Abstract
Frailty is associated with increased mortality among lung transplant candidates. We sought to determine the association between frailty, as measured by the Short Physical Performance Battery (SPPB) and mortality after lung transplantation. In a multicenter prospective cohort study of adults who underwent lung transplantation, pre-operative frailty was assessed with the SPPB (n=318) and, in a secondary analysis, the Fried Frailty Phenotype (FFP; n=299). We tested the association between pre-operative frailty and mortality following lung transplantation with propensity score adjusted Cox models. We calculated post-estimation marginalized standardized risks for one-year mortality by frailty status using multivariate logistic regression. SPPB frailty was associated with an increased risk of both one- and four-year mortality (adjusted hazard ratio [aHR]: 7.5; 95%CI: 1.6–36.0 and aHR 3.8; 95%CI: 1.8–8.0, respectively). Each one-point worsening in SPPB was associated with a 20% increased risk of death (aHR: 1.20; 95%CI: 1.08–1.33]). Frail subjects had an absolute increased risk of death within the first year after transplantation of 12.2% (95%CI: 3.1–21%). In secondary analyses, FFP frailty was associated with increased risk of death within the first post-operative year (aHR: 3.8; 95%CI: 1.1–13.2) but not over longer follow-up. Pre-operative frailty is associated with an increased risk of death after lung transplantation.
INTRODUCTION
Lung transplantation aims to extend survival, reduce disability, and improve health-related quality of life for persons suffering from advanced lung diseases. Despite stringent selection criteria and advances in surgical and medical therapies, mortality after lung transplantation is high and has not improved in the last 5 years (1).
A 2005 overhaul in the U.S. donor lung organ allocation system aimed to reduce an unacceptably high waitlist mortality (2). While achieving its primary aim, a consequence of the Lung Allocation Score (LAS) system is that older and sicker candidates are prioritized for transplantation. As a result, older adults (age ≥65) are the fastest growing group of lung transplant candidates in the U.S., now accounting for nearly 30% of new transplant recipients compared to only 4% in 2002(1). After lung transplantation, those 65 years and older have a median survival of only 3.6 years, fully three years less than those 35–49 years old (3). This rapid trend has outpaced the evidence base and data are needed to identify which candidates will do well after lung transplantation. Absent better approaches, transplant programs have adopted admittedly arbitrary age cutoffs or “eyeball tests” of fitness to identify suitable candidates. Although known risk factors for death such as age, functional status, oxygen use, and others are already incorporated into LAS, increasing waitlist mortality rates and persistently high mortality after lung transplantation underscore the need to identify novel, ideally modifiable, risk factors for poor outcomes (1). Doing so will help to maximize the individual and societal benefit of a scarce resource such as lung transplantation (2).
We recently identified physical frailty as a novel risk factor for disability and death prior to transplant in lung transplant candidates (4). Originally a geriatric construct, frailty reflects accumulated aging-related deficits across multiple systems that attenuate the body’s physiologic reserve. This results in a “state-of-risk” for disproportionate declines in health status upon exposure to an additional physiologic stressor (5). Frailty is a risk factor for disability, perioperative complications, and mortality in older medical and surgical populations (6–14) including abdominal organ transplantation (4, 15–19). Further, frailty, defined by an accumulation of deficits, is associated with mortality after lung transplantation (19). Whether pre-operative physical frailty is a novel risk factor for mortality following lung transplantation, however, is unknown. In this multi-center prospective cohort study, we aimed to determine whether pre-operative physical frailty quantified primarily by the Short Physical Performance Battery and, secondarily, by the Fried Frailty Phenotype, was associated with an increased risk of death after lung transplantation.
METHODS
Study design, Participants, and Setting
We analyzed participants in the Lung Transplant Body Composition (LTBC) study. LTBC is a multicenter observational prospective cohort study investigating the impact of pre-operative body composition and physical frailty on outcomes in lung transplant candidates and recipients. We excluded candidates for multi-organ or redo lung transplantation. For this analysis, candidates for lung transplantation age ≥18 years at the University of California, San Francisco (UCSF), Columbia University Medical Center (CUMC), and the University of Pennsylvania were recruited. As reported previously, in the early phase of this study (2010–2012), UCSF was utilizing the Short Physical Performance Battery (SPPB) and CUMC was utilizing the Fried Frailty Phenotype (FFP) as to quantify physical frailty (detailed below) (4). After 2012, centers began collecting both measures; as a result, not all subjects completed both frailty measures. The overall study period ranged between February, 2010 and May, 2017. Institutional Review Boards at each center approved this study and participants provided written informed consent for participation.
Frailty Assessment
We tested two well-validated frailty measures that emphasize physical functioning and that we previously found to be associated with death or removal from the waitlist in lung transplant candidates (4). Although our previous work and new findings in kidney transplantation suggest that the SPPB may be superior to the FFP, there is equipoise in regards to survival after lung transplantation (4, 20). Details on the frailty measures and scoring are in Supplemental Table S1. For this analysis, we defined the SPPB as our primary measure of frailty and, in secondary analyses, defined frailty by the FFP. Frailty assessments were performed around the time of listing and, as possible, every three months while subjects were listed. Those assessments most proximal to the time of transplant were used for this analysis. The SPPB is a 3-component battery that includes gait speed, chair stands, and balance (6, 7). Each measure is scored from 0–4 with an aggregate score ranging from 0–12. Lower SPPB scores reflect increased frailty. Consistent with established definitions and our prior work, we defined frailty as an SPPB score of ≤ 7 (4, 6).
The FFP is an aggregate score of five constructs: low physical activity, slowness, weakness, shrinking, and exhaustion (8). Each construct is assigned “1” if present or “0” if absent, standardized to sex, height, and weight. The FFP ranges from 0–5, In contrast to the SPPB, higher scores reflect increased frailty. Consistent with established definitions, we defined frailty as a FFP score of ≥3 (8). To minimize missing data and maximize our FFP sample size, we defined frailty by FFP using a “best-measure” approach. We have previously shown that replacing the operational measure of the FFP low activity domain (the Minnesota Leisure Time Activity Questionnaire [MLTA] (21)) with a measure validated in cardiopulmonary disease (the Duke Activity Status Index questionnaire [DASI] (22)), better quantified frailty-attributable low activity in lung transplant candidates by improving multiple tests of validity, including predicting waitlist delisting or death (23). Over the study period, early participants completed only the MLTA survey, others both the MLTA and DASI, and later subjects only the DASI. For subjects who had completed the DASI survey, we defined low activity by this metric (termed “FFP-DASI”); for earlier subjects who had only completed the MLTA survey, we defined low activity by MLTA (“FFP-MLTA”).
Confounding and precision variables
Demographic and clinical variables at the time of frailty assessments were abstracted from medical records. Variables included age, gender, body mass index (BMI; kg/m2), race/ethnicity, diagnostic indication for transplant, forced expiratory volume in one second (FEV1; liters) forced vital capacity (FVC; liters), six-minute walk distance (6MWD; meters). We also abstracted each subject’s LAS (range 0 – 100; higher scores are prioritized for organ allocation) at the time of listing and at the time of lung transplantation. All subjects in this analysis underwent lung transplantation, with mandatory reporting of the demographic and clinical factors to a national registry overseen by the Department of Health and Human Services. As a result, we had no missing data for any of the covariates.
Outcome measures
Since we hypothesized that pre-operative frailty was most likely to influence early death, we defined survival time within the first post-operative year as our primary outcome. Dates of death were obtained through medical record review. Survival time was calculated as the number of days from the date of lung transplantation until the date of death. For one-year survival, survival time was right-censored at 365 days. We also performed secondary analyses evaluating whether pre-operative frailty – as either a binary (frail or not) or continuous variable – was associated with longer-term mortality. Due to few subjects with follow up time greater than four years, we restricted our longer-term survival analysis to four years and right-censored survival time at 1460 days. We also tested whether pre-operative frailty was associated with length of index hospitalization stay.
Analysis Approach
The unadjusted associations of frailty with death after transplantation were visually assessed with Kaplan-Meier methods and compared using the log rank test. For subsequent adjusted models, a modest number of deaths within the first year after transplant could have required us to consider a parsimonious list of covariates. To address this, we controlled for potential confounders and precision variables by generating propensity scores predicting frailty status. Variables used to generate the propensity score included age, gender, race/ethnicity, diagnosis, FVC, BMI, 6MWD, and LAS. We utilized the STATA “pscore.ado” program to generate propensity scores and confirm that the covariate balancing hypothesis was satisfied (24, 25). We used multivariable stratified Cox proportional hazard models to estimate the association between pre-operative frailty and death within the first year after transplant. We first examined the unadjusted association between frailty and mortality with one stratum for each transplant center. Next, we included the propensity score in the subsequent adjusted models. Lastly, we conducted a series of a sensitivity analyses. In one analysis, we included the LAS at the time of lung transplantation as a covariate in the propensity score adjusted models. In a subsequent analysis we included change in LAS from the time of listing to the time of transplant as a covariate in the propensity score adjusted models. Next, we tested the association between frailty and mortality after transplant differed in the era before or after the February 15, 2015 update to the LAS scoring algorithm. In a fourth sensitivity analysis, we excluded subjects (n = 17) with frailty assessments that were more than nine months from the date of transplant. Finally, we included the need for extra-corporeal membrane oxygenation or invasive mechanical ventilation as a bridge to lung transplantation as a covariate in the propensity score adjusted models. To evaluate the proportionality of hazards, we plotted scaled Schoenfeld residuals with respect to time, assessed log-negative-log plots, and employed the Schoenfeld test. Length of incident hospital stay was compared with Wilcoxon rank sum.
We calculated post estimation marginalized standardized risks for death within the first-year using logistic regression models with frailty as an independent variable and death as the dependent variable, controlling for center and the propensity score that did not include BMI or 6MWD.
Analyses were performed using Stata (15.1, StataCorp, College Station, TX). For all analyses, we used a p-value of < 0.05 as the threshold for statistical significance.
RESULTS
A total of 386 subjects with pre-operative frailty assessments underwent lung transplantation and were included in this cohort. Of the cohort, 318 completed SPPB frailty assessments and 299 completed FFP assessments, and 231 completed both. The median time between frailty assessment and the date of transplant was 2.8 months (interquartile range [IQR]: 1.1, 6.1). The cohort was 56% male, median age was 59 years (IQR: 50, 65), and median LAS was 42.6 (IQR: 36.2, 54.3; Table 1); 28% of the cohort had more than one frailty assessment before transplant (median 1, IQR: 1, 1; range 1 – 4). Most subjects (68%) underwent lung transplantation for pulmonary fibrosis, followed by 20% for non-suppurative obstructive lung disease (predominantly chronic obstructive pulmonary disease [COPD]), 9% for suppurative lung diseases (predominantly cystic fibrosis), and 3% for pulmonary arterial hypertension. In comparison to all patients undergoing lung transplantation in the U.S., our cohort had fewer Caucasians, more subjects with pulmonary fibrosis, and fewer subjects with LAS scores <35. The median duration of follow-up was 2.5 years (IQR: 1.2, 4.3 and range 1 day to 7.1 years) with 1,096 person-years of observation. No subjects were lost to follow up.
Table 1.
Demographics and baseline clinical characteristics by frailty status
| Short Physical Performance Battery | Fried Frailty Phenotype | |||
|---|---|---|---|---|
|
|
|
|||
| Frail | Not Frail | Frail | Not Frail | |
| N | 65 | 253 | 100 | 199 |
| Age, years | 58 (47, 65) | 59 (52, 66) | 59 (47, 65) | 60 (38, 66) |
| Men | 29 (16%) | 153 (84%) | 48 (27%) | 129 (73%) |
| Women | 36 (26%) | 100 (74%) | 52 (43%) | 70 (57%) |
| Race/Ethnicity | ||||
| White, Non-Hispanic | 39 (61%) | 192 (76%) | 63 (64%) | 160 (80%) |
| Black | 6 (9%) | 22 (9%) | 12 (12%) | 18 (9%) |
| Asian | 7 (11%) | 14 (6%) | 10 (10%) | 9 (5%) |
| Hispanic | 10 (16%) | 22 (9%) | 12 (12%) | 10 (5%) |
| Other | 2 (3%) | 1(0%) | 1 (1%) | 1 (1%) |
| Diagnostic Category* | ||||
| Group A (COPD) | 11 (19%) | 47 (81%) | 18 (31%) | 40 (69%) |
| Group B (PAH) | 2 (18%) | 9 (82%) | 4 (36%) | 7 (64%) |
| Group C (CF) | 5 (16%) | 27 (84%) | 8 (36%) | 14 (63%) |
| Group D (PF) | 47 (22%) | 170 (78%) | 70 (34%) | 138 (66%) |
| Body Mass Index, kg/m2 | 25.5 ± 0.6 | 25.8 ± 0.3 | 24.1 ± 0.4 | 26.4 ± 0.3 |
| FEV1, Liters | 1.1 (0.8, 1.7) | 1.3 (0.9, 1.9) | 1.3 (0.8, 1.9) | 1.4 (0.9, 2.0) |
| FVC, Liters | 1.7 (1.3, 2.2) | 2.0 (1.5, 2.6) | 1.8 (1.3, 2.4) | 2.0 (1.5, 2.6) |
| 6 Minute Walk Distance, m | 136 (35, 203) | 310 (226, 383) | 246 (155, 322) | 317 (229, 398) |
| LAS | 63 (44, 90) | 40 (36, 51) | 46 (40, 63) | 40 (35, 48) |
| ECMO | 9 (14%) | 18 (7%) | 5 (5%) | 14 (7%) |
| Mechanical ventilation | 13 (20%) | 20 (8%) | 10 (10%) | 13 (7%) |
| Bilateral lung transplant | 56 (88%) | 204 (84%) | 80 (83%) | 132 (70%) |
A total of 20% of the subjects were frail by our primary definition employing the SPPB (95% Confidence Interval [CI]: 16–25; Table 1). The prevalence of frailty was similar in older and younger recipients (age <65: 21% [95%CI: 17–27] versus age ≥ 65: 17% [95%CI: 11–25]; p = 0.35). Consistent with other populations, frailty was more prevalent in women (8). The distribution of frailty across diagnostic groups was similar, despite heterogeneous age distributions across the groups (for example, median age of those with pulmonary fibrosis was 60 years [IQR: 53–66] versus 30 years [IQR: 26–42] for those with suppurative lung diseases). Those who were frail walked substantially shorter distances in six minutes and had higher LAS scores at the time of lung transplantation (Table 1).
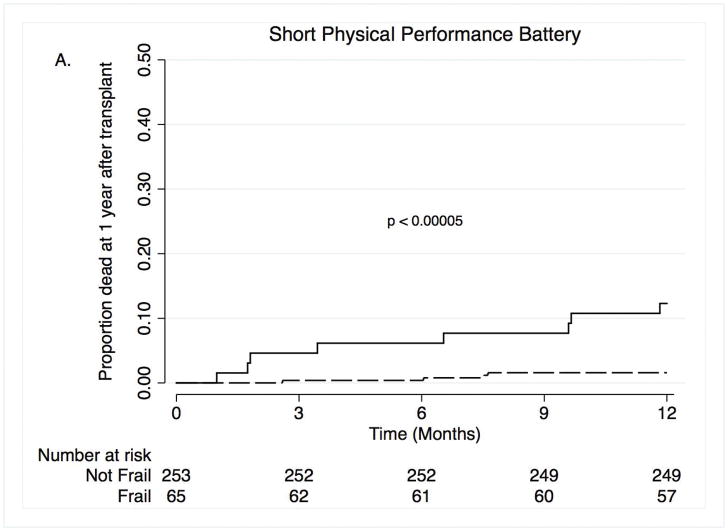
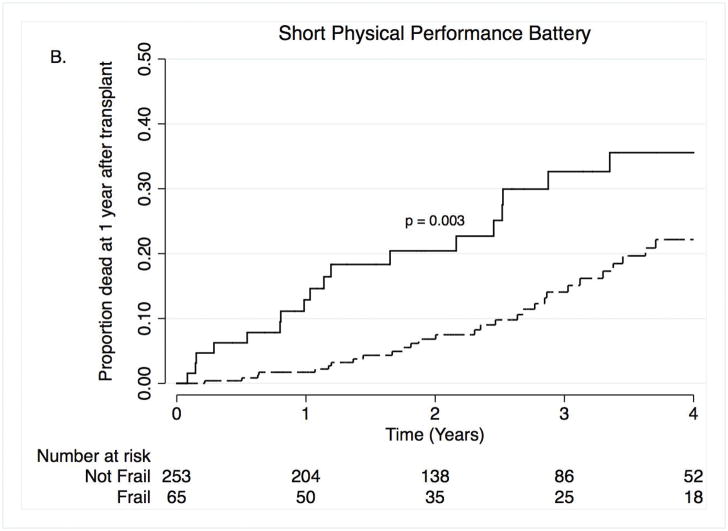
Nineteen subjects died within the first year after lung transplantation; of these 10 were women. Causes of death included infection (n =7), primary graft dysfunction (n = 4), acute rejection or chronic allograft dysfunction (n =3), cardiovascular events (n =2), and other (n =2). In unadjusted analyses, pre-operative frailty was associated with a ten-fold increase in the risk of death during the first-year after lung transplantation (Hazard Ratio [HR]: 10.5; 95%CI: 2.9–37.9; Table 2; Figure 1A). In the propensity score-adjusted model, frailty was associated with a nine and a half-fold increased risk of death (HR 9.7; 95%CI: 2.4–38.9; Table 2). Over the entire study period, 70 subjects died with 65 of these deaths occurring within the first four years after transplant (mortality rate: 6.5 deaths per 100 person years). Causes of death included infection (n = 21), primary graft dysfunction (n = 4), acute rejection or chronic allograft dysfunction (n = 20), cardiovascular events (n = 3), cancer, (n = 6), and other (n = 16). In an unadjusted model, frailty was associated with a two and a half-fold increased risk of death (Figure 1B; HR: 2.5; 95%CI: 1.3–4.8). In the propensity score-adjusted model, frailty was associated with a greater than three and a half-fold increased risk of death (HR 3.8; 95%CI: 1.8–8.0). Furthermore, each one-point worsening in frailty was associated with a 10–20% increased risk of longer-term mortality (HR 1.12; 95%CI: 1.03–1.21 for unadjusted model; HR 1.20; 95%CI: 1.08–1.33 for propensity score-adjusted model). Adding LAS as a covariate to the propensity-score adjusted models did not appreciably change our findings (Table 2). Our findings were also generally not substantially different across five sensitivity analyses (Supplemental Tables S2–5). The exception was a stronger association between FFP frailty and one-year mortality when controlling for the need for pre-operative ECMO or invasive mechanical ventilation as a bridge to lung transplantation (Supplemental Table S5).
Table 2.
Association between Short Physical Performance Battery frailty and death (n = 318)
| Death within the first year after lung transplant | Hazard ratio for frail vs. non-frail (95% confidence interval) | Hazard ratio per 1-unit worsening in SPPB (95% confidence interval) |
|---|---|---|
| Unadjusted, stratified by center | 10.5 (2.9 – 37.9) | 1.19 (1.05 – 1.36) |
| Adjusted for propensity score* | 7.5 (1.6 – 36.0) | 1.05 (0.86 – 1.27) |
| Adjusted for propensity score and LAS | 7.5 (1.6 – 36.6) | 1.05 (0.86 – 1.27) |
|
| ||
| Death over up to 4 years of follow-up | Hazard ratio for frail vs. non-frail (95% confidence interval) | Hazard ratio per 1-unit worsening in SPPB (95% confidence interval) |
| Unadjusted, stratified by center | 2.5 (1.3 – 4.8) | 1.12 (1.03 – 1.21) |
| Adjusted for propensity score* | 3.8 (1.8 – 8.0) | 1.20 (1.08 – 1.33) |
| Adjusted for propensity score and LAS | 3.8 (1.8 – 8.0) | 1.20 (1.08 – 1.34) |
Effect estimates are hazard ratios (95% confidence intervals) for mortality associated with frailty. Propensity score includes age, gender, race/ethnicity, diagnosis, forced vital capacity, body mass index and six minute walk distance. SPPB = Short Physical Performance Battery.
Figure 1. Time to death by Short Physical Performance Battery within the first year after transplant (A) and over four years after transplant (B).
Solid lines = frail, dashed lines = not frail.
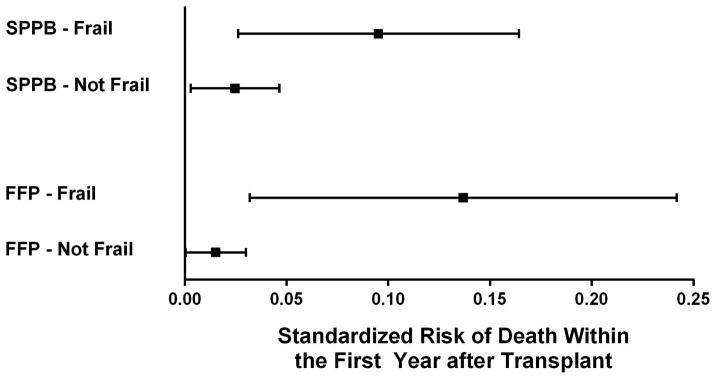
Based on the calculated standardized predicted risk of death in the first year after lung transplantation, frail subjects had a propensity score adjusted 13.7% absolute risk of death (95%CI: 3.2–24%) compared to 1.5% in those who were not frail (95%CI: 0.03–3.0%) – an absolute risk increase of 12.2% (95%CI: 3.1–21%) (Figure 3).
Figure 3. Standardized mortality risk by SPPB and FFP Frailty status.
The risk reflects the post-estimation marginalized standardized risk derived from a logistic regression model controlling for age, gender, diagnosis, forced vital capacity, Lung Allocation Score, and transplant center. The dots represent the point estimates for the adjusted standardized risk and the bars represent the 95% confidence intervals.
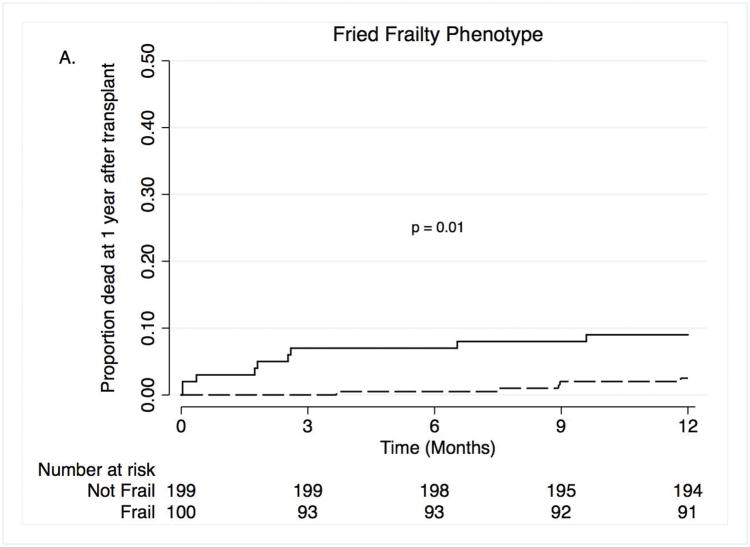
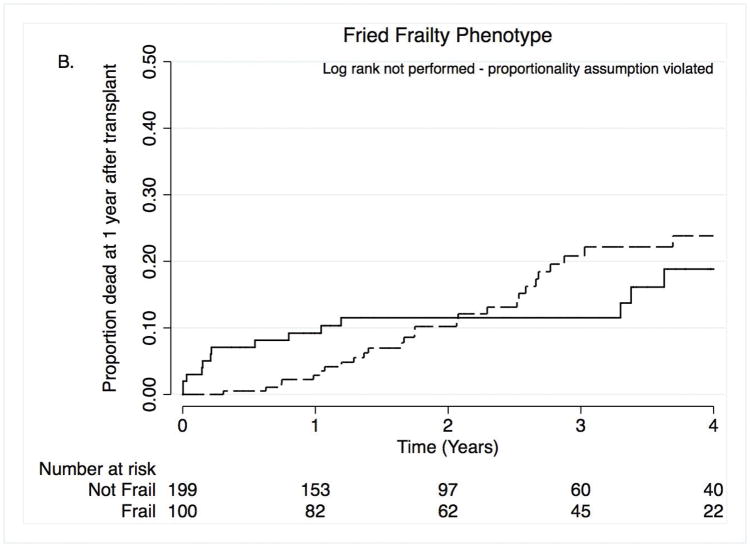
In our secondary analysis in which we defined frailty by the FFP, 33% of subjects were frail before transplantation (95%CI: 28, 39%). FFP frailty was associated with a greater than three and a half-fold increased risk of death within the first year after transplant, adjusting for propensity score (HR: 3.8; 95%CI: 1.1–13.2) (Figure 2A and Table 3). In contrast to the SPPB, the association between FFP frailty and death over four years of follow-up varied over time and violated the proportionality assumption, precluding log rank testing or using cox proportional hazard models (Figure 2B and Table 3). It should be noted that none of the subjects identified as frail by FFP died between 1.2 and 3.3 years after lung transplantation. Finally, based on the calculated standardized predicted risk of death in the first year after lung transplantation, frail subjects measured by FFP had a 7% (95%CI: 2.3–11.8%) absolute increased risk of death after controlling for age, diagnosis, lung function, LAS, and transplant center (standardized predicted risk for those who were frail was 9.5% [95%CI: 2.6–16%] versus 2.5% [95%CI: 0.3–4.6%] for those who were not). Supplemental Table S5 illustrates that the point estimates for the association between FFP-DASI and FFP-MTLA defined frailty and one-year mortality were similar to our composite FFP measure.
Figure 2. Time to death by Fried Frailty Phenotype within the first year after transplant (A) and over four years after transplant (B).
Solid lines = frail, dashed lines = not frail.
Table 3.
Association between Fried Frailty Phenotype frailty and death within the first year after lung transplantation (n = 299)*
| Death within the first year after lung transplant | Hazard ratio for frail vs. non-frail (95% confidence interval) | Hazard ratio per 1-unit worsening in FFP (95% confidence interval) |
|---|---|---|
| Unadjusted, stratified by center | 6.3 (2.2 – 18.0) | 2.1 (1.3 – 3.3) |
| Adjusted for propensity score** | 3.8 (1.1 – 13.2) | 1.7 (1.04 – 2.7) |
| Adjusted for propensity score and LAS | 4.3 (1.1 – 16.3) | 1.6 (1.01 – 2.5) |
Effect estimates are hazard ratios (95% confidence intervals) for mortality associated with frailty.
The association of frailty and death over up to 4 years of follow-up violated the assumption of proportional hazards so hazard ratios were not estimated.
Propensity score includes age, gender, race/ethnicity, diagnosis, forced vital capacity, body mass index and six minute walk distance.
DISCUSSION
In this U.S.-based multicenter prospective cohort study, we found that frailty, defined by the Short Physical Performance Battery (SPPB), was common among lung transplant candidates and was independently associated with an increased risk for early mortality following lung transplantation. Beyond the increased relative risk, those who were frail before lung transplantation had an absolute increased risk of death within the first post-operative year of 12% compared to those who were not frail. Additionally, each one point worsening in pre-operative SPPB was associated with a 20% increased risk of longer-term death after lung transplantation. In secondary analyses, we found that frailty defined by the Fried Frailty Phenotype (FFP) was independently associated with one-year, but not longer-term, mortality following lung transplantation.
Given the scarcity of donor organs, especially in lung transplantation in which only <30% of donor lungs are accepted, efforts to reduce mortality after transplantation are fundamental to maximizing the individual and societal benefit of transplantation (26). Applying concepts and principles from the aging literature to lung and other solid organ transplantation is becoming increasingly relevant to clinical care and, potentially, organ allocation policy. Adults ≥65 years of age now represent nearly 30% of new lung transplant recipients, up from 4% in 2002 (1). Similar trends have been observed in heart, liver, and kidney transplantation (27–29). In the last five years, frailty measured by the SPPB and/or FFP has been shown to be associated with an increased risk of death among lung, heart, and liver transplant candidates (4, 16, 18). In addition, frailty is associated with delayed graft function, length of stay following transplant surgery, hospital readmission, and mortality after kidney transplantation (15, 17, 30–32). Also, in a single center retrospective study, frailty quantified by the cumulative index model was associated with mortality after lung transplantation (19). This study and our prior work demonstrating that frailty is independently associated with waitlist and post-transplant mortality across age groups adds to this emerging body of work (4).
That the prevalence of frailty in solid organ transplantation ranges from 10–43% inclusive of younger candidates suggests that the biology driving pathologic aging may be operational across the age spectrum (4, 15–18, 31–34). Work starting to tease apart the pathobiology of frailty, including chronic inflammation, neurohormonal failure, sarcopenia, and epigenetics could provide mechanistic insights and, potentially explain part of the association between clinical frailty and mortality (4, 35–39). The putative mechanisms causing frailty are multiple, however, and reflect the complexity of the aging process (40). Understanding the pathobiology driving frailty could also highlight novel targets for intervention and identify whether the causes of frailty are universal across lung diseases with heterogeneous pathophysiology or whether sub-phenotypes of frailty with distinct pathobiology, outcome risks, and treatment responses (so called “endotypes”) exist. For example, higher levels of systemic inflammation (as quantified by interleukin-6 [IL-6], tumor necrosis factor [TNF], C-reactive protein [CRP]) are associated with increased exacerbations, poorer lung function, and death in COPD (41–43). In contrast, patients with idiopathic pulmonary fibrosis (IPF) do not have evidence of systemic inflammation by IL-6, TNF, or CRP (44). As in heterogeneous conditions like asthma or the adult respiratory distress syndrome in which endotype analysis has resulted in major breakthroughs in understanding the pathobiology and treatment responses, considering the relationship between multiple pathobiological factors may advance our understanding of frailty (45–47).
Our findings, considered in the context of work in other solid organ transplant populations, suggests that frailty may represent a universal risk factor for poor outcomes not currently captured by existing risk stratification and U.S. organ allocation systems including the LAS, Model for End-Stage Liver Disease, kidney Estimated Post-Transplant Survival score, and the less granular heart allocation system(48). Given the prevalence of frailty in organ transplant candidates and its strong association with poor outcomes, including measures of frailty in the transplant candidacy evaluation process may ultimately help improve outcomes. While measures of frailty hold promise to improve risk stratification and potentially organ allocation schema, the existing evidence – limited to single or multicenter (but not national) data – is not robust enough to advocate that measures of frailty be immediately incorporated into existing allocation policy.
Before implementing measures of frailty into organ allocation, work is needed to identify the most valid measures of frailty in solid organ transplantation. This study and our prior work has demonstrated that the prevalence and strength of association between frailty and waitlist and post-transplant mortality differs by the specific operational measure of frailty employed (4). Although our data show that the SPPB outperforms the FFP in predicting both waitlist and post-transplant mortality in lung transplantation, numerous operational measures of frailty exist, some of which quantify other conceptual domains of frailty not included in the SPPB (chair stands, gait speed, and balance) or FFP (gait speed, grip strength, exhaustion, low activity, and wasting). Although originally referred to as a measure of lower extremity performance, the SPPB meets the conceptual multidimensional criteria as well as construct and predictive validity criteria needed for valid frailty measures (49). Because its ease of use relative to other frailty measures, SPPB was selected as the measure of physical frailty by Sarcopenia and Physical Frailty in Older People: multicomponent treatment strategies (SPRINTT) Consortium (50). Whether the SPPB is the best measure of frailty in lung transplantation or whether other conceptual domains, such as cognitive impairment, could be informative and improve the predictive validity of frailty quantification is unknown (51, 52). The existing literature evaluating the association between single frailty domains such as malnutrition, low exercise capacity by six-minute walk testing, or CT imaging-based assessments of low muscle mass as a proxy for sarcopenia and outcomes before and after lung transplant provide supportive evidence that additional metrics are likely to be useful in defining frailty in advanced lung disease and lung transplantation (53–57). Thus, work is needed to identify which conceptual domains to include, whether established cut points derived in community dwelling older populations need to be modified for use in lung transplantation, and whether the addition of biomarkers or imaging to better quantify inflammation, sarcopenia, and other putative pathobiological drivers of frailty can further improve existing measures. Furthermore, work is needed to investigate whether a single measure of frailty will perform well across solid organ transplant types. As an early step, the American Society of Transplantation held a consensus conference on frailty in solid organ transplantation in early 2018 to address some of these pressing issues. Finally, any changes to organ allocation policy will require data on large numbers of patients, extensive simulation modeling to understand the impact of incorporating measures of frailty, and input from key stakeholders.
Perhaps equally promising is the emerging literature suggesting that frailty is dynamic and reversible. Several of the putative “upstream” drivers of the frailty phenotype such as micronutrient deficiencies, sarcopenia, physical inactivity and even chronic inflammation should be amenable to intervention. Recent interventional studies of exercise programs (with or without nutritional intervention) in older adults have demonstrated improvements in SPPB frailty scores (58–60). In a study of patients with COPD referred for pulmonary rehabilitation, 26% were frail by the FFP measure. Although frailty was associated with a two-fold higher odds of failing to complete the 8-week program, amongst those who did, 61% were no longer frail on repeat assessment. We performed a pilot home-based exercise and nutrition intervention study to treat frailty in lung transplant candidates using a novel mobile health based program. In 15 subjects with COPD or pulmonary fibrosis, we reported in abstract form that treating frailty was safe and, for some of the participants, SPPB frailty scores improved (61). These studies suggest that implementing targeted interventions before transplantation to optimize pre-operative functional status (i.e., “pre-habilitation”) could reverse components of the frailty phenotype; ideally, doing so would then reduce subsequent morbidity and mortality (62–64).
Our study had limitations and also raises several important questions that identify important areas for future research efforts focused on frailty in advanced lung disease and lung transplantation. The limited number of deaths precluded us from performing clinically important stratified analyses such as by age group or diagnosis, or testing for multiplicative interaction terms. Due to the timing of study initiation at each center, both SPPB and FFP measures were not collected in all subjects. In addition, as evidenced by the wide confidence intervals around estimates of the mortality risk associated with SPPB and FFP frailty, the point estimates of risk should be cautiously interpreted. Additional investigation is needed to understand the specific mechanisms by which frailty increases the risk of death after lung transplant. Further, the one-year mortality rate of 5% is lower than the national average, now at approximately 11% (1). While, in part, this difference may reflect atypically low mortality rates at one of the participating centers in this study, it also raises the possibility that this study may not reflect the spectrum of patients undergoing lung transplantation in the U.S. As a result, our work should be validated across a broader set of transplant centers before frailty is considered in listing decisions or lung allocation schema. It should be noted that the median time between frailty assessments and transplant surgery was close to three months. During the time while listed, subjects may have engaged in various amounts of formal and informal exercise, including pulmonary rehabilitation, that could have impacted the severity of their frailty status. We did not have access to reliable records of the amount and type of exercises subjects were performing during the waitlist period. It is possible that subjects’ frailty status could have changed during this unobserved period. If this were the case, however, such misclassification would tend to bias our findings towards the “null”. Anecdotally, we are aware of a subset of patients whose physical functioning improves or worsens dramatically in the months preceding lung transplantation. Future work should focus on the post-operative implications of this subset of performance trajectories. Although performing assessments at the time of admission for transplant surgery is not likely to be immediately clinically useful, such assessments could be useful for future investigations aimed at most accurately testing the associations between pre-operative frailty and outcomes after transplant. Another limitation is our definition of FFP frailty. During the study period, we found that using a new metric for operationalizing the “low activity” domain for the 5-domain based FFP frailty measure improved the validity of FFP frailty assessment in lung transplant candidates (23). Although we previously demonstrated both the FFP-DASI and FFP-MLTA measures to be valid in advanced lung disease (4) and combining the two enriches our sample size and reduces missing data, it is possible that the hybrid measure could contribute to misclassification of frailty by FFP and thereby influence the differential association between SPPB and FFP frailty and risk of mortality after lung transplantation. While the point estimates of mortality risk associated with either the FFP-DASI and FFP-MTLA were similar to our composite measure in both unadjusted and propensity score models, we cannot exclude the potential for misclassification. Finally, due to the limited number of repeated assessments before lung transplant, we were unable to evaluate whether a change in frailty score before transplant was associated with mortality after lung transplantation. It is plausible that acute worsening in frailty scores, regardless of the absolute value, could identify patients at increased risk of poor outcomes, as has been shown for changes in 6MWD and the LAS.
In conclusion, frailty is prevalent in lung transplant candidates and strongly associated with an increased risk of early mortality following transplant surgery. Since frailty is also associated with mortality before lung transplantation, efforts are needed determine how to include these measures in the assessment of lung transplant candidacy and organ allocation.
Supplementary Material
Acknowledgments
The authors greatly appreciate the hundreds of patients who have participated the Lung Transplant Body Composition Study.
FUNDING: National Heart, Lung, and Blood Institute (Grant K23 HL111115 and R01 HL134851 to JPS) and a Nina Ireland Program in Lung Health Award (JPS); National Heart, Lung, and Blood Institute (Grant K23 HL121406 to JMD); National Heart, Lung, and Blood Institute (Grant K23 HL116656 and R03 HL135227 to EC); National Heart, Lung, and Blood Institute (Grant R01 HL081619, HL096845, HL115354 and HL087115 to JDC; and R01 HL114626 and K24 HL131937 to DJL). A portion of this study was supported by the National Center for Advancing Translational Sciences (previously the National Center for Research Resources) at the National Institutes of Health (UCSF-CTSI UL1 RR024131; CUMC-CTSA UL1 TR000040), and the Rocco Guinta Research Fund
ABBREVIATIONS
- IL-6
interleukin-6
- 6MWD
six-minute walk distance
- BMI
body mass index
- CRP
C-reactive protein
- CUMC
Columbia University Medical Center
- DASI
Duke Activity Status Index questionnaire
- FEV1
forced expiratory volume in one second
- FFP
Fried Frailty Phenotype
- FFP-DASI
Fried Frailty Phenotype utilizing the Duke Activity Status Index questionnaire as the measure of low activity
- FFP-MLTA
Fried Frailty Phenotype utilizing the Minnesota Leisure Time Activity questionnaire as the measure of low activity
- FVC
forced vital capacity
- LAS
Lung Allocation Score
- LTBC
Lung Transplant Body Composition
- MLTA
Minnesota Leisure Time Activity Questionnaire
- SPPB
Short Physical Performance Battery
- SPRINTT
Sarcopenia and Physical Frailty in Older People: multicomponent treatment strategies Consortium
- TNF
Tumor Necrosis Factor
- UCSF
University of California, San Francisco
Footnotes
DISCLOSURE: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Valapour M, Lehr CJ, Skeans MA, Smith JM, Carrico R, Uccellini K, Lehman R, Robinson A, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant. 2018;18(Suppl 1):363–433. doi: 10.1111/ajt.14562. [DOI] [PubMed] [Google Scholar]
- 2.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, Grover FL. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 3.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Singer JP, Diamond JM, Gries C, McDonnough J, Blanc PD, Shah R, Dean YM, Hersch B, Dolan J, Arcasoy S, Ramphal K, Greenland JR, Smith N, Patterson S, Shah L, Golden JA, Blumenthal N, Huang D, Sonett J, Hays S, Oyster M, D’Ovidio F, Katz PP, Robbins H, Brown M, Leard L, Kukreja J, Bacchetta M, Bush E, D’Ovidio F, Rushefski M, Raza K, Christie JD, Lederer DJ. Frailty phenotypies, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. 2015;192:1325–1334. doi: 10.1164/rccm.201506-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Ageing. 2014;9:433–441. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Ambler GK, Brooks DE, Al Zuhir N, Ali A, Gohel MS, Hayes PD, Varty K, Boyle JR, Coughlin PA. Effect of frailty on short- and mid-term outcomes in vascular surgical patients. Br J Surg. 2015;102:638–645. doi: 10.1002/bjs.9785. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Archive Gerontol Geriatrics. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Sur. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, Mohr FW, Walther T. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Lahousse L, Ziere G, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, Tiemeier H, Joos GF, Hofman A, Ikram MA, Franco OH, Brusselle GG, Stricker BH. Risk of Frailty in Elderly With COPD: A Population-Based Study. J Gerontol A Biol Sci Med Sci. 2016;71:689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Sanchez FJ, Rodriguez-Adrada E, Vidan MT, Llopis Garcia G, Gonzalez Del Castillo J, Rizzi MA, Alquezar A, Pinera P, Lazaro Aragues P, Llorens P, Herrero P, Jacob J, Gil V, Fernandez C, Bueno H, Miro O. Impact of Frailty and Disability on 30-Day Mortality in Older Patients With Acute Heart Failure. Am J Cardiol. 2017 doi: 10.1016/j.amjcard.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 15.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, Jain V, Ros RL, James NT, Kucirka LM, Hall EC, Berger JC, Montgomery RA, Desai NM, Dagher NN, Sonnenday CJ, Englesbe MJ, Makary MA, Walston JD, Segev DL. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 16.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, Walston J, Segev DL. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha SR, Hannu MK, Chang S, Montgomery E, Harkess M, Wilhelm K, Hayward CS, Jabbour A, Spratt PM, Newton P, Davidson PM, Macdonald PS. The Prevalence and Prognostic Significance of Frailty in Patients With Advanced Heart Failure Referred for Heart Transplantation. Transplantation. 2016;100:429–436. doi: 10.1097/TP.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 19.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant. 2016;35:173–178. doi: 10.1016/j.healun.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Nastasi AJ, McAdams-DeMarco MA, Schrack J, Ying H, Olorundare I, Warsame F, Mountford A, Haugen CE, Fernandez MG, Norman SP, Segev DL. Pre-Kidney Transplant Lower Extremity Impairment and Post-Transplant Mortality. Am J Transplant. 2017 doi: 10.1111/ajt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 22.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin MR, Singer JP, Huang D, Sell J, Gonzalez WC, Pollack L, Maurer MS, D’Ovidio FF, Bacchetta M, Sonett JR, Arcasoy SM, Shah L, Robbins H, Hays SR, Kukreja J, Greenland JR, Shah RJ, Leard L, Morrell M, Gries C, Katz PP, Christie JD, Diamond JM, Lederer DJ. Refining Low Physical Activity Measurement Improves Frailty Assessment in Advanced Lung Disease and Survivors of Critical Illness. Ann Am Thorac Soc. 2017 doi: 10.1513/AnnalsATS.201612-1008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehejia RH, Wahba S. Causal Effects in Nonexperimental Studies: Reevaluating the Evaluation of Training Programs. J Am Statistic Assn. 1999;94:1053–1062. [Google Scholar]
- 25.Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. Stata Journal. 2002;2:358–377. [Google Scholar]
- 26.Valapour M, Skeans MA, Heubner BM, Smith JM, Schnitzler MA, Hertz MI, Edwards LB, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant. 2014;14(Suppl 1):139–165. doi: 10.1111/ajt.12584. [DOI] [PubMed] [Google Scholar]
- 27.Colvin M, Smith JM, Skeans MA, Edwards LB, Uccellini K, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Heart. Am J Transplant. 2017;17(Suppl 1):286–356. doi: 10.1111/ajt.14128. [DOI] [PubMed] [Google Scholar]
- 28.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Kucheryavaya A, Woodbury M, Snyder JJ, Kasiske BL, Israni AK. OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant. 2017;17(Suppl 1):21–116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17(Suppl 1):174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, Kucirka LM, Desai NM, Dagher NN, Lonze BE, Montgomery RA, Walston J, Segev DL. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2016 doi: 10.1097/SLA.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, Segev DL. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B, Salter M, Alachkar N, Desai N, Grams M, Walston J, Segev DL. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99:805–810. doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derck JE, Thelen AE, Cron DC, Friedman JF, Gerebics AD, Englesbe MJ, Sonnenday CJ. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation. 2015;99:340–344. doi: 10.1097/TP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 34.McAdams-DeMarco MA, Isaacs K, Darko L, Salter ML, Gupta N, King EA, Walston J, Segev DL. Changes in Frailty After Kidney Transplantation. J Am Geriatr Soc. 2015;63:2152–2157. doi: 10.1111/jgs.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breitling LP, Saum KU, Perna L, Schottker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenetics. 2016;8:21. doi: 10.1186/s13148-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Int Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 38.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 39.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–2361. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 43.Agusti A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, Crim C, Yates JC, Silverman EK, Coxson HO, Bakke P, Mayer RJ, Celli B Evaluation of CLtIPSEI. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PloS one. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley B, Brown KK, Collard HR. Molecular biomarkers in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L681–691. doi: 10.1152/ajplung.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 46.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 47.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, Network NA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Organ Procurement and Transplantation Network Allocation Calculators. 2017 Aug 24; Available from: https://optn.transplant.hrsa.gov/resources/allocation-calculators/
- 49.Cesari M, Landi F, Calvani R, Cherubini A, Di Bari M, Kortebein P, Del Signore S, Le Lain R, Vellas B, Pahor M, Roubenoff R, Bernabei R, Marzetti E Consortium S. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Research. 2017;29:81–88. doi: 10.1007/s40520-016-0716-1. [DOI] [PubMed] [Google Scholar]
- 50.Marzetti E, Calvani R, Landi F, Hoogendijk EO, Fougere B, Vellas B, Pahor M, Bernabei R, Cesari M Consortium S. Innovative Medicines Initiative: The SPRINTT Project. J Frailty Aging. 2015;4:207–208. [PMC free article] [PubMed] [Google Scholar]
- 51.Jha SR, Hannu MK, Gore K, Chang S, Newton P, Wilhelm K, Hayward CS, Jabbour A, Kotlyar E, Keogh A, Dhital K, Granger E, Jansz P, Spratt PM, Montgomery E, Harkess M, Tunicliff P, Davidson PM, Macdonald PS. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced heart failure referred for heart transplantation. J Heart Lung Transplant. 2016 doi: 10.1016/j.healun.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Smith PJ, Blumenthal JA, Carney RM, Freedland KE, O’Hayer CVF, Trulock EP, Martinu T, Schwartz TA, Hoffman BM, Koch GG, Davis RD, Palmer SM. Neurobehavioral functioning and survival following lung transplantation. Chest. 2014;145:604–611. doi: 10.1378/chest.12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–664. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castleberry AW, Englum BR, Snyder LD, Worni M, Osho AA, Gulack BC, Palmer SM, Davis RD, Hartwig MG. The Utility of Preoperative Six-Minute Walk Distance in Lung Transplantation. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201409-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldwin MR, Arcasoy SM, Shah A, Schulze PC, Sze J, Sonett JR, Lederer DJ. Hypoalbuminemia and early mortality after lung transplantation: a cohort study. Am J Transplant. 2012;12:1256–1267. doi: 10.1111/j.1600-6143.2011.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelm DJ, Bonnes SL, Jensen MD, Eiken PW, Hathcock MA, Kremers WK, Kennedy CC. Pre-transplant wasting (as measured by muscle index) is a novel prognostic indicator in lung transplantation. Clin Transplant. 2016;30:247–255. doi: 10.1111/ctr.12683. [DOI] [PubMed] [Google Scholar]
- 57.Weig T, Milger K, Langhans B, Janitza S, Sisic A, Kenn K, Irlbeck T, Pomschar A, Johnson T, Irlbeck M, Behr J, Czerner S, Schramm R, Winter H, Neurohr C, Frey L, Kneidinger N. Core Muscle Size Predicts Postoperative Outcome in Lung Transplant Candidates. Ann Thorac Surg. 2016;101:1318–1325. doi: 10.1016/j.athoracsur.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 58.Latham NK, Harris BA, Bean JF, Heeren T, Goodyear C, Zawacki S, Heislein DM, Mustafa J, Pardasaney P, Giorgetti M, Holt N, Goehring L, Jette AM. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311:700–708. doi: 10.1001/jama.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abizanda P, Lopez MD, Garcia VP, de Estrella JD, da Silva Gonzalez A, Vilardell NB, Torres KA. Effects of an Oral Nutritional Supplementation Plus Physical Exercise Intervention on the Physical Function, Nutritional Status, and Quality of Life in Frail Institutionalized Older Adults: The ACTIVNES Study. J Am Med Dir Assoc. 2015;16:439 e439–439 e416. doi: 10.1016/j.jamda.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Fragala MS, Dam TT, Barber V, Judge JO, Studenski SA, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Kiel DP, Kritchevsky SB, Shardell MD, Vassileva MT, Kenny AM. Strength and function response to clinical interventions of older women categorized by weakness and low lean mass using classifications from the foundation for the national institute of health sarcopenia project. J Gerontol A Biol Sci Med Sci. 2015;70:202–209. doi: 10.1093/gerona/glu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singer JP, Soong A, Bruun A, Hays S, Kukreja J, Bracha A, Chin G, Wolters PJ, Peters M, Garvey CM. Pre-Habilitation” of Frail Candidates for Lung Transplantation Using a Mobile Health Enabled Home-Based Intervention Is Feasible and Safe. Am J Resp Crit Care Med. 2017;195:A2342. (abstract) [Google Scholar]
- 62.Cheng XS, Myers JN, Chertow GM, Rabkin R, Chan KN, Chen Y, Tan JC. Prehabilitation for kidney transplant candidates: Is it time? Clinical transplantation. 2017:31. doi: 10.1111/ctr.13020. [DOI] [PubMed] [Google Scholar]
- 63.Abdullah HR, Lien VP, Ong HK, Er PL, Hao Y, Khan SA, Liu CW. Protocol for a single-centre, randomised controlled study of a preoperative rehabilitation bundle in the frail and elderly undergoing abdominal surgery. BMJ Open. 2017;7:e016815. doi: 10.1136/bmjopen-2017-016815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rumer KK, Saraswathula A, Melcher ML. Prehabilitation in our most frail surgical patients: are wearable fitness devices the next frontier? Curr Opin Organ Transplant. 2016;21:188–193. doi: 10.1097/MOT.0000000000000295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.