Abstract
The impact of a new national kidney allocation system (KAS) on access to the national deceased-donor waiting list (waitlisting) and racial/ethnic disparities in waitlisting among U.S. end stage renal disease (ESRD) patients is unknown. We examined waitlisting pre- and post-KAS among incident (N=1,253,100) and prevalent (N=1,556,954) ESRD patients from the United States Renal Data System database (2005–2015) using multivariable time-dependent Cox and interrupted time-series models. The adjusted waitlisting rate among incident patients was 9% lower post-KAS (HR: 0.91; 95% CI: 0.90–0.93), although preemptive waitlisting increased from 30.2% to 35.1% (p<0.0001). The waitlisting decrease is largely due to a decline in inactively waitlisted patients. Pre-KAS, blacks had a 19% lower waitlisting rate vs. whites (HR: 0.81; 95% CI 0.80–0.82); following KAS, disparity declined to 12% (HR: 0.88; 95% CI: 0.85–0.90). In adjusted time-series analyses of prevalent patients, waitlisting rates declined by 3.45/10,000 per month post-KAS (p<0.001), resulting in ~146 fewer waitlisting events/month. Shorter dialysis vintage was associated with greater decreases in waitlisting post-KAS (p<0.001). Racial disparity reduction was due in part to a steeper decline in inactive waitlisting among minorities and a greater proportion of actively waitlisted minority patients. Waitlisting and racial disparity in waitlisting declined post-KAS; however, disparity remains.
Introduction
Kidney transplantation is generally preferred over dialysis for treatment of end-stage renal disease (ESRD) because it is associated with substantially better outcomes than dialysis.(1) However, there is a severe shortage of organs for transplantation, forcing organ allocation policies to balance both equity and utility in their design (2). Many countries established their own deceased kidney organ allocation system to strike a balance between efficient use of and equal access to the deceased organs for deceased donor transplantation (2). In the United States, only ~15% of ESRD patients were waitlisted for kidney transplantation, and ~19,000 patients received a transplant in 2016 among the 103,114 people currently on the waitlist. (3, 4) Despite policy regulations of the Department of Health and Human Services that require organs to be allocated equitably,(5) there are racial and ethnic disparities at each step of the kidney transplant process. Black patients are less likely to be referred for transplant, (6) complete the evaluation process if referred,(7) be placed on the waiting list,(7, 8) and receive a transplant(9–16) compared to white patients. In addition, Hispanics have historically had lower transplant rates after waitlisting(17). Disparities are the result of many potential factors, such as poverty,(18–20) geography, (8, 20, 21) limited education about transplant,(15, 22) physician bias,(23) and other system-level factors, such as federal policies that guide US organ allocation.(24, 25)
The new kidney allocation system (KAS)(26) was implemented by the United Network for Organ Sharing in December 2014 in part to improve equity related to dialysis time, and to the group of patients with high panel reactive antibody.(27) More specifically, changes that were likely to benefit minority patients include the change in the calculation of waiting time, which now starts at dialysis start instead of waitlist, and prioritization of the most sensitized patients, who are disproportionately more likely to be minorities. Changes in allocation priority were intended to improve kidney allocation disparities among different race/ethnicities on the waiting list.(28) Prior to KAS implementation, simulations predicted that black vs. white racial disparities in transplantation among patients already on the transplant waiting list would decrease by 6% within 1 year.(3, 29) Subsequent research has confirmed these hypotheses, where racial/ethnic disparities in deceased-donor transplant among waitlisted patients have been at least temporarily eliminated.(30, 31) However, it is unknown how the new KAS policy may have influenced access to the waiting list. It is possible that the implementation of KAS led to increased referrals for transplant evaluation and higher waitlisting, particularly among patients with longer time on dialysis who were not previously referred or waitlisted for transplant. Because minorities tend to spend longer on dialysis prior to referral for transplantation,(32) the policy change may have differential effects by racial/ethnic group. However, it is also possible that the policy has reduced the sense of urgency to refer some patients who may have only recently started dialysis. Delayed referral to transplant for minority patients could further exacerbate the racial/ethnic disparities in living donor transplantation, the optimal treatment for ESRD patients. (33) Thus, the influence of KAS on waitlisting and waitlisting disparities may differ between the incident and prevalent ESRD populations.
The aims of this study were (1) to assess the impact of the 2014 KAS policy change on waitlisting overall, and (2) to evaluate whether racial/ethnic disparities in waitlisting in the United States changed following the policy’s implementation. We performed two types of analyses to achieve these aims: (1) a time-to-event analysis to examine how KAS affected time from dialysis start to waitlisting among the incident ESRD population, and (2) a trend analysis to examine how the new KAS policy affected monthly waitlisting rates among prevalent (existing) dialysis patients not already on the waiting list.
Materials and Methods
Data sources
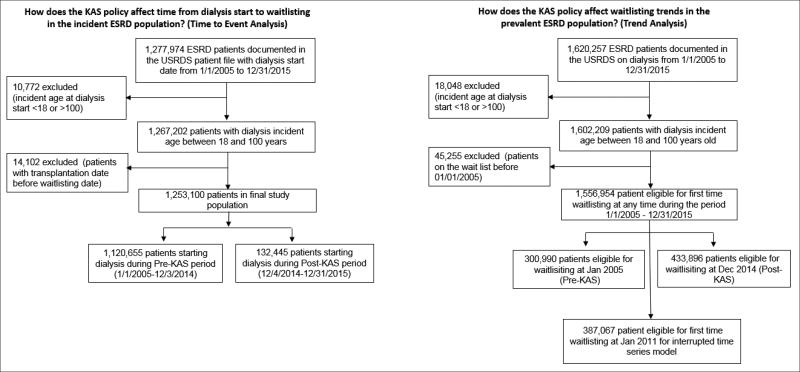
To address both study aims, we constructed both an incident and a prevalent (i.e., patients already on dialysis) ESRD patient cohort using the United States Renal Data System (USRDS) (Figure 1). Because USRDS data do not contain detailed information about active and inactive status of incident waitlisting, we also examined United Network for Organ Sharing (UNOS) system data (34).
Figure 1. Definition of the incident and prevalent U.S. ESRD patient populations used for study aims, 2005–2015.
Abbreviations: ESRD, End stage renal disease; KAS, kidney allocation system
Note: The waitlisting date for preemptive waitlisting patients was considered as the dialysis starting date for both incident and prevalent ESRD patients.
The incident study population included a retrospective longitudinal cohort of 1,267,202 adult (age ≥18 years) patients who started dialysis from 2005 through 2015 (the most recent data available from USRDS). Patients who received a transplant prior to waitlisting were excluded from the cohort (n=14,102; 1.1%). The final cohort included 1,253,100 patients. Six percent of patients were waitlisted before starting dialysis (i.e. “preemptive waitlisting”) and were included in analyses with their time to waitlisting coded as one day. Patients beginning dialysis from 1/1/2005 to 12/03/2014 (policy implementation date) were included in the pre-KAS group (n=1,120,655), while patients starting dialysis from 12/4/2014 to 12/31/2015 were included in the post-KAS group (n=132,445). In a corresponding cohort within the UNOS database (2005–2015), we examined 277,554 newly waitlisted patients for inactive vs. active waitlisting information.
The prevalent dialysis cohort included adult (age ≥18 years) ESRD patients eligible to be waitlisted for the first time between 2005 and 2015, i.e., patients on dialysis and not yet waitlisted at any point during the period (N= 1,556,954). For patients with more than one waitlisting event, we captured only the earliest event.
Study variables
In the incident ESRD population, the main study outcome (event) was placement on the national deceased donor waiting list (i.e., waitlisting) overall, including both active and inactive waitlisting, and the secondary outcome was the racial/ethnic disparity in waitlisting after the KAS policy. Outcomes were obtained from USRDS standard analytic files, which are administratively linked with United Network for Organ Sharing data on waitlisting and transplantation. The main exposure variables were policy era (pre- and post-KAS; defined above) and race/ethnicity. We defined five racial and ethnic groups: white (non-Hispanic), black (non-Hispanic), Hispanic, Asian and other (including American Indian/Alaska native and Native Hawaiian/other Pacific Islander).
Among incident ESRD patients, we examined information collected from the Centers for Medicare & Medicaid Services (CMS) 2728 Form captured within USRDS at time of ESRD start.(35) Variables included age, sex, race/ethnicity, and clinical data, including assigned cause of ESRD; body mass index (BMI); history of tobacco use, cancer, congestive heart failure, atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease, hypertension, diabetes, and chronic obstructive pulmonary disease; and whether the patient was informed of kidney transplant options at ESRD start. Socioeconomic characteristics assessed included health insurance, whether the patient had pre-ESRD nephrology care, and patient neighborhood (ZIP code) poverty derived from the 2007–2011 American Community Survey,(36) which was linked to surveillance data by patient ZIP code at ESRD start. Results for the “other” racial/ethnic group were included in summary statistics for “all patients”, but are not reported separately because this group was too heterogeneous to make meaningful conclusions about the policy impact.
In the prevalent ESRD cohort of patients already on dialysis, we calculated the monthly waitlisting incident rate by dividing the number of first waitlisting events by the number of patients eligible for waitlisting. Patients were defined as eligible for waitlisting in a given month if their ESRD start date was before the end of the month, and the death date, transplant date, or first waitlisting date was after the first day of the month. Results were stratified by dialysis vintage, i.e., the time from dialysis start to earliest date of waitlisting, death or the end of the given month, because we hypothesized that KAS may have a differential impact on those with shorter vs. longer dialysis times.
Statistical Analyses
In the incident ESRD cohort, we compared demographic and clinical characteristics before and after KAS using chi-square tests for categorical variables and t-tests for continuous variables. The incidence rate per person-year was defined as the number of incident ESRD patients waitlisted in each year divided by person-years accumulated before being waitlisted by incident ESRD patients for each year. We used multivariable Cox models with pre- vs. post-KAS modeled as a time-dependent covariate to assess the effect of KAS on time to waitlisting. Patients were censored at death (N=667,509, 53%) or end of follow-up (12/31/2015). To examine whether the effect of KAS on time to waitlisting differed by race/ethnicity, we included an interaction term for race/ethnicity. Missing values for covariates were imputed using the median of each variable (missing proportions detailed in Table 1) in multivariable models; 254,189 (20%) observations had at least one covariate imputed.
Table 1.
Demographic and clinical characteristics of the incident U.S. ESRD patient cohort by race/ethnicity, before (1/1/2005–12/03/2014) and after (12/04/2014–12/31/2015) the Kidney Allocation System (KAS) policy change
| Characteristics at the time of ESRD start | Pre-KAS N=1,120,655 (89.4%) |
Post-KAS N=132,445 (10.6%) |
P-value for KAS difference |
|---|---|---|---|
| Age group, N (%) | <0.001 | ||
| 18–39 | 84,411(7.5) | 9,609(7.3) | |
| 40–49 | 119,888(10.7) | 13,718(10.4) | |
| 50–59 | 222,520(19.9) | 26,147(19.7) | |
| 60–69 | 278,676(24.9) | 36,033(27.2) | |
| >=70 | 415,160(37.0) | 46,938(35.4) | |
| Male, N (%) | 635,986(56.8) | 77,040(58.2) | <0.001 |
| Race/ethnicity | <0.001 | ||
| White, non-Hispanic | 585223 (52.1) | 67,518 (51.0%) | |
| Black, non-Hispanic | 297067 (26.5) | 33171 (25.1) | |
| Hispanic | 153,690(13.7) | 18123(13.7) | |
| Asian | 47033 (4.2) | 6352(4.8) | |
| Other | 37642 (3.4) | 7281(5.5) | |
| Assigned ESRD cause, N (%) | <0.001 | ||
| Diabetes | 495,202(44.2) | 60,335(45.6) | |
| Hypertension | 317,187(28.3) | 37,382(28.2) | |
| Glomerulonephritis | 91,403(8.2) | 9,643(7.3) | |
| Other | 216,863(19.4) | 25,085(18.9) | |
| BMI ≥ 35 kg/m2, N (%) | 206,082(18.4) | 26,141(19.7) | <0.001 |
| History of comorbid conditions | |||
| Congestive heart failure, N (%) | 345,193(31.5) | 36,170(28.6) | <0.001 |
| Atherosclerotic heart disease, N (%) | 206,146(18.8) | 17,556(13.9) | <0.001 |
| Other cardiac disease, N (%) | 185,101(16.9) | 25,088(19.8) | <0.001 |
| Chronic obstructive pulmonary disease, N (%) | 101,289(9.2) | 11,610(9.2) | 0.37 |
| Peripheral vascular disease, N (%) | 143,726(13.1) | 13,000(10.3) | <0.001 |
| Smoker, N (%) | 68,018(6.2) | 7,606(6.0) | 0.005 |
| Diabetes, N (%) | 584,247(53.3) | 73,594(58.1) | <0.001 |
| Cancer, N (%) | 80,555(7.4) | 8,817(7.0) | <0.001 |
| Hypertension, N (%) | 937,681(85.6) | 110,762(87.5) | <0.001 |
| Patient informed of kidney transplant option, N (%) | 792,946(76.4) | 107,389(86.4) | <0.001 |
| Socioeconomic Status Indicators | |||
| Insurance, N (%) | <0.001 | ||
| Medicaid | 410,724(37.5) | 56,071(44.3) | |
| Medicare | 277,876(25.4) | 32,966(26.0) | |
| Employer | 251,765(23.0) | 23,839(18.8) | |
| Other | 76,133(7.0) | 8,419(6.7) | |
| No insurance | 78,693(7.2) | 5,271(4.2) | |
| Pre-ESRD nephrology care, N (%) | 620,617(67.1) | 79,870(73.5) | <0.001 |
| Patient Neighborhood (ZIP Code) poverty category, N (%) | <0.001 | ||
| 0–4% | 155,239(14.1) | 18,456(14.2) | |
| 5%–9% | 262,215(23.8) | 31,265(24.0) | |
| 10%–14% | 223,042(20.2) | 26,723(20.5) | |
| 15%–19% | 174,329(15.8) | 20,832(16.0) | |
| >=20% | 286,800(26.0) | 33,067(25.4) |
Abbreviations: ESRD, End stage renal disease; KAS, kidney allocation system; BMI, body mass index
Note: The proportion of missing values for variables are as follows: pre-ESRD nephrology care (17.6%); informed of kidney transplant option (7.3%); atherosclerotic heart disease (2.48%); congestive heart failure (2.49%); other cardiac disease (2.49%); hypertension (2.49%); diabetes (2.48%); smoking (2.49%); cancer (2.49); insurance (2.5%); patient neighborhood poverty category (1.68%);. Observations with missing values were excluded from the descriptive statistics for each variable. Missing values were imputed using the median of each variable in multivariable models.
In the prevalent patient cohort, we examined the monthly count of new waitlisting events and the monthly incidence rate of new waitlisting events among the study population from 2005 to 2015. We used interrupted time series models(37) to evaluate the change in both the absolute count and rate of new waitlisting events among patients on dialysis pre- and post-KAS. The model includes a pre-KAS time trend, a KAS indicator variable, and a post-KAS time trend. The coefficient for the KAS indicator estimates the immediate change in the waitlisting rate due to KAS. The coefficient for the post-KAS time trend assesses how KAS altered the trend.
Results from the interrupted time series model were sensitive to the length of pre-intervention periods included for modeling. The length of this period should be neither too short, which may not reflect the long-term trend, nor too long, which may mask the relevant pre-intervention trend.(37) We selected 2011 to 2015 for the time-series analysis, but explored other time periods (e.g. 2012–2015) in sensitivity analyses.
Finally, we examined monthly counts and proportions of new waitlisting events from 2005 to 2015, stratified by waitlist status (active vs. inactive at time of first waitlisting) and KAS era using UNOS data.
Sensitivity analyses
Since the implementation of KAS may have had a differential effect on patients who received a living donor transplant after waitlisting, we performed a sensitivity analysis excluding patients who received a living donor transplant within 180 days of waitlisting (N=17,845; 1.4%) among the incident cohort. We conducted a further sensitivity analysis excluding patients with a history of cancer (N=120,635, 9.6%) and excluding those who died within 30 days after dialysis began (N=31,160; 2.48%), since these patients are less likely eligible for transplantation.
Results
Waitlisting among incident ESRD patients
A total of 1,253,100 ESRD patients started dialysis between 2005 and 2015 (Table 1), including 1,120,655 patients (89.4% of study population) in the pre-KAS period (52.1% white, 26.5% black, 13.7% Hispanic and 4.2% Asian) and 132,445 patients in the post-KAS period (51.0% whites, 25.1% black, 13.7% Hispanic, and 4.8% Asian). The annual incidence rate of waitlisting increased from 17.7 per 100 person-years in 2005 to 22.7 per 100 person-years in 2014, but slightly declined in 2015 (22.4 per 100 person years) for all racial/ethnic groups (SFigure 1).
White patients represented about half (47.0%) of incident ESRD patients waitlisted in the pre-KAS era; this declined to 45.1% in the post-KAS period (p<0.001) (STable 1). The proportion of preemptively waitlisted patients (patients waitlisted prior to dialysis) increased after KAS for all racial/ethnic groups (pre-KAS: 30.2% vs post-KAS: 35.1%, p<0.001). There were declines in the share of patients with several comorbidities, such as congestive heart failure (pre-KAS: 13.2% vs post-KAS: 11.2%), atherosclerotic heart disease (pre-KAS: 9.0% vs post-KAS: 6.5%) and peripheral vascular disease (pre-KAS: 5.3% vs post-KAS: 4.0%). The proportion of patients with diabetes (pre-KAS: 42.0% vs post-KAS: 44.4%), pre-ESRD nephology care (pre-KAS: 78.3% vs post-KAS: 80.1%), and patients with Medicaid (pre-KAS: 16.6% vs post-KAS: 21.4%) increased after KAS implementation (STable 1; all p<0.001).
After adjusting for potential confounders (Table 2), the waitlisting rate was 9% lower in the post-KAS era (HR: 0.91; 95% CI: 0.90–0.93) for all ESRD patients. Declines in waitlisting from pre- to post-KAS were observed among all racial/ethnic groups, including white (HR: 0.89; 95% CI 0.87–0.91), black (HR: 0.96; 95% CI 0.93–0.98), Hispanic 0.90 (95% CI 0.87–0.93), and Asian (HR: 0.92; 95% CI 0.87–0.97) patients. A race/ethnicity and KAS era interaction was significant (p<0.0001).
Table 2.
Impact of new Kidney Allocation System (KAS) policy on waitlisting and racial/ethnic disparities among U.S. incident ESRD patients 2005–2015
| Crude Hazard Ratio, (95% CI) |
Adjusted Hazard Ratio1, (95% CI) |
|
|---|---|---|
| Impact of KAS on waitlisting (post-KAS vs pre-KAS) | ||
| Among all patients | 0.93 (0.92–0.94) | 0.91(0.90–0.93) |
| Among racial/ethnic subgroups: | ||
| White | 0.92 (0.90–0.94) | 0.89(0.87–0.91) |
| Black | 0.94(0.92–0.97) | 0.96(0.93–0.98) |
| Hispanic | 0.90(0.87–0.93) | 0.90(0.87–0.93) |
| Asian | 0.90(0.85–0.94) | 0.92(0.87–0.97) |
| Racial/ethnic differences in waitlisting by pre- and post-KAS periods | ||
| Pre-KAS period | ||
| White | Reference [1] | Reference [1] |
| Black | 0.98 (0.97–0.99) | 0.81 (0.80–0.82) |
| Hispanic | 1.21 (1.20–1.23) | 1.07 (1.06–1.09) |
| Asian | 1.60 (1.57–1.63) | 1.20 (1.18–1.22) |
| Post-KAS period | ||
| White | Reference [1] | Reference [1] |
| Black | 1.00 (0.97–1.03) | 0.88 (0.85–0.90) 2 |
| Hispanic | 1.17 (1.13–1.21) | 1.08 (1.04–1.12) 3 |
| Asian | 1.55 (1.48–1.63) | 1.23 (1.17–1.30) 4 |
Abbreviations: KAS, kidney allocation system
The variables included in the time-dependent Cox model included race/ethnicity, age at ESRD start, sex, cause of ESRD, BMI, tobacco use, cancer, congestive heart failure, atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, whether or not a patient had been informed of kidney transplant options, type of health insurance, whether or not a patient had pre-ESRD nephrology care and patient neighborhood (ZIP Code) poverty, Missing values (details in Table 1) were imputed using the median of each variable. A total of 254,189 (20%) of observations in model that had at least one covariate imputed.
The p-value for the black vs. white racial/ethnic disparity reduction between pre-KAS and post-KAS periods: p<0.001
The p-value for the Hispanic vs. white racial/ethnic disparity reduction between pre-KAS and post-KAS periods: p=0.62
The p-value for the Asian vs. white racial/ethnic disparity reduction between pre-KAS and post-KAS periods: p=0.27
Change in racial/ethnic disparity in waitlisting among incident ESRD patients
Prior to KAS, the rate of waitlisting was 19% lower among black vs. white patients (HR: 0.81; 95% CI 0.80–0.82). Following KAS, the racial difference declined (p<0.001) to 12% (HR: 0.88; 95% CI 0.85–0.90). The rate of waitlisting for Hispanics (vs. whites) was 7% higher (HR: 1.07; 95% CI 1.06–1.09) pre-KAS and 8% higher post-KAS (HR: 1.08; 95% CI 1.04–1.12); this difference was not significant (p=0.62). The hazard ratio comparing waitlisting among Asian patients with whites was 1.20 (95% CI 1.18–1.22) pre-KAS, and 1.23 (95% CI 1.17–1.30) post-KAS, but this difference was not significant (p=0.27) (Table 2).
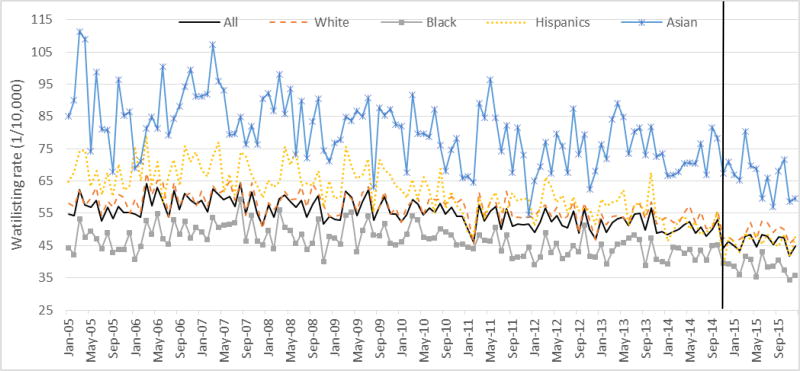
Waitlisting among prevalent dialysis patients
The number of prevalent dialysis patients who had not been waitlisted increased from 300,990 (45% white, 33% black, 13% Hispanic and 4% Asian) in January 2005 to 450,027 (43% white, 31% black, 16% Hispanic and 5% Asian) in December 2015, (Figure 2). On average, the monthly rate of waitlisting was 55.0 per 10,000 prevalent ESRD patients (57.1/10,000 for white, 46.7/10,000 for black, 62.0/10,000 for Hispanic, and 80.6/10,000 for Asian patients; p<0.001) in the pre-KAS era. In the post-KAS era, monthly waitlisting rates declined to 46.1/10,000 (48.9/10,000 for white, 38.4/10,000 for black, 46.4/10,000 for Hispanic, and 66.4/10,000 for Asian patients; p<0.001), respectively.
Figure 2. Monthly rate of new waitlisting events per 10,000 prevalent dialysis patients in the U.S. from 2005 to 2015.
Note: The vertical line indicates the introduction of the new Kidney Allocation System on December 4, 2014
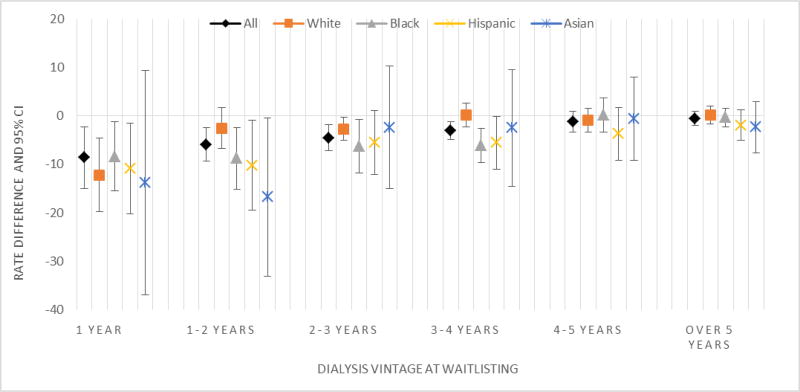
After adjusting for time trends in the interrupted time series analyses, the monthly count of new waitlisting events decreased by 146 events (95% CI: 41–250; p<0.001) from the pre-to post-KAS eras (SFigure2, STable 3). The monthly waitlisting rate also decreased by 3.45/10,000 (95% CI: 0.9–6.0) prevalent ESRD patients overall (p<0.001), including 3.57/10,000 for whites (95% CI: 0.62–6.51; p=0.017), 3.50/10,000 for blacks (95% CI: 0.79–6.21; p=0.011), 4.56/10,000 for Hispanics (95% CI: 0.38–8.74; p=0.03) and 5.43/10,000 for Asians (95% CI: −1.82–12.69; p=0.13) from the pre-to post-KAS eras (STable 3). Shorter dialysis vintage was associated with greater decreases in waitlisting post- vs. pre-KAS, and declined in a stepwise manner (p<0.001). For example, the average adjusted monthly waitlisting rate among prevalent patients whose dialysis vintage was >5 years was similar before and after KAS (−0.51/10,000; 95% CI: −1.98, 0.95) but among patients with < 1 year on dialysis, rates were significantly lower than those prior to KAS (−8.59/10,000; 95% CI: −14.95, −2.23) (Figure 3).
Figure 3. Change in adjusted rate of waitlisting among prevalent ESRD patients in the United States post-KAS (vs pre-KAS) by patient time on dialysis, derived from interrupted time series models, 2011–2015.
Abbreviations: ESRD, End stage renal disease; KAS, kidney allocation system
Note: The interrupted time series regression model adjusted for time trends. The coefficient represented in the Y axis represents the estimated change in waitlisting rate associated with KAS, after adjusting for time trends. Negative coefficients indicate declines in waitlisting. Patients who were waitlisted prior to dialysis start were excluded from these analyses. P-values for trend test for the association of change in rate of waitlisting by dialysis vintage is as follows: all patients (p<0.0001); white (p=0.058); black (p=0.011); Hispanic (p=0.002); and Asian (p=0.052).
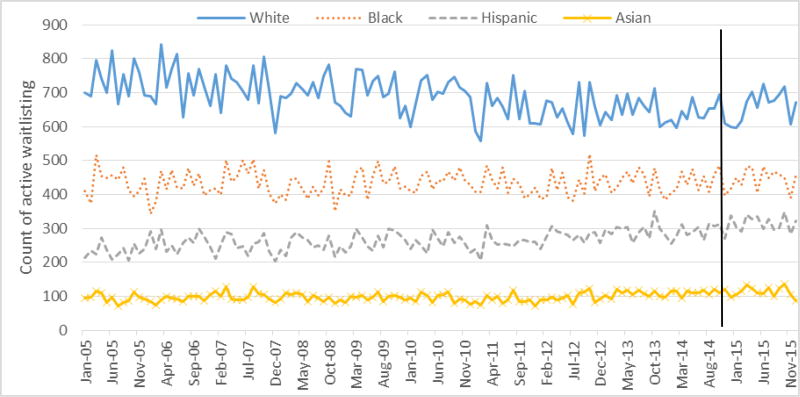
Active vs. Inactive Waitlisting
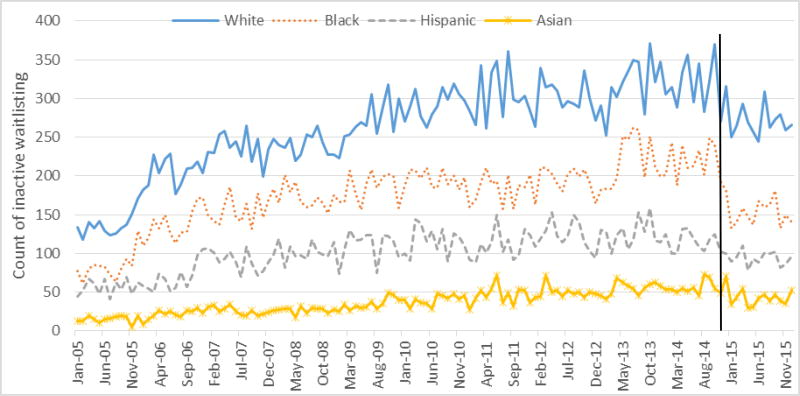
A total of 277,554 (90.7% for pre-KAS and 9.3% for post-KAS) first-time adult waitlisting events were reported in UNOS data from 2005 to 2015. The proportion of new actively waitlisted patients in the pre-KAS era was 72.1% (72.3% for white, 71.3% for black, 72.2% for Hispanics, and 72.7 for Asian, p<0.001), and this increased to 73.5% post-KAS (71.4% for white, 76.3% for black, 78.0% for Hispanics, and 73.5 for Asian, p<0.001). Active waitlisting counts were similar pre- and post-KAS (Figure 4; p=0.601). Inactive waitlisting counts were significantly lower post- vs. pre-KAS (Figure 5, p<0.001), and there was a greater decline in inactive waitlisting counts among black and Hispanic patients (p<0.0001).
Figure 4. Monthly time series of the count of new active waitlisting events by racial/ethnic groups from United Network for Organ Sharing Data, 2005 to 2015.
Note: The vertical line indicates the introduction of the new Kidney Allocation System on December 4, 2014.
Figure 5. Monthly time series of the count of new inactive waitlisting events by racial/ethnic groups from United Network for Organ Sharing Data, 2005 to 2015.
Note: The vertical line indicates the introduction of the new Kidney Allocation System on December 4, 2014.
Sensitivity analysis
In the sensitivity analyses excluding the 1.4% of patients who had a living donor transplant within 180 days after waitlisting, the sensitivity analyses excluding patients with a history of cancer (N=120,635, 9.6%), and the sensitivity analysis excluding patients who died within 30 days after dialysis began (N=31,160; 2.48%), results were similar to main analyses (STable 2).
Discussion
After the implementation of a major change in the national KAS in December 2014, the rate of waitlisting declined among both incident and prevalent dialysis patients. There was a 9% decline in overall waitlisting among incident ESRD patients, including an 11% decline among whites, a 10% decline among Hispanics, an 8% decline among Asians and a 4% decline among African Americans. There were nearly 150 fewer waitlisting events per month among prevalent dialysis patients across the United States. This decline in waitlisting was primarily due to a decline in inactive waitlisting, likely due to the limited incentive under KAS to waitlist patients as early as possible. This could be interpreted as a positive result of KAS, as it could result in a reduction of both patient and clinician time as well as a reduction in costs and limited resources of transplant centers. However, it is also possible that a decline in overall waitlisting could indicate reduced referral for transplantation, which could be problematic if this limits the early exploration of living donor transplantation as a treatment option. We also observed that the black vs. white racial disparity in waitlisting declined from 19% lower rate of waitlisting pre-KAS to 12% lower rate of waitlisting in the post-KAS era, in part due to a higher proportion of new active waitlisting among black but not white patients. However, black vs. white racial disparities in waitlisting still persist in the United States, suggesting that additional interventions may be needed to ensure that all patients, and particularly minorities, are encouraged to pursue kidney transplantation as a treatment option.
A major goal of the new national kidney allocation policy was to increase equity in kidney transplantation across racial/ethnic groups. The share of minority transplant recipients increased after KAS.(30, 38) Our study extends these previous findings by showing how the policy influenced waitlisting. There are several potential explanations for why there was a reduction in the black vs. white disparity in waitlisting following the policy change. For the patients with prevalent ESRD, black patients accounted for a larger proportion of prevalent patients with long dialysis times who were yet to be waitlisted or waitlisted long after starting renal replacement therapy and thus had a higher chance of transplantation under the new KAS policy prioritization. For incident patients, the new allocation system reduces the urgency for transplant centers to waitlist incident dialysis patients, and this may disproportionately impact white patients who have traditionally been more likely to be waitlisted soon after starting dialysis. Our data suggest that there was a larger increase in the proportion of minority patients actively waitlisted post-KAS, which may partially explain these findings. Moreover, education and pre-ESRD nephrology care as reported on the CMS-2728 form improved after KAS implementation in a similar fashion for both racial groups. The proportion of patients informed of kidney transplant as a treatment option increased from pre- to post-KAS for both white (73.9% to 84.4%) and black (79.0% to 88.5%) patients. Pre-ESRD nephrology care also increased among both white (70.5% to 76.4%) and black (63.5% to 70.4%) patients. The similar improvement in these factors for both groups would be expected to decrease the waitlisting disparity, because the disparity was assessed as a ratio (a hazard ratio) in this analysis rather than a difference. It also may be that a per-unit increase in these factors is stronger at lower levels, leading to a greater increase in waitlisting among blacks compared with whites.
In other group-wise comparisons, we found that waitlisting for Hispanics was higher than for whites both before and after KAS. These results are similar to previous findings showing no ethnic disparities in waitlisting, but lower rates of transplantation among Hispanics once waitlisted.(17, 32) In our study, Hispanics had a higher overall incidence of waitlisting but a slower time to waitlisting than white patients. Other analyses(30) have also reported that KAS increased transplant rates for Hispanics after waitlisting, thus reducing the overall ethnic disparity in transplant access. We also report that waitlisting for Asian patients decreased after KAS, but this group still had the highest waitlisting rate among all racial/ethnic groups, as previously reported.(3) Importantly, when comparing characteristics of waitlisted patients in pre- and post- KAS eras, we found that more ESRD patients with Medicaid were waitlisted after KAS (21.4%) than before (16.6%). This result is consistent with previous reports showing that Medicaid expansion was associated with higher levels of waitlisting and transplantation within 1 year of ESRD,(39) suggesting that disparities in waitlisting by health insurance status may also have been reduced.
Although early referral after the initiation of dialysis no longer gives patients an advantage on the waiting list, it can lead to earlier transplantation for patients with living donors and those who are highly sensitized. This is particularly important for minorities, who have substantially lower rates of living donor transplantation(40) and higher rates of sensitization(41) than white patients. More investment should be made in the early interventions leading to appropriate referral for transplantation. Additional years of follow-up data are needed to examine how declines in waitlisting following KAS may influence access to or delay living donor transplant for patients, and any impact on disparities in living donor transplant rates.
Without provider-level data, it is difficult to know the extent to which KAS has changed clinician behavior. In a recent study of ~650 dialysis facility providers, nearly 30% of dialysis social workers and 15% of dialysis medical directors were unaware of the KAS policy change,(42) but it is unclear how KAS may have impacted transplant center waitlisting practices not captured in surveillance data. Future research should examine how KAS influenced physician referral of patients for transplant evaluation.
This study has limitations. First, the study has only approximately 1 year of follow-up data after KAS implementation, which is the most recent data available from USRDS. The other major national kidney registry, UNOS, has more recent data on waitlisting events, but does not take into account the changing denominator population of incident or prevalent dialysis patients prior to waitlisting. In addition, the decline in waitlisting for ESRD patients has been further confirmed by the recent release of the national United States Renal Data System report.(43) Additional follow-up data are needed to confirm longer-term effects of the policy. Second, the national surveillance data used for the study did not have contextual information such as dialysis facility staff perspectives on the impact of the policy on their referral patterns, transplant center waitlisting policies, and patient perspectives. These data also do not contain information on which patients were eligible for transplantation. Our study thus assumes there was no racial/ethnic difference in transplant eligibility from pre- to post-KAS. Third, we were unable to assess the impact of the policy on smaller racial/ethnic groups, as the “other” category of patients was too small and heterogeneous to make meaningful conclusions about the policy’s impact. Other limitations include missing covariate data and the potential for residual confounding due to unavailable covariate data in the USRDS database, such as income, that could influence access to waitlisting and racial/ethnic disparities. In addition, unmeasured time-varying confounding could have occurred if other factors impacted access to waitlisting at around the same time as KAS. These factors may have included transplant center behavior change due to increased monitoring of waitlist deaths by the Scientific Registry of Transplant Recipients (SRTR).
Our study also has several strengths. The study population was large and diverse and examines the impact of a major policy change for both incident and prevalent ESRD patients. Results are generalizable to all treated U.S. ESRD patients, as USRDS is a near complete representation of these patients. Second, follow-up data on outcomes of waitlisting are nearly complete, so validity is unlikely to be threatened by selection bias. Third, availability of individual-level follow-up data allowed for estimation of individual-level time to waitlisting and its potential variation by important socio-demographic factors.
Overall, the likelihood of waitlisting for all racial/ethnic groups significantly declined after the new national KAS, and this was primarily due to declines in inactive waitlisting and waitlisting among those with lower time on dialysis. Racial disparities between in waitlisting for kidney transplantation were reduced between black and white ESRD patients after the implementation of KAS in December of 2014 in part to a steeper decline in inactive waitlisting among minorities and a greater proportion of actively waitlisted minority patients; however, black vs. white disparities in waitlisting remain.
Supplementary Material
Table S1: Waitlisting among incident ESRD patients by race/ethnicity, before (01/01/2005–12/03/2014) and after (12/04/2014–12/31/2015) the Kidney Allocation System (KAS) policy change
Table S2: Sensitivity analysis of effect of KAS and race/ethnicity on waitlisting among the incident ESRD patient cohort when (1) patients with a living donor transplant within 180 days were excluded, (2) patients with a history of cancer, (3) patients who die within the 30 days after dialysis start were excluded, 2005–2015
Table S3: Change in monthly count of incident waitlisting and rate of waitlisting among prevalent ESRD patient cohorts after KAS (vs pre-KAS) derived from interrupted time series models, 2011–2015
Figure S1: Change in the rate of waitlisting among incident ESRD patients in the United States from 2005 to 2015
Figure S2: Monthly time series of the count of new waitlisting events among prevalent dialysis patients from 2005 to 2015
Acknowledgments
This work was supported by the National Institute on Minority Health and Health Disparities (Grant #R01MD010290).
Abbreviations
- BMI
Body mass index
- CMS
Centers for Medicare & Medicaid Services
- ESRD
End-stage renal disease
- KAS
Kidney allocation system
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
- USRDS
United States Renal Data System
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Danovitch GM. Handbook of kidney transplantation. Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 2.Wu DA, Watson CJ, Bradley JA, Johnson RJ, Forsythe JL, Oniscu GC. Global trends and challenges in deceased donor kidney allocation. Kidney International. 2017 doi: 10.1016/j.kint.2016.09.054. [DOI] [PubMed] [Google Scholar]
- 3.USRDS. United States Renal Data System, 2017 Annual Data Report: An overview of the epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017. [Google Scholar]
- 4.Network OPaT. Current United States Kidney Transplant Waiting List Data. 2017 [Google Scholar]
- 5.Medicine Io. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: The National Academies Press; 1999. [PubMed] [Google Scholar]
- 6.Garg PP, Frick KD, Diener-West M, Powe NR. Effect of the ownership of dialysis facilities on patients' survival and referral for transplantation. New England Journal of Medicine. 1999;341(22):1653–1660. doi: 10.1056/NEJM199911253412205. [DOI] [PubMed] [Google Scholar]
- 7.Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. American journal of kidney diseases. 2005;46(4):734–745. doi: 10.1053/j.ajkd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Saunders MR, Lee H, Alexander GC, Tak HJ, Thistlethwaite JR, Ross LF. Racial disparities in reaching the renal transplant waitlist: is geography as important as race? Clinical transplantation. 2015;29(6):531–538. doi: 10.1111/ctr.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant. 2007;7(5 Pt 2):1412–1423. doi: 10.1111/j.1600-6143.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 10.Stolzmann KL, Bautista LE, Gangnon RE, McElroy JA, Becker BN, Remington PL. Trends in kidney transplantation rates and disparities. J Natl Med Assoc. 2007;99(8):923–932. [PMC free article] [PubMed] [Google Scholar]
- 11.Waterman AD, Rodrigue JR, Purnell TS, Ladin K, Boulware LE. Addressing racial and ethnic disparities in live donor kidney transplantation: priorities for research and intervention. Seminars in nephrology. 2010;30(1):90–98. doi: 10.1016/j.semnephrol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng FL, Reese PP, Mulgaonkar S, Patel AM. Barriers to Living Donor Kidney Transplantation among Black or Older Transplant Candidates. Clinical Journal of the American Society of Nephrology. 2010;5(12):2338–2347. doi: 10.2215/CJN.03040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soucie JM, Neylan JF, W M. Race and sex differences in the identification of candidates for renal transplantation. AMerican Journal of Kidney Diseases. 1992;19(5):414–419. doi: 10.1016/s0272-6386(12)80947-4. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, et al. Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis. 2000;36(5):1025–1033. doi: 10.1053/ajkd.2000.19106. [DOI] [PubMed] [Google Scholar]
- 15.Young CJaG, R S. Renal transplantation in black Americans. N Engl J Med. 2000;343(21):1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 16.Young CJaRSG. African Americans and renal transplantation: dispoportionate need, limited access, and impaired outcomes. Am J Med Sci. 2002;323(2):94–99. doi: 10.1097/00000441-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Arce CM, Goldstein BA, Mitani AA, Lenihan CR, Winkelmayer WC. Differences in access to kidney transplantation between Hispanic and non-Hispanic whites by geographic location in the United States. Clinical Journal of the American Society of Nephrology. 2013 doi: 10.2215/CJN.01560213. CJN. 01560213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patzer RE, Amaral S, Klein M, Kutner N, Perryman JP, Gazmararian JA, et al. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(2):369–378. doi: 10.1111/j.1600-6143.2011.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med. 1995;155(11):1201–1208. [PubMed] [Google Scholar]
- 20.Mohan S, Mutell R, Patzer RE, Holt J, Cohen D, McClellan W. Kidney transplantation and the intensity of poverty in the contiguous United States. Transplantation. 2014;98(6):640–645. doi: 10.1097/TP.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelrod DA, Lentine KL, Xiao H, Bubolz T, Goodman D, Freeman R, et al. Accountability for end-stage organ care: implications of geographic variation in access to kidney transplantation. Surgery. 2014;155(5):734–742. doi: 10.1016/j.surg.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Reddan DN, Szczech LA, Klassen PS, Owen WF., Jr Racial inequity in America's ESRD program. Semin Dial. 2000;13(6):399–403. doi: 10.1046/j.1525-139x.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Ryn Ma JB. The effect of patient race and socioeconomic status on physicians' perceptions of patients. Soc Sci Med. 2000;50(6):813–828. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- 24.Ashby VB, Port FK, Wolfe RA, Wynn JJ, Williams WW, Roberts JP, et al. Transplanting kidneys without points for HLA-B matching: consequences of the policy change. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(8):1712–1718. doi: 10.1111/j.1600-6143.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 25.Amaral S, Patzer RE, Kutner N, McClellan WM. Racial Disparities in Access to Pediatric Kidney Transplantation Since Share 35. Journal of American Society of Nephrology. 2012 doi: 10.1681/ASN.2011121145. [DOI] [PubMed] [Google Scholar]
- 26.Hampton T. New Kidney Allocation System. Jama. 2015;313(4):346–346. [Google Scholar]
- 27.Procurement O, Network T. The new kidney allocation system (KAS) frequently asked questions. 2014 [Google Scholar]
- 28.Wang CJ, Wetmore JB, Israni AK. Old versus new: Progress in reaching the goals of the new kidney allocation system. Human immunology. 2017;78(1):9–15. doi: 10.1016/j.humimm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Policy IoMCoOPaT. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington (DC): 1999. [PubMed] [Google Scholar]
- 30.Melanson TA, Hockenberry JM, Plantinga L, Basu M, Pastan S, Mohan S, et al. New Kidney Allocation System Associated With Increased Rates Of Transplants Among Black And Hispanic Patients. Health Affairs. 2017;36(6):1078–1085. doi: 10.1377/hlthaff.2016.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massie AB, Luo X, Lonze BE, Desai NM, Bingaman AW, Cooper M, et al. Early changes in kidney distribution under the new allocation system. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015080934. ASN. 2015080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall YN, Choi AI, Xu P, O'Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. Journal of the American Society of Nephrology. 2011 doi: 10.1681/ASN.2010080819. ASN. 2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe Rudow D, Baliga P, et al. Living Donor Kidney Transplantation: Overcoming Disparities in Live Kidney Donation in the US--Recommendations from a Consensus Conference. Clin J Am Soc Nephrol. 2015;10(9):1687–1695. doi: 10.2215/CJN.00700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OPTN. United Network for Organ Sharing Data. 2017 Available from: https://unosorg/data/
- 35.Services USCfMM. ESRD MEDICAL EVIDENCE REPORT MEDICARE ENTITLEMENT AND/OR PATIENT REGISTRATION [Google Scholar]
- 36.Becker BN, Becker YT. Evaluating the Emergency Department as a Site of Transplant Care. Transplantation. 2015;99(8):1549–1550. doi: 10.1097/TP.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 37.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. International journal of epidemiology. 2017;46(1):348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schold JD, Reese PP. Simulating the new kidney allocation policy in the United States: modest gains and many unknowns. Journal of the American Society of Nephrology. 2014;25(8):1617–1619. doi: 10.1681/ASN.2014030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurella-Tamura M, Goldstein BA, Hall YN, Mitani AA, Winkelmayer WC. State Medicaid coverage, ESRD incidence, and access to care. Journal of the American Society of Nephrology. 2014;25(6):1321–1329. doi: 10.1681/ASN.2013060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handbook of Kidney Transplantation. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 41.Malek SK, Keys BJ, Kumar S, Milford E, Tullius SG. Racial and ethnic disparities in kidney transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2011;24(5):419–424. doi: 10.1111/j.1432-2277.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, MB, Plantinga L, Escoffery C, Pastan S, Patzer R. Dialysis Facility Providers' Awareness of Racial Disparities in Kidney Transplantation. American Journal of Transplantation. 2017;17(suppl 3) [Google Scholar]
- 43.OPTN. Data Reports: National Data. US Department of Health and Human Services; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Waitlisting among incident ESRD patients by race/ethnicity, before (01/01/2005–12/03/2014) and after (12/04/2014–12/31/2015) the Kidney Allocation System (KAS) policy change
Table S2: Sensitivity analysis of effect of KAS and race/ethnicity on waitlisting among the incident ESRD patient cohort when (1) patients with a living donor transplant within 180 days were excluded, (2) patients with a history of cancer, (3) patients who die within the 30 days after dialysis start were excluded, 2005–2015
Table S3: Change in monthly count of incident waitlisting and rate of waitlisting among prevalent ESRD patient cohorts after KAS (vs pre-KAS) derived from interrupted time series models, 2011–2015
Figure S1: Change in the rate of waitlisting among incident ESRD patients in the United States from 2005 to 2015
Figure S2: Monthly time series of the count of new waitlisting events among prevalent dialysis patients from 2005 to 2015