Abstract
Background:
Type 1 diabetes mellitus (T1DM) is associated with skeletal fragility. While previous meta- analyses have demonstrated an increased risk of fracture in individuals with T1DM, little is known about fracture risk in T1DM, in the absence of age- related confounders.
Aims:
To determine the risk of fracture in young and middle- aged adults with T1DM aged 18– 50 years old.
Design:
Systematic review and meta- analysis.
Data sources:
Ovid MEDLINE, PubMed, EMBASE, EBM reviews and relevant conference abstracts.
Study inclusion criteria:
Studies of adults aged between 18– 50 years with type 1 diabetes mellitus, with reported fracture outcomes.
Primary outcomes:
Incident or prevalent fracture.
Results:
Six studies were included in the meta- analysis. A total of 1724 fractures occurred in 35 925 patients with T1DM and 48 253 fractures occurred in 2 455 016 controls. RR for all fractures was 1.88 (95% CI 1.52– 2.32, P < .001). Fifty- six hip fractures occurred among 34 707 patients with T1DM and 594 hip fractures occurred in 2 295 177 controls. The RR of hip fractures was 4.40 (95% CI 2.58– 7.50, P < .001). Females and males with T1DM had a RR of 5.79 (95% CI 3.55– 9.44, P < .001) and 3.67 (95% CI 2.10– 6.41, P < .001), respectively.
Conclusions:
In the absence of age- related comorbidities, fracture risk remains significantly elevated in young and middle- aged adults with T1DM. Younger age does not mitigate against hip fracture risk in T1DM, and health professionals need to be aware of this risk. Further studies are needed to evaluate the mechanisms of fracture in T1DM.
Keywords: bone metabolism, fracture, hip fracture, type 1 diabetes mellitus, young adults
1 |. INTRODUCTION
Both diabetes mellitus and osteoporosis represent important public health concerns associated with rising mortality, morbidity and healthcare costs. Current projections estimate that by 2030, the number of individuals with diabetes will grow to 366 million worldwide. The burgeoning global prevalence of diabetes appears to occur in parallel with the increase in osteoporotic fractures, with a projected 2.6 million hip fractures occurring by 2025.1 Emerging evidence from large observational studies, particularly in the last decade, supports a direct detrimental impact of diabetes on skeletal health, particularly in type 1 diabetes mellitus (T1DM).
Type 1 diabetes mellitus is an autoimmune condition characterized by destruction of pancreatic islet cells, leading to absolute insulin deficiency. Several mechanisms have been identified to account for skeletal fragility in T1DM2 and deficits in bone mineral density (BMD),3,4 bone geometry,5 bone microarchitecture6 and biomechanical properties7,8 have been identified in humans and animal models of T1DM.9 Previous meta- analyses3,10–12 have demonstrated a four- to sevenfold increased risk of hip fracture in T1DM compared to controls; however, significant heterogeneity exists across study cohorts, with one meta- analysis including studies of children10 and others incorporating predominantly older study cohorts.3,12 Fractures in children are frequent, compared to young and middle- aged adults; this can largely be attributed to a combination of reduced bone mass and size, physical activity and trauma,13 rather than true skeletal fragility. Furthermore, peak bone mass is accrued in adolescence14 and completed by the third decade of life. Hence, fracture risk in children who have yet to achieve maximal growth and bone mass cannot be extrapolated to adults. Conversely, in studies of older adults, potential age- related confounders for bone fragility and falls, such as menopause and sarcopenia, can overestimate the true fracture risk in T1DM.
Type 1 diabetes mellitus disproportionately affects children and young adults, with the peak incidence spanning from birth to 14 years of age.15 Individuals with juvenile- onset T1DM are consequently exposed to disease for a longer duration throughout their lifetime and may be at risk of developing diabetes- related complications earlier in life. In the largest UK- based prospective cohort study of over 30 000 individuals with T1DM and over 300 000 non- diabetic controls, Weber at al14 demonstrated that individuals with T1DM had an increased risk of incident fracture across all ages, from birth to age 89 years, as well as a predilection for lower extremity fractures. Similarly, in a population- based study from Scotland, Hothersall et al16 reported substantially higher hip fracture risks in men and women with T1DM across the ages of 20– 49 years, compared to age- matched controls. Thus, the findings from large cohort studies support the notion that young to middle- aged adults with T1DM are equally vulnerable to fracture as their older, postmenopausal counterparts.
While T1DM is recognized as an established risk factor for osteoporosis and fracture, the recommendations for bone health assessment in this cohort is still unclear. Bone health screening programs are commonly targeted at older populations, given the low absolute numbers of osteoporotic fracture in young adults. In the light of mounting evidence for increased skeletal fragility in young adults with T1DM, we undertook a systematic review and meta- analysis dedicated to evaluating fracture risk in a younger adult cohort, with the aims of addressing the following questions:
What is the risk of overall fracture in young to middle-aged adults (aged 18–50 years) with T1DM, compared to controls?
What is the risk of hip fracture in young to middle-aged adults with T1DM?
Is fracture risk in T1DM different between sexes?
2 |. METHODS
This systematic review and meta- analysis was performed in accordance with the PRISMA statement.17 The protocol has been registered with PROSPERO (CRD42017077850) and is available at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=77850.
2.1 |. Search strategy
We conducted a systematic search in several databases including Ovid MEDLINE, PubMed, EMBASE, all EBM reviews from 1980 to present (28 November 2017).
Abstracts from annual scientific meetings of the American Society for Bone and Mineral Research, American Diabetes Association, European Association for the Study of Diabetes, European Calcified Tissue Society and World Congress of Osteoporosis (from 2005 onwards) were also screened.
All aforementioned databases were searched for keywords including:
“Type 1 diabet*” OR “Type I diabet*” OR “T1DM” OR “TIDM” OR “insulin dependent diabet*”
AND “fracture” OR “bone” OR “bone mineral*”
OR “osteoporo*” OR “osteopaeni*” OR “osteopeni*”
The literature search was limited to studies carried out in humans and published in English.
2.2 |. Study selection
Study selection was performed by two independent reviewers (EPT and MH). Only studies published in English were screened. Abstracts were assessed if the study fulfilled the inclusion criteria of: (i) cases as adults aged 18 years and above with established type 1 diabetes mellitus and controls as non- diabetic subjects; (ii) criteria for classifying individuals with T1DM was clearly defined; (iii) fracture rates were reported. Studies were excluded if cases also included individuals with type 2 diabetes mellitus, and outcomes were not differentiated by diabetes type, or if no control group was included. Studies comprising post- transplant individuals were also excluded. Fractures occurring at sites other than that considered to be typical of major osteoporotic fractures, such as those of the skull, facial, metacarpals, metatarsals, fingers or toes, were excluded. Full texts of all eligible studies were reviewed and consensus achieved between the two reviewers. Disagreements were resolved with face- to- face discussion or adjudication by the senior author.
2.3 |. Data extraction
Information from included studies comprised of: name of first author, publication year, country of origin, study design, study population and recruitment setting, number of participants (cases and controls, male-to-female distribution), mean ages and reported measures of fracture risk, and variables considered in the adjusted fracture risk. If the age range of participants fell outside the 18– 50 years age group, we contacted individual authors of eligible studies to obtain secondary data analyses for individuals with type 1 diabetes mellitus and age- matched controls for the prespecified age range.
2.4 |. Risk of bias assessment
Methodological quality, as determined by bias analysis, was assessed by two independent reviewers (EPT and MH) using criteria established a priori, outlined in the Monash Centre for Health Research and Implementation (MCHRI) Evidence Synthesis Program critical appraisal template.18
Studies were assessed on individual criteria relating to external validity (methodology, inclusion/exclusion criteria, and appropriateness of measured outcomes) and internal validity (selection, attrition, detection and reporting bias and confounders). Studies that fulfilled all, most or few criteria were deemed to have low, moderate and high levels of bias, respectively. Only studies of low- to- moderate bias were included.
2.5 |. Statistical methods
Data were analysed using RevMan version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Fracture outcomes were presented as relative risks (RR) with 95% confidence intervals. RRs were calculated from raw data obtained by authors for the specified age ranges, using Stata statistical software: Release 15 (College Station, TX, Statacorp LLC). Heterogeneity was assessed using the I2 test, where I2 values greater than 50% and 75% are indicative of moderate and high heterogeneity, respectively. A random- effects model was employed in the analysis if moderate or high heterogeneity between studies were observed. Funnel plots, Begg’s adjusted rank correlation test and Egger’s regression test of asymmetry were used to assess publication bias. All statistical tests were two- tailed, with a P- value of <.05 considered to be statistically significant. Subgroup analyses were performed by fracture type (hip) and sex.
3 |. RESULTS
3.1 |. Study selection
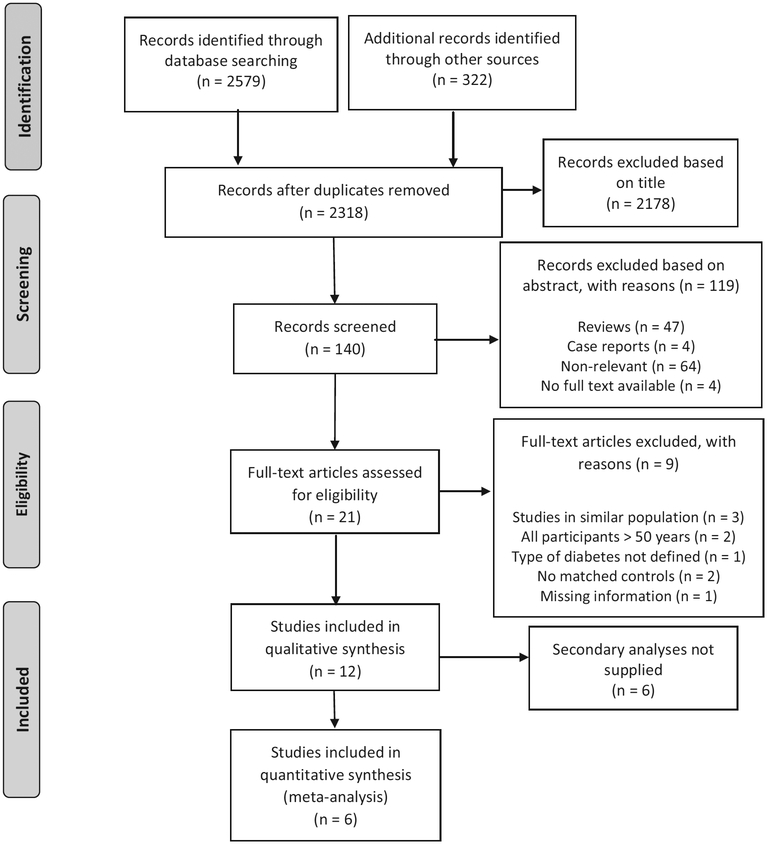
After excluding duplicate records, a total of 2901 publications and conference abstracts were identified in the screening search. A total of 2178 articles were excluded based on title, and a further 119 articles excluded based on abstracts. Twelve studies met eligibility criteria to be included in the qualitative analysis, after full- text review of 21 studies. Corresponding authors of individual studies were contacted by electronic- mail to obtain secondary data analyses of fracture outcomes for the prespecified age range. All authors were contacted a second time if a response had not been received after 4 weeks. Of the 12 studies that met criteria, 6 studies were included in the quantitative analysis (Figure 1).
FIGURE 1.
Flowchart of study selection. Diagram adapted from Moher et al. (2009)17
3.2 |. Study characteristics
Characteristics of the selected studies are described in Table 1. Of the six studies included, two were cohort studies,14,16 one was a case- control study19 and three were cross- sectional studies.20–22 The two largest studies were cohort studies by Weber et al14 and Hothersall et al16 both set in the United Kingdom. The remainder of the studies were conducted in America, Denmark and Belarus. Four studies had fracture events as the primary outcome. Two studies compared BMD as the primary aim, with fracture events being the secondary aim. One study reported solely on vertebral fractures, one reported hip fracture outcomes only, and the rest reported all types of fractures. Fracture ascertainment was determined by vertebral fracture assessment and spinal radiographs in one study,20 by International Statistical Classification of Diseases and Related Health Problems (ICD) codings or discharge codes in two cohort studies,14,19 and from hospital admission data in one study.16 Fractures were self- reported in the two small studies,21,22 with further confirmation of fracture in Danielson et al.21 Classification of individuals with T1DM was clearly defined in all studies. Two studies20,22 were conducted in women only and were included in the analysis despite having participants of a slightly higher age range of participants, up to 55 years, with the justification that only premenopausal or eugonadal women, with no other secondary cause of osteoporosis, were recruited. All studies were population or registry based with the exception of one study, which recruited from a hospital outpatient setting. Overall, the six included studies were heterogenous in terms of study population, classification of T1DM and methodology.
TABLE 1.
Characteristics of studies included for quantitative analysis
| First author and year | Type of study | Setting | Country | Study period | Number of participants | Definition of T1DM | Fracture ascertainment | Age (y) | Total fracture events (fractured/total) | Fracture type | Fracture events by gender in those aged between 18–50 years (fractured/total) | OR/RR/HR | Variables adjusted | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhukouskaya et al., 201320 | Cross-sectional | Hospital outpatient | Belarus | 2007–2011 | 82 T1DM (26M:56F); 82 controls (22M:60F) | American Diabetes Association criteria | Vertebral fracture assessment and spinal radiographs | 20–55 | 20/82 of T1DM vs 5/82 of controls | Vertebral | Males: 6/26 T1DM; 1/22 controls | Females: 14/56 T1DM; 4/60 controls | OR 4.20, 95% Cl 1.40–12.70, P < .01 | Age, sex, BMI, lumbar spine BMD |
| Weber et al., 201514 | Prospective cohort | Population | UK | 1994–2002 | Total 30 394 T1DM (17 074M:13 347F); 303 872 controls (170 421M:133 451F) | Medical diagnoses (read codes) specific to T1DM or diabetes and <35 years old | Diagnosis codes from outpatient electronic medical records consistent with incident fracture, classified by site; surgically-induced fractures or those associated with birth trauma or metastases were excluded | 0–89 | 2615/30 394 of T1DM vs 18 624/30 3872 of controls | Any fracture | Males: 779/10 874 T1DM; 5367/109 016 controlsa | Females: 431/8193 T1DM; 2440/82 156 controlsa | Males: HR 1.55, 95% Cl 1.44–1.67, P < .001a; Females: 1.76 95% Cl 1.58–1.96, P < .001a | Steroid use, prior fracture, CKD |
| Hip | Males: 21/10 983 T1DM; 75/109 927 controlsa | Females: 14/8321 T1DM; 28/82 390 controlsa | Males: HR 2.55, 95% Cl 1.52–4.26, P < .001a; Females: HR 3.36, 95% Cl 1.61–7.10, P = .001a | |||||||||||
| Hothersall et al., 201416 | Retrospective cohort study | Population/registry-based | Scotland | 2005–2007 | 21 033 T1DM/59 585 person-years; controls 3.66million/10 980 599 person-years | Clinical and prescription history (no evidence of lengthy diabetes duration prior to insulin prescription, no co-prescription of oral anti-diabetic drugs except for metformin) | Incident hip fracture admissions in 2005 to 2007 by linkage of diabetes register to national hospital admissions data (ICD codes) | 20–84 | 105/21 033 of T1DM vs 11 733/36 60000 of controls | Hip | Males: 14/8844 of T1DM; 331/1026 350 of controlsb | Females: 7/6649 of T1DM; 160/107 6510 of controlsb | Males: RR 5.4, 95% CI 3.5–8.3, P < .001b; Females: RR 7.9, 95% Cl 3.4–18.5, P < .001b | Age, sex and calendar year |
| Vestergaard et al., 200919 | Case-control | Population | Denmark | 2000 | 4369 T1DM, 484 615 controls | WHO standards | National Hospital Discharge Register & ICD codes | 43 ± 27 | 1703/4369 of T1DM vs 122 952/371 296 of controls | Any fracture | Males: 284/797 of T1DM; 26 201/105 458 of controlsc | Females: 139/344 of T1DM; 13 677/55 132 of controlsc | Males: OR 1.67, 95% Cl 1.45–1.94, P < .001c; Females: OR 2.06, 95% Cl 1.66–2.55, P < .001c | Macro- and micro-vascular complications |
| Danielson et al., 200921 | Matched, nested cross-sectional | Population | USA | 2005 | T1DM females 75; controls 75 | New cases of T1DM defined as diagnosis ≤age 30, clinical symptoms of hyperglycaemia and insulin-requiring | Self-reported fractures confirmed by physician or radiograph | 18–50 | Fracture: 28 T1DM, 18 controls | Any | N/A | Females: 28/75 of T1DM; 18/75 of controls | Females: OR 2.3, 95% Cl 1.0–5.2, P < .05 | Not reported |
| Strotmeyer et al., 200622 | Cross-sectional | Population (volunteer subgroup from prospective study) | USA | Un-known | T1DM females 67; controls 237 | Premenopausal women on diabetes registry diagnosed at age <17 years in hospital | Self-reported | 35–55 | Fracture: T1DM 33.3% vs controls 22.6%, age-adjusted OR 1.89 (95% Cl 1.02–3.49) | Any fracture after age 20 (site not specified) | N/A | Females: 22/67 of T1DM; 54/237 of controls | Females: OR 1.89, 95% Cl 1.02–3.49, P > .05 | Age |
BMD, bone mineral density; T1DM, type 1 diabetes mellitus.
Part of a secondary analysis of dataset initially assembled to describe fracture incidence from birth to 89 years, using data from The Health Improvement Network; data obtained from investigators through personal communication.
Part of a secondary analysis of original data set; data obtained from investigators through personal communication.
Part of a secondary analysis of original data set; data obtained from investigators through personal communication.
3.3 |. Risk of bias across studies
The studies by Vestergaard et al19, Hothersall et al16 and Weber et al14 involved a large population- or registry- based cohort with objective definitions for T1DM and fracture ascertainment and were therefore of higher quality. While Hothersall et al16 captured hip fractures solely from hospital admission records, the potential for under- reporting of fractures from this study was thought to be low, as almost all hip fractures are managed in hospital. The use of self- reported fracture without further validation in the study by Strotmeyer et al22 is subject to recall bias, although the majority of clinically important fractures are likely to be memorable. Unlike the other studies, which were population- based, Zhukouskaya et al20 recruited participants from hospital outpatient clinics, where the selection of patients with potentially more complex or poorly controlled diabetes was thought to account for the fourfold increased odds of vertebral fracture among young adults with T1DM, compared to controls from the community. In addition, the unique methodology of fracture ascertainment in this study, via active screening, led to the detection of asymptomatic vertebral fractures, which would otherwise have been missed clinically. This raises an important consideration of potential under- detection of asymptomatic fractures in the larger registry- linked studies, which relied on clinically detected fractures as outcomes. In the light of significant study heterogeneity, we performed sensitivity analyses, excluding outliers and including only higher quality studies (Table 2).
TABLE 2.
Subgroup and sensitivity analyses
| Variable | Studies included (references) | RR, 95% Cl, P-value | Heterogeneity (I2), P-value |
|---|---|---|---|
| Sex and fracture type | |||
| Men | |||
| All fractures | N = 4 (14,16,19,20) | RR 1.73, 95% Cl 1.50–2.30, P < .001* | 86%, P < .001 |
| Hip fracture | N = 2 (14,16) | RR 3.67, 95% Cl 2.10–6.41, P < .001* | 58%, P = .12 |
| Women | |||
| All fractures | N = 6 (all studies) | RR 1.85 95% Cl 1.37–2.20, P < .001* | 71%, P = .004 |
| Hip fracture | N = 2 (14,16) | RR 5.79, 95% Cl 3.55–9.44, P < .001* | 43%, P = .15 |
| Higher quality studies (all fractures) | N = 3 (14, 16, 19) | RR 2.25, 95% Cl 1.61–3.14, P < .001* | 94%, P < .001 |
| Excluding outliersa(all fractures) | N = 4 (14, 19, 21, 22) | RR 1.53, 95% Cl 1.47–1.61, P < .001* | 0%, P = .85 |
| Ratio of relative risks (RRR) between sexes, by fracture type | |||
| All fractures | N = 6 (all studies) | RRR 0.94, 95% Cl 0.68–1.29, Z = −0.41b | P = .68 |
| Hip fracture | N = 2 (14,16) | RRR 0.63, 95% Cl 0.30–1.33, Z = −1.20b | P = .23 |
Denotes significant P-value.
Fixed effects model used due to low heterogeneity.
Denotes Z-score (test of interaction).
3.4 |. Synthesis of results
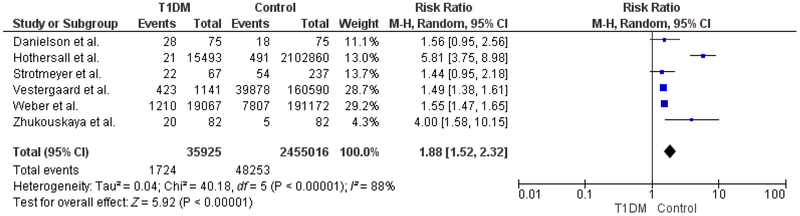
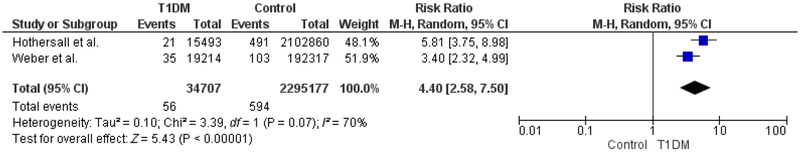
A total of 1724 fractures occurred among 35 925 patients with T1DM (4.8%) and 48 253 fractures occurred among 2 455 016 age- matched controls (2.0%). The pooled relative risk (RR) for all fractures in individuals with T1DM was 1.88 (95% CI 1.52– 2.32, P < .001), compared to controls (Figure 2). The degree of heterogeneity across studies was high (I2 = 88%, P < .001). Fracture outcomes were further stratified by type (hip fracture) and sex, in the pre-specified subgroup analyses. Fifty- six hip fractures occurred among 34 707 patients with T1DM (0.16%) and 594 hip fractures occurred among 2 295 177 controls (0.03%). The pooled RR for hip fracture in individuals with hip fracture was 4.40 (95% CI 2.58– 7.50, P < .001), compared to controls (Figure 3).
FIGURE 2.
All fractures
FIGURE 3.
Hip fractures
Females and males with T1DM had an increased risk of any fracture, compared to their nondiabetic counterparts, with a RR of 1.85 for women with T1DM (95% CI 1.50– 2.30, P < .001) and a RR of 1.73 for men with T1DM (95% CI 1.37– 2.20, P < .001). Women and men with T1DM had a near sixfold (RR 5.79, 95% CI 3.55– 9.44, P < .001) and fourfold (RR 3.67, 95% CI 2.10– 6.41, P < .001) increased risk of hip fracture, respectively, compared to controls. Statistical analyses were also performed to compare RRs for overall and hip fracture, respectively, between sexes, and no significant differences were found (Table 2).
Sensitivity analyses were performed as previously described and presented in Table 2. The RR for any fracture was 1.53 (95% CI 1.47– 1.61, P < .001, I2 = 0%) and 2.25 (95% CI 1.61– 3.14, P < .001, I2 = 94%), after the exclusion of outliers and lower quality studies, respectively. There was no funnel plot asymmetry for T1DM and fracture risk. P- values of .37 and .85 were obtained for Egger’s regression asymmetry test and Begg’s adjusted rank correlation test, respectively, indicating a low probability of publication bias.
4 |. DISCUSSION
The findings from this meta- analysis demonstrate that young and middle- aged adults are twice as likely to fracture, compared to nondiabetic controls. This is somewhat lower than the previously reported RR of 3.16 in the meta- analysis by Shah et al10 but can be reasonably expected in a younger cohort of adults. We report a fourfold increased hip fracture risk, which is slightly lower than the RRs of 5.76 and 6.3 reported in previous meta- analyses of predominantly older cohorts by Fan et al11 and Janghorbani et al12 respectively. Despite the deliberate exclusion of older and post-menopausal females in our analysis, hip fracture risk in younger adult females with T1DM was almost sixfold higher than controls of similar age. Similarly, younger men with T1DM exhibited a similarly elevated hip fracture risk, nearly four times that of controls. Overall, there was no difference observed in hip fracture risk between sexes.
Increased hip fracture risk in T1DM has been established in previous meta- analyses3,10,11 and cohort studies.23–25 However, the majority of such studies have comprised of adults older than 40 years, which could lead to overestimation of fracture risk attributable to T1DM due to confounders such as postmenopausal osteoporosis, increased frailty and susceptibility to falls. Nevertheless, a few cohort studies have demonstrated that hip fracture risk remains elevated in younger adults. A large Swedish prospective registry- based cohort study by Miao et al showed increased standard hospitalization ratios of 7.6 and 3.4 for hip fracture in women and men with T1DM under the age of 40, respectively.26 In a more recent cohort study, Weber et al14 reported an adjusted hazard ratio of 4.16 for hip fracture in women with T1DM aged 30– 39 years, which was comparably higher than that of their counterparts over the age of 60. While hip fractures are generally uncommon events in young adults, the fourfold risk of hip fracture reported in our meta- analysis raises important clinical concerns about fracture mechanisms in young people with T1DM. With only a modest reduction of 0.055 g/cm2 in femoral neck BMD in individuals with T1DM reported in a recent meta- analysis by Shah et al,4 low bone mass alone does not adequately explain the discrepant risk of hip fracture.
Bone geometry and bone microarchitecture are important determinants of bone strength and may provide further insights into the structural mechanisms of hip fracture. Failure to accrue peak bone mass as a result of childhood- onset T1DM may result in smaller and shorter bones in adulthood, which could portend less favourable bone geometry to resist fracture.27 Only a few studies have evaluated hip structure in adults with T1DM thus far. In a small cross- sectional study of middle- aged males, Miazgowski et al found no difference in hip strength indices between those with T1DM and controls, although there was a nonsignificant trend towards decreased cross- sectional area and moment of inertia in males with T1DM on hip structural analysis (HSA).28 However, in a more recent study utilizing quantitative computed tomography, Ishikawa et al were able to demonstrate significantly reduced cortical volumetric bone mineral density (vBMD) and a higher buckling ratio, a marker of cortical instability, in the intertrochanteric region of young to middle- aged males with T1DM compared to controls.29 Cortical bone is a key component of bone tissue in the skeleton, and perturbations of cortical bone integrity may predispose individuals to fracture at sites composed of cortical bone predominantly, such as the femoral neck. In a larger cross- sectional study of middle- aged adult patients with T1DM, Verroken et al found that those with T1DM exhibited cortical bone size deficits at the radial shaft on peripheral quantitative computed tomography (pQCT). These findings were more pronounced at the endosteal envelope, and in association with the finding of lower bone marrow density, are suggestive of a potential role of increased marrow adiposity in the pathogenesis of cortical bone deficits in T1DM.30
Microangiopathy may also serve as a mechanism by which T1DM exerts its effects on bone microstructure.31 Shanbhogue et al6 described reduced trabecular and cortical vBMD, in addition to thinning of the trabeculae and cortex on high- resolution peripheral quantitative computed tomography (HR- pQCT) in patients with T1DM and microvascular disease, while no differences were observed between individuals with T1DM without complications and nondiabetic controls. As bone is highly vascular, disruption of the microvascular circulation in bone may impair osteoblast function and reduce bone remodelling capacity, thereby leading to decreased bone quality. In addition, it is conceivable that the vascular supply to the femoral head may be compromised in diabetic microangiopathy, which could explain the predilection for hip fracture in T1DM.32 Vestergaard et al19 reported a twofold increased risk of fracture in those with T1DM and nephropathy, while Weber et al14 observed a positive association of retinopathy and neuropathy in individuals with T1DM who had sustained a lower extremity fracture. In light of these findings, it is possible that microangiopathy not only has direct effects on bone quality and may also indirectly potentiate the risk of falls and consequent fracture in susceptible individuals, via visual or physical impediments. Microvascular disease is often regarded as a surrogate of longstanding poor glycaemic control, and the impact of hyperglycaemia on bone health may therefore play an important role in fracture pathophysiology. Previous studies evaluating the effect of hyperglycaemia have yielded inconsistent results, owing to differing methods used in the assessment of glycaemic control. Neumann et al33 were able to demonstrate a positive association between poor long- term glycaemic control and prevalent fractures in adults with T1DM, independent of BMD. Chronic hyperglycaemia drives the nonenzymatic glycosylation of proteins, leading to the formation of advanced glycation end- products (AGEs), which can disrupt bone collagen matrix and weaken its biomechanical properties.2 The detrimental effects of AGEs on bone tissue in vivo have been demonstrated by the presence of increased pentosidine, a surrogate marker of AGEs, on bone histomorphometry in individuals with T1DM with prevalent fractures, compared to those without fracture.34
Last but not least, hypoglycaemia is a common adverse effect of insulin therapy, and its contribution to fracture risk should not be overlooked. The sympatho- adrenal response to hypoglycaemia is reduced in the face of recurrent hypoglycaemic episodes, and the threshold at which symptoms develop is subsequently lowered.35 Individuals with hypoglycaemic unawareness are prone to developing severe hypoglycaemia, which can culminate in an altered conscious state predisposing to falls and injury, particularly in the setting of a hypoglycaemic seizure. Several case reports36–38 have described the traumatic component of such seizures, leading to vertebral fractures. In individuals with underlying bone fragility, avoidance of hypoglycaemia is therefore an important component of fracture prevention.
Overall, there is clear evidence for increased skeletal fragility in T1DM, and our findings support a consistent, but unexplained risk of hip fracture in this younger cohort, designed to exclude age- related confounders. We acknowledge that there are several limitations to this meta- analysis. Firstly, only a small number of studies were included, as procurement of data for participants in our prespecified age range of 18– 50 years was limited to authors who had responded. The majority of studies that were excluded in the quantitative analysis were at least a decade old and had small numbers of individuals with T1DM, with the exception of Miao et al26 which included 25 000 individuals with T1DM. As reported previously, the standardized hospitalisation ratios for hip fracture in a subgroup of adults under the age of 40 were 3.4 and 7.6, for men and women, respectively, which are in keeping with our results. While the potential for reporting bias exists, the inclusion of larger and newer studies may be more representative of contemporary fracture risk in T1DM, in the light of current diabetes therapies. Although there were only two studies included for hip fracture, these were the two largest observational studies, with well- characterized cohorts with objective diabetes and fracture classification. We note that the subgroup analysis of hip fracture risk gave rise to point estimates of relative risks which were much higher than that of the pooled overall fracture risk and its confidence interval. A major limitation of this meta- analysis was the heterogeneity in fracture ascertainment and endpoints of the included studies. Virtually all hip fractures are clinical events, and the detection rate of such fractures is likely to be higher than other types of fracture, which may be clinically silent. It is notable that the pooled relative risk and confidence interval for hip fracture in our analysis is quite similar to the relative risk of 4.0 in the study by Zhukouskaya et al20 where vertebral fractures were actively screened for in asymptomatic individuals. The study populations included in these analyses originated from America or Europe with predominantly Caucasian participants, thus the findings from this study may not be generalizable to other ethnic groups. The methodologies of the included studies did not allow for the classification of fracture aetiology, and confounders such as trauma-related or sporting injuries in younger adults may over- estimate the risk of fragility fractures in this cohort. Data on body mass index, glycaemic control, duration of diabetes, presence of microvascular complications and concomitant autoimmune disease were not available for all included studies.
To our knowledge, this is the first meta- analysis of fracture risk specifically targeted at young to middle- aged adults with T1DM, with application of strict selection criteria to reduce heterogeneity and confounding due to age- related factors. We included the two largest cohort studies to date,14,16 comprising over 34 000 individuals with T1DM, and our findings for hip fracture risk are consistent with previous meta- analyses and cohort studies.
5 |. CONCLUSIONS
In a young to middle- aged cohort of type 1 diabetes mellitus (T1DM), we show that hip fracture risk is comparable to previously reported relative risks (RRs) of between 4 and 6, albeit in predominantly older adult study populations.3,11,12 Young adults with T1DM may be at risk of fracture at a younger age compared to their nondiabetic counterparts. The impact of a hip fracture in a young adult is potentially devastating, with ramifications of physical and psychological morbidity, in addition to increased mortality. Although T1DM is widely acknowledged as a risk factor for secondary osteoporosis and fracture, there is no consensus on the timing and modality of bone health assessment in this cohort. However, we show that young adults with T1DM have an increased risk of fracture, and awareness is needed early in diagnosis. While the absolute numbers for hip fracture are low in this younger cohort, we urge clinicians to be cognizant of the diverse risk factors for skeletal fragility, and treat reversible causes where possible. Individuals with poorly controlled T1DM and micro-vascular complications appear to be most at risk of fracture, based on observational studies. Concomitant comorbidities that may contribute to fracture risk, such as hypogonadism, autoimmune conditions and hypoglycaemia, should be identified and managed. Imaging modalities that allow indirect assessment of bone microarchitecture and structural properties, such as trabecular bone score, HR- pQCT and hip structural analysis, may be a useful adjunct to DXA in this cohort, where bone mineral density (BMD) is but one of many contributors to fracture risk. Further research evaluating hip fracture mechanisms in T1DM, screening and assessment, is needed to guide practice.
ACKNOWLEDGEMENTS
The authors acknowledge and thank the following individuals for their help with provision of data analyses: Shona Livingston, Helen Colhoun and the Scottish Care Information- Diabetes Collaboration database; David R. Weber, Michelle Denburg and The Health Improvement Network database, UK; Peter Vestergaard, the Danish Hospital Discharge Register and Psychiatric Central Register.
Funding information
This study was not funded. EPT is a recipient of the Research Training Program Postgraduate Scholarship and FM is supported by an Endocrine Society of Australia Ken Wynne Post- doctoral Research Award. HT is a National Health and Medical Research Council (NHMRC) Fellow.
Footnotes
CONFLICT OF INTEREST
Nothing to declare.
REFERENCES
- 1.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2016;38:S4–S9. [DOI] [PubMed] [Google Scholar]
- 2.Hough FS, Pierroz DD, Cooper C, Ferarri SL. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174:R127–R138. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes - A meta- analysis. Osteoporos Int. 2017;18:427–444. [DOI] [PubMed] [Google Scholar]
- 4.Shah VN, Harrall KK, Shah CS, et al. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta- analysis and review of the literature. Osteoporos Int. 2017;28:2601–2610. [DOI] [PubMed] [Google Scholar]
- 5.Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Pediat. 2013;79:68–74. [DOI] [PubMed] [Google Scholar]
- 6.Shanbhogue VV, Hansen S, Frost M, et al. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR- pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res. 2015;30:2188–2199. [DOI] [PubMed] [Google Scholar]
- 7.Khan TS, Fraser LA. Type 1 diabetes and osteoporosis: from molecular pathways to bone phenotype. J Osteoporos. 2015;2015:174186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhukouskaya VV, Eller-Vanicher C, Shepelkevich AP, Dydyshko Y, Cairoli E, Chiodini I. Bone health in type 1 diabetes: focus on evaluation and treatment in clinical practice. J Endocrinol Invest. 2015;38:941–950. [DOI] [PubMed] [Google Scholar]
- 9.Thrailkill KM, Liu L, Wahl EC, et al. Bone formation is impaired in a model of type 1 diabetes. Diabetes. 2005;54:2875–2881. [DOI] [PubMed] [Google Scholar]
- 10.Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta- analysis and review of the literature. Diabet Med. 2015;32:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y, Wei F, Lang Y, Liu Y. Diabetes mellitus and risk of hip fractures: a meta- analysis. Osteoporos Int. 2016;27:219–228. [DOI] [PubMed] [Google Scholar]
- 12.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. [DOI] [PubMed] [Google Scholar]
- 13.Bishop N, Arundel P, Clark E, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom. 2014;17:275–280. [DOI] [PubMed] [Google Scholar]
- 14.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population- based cohort study using The Health Improvement Network (THIN). Diabetes Care. 2015;38:1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Valencia PA, Bougnères P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health. 2015;15:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta- analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monash Centre for Health Research and Implementation (MCHRI). Evidence Synthesis Program template for critical appraisal of a cohort study 2014. MCHRI – Monash University and Monash Health, Melbourne, Australia (adapted from Critical Appraisal Templates (2010) Centre for Clinical Effectiveness, Southern Health, Melbourne, Australia AND Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. In: Proceedings of the 3rd Symposium on Systematic Reviews Beyond the Basics: Improving Quality and Impact. Oxford: 2000:3–5. [Google Scholar]
- 19.Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84:45–55. [DOI] [PubMed] [Google Scholar]
- 20.Zhukouskaya VV, Eller-Vainicher C, Vadzianava VV, et al. Prevalence of morphometric vertebral fractures in patients with type 1 diabetes. Diabetes Care. 2013;36:1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danielson KK, Elliott ME, LeCaire T, Binkley N, Palta M. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos Int. 2009;20:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strotmeyer ES, Cauley JA, Orchard TJ, Steenkiste AR, Dorman JS. Middle- aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29:306–311. [DOI] [PubMed] [Google Scholar]
- 23.Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes Care. 2006;29:1573–1578. [DOI] [PubMed] [Google Scholar]
- 24.Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord- Trondelag Health Survey. Diabetologia. 1999;42:920–925. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed LA, Joakimsen RM, Berntsen GK, et al. Diabetes mellitus and the risk of non- vertebral fractures: the Tromso study. Osteoporos Int. 2006;17:495–500. [DOI] [PubMed] [Google Scholar]
- 26.Miao J, Brismar K, Nyrén O, Ugarph-Morawski A, Ye W. Elevated hip fracture risk in type 1 diabetic patients: a population- based cohort study in Sweden. Diabetes Care. 2005;28:2850–2855. [DOI] [PubMed] [Google Scholar]
- 27.Sellmeyer DE, Civitelli R, Hofbauer LC, Khosla S, Lecka-Czernik B, Schwartz AV. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes. 2016;65:1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miazgowski T, Pynka S, Noworyta-Zietara M, Krzyzanowska-Swiniarska B, Pikul R. Bone mineral density and hip structural analysis in type 1 diabetic men. Eur J Endocrinol. 2007;156:123–127. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa K, Fukui T, Nagai T, et al. Type 1 diabetes patients have lower strength in femoral bone determined by quantitative computed tomography: a cross- sectional study. J Diabetes Investig. 2015;6:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verroken C, Pieters W, Beddeleem L, et al. Cortical bone size deficit in adult patients with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2017;102:2887–2895. [DOI] [PubMed] [Google Scholar]
- 31.Shanbhogue VV, Hansen S, Frost M, Brixen K, Hermann AP. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 2017;5:827–838. [DOI] [PubMed] [Google Scholar]
- 32.Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone–osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297–305. [DOI] [PubMed] [Google Scholar]
- 33.Neumann T, Sämann A, Lodes S, et al. Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with Type 1 diabetes. Diabet Med. 2011;28:872–875. [DOI] [PubMed] [Google Scholar]
- 34.Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G. Nonenzymatic glycation and degree of mineralization are higher in bone from fractured patients with type 1 diabetes mellitus. J Bone Miner Res. 2016;31:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi T, Tominanga T, Shamoto H, Shimizu H, Yoshimoto T. Seizure- induced thoracic spine compression fracture: case report. Surg Neurol. 2002;58:214–216. [DOI] [PubMed] [Google Scholar]
- 37.Majkowska L, Bohatryrewicz A. Thoracic spine fracture in the course of severe nocturnal hypoglycaemia in young patients with type 1 diabetes mellitus – the role of low bone mineral density. Am J Emerg Med. 2014;32:816 e5–816.e7. [DOI] [PubMed] [Google Scholar]
- 38.Thong EP, Catford SR, Fletcher J, et al. Recurrent vertebral fractures in a young adult: a closer look at bone health in type 1 diabetes mellitus. Endocrinol Diabetes Metab Case Rep. 2018;2018:180010. [DOI] [PMC free article] [PubMed] [Google Scholar]