D, Yang X, Zhao J, Zhao Y, Fan Y, Yuan X, Biomaterials 2013, 34, 7302. [DOI] [PubMed] [Google Scholar]
- [171].Lahtinen M, Blomberg P, Baliulis G, Carlsson F, Khamis H, Zemgulis V, European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2007, 31, 383. [DOI] [PubMed] [Google Scholar]
- [172].Sun Q, Silva EA, Wang A, Fritton JC, Mooney DJ, Schaffler MB, Grossman PM, Rajagopalan S, Pharm Res 2010, 27, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhou M, Liu Z, Wei Z, Liu C, Qiao T, Ran F, Bai Y, Jiang X, Ding Y, Artificial organs 2009, 33, 230. [DOI] [PubMed] [Google Scholar]
- [174].Roh JD, Nelson GN, Udelsman BV, Brennan MP, Lockhart B, Fong PM, Lopez-Soler RI, Saltzman WM, Breuer CK, Tissue engineering 2007, 13, 2743. [DOI] [PubMed] [Google Scholar]
- [175].Mazzucato M, Spessotto P, Masotti A, De Appollonia L, Cozzi MR, Yoshioka A, Perris R, Colombatti A, De Marco L, The Journal of biological chemistry 1999, 274, 3033. [DOI] [PubMed] [Google Scholar]
- [176].Burkel WE, Medical progress through technology 1988, 14, 165. [PubMed] [Google Scholar]
- [177].Yates SG, Barros D’Sa AA, Berger K, Fernandez LG, Wood SJ, Rittenhouse EA, Davis CC, Mansfield PB, Sauvage LR, Annals of Surgery 1978, 188, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Abruzzo A, Fiorica C, Palumbo VD, Altomare R, Damiano G, Gioviale MC, Tomasello G, Licciardi M, Palumbo FS, Giammona G, Lo Monte AI, International Journal of Polymer Science 2014, 2014, 9. [Google Scholar]
- [179].Srinivasan S, Sawyer PN, Journal of colloid and interface science 1970, 32, 456. [DOI] [PubMed] [Google Scholar]
- [180].Sawyer PN, Sprinivasan S, American journal of surgery 1967, 114, 42. [DOI] [PubMed] [Google Scholar]
- Abramson HA, The Journal of experimental medicine 1928, 47, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Scharn DM, Daamen WF, van Kuppevelt TH, van der Vliet JA, European Journal of Vascular and Endovascular Surgery 2012, 43, 66. [DOI] [PubMed] [Google Scholar]
- Owida AA, Do H, Morsi YS, Comput Meth Prog Bio 2012, 108, 689. [DOI] [PubMed] [Google Scholar]
- 182.[] Pennel T, Fercana G, Bezuidenhout D, Simionescu A, Chuang TH, Zilla P, Simionescu D, Biomaterials 2014, 35, 6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Wang XW, Lin PH, Yao QZ, Chen CY, Journal of Cellular and Molecular Medicine 2009, 13, 2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Hermanutz V, Wolf SE, Koveker GB, Journal of Vascular Surgery 1999, 29, 168. [DOI] [PubMed] [Google Scholar]
- Lin QK, Hou Y, Ren KF, Ji J, Thin Solid Films 2012, 520, 4971 [Google Scholar]
- Baguneid MS, Seifalian AM, Salacinski HJ, Murray D, Hamilton G, Walker MG, Brit J Surg 2006, 93, 282. [DOI] [PubMed] [Google Scholar]
- Dai WW, Guo HF, Qian DH, Qin ZX, Lei Y, Hou XY, Wen C, Journal of Materials Chemistry B 2017, 5, 1053. [DOI] [PubMed] [Google Scholar]
- Cardinal KO, Bonnema GT, Hofer H, Barton JK, Williams SK, Tissue Engineering 2006, 12, 3431. [DOI] [PubMed] [Google Scholar]
- Cezeaux JL, Romoser CE, Benson RS, Buck CK, Sackman JE, Nucl Instrum Meth B 1998, 141, 193. [Google Scholar]
- 183.[] Tulloch AW, Chun Y, Levi DS, Mohanchandra KP, Carman GP, Lawrence PF, Rigberg DA, J Surg Res 2011, 171, 317. [DOI] [PubMed] [Google Scholar]
- Heublein B, Ozbek C, Pethig K, J Endovasc Surg 1998, 5, 32. [DOI] [PubMed] [Google Scholar]
- Mayer A, Lee S, Lendlein A, Jung F, Hiebl B, Clin Hemorheol Micro 2011, 48, 57. [DOI] [PubMed] [Google Scholar]
- Fields GB, Lauer JL, Dori Y, Forns P, Yu YC, Tirrell M, Biopolymers 1998, 47, 143. [DOI] [PubMed] [Google Scholar]
- [184].Zhang FM, Li GC, Yang P, Qin W, Li CH, Huang N, Colloid Surface B 2013, 102, 457. [DOI] [PubMed] [Google Scholar]
- Bos GW, Scharenborg NM, Poot AA, Engbers GHM, Terlingen JGA, Beugeling T, Van Aken WG, Feijen J, Tissue Engineering 1998, 4, 267. [DOI] [PubMed] [Google Scholar]
- [185].Antonova LV, Seifalian AM, Kutikhin AG, Sevostyanova VV, Krivkina EO, Mironov AV, Burago AY, Velikanova EA, Matveeva VG, Glushkova TV, Sergeeva EA, Vasyukov GY, Kudryavtseva YA, Barbarash OL, Barbarash LS, Frontiers in pharmacology 2016, 7, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Wang Z, Song L, Zhao Q, Zhang J, Li D, Wang S, Han J, Zheng XL, Yang Z, Kong D, Biomaterials 2012, 33, 2880. [DOI] [PubMed] [Google Scholar]
- [186].Kato R, Kaga C, Kunimatsu M, Kobayashi T, Honda H, Journal of bioscience and bioengineering 2006, 101, 485. [DOI] [PubMed] [Google Scholar]
- [187].Ferrara N, Gerber HP, LeCouter J, Nat Med 2003, 9, 669. [DOI] [PubMed] [Google Scholar]
- [188].Davoudi P, Assadpour S, Derakhshan MA, Ai J, Solouk A, Ghanbari H, Materials science & engineering. C, Materials for biological applications 2017, 80, 213. [DOI] [PubMed] [Google Scholar]
- Antonova LV, Seifalian AM, Kutikhin AG, Sevostyanova VV, Matveeva VG, Velikanova EA, Mironov AV, Shabaev AR, Glushkova TV, Senokosova EA, Vasyukov GY, Krivkina EO, Burago AY, Kudryavtseva YA, Barbarash OL, Barbarash LS, International journal of molecular sciences 2016, 17, 1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Yin M, Yuan Y, Liu C, Wang J, Journal of materials science. Materials in medicine 2009, 20, 1513. [DOI] [PubMed] [Google Scholar]
- Lin Q, Ding X, Qiu F, Song X, Fu G, Ji J, Biomaterials 2010, 31, 4017. [DOI] [PubMed] [Google Scholar]
- Song CL, Li Q, Zhang JC, Wang JP, Xue X, Wang G, Shi YF, Diao HY, Liu B, European review for medical and pharmacological sciences 2016, 20, 311. [PubMed] [Google Scholar]
- [190].Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY, Development 2003, 130, 411. [DOI] [PubMed] [Google Scholar]
- [191].Liu SQ, Tieche C, Alkema PK, Biomaterials 2004, 25, 1869. [DOI] [PubMed] [Google Scholar]
- [192].McCarthy CW, Ahrens DC, Joda D, Curtis TE, Bowen PK, Guillory RJ, Liu SQ, Zhao F, Frost MC, Goldman J, ACS applied materials & interfaces 2015, 7, 16202. [DOI] [PubMed] [Google Scholar]
- [193].Daamen WF, Veerkamp J, Van Hest J, Van Kuppevelt T, Biomaterials 2007, 28, 4378. [DOI] [PubMed] [Google Scholar]
- [194].Blit PH, McClung WG, Brash JL, Woodhouse KA, Santerre JP, Biomaterials 2011, 32, 5790. [DOI] [PubMed] [Google Scholar]
- [195].Liu SQ, Alkema PK, Tieché C, Tefft BJ, Liu DZ, Li YC, Sumpio BE, Caprini JA, Paniagua M, Journal of Biological Chemistry 2005, 280, 39294. [DOI] [PubMed] [Google Scholar]
- [196].Ayad S, Boot-Handford R, Humphries M, Kadler K, Shuttleworth A, The extracellular matrix factsbook, Academic Press, 1998. [Google Scholar]
- [197].Blit PH, Battiston KG, Woodhouse KA, Santerre JP, Journal Of Biomedical Materials Research Part A 2011, 96, 648. [DOI] [PubMed] [Google Scholar]
- [198].Sallach RE, Cui W, Wen J, Martinez A, Conticello VP, Chaikof EL, Biomaterials 2009, 30, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Keeley FW, Bellingham CM, Woodhouse KA, Philosophical Transactions of the Royal Society of London B: Biological Sciences 2002, 357, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham C, Woodhouse K, Robson P, Rothstein S, Keeley F, Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 2001, 1550, 6. [DOI] [PubMed] [Google Scholar]
- [200].Kumar VA, Caves JM, Haller CA, Dai E, Liu L, Grainger S, Chaikof EL, Acta biomaterialia 2013, 9, 8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Yeo GC, Keeley FW, Weiss AS, Advances in colloid and interface science 2011, 167, 94. [DOI] [PubMed] [Google Scholar]
- [202].Wise SG, Yeo GC, Hiob MA, Rnjak-Kovacina J, Kaplan DL, Ng MK, Weiss AS, Acta biomaterialia 2014, 10, 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Yin Y, Wise SG, Nosworthy NJ, Waterhouse A, Bax DV, Youssef H, Byrom MJ, Bilek MM, McKenzie DR, Weiss AS, Biomaterials 2009, 30, 1675. [DOI] [PubMed] [Google Scholar]
- [204].Wise SG, Liu H, Kondyurin A, Byrom MJ, Bannon PG, Edwards GA, Weiss AS, Bao S, Bilek MM, ACS Biomaterials Science & Engineering 2016, 2, 1286. [DOI] [PubMed] [Google Scholar]
- [205].Hiob MA, Wise SG, Kondyurin A, Waterhouse A, Bilek MM, Ng MKC, Weiss AS, Biomaterials 2013, 34, 7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Yokoyama U, Tonooka Y, Koretake R, Akimoto T, Gonda Y, Saito J, Umemura M, Fujita T, Sakuma S, Arai F, Scientific Reports 2017, 7, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Qian Z, Tahtinen M, Yap AH, Yates K, Zhao F, Advanced Healthcare Materials 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Palombo C, Kozakova M, Vascular pharmacology 2016, 77, 1. [DOI] [PubMed] [Google Scholar]
- [208].von Segesser LK, Mueller X, Marty B, Horisberger J, Corno A, Perfusion 2001, 16, 411. [DOI] [PubMed] [Google Scholar]
- [209].Qu Z, Muthukrishnan S, Urlam MK, Haller CA, Jordan SW, Kumar VA, Marzec UM, Elkasabi Y, Lahann J, Hanson SR, Chaikof EL, Advanced Functional Materials 2011, 21, 4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Salvagnini C, Gharbi S, Boxus T, Marchand-Brynaert J, European journal of medicinal chemistry 2007, 42, 37. [DOI] [PubMed] [Google Scholar]
- [211].Dahlbäck B, The Lancet 2000, 355, 1627. [DOI] [PubMed] [Google Scholar]
- [212].Lee CJ, Ansell JE, British Journal of Clinical Pharmacology 2011, 72, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Watanabe T, Kanda K, Ishibashi-Ueda H, Yaku H, Nakayama Y, Journal of Biomedical Materials Research - Part B Applied Biomaterials 2010, 92, 236. [DOI] [PubMed] [Google Scholar]
- [214].Nakayama Y, Ishibashi-Ueda H, Takamizawa K, Cell transplantation 2004, 13, 439. [DOI] [PubMed] [Google Scholar]
- Kikumoto R, Tamao Y, Tezuka T, Tonomura S, Hara H, Ninomiya K, Hijikata A, Okamoto S, Biochemistry 1984, 23, 85. [DOI] [PubMed] [Google Scholar]
- Kumada T, Abiko Y, Thrombosis research 1981, 24, 285. [DOI] [PubMed] [Google Scholar]
- [215].Nishi S, Nakayama Y, Ishibashi-Ueda H, Okamoto Y, Kinoshita Y, Journal of Artificial Organs 2009, 12, 35. [DOI] [PubMed] [Google Scholar]
- Richey T, Iwata H, Oowaki H, Uchida E, Matsuda S, Ikada Y, Biomaterials 2000, 21, 1057. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Arita M, Hamada M, Tomobuchi Y, Hano T, Nishio I, Japanese circulation journal 1997, 61, 256. [DOI] [PubMed] [Google Scholar]
- [216].Nakayama Y, Yamaoka S, Yamanami M, Fujiwara M, Uechi M, Takamizawa K, Ishibashi-Ueda H, Nakamichi M, Uchida K, Watanabe T, Kanda K, Yaku H, Journal of Biomedical Materials Research - Part B Applied Biomaterials 2011, 99 B, 420. [DOI] [PubMed] [Google Scholar]
- [217].Zwaal RF, Comfurius P, Bevers EM, Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes 1998, 1376, 433. [DOI] [PubMed] [Google Scholar]
- [218].Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D, Acs Nano 2009, 3, 1357. [DOI] [PubMed] [Google Scholar]
- [219].Ragaseema V, Unnikrishnan S, Krishnan VK, Krishnan LK, Biomaterials 2012, 33, 3083. [DOI] [PubMed] [Google Scholar]
- [220].Fröhlich E, Current medicinal chemistry 2016, 23, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski KA, Radomski MW, Santos-Martinez MJ, Nanomedicine 2015, 10, 1451. [DOI] [PubMed] [Google Scholar]
- [221].Madhavan RV, Rosemary MJ, Nandkumar MA, Krishnan KV, Krishnan LK, Tissue Engineering Part A 2010, 17, 439. [DOI] [PubMed] [Google Scholar]
- [222].Xing Q, Zhang L, Redman T, Qi S, Zhao F, Journal of Biomedical Materials Research - Part A 2015, 103, 3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Moncada S, Higgs A, New England Journal of Medicine 1993, 329, 2002. [DOI] [PubMed] [Google Scholar]
- [224].Ignarro LJ, Nitric oxide: biology and pathobiology, Academic press, 2000. [Google Scholar]
- [225].Gao J, Wang Y, Chen S, Tang D, Jiang L, Kong D, Wang S, RSC Advances 2017, 7, 18775. [Google Scholar]
- [226].Hirsh J, Raschke R, Warkentin TE, Dalen JE, Deykin D, Poller L, Chest Journal 1995, 108, 258S. [DOI] [PubMed] [Google Scholar]
- [227].Choay J, Petitou M, Medical Journal of Australia 1986, 144, HS7.3945202 [Google Scholar]
- [228].Rosenberg R, Hemostasis and Thrombosis, Basic Principles and Clinical Practice 1994, 837 [Google Scholar]
- Atha D, Lormeau J, Petitous M, Biochemistry1987 1987, 26, 6454 [Google Scholar]
- Ellis V, Scully MF, Kakkar VV, Biochemical Journal 1986, 238, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U, Thunberg L, Bäckström G, Riesenfeld J, Nordling K, Björk I, Journal of Biological Chemistry 1984, 259, 12368. [PubMed] [Google Scholar]
- Choay J, Petitou M, Lormeau J, Sinay P, Casu B, Gatti G, Biochemical and biophysical research communications 1983, 116, 492. [DOI] [PubMed] [Google Scholar]
- Choay J, Lormeau JC, Petitou M, Sinay P, Fareed J, Annals of the New York Academy of Sciences 1981, 370, 644. [DOI] [PubMed] [Google Scholar]
- Casu B, Oreste P, Torri G, Zoppetti G, Choay J, Lormeau J, Petitou M, Sinaɕ P, Biochemical Journal 1981, 197, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RD, Lam L, Proceedings of the National Academy of Sciences 1979, 76, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U, Bäckström G, Höök M, Thunberg L, Fransson L-A, Linker A, Proceedings of the National Academy of Sciences 1979, 76, 3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höök M, Björk I, Hopwood J, Lindahl U, FEBS letters 1976, 66, 90. [DOI] [PubMed] [Google Scholar]
- [229].Brill-Edwards P, Ginsberg JS, Johnston M, Hirsh J, Annals of internal medicine 1993, 119, 104. [DOI] [PubMed] [Google Scholar]
- Harenberg J, “Pharmacology of low molecular weight heparins”, presented at Seminars in thrombosis and hemostasis, 1990 [PubMed] [Google Scholar]
- Andersson L-O, Barrowcliffe T, Holmer E, Johnson E, Söderström G, Thrombosis research 1979, 15, 531. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Mulloy B, Carbohydrate research 1976, 51, 119. [DOI] [PubMed] [Google Scholar]
- [230].Danielsson A, Raub E, Lindahl U, Björk I, Journal of Biological chemistry 1986, 261, 15467. [PubMed] [Google Scholar]
- Lane D, Denton J, Flynn A, Thunberg L, Lindahl U, Biochemical Journal 1984, 218, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D, Majerus DW, Blank MK, Journal of Biological Chemistry 1982, 257, 2162. [PubMed] [Google Scholar]
- Jordan RE, Oosta G, Gardner W, Rosenberg R, Journal of Biological Chemistry 1980, 255, 10081. [PubMed] [Google Scholar]
- Holmer E, Lindahl U, Bäckström G, Thunberg L, Sandberg H, Söderström G, Andersson L-O, Thrombosis research 1980, 18, 861. [DOI] [PubMed] [Google Scholar]
- [231].Sié P, Petitou M, Lormeau J-C, Dupouy D, Boneu B, Choay J, Biochimica et Biophysica Acta (BBA)-General Subjects 1988, 966, 188. [DOI] [PubMed] [Google Scholar]
- Petitou M, Lormeau J, Perly B, Berthault P, Bossennec V, Sie P, Choay J, Journal of Biological Chemistry 1988, 263, 8685. [PubMed] [Google Scholar]
- Maimone MM, Tollefsen DM, Biochemical and biophysical research communications 1988, 152, 1056. [DOI] [PubMed] [Google Scholar]
- Hurst R, Poon M-C, Griffith M, Journal of Clinical Investigation 1983, 72, 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Barrowcliffe T, in Heparin-A Century of progress, Springer, 2012, 3. [Google Scholar]
- [233].Hoshi RA, Van Lith R, Jen MC, Allen JB, Lapidos KA, Ameer G, Biomaterials 2013, 34, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Dorigo W, Pulli R, Castelli P, Dorrucci V, Ferilli F, De Blasis G, Monaca V, Vecchiati E, Pratesi C, P. I. R. Group, Journal of vascular surgery 2011, 54, 1332. [DOI] [PubMed] [Google Scholar]
- Pulli R, Dorigo W, Castelli P, Dorrucci V, Ferilli F, De Blasis G, Monaca V, Vecchiati E, Pratesi C, Group PIR, Journal of vascular surgery 2010, 51, 1167. [DOI] [PubMed] [Google Scholar]
- [235].Li Y, Neoh K, Kang E, Journal of Biomedical Materials Research Part A 2005, 73, 171. [DOI] [PubMed] [Google Scholar]
- Gutowska A, Bae YH, Jacobs H, Mohammad F, Mix D, Feijen J, Kim SW, Journal of Biomedical Materials Research Part A 1995, 29, 811. [DOI] [PubMed] [Google Scholar]
- [236].Lindholt JS, Gottschalksen B, Johannesen N, Dueholm D, Ravn H, Christensen ED, Viddal B, Flørenes T, Pedersen G, Rasmussen M, European Journal of Vascular and Endovascular Surgery 2011, 41, 668. [DOI] [PubMed] [Google Scholar]
- Vicaretti M, Hawthorne W, Ao P, Fletcher J, Cardiovascular Surgery 1998, 6, 268. [DOI] [PubMed] [Google Scholar]
- Drury JK, Ashton TR, Cunningham JD, Maini R, Pollock JG, Annals of vascular surgery 1987, 1, 542. [DOI] [PubMed] [Google Scholar]
- [237].Devine C, McCollum C, Trial TNWF-P, Journal of vascular surgery 2004, 40, 924. [DOI] [PubMed] [Google Scholar]
- [238].Begovac P, Thomson R, Fisher J, Hughson A, Gällhagen A, European Journal of Vascular and Endovascular Surgery 2003, 25, 432. [DOI] [PubMed] [Google Scholar]
- [239].Lindholt JS, Gottschalksen B, Johannesen N, Dueholm D, Ravn H, Christensen ED, Viddal B, Florenes T, Pedersen G, Rasmussen M, Carstensen M, Grondal N, Fasting H, European Journal of Vascular and Endovascular Surgery 2011, 41, 668. [DOI] [PubMed] [Google Scholar]
- [240].Beamish JA, Geyer LC, Haq-Siddiqi NA, Kottke-Marchant K, Marchant RE, Biomaterials 2009, 30, 6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Rolfe BE, Xu ZP, Thomas AC, Campbell JH, Lu GQ, Biomaterials 2010, 31, 5455. [DOI] [PubMed] [Google Scholar]
- [241].Shafiq M, Jung Y, Kim SH, Journal of Biomedical Materials Research - Part A 2016, 104, 1352. [DOI] [PubMed] [Google Scholar]
- Gong W, Lei D, Li S, Huang P, Qi Q, Sun Y, Zhang Y, Wang Z, You Z, Ye X, Zhao Q, Biomaterials 2016, 76, 359. [DOI] [PubMed] [Google Scholar]
- [242].Smith RJ Jr, Koobatian MT, Shahini A, Swartz DD, Andreadis ST, Biomaterials 2015, 51, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Koobatian MT, Row S, Smith RJ Jr, Koenigsknecht C, Andreadis ST, Swartz DD, Biomaterials 2016, 76, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Glynn JJ, Hinds MT, Advanced healthcare materials 2016, 5, 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Xu L, Guo Y, Huang Y, Xiong Y, Xu Y, Li X, Lu J, Wang L, Wang Y, Lu Y, Journal of biomaterials applications 2018, 0885328217752859. [Google Scholar]
- [246].McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, LHeureux N, Lancet 2009, 373, 1440. [DOI] [PubMed] [Google Scholar]
- L’Heureux N, McAllister TN, de la Fuente LM, N Engl J Med 2007, 357, 1451. [DOI] [PubMed] [Google Scholar]
- [247].Wystrychowski W, Cierpka L, Zagalski K, Garrido S, Dusserre N, Radochonski S, McAllister TN, L’Heureux N, J Vasc Access 2011, 12, 67. [DOI] [PubMed] [Google Scholar]
- [248].Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L’Heureux N, Journal of Vascular Surgery 2014, 60, 1353. [DOI] [PubMed] [Google Scholar]
- [249].Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE, Sci Transl Med 2011, 3, 68ra9. [DOI] [PubMed] [Google Scholar]
- [250].Dahl SL, Blum JL, Niklason LE, Trends Cardiovasc Med 2011, 21, 83. [DOI] [PubMed] [Google Scholar]
- [251].Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, Szmidt J, Turek J, Witkiewicz W, Zapotoczny N, Zubilewicz T, Niklason LE, Lancet 2016, 387, 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Melchiorri AJ, Hibino N, Best CA, Yi T, Lee YU, Kraynak CA, Kimerer LK, Krieger A, Kim P, Breuer CK, Fisher JP, Advanced healthcare materials 2016, 5, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang MT, Chen CS, Nature Biomedical Engineering 2017, 1, 0083. [DOI] [PMC free article] [PubMed] [Google Scholar]


