Abstract
Introduction
Electrical impedance myography (EIM) is a non-invasive technique for measuring muscle composition and a potential physiological biomarker for facioscapulohumeral muscular dystrophy (FSHD).
Methods
Thirty-two genetically confirmed and clinically affected FSHD participants underwent EIM in 7 muscles bilaterally. Correlations between EIM and baseline clinical measures were used to select EIM parameters of interest in FSHD, and EIM and clinical measures were followed for 1 year.
Results
There were no significant changes in the EIM parameters. While fifty kHz reactance correlated the strongest to clinical measures at baseline, the 50–211 kHz phase-ratio demonstrated lower within subject 12 month variability, potentially offering sample size savings for FSHD clinical trial planning.
Discussion
EIM did not identify significant disease progression over 12 months. It is currently unclear whether this is due to limitations of the technology, or the slow rate of disease progression in this cohort of FSHD patients over this period of time.
Keywords: Facioscapulohumeral muscular dystrophy, muscular dystrophy, electrical impedance myography, biomarker, outcome measure, muscle composition
Introduction
Facioscapulohumeral muscular dystrophy is a hereditary muscle disorder.1,2 A unifying model of the genetic mechanism for FSHD has been proposed and the increasing knowledge of the pathogenic mechanism provides opportunities to develop targeted therapeutic strategies.3,4 Therefore, there is a high likelihood of clinical trials being initiated in the upcoming years. In preparation for these trials, there is a need to develop outcome measures.5
Development of biomarkers that are sensitive to change will be crucial especially for early phase clinical trials.
Electrical impedance myography (EIM) has been proposed as a biomarker for disease severity and progression in neuromuscular disorders.6,7 EIM is a non-invasive technique that measures changes in muscle composition through bio-impedance measures and thus provides a potential quantitative assessment of structural muscle changes.6 Electrodes are placed on the skin that produce multi-frequency, low intensity alternating electrical currents. These currents are applied to individual muscles or muscle groups and the resulting voltage is measured. Impedance is a measure of obstruction of the flow of the electrical current through the tissue, which differs between various body tissues such as muscle or fat.
In FSHD, muscles undergo structural changes as the disease progresses, including atrophy, fatty infiltration, edema and fibrosis. These changes can all potentially influence the impedance across the muscle tissue.8,9 In a previous cross-sectional study on EIM in FSHD, we showed that EIM is a reliable measure of muscle composition in FSHD, that correlates with functional outcome measures.10 Studies on other neuromuscular disorder like amyotrophic lateral sclerosis, congenital myopathies and Duchenne muscular dystrophy, have shown that EIM is able to capture changes over time11–14, although research is ongoing on what parameters are most suitable to use in each different disorder.
In this study we assessed the changes in EIM measurements in FSHD participants and the potential of EIM as a biomarker for clinical trials using a one year longitudinal study.
Methods
Study design
This was a prospective, observational study. Participants were recruited at the University of Rochester Medical Center from 2012 to 2015. The study was approved by the human subjects committee, and written informed consent was obtained from all participants.
Participants
We included genetically confirmed participants aged 18–75 years, who had clinical symptoms while still being ambulatory.10 Data were obtained during 3 visits: one at baseline (and < 3 weeks later for reliability testing), one at 6 months and one at 12 months. Baseline and reliability data have previously been reported.10
EIM measurements
EIM measurements were obtained using a handheld EIM device from Skulpt, Inc. (Boston, Massachusetts) with a transverse sensor configuration (current passing across the myofibers), as previously described10. Measurements were obtained utilizing standard positioning over the following muscles bilaterally: deltoid, biceps, triceps, abdominals, vastus lateralis, tibialis anterior, and thoracic paraspinals. Investigators underwent a single afternoon training session at the beginning of the study. Reactance, resistance and phase angle were recorded for 41 frequencies ranging from 1 kHz to 10 MHz. For each muscle, 3 consecutive measurements were performed and the 2 most closely aligned measurements were averaged. For analyses 4 different frequencies were chosen based on prior EIM studies in neuromuscular diseases: 50 kHz, 100 kHz, 211 kHz and 300 kHz. In addition, phase ratios were calculated for 50/211 kHz and 100/300 kHz11,15.
Clinical outcome measures
Quantitative strength measurements were collected using fixed dynamometry (QMA system, Gainesville, GA). The maximum voluntary isometric contraction testing (MVICT) was recorded in kilogram-force for each muscle group and then standardized for sex, age and height as previously described16. Composite MVICT scores were calculated by averaging the standardized scores across multiple muscle groups: shoulder abduction, elbow flexors and extensors, ankle dorsiflexors, knee flexors and extensors and handgrip. Regional composite scores were calculated by averaging upper and lower extremity muscle groups only.
The FSHD clinical score assigns severity scores for different body regions, resulting in a total score that ranges from 0–15, in which zero indicates no symptoms and 15 indicates extremely severe symptoms (e.g. requiring a wheelchair for mobility).
The ‘timed up and go’ is a timed functional test that uses the time it takes for a patient to get up from a standard armchair, walk 3 meters, turn, walk back and sit down17.
The domain delta questionnaire is a patient-reported anchor questionnaire that asks participants to indicate whether they got worse, remained stable or improved during the study period on the domains of overall health, mobility and ambulation and upper arm and shoulder function. There is one question for each domain with five answering options: much worse, a little worse, unchanged, a little better, or much better.
Statistical analyses
Statistical analyses were performed using SPSS version 22. Descriptive statistics were calculated for all parameters and are presented as mean and standard deviation unless stated otherwise.
Outliers due to technical EIM errors, such as negative values at low frequencies or extremely high values far outside the normal range, were not included in analysis (the combination of deleted outliers and missing data, resulted in no more than 1.4% of total data being missing). Missing values were considered to be missing at random. For analyses of average scores over multiple muscles, we used a last-observation carried forward approach for missing data.
Spearman rho analyses were used to calculate bivariate correlations with p-values of < 0.05 considered statistically significant. A one-way repeated measures ANOVA was used to test for changes in EIM and clinical results at baseline and after 6 and 12 months. To control for multiple testing we applied the Benjamini-Hochberg false discovery rate procedure, a less conservative method than the Bonferroni correction, in which we accepted the proportion of false discoveries to be 5%18.
Differences in change in EIM outcomes between participants that subjectively did or did not progress over 6 months based on the domain delta questionnaire, was tested using a mixed ANOVA with time as a within-subject factor and outcome on the domain delta questionnaire as a between-subject factor.
Sample size calculations were based on a hypothetical two-armed placebo-controlled clinical trial with an 80% power to detect a 5% difference. G*Power statistical software version 3.1 was used to calculate sample sizes based on the mean baseline EIM values and the standard deviation of the 12 month change19.
Results
Participants
Out of the previously reported 35 participants in our cross-sectional EIM study10, 32 participants had 12 month longitudinal data available for analysis. The majority of participants were male. Baseline characteristics are shown in table 1.
Table 1.
Baseline patient characteristics.
| Parameter | Value (n =32) |
|---|---|
| Sex (n) | 25 male (78.1%) |
| Age (years, SD, [range]) | 52.3 ±11.7 [22–68] |
| FSHD clinical score (mean, SD, [range]) | 6.6 ±3.0 [1–11] |
| BMI (kg/m2, SD, [range]) | 27.6 ±6.0 [17.2–43.3] |
| FSHD type (n) | 30 FSHD1; 2 FSHD2 |
| Repeat length FSHD1 (kb, SD, [range]) | 25.2 ±6.5 [13–41] |
| Time since first symptom (patient reported) (years, SD, [range]) | 24.7 ±12.8 [4.2–47.0] |
SD: standard deviation, BMI: body mass index
Baseline correlations EIM and clinical measures
A comprehensive overview of correlations of EIM measurements to clinical measures is given in supplemental table 1. The 50 kHz reactance showed moderate, statistically significant correlations to all clinical measures. Correlations between clinical measures and phase-ratios were stronger than correlations to single frequency phase values.
EIM changes
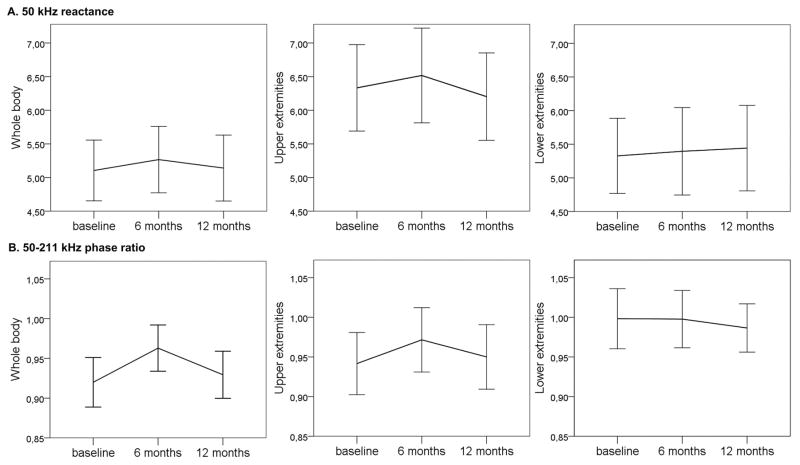
After 12 months of follow-up, no significant changes in EIM parameters were found for the average of all muscles measured, or the average of lower or upper extremity muscles. Mean changes over 12 months in 50 kHz reactance and 50–211 kHz phase-ratio, 2 of the EIM parameters that correlated the strongest with clinical measures at baseline, are shown in figure 1. The mean change of the 50 kHz reactance over all muscles measured was 0.001 after 12 months (95%-CI −0.309 to 0.312), for the 50–211 kHz phase-ratio the mean 12 month change was 0.009 (95%-CI −0.015 to 0.034). Additionally, we assessed changes in 50 kHz reactance and 50–211 kHz phase-ratio over 12 months for all individual muscles and found no significant changes (supplemental table 2). We also assessed 12 months changes in individual muscles for the reactance and phase for 100, 211 and 300 kHz and for the 100–300 kHz phase-ratio and found no significant changes (data not reported).
Figure 1.
50 kHz reactance (A) and 50–211 kHz phase-ratio (B) at baseline, 6 months and 12 months (whole body, upper extremity, and lower extremity averages)
Changes in clinical outcomes
After 12 months there was a change in the composite MVICT for all muscles measured (mean change −0.48, 95%-CI −0.90 to −0.07, p = 0.042), but not in the raw quantitative strength results per muscle (supplemental table 3). The ‘timed up and go’ test did not significantly change over 12 months (mean change 0.11, 95%-CI −0.40 to 0.62, p = 0.663).
Self-reported impression of change
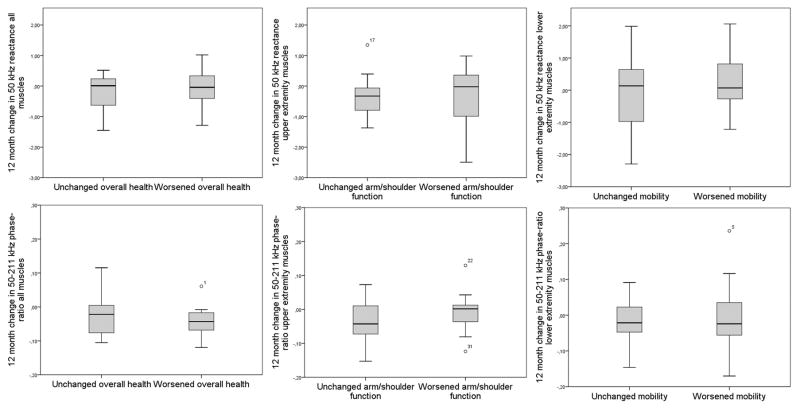
Participants who reported worsening on the domains of overall health (n=12, 37.5%), mobility and ambulation (n=18, 56.3%), and arm and shoulder function (n=15, 46.9%), did not significantly change in their results on mean EIM measurements for the whole body, lower extremities and upper extremities, respectively (figure 2).
Figure 2.
Change in EIM parameters (50 kHz reactance and 50–211 kHz phase-ratio) after 12 months for patients who reported to have remained stable and who reported subjective disease progression on different domains of the domain delta questionnaire.
Clinical trial planning
Estimates of variability of baseline values and changes over time are given in table 2. These estimates of variability were used to calculate the required sample sizes for a two-armed placebo-controlled clinical trial with an 80% power to detect a 5% difference over 12 months. The variability across phase-ratio measures was smaller compared to reactance measures and to clinical measures, resulting in smaller sample sizes with the phase-ratio as a potential outcome parameter (table 2).
Table 2.
Estimates of variability of baseline measurements and of changes over 12 months, and subsequent sample size requirements
| Baseline | 12 months change from baseline | Required sample size (per arm) | |
|---|---|---|---|
| EIM measure | SD | SD | |
| Reactance 50 kHz whole body | 1.25 | 0.61 | 90 |
| Reactance 50 kHz upper extremities | 1.78 | 0.70 | 77 |
| Reactance 50 kHz lower extremities | 1.55 | 0.87 | 168 |
| Phase-ratio 50–211 kHz whole body | 0.09 | 0.06 | 29 |
| Phase-ratio 50–211 kHz upper extremities | 0.11 | 0.06 | 28 |
| Phase-ratio 50–211 kHz lower extremities | 0.11 | 0.07 | 33 |
|
| |||
| Clinical measures | |||
|
| |||
| QMT whole body (kgf) | 4.50 | 1.15 | 140 |
| QMT upper extremities (kgf) | 4.71 | 0.88 | 143 |
| QMT lower extremities (kgf) | 5.72 | 0.93 | 57 |
| Timed up and go test (sec) | 3.55 | 1.42 | 90 |
QMT: standardized quantitative muscle testing; kgf: kilogram-force; sec: seconds
Discussion
In this 12 month follow-up study of 32 FSHD participants, EIM measurements did not change. The absence of change was consistent across all EIM parameters, all frequencies and all (individual) muscles measured. There was a small decrease in the composite MVICT for all muscles measured, and no change in the strength measurements for individual muscles and in the timed up and go test. This indicates that this select group of patients showed only minimal change over one year. This fits with the clinical picture of FSHD as a slowly progressive disorder with functional decline gradually evolving over many years.16 Because of the small or even absent change in the clinical outcome measures, it is not possible to definitively conclude if the absence of change in EIM parameters was due to limitations of this technology as a longitudinal biomarker in this population over this period of time versus the possibility that EIM is a sufficient tool that detected no change in a clinically relatively static subset of patients.
On a cross-sectional level there is a moderate correlation of EIM measurements with clinical measures, especially for 50 kHz reactance, indicating that EIM is able to capture differences in the degree of muscle involvement.10
We analyzed data both on individual muscles or muscle groups and on composite scores. While composite scores have the advantage of smaller variability, the addition of relatively unaffected muscles can decrease the chance of detecting a change. The muscles that were measured by EIM in this study, were all shown to be commonly affected in imaging studies on FSHD.20–22 For future studies, including the hamstrings could be a valuable addition, as these are affected frequently and early in the disease course.21
This study only measured EIM parameters in the setting of a natural history study. Therefore, EIM could potentially be a useful biomarker in clinical trials where therapeutic interventions may have a beneficial effect on the muscles. For example, in boys with Duchenne muscular dystrophy, a change in EIM values was seen shortly after initiating corticosteroid treatment.23
It is still unclear which EIM parameters are best suited to measure changes and the choice for a specific parameter depends on the disease being studied. Consequently, multiple studies over the last years have all used different outcomes including, amongst others, single and multi-frequency measures, single muscle and composite measures, single phase values and phase-ratios.13,11,15,24,14 Prior studies have shown reactance is least susceptible to variability in subcutaneous fat content or electrode configuration.25 Despite this, several studies have used phase ratios as opposed to reactance – this value appears to be optimized for function versus subcutaneous fat in muscle disease, and as a derived measure, utilizes both elements of reactance and resistance.23,26 For FSHD, the reactance correlated the strongest with clinical measures at baseline and seemed least affected by changes in body fat, which is also reflected in the weaker correlations to the body mass index. However, because of the smaller variability in phase-ratio outcomes, this parameter would require much smaller sample sizes to detect differences in a clinical trial. Therefore, the choice of the parameter to be used in FSHD, will depend on the clinical trial design in which a trade-off should be made between the stronger correlation to clinical measures of 50 kHz reactance, and the opportunity to use smaller sample sizes with the 50–211 kHz phase-ratio. In addition, the use of the 50–211 kHz EIM parameter would permit smaller sample sizes compared to the clinical measures. The required sample sizes for QMT are consistent with a natural history study on FSHD, that found sample sizes of 160 patients per group for a two-armed clinical trial with one year of follow-up16.
For future studies, there would be different ways to increase the chance of detecting changes using EIM measurements. First, a larger cohort, a longer follow-up period, or a subset of patients with a more rapid progression could potentially result in differences that can be picked up by EIM. Additionally, advances in the sensor technology make different electrode configurations possible using the same sensor (e.g. longitudinal versus cross sectional, or a combination of the two).27
This was a single site study and for EIM to be used in future studies on FSHD, its results should be validated in a multicenter cohort. To assess changes over time we used a repeated measure ANOVA. Another commonly used option for longitudinal studies is a linear mixed effects model. Since the design of this study was simple and there were few data points missing, the results of both analyses including the sample size calculations are expected to be similar. We did not include a healthy control group, and therefore do not know how longitudinal EIM data would compare to healthy subjects.
Although EIM did not identify statistically significant changes in this FSHD cohort, it is an easily applied, fast and non-invasive technique that provides quantitative measures of muscle composition that can possibly results in sample size savings for clinical trials. Therefore, assessment of its sensitivity to change in larger cohorts or over a longer follow-up period should be considered.
Supplementary Material
Acknowledgments
The project described in this publication was supported by the following: the FSH Society grants (FSHS-22013 and FSHS-82012) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant #U01AR065119. Additional support was provided by the University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Statland’s work on this project was supported by a NCATS grant awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119. Dr. Mul was supported by a grant from the Dr. Jan Meerwaldt Foundation and the Prinses Beatrix Spierfonds (W.OR12-22). Dr. Heatwole’s work was in supported in part by the The Goldberg Nathan Foundation.
List of abbreviations
- EIM
electrical impedance myography
- FSHD
facioscapulohumeral muscular dystrophy
- MVICT
maximum voluntary isometric contraction testing
Footnotes
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of conflicts of interest
KM receives support from the Dr. Jan Meerwaldt Foundation and the Prinses Beatrix Spierfonds. CH has received grant funding from the NIH, FDA, and Cure SMA foundation. He is the founder and CEO of the Neuromuscular Quality of Life Institute. He receives royalties for multiple clinical trial instruments. He has provided expert testimony for neuromuscular cases unrelated to this research. He has provided consultation to Biogen, Ionis, AMO, aTyr, Regeneron, and Acceleron Pharma. JS is a consultant for Acceleron, Strongbridge Biopharma, Regeneron, and Sanofi-Genzyme. BvE reports grants from Prinses Beatrix Spierfonds, Association Francaise contre les Myopathies, Stichting Spieren voor Spieren, FSHD Stichting, and NWO (Dutch Organisation for scientific research). RT has ongoing research support from NIH/NINDS grants 1 U01 NS101944-01 and 1P01 NS069539. The remaining authors have no conflicts of interest.
References
- 1.Statland J, Tawil R. Facioscapulohumeral muscular dystrophy. Neurol Clin. 2014;32(3):721–728. ix. doi: 10.1016/j.ncl.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mul K, Lassche S, Voermans NC, Padberg GW, Horlings CG, van Engelen BG. What’s in a name? The clinical features of facioscapulohumeral muscular dystrophy. Pract Neurol. 2016 doi: 10.1136/practneurol-2015-001353. [DOI] [PubMed] [Google Scholar]
- 3.Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, Dauwerse JG, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329(5999):1650–1653. doi: 10.1126/science.1189044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemmers RJ, Tawil R, Petek LM, Balog J, Block GJ, Santen GW, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet. 2012;44(12):1370–1374. doi: 10.1038/ng.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawil R, Padberg GW, Shaw DW, van der Maarel SM, Tapscott SJ, Participants FW. Clinical trial preparedness in facioscapulohumeral muscular dystrophy: Clinical, tissue, and imaging outcome measures 29–30 May 2015, Rochester, New York. Neuromuscul Disord. 2016;26(2):181–186. doi: 10.1016/j.nmd.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Rutkove SB. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve. 2009;40(6):936–946. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez B, Rutkove SB. Electrical Impedance Myography and Its Applications in Neuromuscular Disorders. Neurotherapeutics. 2017;14(1):107–118. doi: 10.1007/s13311-016-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statland JM, Shah B, Henderson D, van der Maarel S, Tapscott SJ, Tawil R. Muscle pathology grade for facioscapulohumeral muscular dystrophy biopsies. Muscle Nerve. 2015 doi: 10.1002/mus.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen BH, Voet NB, Nabuurs CI, Kan HE, de Rooy JW, Geurts AC, et al. Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One. 2014;9(1):e85416. doi: 10.1371/journal.pone.0085416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statland JM, Heatwole C, Eichinger K, Dilek N, Martens WB, Tawil R. Electrical impedance myography in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2016;54(4):696–701. doi: 10.1002/mus.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidman CM, Wu JS, Kapur K, Pasternak A, Madabusi L, Yim S, et al. Quantitative muscle ultrasound detects disease progression in Duchenne muscular dystrophy. Ann Neurol. 2017;81(5):633–640. doi: 10.1002/ana.24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Lateral Scler. 2012;13(5):439–445. doi: 10.3109/17482968.2012.688837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutkove SB, Gregas MC, Darras BT. Electrical impedance myography in spinal muscular atrophy: a longitudinal study. Muscle Nerve. 2012;45(5):642–647. doi: 10.1002/mus.23233. [DOI] [PubMed] [Google Scholar]
- 14.Nichols C, Jain MS, Meilleur KG, Wu T, Collins J, Waite MR, et al. Electrical impedance myography in individuals with collagen 6 and laminin alpha-2 congenital muscular dystrophy: a cross-sectional and 2-year analysis. Muscle Nerve. 2017 doi: 10.1002/mus.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz S, Geisbush TR, Mijailovic A, Pasternak A, Darras BT, Rutkove SB. Optimizing electrical impedance myography measurements by using a multifrequency ratio: a study in Duchenne muscular dystrophy. Clin Neurophysiol. 2015;126(1):202–208. doi: 10.1016/j.clinph.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): implications for therapeutic trials. The FSH-DY Group. Neurology. 1997;48(1):38–46. doi: 10.1212/wnl.48.1.38. [DOI] [PubMed] [Google Scholar]
- 17.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. The Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 19.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3. 1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 20.Leung DG, Carrino JA, Wagner KR, Jacobs MA. Whole-body magnetic resonance imaging evaluation of facioscapulohumeral muscular dystrophy. Muscle Nerve. 2015 doi: 10.1002/mus.24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mul K, Vincenten SCC, Voermans NC, Lemmers R, van der Vliet PJ, van der Maarel SM, et al. Adding quantitative muscle MRI to the FSHD clinical trial toolbox. Neurology. 2017;89(20):2057–2065. doi: 10.1212/WNL.0000000000004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlqvist JR, Vissing CR, Thomsen C, Vissing J. Severe paraspinal muscle involvement in facioscapulohumeral muscular dystrophy. Neurology. 2014;83(13):1178–1183. doi: 10.1212/WNL.0000000000000828. [DOI] [PubMed] [Google Scholar]
- 23.Rutkove SB, Kapur K, Zaidman CM, Wu JS, Pasternak A, Madabusi L, et al. Electrical impedance myography for assessment of Duchenne muscular dystrophy. Ann Neurol. 2017;81(5):622–632. doi: 10.1002/ana.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, Parsons JA, et al. Electrical impedance myography in Duchenne muscular dystrophy and healthy controls: A multicenter study of reliability and validity. Muscle Nerve. 2015;52(4):592–597. doi: 10.1002/mus.24611. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Li X, Hu H, Shin H, Zhou P. The Effect of Subcutaneous Fat on Electrical Impedance Myography: Electrode Configuration and Multi-Frequency Analyses. PLoS One. 2016;11(5):e0156154. doi: 10.1371/journal.pone.0156154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, Parsons JA, et al. Electrical impedance myography in duchenne muscular dystrophy and healthy controls: A multi-center study of reliability and validity. Muscle Nerve. 2015 doi: 10.1002/mus.24611. [DOI] [PubMed] [Google Scholar]
- 27.Jafarpoor M, Li J, White J, Rutkove S. Optimizing Electrode Configuration for Electrical Impedance Measurements of Muscle via the Finite Element Method. IEEE Trans Biomed Eng. 2013 doi: 10.1109/TBME.2012.2237030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.