Abstract
West Nile Virus (WNV) can be a neuroinvasive pathogen that may produce persistent mild-to-moderate neurocognitive impairments in some infected persons. Intra-individual variability (IIV) is an index of a person’s performance across a neuropsychological test or battery, which is an indicator of neurocognitive control and integrity of prefrontal systems. The present study examined possible associations of IIV to neurological health and well-being in WNV infection. Participants included 84 adults with a range of clinical WNV disease (31 West Nile Encephalitis, 16 West Nile Meningitis, 37 West Nile Fever) who completed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). IIV was operationalized as covariance of variation (CoV), or the intra-individual standard deviation across 5 age-adjusted RBANS standard scores divided by the mean of standard scores. Participants were assessed for health-related quality of life (QoL) using the RAND 36-item short form health survey (SF-36). Analyses revealed that the West Nile Encephalitis group had higher neurocognitive CoV compared to the West Nile Fever group, and this difference was associated with a medium effect size (Cohen’s d = 0.52). Mixed linear models controlling for estimated IQ, activities of daily living, depression, neuroinvasive disease groups, and fatigue showed that higher RBANS CoV was associated with lower physical, but not mental health QoL. In persons with WNV infection, there is a modest association between elevations in IIV and encephalitis, and even subtle disruptions in neuropsychological functioning show relationships with important self-reported functioning as measured by physical health quality of life. Future studies should examine whether IIV predicts long-term health outcomes (e.g., mortality) in individuals infected with WNV.
Keywords: West Nile Virus, neuropsychology, intra-individual variability, neuroinvasive disease, encephalitis, quality of life
Since the initial presentation of West Nile Virus (WNV) in the United States in 1999, there have been over 46,000 cases reported (Centers for Disease Control and Prevention 2016) and an estimated 3+ million infections nationally (Petersen et al. 2013a). WNV incidence has varied annually with rates between 0.15 to 1.0 cases per 100,000 (Centers for Disease Control and Prevention 2016). Reported symptoms and clinical outcomes of WNV can be variable, but most commonly include muscle weakness and fatigue (Patel et al. 2015). Severe neuroinvasive disease (i.e., meningitis, encephalitis, acute flaccid paralysis) affects less than 1% of individuals infected with WNV (Carson et al. 2012).
While prevalence estimates of neuroinvasive disease in WNV are quite low, self-reported neurocognitive problems among individuals with severe neuroinvasive disease in WNV infection have been observed in approximately half to three-quarters of persons with recent infection (Brilla et al. 2004; Jeha et al. 2003; Pepperell et al. 2003). The first study to objectively examine the neurocognitive effects of WNV used the Telephone Interview for Cognitive Status (TICS; Brandt et al. 1988) and found that WNV infected individuals with a neuroinvasive diagnosis had poorer global scores than individuals with WNV fever (Haaland et al. 2006). Subsequent investigations using performance-based neurocognitive measures in WNV generally show mild-to-moderate impairments in verbal learning and memory, executive functions, and psychomotor speed (Sadek et al. 2010; Carson et al. 2006; Samaan et al. 2016), with the most marked impairments found within the psychomotor domain. Notably, the limited number of studies comparing neurocognitive outcomes between WNV-infected individuals with and without neuroinvasivie disease (k = 3), show reliably observed null effects (Carson et al. 2006; Sejvar et al. 2008; Samaan et al. 2016). However, these studies all included small sample sizes (neuroinvasive groups range: n=5 to 19), and in one case (Carson et al. 2006) used a “hospitalized” group of which only a proportion (75%) of cases had neuroinvasive disease. Taken together, these studies are limited in generalizability and power to detect an effect of neuroinvasive disease on neurocognitive outcomes due to study limitations of sample attrition and lacking non-clinical comparison samples. Nevertheless, WNV infection, irrespective of neuroinvasive disease, appears to confer a risk of neurocognitive impairments beyond one year after symptom onset (average length 13 months; Sadek et al. 2010). Furthermore, WNV morbidity has been noted up to 8 years post-infection (Murray et al., 2014; Nolan et al. 2012) with evidence of sustained and even progressive neurological impairment (Hasbun et al. 2016; Weatherhead et al. 2015). Thus, examining the extent to which WNV-associated neurocognitive deficits impact health outcomes and well-being is potentially important.
Health-related quality of life (HRQoL) is defined as an individual’s overall rating of their health and well-being as determined by the extent to which a disease or its treatment impacts everyday functioning, physical or affective symptoms, and interpersonal relationships (Cooley, 1998). The only 3 studies to examine HRQoL and neurocognitive impairment in WNV suggest that the two constructs are at least moderately negatively associated (Carson et al. 2006; Sadek et al. 2010; Sejvar et al. 2008). In 41 individuals with WNV, Sadek et al. (2010) found that processing speed and spatial abilities correlated with physical QoL as measured by the 36-item short form health survey (SF-36), while mental QoL was related to attention, memory, and word reading. Carson et al. (2006) reported that slower fine motor speed was associated with lower physical QoL as measured by the SF-12 (version 2) in 49 individuals with WNV. Finally, Sejvar et al. (2008) found that among 54 individuals recovering from WNV syndromes (fever, meningitis, and encephalitis) physical, but not mental, health scores from the SF-36 were related to neurocognitive measures of processing speed, executive functions, spatial abilities, and memory. While neurocognition appears to be associated with lower physical QoL at the univariate level, important questions remain regarding the extent to which this relationship is independent of critical co-factors, such as depression, neuroinvasive disease, or premorbid IQ in WNV (Trépanier et al. 2005). It is important to control for these co-factors since they may partially or fully explain previously observed associations between cognition and HRQoL in WNV, and this is particulary important considering that reporting of HRQoL can be disproportionately impacted by mood and insight (e.g., Morgan et al. 2012a). As such, the variability in domain-level findings within WNV suggests the need for the examination of intra-individual differences in neurocognition in these samples.
Intra-individual variability (IIV) may be particularly relevant to WNV, especially given its associated non-specific cognitive profile and its possible effects on QoL. IIV is an index of neuropsychological performance that considers the spread or deviation within an individual across tasks or trials, rather than a measure of central tendency (e.g., average performance; Hultsch et al. 2002; Hillborn et al. 2009). IIV can be measured using several methodologies, including the inconsistency of an individual’s cognitive performances across time, deviation of individual test scores within a domain of interest at a given time point, or dispersion of cognitive performances across cognitive domains. IIV is potentially important since it is a unique index of mild neurocognitive changes that may act as an early indicator of frank declines in cognition (Brewster et al. 2012; Holtzer et al. 2008). Processes through which increased IIV may occur in certain disease populations are 1) diminished cognitive (i.e., executive) control, wherein failure is top-down, 2) sustained regulation and 3) attention, which can lead to variable performances across trials or tests. While there are higher rates of neuroinvasive disease in WNV compared to other neuroviruelent diseases (e.g., HIV, Hepatitis C virus; Carson et al., 2012; Hogan & Wilkins, 2011), there is evidence in other infectious disease populations with neurocognitive involvement (e.g., HIV disease) that IIV predicts disease and everyday functioning outcomes, even among individuals defined as “normal” by traditional clinical criteria (Morgan et al. 2012b). IIV has been found to be elevated in individuals infected with Hepatitis C virus (HCV), and in this infectious disease population, IIV is associated with unemployment (Morgan et al. 2012c). IIV also has been studied extensively in the cognitive aging literature and has been shown to increase with age (Burton et al. 2006) and is hypothesized to be a harbinger for more frank longitudinal cognitive declines (Hilborn et al. 2009; Vaughan et al. 2013).
The present study sought to determine the extent to which IIV, a potentially useful index for the relatively non-specific cognitive profile of WNV, is related to important health and well-being outcomes. We hypothesized that IIV will be elevated in individuals with neuroinvasive WNV in samples matched on relevant co-factors (e.g., age, depression, pre-morbid IQ), and that elevated IIV would associated with poorer QoL.
Methods
Participants
A cohort of WNV-positive individuals was established following the introduction of WNV in 2002 in Houston, Texas. This cohort was followed prospectively from the time of acute infection to time of evaluation with a median of 6.8 years (0.1–11 years) post-infection. Participants were originally identified through local health department surveillance or routine screening of the blood supply at local blood donation centers during 2002–2012. All participants were tested during the years 2012–2013. Detailed procedures for diagnosis and confirmation of WNV-positive status were previously described (Hasbun et al. 2016), and inclusion criteria included WNV-positive participants with encephalitis (n = 31), meningitis (n = 16), or fever (n = 37). A total of 84 participants were eligible for the current analyses. The University of Texas Health Science Center and Baylor College of Medicine institutional review boards approved our study protocol and procedures.
Procedure
After providing written informed consent, all participants completed physician-guided comprehensive neuropsychological, psychiatric, and medical research evaluations.
Neuropsychological Examination
The Repeatable Battery for Assessment of Neuropsychological Status (RBANS) test was administered to provide a comprehensive screening of relevant neurocognitive functions (Randolph et al. 1998), including attention, language, visuospatial construction abilities, and immediate and delayed memory. Raw scores were converted to standard scores (M=100, SD=15) for each individual domain (5 domains total) using age-adjusted normative standards from the published manual. Thus, the individual measures that were used to calculate the intra-individual variability index were derived from demographically-adjusted normative standards in the RBANS manual.
Intra-Individual Covariance
The primary predictor for the current study was an index of dispersion, or IIV across neurocognitive domains in a single testing session of the RBANS. Calculation of the dispersion variable in the present study was undertaken using a procedure similar to that which has been employed in previous studies (e.g., Christensen et al. 1999; Hilborn et al. 2009; Morgan et al. 2012b). The 5 RBANS domain scores were used to generate an intra-individual standard deviation (ISD). This ISD was subsequently divided by the mean of all 5 domain standard scores for each participant, which is defined as an individual’s coefficient of variation (CoV). Each individual’s CoV, therefore, indicated the degree of variability in performance across the 5 domains, adjusted for mean performance, such that higher dispersion scores signify greater variability across measures in the battery.
Quality of Life Assessment
All participants were administered the RAND 36-item short form health survey (SF-36) to assess QoL (Ware and Sherbourne 1996). This measure yields eight subscales of HRQoL, including physical functioning, social functioning, role limitations due to physical health, bodily pain, mental health, role limitations due to emotional problems, vitality, and general health perceptions (Ware and Sherbourne 1996). For the purposes of this study, the eight dimensions were summarized into two subscales of HRQoL: physical health and mental health (Ware and Sherbourne 1996). These two component scores have been well validated in HRQoL literature (McHorney et al. 1993). Each subscale was given a score ranging from 0 to 100, with higher scores indicating better HRQoL.
Other Clinical Characteristics
The Barthel Index assessed the ability to perform activities of daily living (ADLs; Huybrechts & Caro, 2006), the Beck Depression Inventory was used to assess recent mood (Shafer et al. 2006), and the Modified Fatigue Impact Scale (MFIS) measured the impact of fatigue on physical, cognitive and psychosocial functioning (Garcia et al. 2014). The Matrix Reasoning (MR) subtest from the Wechsler Abbreviated Scale of Intelligence-II (WASI-II) was administered as an estimate of IQ using age-adjusted scores (Wechsler 2011). All participants underwent a structured neurological examination from which determinations of neuroinvasive disease were derived (Hasbun et al. 2016).
Data Analysis
All analyses were performed using a critical α = .05. CoV scores in the sample were non-normally distributed (Shapiro Wilk W = 0.92, p < .001); therefore, non-parametric analyses were used for primary CoV analyses. Possible covariates for our primary analyses of interest were selected from the demographic and clinical characteristics of the WNV sample displayed in Table 1. Examination of variables that were associated with both the independent variable/predictor and the dependent variable/criterion revealed that no variables listed in Table 1 were significantly related to CoV and neuroinvasive disease. Therefore, a Kruskal-Wallis test was used to examine the three-level (West Nile Encephalitis, West Nile Meningitis, West Nile Fever) clinical WNV variable as the independent variable predicting CoV as the dependent variable. Given the small samples associated with neuroinvasive disease, we elected to utilize Wilcoxon post hoc tests to examine pair-wise comparisons between the three groups and strongly considered sample sizes for these comparisons. For analyses for the SF-36 physical and mental health outcomes, selected variables from Table 1 were included as a priori derived covariates for examining possible associations with CoV, including depression, fatigue, WASI estimated IQ, neuroinvasive diagnosis, and ADLs.
Table 1.
Mean (standard deviation) demographic, comorbidity/medical, and neurocognitive/functional characteristics of patients with West Nile virus infection.
| Variable | WNE (N = 31) | WNM (n = 16) | WNF (n = 37) |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 60.7 (18.8) | 54.7 (11.3) | 52.1 (14.5) |
| Education (years) | 13.5 (2.8) | 14.3 (2.0) | 14.8 (2.4) |
| WASI Estimated IQ1 | 102.5 (17.5) | 101.6 (17.0) | 102.9 (16.2) |
| Gender (% men) | 58.1 | 50.0 | 48.7 |
| Ethnicity (% Caucasian) | 80.7 | 68.8 | 91.9 |
| Comorbidity/Medical Characteristics | |||
| Beck Depression Inventory (of 63) | 9.7 (8.5) | 12.4 (13.2) | 9.8 (10.0) |
| Depression elevated2 (%) | 22.6 | 37.5 | 24.3 |
| Neurocognitive/Functional Characteristics | |||
| RBANS3 Total Standard Score | 90.7 (16.1) | 96.9 (24.7) | 99.1 (15.7) |
| Intra-individual Covariance4 | 0.15 (.07) | 0.13 (.06) | .12 (.05) |
| Immediate Memory5 | 85 (78,100) | 87.5 (70, 120.2) | 97 (85, 103) |
| Visuospatial/Constructional5 | 100 (84,109) | 110.5 (97, 126) | 112 (94, 118.5) |
| Language5 | 95 (88, 101) | 90.5 (89.3, 109.5) | 97 (90.5, 104.5) |
| Attention5 | 100 (88, 118) | 97 (77.5, 115) | 103 (95.5, 118) |
| Delayed Memory5 | 95 (81, 101) | 97 (77.3, 108.8) | 100 (90, 107) |
| Barthel Index6 (of 100) | 95.8 (9.6) | 100.0 (0.0) | 99.9 (0.8) |
| Modified fatigue impact scale (of 84) | 29.6 (22.4) | 24.2 (21.5) | 28.4 (24.2) |
| SF-367 (of 100) | 59.9 (22.7) | 62.7 (30.6) | 65.1 (23.9) |
| Physical Health Summary Score (of 100) | 56.5 (25.7) | 62.3 (34.3) | 65.4 (23.6) |
| Mental Health Summary Score (of 100) | 63.4 (23.6) | 63.1 (29.5) | 64.7 (26.1) |
Note. Data represent means (standard deviation) unless otherwise noted. WNE = West Nile Encephalitis; WNM = West Nile Meningitis; WNF = West Nile Fever;
Wechsler Abbreviated Scale of Intelligence estimated IQ through Matrix Reasoning Subtest;
Beck Depression Inventory Score ≥ 17.
Repeatable Battery for the Assessment of Neuropsychological Status (standard scores);
Standard deviation of all 5 RBANS domain standard scores divided by the mean of all RBANS standard scores;
Median (25%ile, 75%ile) standard scores;
Index of activities of daily living;
Medical Outcomes Study 36-Item Short Form Survey.
Results
Neuroinvasive Diagnosis and IIV
A Kruskal-Wallis test with the three-group clinical WNV variable (West Nile Encephalitis, West Nile Meningitis, West Nile Fever) predicting RBANS CoV fell at a trend level, χ2(2) = 4.82, p = .090. Post hoc pair-wise Wilcoxon tests revealed a significant difference between West Nile Encephalitis and West Nile Fever groups (Z = −2.14, p = .032, d = 0.52), such that individuals with West Nile Encephalitis had higher CoV than individuals with West Nile Fever associated with a medium effect size. No other pairwise differences were observed between these groups and the West Nile Meningitis group (ps >.10; West Nile Fever d = −.21; West Nile Encephalitis d = .33).
Quality of Life and IIV
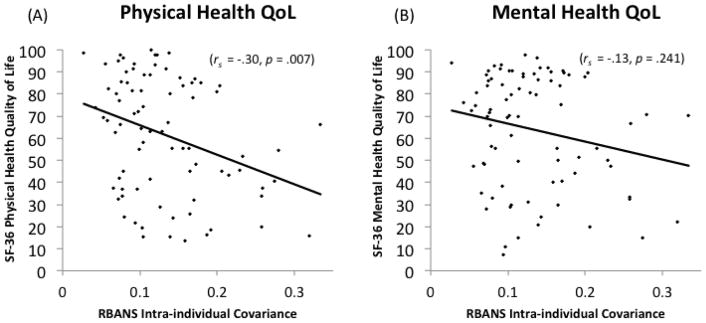
The multiple regression model with RBANS CoV predicting SF-36 physical health summary scores while controlling for BDI, fatigue, WASI IQ, neuroinvasive diagnosis, and ADLs reached overall significance, F(7, 59) = 10.07, p < .001, R2 adjusted = .49. Within this model, significant independent predictors of SF-36 physical health QoL scores were RBANS CoV (β = −2.06, p = .044), ADLs (β = 2.60, p = .012), and BDI (β = −3.06, p = .003), such that lower physical health QoL was associated with higher RBANS CoV, higher BDI depression scores, and more impaired ADLs. WASI IQ, neuroinvasive diagnosis, and fatigue did not reach statistical significance as an independent predictors (ps > .10). Since SF-36 physical health scores were non-normally distributed as determined by a Shapiro-Wilk test of normality at a critical alpha of .05, follow-up analyses were conducted using a spearman rank correlation between RBANS CoV and SF-36 quality of life scores, which showed a significant negative association between these two variables (rs = −.30, p = .007).
For mental health summary scores as the outcome using an identical set of covariates included in the model for physical health summary scores, the regression model reached overall significance, F(7, 59) = 17.99, p < .001, R2 adjusted = .64. Within this model, only BDI was a significant independent predictor of SF-36 mental health summary scores (β = −5.10, p < .001), although fatigue was predictive at the level of a trend (β = −1.95, p = .056). No other variables in the model, including CoV, reached statistical significance (all ps > .10).
Discussion
The present study examined the extent to which neurocognitive IIV – an indicator of mild neurocognitive dyscontrol – is related to important health factors (e.g., neuroinvasive disease history and quality of life) in persons infected with WNV. Compared to WNV fever, encephalitis was associated with significantly higher neurocognitive IIV, which was associated with a medium effect size (d = 0.52). These data suggest that even subtle alterations in cognitive status may be detected in subgroups of WNV-infected individuals with neuroinvasive disease and specifically encephalitis. Independent of disease or comorbid variables (e.g., neuroinvasive disease, depression), higher IIV was associated with poorer physical health, but not mental health, QoL in individuals with WNV. The unique association between IIV and physical health QoL was associated with a small-to-medium effect size (rs = .30), and provides preliminary evidence for subtle disruptions in neuropsychological functioning demonstrating associations with important self-reported functioning as measured by physical health quality of life.
WNV encephalitis patients showed higher variability in performances across a battery of neuropsychological tests compared to WNV fever, even when controlling for mean level of neuropsychological performance. Other neurovirulent viruses including HCV and HIV also have been show to evidence this pattern of elevated neurocognitive performance variability controlling for mean-level performance (Morgan et al. 2011; Morgan et al. 2012c). While these diseases show relatively lower rates of neuroinvasive involvement compared to WNV (Carson et al., 2012; Hogan & Wilkins, 2011), HIV and HCV serve as relevant parallels to WNV since they can be neurovirulent and have previously been examined in intraindividual neurocognitive variability studies. For example, Morgan et al. (2011) found that older age and HIV infection were associated with neurocognitive dispersion, while HCV has shown associations with higher neurocognitive dispersion with subsequent associations with everyday living deficits (Morgan et al. 2012c). The current study provides important evidence showing that neuroinvasive disease in WNV is associated with subtle alterations in neuropsychological functions independent of mean cognitive performance. These findings are similar to those found in other disease populations commonly considered to have impairments in cognitive control (Morgan et al. 2011). Nevertheless, HIV and HCV may not represent ideal parallel disease processes to WNV since they differ in their affinity for the brain, but do highlight potential parallels to the current findings in WNV. The current data are particularly important considering that previous studies examining cognitive outcomes in WNE and WNM groups have failed to detect differences on global measures of cognitive impairment within these clinical groups (e.g., Sejvar et al. 2008). However, there is pathophysiological data in WNV to suggest that these groups may have important pathological differences contributing to the presently observed findings. For example, previous pathology studies have shown that encephalitis is most commonly found in the brainstem, deep gray nuclei, and spinal cord showing perivascular inflammation, necrosis, microglial nodules, and loss of neurons (Kleinschmidt-DeMasters et al. 2004; Guarner et al. 2004). In contrast, meningitis is commonly associated with meningeal inflammation (Petersen et al. 2013b). The present data showing increased subtle disruptions, specifically in encephalitis cases of WNV, are consistent with previous reports of overall poorer outcomes in cases of encephalitis (Tyler et al. 2006). Future studies should examine possible cognitive associations with specific biological markers of specific neuroinvasive diseases (e.g., brain volume) as well as longitudinal data to determine the possible long-term cognitive declines in these subgroups among WNV-infected individuals.
Of clinical relevance, we observed that IIV was related to an important outcome, namely physical health QoL. This observation was associated with a small-to-medium effect size (rs = .27) and was independent of neuroinvasive disease and important co-factors (e.g., depression). These data support the notion that even subtle disruptions as measured by “spread” in cognitive performance is associated with self-reported health outcome quality of life in WNV, a disease population known to have a relatively subtle and non-specific neurocognitive profile. Importantly, IIV was specifically related to QoL for physical, but not mental, health. Notably, previous studies have shown similar patterns of cognitive outcomes relating to physical, but not mental QoL (Sejvar et al. 2008). One possibility for these findings and those of previous studies is that physical health QoL is more strongly affected than mental health QoL in WNV, thus conferring relative differences in the strengths of their relationships with cognitive predictors. However, as shown in Table 1, the current study observed no significant difference between mean-level physical and mental health QoL in our sample of WNV-infected individuals (d = .09). Further, it is noted that physical health QoL has previously demonstrated associations with other comorbid factors (e.g., physical disability, ongoing physical changes, fatigue) that also likely play a role in physical health quality of life (Bakula et al., 2015; Elbers et al., 2014). In our study, both physical functioning and role of limitations of physical health were each significantly associated with physical health QoL (rs range −.25 to −.27), which generally suggests similar strengths in the associations of overall physical health and role limitations with QoL. Another potential explanation for cognitive predictors of QoL remaining specific to physical health QoL is that mental health QoL is relatively restricted as a construct assessing depression. For example, items of mental health QoL (e.g., “Have you felt downhearted and blue?”) may simply be unrelated to broad cognitive functioning show low or non-significant associations with cognitive variables. Indeed, although our inclusion of depression as a covariate is potentially unduly conservative, it remains an important comorbid consideration and its inclusion did not affect the pattern of results as RBANS CoV was not significantly associated with SF-36 mental health QoL even at the binary (unadjusted) level (rs = −.13, p = .240). Post hoc analyses of the BDI depression inventory (Shafer et al. 2006) revealed that all three domains of depression (affective, somatic, cognitive) had strong associations with mental health QoL (rs range .73–.76), indicating that mental health QoL is strongly correlated with multiple symptom manifestations of depression. In contrast, mental health QoL was not related to either WASI IQ or RBANS CoV in our models, further suggesting that mental health QoL may show specific associations with depressive symptoms.
Regardless of cognitive or psychometric mechanisms, the present data suggest that CoV adds incremental predictive validity independent of mean-level cognition (NB. CoV accounts for mean-level cognitive performance) in predicting important health outcomes such as physical health QoL. This pattern of findings is consistent with those found in similar neurovirulent disease populations, such as data showing that individuals with HCV or HIV display associations between neurocognitive dispersion and deficits in everyday functioning in including unemployment (Morgan et al., 2012c) and medication adherence (Morgan et al. 2012b), and these associations can be found even among samples with intact mean-level performance (Morgan et al. 2012b). Especially given the simplicity of computing CoV in addition to mean-level cognitive performance, these findings suggest that subtle disruptions in neuropsychological functioning (e.g., cognitive control) may be an interesting metric that may further elucidate the relationship between cognition and other important health outcomes, such as comorbidity burden or mortality.
Regarding limitations of the current study, the relatively high rate of neuroinvasive disease in the cohort (56% in our WNV sample compared to the <1% in the total WNV population) limits the present results in their applicability to the broader WNV population. However, the presently utilized sample did have a rate that is commensurate with (or significantly less than) those utilized in previous studies of neurocognition (Sadek et al. 2010), and the present data may be more reflective of base rates of neuroinvasive diagnoses observed in neuro-clinic settings compared to previous studies. While our loss to follow-up rate was within acceptable limits (Kristman, Manno & Côté, 2004), attrition does potentially limit the generalizability of findings isnce severe health conditions may have been underrepresented in our sample. However, it is likely that this represents a conservative estimate of the study aims and is consistent with previous studies that also have included samples with loss to follow-up (Sejvar et al., 2008; Samaan et al., 2016). This study was limited by the lack of a non-WNV sample to determine whether neuroinvasive disease and education evidence an interaction on IIV similar to that observed in the current study in populations outside of WNV. Finally, the relatively limited neurocognitive battery (RBANS was developed to be used clinically as a screening and tracking tool for dementia; see Randolph et al. 1998) may limit the scope of the present findings for comprehensive investigations into the neuropsychology of WNV; however, the relatively brief RBANS is easily administered and may be easily administered in diverse clinical settings. While future studies may elect to examine IIV in WNV using more comprehensive neuropsychological batteries or reaction time measures, the present study provides important descriptive evidence for the association between neurocognitive functioning and physical health QoL in WNV.
Fig. 1.
Association between Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) intra-individual covariance and 36-Item Short Form Survey (SF-36) quality of life for (A) physical health and (B) mental health scales among 84 patients with West Nile virus infections
Acknowledgments
This study was supported National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID 1R01AI091816-01) and by the Gillson Longenbaugh Foundation. The authors would like to thank all of the study participants for contributing their time and efforts to this study.
Footnotes
Conflict of Interest
The authors, David P. Sheppard, Steven Paul Woods, Rodrigo Hasbun, Lucrecia Salazar, Melissa S. Nolan, & Kristy O. Murray, declare that they have no conflict of interest.
References
- Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status 1988 [Google Scholar]
- Brewster P, Tuokko H, MacDonald S. Inter-test variability contributes independently to the five-year prediction of Alzheimer’s disease in nondemented older adults. Alzheimers Dement. 2012;8:369. doi: 10.1016/j.jalz.2012.05.1010. [DOI] [Google Scholar]
- Brilla R, Block M, Geremia G, Wichter M. Clinical and neuroradiologic features of 39 consecutive cases of West Nile Virus meningoencephalitis. J Neurol Sci. 2004;220:37–40. doi: 10.1016/j.jns.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, et al. Intraindividual variability as a marker of neurological dysfunction: a comparison of Alzheimer’s disease and Parkinson’s disease. J Clin Exp Neuropsychol. 2006;28:67–83. doi: 10.1080/13803390490918318. [DOI] [PubMed] [Google Scholar]
- Carson PJ, Borchardt SM, Custer B, et al. Neuroinvasive disease and West Nile virus infection, North Dakota, USA, 1999–2008. Emerging Infect Dis. 2012;18:684–686. doi: 10.3201/eid1804.111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson PJ, Konewko P, Wold KS, et al. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin Infect Dis. 2006;43:723–730. doi: 10.1086/506939. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Final Cumulative Maps & Data for 1999–2016. 2016 Retrieved from: www.cdc.gov/westnile/statsmaps/cumMapsData.html.
- Christensen H, MacKinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P. Dispersion in cognitive ability as a function of age: A longitudinal study of an elderly community sample. Aging, Neuropsychology, & Cognition. 1999;6:214–228. [Google Scholar]
- Cooley ME. Quality of life in persons with non-small cell lung cancer: a concept analysis. Cancer Nurs. 1998;21:151–161. doi: 10.1097/00002820-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Garcia MN, Hause AM, Walker CM, Orange JS, Hasbun R, Murray KO. Evaluation of prolonged fatigue post-West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral immunology. 2014;27(7):327–33. doi: 10.1089/vim.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J, Shieh W-J, Hunter S, et al. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol. 2004;35:983–990. doi: 10.1016/j.humpath.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Sadek J, Pergam S, et al. Mental status after West Nile virus infection. Emerging Infect Dis. 2006;12:1260–1262. doi: 10.3201/eid1208.060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbun R, Garcia MN, Kellaway J, et al. West Nile Virus Retinopathy and Associations with Long Term Neurological and Neurocognitive Sequelae. PLoS ONE. 2016;11:e0148898. doi: 10.1371/journal.pone.0148898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. J Clin Exp Neuropsychol. 2009;31:412–424. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, et al. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300:823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Caro JJ. The Barthel Index and modified Rankin Scale as prognostic tools for long-term outcomes after stroke: a qualitative review of the literature. Current medical research and opinion. 2007;23(7):1627–36. doi: 10.1185/030079907X210444. [DOI] [PubMed] [Google Scholar]
- Jeha LE, Sila CA, Lederman RJ, et al. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61:55–59. doi: 10.1212/01.wnl.0000073617.08185.0a. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Marder BA, Levi ME, et al. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- Levi ME, Quan D, Ho JT, et al. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant. 2010;24:223–228. doi: 10.1111/j.1399-0012.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012a;61:341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, et al. Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–654. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Grant I HIV Neurobehavioral Research Program (HNRP) Group. Intra-individual neurocognitive variability confers risk of dependence in activities of daily living among HIV-seropositive individuals without HIV-associated neurocognitive disorders. Arch Clin Neuropsychol. 2012b;27:293–303. doi: 10.1093/arclin/acs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Rooney A, et al. Intra-individual variability across neurocognitive domains in chronic hepatitis C infection: elevated dispersion is associated with serostatus and unemployment risk. Clin Neuropsychol. 2012c;26:654–674. doi: 10.1080/13854046.2012.680912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KO, Garcia MN, Rahbar MH, et al. Survival analysis, long-term outcomes, and percentage of recovery up to 8 years post-infection among the Houston West Nile virus cohort. PLoS ONE. 2014;9:e102953. doi: 10.1371/journal.pone.0102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MS, Hause AM, Murray KO. Findings of long-term depression up to 8 years post infection from West Nile virus. J Clin Psychol. 2012;68:801–808. doi: 10.1002/jclp.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Sander B, Nelder MP. Long-term sequelae of West Nile virus-related illness: a systematic review. Lancet Infect Dis. 2015;15:951–959. doi: 10.1016/S1473-3099(15)00134-6. [DOI] [PubMed] [Google Scholar]
- Pepperell C, Rau N, Krajden S, et al. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ. 2003;168:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Carson PJ, Biggerstaff BJ, et al. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2013a;141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013b;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Pergam SA, Harrington JA, et al. Persistent neuropsychological impairment associated with West Nile virus infection. J Clin Exp Neuropsychol. 2010;32:81–87. doi: 10.1080/13803390902881918. [DOI] [PubMed] [Google Scholar]
- Samaan Z, McDermid Vaz S, Bawor M, et al. Neuropsychological Impact of West Nile Virus Infection: An Extensive Neuropsychiatric Assessment of 49 Cases in Canada. PLoS ONE. 2016;11:e0158364. doi: 10.1371/journal.pone.0158364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–1624. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- Sejvar JJ, Curns AT, Welburg L, et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J Neuropsychol. 2008;2:477–499. doi: 10.1348/174866407x218312. [DOI] [PubMed] [Google Scholar]
- Sejvar JJ, Leis AA, Stokic DS, et al. Acute flaccid paralysis and West Nile virus infection. Emerging Infect Dis. 2003;9:788–793. doi: 10.3201/eid0907.030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of clinical psychology. 2006;62(1):123–46. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Suen WW, Prow NA, Hall RA, Bielefeldt-Ohmann H. Mechanism of West Nile virus neuroinvasion: a critical appraisal. Viruses. 2014;6:2796–2825. doi: 10.3390/v6072796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trépanier LL, Rourke SB, Bayoumi AM, et al. The impact of neuropsychological impairment and depression on health-related quality of life in HIV-infection. J Clin Exp Neuropsychol. 2005;27:1–15. doi: 10.1080/138033990513546. [DOI] [PubMed] [Google Scholar]
- Tyler KL, Pape J, Goody RJ, et al. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology. 2006;66:361–365. doi: 10.1212/01.wnl.0000195890.70898.1f. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Leng I, Dagenbach D, et al. Intraindividual variability in domain-specific cognition and risk of mild cognitive impairment and dementia. Curr Gerontol Geriatr Res. 2013;2013:495793. doi: 10.1155/2013/495793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Weatherhead JE, Miller VE, Garcia MN, et al. Long-term neurological outcomes in West Nile virus-infected patients: an observational study. Am J Trop Med Hyg. 2015;92:1006–1012. doi: 10.4269/ajtmh.14-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. 2. San Antonio, TX: Psychological Corporation; 2011. [Google Scholar]