Abstract
MEG and EEG have identified post-stimulus low frequency and 40Hz steady-state auditory encoding abnormalities in schizophrenia (SZ). Negative findings have also appeared. To identify factors contributing to these inconsistencies, healthy control (HC) and SZ group differences were examined in MEG and EEG source space and EEG sensor space, with better group differentiation hypothesized for source than sensor measures given greater predictive utility for source measures. 55 HC and 41 chronic SZ were presented 500Hz sinusoidal stimuli modulated at 40Hz during simultaneous whole-head MEG and EEG. MEG and EEG source models using left and right superior temporal gyrus (STG) dipoles estimated trial-to-trial phase similarity and percent change from pre-stimulus baseline. Group differences in post-stimulus low-frequency activity as well as the 40Hz steady-state response were evaluated. Several EEG sensor analysis strategies were also examined. Post-stimulus low-frequency group differences were observed across all methods. Given an age-related decrease in left STG 40Hz steady-state activity in HC, HC > SZ 40Hz steady-state group differences were evident only in younger participants’ source measures. Findings thus indicated that optimal data collection and analysis methods depend on the auditory encoding measure of interest. In addition, whereas results indicated that HC and SZ auditory encoding low-frequency group differences are generally comparable across modality and analysis strategy (and thus not dependent on obtaining construct-valid measures of left and right auditory cortex activity), 40 Hz steady-state group-difference findings are much more dependent on analysis strategy, with 40Hz steady-state source-space findings providing the best group differentiation.
Keywords: schizophrenia, superior temporal gyrus, auditory encoding, magnetoencephalography, electroencephalography
1. Introduction
EEG and MEG have long been used to evaluate neural activity in psychiatric and neurologic populations. Comparisons of EEG and MEG findings are sometimes challenging. Such work is limited by differences in data collection and analysis strategies, such as EEG studies usually examining data in sensor space and MEG studies more often examining data in source space. Given different strengths and weakness of EEG and MEG, it is likely that depending on the research question either EEG or MEG may be found most useful, potentially differing in construct validity, ease of measurement or the group effect size. The present study sought to make progress in the above by comparing group differences in EEG and MEG auditory encoding activity (EEG and MEG data simultaneously obtained) in adults with schizophrenia (SZ) and healthy comparison participants (HC). The present study focused on two auditory encoding measures frequently of interest: early transient low-frequency activity and 40 Hz steady-state activity.
In a review of studies examining the early transient 100 ms component of the auditory event-related brain potential, Rosburg et al. (Rosburg, Boutros, & Ford, 2008) concluded that auditory encoding abnormalities in SZ are a robust finding. In a large multisite study (Consortium on the Genetics of Schizophrenia), abnormal 100 ms responses where observed in individuals with SZ as well as unaffected first-degree relatives of individuals with SZ with co-morbid psychiatric or substance use conditions, suggesting 100 ms activity as an endophenotype for SZ (Miller & Rockstroh, 2013; Turetsky et al., 2008). Other studies have shown that decreased 100 ms auditory activity is associated with impairment on tests of attention as well as reduced gray matter (J. C. Edgar et al., 2012; Smith et al., 2010). Studies examining the time-frequency profile of the early transient activity (50ms and 100 ms activity) have repeatedly demonstrated decreased lower-frequency activity in adults with SZ (Blumenfeld & Clementz, 2001; Clementz & Blumenfeld, 2001; J. C. Edgar et al., 2008), with Johannesen et al. (Johannesen et al., 2005) and Jansen et al. (Jansen, Hegde, & Boutros, 2004) concluding that the small response observed in schizophrenia reflects a diminished capacity to “gate-in” relevant signal. Auditory encoding abnormalities may be common to psychosis, with a large multisite EEG study (Bipolar-Schizophrenia Network on Intermediate Phenotypes) examining auditory oddball activity in SZ and psychotic bipolar disorder reporting decreased 100 ms amplitude as well as decreased 11 to 30 Hz activity to standard tones and decreased 3 to 10 Hz activity to target tones in SZ and bipolar disorder than in HC (Ethridge et al., 2015).
Researchers have also used 40Hz auditory driving stimuli to examine dysfunction in these networks in SZ, given hypothesized abnormalities in pyramidal cells and inhibitory interneuron networks in the superficial cortical layers in SZ (for a review see (Gandal, Edgar, Klook, & Siegel, 2012)). Many studies have reported 40Hz auditory steady-state abnormalities in SZ (Brenner, Sporns, Lysaker, & O’Donnell, 2003; Hall et al., 2011; Hamm, Gilmore, & Clementz, 2012; Hong et al., 2004; Koenig, van Swam, Dierks, & Hubl, 2012; Krishnan et al., 2009; Kwon et al., 1999; Lenz, Fischer, Schadow, Bogerts, & Herrmann, 2011; Light et al., 2006; Maharajh, Teale, Rojas, & Reite, 2010; Rass et al., 2012; Spencer, Niznikiewicz, Nestor, Shenton, & McCarley, 2009; Teale et al., 2008). Using 40Hz auditory steady-state stimuli and relatively long inter-stimulus intervals (e.g., greater than 1 second) allows examination of both early transient and steady-state activity (Jacobson & Fitzgerald, 1997; Pantev, 1995). As left and right superior temporal gyrus (STG) regions are the primary generators of the early transient responses (Hari, 1990; Hari, Aittoniemi, Jarvinen, Katila, & Varpula, 1980; Huotilainen et al., 1998; Makela, Hamalainen, Hari, & McEvoy, 1994; Naatanen & Picton, 1987; Pelizzone et al., 1987; Reite, Teale, Zimmerman, Davis, & Whalen, 1988; Yvert, Crouzeix, Bertrand, Seither-Preisler, & Pantev, 2001) as well as the 40Hz steady-state response (Herdman et al., 2003; Ross, Herdman, & Pantev, 2005; Ross, Picton, & Pantev, 2002), some studies have used source localization to directly assess the left and right STG cortical microcircuits involved in auditory encoding. As an example, using a 40Hz steady-state task with a relatively long inter-stimulus interval, Edgar et al. (J. C. Edgar et al., 2014) showed multiple disruptions in STG auditory areas in SZ, including STG post-stimulus low-frequency abnormalities (4 to 16 Hz) as well as 40Hz steady-state abnormalities.
In a recent study examining the construct validity of 40Hz steady-state MEG and EEG measures, Edgar et al. (J. C. Edgar et al., 2017) demonstrated limitations examining 40Hz steady-state activity in EEG sensor space (e.g., inability to assess hemisphere differences in the strength of the 40Hz steady-state response) and thus advantages of directly assessing 40Hz steady-state activity in left and right STG. Examining a variety of head models (spherical and realistic) and a variety of dipole source modeling strategies (dipole source localization and dipoles fixed to Heschl’s Gyrus), Edgar et al. demonstrated generality in results across head and source modeling strategies, and concluded that the use of a MEG or EEG spherical head model with sources manually fixed to left and right Heschl’s Gyrus is an effective and efficient method for measuring the left and right STG 40Hz steady-state response.
The present study builds upon Edgar et al. (J. C. Edgar et al., 2017), using MEG and EEG spherical head models with sources manually fixed to left and right Heschl’s gyri, to compare MEG and EEG in identifying control and SZ group differences in post-stimulus low-frequency (4 to 16 Hz) and 40 Hz steady-state processes. Also of interest was comparing source modeling results to EEG sensor results, with the hypothesis of better group differentiation for EEG source and MEG source than EEG sensor measures given greater predictive utility for source measures.
2. Method
2.1 Participants
Fifty-five HC (36 males; mean age 39.6 ± 11.9 years; range 21 to 58 years), and 41 patients with chronic SZ (33 males, mean age 40.3 ± 11.6 years; range 20 to 60 years) were recruited. The HC in this study largely overlapped with the participants in Edgar et al. (J. C. Edgar et al., 2017). Selection criteria for SZ were: (1) diagnosis of schizophrenia with no other Axis I diagnosis, determined by the Structured Clinical Interview for DSM-IV-Patient Edition (SCID-DSM-IV; American Psychiatric Association, 1994); (2) stable, continuous treatment with antipsychotic medication for at least 3 months; (3) no history of substance dependence (determined via SCID); (4) no history of alcohol or other substance abuse in the past 3 months (determined via SCID); (5) no history of head injury with loss of consciousness for more than 5 minutes; and (6) no psychiatric hospitalization in the last 3 months. Selection criteria for HC were: (1) no history of Axis I psychiatric dysfunction (via SCID), (2) no history of substance dependence in the last 3 years, (3) no history of alcohol or other substance abuse in the last 3 months, (4) no history of head injury with loss of consciousness for more than 5 minutes or other neurological disease, and (5) no family history of a psychotic disorder in first-degree relatives by self-report. As shown in Table 1, groups did not differ in age or parental socioeconomic status (SES (Oakes & Rossi, 2003)). Patients’ mean total scores on the Positive and Negative Syndrome Scale (PANSS)(Kay, Fiszbein, & Opler, 1987) were 17.05 for positive symptoms and 15.10 for negative symptoms. As is typical in SZ studies, patients’ IQ, education, and SES were significantly lower than those of HC. Groups did not differ in gender distribution (Chi-square = 2.81; p > 0.05). Nine HC and 15 SZ were smokers. Thirty of the 55 SZ were treated with 2nd generation antipsychotics, three with the 1st generation antipsychotic haloperidol, and six with more than one antipsychotic. Two participants were not taking medications.
Table 1.
Group demographics
| HC (N = 55) | SZ (N = 41) | |||
|---|---|---|---|---|
| mean | SD | mean | SD | |
| Age (years) | 39.6 range 21 to 58 |
11.9 | 40.3 range 20 to 60 |
11.6 |
| WISC IQ** | 101.4 | 11.9 | 92.2 | 19.2 |
| Education** (years) | 14.8 | 1.5 | 13.0 | 2.0 |
| SES** | 49.3 | 16.8 | 66.5 | 8.7 |
| Parental SES | 43.4 | 15.5 | 47.8 | 19.5 |
2.2 Steady-state Task
The amplitude of a 500 Hz stimulus was modulated at 40 Hz, the modulation depth 100%. Stimuli of 1 s duration were binaurally presented with a 4 s offset-to-onset inter-stimulus interval (ISI, ±2 s) and delivered using a sound pressure transducer through conduction tubing to the ear canal via ear-tip inserts (ER3A; Etymotic Research, Elk Grove Village, Ill., USA). Prior to data acquisition, 500 Hz tones of 300 ms duration and 12.5 ms rise time were used to obtain auditory thresholds for each ear. Auditory thresholds were initially estimated via stepwise amplitude reduction until participants stopped verbally identifying the presence of the tone. For fine tuning, tone loudness was then adjusted within ±10 dB of the preliminary threshold until a final threshold was confirmed (approximately 50% accuracy). For the 40Hz steady-state task, for each ear, the peak intensity of the steady-state stimulus was presented 35 dB above each participant’s hearing threshold. The number of steady-state stimuli presented depended on the MEG recording time available, but the mean number of HC (mean = 92, SD = 22) and SZ (mean = 87, SD = 18) artifact-free trials (artifact criteria describe below) did not differ, t(104) = 1.26, p > 0.05.
2.3 Structural Magnetic Resonance Imaging (sMRI)
T1-weighted MPRAGE structural MRIs were collected on a Siemens 3T TIM Trio scanner at the Mind Research Network. Images were collected with a field of view = 256 × 256 mm, 192 sagittal slices, and 1 × 1 × 1 mm spatial resolution. This was a five-echo sequence with echo times of 1.64, 3.5, 5.36, 7.22, and 9.08 ms, a repetition time = 2,530 ms, a gray-white matter contrast enhancement inversion recovery time of 1,200 ms, and 7° flip angle.”
2.4 MEG Data Acquisition and Coregistration
MEG data were recorded using a 306-channel Vector-View MEG system (Elekta-Neuromag, Helsinki, Finland). During MEG recording, the participant’s head position was monitored using four HPI coils attached to the scalp. EEG data were collected with Ag/AgCl electrodes from 60 equidistant sites (Falk Minow Customized Easy Cap®). EEG data were collected simultaneously with MEG data using Elekta-Neuromag EEG amplifiers. The left mastoid served as the EEG reference at data collection. All EEG scores were obtained using an average reference (see below). Electro-oculogram (EOG) (bipolar vertical EOG above and below left eye) and electrocardiogram (ECG) (bipolar channel at the collarbone) were also obtained.
To coregister MEG/EEG and sMRI data for each participant, three anatomical landmarks (nasion and right and left preauriculars) as well as an additional 200+ points on the scalp (including the position of each EEG electrode) and face were digitized for each participant using the Probe Position Identification (PPI) System (Polhemus, Colchester, VT). The three fiducials were identified in the participant’s sMRI, and a 6-degree rigid-body transformation matrix that involved rotation and translation between the MEG/EEG and sMRI coordinate systems was obtained by matching the 200+ points from the PPI measurements to the surface of the scalp and face to the structural MRI using BESA MRI 2.0.
2.5 Magnetic Source Analysis
MEG raw signals were first processed with Signal Space Separation (SSS; (Taulu, Kajola, & Simola, 2004)) using Maxfilter (Elekta Maxfilter™; Elekta Oy). SSS separates neuronal magnetic signals arising from inside the MEG sensor array from external magnetic signals arising from the surrounding environment to reduce environmental noise and artifacts. Following SSS, MEG and EEG eye-blink activity was corrected using the methods outlined in Berg and Scherg (Berg & Scherg, 1994). Using BESA 6.0, epochs −1000 ms pre-stimulus to 1200 ms post-stimulus onset were identified, and within this interval artifacts other than blinks were rejected by amplitude and gradient criteria (amplitude > 1200fT, gradient > 800fT/cm). Epochs (trials) without artifact were then averaged from −1000ms pre-stimulus to 1200ms post-stimulus onset. MEG analyses were performed using the planar gradiometer sensors.
For source localization, a BESA 6.0 bandpass filter (Butterworth) was applied with a center frequency of 40Hz and a 20Hz width (a bandpass filter superior to using separate low- and high-pass filters for extracting MEG/EEG activity in narrow frequency bands) with 100% of the activity passed at 40Hz and 50% amplitude cutoffs at 30Hz and 50Hz. For modeling the 40Hz steady-state response, data 500 to 1000ms post-stimulus were selected, with a 500ms starting point as the amplitude modulated 40Hz steady-state response does not fully develop until after 250 to 300ms (Ross et al., 2002).
Source localization and scoring were done blind to group. The source modeling approach applied in the present study relied on the findings reported in Edgar et al. (J. C. Edgar et al., 2017), where it was concluded that the use of a MEG or EEG spherical head model with sources manually fixed to left and right Heschl’s Gyrus is an effective and efficient method for measuring the left and right STG 40Hz steady-state response. Other studies also note the advantages of using prior knowledge of the location of source generators in brain electromagnetic source analysis (Scherg & Berg, 1991). Thus, for MEG and EEG, a spherical head model with sources manually fixed to left and right Heschl’s gyrus and an eye-blink artifact source was created for each participant (same left and right Heschl’s Gyrus location for EEG and MEG). Specifically, in each participant the left and right Heschl’s gyri were visually identified and a dipole source was placed approximately a third of the length along Heschl’s gyrus (with the most medial aspect of Heschl’s gyrus the starting point). If two Heschl’s gyri were present within a hemisphere, the dipole was placed between the two Heschl’s gyri. For both the MEG and EEG source models, left and right regional sources (a source with three (EEG) or two (MEG) single dipoles at the same location but with orthogonal orientations) were used to determine a single optimal orientation for the left and a single optimal orientation for the right source over the 500 to 1000ms period. The non-primary orientations were then removed to obtain a MEG source model and an EEG source model with single dipoles in left and right Heschl’s Gyrus (Scherg & Ebersole, 1993).
For MEG, the spherical head model of Sarvas (Sarvas, 1987) was used. MEG data were analyzed only in source space. For EEG source analyses, a multi-shell spherical head model containing four homogeneous shells (brain, CSF, bone, and skin) was used, with the shells distorted slightly into an ellipsoid that best fit the participant’s EEG electrode cloud (EEG coordinates digitized using Polhemus), and with the center of the sphere determined from the EEG electrode locations (Berg & Scherg, 1994). For the EEG spherical head model, the BESA default conductivity values were used: 0.33 S/m for scalp and brain tissue, 0.0042 S/m for skull, and 1.79 S/m for cerebral spinal fluid. All EEG analyses were done using an average reference. In addition to the EEG source models, pre-stimulus power measures and 40 Hz steady-state and low-frequency activity measures were obtained at EEG sensors Cz and Fz (average reference). Using all EEG sensors, measures were also obtained via applying principal component analysis (PCA) to the whole-head EEG data and then using the time course associated with the first component of the PCA (computed over a 500 to 1000ms interval) to compute the needed time-frequency measures.
2.6 Source Time-frequency Analysis
The calculation of single-trial phase and magnitude for the left and right STG sources used procedures outlined in Hoechstetter et al. (Hoechstetter et al., 2004), where for each participant the derived source model was applied to the raw unfiltered data. Transformation from the time domain to the time-frequency domain used complex demodulation procedures (Papp & Ktonas, 1977) implemented in BESA 6.0, using frequencies between 4 and 60Hz in steps of 2Hz. 40Hz steady-state and post-stimulus low-frequency total power and phase-locking were both examined. Total power and phase-locking measures were extracted from the single-trial complex time-frequency matrix. Total power (TP) was calculated by averaging the time-frequency spectra of each MEG/EEG epoch, thus providing a measure of the magnitude or power of oscillatory activity. TP was computed as percent-change score: (100*(post-stimulus activity – pre-stimulus activity)/pre-stimulus activity). For TP calculations, the pre-stimulus period was −800 to −200 ms. A measure of phase-locking referred to as inter-trial coherence (ITC) was also computed. ITC is a normalized measure with ITC = 1 reflecting no trial-to-trial phase variability and ITC = 0 reflecting maximal phase variability across trials.
As discussed in the Introduction, two a priori auditory encoding processes were examined: post-stimulus low-frequency activity (50 - 200ms, 4 - 16Hz) and 40Hz steady-state activity (500 - 1000ms, 38 - 42Hz). Specifically, for each participant, a single value was obtained for each of low-frequency and 40Hz steady-state and TP and ITC, the average TP and ITC within a 50 to 200 ms and 4 to 16Hz interval, and the average TP and ITC within a 500 to 1000 ms and 38 to 42Hz interval.
Given that pre-stimulus group differences may affect interpretation of post-stimulus group findings (J. C. Edgar, Khan, S.Y., Blaskey, L., Chow, V.Y., Rey, M., Gaetz, W., Cannon, K.M., Monroe, J.F., Cornew, L., Qasmieh, S., Liu, S., Welsh, J.P., Levy, S.E., Roberts, T.P., 2013), and given studies indicating elevated pre-stimulus activity in SZ (J. C. Edgar et al., 2014; Gandal et al., 2012; Hong, Summerfelt, Mitchell, O’Donnell, & Thaker, 2012), pre-stimulus group differences as well as the effect of pre-stimulus activity on post-stimulus group differences were examined. Although pre-stimulus group differences were observed, after accounting for the influence of pre-stimulus activity on post-stimulus activity (assessed via hierarchical regression), the pattern of post-stimulus findings did not change(Miller & Chapman, 2001). As such, and given that the primary focus of the study is post-stimulus activity, pre-stimulus analyses and findings are reported in the Online Supporting information.
Given that left 40Hz steady-state activity may decrease as a function of age in controls (Berman et al., 2016), and given the observation in a recent meta-analysis of 40Hz steady-state studies of a trend for larger 40Hz steady-state group differences for patients with SZ younger than 39.8 years versus patients older than 39.8 years (Thune, Recasens, & Uhlhaas, 2016), the effect of age on group differences was examined, with the hypothesis that an age-related decrease in left STG 40Hz steady-state activity in HC would mask 40Hz steady-state group differences.
Finally, analyses of the time-domain evoked signal examined group differences with respect to the ability to maintain a stable 40Hz steady-state response. In particular, the T2circ procedure described in Victor and Mast (Victor & Mast, 1991) determined whether a 40Hz steady-state response was present in each participant, via a quantitative analysis of a steady-state response against a background of additive noise. The evoked time-domain 40Hz steady-state response was used for T2circ analyses, using as input epochs of 125 ms (= 5 cycles of 40Hz activity), starting at 300 ms, and thus obtaining 5 epochs over a 300 to 925 ms period for each evoked 40Hz steady-state response (an earlier start time for these analyses selected given the need to obtain sufficient epochs).
2.7 Statistical Analyses
ANOVAs on the full sample examined group differences in the two post-stimulus intervals, with hemisphere as a repeated measure. Given a slightly skewed distribution of TP and T2circ values in both groups, TP and T2circ analyses were performed on log-transformed values. In each analysis, participants more than 2.5 standard deviations from the sample mean were excluded (generally none and at most 1 participant per analysis).
3. Results
Analyses presented in the Online Supporting information showed no differences between SZ and HC in goodness-of-fit (GOF) or dipole orientation.
3.1 Time-frequency ANOVAs
Figure 1 shows TP and ITC findings.
Figure 1.
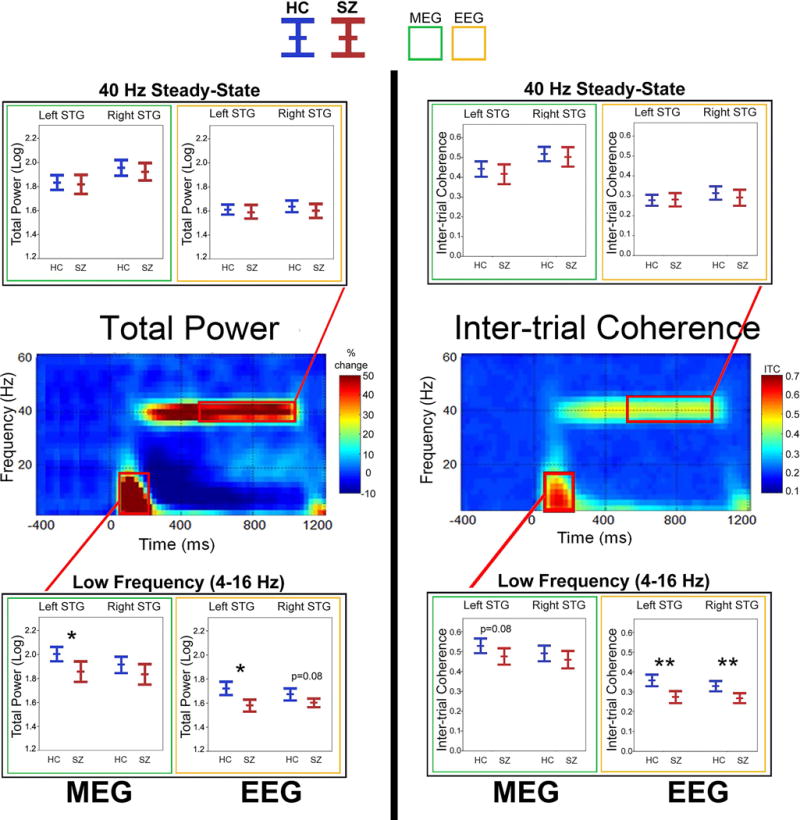
Total power and inter-trial coherence time-frequency plots (grand average of controls shown), with boxplots (MEG = green border; EEG = yellow border) showing the mean and 95% confidence intervals for each group (HC = blue; SZ = red) for left and right STG post-stimulus low-frequency activity (4 to 16Hz activity averaged from 50 to 200 ms) and 40Hz steady-state activity (38 to 42Hz activity averaged from 500 to 1000 ms). In the boxplots, significant group differences are indicated (*p<0.05; **p<0.001; trends below p = 0.10 reported with p-value).
3.1a Post-stimulus low-frequency activity
MEG ANOVAs showed an effect of Hemisphere (right > left) for TP, F(1,94) = 5.02, p < 0.05, and a marginal effect (right > left) for ITC, F (1,94) = 3.27, p = 0.07. A Group effect (HC > SZ) was observed for TP, F (1,94) = 4.92, p < 0.05, trending (HC > SZ) for ITC, F (1,94) = 2.80, p = 0.10. For EEG, Hemisphere was not significant for TP or ITC (ps > 0.05), but a Group main effect showed HC > SZ for TP, F (1,93) = 4.90, p < 0.05, and for ITC, F (1,94) = 16.56, p < 0.001.
3.1b 40 Hz steady-state activity
MEG ANOVAs showed Hemisphere effects (right > left) for TP, F (1,94) = 34.15, p < 0.001, and for ITC, F (1,94) = 43.82, p < 0.001. EEG ANOVAs showed a marginal effect of Hemisphere (right > left) only for ITC, F (1,94) = 3.45, p = 0.07. No effects involving Group were significant.
3.1c Summary
Group differences were observed only for low-frequency activity. For comparison with previous and future studies, Table 2 provides TP and ITC mean and standard deviation values as well as Cohen’s d group effect sizes. For comparison with other studies, Online Supplement Figure 2 provides time-frequency plots using an earlier 0 to 500 ms interval for 40Hz steady-state activity (see (Hamm et al., 2015)). Findings using the earlier 40Hz steady-state interval were analogous to those for the above interval (with no 40Hz steady-state group difference). Finally, in SZ, none of the MEG or EEG post-stimulus measures above was associated with medication (olanzapine equivalent, calculated based on the clinical dosing equivalencies provided in Gardner et al. (Gardner, Murphy, O’Donnell, Centorrino, & Baldessarini, 2010)). Average olanzapine equivalent dose was 15.65 mg (SD = 9.56).
Table 2.
Auditory encoding: TP and ITC mean and standard deviation values for each group and hemisphere
| Left STG | Right STG | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency Interval | Controls | Patients | Controls | Patients | |||||
| Low-Frequency (4-16 Hz) | Mean (SD) | Mean (SD) | p-value | Cohen's D | Mean (SD) | Mean (SD) | p-value | Cohen's D | |
| TP(log) | MEG | 2.00 (.24) | 1.86 (.28) | 0.01 | 0.58 | 1.91 (.26) | 1.83 (.28) | 0.16 | 0.30 |
| EEG | 1.70 (.07) | 1.58 (.17) | 0.02 | 0.53 | 1.66 (.21) | 1.60 (.13) | 0.08 | 0.29 | |
|
| |||||||||
| ITC | MEG | 0.52 (.13) | 0.47 (.13) | 0.08 | 0.38 | 0.49 (.13) | 0.46 (0.15) | 0.3 | 0.21 |
|
|
|||||||||
| EEG | 0.35 (.12) | 0.27 (.10) | 0.001 | 0.72 | 0.33 (.10) | 0.27 (.09) | 0.002 | 0.63 | |
|
| |||||||||
| Steady-State (38-42 Hz) | |||||||||
|
| |||||||||
| TP(log) | MEG | 1.83 (.24) | 1.81 (.27) | 0.85 | 0.08 | 1.94 (.26) | 1.92 (.25) | 0.63 | 0.08 |
| EEG | 1.59 (.21) | 1.59 (.18) | 0.92 | 0.00 | 1.63 (.19) | 1.60 (.20) | 0.45 | 0.15 | |
|
| |||||||||
| ITC | MEG | 0.44 (.15) | 0.41 (.17) | 0.47 | 0.19 | 0.51 (.15) | 0.50 (.16) | 0.8 | 0.06 |
| EEG | 0.28 (.11) | 0.28 (.12) | 0.8 | 0.00 | 0.31 (.13) | 0.29 (.13) | 0.44 | 0.15 | |
3.2 Regression Analyses
Regressions with age entered first, Group second, and the interaction term last examined associations between age and post-stimulus dependent measures.
3.2a Predicting post-stimulus low-frequency activity
As shown in Table 3a and 3b for MEG and EEG, no effect of age on post-stimulus low-frequency group differences was observed. Whereas MEG analyses indicated group differences in left STG (significant for TP and marginally significant for ITC), EEG analyses indicated low-frequency group differences bilaterally.
Table 3a.
Age and MEG post-stimulus low-frequency and 40 Hz steady-state activity
| Age | Group | Interaction Term | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Frequency Interval | R2 | p-value | R2 change | p-value | Effect | R2 change | p-value | |
| TP(log) | Low-Frequency Left | 0.00 | p>0.05 | 0.07 | p<0.01 | HC > SZ | 0.01 | p>0.05 |
|
|
||||||||
| Low-Frequency Right | 0.00 | p>0.05 | 0.02 | p>0.05 | NA | 0.02 | p>0.05 | |
|
| ||||||||
| ITC | Low-Frequency Left | 0.00 | p>0.05 | 0.03 | p=0.08 | HC > SZ | 0.02 | p>0.05 |
|
|
||||||||
| Low-Frequency Right | 0.00 | p>0.05 | 0.01 | p>0.05 | NA | 0.02 | p>0.05 | |
|
| ||||||||
| TP(log) | 40 Hz Left | 0.00 | p>0.05 | 0.00 | p>0.05 | NA | 0.04 | p=0.07 |
|
|
||||||||
| 40 Hz Right | 0.00 | p>0.05 | 0.00 | p>0.05 | NA | 0.02 | p>0.05 | |
|
| ||||||||
| ITC | 40 Hz Left | 0.01 | p>0.05 | 0.01 | p>0.05 | NA | 0.05 | p<0.05 |
|
|
||||||||
| 40 Hz Right | 0.00 | p>0.05 | 0.00 | p>0.05 | NA | 0.02 | p>0.05 | |
Age as the independent measure and post-stimulus low-frequency and 40 Hz steady-state total power (TP) as the dependent measure.
Table 3b.
Age and EEG post-stimulus low-frequency and 40 Hz steady-state activity
| Age | Group | Interaction Term | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Frequency Interval | R2 | p-value | R2 change | p-value | Effect | R2 change | p-value | |
| TP(log) | Low-Frequency Left | 0.01 | p>0.05 | 0.11 | p=0.001 | HC > SZ | 0.01 | p >0.05 |
|
|
||||||||
| Low-Frequency Right | 0.00 | p>0.05 | 0.03 | p=0.09 | HC > SZ | 0.00 | p >0.05 | |
|
| ||||||||
| ITC | Low-Frequency Left | 0.00 | p>0.05 | 0.12 | p<0.001 | HC > SZ | 0.00 | p >0.05 |
|
|
||||||||
| Low-Frequency Right | 0.00 | p>0.05 | 0.10 | p <0.01 | HC > SZ | 0.01 | p >0.05 | |
|
| ||||||||
| TP(log) | 40 Hz Left | 0.13 | p<0.001 | 0.00 | p >0.05 | NA | 0.00 | p >0.05 |
|
|
||||||||
| 40 Hz Right | 0.01 | p>0.05 | 0.01 | p >0.05 | NA | 0.00 | p >0.05 | |
|
| ||||||||
| ITC | 40 Hz Left | 0.08 | p<0.01 | 0.00 | p >0.05 | NA | 0.03 | p >0.05 |
|
|
||||||||
| 40 Hz Right | 0.00 | p>0.05 | 0.01 | p >0.05 | NA | 0.00 | p >0.05 | |
Age as the independent measure and post-stimulus low-frequency and 40 Hz steady-state total power (TP) as the dependent measure.
3.2b Predicting 40Hz steady-state activity
As shown in Table 3a, MEG regression analyses showed left STG Age × Group interactions (marginally significant for left STG TP and significant for left STG ITC). The Figure 2 MEG scatterplots (far left panels) show a negative association between age and left STG 40Hz steady-state activity in HC but not SZ. For EEG, although regression analyses showed a main effect of Age for left STG TP and ITC and no Group × Age interaction, the Figure 2 EEG ITC scatterplots generally mirror the MEG findings, with the EEG left STG scatterplots indicating a more prominent age-related decrease in 40Hz steady-state activity in HC, and with the scatterplots suggesting in some instances possibly lower 40Hz steady-state activity in older HC versus older SZ (e.g., see MEG and EEG left ITC scatterplots).
Figure 2.
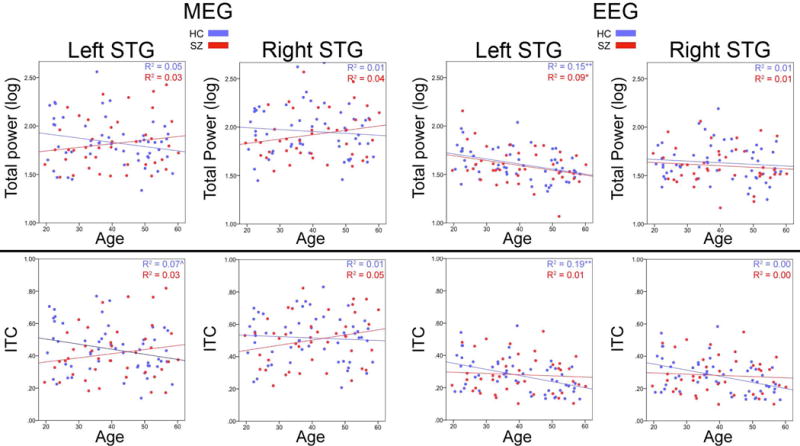
Scatterplots showing correlations between age and left and right STG 40Hz steady-state activity for MEG (left panels) and EEG (right panels), and for total power (TP, upper row) and inter-trial coherence (ITC, lower row). HC shown in blue and SZ in red. R2 values show the percent variance explained.
To explore this issue, t-tests were run separately for younger and older participants via a median split on age = 40 years (sample size for younger and older groups differed slightly given multiple participants at the same median age of 40 years as well as different numbers of HC and SZ above and below the total sample median age). Online Supplement Table 2 shows that the pattern of HC and SZ group effects of age, IQ, education, and SES observed in the full sample was also observed in the younger and older participants. Exploratory t-tests showed no differences in IQ, education, or self or parental SES between the younger and older HC or younger and older SZ.
Figure 3 shows 40 Hz group difference findings separately for younger and older participants (for comparison, younger and older group differences for pre-stimulus activity and post-stimulus low-frequency activity are also shown). HC > SZ 40Hz steady-state group differences were observed only for MEG left STG 40Hz steady-state ITC in the younger participants. As suggested by the EEG 40Hz steady-state ITC and age scatterplots in Figure 2 and as shown in Figure 3, in the older participants significant EEG SZ > HC 40Hz steady-state ITC group differences were observed in left STG. For comparison with previous and future studies, Online Supplement Tables 3 and 4 provide TP and ITC mean and standard deviation values as well as Cohen’s d group difference effect sizes for the younger and older participants. For comparison with the 40 Hz steady-state younger and older analyses, Online Supplement Tables 3 (younger participants) and 4 (older participants) provide low-frequency TP and ITC mean and standard deviation values as well as Cohen’s d group difference effect sizes.
Figure 3.
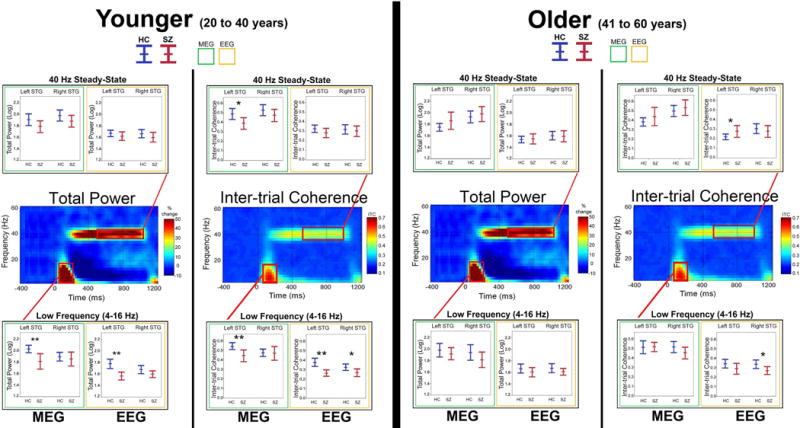
The same as Figure 1 but showing findings for the younger and older participants. In the boxplots, significant pairwise group differences are indicated (*p<0.05; **p<0.001; trends below p = 0.10 reported with p-value).
3.3 EEG Sensor and PCA Measures
Table 4 shows sensor-space PCA and EEG Cz and Fz group differences for each ROI in the full sample. Analogous to the source-space analyses, all sensor measures except PCA TP indicated higher post-stimulus low-frequency TP and ITC in HC than in SZ. Also, analogous to the source-space analyses, no 40Hz steady-state group differences were observed in the full sample.
Table 4.
Group difference for EEG sensor measures
| Frequency Interval | TP (Log) | ITC | ||||||
|---|---|---|---|---|---|---|---|---|
| Low-Frequency Activity | Controls | Patients | Controls | Patients | ||||
| Mean (SD) | Mean (SD) | p-value | Cohen's D | Mean (SD) | Mean (SD) | p-value | Cohen's D | |
| EEG PCA | 1.65 (.19) | 1.61 (.22) | 0.30 | 0.19 | 0.36 (.11) | 0.29 (.10) | p < 0.01 | 0.67 |
| EEG Cz | 1.81 (.21) | 1.62 (.21) | p < 0.001 | 0.90 | 0.39 (.12) | 0.33 (.10) | p < 0.01 | 0.54 |
| EEG Fz | 1.71 (.16) | 1.56 (.19) | p < 0.001 | 0.85 | 0.34 (.10) | 0.26 (.08) | p < 0.001 | 0.78 |
| 40 Hz Steady-State Activity | TP (Log) | ITC | ||||||
| EEG PCA | 1.80 (.28) | 1.76 (.24) | 0.46 | 0.15 | 0.45 (.18) | 0.43 (.16) | 0.74 | 0.12 |
| EEG Cz | 1.70 (.21) | 1.66 (.19) | 0.32 | 0.20 | 0.36 (.12) | 0.35 (.13) | 0.69 | 0.08 |
| EEG Fz | 1.66 (.20) | 1.68 (.22) | 0.75 | 0.10 | 0.35 (.12) | 0.39 (.14) | 0.20 | 0.31 |
3.4 Time-domain 40Hz Steady-state T2circ Measures
Given a skewed distribution (some individuals with very high T2circ values), group differences in T2circ were computed on log transformed T2circ values. Similar to the time-frequency TP and ITC 40Hz steady-state findings, in the full sample no group differences were observed for T2circ for any source space or sensor measure (all ps > 0.15). Exploratory analyses also examined younger and older T2circ group differences. In the younger participants, HC > SZ T2circ values were observed for MEG left STG (p < 0.05) and EEG left STG (p < 0.05). In the older sample, no T2circ group differences were observed.
4. Discussion
The literature on neural oscillatory auditory responses in SZ has been plagued by inconsistencies in findings that may be driven in part by inconsistencies in measurement, constituting an obstacle to identifying neural mechanisms contributing to perceptual abnormalities in SZ. Present results indicate that, whereas HC and SZ auditory encoding low-frequency effects are generally comparable across modality and analysis strategies, 40 Hz steady-state difference findings are much more dependent on modality and analysis strategy. In particular, low-frequency analyses did not support the hypothesis of better group differentiation for source than sensor measures, with post-stimulus low-frequency group differences observed across all recording strategies (source and sensor), indicating that the detection of low-frequency group differences is generally not reliant on recording or analysis strategy. In contrast, the 40Hz steady-state analyses supported the study hypothesis, although given an age-related decrease in left STG 40Hz steady-state activity in HC, the expected HC > SZ 40Hz steady-state group differences were evident only in the younger participants’ source-space measures (MEG left STG 40 Hz ITC and MEG and EEG left STG T2circ). For EEG, given strong age-related 40Hz steady-state changes in HC, 40Hz steady-state group differences were in the unexpected direction in older participants (left STG ITC). As detailed below, these results help make sense of the 40Hz steady-state inconsistencies in the literature - in some studies 40 Hz steady-state activity is greater in controls than individuals with schizophrenia, in some the converse, and in some studies no group differences are observed.
4.1 Age Confound in Assessment of Auditory Encoding Processes
4.1a Post-stimulus low-frequency activity
As in previous studies, post-stimulus low-frequency TP and ITC auditory encoding activity was lower in SZ than in HC (Blumenfeld & Clementz, 2001; Clementz & Blumenfeld, 2001; J. C. Edgar et al., 2008; Jansen et al., 2004; Johannesen et al., 2005). Although as in previous studies there was some evidence for larger post-stimulus low-frequency group differences in the left than right STG (J. C. Edgar et al., 2014; Hall et al., 2011), in the present study ANOVA analyses showed only main effects of Group and no Group × Hemisphere interaction. Age did not predict post-stimulus low-frequency activity, but Online Tables 3 and 4 indicated larger group difference effects for post-stimulus low-frequency activity in younger (left STG Cohen’s d ranging from 0.76 to 1.08) than older participants (left STG Cohen’s d ranging from (0.15 to 0.38).
4.1b 40Hz steady-state activity
In the full sample, no SZ/HC group difference in 40Hz steady-state activity was observed. This null finding was somewhat surprising in light of many studies reporting less 40Hz steady-state activity in SZ (Brenner et al., 2003; Krishnan et al., 2009; Kwon et al., 1999; Light et al., 2006; Spencer, Salisbury, Shenton, & McCarley, 2008; Teale et al., 2008; Tsuchimoto et al., 2011; Vierling-Claassen, Siekmeier, Stufflebeam, & Kopell, 2008). As outlined in the Introduction, however, given an age-related decrease in left 40Hz steady-state TP and ITC in HC, it was hypothesized that HC and SZ left STG 40Hz steady-state group differences would be more prominent in younger than in older participants. This hypothesis received support. As shown in Tables 3a,b and Figure 2, older age was associated with less left STG 40Hz steady-state activity, and the 40Hz steady-state analyses splitting by age showed more robust left 40Hz steady-state activity in HC than in SZ only in younger participants (MEG left ITC and EEG and MEG left T2circ). In contrast, higher EEG left 40Hz steady-state ITC in SZ than in HC was observed in the older participants (see Figure 3 and Online Supplement Table 4).
In a meta-analysis of 40Hz steady-state studies of SZ, Thuné et al. (Thune et al., 2016) observed a trend for an effect of age on group differences (p=0.09), with HC versus SZ effect sizes 0.52 for patients older than 39.8 years and 0.77 for patients younger than 39.8 years. Present findings extend Thuné et al. (Thune et al., 2016) by showing that this effect may largely be due to an age-related decrease in left STG 40Hz steady-state activity in HC. Present findings and the Thuné et al. review thus demonstrate that studies examining HC and SZ group differences in STG 40Hz steady-state activity are more likely to observe group differences when examining younger participants, such as comparing first-episode SZ and age-matched controls. Several studies provide support for this claim. In particular, studies examining controls and individuals with SZ with a mean age in the late 30s (Rass et al., 2012) and early 40s (Hong et al., 2004) have reported only trend-level group differences (Rass et al., 2012) or no group differences (Hong et al., 2004). Hamm et al. (Hamm et al., 2012), reporting auditory steady-state activity in HC and SZ participants with a mean age of ~40 years (SD ~= 8.5 years), observed greater steady-state activity in SZ than in HC, with the greater 40Hz steady-state response in SZ perhaps due to the age-related decrease in 40Hz steady-state activity present in controls. In contrast, studies examining first-episode and early onset SZ have reported HC versus SZ 40Hz steady-state group differences (Spencer et al., 2009; Wilson et al., 2008). Nevertheless, some studies examining older participants (mean age greater than 40 years) have reported HC versus SZ group differences (Hirano et al., 2015; Krishnan et al., 2009; Kwon et al., 1999). (It is of note that HC and SZ samples sizes for the separate younger and older participant analyses in the present study are similar to or larger than the majority of previous studies examining 40Hz steady-state activity in SZ (see Table 1 in Thune et al. ref (Thune et al., 2016)), and thus the above comparisons are reasonable.)
The present finding of age-related changes in 40Hz steady-state activity can be interpreted within the context of other studies, with a review of the 40Hz steady-state literature indicating an inverted-U shape function for 40Hz steady-state activity in typically developing participants, with absent or very weak 40Hz steady-state activity in children and young adolescents (Cho et al., 2015; J. C. Edgar et al., 2016; Rojas et al., 2006), relatively large 40Hz steady-state responses through late adolescents and middle age, and then weaker 40Hz steady-state responses in older age. The above pattern indicates that 40Hz auditory driving stimuli may not be optimal for examining STG 40Hz auditory neural circuits in younger populations (e.g., children with autism spectrum disorder) or in elderly populations.
4.2 Comparing Low-frequency and 40Hz Steady-state Group Differences
Differences between the pattern of 40Hz steady-state and post-stimulus low-frequency source and sensor group effects may be due to the fact that the latter are less lateralized and thus make reliance on focused measurement of left and right STG post-stimulus low frequency activity less critical. In addition to low-frequency oscillatory activity being larger than 40Hz oscillatory activity (thus low-frequency activity having a higher signal-to-noise ratio), low-frequency oscillatory abnormalities in SZ are observed in multiple brain regions (Y. H. Chen et al., 2016; Fehr et al., 2001; Pascual-Marqui et al., 1999; Sponheim, Clementz, Iacono, & Beiser, 1994; Winterer et al., 2000). As a consequence, assessment of low-frequency auditory abnormalities in SZ may be less dependent on recording strategy. Perhaps more importantly, whereas local networks of pyramidal cells and inhibitory interneurons in superficial cortical layers are likely sufficient to maintain the 40Hz driving response (Cardin et al., 2009; Rubenstein & Merzenich, 2003; Tamas, Buhl, Lorincz, & Somogyi, 2000; Tiesinga & Sejnowski, 2009), low-frequency post-stimulus auditory activity (associated with the 50ms and 100ms auditory responses) reflects input to primary/secondary auditory areas via thalamocortical interactions in deep cortical layers as well as interactions between temporal and frontal areas (Buffalo, Fries, Landman, Buschman, & Desimone, 2011; Y. Chen et al., 2013; Knight, Staines, Swick, & Chao, 1999), with these more spatially distributed post-stimulus low-frequency neural processes perhaps adequately assessed across recording and analyses methods.
Regarding specific EEG sensor-space strategies, PCA measures did not appear to show a clear advantage over EEG Cz or Fz sensor measures. 40Hz steady-state group differences were not observed at either Cz or Fz, but p-values suggested larger 40Hz steady-state group differences at Fz than Cz. Of note, however, the MEG and EEG one and two standard deviation lines in supplement Figure S1 show that in many participants the 40Hz steady-state peak source/sink “hotspots” are not precisely at EEG Cz or Fz.
4.3 Study Limitations
Study findings must be interpreted within the context of stimuli and task, with future studies needed to determine whether similar findings are observed using click stimuli popular in the SZ literature, rather than 40Hz amplitude-modulated tones, as well as using shorter ISIs. Whereas use of a shorter ISI would reduce task time, too short an ISI would likely attenuate post-stimulus low-frequency activity and thus may reduce the ability to detect post-stimulus low-frequency group differences.
The present study has several limitations. First, most participants were chronic patients, almost all on medication. As such, it is not possible to determine whether the observed abnormalities were due to the disorder or an effect of medication. As previously noted, however, several studies have reported that 40Hz steady-state abnormalities in SZ are present at first onset and in first-degree relatives (Rass et al., 2012; Spencer et al., 2008), arguing against a medication confound. In addition, in a review of 100 ms auditory studies Rosburg et al. (Rosburg et al., 2008) concluded that medication did not seem to account for group differences in N100 activity (related to the post-stimulus low-frequency activity examined in this study). As noted in Results, no associations between EEG and MEG auditory encoding measures and medication were observed (analogous findings for 40 Hz steady-state activity reported by Hirano et al. (Hirano et al., 2015)), again suggesting that present group difference findings were not due to medication.
Another limitation of the present study is that the orientation of the left and right STG dipoles was determined based on 40Hz steady-state activity (see Online Supplement Figure 1). As a result, estimates of post-stimulus low-frequency activity may not have been optimal. However, given general similarity in the topography of the 40Hz steady-state and the P50/M50 and N100/M100 ERP/ERF, it is unlikely that this resulted in substantial error in estimates of post-stimulus low-frequency activity (and with similar error for HC and SZ), especially given very similar orientation in the 40Hz steady-state and M100 generators(Weisz, Keil, Wienbruch, Hoffmeister, & Elbert, 2004). In addition, the fact that EEG sensor measures identified post-stimulus low-frequency abnormalities in SZ suggests that accurate dipole orientation is less of a concern for post-stimulus low-frequency activity than 40 Hz steady-state activity. Nonetheless, the bias in the study for better estimating 40Hz steady-state activity is noted.
Given a primary goal of comparing MEG and EEG source findings and EEG source and sensor findings, no correction was applied to control family-wise error. Of note, however, where marginally significant findings were observed, significant findings were generally observed in a closely related measure (e.g., marginally significant for TP but significant for ITC), indicating general consistency in findings. Although the number of tests could have been reduced by combining EEG and MEG to identify a combined measure that best differentiated SZ and HC, this line of investigation, although of interest, was outside the scope of the present study.
4.3 Conclusions
Post-stimulus low-frequency group differences were robustly observed across all recording methods (MEG and EEG source and sensor space), indicating that the identification of low-frequency pathology in SZ is generally robust to recording method. In contrast, auditory 40Hz steady-state group differences were best observed using source-space analyses and better observed in younger than older adult participants. Present findings thus suggest that optimal study design, data collection, and analysis methods depend on the auditory encoding measure of interest. Future work replicating these findings as well as extending this multimodal approach to compare other EEG and MEG clinical markers is needed so that such EEG and MEG work can move forward with an optimal data collection and analysis approach derived for each neural measure of interest as well as a head-to-head comparison of modality and analysis strategies.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01 MH65304 to Dr. José M. Cañive, K08 MH085100 to Dr. J. Christopher Edgar, K01 MH108822 to Dr. Yuhan Chen, a VA Merit grant (VA Merit CSR&D: IIR-04-212-3 to Dr. José M. Cañive), a COBRE grant to UNM (P20 RR021938), and a University of California, San Diego, Merit Review Grant from the Department of Veterans Affairs to Dr. Mingxiong Huang. The authors would like to thank the participants who enrolled in this study and Megan Schendel, Kim Paulson, and Emerson Epstein, who helped with data collection, and Lawrence Calais, Gloria Fuldauer, and Nickolas Lemke for their help with participant recruitment and administrative support related to this project.
Footnotes
Financial Disclosure
The authors have no biomedical financial interests or potential conflicts of interest to report.
References
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994;90(3):229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Berman JI, Edgar JC, Blaskey L, Kuschner ES, Levy SE, Ku M, Roberts TP. Multimodal Diffusion-MRI and MEG Assessment of Auditory and Language System Development in Autism Spectrum Disorder. Front Neuroanat. 2016;10:30. doi: 10.3389/fnana.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clin Neurophysiol. 2001;112(9):1650–1659. doi: 10.1016/S1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160(12):2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci U S A. 2011;108(27):11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Edgar JC, Huang M, Hunter MA, Epstein E, Howell B, Cañive JM. Frontal and superior temporal auditory processing abnormalities in schizophrenia. Neuroimage: Clinical. 2013;2:695–702. doi: 10.1016/j.nicl.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Stone-Howell B, Edgar JC, Huang M, Wootton C, Hunter MA, Canive JM. Frontal slow-wave activity as a predictor of negative symptoms, cognition and functional capacity in schizophrenia. Br J Psychiatry. 2016;208(2):160–167. doi: 10.1192/bjp.bp.114.156075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen CM, Lewis DA. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex. 2015;25(6):1509–1518. doi: 10.1093/cercor/bht341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139(4):377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Chen YH, Lanza M, Howell B, Chow VY, Heiken K, Canive JM. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin. 2014;4:122–129. doi: 10.1016/j.nicl.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Fisk CLt, Liu S, Pandey J, Herrington JD, Schultz RT, Roberts TP. Translating Adult Electrophysiology Findings to Younger Patient Populations: Difficulty Measuring 40-Hz Auditory Steady-State Responses in Typically Developing Children and Children with Autism Spectrum Disorder. Dev Neurosci. 2016;38(1):1–14. doi: 10.1159/000441943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Fisk Iv CL, Chen YH, Stone-Howell B, Hunter MA, Huang M, Miller GA. By our bootstraps: Comparing methods for measuring auditory 40 Hz steady-state neural activity. Psychophysiology. 2017;54(8):1110–1127. doi: 10.1111/psyp.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Hanlon FM, Huang MX, Weisend MP, Thoma RJ, Carpenter B, Miller GA. Superior temporal gyrus spectral abnormalities in schizophrenia. Psychophysiology. 2008;45(5):812–824. doi: 10.1111/j.1469-8986.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Hunter MA, Huang M, Smith AK, Chen Y, Sadek J, Canive JM. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr Res. 2012;140(1–3):250–257. doi: 10.1016/j.schres.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, Cannon KM, Monroe JF, Cornew L, Qasmieh S, Liu S, Welsh JP, Levy SE, Roberts TP. Neruomagnetic noise predicts evoked-response delays and core language deficits in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1904-x. [DOI] [PMC free article] [PubMed]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2015;77(2):127–136. doi: 10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T, Kissler J, Moratti S, Wienbruch C, Rockstroh B, Elbert T. Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenic patients. Biol Psychiatry. 2001;50(2):108–116. doi: 10.1016/s0006-3223(01)01122-2. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62(3):1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. 2011;37(4):778–787. doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Bobilev AM, Hayrynen LK, Hudgens-Haney ME, Oliver WT, Parker DA, Clementz BA. Stimulus train duration but not attention moderates gamma-band entrainment abnormalities in schizophrenia. Schizophr Res. 2015;165(1):97–102. doi: 10.1016/j.schres.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Clementz BA. Augmented gamma band auditory steady-state responses: support for NMDA hypofunction in schizophrenia. Schizophr Res. 2012;138(1):1–7. doi: 10.1016/j.schres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R. The neuromagnetic method in the study of the human auditory cortex. In: Grandori MHF, Romani G, editors. Auditory evoked magnetic fields and potentials advances in audiology. Basel: Karger; 1990. [DOI] [Google Scholar]
- Hari R, Aittoniemi K, Jarvinen ML, Katila T, Varpula T. Auditory evoked transient and sustained magnetic fields of the human brain. Localization of neural generators. Exp Brain Res. 1980;40(2):237–240. doi: 10.1007/bf00237543. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Wollbrink A, Chau W, Ishii R, Ross B, Pantev C. Determination of activation areas in the human auditory cortex by means of synthetic aperture magnetometry. Neuroimage. 2003;20(2):995–1005. doi: 10.1016/s1053-8119(03)00403-8. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous Gamma Activity in Schizophrenia. JAMA Psychiatry. 2015;72(8):813–821. doi: 10.1001/jamapsychiatry.2014.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16(4):233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Thaker GK. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70(2–3):293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, O’Donnell P, Thaker GK. A shared low-frequency oscillatory rhythm abnormality in resting and sensory gating in schizophrenia. Clin Neurophysiol. 2012;123(2):285–292. doi: 10.1016/j.clinph.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi RJ, Naatanen R. Combined mapping of human auditory EEG and MEG responses. Electroencephalogr Clin Neurophysiol. 1998;108(4):370–379. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Fitzgerald MB. Auditory evoked gamma band potential in normal subjects. J Am Acad Audiol. 1997;8(1):44–52. [PubMed] [Google Scholar]
- Jansen BH, Hegde A, Boutros NN. Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2004;115(3):523–533. doi: 10.1016/j.clinph.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res. 2005;78(2–3):269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999;101(2–3):159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Koenig T, van Swam C, Dierks T, Hubl D. Is gamma band EEG synchronization reduced during auditory driving in schizophrenia patients with auditory verbal hallucinations? Schizophr Res. 2012;141(2–3):266–270. doi: 10.1016/j.schres.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47(4):1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz D, Fischer S, Schadow J, Bogerts B, Herrmann CS. Altered evoked gamma-band responses as a neurophysiological marker of schizophrenia? Int J Psychophysiol. 2011;79(1):25–31. doi: 10.1016/j.ijpsycho.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60(11):1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Maharajh K, Teale P, Rojas DC, Reite ML. Fluctuation of gamma-band phase synchronization within the auditory cortex in schizophrenia. Clin Neurophysiol. 2010;121(4):542–548. doi: 10.1016/j.clinph.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela JP, Hamalainen M, Hari R, McEvoy L. Whole-head mapping of middle-latency auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol. 1994;92(5):414–421. doi: 10.1016/0013-4694(94)00107-v. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand? Annu Rev Clin Psychol. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–784. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- Pantev C. Evoked and induced gamma-band activity of the human cortex. Brain Topogr. 1995;7(4):321–330. doi: 10.1007/bf01195258. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999;90(3):169–179. doi: 10.1016/s0920-9964(97)88607-0. [DOI] [PubMed] [Google Scholar]
- Pelizzone M, Hari R, Makela JP, Huttunen J, Ahlfors S, Hamalainen M. Cortical origin of middle-latency auditory evoked responses in man. Neurosci Lett. 1987;82(3):303–307. doi: 10.1016/0304-3940(87)90273-4. [DOI] [PubMed] [Google Scholar]
- Rass O, Forsyth JK, Krishnan GP, Hetrick WP, Klaunig MJ, Breier A, Brenner CA. Auditory steady state response in the schizophrenia, first-degree relatives, and schizotypal personality disorder. Schizophr Res. 2012;136(1–3):143–149. doi: 10.1016/j.schres.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50 msec latency auditory evoked field component. Electroencephalogr Clin Neurophysiol. 1988;70(6):490–498. doi: 10.1016/0013-4694(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale PD, Kleman MR, Benkers TL, Carlson JP, Reite ML. Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin Neurophysiol. 2006;117(1):110–117. doi: 10.1016/j.clinph.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia–a critical review. Psychiatry Res. 2008;161(3):259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Ross B, Herdman AT, Pantev C. Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cereb Cortex. 2005;15(12):2029–2039. doi: 10.1093/cercor/bhi078. [DOI] [PubMed] [Google Scholar]
- Ross B, Picton TW, Pantev C. Temporal integration in the human auditory cortex as represented by the development of the steady-state magnetic field. Hear Res. 2002;165(1–2):68–84. doi: 10.1016/s0378-5955(02)00285-x. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol. 1987;32(1):11–22. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Scherg M, Berg P. Use of prior knowledge in brain electromagnetic source analysis. Brain Topogr. 1991;4(2):143–150. doi: 10.1007/bf01132771. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ebersole JS. Models of brain sources. Brain Topogr. 1993;5(4):419–423. doi: 10.1007/bf01128700. [DOI] [PubMed] [Google Scholar]
- Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, Canive JM. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry. 2010;167(10):1264–1275. doi: 10.1176/appi.ajp.2010.09071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci. 2011;5:190. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64(5):369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31(1):37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3(4):366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M, Simola J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 2004;16(4):269–275. doi: 10.1023/b:brat.0000032864.93890.f9. [DOI] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42(4):1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thune H, Recasens M, Uhlhaas PJ. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA Psychiatry. 2016;73(11):1145–1153. doi: 10.1001/jamapsychiatry.2016.2619. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63(6):727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto R, Kanba S, Hirano S, Oribe N, Ueno T, Hirano Y, Onitsuka T. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr Res. 2011;133(1–3):99–105. doi: 10.1016/j.schres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Calkins ME. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64(12):1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78(5):378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99(5):2656–2671. doi: 10.1016/s0920-9964(08)70021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Keil A, Wienbruch C, Hoffmeister S, Elbert T. One set of sounds, two tonotopic maps: exploring auditory cortex with amplitude-modulated tones. Clin Neurophysiol. 2004;115(6):1249–1258. doi: 10.1016/j.clinph.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18(2):371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann WM. Frontal dysfunction in schizophrenia–a new electrophysiological classifier for research and clinical applications. Eur Arch Psychiatry Clin Neurosci. 2000;250(4):207–214. doi: 10.1007/s004060070026. [DOI] [PubMed] [Google Scholar]
- Yvert B, Crouzeix A, Bertrand O, Seither-Preisler A, Pantev C. Multiple supratemporal sources of magnetic and electric auditory evoked middle latency components in humans. Cereb Cortex. 2001;11(5):411–423. doi: 10.1093/cercor/11.5.411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


