Abstract
Introduction
Limited data are available describing indications for and outcomes of therapeutic plasma exchange (TPE) in cardiac transplantation.
Methods
In a retrospective study of patients who underwent cardiac transplantation at Duke University Medical Center from 2010 to 2014, we reviewed 3 TPE treatment patterns: a single TPE procedure within 24 hours (h) of transplant; multiple TPE procedures initiated within 24h of transplant; and one or more TPE procedures beginning >24h post-transplant. Primary and secondary outcomes were overall survival and TPE survival (OS and TS), respectively.
Results
Of 313 patients meeting study criteria, 109 (35%) underwent TPE. TPE was initiated in 82 patients within 24h, 40 (37%) receiving a single procedure (Single TPE) and 42 (38%) multiple procedures (Multiple TPE). Twenty-seven (25%) began TPE >24h after transplant (Delayed TPE). The most common TPE indication was elevated/positive panel reactive or human leukocyte antigen antibodies (32%). With a median follow-up of 49 months, the non-TPE treated and Single TPE cohorts had similar OS (HR 1.08 [CI, 0.54, 2.14], p = 0.84), while the Multiple and Delayed TPE cohorts had worse OS (HR 2.62 [CI, 1.53, 4.49] and HR 1.98 [CI, 1.02, 3.83] respectively). The Multiple and Delayed TPE cohorts also had worse TS (HR 2.59 [CI, 1.31, 5.14] and HR 3.18 [CI, 1.56, 6.50] respectively). Infection rates did not differ between groups but was independently associated with OS (HR 2.31 [CI, 1.50, 3.54]).
Conclusions
TPE is an important therapeutic modality in cardiac transplant patients. Prospective studies are needed to better define TPE’s different roles in this patient population.
Keywords: Plasmapheresis, heart transplant rejection, allograft dysfunction
INTRODUCTION
An important and commonly used treatment modality in cardiac transplantation is therapeutic plasma exchange (TPE).1–3 Although primarily used to preserve allograft function, TPE in cardiac transplantation can be used in one or more of the following ways: to facilitate transplant across a positive crossmatch; treat antibody-mediated rejection (AMR); treat primary graft dysfunction; reduce donor-specific human leukocyte antigen (HLA) antibody titers (DSA); and, in highly-sensitized patients, decrease panel reactive antibody (PRA) titers.2,4–9 Of these indications, the only ones categorized in the American Society for Apheresis (ASFA) national guidelines are desensitization (ASFA category II; Grade 1C) and AMR (ASFA category III; Grade 2C).10 Categorization of additional indications within ASFA guidelines is constrained by the limited availability of outcomes data.2 Therefore, we undertook a retrospective study of cardiac transplant patients at a single center to define TPE indications and assess clinical outcomes.
MATERIALS AND METHODS
Patients and Study Design
Under an institutional review board-approved protocol, we retrospectively reviewed a prospectively maintained clinical therapeutic apheresis database. We identified all consecutive patients who received TPE between January 1, 2010, and December 31, 2014, for a cardiac transplant indication such as 1) perioperative TPE for suspected positive crossmatch or heparin induced thrombocytopenia or 2) postoperative TPE for positive HLA antibodies, elevated PRA, positive crossmatch, primary graft dysfunction, and/or AMR. Simultaneously, using an institutional electronic search resource, the Duke Enterprise Data Unified Content Explorer (DEDUCE),11 we identified all consecutive patients who received a cardiac transplant from 2010 – 2014. We included in our study cohort all patients identified as having received both a cardiac transplant and TPE within the 2010 – 2014 study period. We excluded any patients who were transplanted at a center other than Duke University Medical Center (DUMC) or whose 2010 – 2014 cardiac transplant surgery represented a second or third transplant (re-transplant). We also excluded all TPE procedures performed prior to transplantation.
Based on 1) the timing of TPE relative to transplantation and 2) number of TPE procedures, TPE-treated patients were categorized into the following cohorts: cohort 1 received a single TPE procedure within 24 hours of transplantation and was designated the “Single TPE” cohort; cohort 2 received more than 1 TPE procedure within 24 hours of transplantation and was designated the “Multiple TPE” cohort; and cohort 3 received one or more TPE procedures beginning more than 24 hours post transplantation and was designated the “Delayed TPE” cohort. Cardiac transplant patients who did not receive TPE within the study period were designated the non-TPE treated or “Zero TPE” cohort and used as the control group for assessment of overall survival.
Therapeutic Plasma Exchange Protocol
Patients were treated with a standardized TPE protocol to exchange 1.0 plasma volume (PV), calculated using Nadler’s formula.12 The default TPE exchange fluid was Albumin (Albutein® 5% Solution; Grifols, Durham NC). A 100% albumin exchange was typically performed except in the following cases: when TPE was done within 3 days of a surgical procedure; when pre-TPE fibrinogen was < 100 mg/dL; on the 3rd consecutive day of daily TPE; and in any patient in whom clinical bleeding risk was felt to be increased due to ongoing therapeutic anticoagulation. In these cases, plasma or a combination of albumin and plasma was used. The primary anticoagulant was Anticoagulant Citrate Dextrose Solution, Solution A® (ACD-A; Citra Labs, Braintree MA) at a ratio of 1:15. From 2010 – 2012, all patients were treated on the COBE® Spectra (Terumo BCT, Lakewood CO) and, from 2013 – 2014 on either the COBE Spectra or Spectra Optia® (Terumo BCT, Lakewood CO) apheresis systems.
Data Collection and Statistical Analysis
We abstracted patient-level study data from the electronic medical record into Research Electronic Data Capture (REDCap), a secure web-based application designed to support data capture for research studies.13 We recorded demographic information such as age, sex, race, transplant date, and transplant indication. We also recorded medications given on a day that TPE was performed and, for all patient cohorts, documented the presence of infection (positive tissue, blood, urine or stool cultures) within the first 30 days of transplantation. We recorded TPE indication, dates of TPE procedures, and number of TPE episodes (defined as 1 or more TPE procedures constituting a treatment encounter). For the purposes of the study, if a patient received TPE in more than one treatment episode, only the first TPE episode was analyzed. We recorded time from transplant to the first TPE episode and time from the first TPE episode to the next TPE episode, re-transplant, death, or end of the follow up period. These outcomes were measured until the end of the follow up period, August 31, 2017.
Descriptive statistics were used to summarize patient-level demographics, transplant-related variables, and clinical outcomes. Standard χ2 tests were performed to examine the association between the demographic variables in the TPE-treated (Single, Multiple, and Delayed) vs. non TPE-treated (Zero TPE) cohorts. The primary outcome was overall survival with endpoints of cardiac re-transplantation or death from the date of initial transplant. The secondary outcome was TPE survival with endpoints of repeat TPE episode, re-transplantation, or death, from the date of the first TPE episode. Kaplan-Meier plots, log rank tests, and Cox proportional hazards regression modeling were used to examine associations between each survival outcome and the cohorts of interest (non TPE vs. TPE cohorts for overall survival and TPE cohorts for TPE survival). Regression models considered adjustment variables for inclusion using a forward selection technique with p-value for entry of 0.25 or less. Based on known risk factors for post cardiac transplant mortality, variables considered for inclusion included age, sex, race (white vs non-white), transplant indication (non-ischemic cardiomyopathy, ischemic cardiomyopathy, other), infection status (yes/no), and documentation of AMR (yes/no; only considered for TPE survival model).14,15
Results of multivariable modeling are presented as hazard ratios (HR), 95% confidence intervals, and p-values. All p-values were two-sided and considered significant at the nominal 0.05 level (i.e., p-value < 0.05). Statistical analyses were performed using GraphPad prism® (GraphPad Software, Inc., La Jolla, CA, v7.0b) and R 3.4.0 (R Core Team (2017), https://www.R-project.org) using the Survival Package.
RESULTS
Patient Characteristics
Of 333 orthotopic heart transplant (OHT) patients, 313 were eligible for analysis and 109 were treated with TPE. The flowchart for patient selection and outcomes reporting is shown in Figure 1. Demographics of the non TPE vs. TPE-treated study cohorts are shown in Table I. When compared to the non TPE-treated cohort, the TPE-treated cohort had a higher percentage of females (46 vs. 23%; p < 0.0001) and Blacks/African Americans (44 vs. 23%; p = 0.0009).
Figure 1. Schema of patient selection.
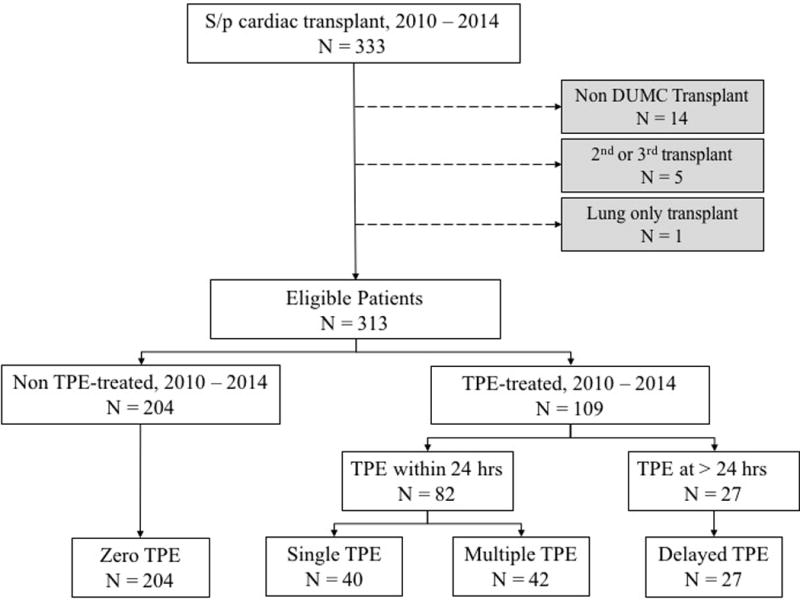
Of 333 patients transplanted within the study period, 1 incorrectly classified lung only transplant patient was excluded. Also excluded were 14 patients transplanted at a center other than Duke University Medical Center (DUMC) and 5 patients who received a second or third heart transplant. Of the 313 evaluable patients, 204 did not receive TPE within the study period (Zero TPE cohort) and 109 did. Of these 109 TPE-treated patients, 82 received TPE within 24 hours of transplantation with 40 patients receiving a single TPE procedure (Single TPE cohort) and 42 patients receiving multiple TPE procedures (Multiple TPE cohort); An additional 27 patients received one or more TPE procedures beginning more than 24 hours post transplantation (Delayed TPE cohort).
Table I.
Demographics of TPE vs. Non-TPE Treated Cardiac Transplant Patients
| TPE-treated N = 109 N (%) |
Non-TPE Treated N = 204 N (%) |
p | |
|---|---|---|---|
|
| |||
| Age (years), mean, SD | 48, 19 | 51, 19 | 0.45 |
| Range | 4 mos. – 76 yrs. | 2 mos. – 74 yrs. | |
|
| |||
| Pediatric patients (Age < 18) | 11 (10) | 20 (10) | 0.94 |
|
| |||
| Sex | < 0.0001 | ||
| Males | 59 (54) | 158 (77) | |
| Females | 50 (46) | 46 (23) | |
|
| |||
| Race | 0.0009 | ||
| White/Caucasian | 56 (51) | 142 (70) | |
| Black/African American | 48 (44) | 48 (23) | |
| Other | 5 (5) | 14 (7) | |
|
| |||
| Transplant year | 0.06 | ||
| 2010 | 22 (20) | 36 (18) | |
| 2011 | 28 (26) | 32 (16) | |
| 2012 | 17 (16) | 47 (23) | |
| 2013 | 24 (22) | 37 (18) | |
| 2014 | 18 (16) | 52 (25) | |
|
| |||
| Transplant indication | |||
| Non-ischemic cardiomyopathy | 58 (53) | 90 (44) | |
| Ischemic cardiomyopathy | 34 (31) | 86 (42) | 0.02 |
| Congenital Heart Defect | 12 (11) | 9 (5) | |
| Other | 5 (5) | 19 (9) | |
|
| |||
| Post-transplant infection present | 42 (39) | 76 (37) | 0.82 |
TPE = Therapeutic plasma exchange; N = Number; SD = Standard deviation; mos. = months; yrs. = years; p-values calculated using two-sample t-test and chi-square test of independence as appropriate.
Three patients in the non TPE-treated cohort underwent combined heart/lung transplant compared to 1 patient in the TPE-treated cohort. One patient in the TPE-treated cohort underwent a combined heart/kidney transplant.
Outcomes by TPE Cohort
As illustrated in Figure 1, there were 40, 42, and 27 patients in the Single, Multiple, and Delayed TPE cohorts, respectively. These 3 cohorts were similar in respect to age, sex, and transplant indication (see Table II).
Table II.
Characteristics of the Three (Single, Multiple, and Delayed) TPE Cohorts
| TPE Cohorts | |||
|---|---|---|---|
| Single TPE (N = 40) | Multiple TPE (N = 42) | Delayed TPE (N = 27) | |
| N (%) | N (%) | N (%) | |
|
| |||
| *Time from transplant (days) | |||
| Mean | 0 | 0 | 300 |
| Median (Q1, Q3) | 245 (9, 517) | ||
| Range | 2 - 1368 | ||
|
| |||
| Age (mean, SD) | 51, 16 | 50, 18 | 42, 22 |
|
| |||
| Pediatric (Age < 18) | 2 (5) | 3 (7) | 6 (22) |
|
| |||
| Sex | |||
| Male | 28 (70) | 16 (38) | 15 (56) |
| Female | 12 (30) | 26 (62) | 12 (44) |
|
| |||
| Race | |||
| Other | 1 (3) | 2 (5) | 2 (7) |
| Black/African American | 12 (30) | 25 (59) | 11 (41) |
| White/Caucasian | 27 (67) | 15 (36) | 14 (52) |
|
| |||
| Transplant Indication | |||
| Non-ischemic cardiomyopathy | 18 (45) | 26 (62) | 14 (52) |
| Ischemic cardiomyopathy | 17 (43) | 10 (24) | 7 (26) |
| Congenital heart defect | 1 (2) | 5 (12) | 6 (22) |
| Other | 4 (10) | 1 (2) | 0 (0) |
|
| |||
| TPE Indication | |||
| Graft dysfunction and/or AMR | 0 (0) | 7 (17) | 27 (100) |
| +PRA, +HLA Ab | 27 (68) | 8 (19) | 0 (0) |
| +Xmatch | 0 (0) | 26 (62) | 0 (0) |
| Other | 13 (32) | 1 (2) | 0 (0) |
|
| |||
| Medications | |||
| Steroids | 40 (100) | 42 (100) | 27 (100) |
| Monoclonal antibody | 39 (98) | 39 (93) | 6 (22) |
| Immune globulin | 1 (3) | 11 (26) | 16 (59) |
| Standard immunosuppression | 22 (55) | 42 (100) | 27 (100) |
|
| |||
| Post-transplant infection | 16 (40) | 17 (40) | 9 (33) |
Time from transplant is measured in days. TPE = Therapeutic plasma exchange; N = Number; Q1 = First Quartile; Q3 = Third Quartile; SD = Standard deviation; +PRA = positive panel reactive antibodies; +HLA Ab = positive human leukocyte antigen antibodies; AMR = antibody-mediated rejection.
Single TPE Cohort
For all patients, perioperative TPE was performed once at the time of transplantation. Most patients underwent TPE due to an elevated PRA or positive HLA antibodies (N = 27; 68%), followed by a variety of other causes, including 5 patients (12%) with suspected HLA antibodies but eventual negative crossmatch, 5 patients (12%) with heparin-induced thrombocytopenia (HIT), and 3 patients (8%) with unclear indication.
With regards to medical therapy on the day of the TPE procedure, all patients received methylprednisolone. Thirty-eight patients (95%) received basiliximab. Of the remaining 2 patients not treated with basiliximab, one (3%) received rabbit Antithymocyte Globulin (rATG) and the other rituximab. Twenty-two patients (55%) received a dose of standard immunosuppression (21 patients received mycophenolate and 1 patient received cyclosporine).
Thirty-day post-transplant infection occurred in 16 patients (40%). Ten patients had only 1 infection (6 with CMV viremia and 4 with positive bacterial cultures) and 6 patients had 2 infections (5 with CMV viremia and positive bacterial cultures and 1 with CMV viremia and Clostridium difficile).
Multiple TPE Cohort
TPE was used to treat a positive crossmatch in 26 patients (62%), elevated PRA or positive HLA antibodies in 8 patients (19%), primary graft dysfunction/AMR in 7 patients (17%), and suspected but eventually negative crossmatch in 1 patient (2%).
The most common TPE schedule was daily in 36 patients (86%) followed by daily with one skipped/missed day in 6 patients (14%). With regards to number of TPE procedures, most patients (N = 28; 67%) received 5 TPE. In descending order of number of TPE procedures, six patients (14%) received 6 TPE, 2 patients (5%) received 4 TPE, 1 patient (2%) received 3 TPE and 5 patients (12%) received 2 TPE. Of the 5 patients who received only 2 TPE, 4 were stopped early due to negative crossmatch results and 1 died after the second TPE procedure.
We also analyzed the number of procedures by TPE indication. The 7 patients with primary graft dysfunction/AMR were all treated the same with an average of 5 TPE procedures (all but one patient who died after the 2nd procedure received 5 TPE). The 26 patients with a positive crossmatch received an average of 5 procedures (19 patients received 5 TPE, 6 patients received 6 TPE, and 1 patient received 4 TPE). The greatest variation in procedure number occurred in the 8 patients treated for positive PRA or HLA antibodies with an average of 3.5 TPE procedures (3 patients each received 2 and 5 TPE procedures, while 1 patient each received 3 and 4 TPE procedures). Finally, the 1 patient with suspected antibodies stopped TPE after only 2 procedures when antibody testing returned negative.
All patients received methylprednisolone and standard immunosuppression with 33 patients (79%) receiving mycophenolate + tacrolimus, 8 patients (19%) receiving mycophenolate alone, and 1 patient (2%) receiving tacrolimus alone. Thirty-eight patients (90%) received basiliximab and 1 patient (2%) received rituximab. Eleven patients (26%) were treated with an immune globulin, with 8 receiving IVIG and 3 receiving rATG.
Thirty-day post-transplant infection occurred in 17 patients (40%) as follows: ten patients had only one infection (8 with positive bacterial cultures, 1 with CMV viremia, and 1 with Clostridium difficile); 6 patients had 2 infections (3 with positive bacterial cultures and CMV viremia, 2 with positive bacterial cultures and sternal wound infection, and 1 with positive bacterial cultures and Clostridium difficile); 1 patient had three infections (positive bacterial cultures, sternal wound infection, and Clostridium difficile).
Delayed TPE Cohort
In all patients, TPE was primarily used to treat AMR. The median time from transplantation to first TPE episode was 245 days (range 2 – 1368 days).
The most common TPE schedule was daily in 20 patients (74%), followed by daily with one skipped/missed day in 3 patients (11%), and other (TPE every 1-3 days) in 4 patients (15%).
Most patients received 5 TPE (N = 20; 74%); 3 patients (11%) received 4 TPE; 2 patients (7%) received 3 TPE; and one patient (4%) received 7 TPE. One patient (4%) who was planned for daily TPE died after the first procedure.
All patients were treated with steroids with the most common agent being methylprednisolone in 25 patients (93%). Additionally, all patients received standard immunosuppressive therapy as follows: mycophenolate + tacrolimus in 16 patients (59%); tacrolimus alone in 7 patients (26%); and, in one patient each, azathioprine + mycophenolate + tacrolimus; azathioprine + tacrolimus; mycophenolate + cyclosporine; or mycophenolate alone. Sixteen patients (59%) were treated with an immune globulin with 10 patients receiving rATG alone (37%) and 3 patients each receiving rATG + IVIG (11%) or IVIG alone (11%). Four patients (15%), who were within 5 days of transplantation, received basiliximab and 2 patients (7%) received rituximab.
Post-transplant infection occurred in 9 patients (33%). Eight patients had only one infection (6 with a positive bacterial culture, 1 with CMV viremia, and 1 with Clostridium difficile) and 1 patient had 2 infections (positive bacterial culture and CMV viremia).
Overall and TPE Survival Outcomes
The median follow-up period was 49 months (4 years). Kaplan-Meier plots for each outcome are shown in Figures 2 and 3, respectively. Adjusted overall and TPE survival outcome model results are shown in Table III.
Figure 2.
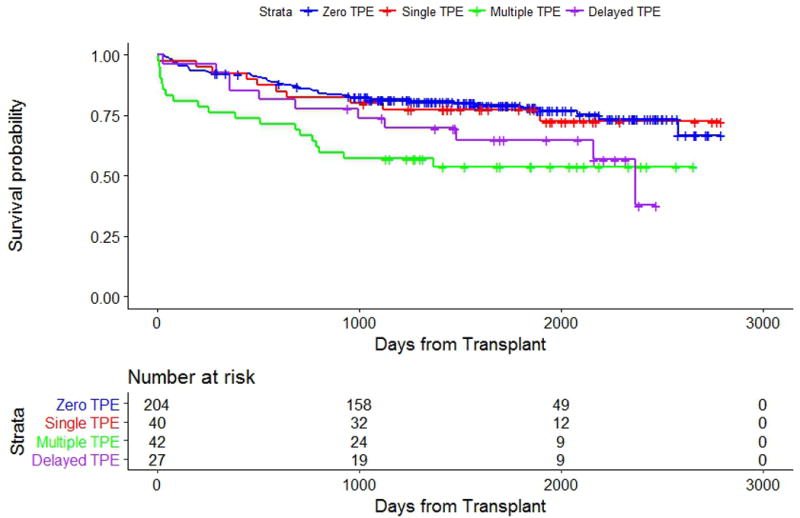
Kaplan Meier curves of overall survival of the Zero, Single, Multiple, and Delayed TPE cohorts. The number of events in the Zero, Single, Multiple, and Delayed TPE cohorts respectively was 45/204, 10/40, 19/42, and 11/27 (log-rank p-value = 0.003).
Figure 3.
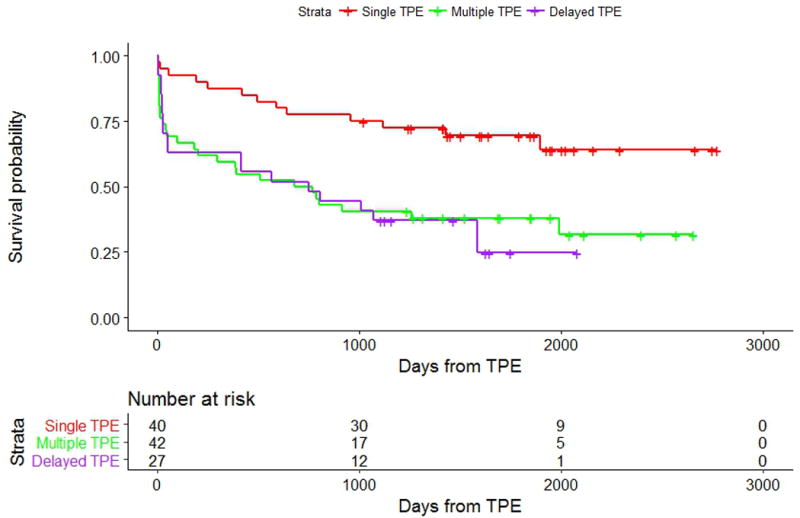
Kaplan Meier curves of TPE survival between the 3 TPE cohorts (Single, Multiple, and Delayed). The number of events in the Single, Multiple and Delayed TPE cohorts respectively was 13/40, 27/42, and 19/27 (log-rank p-value = 0.001).
Table III.
Overall & TPE Adjusted Hazard Ratios*
| Overall survival | TPE survival | |||||
|---|---|---|---|---|---|---|
| Variable Name | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p |
| Cohorts | 0.005 | 0.002 | ||||
| Zero TPE | 1 | |||||
| Single TPE | 1.08 | 0.54, 2.14 | 0.84 | 1 | ||
| Multiple TPE | 2.62 | 1.53, 4.49 | 0.0004 | 2.59 | 1.31, 5.14 | 0.006 |
| Delayed TPE | 1.98 | 1.02, 3.83 | 0.04 | 3.18 | 1.56, 6.50 | 0.002 |
| Age | 1.01 | 0.99, 1.02 | 0.23 | |||
| Race - White | 0.64 | 0.37, 1.11 | 0.11 | |||
| Infection Present - Yes | 2.31 | 1.50, 3.54 | 0.0001 | 1.64 | 0.97, 2.76 | 0.06 |
Cox regression models considered adjustment variables for inclusion using a forward selection technique with p-value for entry of 0.25 or less. Variables considered for inclusion included age, sex, race (white vs non-white), transplant indication (non-ischemic cardiomyopathy, ischemic cardiomyopathy, other), 30-day post-transplant infection status (yes/no), and documentation of AMR (yes/no; only considered for TPE survival model).
CI = confidence interval.
For the primary outcome, 0 patients underwent a re-transplant and 40 patients (37%) died during follow-up. There was a significant overall survival difference between all patient cohorts (Zero, Single, Multiple, and Delayed TPE; p-value = 0.005). When compared to the Zero TPE cohort (reference group), there was no significant survival difference for patients in the Single TPE cohort (HR 1.08 [CI, 0.54, 2.14], p = 0.84; see Table III). However, there were significant declines in overall survival for both the Multiple and Delayed TPE cohorts (HR 2.62 [CI, 1.53, 4.49], p = 0.0004 and HR 1.98 [CI, 1.02 to 3.83], p = 0.04, respectively).
For the secondary outcome, 34 patients (31%) had a repeat TPE episode, 0 patients underwent a re-transplant, and 25 patients (23%) died. There were significant differences between the three TPE groups (p = 0.002). When compared to the Single TPE cohort (reference group), the Multiple TPE cohort had worse TPE survival (HR 2.59 [CI, 1.31 to 5.14], p = 0.006). The Delayed TPE cohort also had worse TPE survival (HR 3.18 [CI, 1.56, 6.50], p = 0.002).
Impact of Post-Transplant Infection
Thirty-day post-transplant infection was independently associated with overall survival (HR 2.31 [CI, 1.50, 3.54], p = 0.0001) but did not achieve statistical significance for TPE survival (HR 1.64 [CI, 0.97, 2.76], p = 0.06). As shown in Table I, infection rates were not different between TPE-treated and non TPE-treated cohorts (39 vs 37%; p = 0.82). Further, as shown in Table II, infection rates among the TPE-treated cohorts were similar (40% vs 40% vs 33% in the Single, Multiple, and Delayed TPE cohorts, respectively).
DISCUSSION
In our study, we show that, at our institution, the top 3 indications for TPE in cardiac transplant patients are 1) elevated PRA or positive HLA antibodies, 2) post-transplant graft dysfunction and/or AMR, and 3) positive crossmatch. Patients who received TPE once perioperatively at transplant (Single TPE cohort) were not found to have different overall survival compared to patients who did not receive TPE (Zero TPE cohort), while patients who received postoperative TPE (Multiple and Delayed TPE cohorts) had worse overall survival compared to those who did not receive TPE (Figure 2). Additionally, when compared to the Single TPE cohort, patients in the Multiple and Delayed TPE cohorts had worse TPE survival (Figure 3). Finally, we show that thirty-day post-transplant infection rates did not differ between the groups but was independently associated with overall survival.
It is pertinent to note that the overall survival of the Zero TPE cohort is similar to that of the Single TPE cohort. Patients in the Single and, to some extent, Multiple TPE cohorts represent a group of patients for whom TPE is initiated pending final crossmatch results. This practice highlights our tendency within DUMC to utilize organs from donors to whom the recipient may have anti-HLA antibodies at a low concentration, thus allowing for a larger donor pool for pre-sensitized recipients. When these antibodies become dilute to a mean fluorescent intensity (MFI) of < 1000 at a 1:16 dilution, they are not considered unacceptable in the virtual crossmatch used for donor selection. However, given the possibility of a positive crossmatch, we utilize a single TPE procedure until final crossmatch results become available. With the high likelihood that the final crossmatch results could be negative, the Single TPE cohort potentially represents a group of patients for which TPE may not be required. Strategies aimed at expediting cytolytic crossmatch results may thus save an unnecessary TPE procedure. While it remains plausible that TPE in this cohort exerts a protective effect, as the sensitivity of HLA testing increases, the risk of the low concentration antibodies would need to be better defined. Ultimately, a prospective study randomizing patients to empiric TPE or no TPE would help clarify TPE’s role in this subpopulation.
Our observation of worse overall survival in the Multiple and Delayed TPE cohorts (Figure 2) is consistent with our expectations for the associated TPE treatment indications. Both diminished allograft and patient survival are associated with the presence of positive crossmatch, primary graft dysfunction, AMR, or elevated PRA/positive HLA antibodies.16,17 As shown in a retrospective study of 8,160 cardiac transplant patients, elevated PRA is a significant predictor of mortality.18 Compared to a PRA of 0%, PRA > 25% conferred worse overall survival at both 1 year (94 vs. 89%; p < 0.001) and 5 years (71 vs. 65%; p < 0.001). In addition to an elevated PRA at transplant, development of AMR post-transplant decreases survival. As shown in a retrospective study of 68 heart transplant patients, survival is worst when both late AMR and graft dysfunction are present. Compared to patients with early AMR, the development of late AMR with graft dysfunction confers worse post-AMR survival at 1 and 5 years (93 vs. 64% at 1 year; 73 vs. 36% at 5 years; p < 0.006).19 Further study is required to determine whether the overall survival of these subpopulations may be better improved by better monitoring tools for the presence or recurrence of AMR.
In spite of the decreased survival outcomes of the Multiple and Delayed TPE cohorts, we consider these survival rates to be acceptable when compared to the survival rates of decompensated heart failure without transplant.20,21 The ability to accept a high-risk transplant with TPE support, rather than the certainty of death, increases the likelihood that a patient who is highly sensitized will be able to undergo a heart transplant.22,23
In light of the above, it is most likely that our TPE-treated patients did worse, not because they received TPE but rather, as a result of the underlying indication for which they received TPE. The role of TPE as a tool to minimize survival differences between the cohorts should be examined in future studies. To better understand TPE’s impact, studies are needed to develop biomarkers of allograft improvement. These biomarkers will help tailor treatment to individual patients so that, rather than treating patients with a set number of TPE procedures, patients can be treated to maximum response.
In our study, we defined a new outcome of TPE survival. TPE survival may help us better assess the long-term success of TPE treatment because it allows us to capture a subpopulation of patients who may not have been optimally treated with the first TPE episode. In our study population, we observed that the Multiple and Delayed TPE cohorts had worse TPE survival (Figure 3). While worse TPE survival may imply suboptimal TPE treatment, it may also suggest treatment refractoriness. Treatment refractoriness could be explained by the presence of AMR, which predisposes patients to future episodes of AMR and, ultimately, cardiac allograft vasculopathy.24,25 Noting that AMR is notoriously difficult to diagnose,26 allograft vasculopathy may already be present in patients presenting with clinically apparent AMR and may contribute to lower treatment responsiveness. Further study may help clarify whether earlier TPE treatment of high risk patients may decrease future presentation with clinically-significant AMR.
The only variable we found to be independently associated with overall survival was 30-day post-transplant infection (Table III). Other studies have similarly shown that infection increases post-transplant morbidity and mortality.27,28 Infection may also increase predisposition towards AMR.29,30 Interestingly, infection rates were similar across groups, suggesting that the use of TPE did not confer additive infectious risk. Post-transplant infectious risk is likely mediated by cardiac transplantation surgery and its associated immunosuppressive drug regimen, need for mechanical circulatory support, presence of foreign bodies, prolonged ICU stay, and ventilator dependence.31–35 Although there is concern that post-transplant infectious risk may be mediated by hypogammaglobulinemia,36 our study suggests that TPE contributes minimal risk, if any, to the already high baseline infectious risk of the cardiac transplant patient.
Our study showed that in the TPE-treated cohort, both females and Blacks/African Americans were disproportionately increased. These findings are consistent with other studies that suggest that female sex is a risk factor for both AMR and decreased survival and that Black/African American race is associated with decreased survival.37–39 Although females and African Americans were overrepresented in the TPE cohort, in our statistical model, race and sex were not found to be associated with survival (Table III). This negative finding may be a function of our small numbers. However, our findings may suggest that TPE treatment of females and Blacks/African Americans at increased risk decreases transplant-associated mortality. To assess this possibility, prospective studies are needed.
Of the cardiac transplantation TPE indications in our study, the only one categorized in the ASFA guidelines is AMR.10 Across many studies of cardiac transplantation, AMR is a common use of TPE2; however, as we found in our study, TPE is also frequently used for treatment of elevated PRA antibodies or positive HLA antibodies and positive crossmatch.6,7,40 Given the high incidence of TPE use for these indications, TPE use for positive crossmatch status and positive HLA antibodies/PRA should be considered in the next iteration of the ASFA guidelines.
Although the two most common schedules in our cohort were once perioperatively and five daily procedures, our study showed some variation in TPE procedure number and treatment schedule. This variation may primarily be explained by TPE indication. However, it also highlights the difficulty in determining the optimal treatment schedule and number of TPE procedures. In the literature, with regards to AMR treatment, as few as 2 and as many as 19 TPE procedures have been used.7,41 Additional studies are needed to determine the optimal number of TPE procedures for each TPE cardiac transplant indication and to determine whether a more individualized approach to TPE is warranted.
The primary limitation of our study is that it is a retrospective analysis and cannot adequately account for all the factors that may have contributed to patient outcomes Additionally, parameters assessing TPE response, such as post-TPE HLA or PRA levels, left ventricular ejection fraction, and biopsy evaluation were not uniformly assessed across all patients, thereby limiting our ability to directly measure the impact of TPE. Finally, because TPE is used in the post-cardiac transplant setting for indications that are associated with increased morbidity and mortality, the impact that TPE has on these outcomes cannot be determined from this analysis. Prospective studies are needed to clarify the impact and benefit of TPE in this complex population.
CONCLUSIONS
In our retrospective study of cardiac transplant patients, the most common TPE indication was elevated PRA or positive HLA antibodies. Although post-transplant infection was independently associated with overall survival, TPE did not appear to contribute to infectious risk. Prospective studies to define the role of TPE in cardiac transplant subpopulations may save unnecessary TPE procedures and help develop tailored treatment approaches to improve survival.
Acknowledgments
This publication was made possible by support from funding from National Institutes of Health (NIH) K12HL087097 and Duke Office of Clinical Research (DOCR) funding from Grant Number UL1TR001117 from the National Center for Research Resources (NCRR), a component of NIH and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
AUTHOR DISCLOSURES
S.C.G. receives consulting fees from Gilead Sciences for serving on multiple Data and Safety Monitoring Boards. Although the relationship is not perceived to represent a conflict with the present work, it has been included in the spirit of full disclosure.
References
- 1.Chou HW, Chi NH, Lin MH, Chou NK, Tsao CI, Yu HY, Chen YS, Wang SS. Steroid pulse therapy combined with plasmapheresis for clinically compromised patients after heart transplantation. Transplantation proceedings. 2012;44(4):900–902. doi: 10.1016/j.transproceed.2012.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Colvin MM, Cook JL, Chang P, Francis G, Hsu DT, Kiernan MS, Kobashigawa JA, Lindenfeld J, Masri SC, Miller D, O’Connell J, Rodriguez ER, Rosengard B, Self S, White-Williams C, Zeevi A. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2015;131(18):1608–1639. doi: 10.1161/CIR.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 3.Kaczorowski DJ, Datta J, Kamoun M, Dries DL, Woo YJ. Profound hyperacute cardiac allograft rejection rescue with biventricular mechanical circulatory support and plasmapheresis, intravenous immunoglobulin, and rituximab therapy. Journal of cardiothoracic surgery. 2013;8:48. doi: 10.1186/1749-8090-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Issitt RW, Crook RM, Cross NT, Shaw M, Robertson A, Burch M, Hsia TY, Tsang VT. Incompatible ABO-plasma exchange and its impact on patient selection in paediatric cardiac transplantation. Perfusion. 2012;27(6):480–485. doi: 10.1177/0267659112453076. [DOI] [PubMed] [Google Scholar]
- 5.Ius F, Sommer W, Tudorache I, Kuhn C, Avsar M, Siemeni T, Salman J, Hallensleben M, Kieneke D, Greer M, Gottlieb J, Kielstein JT, Boethig D, Welte T, Haverich A, Warnecke G. Preemptive treatment with therapeutic plasma exchange and rituximab for early donor-specific antibodies after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(1):50–58. doi: 10.1016/j.healun.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Jackups R, Jr, Canter C, Sweet SC, Mohanakumar T, Morris GP. Measurement of donor-specific HLA antibodies following plasma exchange therapy predicts clinical outcome in pediatric heart and lung transplant recipients with antibody-mediated rejection. Journal of clinical apheresis. 2013;28(4):301–308. doi: 10.1002/jca.21270. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Vanlandingham S, Halverson C, Marques MB, Tallaj J, Kirklin J, Adamski J. Therapeutic plasma exchange rapidly improves cardiac allograft function in patients with presumed antibody-mediated rejection. Journal of clinical apheresis. 2014;29(6):316–321. doi: 10.1002/jca.21338. [DOI] [PubMed] [Google Scholar]
- 8.Scott V, Williams RJ, Levi DS. Outcomes of cardiac transplantation in highly sensitized pediatric patients. Pediatric cardiology. 2011;32(5):615–620. doi: 10.1007/s00246-011-9928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onwuemene OA, Heath DM, Hartman C, Wong ECC. Role of C1q complement fixing antibody assay in therapeutic plasma exchange management of pediatric cardiac antibody mediated rejection. Journal of clinical apheresis. 2017;32(4):279–281. doi: 10.1002/jca.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. Journal of clinical apheresis. 2016;31(3):149–162. doi: 10.1002/jca.21470. [DOI] [PubMed] [Google Scholar]
- 11.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query Tool: Providing Simplified Access to Clinical Data for Research and Quality Improvement. Journal of biomedical informatics. 2011;44(2):266–276. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almenar L, Cardo ML, Martinez-Dolz L, Garcia-Palomar C, Rueda J, Zorio E, Arnau MA, Osa A, Palencia M. Risk factors affecting survival in heart transplant patients. Transplantation proceedings. 2005;37(9):4011–4013. doi: 10.1016/j.transproceed.2005.09.160. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo MR, Naftel DC, Pritzker MR, Heilman JK, 3rd, Boehmer JP, Brozena SC, Dec GW, Ventura HO, Kirklin JK, Bourge RC, Miller LW. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1998;17(8):744–753. [PubMed] [Google Scholar]
- 16.Coutance G, Ouldamar S, Rouvier P, Saheb S, Suberbielle C, Brechot N, Hariri S, Lebreton G, Leprince P, Varnous S. Late antibody-mediated rejection after heart transplantation: Mortality, graft function, and fulminant cardiac allograft vasculopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(8):1050–1057. doi: 10.1016/j.healun.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Kfoury AG, Renlund DG, Snow GL, Stehlik J, Folsom JW, Fisher PW, Reid BB, Clayson SE, Gilbert EM, Everitt MD, Bader FM, Singhal AK, Hammond ME. A clinical correlation study of severity of antibody-mediated rejection and cardiovascular mortality in heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28(1):51–57. doi: 10.1016/j.healun.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84(5):1556–1562. doi: 10.1016/j.athoracsur.2007.05.095. discussion 1562-1553. [DOI] [PubMed] [Google Scholar]
- 19.Clerkin KJ. The Impact of Timing and Graft Dysfunction on Survival and Cardiac Allograft Vasculopathy in Antibody Mediated Rejection. 2016;35(9):1059–1066. doi: 10.1016/j.healun.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 21.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. Journal of the American College of Cardiology. 1992;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 22.Reinsmoen NL, Patel J, Mirocha J, Lai CH, Naim M, Ong G, Wang Q, Zhang X, Liou F, Yu Z, Kobashigawa J. Optimizing transplantation of sensitized heart candidates using 4 antibody detection assays to prioritize the assignment of unacceptable antigens. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35(2):165–172. doi: 10.1016/j.healun.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Asante-Korang A, Amankwah EK, Lopez-Cepero M, Ringewald J, Carapellucci J, Krasnopero D, Berg A, Quintessenza J, Jacobs JP. Outcomes in highly sensitized pediatric heart transplant patients using current management strategies. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(2):175–181. doi: 10.1016/j.healun.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Uretsky BF, Murali S, Reddy PS, Rabin B, Lee A, Griffith BP, Hardesty RL, Trento A, Bahnson HT. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- 25.Radovancevic B, Poindexter S, Birovljev S, Velebit V, McAllister HA, Duncan JM, Vega D, Lonquist J, Burnett CM, Frazier OH. Risk factors for development of accelerated coronary artery disease in cardiac transplant recipients. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1990;4(6):309–312. doi: 10.1016/1010-7940(90)90207-g. discussion 313. [DOI] [PubMed] [Google Scholar]
- 26.Kobashigawa J, Crespo-Leiro MG, Ensminger SM, Reichenspurner H, Angelini A, Berry G, Burke M, Czer L, Hiemann N, Kfoury AG, Mancini D, Mohacsi P, Patel J, Pereira N, Platt JL, Reed EF, Reinsmoen N, Rodriguez ER, Rose ML, Russell SD, Starling R, Suciu-Foca N, Tallaj J, Taylor DO, Van Bakel A, West L, Zeevi A, Zuckermann A. Report from a consensus conference on antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(3):252–269. doi: 10.1016/j.healun.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupeli E, Ulubay G, Akkurt ES, Oner Eyuboglu F, Sezgin A. Long-term pulmonary infections in heart transplant recipients. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2015;13(Suppl 1):356–360. doi: 10.6002/ect.mesot2014.p205. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez R, Vazquez-Guillamet MC, Suarez J, Mooney J, Montoya JG, Dhillon GS. Invasive mold infections in lung and heart-lung transplant recipients: Stanford University experience. Transplant infectious disease : an official journal of the Transplantation Society. 2015;17(2):259–266. doi: 10.1111/tid.12362. [DOI] [PubMed] [Google Scholar]
- 29.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. Jama. 1989;261(24):3561–3566. [PubMed] [Google Scholar]
- 30.Cainelli F, Vento S. Infections and solid organ transplant rejection: a cause-and-effect relationship? The Lancet Infectious diseases. 2002;2(9):539–549. doi: 10.1016/s1473-3099(02)00370-5. [DOI] [PubMed] [Google Scholar]
- 31.Carbone J. The Immunology of Posttransplant CMV Infection: Potential Effect of CMV Immunoglobulins on Distinct Components of the Immune Response to CMV. Transplantation. 2016;100(Suppl 3):S11–18. doi: 10.1097/TP.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sayed Ahmed MM, Almanfi A, Aftab M, Singh SK, Mallidi HR, Frazier OH. Aspergillus Mediastinitis after Orthotopic Heart Transplantation: A Case Report. Texas Heart Institute journal. 2015;42(5):468–470. doi: 10.14503/THIJ-14-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy E, Vanichanan J, Rajapreyar I, Gonzalez B, Nathan S, Gregoric I, Kar B, Loyalka P, Weeks P, Chavez V, Wanger A, Ostrosky Zeichner L. A pseudo-outbreak of disseminated cryptococcal disease after orthotopic heart transplantation. Mycoses. 2016;59(2):75–79. doi: 10.1111/myc.12433. [DOI] [PubMed] [Google Scholar]
- 34.Koerner MM, El-Banayosy A, Schulz U, Zeriouh M, Koerfer R, Tenderich G, Ghodsizad A. Nocardiosis in Heart Transplant Recipients. The heart surgery forum. 2015;18(6):E250–252. doi: 10.1532/hsf.1372. [DOI] [PubMed] [Google Scholar]
- 35.Veitch D, Abioye A, Morris-Jones S, McGregor A. Propionibacterium acnes as a cause of lung abscess in a cardiac transplant recipient. BMJ case reports. 2015;2015 doi: 10.1136/bcr-2015-212431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarmiento E, Diez P, Arraya M, Jaramillo M, Calahorra L, Fernandez-Yanez J, Palomo J, Sousa I, Hortal J, Barrio J, Alonso R, Munoz P, Navarro J, Vicario J, Fernandez-Cruz E, Carbone J. Early intravenous immunoglobulin replacement in hypogammaglobulinemic heart transplant recipients: results of a clinical trial. Transplant infectious disease : an official journal of the Transplantation Society. 2016;18(6):832–843. doi: 10.1111/tid.12610. [DOI] [PubMed] [Google Scholar]
- 37.Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, Reed EF, Fishbein MC. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2003;22(1):58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 38.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, Stehlik J. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report–2014; focus theme: retransplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33(10):996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Morris AA, Kransdorf EP, Coleman BL, Colvin M. Racial and ethnic disparities in outcomes after heart transplantation: A systematic review of contributing factors and future directions to close the outcomes gap. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35(8):953–961. doi: 10.1016/j.healun.2016.01.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daly KP, Chandler SF, Almond CS, Singh TP, Mah H, Milford E, Matte GS, Bastardi HJ, Mayer JE, Fynn-Thompson F, Blume ED. Antibody depletion for the treatment of crossmatch-positive pediatric heart transplant recipients. Pediatric transplantation. 2013;17(7):661–669. doi: 10.1111/petr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crespo-Leiro MG, Veiga-Barreiro A, Domenech N, Paniagua MJ, Pinon P, Gonzalez-Cuesta M, Vazquez-Martul E, Ramirez C, Cuenca JJ, Castro-Beiras A. Humoral heart rejection (severe allograft dysfunction with no signs of cellular rejection or ischemia): incidence, management, and the value of C4d for diagnosis. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(10):2560–2564. doi: 10.1111/j.1600-6143.2005.01039.x. [DOI] [PubMed] [Google Scholar]


