Abstract
Introduction
Propofol is an effective sedative for Magnetic Resonance Imaging (MRI). Nevertheless, it may cause hemodynamic and respiratory complications in a dose dependent fashion. We investigated the role of low-dose dexmedetomidine (0.5 mcg/kg) as an adjuvant to propofol sedation for children undergoing MRI. We hypothesized that dexmedetomidine would decrease the propofol dose required, airway complications, and hemodynamic instability.
Methods
We performed a retrospective chart review of patients’ age of 1 month to 20 years. Children were divided into two groups; group P received only propofol; group D+P received intravenous bolus of dexmedetomidine (0.5mcg/kg) and propofol.
Results
We reviewed 172 children in P and 129 in D+P (dexmedetomidine dose, median: 0.50 mcg/kg (IQR: 0.45 – 0.62). An additional dexmedetomidine bolus was given to 17 children for sedation lasting longer than 2 hours. Total propofol dose (mcg/kg/min) was significantly higher in group P than D+P; 215.0 (182.6 – 253.8) vs. 147.6 (127.5 – 180.9); Median Diff= −67.8; 95%CI=, −80.6, −54.9; p<0.0001. There was no difference in recovery time (minutes); P: 28 (17 – 39) vs. D+P: 27 (18-41); Median Diff = −1; 95%CI= −6.0, 4.0; p=0.694. The need for airway support was significantly greater in P compared to D+P; 15/172 vs. 3/129; OR= 0.25; 95%CI= 0.07 to 0.90; p=0.02 (two-sample proportions test). Mean arterial pressure was significantly lower in P compared to D+P across time over 60 minutes after induction (coef= −0.06, 95%CI= −0.11, −0.02, p= 0.004).
Discussion & Conclusion
A low dose bolus of dexmedetomidine (0.5 mcg/kg) used as an adjuvant can decrease the propofol requirement for children undergoing sedation for MRI. This may decrease the need for airway support and contribute to improved hemodynamic stability without prolonging recovery time.
Keywords: dexmedetomidine, propofol, pediatric sedation, MRI
Introduction
A propofol intravenous infusion is a common means to provide sedation for children undergoing MRI (1–5). Propofol enables both a smooth induction of sedation and a rapid recovery. Nevertheless, propofol is known to diminish systemic vascular resistance and decrease mean arterial pressure (6). It may also cause decreased respiratory drive and upper airway obstruction (6, 7). Propofol infusions may cause delayed emergence in higher doses over a prolonged time (8). Occasionally, deeper sedation is needed to avoid emergence during scans with higher Tesla MRI machines due to noise (1). Larger doses of propofol in these settings may increase the side effects.
Dexmedetomidine is a selective alpha-2 adrenergic agonist that can be used as a primary anesthetic as well as an adjuvant to propofol or volatile anesthetics. As a single sedative agent for MRI, multiple reports have elucidated the superior features of dexmedetomidine over propofol with regard to hemodynamic stability and decreased risk of oxygen desaturation. However, when used as a sole sedative, large doses of dexmedetomidine are required and this may contribute to delayed recovery after sedation (9–14). High-dose dexmedetomidine may also cause hemodynamic instability including bradycardia and hypertension (13, 15, 16). Dexmedetomidine has been reported to reduce propofol plasma concentrations and dose (17) required for sedation and suppression of motor response in healthy subjects. In a pediatric study, dexmedetomidine combined with propofol infusion reduced total propofol dose and decreased the incidence of airway complications (18). We also wanted to explore the effect of low dose dexmedetomidine on hemodynamic parameters from pre-induction to up to 60 minutes after induction. In this study, we compared intravenous propofol infusion with or without a low-dose dexmedetomidine bolus prior to induction. We hypothesized that low-dose dexmedetomidine as an adjuvant would decrease the amount of propofol required for sedation, reduce the need for airway support, and improve hemodynamic stability.
Materials and Methods
After approval by the Institutional Review Board, we performed a retrospective chart review of all patients, age from 1 month old to 20 years old with an American Society of Anesthesiologists Physical Status Classification of 1–3, who received sedation for MRI between November 1, 2016 and December 31, 2016. Exclusion criteria were: absence of pre-induction hemodynamic or respiratory vital signs, planned inhalational general anesthesia, or cases shorter than 30 minutes. We also excluded cases converted from use of intravenous infusion to inhalational general anesthesia. Children were divided into two groups; group P received propofol only; group D+P received intravenous bolus of dexmedetomidine followed by propofol bolus and infusion.
All the children were induced to a Ramsay Sedation Scale (19) status of at least 5 or 6 (sluggish or no response to light glabellar tap or loud auditory stimulus) suited for 1.5 Tesla (T) or 3T MRI. Then, sedation was maintained at a level in which noise and vibration in MRI does not cause movement of children. As the patient’s were not stimulated during the procedure it is possible that the level of sedation was deeper than 6 but with maintenance of appropriate vital signs. During the study period, our sedation using propofol without dexmedetomidine bolus (group P) consisted of an intravenous bolus of 2-3 mg/kg followed by a maintenance infusion started at 150 – 300 (median 250) mcg/kg/min. For the sedation with dexmedetomidine (group D+P), a single intravenous bolus of dexmedetomidine (0.5mcg/kg) over 5 minutes was followed by the same intravenous bolus of propofol (2-3 mg/kg), then a maintenance infusion of propofol was started at 50-300 (median 150) mcg/kg/min (Table 2). In cases exceeding two hours, some patients received an additional dexmedetomidine bolus (less than 0.5 mcg/kg) at least 60 minutes after the initial bolus.
Table 2.
Comparison of administered propofol and recovery time in both groups
| Propofol N = 172 |
Dex + Propofol N = 129 |
Median Diff (95% CI) | Difference P-value |
|
|---|---|---|---|---|
| Propofol bolus (mg/kg) | 3.13 (2.4 – 4.0) | 3.05 (2.3 – 3.8) | −0.08 (−0.4, 0.3) | 0.659 |
| propofol infusion rate | ||||
| start: median (min-max) | 250 (150-300) | 150 (50-300) | −100 (−116.9. −83.1) | <0.0001 |
| end: median (min-max) | 200 (150-300) | 125 (10-300) | −75 (−96.4, −53.6) | <0.0001 |
| Anesthesia time (minutes) | 89 (66 – 114) | 90 (67 – 126) | 0 (−11.0, 11.0) | 0.998 |
| Total Propofol infused (mcg/kg/min) | 176.9 (145.7 – 205.9) | 114.1 (97.8 – 138.3) | −62.3 (−71.4, −53.2) | <0.0001 |
| Total Propofol given (mcg/kg/min) | 215.0 (182.6 – 253.8) | 147.6 (127.5 – 180.9) | −67.8 (−80.6, −54.9) | <0.0001 |
| Recovery time (minutes) | 28 (17-39) | 27 (18 – 41) | −1 (−6.0, 4.0) | 0.694 |
group P, propofol only; group D+P, propofol infusion with dexmedetomidine bolus.
Values are expressed as median (IQR) except Propofol infusion rate at the start and the end.
+ Statistical comparison is based on Wilcoxon ranksum test.
All anesthesiologists who provided sedation in MRI were pediatric anesthesiology fellowship trained with experience that ranged from one year to over 10 years (1-5 years, 10 anesthesiologists; 5-10 years, 3; longer than 10 years, 6). We surveyed these anesthesiologists to determine our routine care. Our overall response rate was 89.5% (17/19). Summary of survey results are as follows.
Initial airway management is Nasal cannula oxygen (2LPM) with endtidal CO2 monitoring (EtCO2) via side-port (consistently reported in 94% of surveys). All providers employ a shoulder roll. We assess of depth of sedation via continuous observation of vital signs, oxygen saturation, EtCO2 waveform, and movement noticed through window or disturbed MRI image (consistency 93%). We consider noise and vibration in MRI to be strong enough to wake up children unless sedated adequately (consistency 100%). Titration of propofol infusion rate is judged by vital signs, changed of SO2, EtCO2 waveform, direct observation of movement and MRI imaging disturbance. Deepening sedation is achieved by bolus of propofol, increase of propofol infusion and/or, bolus of dexmedetomidine. Indications for airway adjuncts (oral airway, nasal airway, O2 face mask, LMA) as indicated by our survey were upper airway obstruction as shown by paradoxical movement of chest, loss of breath sounds, loss of EtCO2 waveform, or head movement that may interfere with MRI image. The indication to increase the flow of oxygen was decreasing oxygen saturations (consistency 83%). Hypoxia (SO2 < 90%) that is unresolved by the use of airway adjuncts and increases in O2 flow is managed with an LMA or endotracheal tube (consistency 85%). More than a 20% drop of mean blood pressure (MAP) from baseline (pre-anesthesia measurement) is considered clinically significant hypotension and is an indication for intervention such as decreasing propofol infusion rate and/or fluid therapy (consistency 66%).
Study data obtained for analysis includes age, weight, gender, ASA classification, diagnosis by service, pre-existing conditions which may affect respiration, type of MRI (site), MRI tesla, and anesthesia time. Dexmedetomidine dose (bolus), and propofol dose (bolus plus infused dose) were examined. Infused propofol dose and total administered propofol dose (bolus plus infused dose) were expressed as (dose administered; mcg)/(body weight; kg)/(anesthesia time; minutes). Hemodynamic and respiratory parameters including HR, RR, MAP and SO2 were obtained from the pre-induction period as baseline, then at 5, 30, and 60 minutes after induction. Complications analyzed during MRI were: airway interventions (none, oral/nasal airway, LMA), increase of O2 flow from default (2 L/minutes), deepening sedation (timing after starting MRI), fluid therapy and clinical hypotension (MAP drop more than 20% from pre anesthesia measurement). Clinically significant hypotension was assessed at 3 time points (5, 30, 60 minutes after induction) and recorded as times from 0 to 3. Post Anesthesia Care Unit (PACU) recovery score was assessed by PACU nursing staff using the Modified Aldrete Scoring System (20). Recovery time was determined as the duration from the anesthesia finish time (the time of first vital signs check in the PACU) to discharge ready time by PACU recovery score over 8 out of 10.
The two groups were compared with respect to age, weight, gender, ASA classification, diagnosis by service, pre-existing conditions which may affect respiration, type of MRI (site), MRI tesla, propofol bolus dose, propofol infusion dose, propofol total dose, anesthesia time, recovery time, airway intervention, increase of O2 flow, deepening sedation, fluid therapy and incidence of clinical hypotension (MAP drop more than 20% from pre anesthesia measurement), and vital signs including HR, RR, MAP, and SO2. The measure of association of demographic data and complications of the two groups were analyzed with Chi-Square test. Anesthesia time, propofol bolus dose, propofol infusion dose, propofol total dose, and recovery time of the two groups were expressed as median and interquartile range (IQR), then compared using Wilcoxon rank sum test. Generalized estimating equation based on linear regression with repeated measure adjustment was utilized to examine hemodynamic and respiratory parameters over time between the two treatment groups. The results are summarized as beta coefficient with 95% confidence intervals and p-values. All analyses were two-sided with significance set at p<0.05. Statistical computations were performed using Stat/IC 13.1(StataCorp, College Station, TX).
The quality of MRI was blindly assessed by senior pediatric radiologist. Ten cases from each group were randomly selected.
Results
A total of 306 children were included in this study. One hundred-seventy-two children received propofol infusion only (group P). One hundred-twenty-nine children received propofol and intravenous bolus of dexmedetomidine (group D+P). Administered dexmedetomidine dose in group D+P was 0.50 (IQR: 0.45 – 0.62) mcg/kg. There were 17 children whose MRI lasted longer than 2 hours received a second bolus of dexmedetomidine. The demographic data is shown in Table 1. Details of pre-existing conditions (19 in group P, 14 in D+P) that may affect respiration were obstructive sleep apnea (OSA) diagnosed by sleep study (5 in group P, 6 in D+P), obesity (BMI higher than 99 percentile by age prediction, 2 in group P), upper respiratory infection (URI) within one month (3 in P), chronic lung disease (2 in P, 1 in D+P), pulmonary edema (1 in P), airway compression by mass (4 in P, 1 in D+P), airway obstruction by craniofacial anomaly (1 in D+P), vocal cord paralysis (1 in D+P), severe asthma (1 in D+P), hypotonia (2 in P, 2 in D+P), and Down syndrome (1 in D+P). There were two cases with pre-existing conditions (OSA, obesity) who were converted to LMA by provider’s judgment.
Table 1.
Demographic and baseline data between P and D+P groups
| P N = 172 |
D+P N = 129 |
||
|---|---|---|---|
| Gender | Male | 87 (50.6%) | 61 (47.3%) |
| Female | 85 (49.4%) | 68 (52.7%) | |
|
| |||
| Age group (years) | < 1 | 21 (12.2%) | 10 (7.8%) |
| 1 – 5 | 74 (43.0%) | 53 (41.1%) | |
| 6 – 10 | 53 (30.8%) | 43 (33.3%) | |
| >10 | 24 (14.0%) | 23 (17.8%) | |
|
| |||
| Weight group (kg) | <10 | 19 (11.1%) | 12 (9.3%) |
| 10 – 19 | 87 (50.6%) | 61 (47.3%) | |
| 20 – 40 | 42 (24.4%) | 36 (27.9%) | |
| >40 | 24 (14.0%) | 20 (15.5%) | |
|
| |||
| ASA classification | 1 | 16 (9.3%) | 16 (12.4%) |
| 2 | 83 (48.3%) | 47 (36.4%) | |
| 3 | 73 (42.4%) | 66 (51.2%) | |
|
| |||
| Diagnosis by service | Endocrine | 2 (1.2%) | 0 (0%) |
| ENT | 7 (4.1%) | 4 (3.1%) | |
| GastroIntestinal | 10 (5.8%) | 3 (2.3%) | |
| Hematology/Oncology | 12 (7.0%) | 12 (9.3%) | |
| NeuroSurgery | 56 (32.6%) | 34 (26.4%) | |
| Neurology | 51 (29.7%) | 38 (29.5%) | |
| Opthalmology | 10 (5.8%) | 12 (9.3%) | |
| Orthopedics | 20 (11.6%) | 17 (13.2%) | |
| Urology | 4 (2.3%) | 4 (3.1%) | |
| other | 0 (0%) | 5 (3.9%) | |
|
| |||
| pre-existing condition affecting respiration | Yes | 19 (11.1%) | 14 (10.9%) |
| No | 153 (89.0%) | 115 (89.1%) | |
|
| |||
| MRI site | Head | 96 (55.8%) | 69 (53.5%) |
| Spine | 15 (8.7%) | 16 (12.4%) | |
| Trunk | 17 (9.9%) | 12 (9.3%) | |
| Extremity | 21 (12.1%) | 11 (8.5%) | |
| Head & Spine | 19 (11.0%) | 20 (15.5%) | |
| Head & Trunk | 1 (0.6%) | 0 (0%) | |
| Trunk & Spine | 2 (1.2%) | 0 (0%) | |
| Extremity & Spine | 1 (0.6%) | 0 (0%) | |
| Trunk & Extremity | 0 (0%) | 1 (0.8%) | |
|
| |||
| MRI Tesla | 1.5T | 104 (60.5%) | 82 (63.6%) |
| 3T | 68 (39.5%) | 47 (36.4%) | |
group P, propofol only; group D+P, propofol infusion with dexmedetomidine bolus.
Statistical comparison is based on Chi-Square test.
The comparisons of administrated propofol were shown in Table 2. There was no difference in bolus propofol dose (mg/kg) between groups P and D+P; 3.1 (2.4 – 4.0) vs. 3.1 (2.3 – 3.9). The infused propofol dose (mcg/kg/min) was significantly higher in group P than group D+P; 176.9 (145.7 – 205.9) vs. 114.1 (97.8 – 138.3), p<0.0001). Total propofol (mcg/kg/min) was also significantly greater in P than D+P; 215.0 (182.6 – 253.8) vs. 147.6 (127.5 – 180.9), p<0.0001. There was no significant difference in anesthesia time (minutes) between groups P and D+P; 90 (66 – 114) vs. 90 (67 – 126), p=0.99. There was no significant difference in recovery time (minutes) between groups P and D+P; 28 (17 – 39) vs. 27 (18-41), p=0.694.
The comparison of complications during the MRI is shown in Table 3. In group P, fifteen out of 172 (8.7%) children required airway support (oral or nasal airway 10 children and LMA 5 children). In contrast in the D+P group, only two out of 129 (2.3%) children required a nasal or oral airway. The difference was statistically significant (p=0.02, Chi-square test). More children in group P needed an increase in O2 flow than those in group D+P but this was not statistically significant; 15 (8.7%) vs. 5 (3.9%), p=0.1. There was no relevant difference in the percentage of children in group P needed deepening sedation compared to those in group D+P, 27 (15.7%) vs. 19 (14.7%), p=0.94. Focusing on first 60 minutes after MRI start, a greater percentage of children in in group P needed deepening sedation compared to those in group D+P but this was not statistically significant; 18 (10.5%) vs. 8 (6.2%), p= 0.26. Fluid therapy was given to two children (1.2%) in group P and two (1.6%) in group D+P. In comparing the number of episodes of clinically significant hypotension at 5, 30, 60 minutes after induction, 12 (7.0%) children in group P had no episodes, 21 (12.2%) had one, 42 (24.4%) had two and 97 (56.4%) had three. In comparison, 22 (17.0%) children in group D+P had no epidsodes, 28 (21.7%) had one, 50 (38.8%) had two, and 29 (22.5%) had three. The difference in two groups was statistically significant (p<0.0001, Chi-square test).
Table 3.
Comparison of complications in both groups
| P N = 172 |
D+P N = 129 |
OR (95% CI) (95% CI) |
Difference P-value |
|
|---|---|---|---|---|
| Airway support | ||||
| No | 157 (91.3%) | 126 (97.7%) | Ref | 0.02 |
| Yes | 15 (8.7%) | 3 (2.3%) | 0.25 (0.07, 0.90) | |
|
| ||||
| O2 flow increase | ||||
| No | 157 (91.3%) | 124 (96.1%) | Ref | 0.1 |
| Yes | 15 (8.7%) | 5 (3.9%) | 0.42 (0.15, 1.21) | |
|
| ||||
| Deepening sedation | ||||
| No | 145 (84.3%) | 110 (85.3%) | Ref | 0.94 |
| Yes | 27 (15.7%) | 19 (14.7%) | 0.98 (0.51, 1.86) | |
|
| ||||
| Deepening sedation within 60 min after MRI start | ||||
| No | 154 (89.5%) | 121 (93.8%) | Ref | 0.26 |
| Yes | 18 (10.5%) | 8 (6.2%) | 1.73 (0.66, 4.54) | |
|
| ||||
| Fluid therapy | ||||
| No | 170 (98.8%) | 127 (98.4%) | Ref | 0.77 |
| Yes | 2 (1.2%) | 2 (1.6%) | 1.35 (0.19, 9.72) | |
|
| ||||
| Clinical hypotension at 5, 30, 60 minutes after induction (times) | ||||
| 0 | 12 (7.0%) | 22 (17.0%) | Ref | <0.0001 |
| 1 | 21 (12.2%) | 28 (21.7%) | 0.73 (0.29, 1.81) | |
| 2 | 42 (24.4%) | 50 (38.8%) | 0.65 (0.29, 1.48) | |
| 3 | 97 (56.4%) | 29 (22.5%) | 0.16 (0.07, 0.39) | |
group P, propofol only; group D+P, propofol infusion with dexmedetomidine bolus.
The airway support includes oral/nasal airway and LMA under TIVA.
O2 flow increase from default value (2 L/minutes).
Deepening sedation is defined as propofol bolus, increase of propofol infusion rate, or dexmedetomidine bolus after MRI start.
Clinical hypotension is defined as MAP drop >20% from baseline (pre-anesthesia values).
Statistical comparison is based on Chi-Square test.
Line graphs (Figure 1) and linear regression of hemodynamic and respiratory parameters (HR, RR, MAP, SO2) in relation to treatment group over time based generalized estimating equation are demonstrated in Table 4. There was a significant difference in HR between P and D+P group (coef= −7.68, 95%CI= −11.46, −3.91, p=0.0001). HR was consistently lower across time in D+P group compared to P group (Figure 1A). There was no significant interaction in HR between two groups over time (coef= −0.05, 95%CI= −0.11, 0.001, p= 0.06). There was no difference in RR between P and D+P group (coef= 0.70, 95%CI= −0.47, 1.87, p=0.24). There was no significant interaction in RR between two groups across time (coef= −0.02, 95%CI= −0.04, 0.007, p=0.17). At the earlier time point (5 minutes after induction), RR was higher in D+P group compared to the P group but both groups started to be comparable at 30 minutes after induction (Figure1B). There was a significant difference in MAP between P group and D+P group across time (coef= −0.06, 95%= −0.11, −0.02, p= 0.004). As shown in Figure1C, the two groups were different at the earlier time point (5 minutes after induction) but they were comparable at time point 30 minutes after induction. There is no difference between P and D+P group in SO2 across time points (coef= 0.001, 95%CI= −0.01, 0.01, p= 0.76). Both group showed the effects are similar (Figure1 D).
Figure 1.
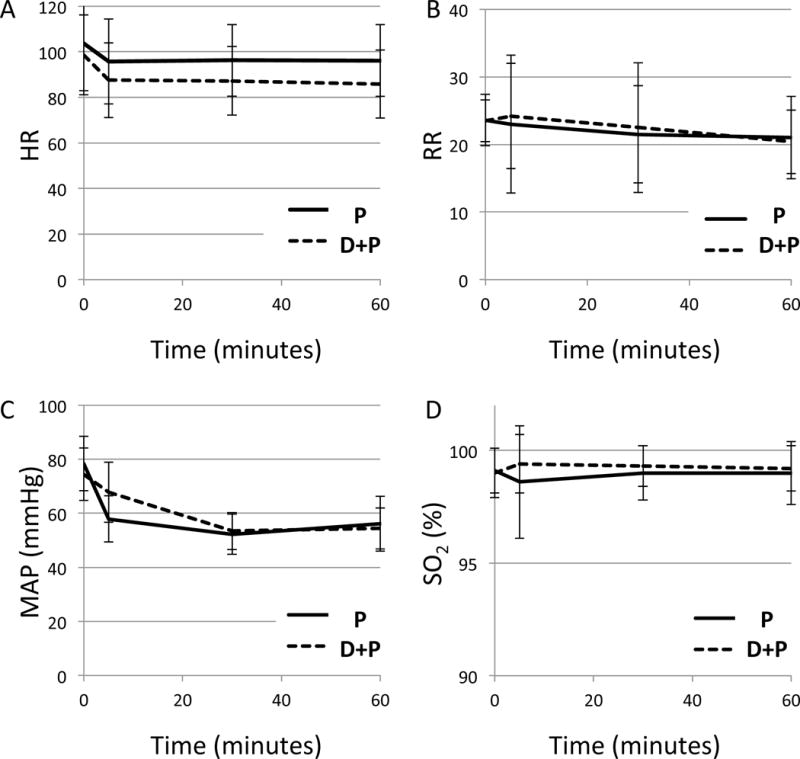
Hemodynamic and respiratory parameters during the sedation. A, heart rate (HR,/min); B, respiratory rate (RR,/min); C, mean arterial pressure (MAP, mmHg); D, oxygen saturation (SO2, %). Time starts at the time of induction (0).
Group P (propofol only); Group D+P (propofol with dexmedetomidine bolus)
Error bars represent standard deviation.
A. There was a significant difference in two groups across time points (p<0.0001)
B. There was no difference between two groups (p=0.17)
C. There was a significant difference in MAP across the time (p= 0.004). Two groups were different at 5 minutes after induction.
D. There was no difference between two groups in SO2 (p=0.76).
Table 4.
Linear regression of HR, RR, MAP, and SO2 in relation to treatment group over time based generalized estimating equation model
| HR | Coef (SE) | 95% CI | P-value |
|---|---|---|---|
| Time | −0.03 (0.04) | (−0.11, −0.53) | 0.49 |
| treatment group | |||
| P | Reference | – | – |
| D+P | −7.68(1.93) | (−11.46, −3.91) | <0.0001 |
| Time*treatment group | −0.05(0.03) | (−0.11, 0.001) | 0.06 |
|
| |||
| RR | Coef (SE) | 95% CI | P-value |
|
| |||
| Time | −0.01 (0.02) | (−0.05, −0.02) | 0.48 |
| Treatment group | |||
| P | Reference | – | – |
| D+P | 0.70 (0.60) | (−0.47, 1.87) | 0.24 |
| Time*treatment group | −0.02 (0.01) | (−0.04, 0.007) | 0.17 |
|
| |||
| MAP | Coef (SE) | 95% CI | P-value |
|
| |||
| Time | −0.26 (0.02) | (−0.29, −0.23) | <0.0001 |
| Treatment group | |||
| P | Reference | – | – |
| D+P | 2.94 (0.96) | (1.05, 4.83) | 0.002 |
| Time*treatment group | −0.06 (0.02) | (−0.11, −0.02) | 0.004 |
|
| |||
| SO2 | Coef (SE) | ||
|
| |||
| Time | 0.002 (0.002) | (−0.003, 0.006) | 0.48 |
| Treatment group | |||
| P | Reference | – | – |
| D+P | 0.26 (0.12) | (0.01, 0.50) | 0.04 |
| Time*treatment group | 0.001 (0.003) | (−0.01, 0.01) | 0.76 |
group P, propofol only; group D+P, propofol infusion with dexmedetomidine bolus
In MRI image quality evaluation, nine out of ten cases contained no motion artifact in either group. One case in each group contained minimal motion artifact that did not impede the ability of the radiologist to provide interpretation.
Discussion and conclusion
We found that a single low-dose dexmedetomidine bolus (0.5 mcg/kg with a second bolus for prolonged scans) as an adjuvant to propofol infusion decreased the propofol requirement for children undergoing MRI. There was also a significant reduction in the number of children who required airway support and they remained hemodynamically stable. The ideal medications for sedation for children undergoing MRI should maintain spontaneous ventilation, provide hemodynamic stability, ensure patient immobility, allow easy drug titration and no prolong recovery time. As a single agent for sedation, propofol has been popular as it provides rapid and smooth induction to deep sedations levels and ensures patient immobility for non-painful procedures (2, 3). However, propofol may cause hemostatic instability due to diminished systemic vascular resistance, hypoventilation due to decreased respiratory drive, and upper airway obstruction due to decreased tone of pharyngeal muscles in dose dependent fashion (6, 7). Decreasing the propofol infusion rate may reduce these side effects, but the risk of emergence during the procedure may increase. On the contrary, high dose dexmedetomidine as a single sedative agent (2-4 mcg/kg loading dose followed by 2-3 mcg/kg/min maintenance) provides better respiratory stability but there is the risk of bradycardia and occasional hypertension. Further, dexmedetomidine alone carries a higher risk of unwanted emergence during the procedure due to difficulty in titration, as well as prolonged of recovery time (9, 12, 13, 21). Other authors have shown that dexmedetomidine can be added to propofol sedation and will decrease the propofol requirement to achieve optimal sedation or total intravenous anesthesia for surgical procedures. In these reports, the recommended loading dose (1 mcg/kg infusion over 10 minutes) with or without maintenance (0.4 – 1.0 mcg/kg/hour) was combined with a propofol infusion (17, 22–24). Le Guen reported that dexmedtomidine decreased the propofol requirement by 30% (22). A common practice in our institution is to administer a half of the recommended loading dose of dexmedetomidine. Our rational for the lower dose of dexmedetomidine was to reduce the risk of bradycardia that can occur when it is administered as bolus. In our study, there was still a propofol-sparing effect with low-dose dexmedetomidine (initial 0.5mcg/kg bolus with or without second bolus 0.5 mcg/kg for scans > 2 hours). The incidence of deepening sedation was similar in both groups (Table 3). Deepening of sedation occurred more frequently in the propofol only group than the low dose dexmedetomidine group within 60 minutes after start of MRI (difference was not significant). The propofol-sparing effect could be expected given the lower starting dose of the propofol infusion. However, the clinicians adjusted the propofol infusion during the case based upon clinical findings and could have increased it if needed. In our study the decrease in propofol was approximately 31% total dose and 35% in the infusion rate (Table 2). This result is comparable to other studies using dexmedetomidine (1 mcg/kg) bolus (18, 22). In a recent study reported by Borosi, the addition of dexmedetomidine (1 mcg/kg) significantly prolonged discharge time. In our study, the low dose dexmedetomidine bolus did not affect recovery time, even with second bolus (Table 2).
One of the greatest clinical differences between our groups was the reduced need for airway support in the D+P group compared to propofol alone. Although the incidence of airway support was low in both groups, it was significantly lower in dexmedetomidine bolus group. Due to a decreased propofol requirement, a low-dose dexmedetomidine bolus seemed associated with improved airway patency and maintenance of ventilation (Table 3). It is notable that incidence of O2 flow increase happened more often in propofol only group (difference was not significant) (Table 3).
There was a decrease in heart rate after induction throughout the duration of the measurements in both groups (Figure 1A). Heart rate in the propofol was significantly higher than in the dexmedetomidine bolus group through 60 minutes. However, there was no significant difference between two groups over time based generalized estimating equation. The maximal decrease in heart rate from baseline with the addition of low-dose dexmedetomidine (0.5 mcg/kg) was only 10-12% which compares favorably to the heart rate decrease of 15-25% reported in high-dose dexmedetomidine studies (9, 12, 13, 25). The influence of low-dose dexmedetomidine on mean arterial pressure (MAP) were seen over time in 60 minutes. In fact, the decrease in MAP from pre-induction values was larger in the propofol only group at 5 minutes after induction as compared to the low-dose dexmedetomidine adjuvant group (25% vs. 8% decrease). We did find a decrease in MAP for the propofol only group that is comparable to the 15-31% occurrence seen in other studies (2, 9). As for clinically significant hypotension defined as more than 20% drop from baseline (pre-anesthesia) MAP, the number of episodes is significantly higher in the propofol only group compared to low-dose dexmedetomidine group (Table 3). Based on our survey amongst our department anesthesiologists, we anticipated far more cases in both groups (1.2% and 1.6% in each group) to be given fluid therapy for hypotension. This may be a dissociation of actual practice from our principle.
The results of our study need to be considered in light of its limitations. As a retrospective study, the use of dexmedetomidine was not randomized but at the discretion of the anesthesiologist. Upon review we do believe that the two groups were similar (Table 1). We have a common management of this patient population in our institution as shown in the summary of our survey in materials and methods. However, there could be alterations that we could not elicit from reviewing the medical records. The propofol infusion used for maintenance of sedation is higher than some groups may be comfortable with, but these doses have certainly been reported in the literature. The airway interventions reported do not include brief episodes of obstruction that required repositioning. Those reported are more significant interventions of placing an airway device. Further, events in both groups were identified and recorded in a similar fashion. Although statistically significant difference was identified, the incidence of airway interventions was quite low in both groups.
There may be one additional theoretical benefit of using the combination of dexmedetomidine and propofol given the recent Food and Drug Administration (FDA) warning regarding general anesthetics and sedation drugs for young children and pregnant women due to the potential for neurotoxicity. Although supporting clinical data were limited for the warning, the FDA stressed the importance of avoiding prolonged use (longer than 3 hours) and repeated use of these drugs in younger age (less than 3 years old). Propofol is listed in the warning, whereas dexmedetomidine is not. In theory, neurotoxicity is dependent on the amount of anesthetics/sedatives given through the course of a procedure. It is reasonable to limit the amount of propofol given to younger children, especially those who may undergo sedation repeatedly.
We concluded that low-dose dexmedetomidine single bolus (0.5 mcg/kg) as an adjuvant to propofol sedation can decrease the propofol requirement for optimal sedation of children undergoing MRI less than 2 hours. For the MRI longer than 2 hours, a second bolus may be administered. This modified technique can decrease the need for airway support and contribute to better hemodynamic stability without prolonging recovery time.
Clinical implications.
a. What is already known
Propofol infusion for sedation of children in MRI may cause hemodynamic and respiratory complications in a dose dependent fashion. Addition of dexmedetomidine (1 mcg/kg or more) can decrease propofol demands but can prolong recovery time.
b. What this article adds
Low-dose dexmedetomidine bolus (0.5 mcg/kg) used as an adjuvant can decrease the propofol requirement without prolonging recovery time. This technique may decrease the need for airway support and improve hemodynamic stability.
c. Implication for translation
Low-dose dexmedetomidine bolus (0.5 mcg/kg) can decrease the propofol requirement without prolonging recovery time. These findings are novel when compared to studies using higher dose dexmedetomidine (1 mcg/kg or more) which result in prolonged recovery time.
Acknowledgments
This work was supported in part by grants UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
The author gratefully acknowledge the contribution of Jennifer Belzer, MD, Clinical Fellow, and Marla Matar MD, Anthony Romo MD, Samuel Yanofsky MD, Sam Saied MD, Andrew Costandi, Emily Chen MD, Karen Flotildes DO, Abhishek Karnwal MD, Neha Patel MD, Carl Lo MD, Jennifer Lau MD, Sang Le MD, Tiffany Frazee MD, Lydia Andras MD, and Elizabeth Bragg MD, Attending Anesthesiologists at Children’s Hospital Los Angeles, CA USA for research support and collecting clinical data. The authors also gratefully acknowledge the contribution of Choo Phei Wee for biostatics analysis.
Disclosure
The study was approved by the Children’s Hospital Los Angeles institutional review board (IRB; CHLA-16-00549 and CHLA-17-00013).
Footnotes
DR MAKOTO NAGOSHI (Orcid ID : 0000-0002-8430-2203)
Handling Section Editor: Dr Joseph Cravero
References
- 1.Usher AG, Kearney RA, Tsui BC. Propofol total intravenous anesthesia for MRI in children. Paediatr Anaesth. 2005;15(1):23–8. doi: 10.1111/j.1460-9592.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 2.Hasan RA, Shayevitz JR, Patel V. Deep sedation with propofol for children undergoing ambulatory magnetic resonance imaging of the brain: experience from a pediatric intensive care unit. Pediatr Crit Care Med. 2003;4(4):454–8. doi: 10.1097/01.PCC.0000090013.66899.33. [DOI] [PubMed] [Google Scholar]
- 3.Frankville DD, Spear RM, Dyck JB. The dose of propofol required to prevent children from moving during magnetic resonance imaging. Anesthesiology. 1993;79(5):953–8. doi: 10.1097/00000542-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Vangerven M, Van Hemelrijck J, Wouters P, Vandermeersch E, Van Aken H. Light anaesthesia with propofol for paediatric MRI. Anaesthesia. 1992;47(8):706–7. doi: 10.1111/j.1365-2044.1992.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 5.Lefever EB, Potter PS, Seeley NR. Propofol sedation for pediatric MRI. Anesth Analg. 1993;76(4):919–20. doi: 10.1213/00000539-199304000-00071. [DOI] [PubMed] [Google Scholar]
- 6.Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH, Pediatric Sedation Research C The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108(3):795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 7.Evans RG, Crawford MW, Noseworthy MD, Yoo SJ. Effect of increasing depth of propofol anesthesia on upper airway configuration in children. Anesthesiology. 2003;99(3):596–602. doi: 10.1097/00000542-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair RCF, F RJ. Delayed recovery of consciousness after anaesthesia. Contin Educ Anaesth Crit Care Pain. 2006;6(3):114–8. [Google Scholar]
- 9.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103(1):63–7. doi: 10.1213/01.ANE.0000219592.82598.AA. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Fang H, Yang L, Wang X, Zhu H. Clinical efficacy of dexmedetomidine versus propofol in children undergoing magnetic resonance imaging: a meta-analysis. Int J Clin Exp Med. 2015;8(8):11881–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Young ET. Dexmedetomidine sedation in a pediatric cardiac patient scheduled for MRI. Can J Anaesth. 2005;52(7):730–2. doi: 10.1007/BF03016562. [DOI] [PubMed] [Google Scholar]
- 12.Siddappa R, Riggins J, Kariyanna S, Calkins P, Rotta AT. High-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth. 2011;21(2):153–8. doi: 10.1111/j.1460-9592.2010.03502.x. [DOI] [PubMed] [Google Scholar]
- 13.Mason KP, Zurakowski D, Zgleszewski SE, Robson CD, Carrier M, Hickey PR, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18(5):403–11. doi: 10.1111/j.1460-9592.2008.02468.x. [DOI] [PubMed] [Google Scholar]
- 14.Heard CM, Joshi P, Johnson K. Dexmedetomidine for pediatric MRI sedation: a review of a series of cases. Paediatr Anaesth. 2007;17(9):888–92. doi: 10.1111/j.1460-9592.2007.02272.x. [DOI] [PubMed] [Google Scholar]
- 15.Mason KP, Zurakowski D, Zgleszewski S, Prescilla R, Fontaine PJ, Dinardo JA. Incidence and predictors of hypertension during high-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth. 2010;20(6):516–23. doi: 10.1111/j.1460-9592.2010.03299.x. [DOI] [PubMed] [Google Scholar]
- 16.Mason KP, Zgleszewski S, Forman RE, Stark C, DiNardo JA. An exaggerated hypertensive response to glycopyrrolate therapy for bradycardia associated with high-dose dexmedetomidine. Anesth Analg. 2009;108(3):906–8. doi: 10.1213/ane.0b013e3181948a6f. [DOI] [PubMed] [Google Scholar]
- 17.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90(2):172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Boriosi JP, Eickhoff JC, Klein KB, Hollman GA. A retrospective comparison of propofol alone to propofol in combination with dexmedetomidine for pediatric 3T MRI sedation. Paediatr Anaesth. 2017;27(1):52–9. doi: 10.1111/pan.13041. [DOI] [PubMed] [Google Scholar]
- 19.Dawson R, von Fintel N, Nairn S. Sedation assessment using the Ramsay scale. Emerg Nurse. 2010;18(3):18–20. doi: 10.7748/en2010.06.18.3.18.c7825. [DOI] [PubMed] [Google Scholar]
- 20.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 21.Kuyrukluyildiz U, Binici O, Onk D, Ayhan Celik S, Torun MT, Unver E, et al. Comparison of dexmedetomidine and propofol used for drug-induced sleep endoscopy in patients with obstructive sleep apnea syndrome. Int J Clin Exp Med. 2015;8(4):5691–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Le Guen M, Liu N, Tounou F, Auge M, Tuil O, Chazot T, et al. Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index-guided closed-loop anesthesia: a double-blind, placebo-controlled trial. Anesth Analg. 2014;118(5):946–55. doi: 10.1213/ANE.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 23.Ulgey A, Aksu R, Bicer C, Akin A, Altuntas R, Esmaoglu A, et al. Is the addition of dexmedetomidine to a ketamine-propofol combination in pediatric cardiac catheterization sedation useful? Pediatr Cardiol. 2012;33(5):770–4. doi: 10.1007/s00246-012-0211-1. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud M, Radhakrishman R, Gunter J, Sadhasivam S, Schapiro A, McAuliffe J, et al. Effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children. Paediatr Anaesth. 2010;20(6):506–15. doi: 10.1111/j.1460-9592.2010.03311.x. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, Liu J, Wu C. Impact of dexmedetomidine versus propofol on cardiac function of children undergoing laparoscopic surgery. Int J Clin Exp Med. 2014;7(12):5882–5. [PMC free article] [PubMed] [Google Scholar]


