Abstract
Background
We reviewed the effect of ovarian transposition (OT) on ovarian function among long-term survivors of childhood Hodgkin lymphoma (HL) treated with pelvic radiotherapy.
Procedure
Female participants (age 18+ years) with HL in the St. Jude Lifetime Cohort Study (SJLIFE) were clinically evaluated for premature ovarian insufficiency (POI) 10 or more years after pelvic radiotherapy. Reproductive history including age at menopause and pregnancy/live births was available on all patients.
Results
Of 127 eligible females with HL, 90 (80%) participated in SJLIFE including 49 who underwent OT before pelvic radiotherapy. Median age at SJLIFE evaluation was 38 years (range 25-60). In a multiple regression adjusted for age at diagnosis, pelvic radiotherapy doses > 1,500 cGy (HR=25.2, 95% CI=3.1 to 207.3; p=0.0027) and cumulative cyclophosphamide equivalent doses of alkylating agents > 12,000 mg/m2 (HR=11.2, 95% CI=3.4 to 36.8; p<0.0001) were significantly associated with POI. There was no significant association between OT and occurrence of POI (HR=0.6, 95% CI=0.2 to 1.9; p=0.41).
Conclusions
OT did not appear to modify risk of POI in this historic cohort of long-term survivors of HL treated with gonadotoxic therapy. Modern fertility preservation modalities such as mature oocyte cryopreservation should be offered to at-risk patients whenever feasible.
Keywords: late effects, Hodgkin lymphoma, premature ovarian insufficiency, ovarian transposition
INTRODUCTION
Premature ovarian insufficiency (POI) is defined by the absence or interruption of spontaneous pubertal development in children and adolescents or by the onset of menopause before age 40 as a result of primary gonadal failure [1]. Up to 11% of female survivors of childhood cancer experience POI [2–4]. Lower ovarian reserve in young adulthood has been observed in women treated with chemotherapy and radiation therapy during childhood for Hodgkin lymphoma (HL) [5,6]. Exposure to high dose alkylating agents, quantified by cyclophosphamide equivalent dose (CED) ≥8,000 mg/m2 has been associated with POI further highlighting the ovarian toxicity of these agents [2]. Pelvic radiotherapy can also cause irreversible ovarian damage and lead to infertility and other long-term health concerns such as decreased bone mineral density and frail health [2]. The estimated dose of radiation at which half of the follicles are lost in humans is 4-6 Gy in adults and 10-20 Gy in children [7–9]. Older age at radiotherapy has been associated with a higher risk of POI, likely because of the natural decline of ovarian reserve with age [3].
Ovarian transposition (OT) was proposed in 1958 as a means to preserve fertility in girls with tumors requiring pelvic irradiation [10]. This procedure involves relocation of the ovaries out of the irradiation field to minimize exposure of the ovaries to radiation, thereby preserving ovarian function [11]. Studies reporting on ovarian function in childhood cancer survivors who underwent OT have generally been limited and confined to studies with small sample size and short follow-up.
The aim of this study was to evaluate the risk of POI and describe patterns of pregnancy and live births in a cohort of long-term female survivors of HL exposed to pelvic radiotherapy including a subset who underwent OT before radiation.
MATERIAL AND METHODS
Study participants
Eligibility criteria for inclusion included: (1) females treated with pelvic radiotherapy during childhood for HL at St. Jude Children’s Research Hospital (SJCRH); (2) survival of >10 years from diagnosis; and (3) attained age ≥18 years of age at follow-up [12,13]. Eligible survivors underwent a clinical assessment on-campus and completed a series of questionnaires pertaining to reproductive health prior to June 30, 2015. Survivors with a history of surgical menopause or bilateral oophorectomy before age 40 years, or hypothalamic or pituitary area irradiation were excluded. The study was approved by the institutional review board and the participants provided written informed consent prior to assessment.
Study outcomes
Premature ovarian insufficiency was defined as absence of menses five years post cancer diagnosis, or loss of spontaneous menses prior to 40 years of age with laboratory or historic evidence of primary (ovarian) origin. The diagnosis of POI was based on the medical history provided by the patients in regards to puberty, menarche, menstrual cycles, pregnancies, childbirth, use of hormonal therapies including contraception and timing of menopause, and supplemented by clinical and laboratory data from the SJLIFE evaluation [2]. In the absence of treatment with oral contraceptive pills or sex-hormone replacement therapy (HRT) at the time of SJLIFE participation, individuals < 40 years old experiencing amenorrhea > 6 months and having plasma estradiol levels < 17 pg/mL coinciding with FSH ≥30 IU/L were considered to have POI. The diagnosis of POI was based on historical medical information only in participants treated with oral contraceptives or HRT [2]. Additional information on pregnancy and live births was obtained from questionnaires.
Study variables
Exposure to alkylating agents was quantified using the validated cyclophosphamide equivalent dose (CED) [14]. History of OT was abstracted from the medical record. The technique used in SJCRH involved transposition of the ovaries to a midline position behind the uterus by either an open procedure or laparoscopy.
Statistical analyses
Descriptive statistics were used to characterize participants and non-participants, as well as those who underwent OT and those who did not. Characteristics were compared between groups with Fisher’s exact test or Wilcoxon rank sum test as appropriate. Cox proportional hazard regression was used to examine associations between treatments (OT, CED and pelvic radiation) and POI after adjusting for age at diagnosis. Time-to-event analysis was used to compare pregnancies and live birth deliveries between survivors with and without OT, with POI considered a competing risk. Analyses were completed in SAS version 9.4 (Cary, NC) and R 3.4.2.
RESULTS
Characteristics of study participants
Of 127 eligible females with HL, 90 (80%) participated in SJLIFE including 49 who underwent OT before pelvic radiotherapy (Figure 1). Participants did not differ from non-participants by race/ethnicity, age at diagnosis of HL, age at OT, pelvic radiation dose, CED, nitrogen mustard dose or procarbazine dose (Table 1). However, participants who underwent OT were younger when diagnosed with HL (P=0.04), received significantly higher doses of pelvic radiation (P<0.0001) and more alkylating agents (P=0.04) compared to survivors who did not undergo OT (Table 2). Among survivors who underwent an OT, an open surgical technique was used in 48 (98%) and a laparoscopic approach in 1 (2%). Bilateral midline OT was performed in all cases.
Figure 1.
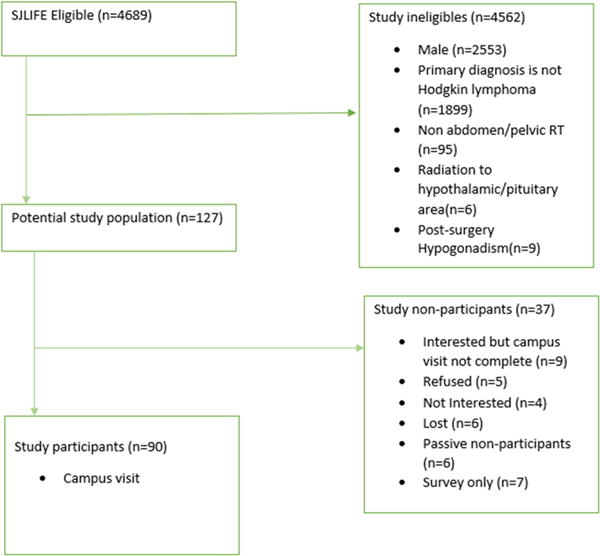
Consort diagram
Table 1.
Comparison of presenting features and treatments between study participants and non-participants
| Participants (N=90) | Non-Participants (N=37) | P value | |
|---|---|---|---|
| Age at diagnosis, Median (range) | 16 (4-22) | 15 (6-20) | 0.3079 |
| Age at Ovarian transposition, Median (range) | 15 (4-24) | 14.5 (7-22) | 0.9364 |
| Race/Ethnicity (N, %) | 0.2277 | ||
| White | 77 (85.6) | 35 (94.6) | |
| Non-white | 13 (14.4) | 2 (5.4) | |
| Ovarian transposition (N, %) | 0.2804 | ||
| Yes | 49 (54.4) | 24 (64.9) | |
| No | 41 (45.6) | 13 (35.1) | |
| Pelvic radiation dose (N, %), cGy | 0.2932 | ||
| ≤ 1500 | 32 (36.8) | 9 (25.0) | |
| > 1500 | 55 (63.2) | 27 (75.0) | |
| Alkylating Agent Cyclophosphamide Equivalent Dose (N, %), mg/m2 | 0.5815 | ||
| 0 to <= 8000 | 45 (51.7) | 21 (58.3) | |
| 8000 to <= 12000 | 18 (20.7) | 7 (19.4) | |
| 12000 to <= 20000 | 20 (23.0) | 5 (13.9) | |
| > 20000 | 4 (4.6) | 3 (8.3) | |
| Median (range) | 8818.2 (1800.0-28980.0) | 8034.4 (2339.0-31625.9) | |
| Nitrogen mustard (N, %), mg/m2 | 3 (3.3) | 2 (5.4) | |
| Median (range) | 18.4 (18.1-36.7) | 20.7 (17.5-23.9) | 0.7728 |
| Procarbazine (N, %), mg/m2 | 65 | 31 | |
| Median (range) | 4433.3 (857.1-15633.3) | 4114.3 (714.3-17710.5) | 0.6779 |
Table 2.
Comparison of presenting features and treatments between survivor participants with or without ovarian transposition
| Ovarian
transposition (N=49) |
Non-ovarian
transposition (N=41) |
P Value | |
|---|---|---|---|
| Age at diagnosis, Median (range) | 15 (4-19) | 16 (6-22) | 0.0417 |
| Age at Questionnaire, Median (range) | 38 (25-51) | 39 (26-60) | 0.9353 |
| Age at primary ovarian insufficiency, Median (range) | 22.7 (15.3-38.2) | 17.9 (15.5-35.0) | 0.2629 |
| Race | 0.9626 | ||
| White | 42 (85.7) | 35 (85.4) | |
| Non-white | 7 (14.3) | 6 (14.6) | |
| Alkylating Agent Cyclophosphamide Equivalent Dose (N, %), mg/m2 | 0.0445 | ||
| 0 to <= 8000 | 19 (39.6) | 26 (66.7) | |
| 8000 to <= 12000 | 12 (25.0) | 6 (15.4) | |
| 12000 to <= 20000 | 13 (27.1) | 7 (18.0) | |
| > 20000 | 4 (8.3) | 0 (0.0) | |
| Median (range) | 10151.7 (1806.2-28980.0) | 7434.6 (1800.0-16486.8) | |
| Pelvic radiation dose (N, %), cGy | <.0001 | ||
| ≤ 1500 | 9 (18.4) | 23 (60.5) | |
| > 1500 | 40 (81.6) | 15 (39.5) | |
| Pregnancy | 0.1459 | ||
| Yes | 30 (61.2) | 31 (75.6) | |
| No | 19 (38.8) | 10 (24.4) | |
| Age at first pregnancy, Median (range) | 23.5 (17.0-38.0) | 22.0 (13.0-34.0) | 0.1693 |
| Live birth delivery* | 0.9663 | ||
| Yes | 27 (90.0) | 28 (90.3) | |
| No | 3 (10.0) | 3 (9.7) | |
| Age at first live birth delivery, Median (range) | 24.0 (17.0-36.0) | 22.5 (16.0-34.0) | 0.3272 |
Analysis for the live birth delivery was based on patients who have reported pregnancy (N=61, 30 in OT and 31 in non-OT group)
Risk factors associated with POI
In multivariable models, there was no significant association between OT and occurrence of POI (hazard ratio [HR]=0.6, 95% confidence interval [CI]=0.2 to 1.9; p=0.41). However, pelvic radiotherapy doses > 1,500 cGy (HR=25.2, 95% CI=3.1 to 207.3; p=0.0027) and exposure to alkylating agents at cumulative CED > 12,000 mg/m2 (HR=11.2, 95% CI=3.4 to 36.8; p<0.0001) were associated with an increased risk of POI (Table 3). A multivariable sub-analysis was conducted to examine associations between OT, pelvic radiation dose and POI among survivors who received lower CED (<12000 mg/m2). After adjusting for age at diagnosis, there was a significant association between POI and pelvic radiation dose (HR=17.8, 95% CI=2.3 to 136.5; P=0.0057) but not with OT (HR=1.1, 95% CI=0.5 to 2.7; P=0.8052) (Table 4).
Table 3.
Multiple regression to investigate the association between OT and POI
| Hazard Ratio (95% CI) | P | ||
|---|---|---|---|
| Alkylating agent cyclophosphamide equivalent dose, mg/m2 | <= 8000 | Ref. | |
| 8001-12000 | 3.3 (0.7, 16.0) | 0.1461 | |
| 12001-2000 | 11.2 (3.4, 36.8) | <0.0001 | |
| >20000 | 36.9 (5.2, 260.5) | 0.0003 | |
|
| |||
| Ovarian transposition | No | Ref. | |
| Yes | 0.6 (0.2, 1.9) | 0.4069 | |
|
| |||
| Pelvic radiotherapy dose, cGy | ≤ 1500 | Ref. | |
| >1500 | 25.2 (3.1, 207.3) | 0.0027 | |
Table 4.
Multiple regression to investigate the association between OT and POI among patients who had low dose CED (<=12000 mg/m2)
| Hazard Ratio (95% CI) | P | ||
|---|---|---|---|
| Ovarian transposition | No | Ref. | |
| Yes | 1.1 (0.5, 2.7) | 0.8052 | |
|
| |||
| Pelvic radiotherapy dose, cGy | ≤ 1500 | Ref. | |
| >1500 | 17.8 (2.3, 136.5) | 0.0057 | |
Ovarian function between survivors who did or did not undergo OT
The median age at POI for survivor participants with or without OT was not significantly different (22.7 [range, 15.3-38.2] vs. 17.9 [range, 15.5-35.0] years, p=0.2629) (Table 2). Among the 49 survivors who had OT, 30 (61%) reported at least one pregnancy, and for those who reported pregnancy, 27 (90%) reported a live birth delivery at least once. The probability of a first pregnancy or a live birth before age 40 were did not differ between OT and non-OT groups (P=0.1360 and P=0.4970, respectively) (Figure 2).
Figure 2.
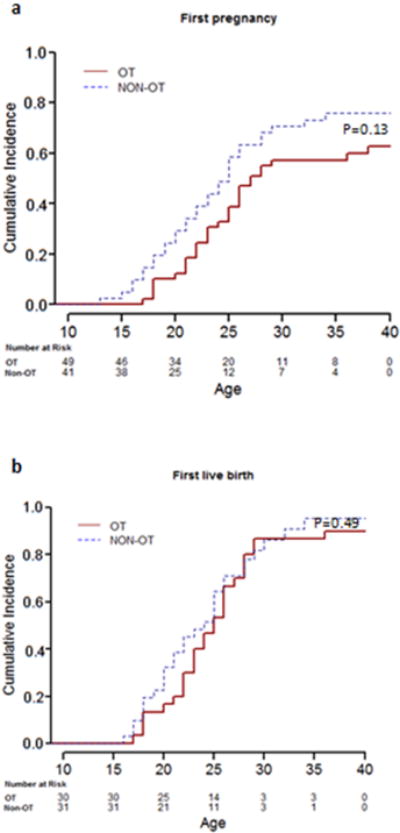
Cumulative incidence plot for the probability of first pregnancy (2a) and live birth over time (2b)
DISCUSSION
Our study provides long-term follow-up data on the effect of OT on ovarian outcomes (POI, pregnancy and live births) in a cohort of clinically assessed HL survivors treated with pelvic radiotherapy. The study is unique in its ability to assess the effect of OT in relation to gonadotoxic therapy that may impact ovarian function, and in the extended duration of follow-up, which is among the longest reported to date. This study is timely given the increased availability of modern fertility preservation techniques, and the need for data to assist childhood cancer survivors and their families to make the best decisions possible in regards to future fertility and family planning [15].
Prior studies have reported variable success rates of OT performed at staging laparotomy for female HL patients in regards to preservation of ovarian function, ranging from 0% to 66% [16–21]. In this study, OT did not appear to modify the long-term risk of POI among female survivors of HL. However, it is important to note that OT was performed in a higher proportion of individuals treated with the highest doses of pelvic irradiation and exposure to higher doses of alkylating agents, which could have been a source of substantial bias. In addition, the effects of gonadotoxic treatments may have overwhelmed potential long-term gains from OT. Notably, age at first pregnancy and pregnancy/live birth history were not significantly different between patients with or without OT, despite of the fact that survivors with OT were treated with higher dose of pelvic irradiation.
Our study indicates that OT may not prevent ovarian injury with use of combined modality therapy including pelvic radiotherapy doses > 1,500 cGy and alkylating agents exceeding a CED > 12,000 mg/m2. Prior studies with smaller numbers of patients, and shorter follow-up, have observed variable results in regards to preservation of ovarian function following OT and pelvic irradiation. Pregnancies have been reported in women who had OT, but small cohort sizes precluded inclusion of patient and treatment factors that may confound or modify the association between OT and preserved ovarian function. Ray et al. [17] reported preservation of ovarian function in 59% of 22 patients who underwent OT, one of whom gave birth to a normal baby girl. Le Floch et al. [18] reported on nine patients who underwent OT prior to pelvic radiation therapy and became pregnant; six patients had given birth to eight babies. An additional two patients had therapeutic abortions and one a spontaneous abortion. Thomas et al. [19] reported reproductive and endocrine function in 22 women with HL who had bilateral mid-line OTs performed at staging laparotomy. OT was only partially successful, preserving fertility in 30% of the patients. Terenziani et al. [20] reported reproductive patterns in 11 HL patients who underwent open bilateral OT at a median age of 13 years. Fourteen pregnancies were recorded among these 11 women, with 12 live births (1 pair of twins) and 3 miscarriages. The median age at the time of first pregnancy was 31 years, and the median time since OT was 14 years. In this series, only 4 patients received both chemotherapy and radiation. Five patients received radiation exclusively, and 2 patients did not receive radiation. In our long-term follow-up study, 68% of study participants reported at least one pregnancy, with no significant difference between OT (61%) and non-OT (76%) groups. In a meta-analysis of 1189 premenopausal women with cancer requiring primary or postoperative radiotherapy (median age of 32 years), ovarian function following OT was preserved in 70% of women [21]. However, patients were only followed for a median of four years (range, 2-16), and the study only included women exposed to pelvic radiation. The median age at OT in our study was 15 years (range, 4-24), and the median age at evaluation was 38 years (range 25-60). Our findings highlight the importance of long-term follow-up to better define the role of OT in delaying the onset of menopause in cancer survivors.
The transposition of ovaries during surgical staging for HL was first reported in 1970 by Stanford investigators [22]. This procedure can be performed by laparotomy, laparoscopy or percutaneous needle transposition. All but one of our patients underwent an open procedure, therefore we were unable to evaluate the theoretical benefit of a less invasive surgical approach. Complications associated with OT include increased ovarian cyst formation, postoperative adhesions, chronic pelvic pain, migration of the ovaries back to their native position, damaged or dysfunctional fallopian tubes and spread of unnoticed metastatic disease within the ovaries. Also, OT does not protect the ovaries from the effects of gonadotoxic chemotherapy. For all these reasons, treatment with systemic gonadotoxic drugs should be taken into consideration when assessing the risk of the procedure.
There are several limitations of this study. First, the small number of patients with OT limited our ability to adjust for confounding variables in analysis. Our non-inclusion of etiologies other than HL limits our ability to relate our findings to the rest of the literature as treatment exposures vary by type and stage of cancer. Second, because a substantially higher percentage of our patients exposed to high dose irradiation were treated with OT, our ability to detect the protective effect of OT for those with lower dose exposure may have been limited. Third, techniques for radiation delivery have changed from 1962 to 2005. Data from older female survivors may not apply to girls treated today. In addition, this study does not incorporate dosimetry calculations based on the position of the ovaries after they were moved. Therefore, the findings do not allow the quantification of the radiation dose reduction brought about by the procedure and that this is subject to variations depending on the individual’s anatomy and the success of the procedure itself. Fourth, data pertaining to pregnancy and live birth history depended on accurate completion of relevant questionnaires. Self-report data is always subject to recall bias, although maternal recall of reproductive history is generally accurate [23]. Fifth, the observed results in survivors who underwent OT may be a result of an operative failure or procedural complication that was not recorded. This study does not address potentially pre-existing conditions of the ovary and that may have already affected follicular reserve, such as unilateral oophorectomy. Further discussion of operative complications is warranted. Finally, the cross-sectional design of this study poises specific challenges in reporting the outcomes of interest given that a subset of patients may still go on to experience either POI or pregnancies with further follow-up.
In summary, OT did not appear to modify the long-term risk of POI and the pregnancy/live birth events in this historic cohort of long-term survivors of HL treated with combined pelvic radiation and alkylating agents. Modern fertility preservation modalities such as mature oocyte cryopreservation should be offered to at-risk patients whenever feasible.
Acknowledgments
Funding
This study was funded by Grant CA 21765 from the National Cancer Institute and ALSAC.
ABBREVIATION KEY
- CED
Cyclophosphamide Equivalent Dose
- CI
Confidence Interval
- FSH
Follicle Stimulating Hormone
- HL
hodgkin lymphoma
- hr
hazard ratio
- hrt
sex hormone replacement Therapy
- ot
ovarian transposition
- poi
Premature Ovarian Insufficiency
- sjlife
St. Jude Lifetime Cohort Study
- Sjcrh
St. Jude Children’s Research Hospital
Footnotes
Presentation: This study was presented as an oral presentation at the annual meeting of the International Society of Paediatric Oncology (SIOP), Washington DC (USA) in October 2017.
Conflict of interest Statement
None of the authors has a conflict of interest to declare.
References
- 1.Committee on Adolescent Health Care. American College of Obstetrics and Gynecology opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124:193–7. doi: 10.1097/01.AOG.0000451757.51964.98. [DOI] [PubMed] [Google Scholar]
- 2.Chemaitilly W, Li Z, Krasin MJ, Brooke RJ, Wilson CL, Green DM, Klosky JL, Barnes N, Clark KL, Farr JB, Fernandez-Pineda I, Bishop MW, Metzger M, Pui CH, Kaste SC, Ness KK, Srivastava DK, Robison LL, Hudson MM, Yasui Y, Sklar CA. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017;102:2242–2250. doi: 10.1210/jc.2016-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91:1723–8. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 4.Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, Mulder J, Green D, Nicholson HS, Yasui Y, Robison LL. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–6. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 5.Papadakis V, Vlachopapadopoulou E, Van Syckle K, Ganshaw L, Kalmanti M, Tan C, Sklar C. Gonadal function in young patients successfully treated for Hodgkin disease. Med Pediatr Oncol. 1999;32:366–72. doi: 10.1002/(sici)1096-911x(199905)32:5<366::aid-mpo10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, van den Bos C, van den Berg H, Pieters R, de Muinck Keizer-Schrama SM. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–74. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 7.Wallace WHB, Shalet SM, Hendry JH, et al. Ovarian Failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol. 1989;62:995–998. doi: 10.1259/0007-1285-62-743-995. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Hoppe RT, Kaplan HS, Rosenberg SA. Female reproductive potential after treatment for Hodgkin’s disease. N Eng J Med. 1981;304:1377–1382. doi: 10.1056/NEJM198106043042301. [DOI] [PubMed] [Google Scholar]
- 9.Damewood MD, Grochow LB. Prospects for fertility after chemotherapy or radiation for neoplastic disease. Fertil Steril. 1986;45:443–459. doi: 10.1016/s0015-0282(16)49268-x. [DOI] [PubMed] [Google Scholar]
- 10.McCall M, Keatye C, Thompson JD. Conservation of ovarian tissue in the treatment of carcinoma of the cervix with radical surgery. Am J Obstet Gynec. 1958;75:590. doi: 10.1016/0002-9378(58)90614-8. [DOI] [PubMed] [Google Scholar]
- 11.Thibaud E, Ramirez M, Brauner R, Flamant F, Zucker JM, Fekete C, Rappaport Preservation of ovarian function by ovarian transposition performed before pelvic irradiation in childhood. J Pediatr. 1992;12:880–884. doi: 10.1016/s0022-3476(05)80332-4. [DOI] [PubMed] [Google Scholar]
- 12.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–64. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, Neglia JP, Sklar CA, Kaste SC, Hudson MM, Diller LR, Stovall M, Donaldson SS, Robison LL. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Practice Committees of American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Williams RS, Littell RD, Mendenhall NP. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer. 1999;86:2138–42. [PubMed] [Google Scholar]
- 17.Ray GR, Trueblood HW, Enright L, Kaplan HS, Nelson TS. Oophoropexy: a means of preserving ovarian function following pelvic megavoltage radiotherapy for Hodgkin’s disease. Radiology. 1970;96:175–80. doi: 10.1148/96.1.175. [DOI] [PubMed] [Google Scholar]
- 18.Le Floch O, Donaldson SS, Kaplan HS. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer. 1976;38:2263–8. doi: 10.1002/1097-0142(197612)38:6<2263::aid-cncr2820380612>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Thomas PR, Winstanly D, Pechham MJ, Austin DE, Murray MA, Jacobs HS. Reproductive and endocrine function of inpatients with Hodgkin’s disease: effects of oophoropexy and irradiation. Br J Cancer. 1976;33:226–31. doi: 10.1038/bjc.1976.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terenziani M, Piva L, Meazza C, Gandola L, Cefalo G, Merola M. Oophoropexy: A relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril. 2009;91:935. doi: 10.1016/j.fertnstert.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Mossa B, Schimberni M, Di Benedetto L, Mossa S. Ovarian transposition in young women and fertility sparing. Eur Rev Med Pharmacol Sci. 2015;19:3418–25. [PubMed] [Google Scholar]
- 22.Trueblood HW, Enright LP, Ray GR, Kaplan HS, Nelsen TS. Preservation of ovarian function in pelvic radiation for Hodgkin’s disease. Arch Surg. 1970;100:236–7. doi: 10.1001/archsurg.1970.01340210012004. [DOI] [PubMed] [Google Scholar]
- 23.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Willett WC, Buka SL. Reproducibility and Validity of Maternal Recall of Pregnancy-Related Events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]


