Abstract
Working memory (WM) is a critical component of many neurocognitive functions. The literature has demonstrated consistently that WM impairment is more frequent and severe among substance dependent individuals (SDIs) infected with HIV compared with uninfected SDIs; however, the SDIs who participated in these previous studies were primarily male. There are few published data on WM performance among HIV+ women with or without substance use disorders, and essentially no direct comparisons of WM performance between HIV+ men and women, regardless of substance use. We investigated potential sex and serostatus effects on WM among a sample of 360 SDIs (114 with HIV; 66% female) verified abstinent from alcohol and drugs of abuse at testing and generally comparable on substance use and comorbid characteristics. Participants were tested with the n-back task, a well-established WM measure that is sensitive to HIV associated cognitive impairment. HIV+ men and women performed spatial and verbal versions of the n-back significantly less accurately compared with HIV− participants. Women showed slower response times compared with men on both versions, regardless of HIV serostatus. Individuals dependent on cocaine showed faster RTs compared with non-dependent users, but this effect was not apparent among opioid or alcohol-dependent groups. Findings on n-back accuracy are consistent with our previous proposal that WM impairment represents a signature deficit among HIV+ SDIs; however, WM impairment appears less common among HIV+ women without a substance use history. The pattern of sex differences in response speed but serostatus effects on response accuracy is comparable to a recent report by our group of sex differences in learning speed but serostatus effects on delayed recall.
Keywords: HIV, drug use, sex differences, n-back, working memory, prefrontal cortex
Introduction
Executive functions, such as decision making and assessing risk, are often impaired among HIV-seropositive (HIV+) individuals, reflecting disrupted activity in prefrontal-striatal circuitry (Walker & Brown, 2017). Deficits in working memory, broadly defined as the executive capacity to maintain and process information on a temporary basis (Bartok et al., 1997; Goldman-Rakic, 1991; Goldman-Rakic, 1995), are particularly common among individuals living with HIV/AIDS (Chang et al., 2001; Bartok et al., 1997; Hinkin et al., 2002; Stout et al., 1995) and impede the capacity to engage in everyday functioning, manage finances, and maintain employment.
Working memory is frequently impaired among substance dependent individuals (SDIs) (Hester & Garavan, 2004; Rapeli et al., 2006; Yi et al, 2017) but these deficits are more common and severe among SDIs infected with HIV. In a series of cognitive neuropsychological studies, our group has shown that HIV+ SDIs are consistently impaired compared with HIV-seronegative (HIV−) SDIs on a range of verbal and spatial tasks that engage working memory by varying processing demands and information load (e.g., Bartok et al, 1997; Farinpour et al., 1997; Martin et al., 2001). Based on these findings, we have posited that impaired working memory might be a signature deficit among HIV+ SDIs (Martin el al., 2001), a finding with considerable clinical significance, as recent studies have employed working memory successfully as a target for cognitive training for substance use disorders (Wesley & Bickel, 2014; Bickel, Moody & Quisenberry, 2014) and for HIV-associated neurocognitive disorder (Chang et al., 2017).
Previous studies of HIV serostatus effects on working memory among SDIs were conducted with largely male study samples. However, increasing evidence indicates that neurocognitive profiles are not identical for HIV-infected men and women (Maki & Martin-Thormeyer, 2009; Martin et al., 2011, 2016; Hestad et al., 2012; Royal et al., 2016; Keutmann et al., 2016; but cf Behrman-Lay et al., 2016; Robertson et al., 2004); further, sex differences have been found in multiple components of the addictive processes (Wetherington, 2010), suggesting that sex differences in neurocognitive profiles among HIV+ SDIs might be more pronounced. We recently reported that HIV+ female but not male SDIs performed more poorly compared with HIV− SDIs on a measure of decision making under risk (Martin et al., 2015). This finding suggests that obtaining a more comprehensive profile of sex and serostatus effects on executive function is critically important as a first step toward identifying possible sex differences in HIV’s disruptive effects on striatal-prefrontal cortical systems.
In the current study, we administered computerized verbal and spatial versions of the n-back task, a well-established working memory measure with known sensitivity to HIV-associated neurocognitive deficits (e.g., Hinkin et al., 2002; Chang et al., 2001), to a sample of 360 SDIs with and without a positive HIV serostatus. The primary goal of this study was to investigate potential sex and HIV serostatus effects on n-back performance by SDIs. We hypothesized that HIV+ participants would perform both verbal and spatial n-back tasks significantly more poorly compared with HIV− participants.
Working memory has not been well-studied among HIV+ women (but cf Maki et al., 2015; Sundermann et al, 2015). However, to our knowledge n-back performance between HIV+ and HIV− men and women has not been compared directly. Consequently, we formulated a more conservative hypothesis regarding potential sex differences. Based on the non-clinical literature (e.g., Reed et al., 2017; Duff & Hampson, 2001), we predicted that HIV+ and HIV− female participants would perform significantly more poorly compared with HIV− and HIV+ men on the spatial n-back; and that the magnitude of this male advantage, (i.e., the extent of the sex difference) would differ significantly between HIV+ men and women compared with HIV− men and HIV− women, resulting in a significant Sex x HIV Serostatus interaction.
Method
Participants
We tested 114 HIV+ (n = 51 females) and 246 (n = 187 females) enzyme immunoassay (EIA) - verified HIV− SDIs enrolled in a larger study of sex and HIV serostatus effects on neurocognition. Participants were recruited from Infectious Disease Clinics at Rush, the University of Illinois-Chicago (UIC) and the Ruth M. Rothstein CORE Center at Stroger (formerly Cook County) Hospital, community agencies and by word of mouth. All participants were age 18–60 years, fluent in English, had 8 or more years of education, and met DSM-IV criteria for dependence on at least one substance other than caffeine or nicotine. Exclusion criteria included AIDS-defining or other CNS disorders, closed head injury with loss of consciousness greater than 30 minutes, open head injury of any kind, schizophrenia, or current antipsychotic medications. Participants were informed at recruitment that a breathalyzer and rapid urine toxicology screen would be administered at each study visit and that the visit would be terminated without payment if either test was positive. The study was approved by Institutional Review Boards at Rush, the Core Center, and the UIC.
Procedure
Tests administered were part of a larger study protocol administered over two 120–180-minute visits to the Department of Psychiatry at Rush University Medical Center. Testing was conducted by bachelor’s level research assistants under the supervision of a board-certified clinical neuropsychologist (EMM). Written informed consent was obtained on arrival for the first study visit. To ensure abstinence from drug and alcohol at the time of testing, on both study visits the participant provided a urine sample for a 10-panel rapid toxicology screen (DrugCheck® NxStep) that tested for 10 street and prescription drugs, including heroin, cocaine, cannabis, benzodiazepines, and amphetamines, as well as a breathalyzer test for alcohol. If a potential participant tested positive, the visit was terminated, the participant received no payment, and the visit was rescheduled1. All participants were informed of these contingencies prior to the testing visit. They received $75 cash compensation for their time and transportation costs at the completion of each study visit. Participants also received a $10 bonus for passing both breathalyzer and urine drug screen tests and arriving at all appointments on time.
Measures
Clinical and personality measures
Each subject was administered the Wechsler Test of Adult Reading (WTAR: Wechsler, 2001) to estimate premorbid verbal intellectual function. Subjects also completed a series of paper-and-pencil measures of conditions comorbid with substance use disorders with potentially confounding effects on neurocognition including post-traumatic stress symptoms (PTSD Checklist—Civilian Version; Keane et al., 1987), attention deficit disorder (Wender Utah Rating Scale; Stein et al., 1995), and antisocial personality traits (Levenson Self-Report Psychopathy Scale; Levenson et al., 1995).
Substance use
Trained interviewers administered a Web-based computer-assisted version (NetSCID; TeleSage, http://www.telesage.com/products/netscid.html) of the Affective Disorders and Substance Abuse Modules of the Structured Clinical Interview for DSM-IV (SCID-SAM; First & Gibbon, 2004), and the Addictions Severity Index (McLellan et al., 1980). Participants also completed the Kreek-McHugh-Schluger-Kellogg scale (Kellogg et al., 2003), a paper and pencil measure that indexes the severity of peak lifetime use of alcohol, cocaine and opioids, derived from the participant’s estimate of the amount of money spent, time duration, and frequency of use at the time of their maximum use of each substance. Finally, CNS Penetration Effectiveness (CPE) scores (Letendre et al. 2008), which index the capacity of specific antiretroviral compounds to cross the blood brain barrier) were computed for all participants prescribed cART. Table 1 contains demographic, substance use, and comorbidity data for the individual groups (HIV− men, HIV+ men, HIV− women, HIV+ women).
Table 1.
Demographic, substance use, comorbidity and HIV disease data
| HIV− Men | HIV+ Men | HIV− Women | HIV+ Women | Statistic | p | |
|---|---|---|---|---|---|---|
| n | 59 | 63 | 187 | 51 | ||
| Age+ | 51.2 (5.7)* | 48.6 (7.1) | 47.9 (8.7)* | 47.9 (8.9) | 2.69 | .046 |
| Education | 12.5 (1.6) | 13.0 (1.8)* | 12.3 (2.0)* | 11.3 (2.4)* | 7.27 | <.001 |
| WTAR FSIQ | 88.2 (8.6)* | 88.7 (9.5)* | 86.6 (9.7) | 83.1 (8.0*) | 4.05 | .008 |
| %African American++ | 97 | 87 | 79 | 94 | 15.2 | .002 |
| %HCV | 10 | 25 | 14 | 16 | 6.34 | .096 |
| ASI-Alcohol | .09 (.18)* | .06 (.11) | .03 (.09)* | .03 (.10) | 4.97 | .002 |
| ASI-Drug | .03 (.08) | .03 (.06) | .03 (.06) | .03 (.06) | 0.10 | .962 |
| KMSK-Alcohol | 10.6 (3.2) | 11.1 (2.4) | 10.0 (3.7) | 10.5 (3.3) | 2.09 | .102 |
| KMSK-Cocaine | 12.2 (5.1) | 13.2 (4.3) | 12.7 (4.8) | 13.9 (4.2) | 1.49 | .218 |
| KMSK-Heroin | 5.8 (5.5) | 3.8 (4.9)* | 6.7 (5.3)* | 5.4 (5.4) | 5.15 | .002 |
| % MDD | 22 | 30 | 37 | 33 | 5.10 | .165 |
| PCL-C | 37.4 (13.2) | 35.0 (13.3) | 38.0 (13.8) | 38.2 (14.2) | 0.80 | .494 |
| WURS | 28.2 (17.4) | 30.1 (21.3) | 29.2 (20.4) | 33.2 (23.7) | 0.63 | .594 |
| SRPS | 48.1 (9.9) | 50.2 (9.3) | 48.2 (9.7) | 48.8 (7.7) | 0.77 | .512 |
| CNS Penetrance | 8.1 (2.0) | 7.3 (1.2)* | 5.09 | .026 | ||
| Md current CD4 | 491 | 537 | −0.70 | .482 | ||
| Md nadir CD4 | 182 | 227* | −1.93 | .054 | ||
| % CD4 < 200 | 14 | 6 | 2.12 | .150 | ||
| % Undetectable VL | 75 | 84 | 1.47 | .225 |
Means with (standard deviations).
Significant post-hoc group comparisons.
Chi-square tests were used for categorical data (%) and Mann Whitney statistic with Z correction for nonnormally distributed data (Md). All other analyses used one-way analysis of variance (ANOVA).
WTAR = Wechsler Test of Adult Reading; FSIQ = Full Scale Intelligence Quotient; ASI = Addiction Severity Index; KMSK = Kreek McHugh Schluger Kellogg Scale; HCV = hepatitis C virus; MDD = major depressive disorder; PCL C = PTSD Checklist Civilian Version; WURS = Wender Utah Rating Scale; SRPS = Levenson Self-Report Psychopathy Scale; CPE = CNS Penetration Effectiveness; CD4 = T4 lymphocyte count (200 cells/μL); VL = viral load (copies/ml).
N-back task
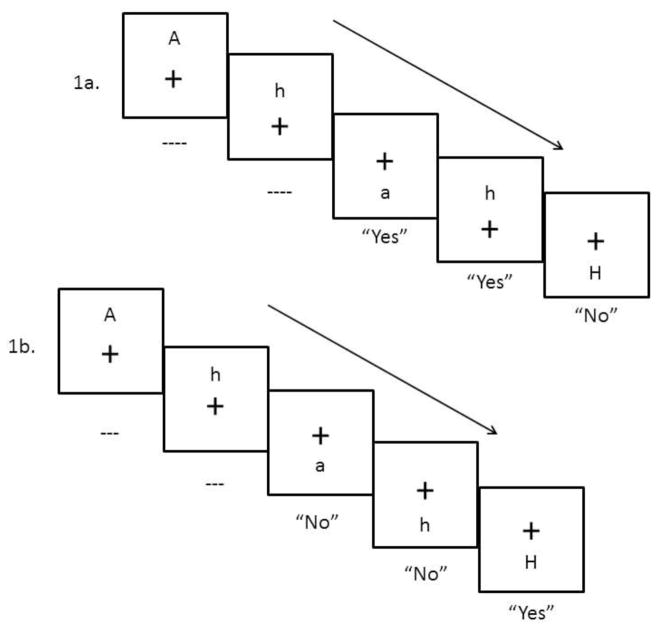
Verbal and spatial working memory performance was assessed using a modified version of the computerized n-back task employed previously by Hinkin et al. (2002). This version is advantageous in that verbal and spatial conditions presented identical stimulus displays in the same order of appearance with the goal of minimizing extraneous variance associated with visual or perceptual processing.
The task was programmed by one of the authors (DLH) using SuperLab® version 4.5 software and administered on a Hewlett-Packard PC (Elite 8300). In this n-back task, participants were presented with a pseudo-random series of letters (A, a, G, g, H, h, T, and t) one at a time on a computer monitor. Letters were black on a white background and subtended 1.9 × 1.6 degrees (with an average viewer distance of 60 cm from the computer screen). Location of each target letter was pseudo-randomly assigned to 2.5 degrees above, below, left, or right of a central fixation (+). Thus, at the start of each trial, a small cross appeared at central fixation, 2500 ms later a target letter appeared. The fixation and target letter remained on the screen until a participant response (a button press) was made. After the response (and disappearance of the fixation and target letter), there was a blank 1000 ms interstimulus interval, whereupon the fixation cross appeared for the next trial, followed by a target letter, and so on (see Figure 1). Working memory difficulty was manipulated via 1-back and 2-back versions of the task. In the 1-back task, each letter had to be compared with the prior presented letter. In the verbal condition, the verbal identity of the presented letter was compared to the verbal identity of the previous letter. If the verbal identity matched (e.g., a currently presented H was preceded by an H or h, note that case of letter does not matter), then a “yes” response was required (by pressing a green left button on the response box with the left forefinger). A non-match required a “no” response (a right red button press with the right forefinger). In the spatial condition, the location of the presented letter had to be compared with the location of the previous letter. If the location matched (e.g., the currently presented letter was to the right of the fixation, as was the preceding letter, note that verbal identity of the letter does not matter), then a “yes” response was required. If not, then a “no” response was required. Both the verbal and spatial task conditions each consisted of 10 practice trials followed by one block of 32 trials. Identical stimulus displays were employed for the verbal and spatial task conditions, where each letter and location were presented an equal number of times. Practice trials included feedback. In the more difficult 2-back task, each letter had to be compared with the letter presented two trials back. Otherwise, verbal and spatial task conditions were identical to the 1-back task.
Figure 1.
1a. 2-back stimulus display in Verbal condition.
1b. 2-back stimulus display in Spatial condition
Participants completed the 1-back first, then the 2-back. The order of verbal and spatial conditions was counterbalanced across participants. Instructions for each task and condition were presented on the computer monitor and explained by the researcher administering the task. Response accuracy and reaction time data were recorded for each trial. The computer recorded RTs for error and no-response trials, but these were excluded from analysis. Mean accuracy (total correct) for each of the four trial blocks (1-Back Spatial, 2-back Spatial, 1-back Verbal, 2-back Verbal) was the dependent variable of primary interest for n-back performance, with response speed (reaction times, RTs) of secondary interest.
Statistical analyses
We compared demographic, substance abuse and comorbidity data from the four participant groups (HIV− men; HIV+ men; HIV− women; HIV+ women) to identify potential confounds, using one-way analyses of variance (ANOVA) and post-hoc Tukey HSD tests for parametric data, Kruskal-Wallis tests for non-parametric data2, and χ2 tests for categorical data. An α level was set at .01 for all four-group comparisons to decrease likelihood of capitalizing on chance findings. Pearson and Spearman correlations were employed to index the strength of associations between n-back data and participant characteristics for parametric and non-parametric data, respectively.
N-back accuracy and RT data were analyzed separately for Spatial and Verbal conditions using mixed model analyses of covariance (ANCOVA), with Sex and HIV Serostatus as between-subjects factors and Task Demand (1 vs. 2 back) as the within-subjects factor, controlling for age and education.
Results
Table 1 shows demographic, comorbidity and HIV disease characteristics for the four participant groups. The overall sample was 86.3% African-American and 66% female. The mean age was 49 (SD = 8.1) and mean years of education was 12.3 (SD = 2.0). Mean estimated Verbal IQ using the Wechsler Test of Adult Reading (WTAR) was 87.4 (SD = 9.4). All HIV+ participants were ambulatory and able to tolerate 2–3 hours of testing. Their median CD4 lymphocyte count was 497 at time of testing, IQR = [285,771] and median nadir CD4 count was 195, IQR = [100, 360]. Approximately 11% of the group had AIDS-defining CD4 counts (< 200 cells/μL) at testing. Plasma viral load was undetectable at < 40 copies/ml for 79% of HIV+ participants. A total of 97.4% were prescribed antiretroviral therapy at testing: 96.2% with combination antiretroviral therapy (cART).
A significantly higher percentage of HIV− men were African-American compared with HIV− women (χ2 = 15.24, p = .002). Mean years of education was significantly lower for HIV+ women compared with the other three study groups (F(3,356) = 7.27, p < .001; HSD p ≤.01 for all tests). Mean WTAR-estimated IQ was significantly lower for HIV+ women compared with HIV− and with HIV+ men, F(3,353) = 4.05, p = .008; HSD p =.02 and .01, respectively). There were small but significant correlations between years of age and education with mean n-back accuracy (age: r = −.15, p = .004; education: r = .19, p < .001) and overall RTs (age: r = .23, p < .001; education: r = −.11. p = .04), so both variables were employed as covariates for the analyses of n-back performance3.
Tables 1 and 2 show substance use data for all participants. Most participants met DSM-IV criteria for substance dependence in early or sustained remission (alcohol 91%, cocaine 92%, opioids 70%). The groups were generally comparable on substance abuse characteristics, with no significant group differences noted on mean ASI-Drug, KMSK-Alcohol and KMSK-Cocaine subscores, or in prevalence of DSM-IV-diagnosed alcohol, opioids, cocaine or cannabis dependence (all ps > .05). Mean KMSK-Heroin scores were significantly higher (p = .001) among HIV− women compared with HIV+ men. Finally, HIV− women reported significantly more days since their last use of cocaine compared with HIV− and HIV+ men, both ps < .01. Mean years of alcohol use were significantly higher and use of alcohol in the past 30 days was significantly more common among the male compared with the female participants, ps = .01; and ASI-Alcohol scores were significantly higher among HIV− men compared with HIV− women (p = .002).
Table 2.
Substance abuse characteristics
| HIV− Men | HIV+ Men | HIV− Women | HIV+ Women | Statistic | p | |
|---|---|---|---|---|---|---|
| Years Used+ | ||||||
| Alcohol | 17.3 (13.2)* | 15.0 (13.5)* | 10.8 (10.9) | 10.6 (9.4) | 6.05 | .001 |
| Opioids | 10.1 (13.0) | 4.9 (10.1) | 9.0 (11.3) | 7.4 (11.3) | 2.93 | .034 |
| Cocaine | 14.2 (10.7) | 14.5 (10.7) | 12.6 (9.9) | 15.3 (9.9) | 1.26 | .289 |
| Cannabis | 13.8 (12.8) | 15.3 (12.6)* | 9.0 (11.8)* | 10.1 (11.8) | 5.95 | .001 |
| Md Days Since Use++ | ||||||
| Alcohol | 491* | 361* | 693 | 732 | 13.5 | .004 |
| Opioids | 496 | 1448 | 676 | 768 | 2.64 | .450 |
| Cocaine | 966 | 730 | 1164* | 768 | 11.9 | .008 |
| Cannabis | 4798 | 5153 | 4182 | 1460* | 10.5 | .020 |
| % Use Last 30 Days | ||||||
| Alcohol | 42* | 40* | 24 | 20 | 13.39 | .004 |
| Opioids | 3 | 2 | 3 | 8 | 3.44 | .328 |
| Cocaine | 10 | 18 | 5 | 6 | 9.84 | .015 |
| Cannabis | 5 | 14 | 4 | 8 | 7.80 | .050 |
| % DSM-IV SUDs | ||||||
| Alcohol | 52 | 71 | 52 | 59 | 7.48 | .060 |
| Opioid | 37 | 29 | 49* | 41 | 8.60 | .035 |
| Cocaine | 81 | 84 | 86 | 90 | 1.78 | .618 |
| Cannabis | 70 | 59 | 52 | 43 | 8.98 | .030 |
| %IDU | 17 | 25 | 17 | 20 | 2.29 | .515 |
| %Overdose | 17 | 14 | 25 | 20 | 3.83 | .280 |
Means with (standard deviations).
Significant post-hoc group comparisons.
Chi-square tests were used for categorical data (%) and Kruskal-Wallis tests for nonnormally distributed data (Md). All other analyses used one-way analysis of variance (ANOVA).
DSM IV = Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; SUDs= Substance Use Disorders; IDU= injection drug use
No significant group differences were observed in prevalence of DSM-IV Major Depression or mean scores on measures of PTSD, ADHD, or antisocial personality traits (p > .17 for all tests). Compared with HIV+ women, median nadir CD4 counts were significantly lower for HIV+ men (z = −1.93, p = .05) and mean CNS Penetration scores were significantly higher (F(1,103) = 5.09, p = .03). HIV+ men and women showed no significant differences in median current CD4 counts, or prevalence of AIDS-defining CD4 counts, undetectable HIV RNA levels, or currently prescribed cART (p ≥ .23 for all tests).
N-Back Task
Approximately 3% of participants obtained at least one RT greater than 3 SDs above the mean. They were considered outliers and their RT data were not analyzed further. Both accuracy and response speed were skewed and were therefore natural log-transformed. Mean error rates for the spatial and verbal task RTs did not differ significantly among the four subject groups (Spatial: p = .12, η2 = .02; Verbal: p = .08, η2 = .02). There were no significant inverse correlations between errors and overall RTs for either spatial or verbal data, indicating no significant speed/accuracy tradeoffs.
Preliminary analyses of each of the four dependent variables (spatial and verbal accuracy, spatial and verbal reaction times) showed expected significant main effects for Task Demand (all ps < .001), verifying that response accuracy was lower, and RTs were slower for the 2-back compared with 1-back conditions. All interactions involving the Task Demand factor were non-significant (ps ≥.09).
Accuracy
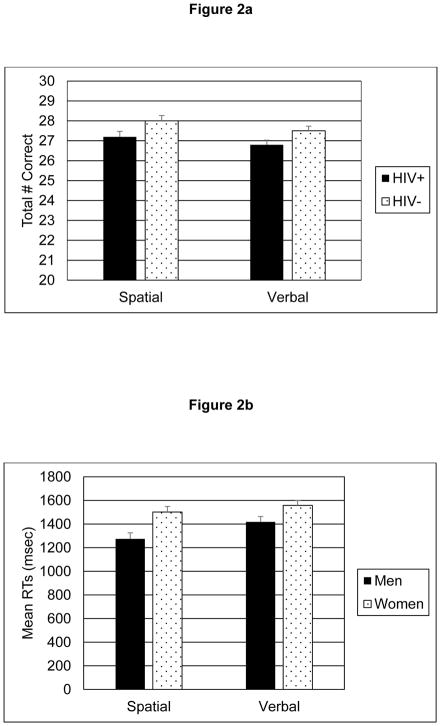
Analyses of spatial and verbal accuracy data revealed significant main effects for Serostatus (Spatial: F(1, 354) = 5.82, p = .02, Cohen’s d = .23; Verbal: F(1, 354) = 4.86, p =.03, d = .21); and inspection of the means indicated that the HIV− participants made significantly more correct responses compared with the HIV+ groups in both stimulus conditions. There were no significant main effects for Sex or for the Sex x HIV Serostatus interactions (ps > .26) for either stimulus condition. Mean spatial and verbal accuracy scores for HIV− and HIV+ groups are represented in Figure 2a.
Figure 2.
2a. Mean n-back spatial and verbal accuracy scores for HIV+ compared with HIV− participants. Mean accuracy was significantly poorer among HIV+ compared with HIV− participants in both verbal and spatial conditions.
2b. Mean spatial and verbal reaction times for male compared with female participants (high score = slower performance). Reaction times were significantly slower among women compared with men in both verbal and spatial conditions.
Response speed
In contrast to accuracy scores, analyses of both spatial and verbal RTs showed significant main effects for Sex (Spatial: F(1,349) = 7.64, p = .006, d = −.43; Verbal: F(1,354) = 7.16, p = .008, d = −.30), with slower response times for women compared with men. The main effects for HIV Serostatus and the Sex x HIV interactions did not reach statistical significance for either analysis (ps ≥.61). Mean spatial and verbal RTs for male and female groups appear in Figure 2b.
HIV disease
To limit the number of group comparisons, only accuracy scores were used to investigate potential associations of n-back performance with HIV disease indicators. We found no significant group differences between verbal or spatial accuracy scores for HIV+ participants with or without AIDS-defining CD4 counts or undetectable HIV RNA levels (p ≥.15) and no significant correlations between accuracy scores with current and nadir CD4 counts or mean CNS Penetrance Effectiveness scores (ps ≥.36).
Additional analyses
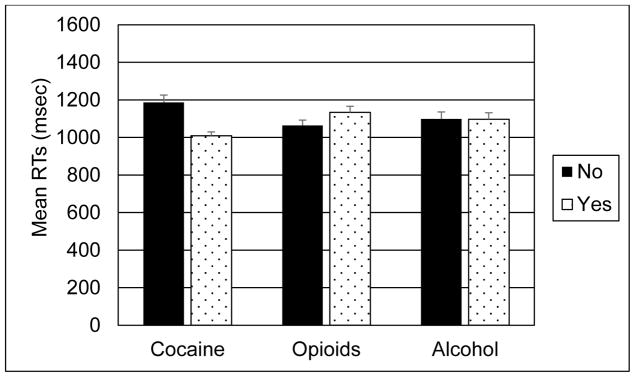
Finally, we conducted a series of exploratory analyses to compare our results with published findings on HIV and n-back. Previous studies (Meyer et al., 2013; Keutmann et al., 2016) have reported that risk of cognitive impairment is increased among HIV+ women with a history of cocaine dependence. We repeated the primary Sex x HIV Serostatus analyses adding Cocaine Dependence as an additional between-subjects factor. There were no significant main effects or interactions involving Cocaine Dependence for spatial or verbal accuracy scores, p > .40 for each comparison. Spatial and verbal RTs were both significantly faster among the cocaine-dependent compared with non-dependent participants (Spatial: F(1,350) = 22.3, p < .001, d = .62; Verbal: F(1,350) = 5.0, p = .03, d = .30). There were no significant inverse correlations between RTs and total number of errors, indicating no speed-accuracy tradeoffs; thus, faster RTs for the cocaine-dependent participants could not be attributed to group differences in errors of commission. There were no significant differences in speed of responding between participants with and without opioid dependence, or alcohol dependence. Figure 3 shows mean spatial RTs for groups of cocaine-dependent, opioid-dependent and alcohol-dependent participants. Hinkin et al. (2002) reported that HIV+ and HIV− groups performed significantly more accurately on the spatial compared with the verbal 2-back. We compared participants’ spatial and verbal accuracy using mixed model ANCOVA, adding Test Stimulus (Spatial vs Verbal) to the primary Sex and HIV Serostatus analyses. There were no significant main effects or interactions involving the Test Stimulus factor (ps ≥.17). Caldwell and colleagues (Caldwell et al., 2014) reported that participants dually infected with HIV and hepatitis C virus (HCV) showed slower RTs and increased errors of commission on the verbal 2-back compared with HIV monoinfected and uninfected groups. We compared verbal 2-back performance of uninfected, monoinfected (HIV+ or HCV+ but not both), and dually infected groups using univariate ANCOVA with Infection Status as the between-subjects factor. We found a significant linear trend toward poorer verbal accuracy and higher total errors (accuracy: F(1,355) = 4.5, p = .01; Errors: F(1,355) = 4.7, p = .01) among groups ordered by infection status. No significant group differences in mean verbal RTs were observed.
Figure 3.
Mean spatial reaction times for participants with and without cocaine dependence, opioid dependence, and alcohol dependence (high score = slower performance). Reaction times were significantly faster among participants with cocaine dependent compared with participants without cocaine dependence.
Note. All n-back measures were natural log-transformed prior to data analyses; data for each figure were back-transformed for clarity of presentation.
Discussion
Working or “representational” memory (WM) is an essential component of many complex cognitive functions, and critically dependent on the integrity of neural circuitry including prefrontal cortex and neostriatum. WM is frequently impaired among individuals infected with HIV, particularly HIV+ substance dependent individuals (SDIs); but previous investigations of working memory among HIV+ SDIs generally involved all- or primarily male participant samples. In the current study, we administered a modified version of the n-back task with known sensitivity to HIV serostatus (Hinkin et al., 2002) to a group of 360 HIV+ and HIV− drug using men and women verified abstinent from alcohol and drugs of abuse at testing. Both HIV-infected men and women performed significantly less accurately compared with HIV− controls on verbal and spatial versions of the n-back, findings which replicate and extend Hinkin et al.’s earlier results from a primarily male study sample (2002). Participant groups were generally comparable on substance use and comorbid characteristics, and most participants met DSM-IV criteria for substance use disorders in remission, indicating that group differences in n-back performance could not be readily attributed to nonspecific effects of recent drug use.
In a recent meta-analysis of studies of executive function and HIV serostatus, Walker and Brown (2017) concluded that working memory was the most commonly affected “cognitive” executive function among HIV+ individuals. Furthermore, among the working memory measures reviewed, the 2-back was most sensitive to HIV effects. Their report and our current findings support our earlier proposal (Martin et al., 2001) that working memory impairment represents a “signature” deficit among HIV+ SDIs, who perform more poorly compared to HIV− SDIs on essentially all measures that engage working memory mechanisms through various task characteristics, such as response requirements, time delay, and memory load. These findings suggest that working memory represents a critically vital component of neurocognitive screening for HAND, especially for the assessment of HIV+ individuals with a history of substance dependence
We found that both HIV+ men and women performed the n-back significantly less accurately compared with HIV− participants, but the magnitude of this serostatus effect did not differ by sex. Although neurocognitive risk factors are more common among HIV+ women (Maki & Martin-Thormeyer, 2009), the current findings indicate that HIV+ women’s neurocognitive performance is not invariably poorer compared with HIV+ men. Additionally, although reports of female-specific impairment in visual memory and in decision making under risk support the hypothesis that neurocognitive profiles are not identical among HIV+ women and men (Keutmann et al., 2016; Martin et al., 2016), deficits in other neurocognitive functions are not necessarily sex-specific. In this regard, we recently reported a similar pattern of poorer performance on a spatial and navigational memory among both HIV+ men and women compared with HIV− controls, but no evidence of sex differences among HIV+ groups (Fogel et al., 2017). Taken together, these findings and Maki et al.’s report that verbal memory is more prominently affected among HIV+ women (2015), might contribute to characterizing sex-specific phenotypes of HIV-associated neurocognitive impairment and affected neural circuitry.
We found no evidence of slower reaction times (RTs) among HIV+ compared with HIV− groups. The clinical significance of psychomotor slowing as the predominant feature of HIV-associated neurocognitive impairment has declined since the introduction of cART; however, results of RT-based studies among individuals living with HIV/AIDS have historically been variable. Some early results suggested that RT tasks might be sensitive to mild cognitive slowing among asymptomatic HIV+ individuals (Martin et al., 1992), but a comprehensive review of RT studies at the time revealed that while fairly common, there was large variability in RT findings, with some but not all studies showing mental slowing in HIV+ groups (Hardy & Hinkin, 2002). Some of the variability in these early findings may be attributable to differences in HCV serostatus or other variables not yet identified as potential contributors to HIV effects on neurocognition. However, at a more conceptual level the assessment of specific cognitive functions using RTs remains complicated: the task response requirements can engage multiple cognitive processes in addition to response speed when participants are instructed to respond as quickly and as accurately as possible, which complicate interpretation of RT performance.
We had hypothesized tentatively that female SDIs would perform the spatial n-back more poorly compared with male SDIs. We found no evidence of sex differences in spatial accuracy. Women performed the spatial n-back more slowly compared to men; however, verbal RTs were also slower for women compared with men, suggesting that the RT results might be attributed to more generalized slowing. Our findings are not incompatible with available literature; although a male advantage on spatial tasks is the most commonly reported sex-specific effect (Miller & Halpern, 2014), evidence for sex differences in n-back performance has been less consistent (Anderson-Schmitt et al.; Haut & Barch, 2006; Lejbak, Crossely & Verbancic, 2011; Evans & Hampson, 2011).
In contrast with our findings, studies from the WIHS have not shown consistent WM impairment among HIV+ compared with HIV− women. Maki and colleagues (2015) reported that cross-sectional performance on the Letter Number Span Task (LNST) did not differ significantly between HIV+ and HIV− women; similarly, Sundermann and colleagues (2015) found no overall differences in n-back performance among HIV+ compared with HIV− women. However, Rubin et al. (2017) reported that HIV+ women with viral suppression performed the LNST significantly more poorly compared with HIV− women at baseline and at follow-up. These differing results might reflect the relatively low prevalence of substance dependence among the WIHS participants, suggesting a positive substance use disorder elevated the risk of WM impairment among HIV+ women.
In contrast with previous findings (Keutmann et al., 2016; Meyer et al., 2013) we found no evidence of poorer n-back performance specifically among cocaine dependent HIV+ women; rather, RTs were faster among cocaine-dependent men and women, regardless of HIV serostatus. Additional neurocognitive and neuroimaging studies are necessary to determine if cocaine affects systematically a particularly subset of neurocognitive functions among HIV+ women and how these might be characterized further.
The pattern of sex differences in n-back response speed, but serostatus differences in response accuracy is roughly comparable with a recent report by our group (Fogel et al., 2017) that both HIV+ and HIV− women performed Memory Island, a spatial-navigational learning task, more slowly on the immediate learning trials compared with men; but delayed recall was significantly poorer for both HIV+ men and women compared with HIV− participants. In both studies, women performed more poorly on the speeded component of the tasks compared with men, but HIV+ men and women showed impairment on the variables of primary importance (n-back accuracy and spatial delayed recall). These results illustrate the importance of considering time-based and accuracy-based components separately, since these two cognitive operations can be affected differentially by sex or HIV serostatus.
In an exploratory analysis, we found a linear trend toward poorer verbal accuracy among participants rank ordered as uninfected, monoinfected, or dually infected with HIV and hepatitis C virus (HCV). In a previous report, we found a monotonic trend toward slower RT Stroop performance among SDIs characterized by HIV and HCV serostatus, suggesting that deficits in multiple executive functions are amplified among HIV+ individuals in the presence of a positive HCV serostatus.
Findings from this study are necessarily limited. All study participants met criteria for a current or lifetime DSM-IV diagnosis of dependence on at least one substance. We assumed a priori that study results would not necessarily generalize to non-substance dependent men and women, regardless of serostatus: although not definitive, study results were consistent with this assumption. Additionally, the majority of participants were dependent on more than one substance, which is by far the most common pattern of substance use observed in North America studies of neuroAIDS and drug abuse so specific neurocognitive effects of different substances cannot be isolated (Martin-Thormeyer and Paul, 2009). On the other hand, the n-back procedure was advantageous by its administration at the second study visit; one might speculate that living situations were more stable and neurocognitive performance less vulnerable to potential confounds among those participants who kept both appointments.
In summary, results from the current study identify new details of the neurocognitive picture among HIV+ women compared with HIV+ men as well as HIV− women; suggest that working memory deficits among HIV+ women are critically affected by current or previous substance dependence; provide further support that working memory impairment may be a signature neurocognitive deficit among HIV+ substance dependent individuals; and suggest that HIV+ female SDIs in substance abuse treatment are likely to derive benefit from working memory training.
Acknowledgments
We thank Stan Chen, Christine Franco, Haley Sullins and Leslie Ladd for data collection, and Benjamin Spielberg for processing and managing the n-back data. Supported by the National Institute on Drug Abuse R01 DA12828 to Eileen Martin.
Footnotes
These procedures were followed in all instances with the single exception that participants who tested positive for cannabis were not excluded if testing was negative for all other substances. The presence of THC metabolites in the urine did not necessarily indicate cannabis use within 1–2 days prior to testing due to its much longer half-life.
Mann-Whitney tests with z approximations were used to compare HIV disease variables among HIV+ men and women.
There was a highly significant correlation between education and mean WTAR IQ estimate, r = .52, p < .001, so mean WTAR scores were not included as a covariate.
Authors’ contributions. All authors contributed scientifically to the manuscript and have read and approved the final version.
Compliance with ethical standards. Written informed consent was obtained upon arrival at the first study visit.
Conflict of interest. The authors declare that they have no conflicts of interest.
References
- Anderson-Schmidt H, Jogia J, Fast K, Christodoulou T, Haldane M, Kumari V, Frangou S. No gender differences in brain activation during the N-back task: An fMRI study in healthy individuals. Hum Br Map. 2009;30:3609–3615. doi: 10.1002/hbm.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok JA, Martin EM, Pitrak DL, Novak RM, Pursell KJ, Mullane KM, Harrow M. Working memory deficits in HIV-seropositive drug users. J Int Neuropsychol Soc. 1997;3:451–456. [PubMed] [Google Scholar]
- Behrman-Lay AM, Paul RH, Heaps-Woodruff J, Baker LM, Usher C, Ances BM. Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. J NeuroVirol. 2016;22:93–103. doi: 10.1007/s13365-015-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Moody L, Quisenberry A. Computerized working-memory training as a candidate adjunctive treatment for addiction. Alc Res. 2014;36:123–126. doi: 10.35946/arcr.v36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Løhaugen GC, Andres T, Jiang CS, Douet V, Tanizaki N, Walker C, Castillo D, Lim A, Skranes J, Otoshi C, Miller EN, Ernst TM. Adaptive working memory training improved brain function in human immunodeficiency virus–seropositive patients. Ann Neurol. 2017;81:17–34. doi: 10.1002/ana.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Br Cog. 2001;47:470–493. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- Evans KL, Hampson E. Sex differences on prefrontally-dependent cognitive tasks. Br Cog. 2015;93:42–53. doi: 10.1016/j.bandc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Harrow M. Verbal working memory in HIV-seropositive drug users. J Int Neuropsychol Soc. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) New York State Psychiatric Institute; New York: 2004. [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Br Res. 1991;83:325–336. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann NY Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH. Reaction time performance in adults with HIV/AIDS. J Clin Exp Neuropsychol. 2002;24:912–929. doi: 10.1076/jcen.24.7.912.8391. [DOI] [PubMed] [Google Scholar]
- Haut KM, Barch DM. Sex influences on material-sensitive functional lateralization in working and episodic memory: Men and women are not all that different. Neuroimage. 2006;32:411–422. doi: 10.1016/j.neuroimage.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR, Imasiku ML, Kalima K, Heaton RK. Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. J Nerv Ment Dis. 2012;200:71–83. doi: 10.1097/NMD.0b013e31824cc225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Lam MN, Stefaniak M, Zolnikov B. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc. 2002;8:532–538. doi: 10.1017/s1355617702814278. [DOI] [PubMed] [Google Scholar]
- Keane TM, Wolfe J, Taylor KL. Post-traumatic stress disorder: Evidence for diagnostic validity and methods of psychological assessment. J Clin Psychol. 1987;43:32–43. doi: 10.1002/1097-4679(198701)43:1<32::aid-jclp2270430106>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek–McHugh–Schluger–Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alc Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Lejbak L, Crossley M, Vrbancic M. A male advantage for spatial and object but not verbal working memory using the n-back task. Br Cog. 2011;76:191–196. doi: 10.1016/j.bandc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. J Pers Soc Psychol. 1995;68:151. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E. HIV, cognition and women. Neuropsychol Rev. 2009;19:204–214. doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber K, Manly J, Richardson J, Alden C, Anastos K. Cognitive function in women with HIV: Findings from the Women’s Interagency HIV Study. Neurology. 2015;8:231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. J Clin Exp Neuropsychol. 2011;33:112–120. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Weddington W, Harrow M. Auditory working memory in HIV-1 infection. J Intl Neuropsychol Soc. 2001;7:20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV Associated Neurocognitive Disorder: Neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’ Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Valcour V, Young MA, Crystal H, Anastos K, Aouizerat BE. HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr. 2013;63:67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DI, Halpern DF. The new science of cognitive sex differences. Trends in Cog Sci. 2014;18:37–45. doi: 10.1016/j.tics.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Rapeli P, Kivisaari R, Autti T, Kähkönen S, Puuskari V, Jokela O, Kalska H. Cognitive function during early abstinence from opioid dependence: a comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry. 2006;6:9. doi: 10.1186/1471-244X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Gallagher NM, Sullivan M, Callicott JH, Green AE. Sex differences in verbal working memory performance emerge at very high loads of common neuroimaging tasks. Brain Cog. 2017;113:56–6. doi: 10.1016/j.bandc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Kapoor C, Robertson WT, Fiscus S, Ford S, Hall CD. No gender differences in the progression of nervous system disease in HIV infection. J Acquir Immune Defic Syndr. 2004;36:817–822. doi: 10.1097/00126334-200407010-00008. [DOI] [PubMed] [Google Scholar]
- Royal W, III, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA. Associations between cognition, gender and monocyte activation among HIV infected individuals in Nigeria. PloS One. 2016;11:e0147182. doi: 10.1371/journal.pone.0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z. Psychometric characteristics of the Wender Utah Rating Scale (WURS): reliability and factor structure for men and women. Psychopharmacol Bull. 1995;31:425–433. [PubMed] [Google Scholar]
- Stout JC, Salmon DP, Butters N, Taylor M, Peavy G, Heindel WC, Delis DC, Ryan L, Atkinson JH, Chandler JL, Grant I the HNRC Group. Decline in working memory associated with HIV infection. HNRC Group. Psychol Med. 1995;25:1221–1232. doi: 10.1017/s0033291700033195. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Bishop JR, Rubin LH, Little DM, Meyer VJ, Martin E, Weber K, Cohen M, Maki PM. Genetic predictor of working memory and prefrontal function in women with HIV. J Neuro Virol. 2015;21:81–91. doi: 10.1007/s13365-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Brown GG. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta-analysis. J Clin Exp Neuropsychol. 2017 Jul;9:1–20. doi: 10.1080/13803395.2017.1349879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75:435–438. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington CL. Sex differences and gonadal hormone influences in drug addiction and sexual behavior: progress and possibilities. Hormones Behav. 2010;58:2–7. doi: 10.1016/j.yhbeh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Yi JY, Bell RP, Ross T, Stein E, Daughters SB. Poorer working memory performance among cocaine users accounted for by increased activation in the middle frontal gyrus. Drug Alc Depend. 2017;171:e220–e221. [Google Scholar]