Abstract
Pseudohypoparathyroidism type 1A (PHP1A), pseudoPHP (PPHP), and PHP type 1B (PHP1B) are caused by maternal and paternal GNAS mutations, and abnormal methylation at maternal GNAS promoter(s), respectively. Adult PHP1A patients are reportedly obese and short, whereas most PPHP patients are born small. In addition to PTH resistance, PHP1A and PHP1B patients may display early-onset obesity. As early-onset and severe obesity and short stature are daily burdens for PHP1A patients, we aimed at improving knowledge on the contribution of the GNAS transcripts to fetal and postnatal growth and fat storage. Through an international collaboration, we collected growth and weight data from birth until adulthood for 306 PHP1A/PPHP and 220 PHP1B patients. PHP1A/PPHP patients were smaller at birth than healthy controls, especially PPHP (length z-score PHP1A: −1.1±1.8; PPHP: −3.0±1.5). Short stature is observed in 64% and 59% of adult PHP1A and PPHP patients. PHP1B patients displayed early post-natal overgrowth (height z-score at 1 year: 2.2±1.3 and 1.3±1.5 in autosomal dominant and sporadic PHP1B) followed by a gradual decrease in growth velocity resulting in normal adult height (z-score for both: −0.4±1.1). Early-onset obesity characterizes GNAS alterations and is associated to significant overweight and obesity in adults (BMI z-score: 1.4±2.6, 2.1±2.0, and 1.4±1.9 in PPHP, PHP1A, and PHP1B, respectively), indicating that reduced Gsα expression is a contributing factor.. The growth impairment in PHP1A/PPHP may be due to Gsα haploinsufficiency in the growth plates; the paternal XLαs transcript likely contributes to prenatal growth; for all disease variants, a reduced pubertal growth spurt may be due to accelerated growth plate closure. Consequently, early diagnosis and close follow-up is needed in patients with GNAS defects to screen and intervene in case of early-onset obesity and decreased growth velocity.
Keywords: pseudohypoparathyroidism, pseudopseudohypoparathyroidism, GNAS, early-onset obesity, growth
INTRODUCTION
Pseudohypoparathyroidism (PHP) was defined initially by Fuller Albright as the resistance to the parathyroid hormone (PTH) characterized by hypocalcemia and hyperphosphatemia in association with different features of Albright Hereditary Osteodystrophy (AHO), including stocky build, brachydactyly, and short stature. These patients often present with additional features such as early-onset obesity, subcutaneous ossifications, neurocognitive deficiency, and resistance to other hormones which mediate their actions through the alpha-subunit of the stimulatory G protein (Gsα) such as thyroid-stimulating hormone (TSH)(1,2). Differently, pseudoPHP (PPHP) is characterized in most patients by physical symptoms of AHO, yet no resistance to PTH or TSH. Binding of these hormones to their receptors, promotes the coupling to the G protein and cAMP generation by adenylyl cyclase. Gsα is encoded by exons 1–13 of GNAS, part of a complex imprinted locus on chromosome 20q13.3. GNAS encodes at least four additional transcripts using alternative first exons and their promoters that undergo parent-specific methylation. These include NESP55 (NeuroEndocrine Secretory Protein 55), derived from the non-methylated maternal allele, while XLαs (the eXtra Large variant of Gsα), the A/B transcript, and an antisense (AS) transcript are transcribed from the non-methylated paternal GNAS allele(3) (figure 1). While Gsα is derived in most tissues from both parental alleles, its expression from the paternal GNAS allele can be reduced or abolished in some tissues such as renal proximal tubules, brown adipose tissue, pituitary, gonads, thyroid and hypothalamus through an as-yet undefined tissue-specific silencing mechanism (4,5). Maternal loss-of-function mutations at GNAS exons 1–13 cause PHP type 1A (PHP1A). Interestingly, the same or similar mutations on the paternal allele cause PPHP(6).
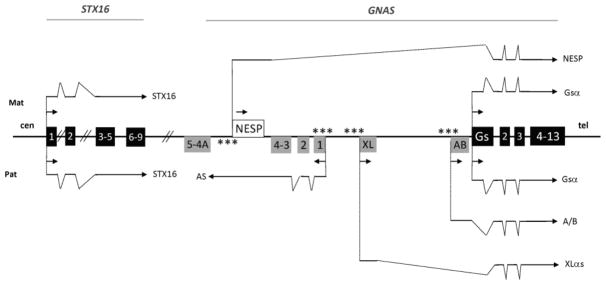
Figure 1.
Schematic drawing of the GNAS locus
The imprinted human GNAS locus (not to scale, Hg19-chr20:57,414,795–57,486,250) and the STX16 gene (Hg19-chr20:57,226,309–57,254,5812) on chromosome 20. The centromeric/telomeric (cen/tel) orientation of the chromosome is indicated. Boxes indicate coding exons. Black boxes: transcripts expressed from both parental allele; grey boxes: transcripts expressed only from the paternal allele; white box: transcript expressed predominantly from the maternal allele. Stars indicate the differentially methylated regions. Arrows: transcription (direction and parental origin). Transcripts are drawn above (mat: maternal) or below (pat: paternal) the GNAS locus and the STX16 gene; for clarity, the N1 exon that is located between exon 3 and 4 of GNAS, and is alternatively spliced, has not been included in this scheme. Sense transcripts (NESP, XLαs, A/B and Gsα) have their own first exon and share GNAS exons 2 to 13.
In contrast, PHP type 1B (PHP1B) encompasses patients with methylation defects at one or more GNAS promoters (7). About 20% of PHP1B patients present with an autosomal form of the disease (AD-PHP1B) characterized by loss-of-methylation restricted to the maternal exon A/B DMR (GNAS A/B:TSS-DMR)(8). Most of these patients display a maternal 3-kb microdeletion within STX16, located approximately 220 kb upstream of GNAS(8). Patients with the sporadic form of PHP1B (spor-PHP1B) - about 70% of all PHP1B patients - present with abnormal maternal methylation involving at least one GNAS DMR in addition to the GNAS A/B:TSS-DMR(7,8). The mechanism(s) underlying these methylation changes has not yet been defined. Lastly, approximately 10% of all spor-PHP1B cases are caused by paternal uniparental isodisomy of chromosome 20q [iUPD(20q)pat], which includes the GNAS locus(9). Resistance to PTH is the most prominent finding in patients affected by PHP1B, but mild resistance to TSH occurs frequently(10).
PHP may easily be confused with other diseases due to clinical, biochemical and/or molecular overlap. We note that a recently proposed alternate classification uses the term “inactivating PTH/PTHrP Signaling Disorder”(11) to describe GNAS loss-of-function mutations. PHP1A and PPHP, which involve mutations of either the maternal or the paternal allele, are referred to as iPPSD2, and PHP1B variants caused by GNAS methylation defects are referred to as iPPSD3 defects upstream of Gsα, e.g., inactivating PTH1R mutations, are referred to as iPPSD, and mutations in PRKAR1A, PDE4D, and PDE3A are referred to as iPPSD4, iPPSD5, and iPPSD6, respectively).
It has been frequently reported that patients affected by PHP1A and PPHP have adult heights that are approximately −2.5 and −2.3 SD, respectively, below average(12,13); growth delay is already present at birth in both diseases, particularly in PPHP patients(14). By contrast, final adult heights of AD-PHP1B and spor-PHP1B patients are described as “normal” in most reports despite recent descriptions of enhanced fetal growth(15) and case reports of post-natal overgrowth(16,17) (a summary of the evidence available in the literature for pre- and postnatal growth in PHP1A, PPHP and PHP1B is provided in the supplemental appendix 1). Obesity is well known to be associated with PHP1A as exemplified by the largest study to-date conducted in adults affected by this disorder, showing an average BMI z-score of 1.7±0.2 with two thirds of the patients meeting the criteria for obesity(13). In addition, early-onset obesity has been observed in case reports and small series of patients with PHP1B(16,17).
Besides fetal growth data, longitudinal trajectories of growth and weight and their determinants are currently unknown for patients affected by the different PHP variants, and final heights and BMI have not been systematically evaluated in either disorder. Obesity and short stature represent heavy burdens in the daily life for many of these patients. Therefore, the goals of our study were to: i) determine the longitudinal pattern of growth and weight gain for large cohorts of patients affected by PHP1A and PPHP (carrying a GNAS loss-of-function mutation), and by AD-PHP1B and spor-PHP1B (carrying a GNAS methylation defect), ii) determine adult height, weight and BMI of patients affected by these disorders, and iii) enhance our understanding of the mechanisms and the roles of the different GNAS-derived transcripts in fetal and postnatal growth and weight gain. We strongly believe that a thorough analysis and understanding of these exemplary rare diseases will contribute to a better management and care of affected patients, and will help generate hypotheses regarding the underlying mechanisms.
MATERIALS AND METHODS
Patients
We collected the GNAS molecular defect, gestational age, length/height and weight parameters from birth until the most recent available data or the age of 18 years (longitudinal data) of patients affected with a documented molecular defect at the GNAS locus, hence a diagnosis of either PHP1A, PPHP, AD-PHP1B or spor-PHP1B. We gathered the data available at the following time points: birth, 0.5, 1, 1.5, 2, 3, 6, 8, 10, 12, 14, 16 and 18 years. Data were obtained from patients in France, Spain, Italy, and USA. Some growth and weight aspects have been partially reported for a subset of these patients (supplemental appendix 1). Patients affected with PHP1B due to iUPD(20q)pat were excluded from the study because the duplication of the paternal chromosome may affect other genes on chromosome 20q with an unknown impact on growth and weight gain. Growth and weight data of patients treated with growth hormone were included only until recombinant human growth hormone (rhGH) therapy was initiated. Ethnicity and average measurement per country, per patient, and per age group are available in supplemental appendix 2. We calculated BMI, using the formula weight(kg)/height(m)2. Z-scores for length/height, weight and BMI were derived from the World Health Organization (WHO) growth charts(18). Small for gestational age (SGA) and macrosomia are defined by z-scores of birth weight and/or birth length for gestational age below −1.28 and above 2.0 (<10th and >97th centile), respectively(19). When term was defined in medical files as “full term”, z-scores were calculated using 39 weeks of gestation, however, these patients were not included in the calculation of mean gestational age. Short and tall stature are defined by z-scores of adult height (measure shown at 18 years in the present report) below −2.0 and above 2.0 (<3rd and >97th centile), respectively. Overweight and obesity were defined as BMI above 25 and 30 kg/m2, respectively. After establishing the diagnosis, patients were regularly monitored for PTH and TSH resistance; LdS, PK, AR, DT, ST, AHS, HJ, GM and AL managed most of the patients.
In the figures and legends, iPPSD2 refers to patients affected with a GNAS loss-of-function mutation whereas iPPSD3 refer to patients with a GNAS methylation defect(11). To address specifically maternal or paternal GNAS mutations, or the different subtypes of methylation defects, the terms of PHP1A, PPHP, AD-PHP1B or spor-PHP1B have been preferred.
The study was approved by the comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé (CCTIRS, #13-028) under the promotion of the INSERM (Institut national de la santé et de la recherche médicale) (#DC-2013-1762). Similarly, institutional review boards approved the use of data for the study in USA, Italy and Spain (Vanderbilt University Medical Center, IRB#130735, Massachusetts General Hospital, IRB#2001P000648, OSI Araba University IRB #PI2013214 and PI2017018).
Molecular characterization of the GNAS gene/locus
The diagnosis of PHP1A was based on the presence of PTH-resistance and AHO features associated with maternal loss-of-function mutations involving GNAS exons 1 to 13 documented DNA sequencing. The parental origin of the mutation was determined by investigating the parents or, in the case of de novo mutations, and if parental DNA was available, through the cloning of PCR amplicons and the analysis of informative intragenic polymorphisms that reside on the same allele as the disease-causing mutation and assigned to one parent. In some patients for whom it was not possible to determine the allelic origin through disease transmission or polymorphism transmission, the GNAS transcripts were amplified from blood lymphocytes (GNAS exon A/B to exon 13) as described by Turan and colleagues(20). Finally, in few remaining patients, the diagnosis of PHP1A was based on the presence of PTH-resistance, AHO features, and a disease-causing GNAS mutation. The diagnosis of PPHP was based on the presence of a GNAS mutation on the paternal allele and AHO features without hormonal resistance. In very few PHP1A patients (n=10), the exact mutation could not be retrieved from medical notes.
To explore the possibility of genotype/phenotype relationships, GNAS variants were divided as follows: 1) variants that severely impact Gsα function, i.e. premature stop codons and insertions/deletions (indels) leading to a shift in the open reading frame versus variants predicted to impact Gsα function less profoundly, i.e. amino acid substitutions and in-frame indels and 2) variants located within GNAS exon 1 predicted to preserve the XLαs function in affected PHP1A patients versus variants located within GNAS exons 2–13 predicted to affect the function of both Gsα and XLαs in PHP1A (figure 1).
The diagnosis of PHP1B was based on the presence of PTH-resistance without obvious AHO features in association with abnormal methylation at the GNAS A/B:TSS-DMR alone or including other GNAS DMRs. Methylation defects were documented by pyrosequencing or MS-MLPA, as described(21). AD-PHP1B was defined by the association of a methylation defect restricted to the GNAS A/B:TSS-DMR, the recurrent 3-kb STX16 deletion or the 4.4-kb STX16 deletion in one family. Spor-PHP1B was defined by a methylation defect affecting GNAS B:TSS-DMR and at least one other GNAS DMR. In these patients iUPD(20q)pat was excluded by showing, through SNP array or microsatellite analysis of genomic DNA, that the patient is heterozygous throughout the GNAS/STX16 locus. Patients affected by AD-PHP1B due to GNAS deletions removing NESP or antisense exons were not included.
Statistical analysis
Statistical analyses were performed using Prism software 6.0.
Length/height, weight and BMI at birth, 1 year, 3 years, 10 years, and 18 years, are presented as z-scores (mean±SD; number of independent samples; p-value). Longitudinal growth patterns of patients are presented as a function of age (mean±SD) and are grouped by diseases PHP1A and PPHP versus AD-PHP1B and spor-PHP1B. When three or fewer values are available per age group, data are not averaged thus do not appear on the figures.
The one-way ANOVA test followed by a Tukey’s multiple comparisons test was used to compare the results for different types of disease variants (alpha=0.05). T-tests were performed to compare whether data (z-score) of patients were significantly different from the hypothetical value of zero (mean of the population) (alpha=0.05). We used the unpaired t-test to compare data between the two subtypes of PHP1B.
Role of the funding sources
The funding sources had no roles regarding the study or the publication.
RESULTS
Patients
Data were collected from 526 patients affected either by PHP1A, n=242 [107M/135F], PPHP, n=64 [19M/45F], AD-PHP1B, n=92 [46M/46F] or spor-PHP1B, n=128 [60M/68F] (table 1). Altogether, 306 patients carry a mutation on their GNAS gene, whereas 220 patients exhibit methylation defects at the GNAS locus.
Table 1.
Birth length, birth weight, final height, weight and BMI at 18 years in the different GNAS-related disorders grouped by gender or genotype. WHO growth charts do not permit calculate weight z-scores above the age of 10 years.
| Birth length (cm) mean [SD; n] |
Birth weight (kg) mean [SD; n] |
Final height (cm) mean [SD; n] |
Weight at 18 years (kg) mean [SD; n] |
BMI at 18 years (kg/m2) mean [SD; n] |
||
|---|---|---|---|---|---|---|
| PHP1A (iPPSD2) |
F n= 135 |
47.1 [2.8; 71] | 2.9 [0.6; 85] | 146.3 [8.0; 56] | 62.2 [15.1; 41] | 29.2 [6.2; 42] |
|
M n=107 |
47.7 [3.8; 72] | 3.0 [0.8; 84] | 158.1 [6.7; 28] | 65.9 [12.6; 26] | 26.2 [4.7; 25] | |
|
Severe impact n=153 |
−1.1* [1.9; 71] | −0.4* [1.7; 85] | −2.3* [1.1; 44] | 2.0* [1.9; 33] | ||
|
Mild impact n=79 |
−1.2* [1.6; 44] | −0.7* [1.4; 51] | −2.6* [1.0; 22] | 2.6* [2.2; 16] | ||
|
Exon 1 n=44 |
−1.3* [0.7; 18] | −1.1* [0.8; 23] | −2.8* [1.1; 11] | 3.1* [2.3; 10] | ||
|
exons 2–13 n=189 |
−1.0* [1.9; 126] | −0.5* [1.7; 150] | −2.4* [1.1; 75] | 2.0* [1.8; 39] | ||
| PPHP (iPPSD2) |
F n= 45 |
43.3 [2.7; 23] | 2.1 [0.5; 26] | 151.3 [7.8; 28] | 59.1 [18.2; 17] | 26.0 [8.4; 16] |
|
M n=19 |
44.4 [3.0; 15] | 2.2 [0.5; 16] | 159.2 [11.4; 6] | 62.0 [15.7; 4] | 24.0 [5.0; 4] | |
|
Severe impact n=48 |
−3.0* [1.5; 31] | −2.6* [1.3; 35] | −1.7* [1.3; 23] | 0.5* [2.0; 16] | ||
|
Mild impact n=15 |
−3.1* [1.5; 7] | −2.9* [1.4; 7] | −2.3* [1.1; 10] | 4.3* [1.6; 3] | ||
|
Exon 1 n=16 |
−2.8* [0.9; 9] | −1.9* [0.9; 12] | −2.4* [0.9; 8] | 0.8* [1.2; 5] | ||
|
exons 2–13 n=47 |
−3.1* [1.6; 28] | −3.0* [1.3; 29] | −1.8* [1.3; 25] | 1.2* [2.7; 14] | ||
| AD-PHP1B (iPPSD3) |
F n=46 |
50.2 [2.1; 13] | 3.7 [0.9; 16] | 160.4 [7.4; 24] | 72.9 [21.3; 21] | 28.8 [8.9; 21] |
|
M n=46 |
51.2 [2.5; 20] | 3.8 [0.5; 23] | 172.7 [8.4; 12] | 72.7 [13.2; 11] | 24.4 [3.7; 11] | |
| spor-PHP1B (iPPSD3) |
F n=68 |
49.4 [2.8; 21] | 3.4 [0.7; 28] | 160.7 [8.4; 41] | 66.7 [12.8; 39] | 26.2 [4.7; 37] |
|
M n=60 |
50.4 [3.5; 24] | 3.5 [0.6; 34] | 172.0 [6.2; 34] | 68.8 [11.3; 33] | 25.0 [6.4; 13] | |
Values reported as the mean z-score [SD; n]
Among the PHP1A and PPHP patients, 66% and 76%, respectively, carry a mutation that likely impacts Gsα function severely. GNAS exon 1 mutations were identified in 19% and 25% of PHP1A and PPHP patients, respectively. In PHP1A patients, the different maternal GNAS mutations are not expected to alter expression -or function- of the paternal XLαs transcript. In PPHP patients, the paternal exon 1 mutations should not affect XLαs protein (figure 1).
Gestational age and birth parameters (table 1, supplemental appendix 3 and 4)
PHP1A patients
21% (21/99) of the patients affected by PHP1A were born prematurely, i.e. before the 37th week of gestation (average gestational age: 38.3±2.6 weeks). Compared to healthy newborns, intrauterine growth was moderately impaired for PHP1A patients as the z-scores for birth length and birth weight were −1.1±1.8 (n=143) and −0.6±1.6 (n=169), respectively (p<0.0001 for both). 37% of the patients were born small for gestational age (SGA). No differences were observed when grouping the patients based on the type of GNAS mutations, i.e. mutations that are predicted to impair Gsα function severely or mildly. At birth, PHP1A patients with mutations involving GNAS exon 1 (n=29) had slightly lower weight z-scores as compared to PHP1A patients with mutations in GNAS exons 2–13 (n=135) (−1.1±0.9 vs −0.5±1.8; p=0.0074). Birth length z-scores of both PHP1A cohorts were not different (−1.2±0.7 [n=23] vs −1.1±1.9 [n=115]; p=0.7124).
PPHP patients
Data regarding the duration of pregnancy were available for about half of the PPHP patients (n=34). The mean gestational age was 37.6±2.5 weeks, which is not different from that of the PHP1A patients (p=0.2096); approximately one fifth of the patients (20%) was born prematurely. Intrauterine growth of the PPHP cohort was severely altered in comparison to the reference population, i.e. the mean z-scores for birth length and weight were −3.0±1.5 (n=38; p<0.0001) and −2.7±1.3 (n=42; p<0.0001), respectively. Overall, 95% of the PPHP patients were SGA at birth. Patients with a mutation involving GNAS exon 1 were less severely affected than those with a mutation in GNAS exon 2–13 (birth weight z-score −1.9±0.9 [n=12] vs −3.0±1.3 [n=29]; p=0.0049).
PHP1B patients
None of the AD-PHP1B patients for whom sufficient information regarding their pregnancy was available (n=32) was born prematurely, while 12% of the spor-PHP1B cases (8 of 64) were born before 37 weeks of gestation. Among PHP1B patients, only those affected by AD-PHP1B showed evidence of increased fetal growth in comparison to healthy controls (birth length z-score 0.7±1.2, n=33; p=0.0039). Both AD-PHP1B and spor-PHP1B had increased birth weight z-scores in comparison to the population of reference (AD-PHP1B: 1.2±1.6, n=39; p<0.0001; spor-PHP1B: 0.5±1.6, n=62; p=0.0172). Overall, 28% of the AD-PHP1B and 18% of the sporPHP1B cases presented with macrosomia at birth.
Post-natal growth
We next determined whether post-natal growth was affected depending on the disease-causing genetic and/or epigenetic defect at the GNAS locus.
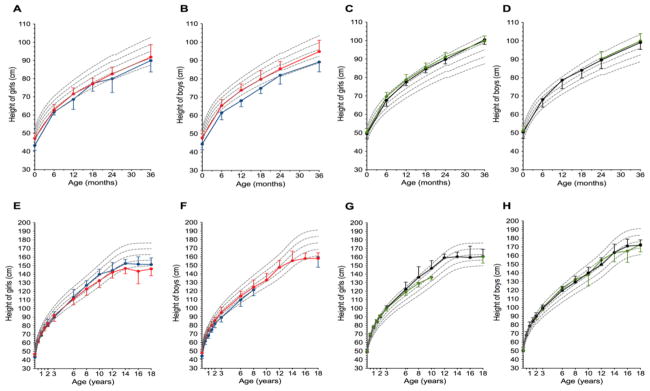
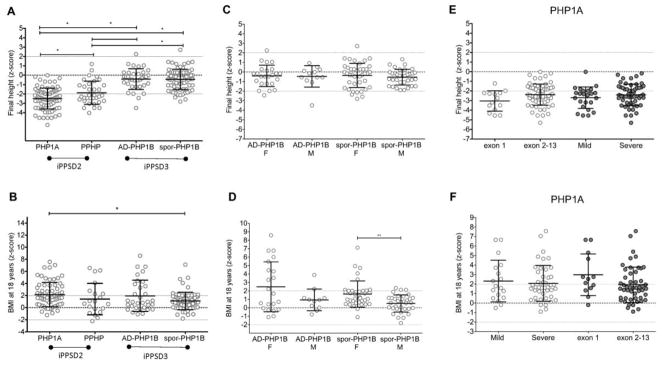
After birth, both PHP1A and PPHP patients displayed a moderate catch-up growth (figure 2A and 2B), but showed a progressive decline in growth velocity and lack of pubertal spurt leading to a final short stature (figure 2E and 2F, figure 3A) as indicated by the height z-scores over time (PHP1A: −0.6±1.7 at 3 years, −0.9±1.3 at 10 years, −2.5±1.1 at 18 years; PPHP: −1.5±1.5 at 3 years, −0.2±1.1 at 10 years, −1.9±1.2 at 18 years). Final heights below the 3rd percentile were found in 64% and in 59% of the PHP1A (n=84) and PPHP (n=34) patients, respectively (supplemental appendix 3 and figure 3A). A similar pattern of longitudinal growth was observed for girls and boys affected by PHP1A, as shown in figure 2A, B, E and F. For PPHP, we had too few data for male patients preventing any comparison of gender-specific growth patterns. Importantly, we found no influence of the GNAS genotype (mild versus severe impact on the Gsα function; exon 1 versus exon 2–13 of Gsα) on birth length and final adult heights of PHP1A and PPHP patients (table 1, figure 3E and supplemental appendix 4 and 5).
Figure 2.
Longitudinal evolution of length/height depicted for the different disorders. Boys and girls affected with inactivating PTH/PTHrP Signaling Disorder or iPPSD2 [PHP1A (red dots and lines) or PPHP (blue dots and lines)] or iPPSD3 [AD-PHP1B (green dots and lines) or spor-PHP1B (black dots and lines)] are plotted on the WHO growth charts. Panels A, B, C and D zoom in the first years of life. Panels E, F, G and H depict the pattern of growth from birth to 18 years (adult height). Note the moderate acceleration of growth in the first years of life in iPPSD2 while with growth velocity is dramatically increased from birth in iPPSD3.
Figure 3.
Z-scores of adult height and BMI of patients affected with the different GNAS genetic and epigenetic lesions.
Raw data (dots), means and SD (lines) are presented. A and B: comparison of the final heights and BMI z-scores between the different diseases. Statistically different means (p<0.05) are indicated by *, (p<0.001) are indicated by **, (p<0.0001) are indicated by ***. Note that the scale differs between the panels. C and D represent the data divided according to the sex; E and F represent the data divided according to the genotype of the PHP1A patients.
In contrast to patients with inactivating GNAS mutations, patients with GNAS methylation defects displayed a pattern of overgrowth in their first years of life (figure 2C and 2D), followed by a shortened or absent pubertal spurt leading to a near normal final height (figure 2G and 2H, figure 3A). The dramatic increase in growth velocity was obvious very early in life, as soon as one year of age. Then, we observed a gradual decline of the height z-scores (AD-PHP1B: 2.2±1.3 at 1 year, 1.2±0.9 at 3 years, −0.2±1.3 at 10 years, and −0.4±1.1 at 18 years; spor-PHP1B: 1.3±1.5 at 1 year, 1.1±0.9 at 3 years, 0.8±1.3 at 10 years, and −0.4±1.1 at 18 years). Growth patterns and final heights were similar for males and females (see figure 2G and H and figure 3C).
Post-natal weight gain
We observed an excessive weight gain in all disease subtypes as shown in figures 3B, D and F and figure 4.
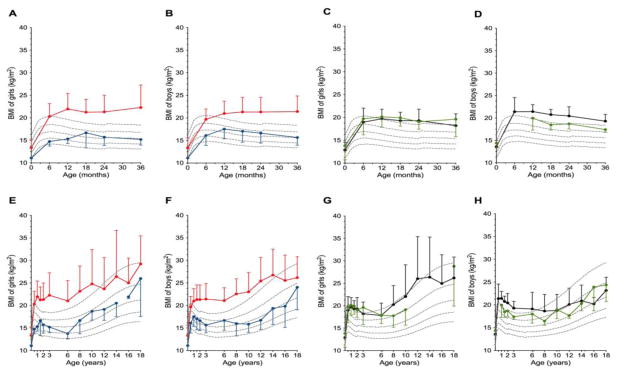
Figure 4.
Longitudinal evolution of BMI (kg/m2) depicted for the different disorders. Boys and girls affected with iPPSD2 [PHP1A (red dots and lines) or PPHP (blue dots and lines)] or iPPSD3 [AD-PHP1B (green dots and lines) or spor-PHP1B (black dots and lines)] are plotted on the BMI WHO charts. Panels A, B, C and D show the first three years of life. Panels E, F, G and H depict the pattern of growth from birth to 18 years (adulthood). Note the dramatic increase in weight gain occurring very early in life in the four diseases variants.
PHP1A patients showed evidence for early-onset obesity with a dramatic increase of the BMI z-score at 1 year of life to 3.5±2.3 (p<0.0001). Most patients continued to gain weight leading by the age of 3 years to a BMI z-score of 5.0±3.4 (figure 4A and B). The final BMI z-score determined at 18 years was 2.2±2.0, including 29/66 patients having a z-score above 2. Men and women showed a similar pattern of weight gain; however, adult PHP1A women were significantly heavier than adult PHP1A men (z-scores of BMI of 2.6±2.1 [n=41] vs 1.7±1.6 [n=25], p=0.0254), respectively (figure 4E and F). Among adult PHP1A patients, 41% were overweighed and 29% were obese. The weight gain was not influenced by the GNAS genotype (figure 3F).
Our PPHP cohort was too small to calculate reliable BMI data. However, we observed that, on average, patients maintained their BMI above the 50th centile during childhood (figure 4A, B, E and F). The BMI z-scores of adult PPHP men (0.8±1.8 [n=4]) and adult PPHP women (1.6±2.8 [n=16]) did not differ significantly (p=0.4458) (figure 4E and F).
Interestingly, all patients affected by PHP1B, including AD-PHP1B and spor-PHP1B showed a dramatic early-onset weight gain (figure 4C, D, G and H). By the age of 1 year, both AD-PHP1B and spor-PHP1B patients revealed significant overweight with BMI z-scores of 2.5±1.6 (n=12) and 2.9±1.4 (n=17), respectively. Thereafter, the mean BMI z-scores of AD-PHP1B and spor-PHP1B patients declined progressively and, by adulthood, were still 2.0±2.6 (n=32) and 1.1±1.4 (n=70) above average, respectively. The women, especially affected with spor-PHP1B, were more overweight than male patients. In fact, the BMI z-scores of adult AD-PHP1B and spor-PHP1B women were higher than those of men (AD-PHP1B women 2.5±3.0 [n=21] vs men 0.9±1.3 [n=11], p=0.1083; spor-PHP1B women 1.6±1.6 [n=37] vs men 0.5±1.0 [n=33], and p=0.0020) (figure 3D). Nine percent of the AD-PHP1B and 44% of the spor-PHP1B women were overweight, and 43% and 13%, respectively, were obese. In contrast, only 18% of the AD-PHP1B and 27% of the spor-PHP1B men were overweight, while only 9% of the AD-PHP1B and none of the spor-PHP1B men were obese.
DISCUSSION
The lack of longitudinal data on growth and weight gain of patients affected with GNAS molecular defects prompted us to launch a large European and North American collaborative study, a necessary step to gather large patient cohorts affected by these rare diseases, and generate comprehensive data. Our findings should help to generate hypotheses regarding the biological roles of the different GNAS-derived transcripts and to support future guidelines for the management of obesity and short stature in patients affected by GNAS molecular defects.
Gsα haploinsufficiency and impaired growth
Gsα is biallelically expressed in the growth plate and it is pivotal for mediating the actions of the PTH/PTHrP receptor in growth plate chondrocytes(22). In humans, monoallelic Gsα expression appears to cause accelerated chondrocyte differentiation and premature fusion of the growth plate, ultimately leading to short stature. An extreme form of accelerated chondrocyte differentiation is also found in mice and humans lacking both copies of the PTH/PTHrP receptor(23). In addition, postnatal inactivation of Gsα in chondrocytes alters the behavior of stem cell-like chondrocyte located in the resting layer of the growth plate and leads to a non-expanding, disorganized cartilage(24). It is therefore likely that Gsα haploinsufficiency in the growth plate - and likely other tissues - is responsible for the mild growth restriction at birth of PHP1A patients, and possibly for the lack of pubertal spurt and consequently for the short stature identified in both PHP1A and PPHP patients. In particular, the impact of Gsα deficiency on stem cell-like chondrocytes may account for an inability to increase growth velocity during puberty.
Lack of paternal XLαs impairs pre-natal growth
XLαs is a developmentally regulated large Gsα variant which is expressed only from the paternal GNAS allele in all tissues; its expression level decreases dramatically after birth(25). In mice, germline disruption of Xlαs leads to a neonatal phenotype characterized by thin and narrow bodies and reduced weight(25). Our data support the role of XLαs for intra uterine growth as we found severely diminished birth weight and length in patients affected with paternal GNAS mutations. The combination of XLαs deficiency and Gsα haploinsufficiency may explain the severe antenatal growth restriction observed in PPHP patients, especially in patients with mutations involving exons 2–13, i.e. regions shared between the paternally-derived transcripts for Gsα and XLαs.
Reduced maternal Gsα expression contributes to obesity
In contrast to the chondrocyte where Gsα is most likely expressed from both parental alleles, this signaling protein appears to be derived in the dorsomedial hypothalamus (DMH), at least in mice, primarily from the maternal Gnas allele(26). Therefore, all maternal GNAS-related disorders likely impair expression and Gsα-dependent actions at different G-protein coupled receptors, including MC4R in the DMH.
We have hereby demonstrated that any disease variant caused by a maternal GNAS molecular defect, i.e. PHP1A, AD-PHP1B and spor-PHP1B, can be associated with impressive early-onset obesity. Throughout childhood and in adults, however, excessive weight gain was more severe and more frequent in patients affected by PHP1A than by PHP1B. Earlier studies have suggested that the abnormal weight gain is likely caused by a defect in the central nervous system rather than in adipose tissue(27). In fact, obese children with PHP1A have low sympathetic nervous system activity, decreased lipolysis(28), low metabolic rates and low resting energy expenditure(29,30). Growth hormone deficiency due to growth hormone-releasing hormone resistance in the pituitary may be another contributing factor to this central nervous system defect(31). Thus, a combination of different tissue-specific events may contribute to obesity in PHP1A and PHP1B patients(27,28,30).
GNAS transcripts and growth evolution
The specific longitudinal pattern of growth and weight gain in AD-PHP1B and spor-PHP1B patients, particularly in early life, could help clarify the relative contribution of the different GNAS-derived transcripts.
In accordance with our previous findings in much smaller cohorts(15), PHP1B patients, including both AD-PHP1B and spor-PHP1B, display enhanced fetal growth. This phenotype is even extended to the first years of life. Because of the epigenetic molecular mechanism that causes PHP1B, it is likely that growth patterns encountered in PHP1B are, at least in part, controlled by GNAS-derived transcripts different from Gsα. In patients affected by spor-PHP1B (note that patients with AD-PHP1B due to deletions within GNAS are excluded in the herein study because only few cases have been described(32)), XLαs expression in growth plates and other tissues is predicted to be biallelic early in life, thus potentially contributing to our observations of enhanced growth during fetal life and infancy. However, patients with AD-PHP1B due to STX16 deletions express XLαs only from their paternal allele, making it unlikely that XLαs contributes to the postnatal overgrowth. In fact, both groups of patients, AD-PHP1B due to STX16 deletions and spor-PHP1B display very similar early overgrowth, followed by a gradual decline in growth velocity and subsequent normal adult height. XLαs, which is biallelically expressed only in spor-PHP1B patients, is therefore unlikely to be uniquely responsible for the overgrowth in all forms of PHP1B. Instead the A/B transcript (or another unknown transcript) may contribute through an as-yet unknown mechanism to the much enhanced growth. In addition, we propose that the early-onset obesity, in the context of open epiphyses, combined with biallelic expression of Gsα in chondrocytes, is likely a major driver of the enhanced growth in these patients.
Alternate GNAS-derived transcripts have distinct roles during pubertal development
The cause of the insufficient pubertal spurt in the different disease variant remains to be investigated. Affected patients may present with lower Gsα expression in stem cell-like chondrocytes, which could impede the increase in growth velocity during puberty(33). Interestingly, XLαs appears to have no major role in postnatal growth plates as indicated by mice with ablation of Gnas exon 1 from the paternal allele thus lacking paternal Gsα, but not Xlαs(24). This is concordant with our observations in PPHP patients with paternal GNAS mutations involving exons 2–13, i.e. lacking XLαs and paternal Gsα, who display a severe antenatal growth restriction, yet post-natal and adult heights that are similar to those in PHP1A patients bearing maternal GNAS exons 2–13 mutations.
In addition, obesity may accelerate the bone maturation, as commonly observed(34). Furthermore, some patients may show early pubertal development or early adrenarche, in the context of advanced bone age, as observed in other imprinting disorders(35).
Limitations of our studies
Weaknesses of our study include the lack of parental heights, heights of healthy siblings, age at puberty and menarche, and additional data such as age at diagnosis, thyroid or growth hormone status, which could help elucidate further the mechanisms underlying growth and weight gain in GNAS-related disorders. To better understand the underlying determinants of growth, weight gain and puberty, including the sex-differences, prospective studies and follow-up need to be pursued in patients affected by the different GNAS-related disorders.
Conclusions
From our observations, we conclude that i) for the majority of PHP1A and PPHP patients, i.e. patients carrying a GNAS loss of function mutation, normal length/height during childhood does not predict a normal adult height as short stature becomes apparent only gradually in these disorders, ii) close follow-up of growth, pubertal development and bone maturation is recommended by mid childhood especially for patients with inactivating GNAS mutations, iii) the maternal mutations or methylation defects of GNAS, as in patients affected with PHP1A, AD-PHP1B and spor-PHP1B, can cause impressive early-onset obesity that persists through adulthood especially in women; specific interventions should therefore be instated as soon as the diagnosis is made to limit obesity, thus long-term metabolic consequences; iv) large collaborative studies are of major importance to gather representative data for these rare diseases and to better understand the impact of GNAS imprinting on major development processes including growth, fat storage and pubertal development.
Supplementary Material
Acknowledgments
We are very grateful to the Spanish patient association (Asociacion Española de PHP, AEPHP), patients and their families for their contribution to the study. We thank Luigi Di Nicola for his support for the Ipsen external sponsored study.
PH, VG, GPdN, GM and AL conducted the study. GPdN, LdS, PK, AR, ST, AS, MLK, HJ, GM and AL recruited patients. All authors collected data. PH, GPdN, HJ, GM and AL analyzed the results and wrote the first and revised version of the manuscript. All authors contributed to the writing and edition of the manuscript. AL, as corresponding author, endorses responsibility for the integrity of the data analysis.
Funding statement
This work was supported by recurrent INSERM funding (to A.L.), an external sponsored study research grant from Ipsen (#8NL7 to A.L.), a crowdfunding project precipita.es (FECYT, MINECO to GPdN), National Institutes of Health grants (RO1 DK46718 and PO1 DK11794, subproject 4, to H.J. and K23 DK101689 to A.S.), a grant from the Instituto de Salud Carlos III [Institute of Health Carlos III] of the Ministry of Economy and Competitiveness [Spain] (to GPdN and A.P.), co-financed by the European Regional Development Fund (PI16-00073), a grant from the Department of Health of the Basque Government (GV2016/111105 to J.E.), a grant from the French Society of Pediatric Endocrinology and Diabetology [SFEDP] and Sandoz (to V.G), a grant from the SFEDP and Pfizer (to P.H.), a grant from the SFEDP and Lilly (to L.C.T.).
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.3450]
Disclosure Summary
PH, VG, GPdN, LCT; LdS, FE, JE, BF, PK, LL, AP, AR, DT, ST, AU, AS, MLK, HJ and GM have nothing to disclose in relation to the study. AL received a research grant for the investigator initiated study from IPSEN (#8NL7).
Additional Supporting Information may be found in the online version of this article.
References
- 1.Albright F, Burnett CH, Smith PH, Parson W. Pseudohypoparathyroidism - an example of “Seabright-Bantam syndrome”. Endocrinology. 1942;30:922–32. [Google Scholar]
- 2.Mantovani G, Spada A, Elli FM. Pseudohypoparathyroidism and Gsα-cAMP-linked disorders: current view and open issues. Nat Rev Endocrinol. 2016 Jun;12(6):347–56. doi: 10.1038/nrendo.2016.52. [DOI] [PubMed] [Google Scholar]
- 3.Hayward BE, Moran V, Strain L, Bonthron DT. Bidirectional imprinting of a single gene: GNAS1 encodes maternally, paternally, and biallelically derived proteins. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15475–80. doi: 10.1073/pnas.95.26.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein LS. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8715–20. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. The gsalpha gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab. 2002 Oct;87(10):4736–40. doi: 10.1210/jc.2002-020183. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein LS, Gejman PV, Friedman E, Kadowaki T, Collins RM, Gershon ES, Spiegel AM. Mutations of the Gs alpha-subunit gene in Albright hereditary osteodystrophy detected by denaturing gradient gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8287–90. doi: 10.1073/pnas.87.21.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, Weinstein LS. A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest. 2000 Nov;106(9):1167–74. doi: 10.1172/JCI10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elli FM, Linglart A, Garin I, de Sanctis L, Bordogna P, Grybek V, Pereda A, Giachero F, Verrua E, Hanna P, Mantovani G, Perez de Nanclares G. The Prevalence of GNAS Deficiency-Related Diseases in a Large Cohort of Patients Characterized by the EuroPHP Network. J Clin Endocrinol Metab. 2016 Oct;101(10):3657–68. doi: 10.1210/jc.2015-4310. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Rebollo E, Lecumberri B, Garin I, Arroyo J, Bernal-Chico A, Goñi F, Orduña R, Castaño L, de Nanclares GP Spanish PHP Group. New mechanisms involved in paternal 20q disomy associated with pseudohypoparathyroidism. Eur J Endocrinol Eur Fed Endocr Soc. 2010 Dec;163(6):953–62. doi: 10.1530/EJE-10-0435. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani G, de Sanctis L, Barbieri AM, Elli FM, Bollati V, Vaira V, Labarile P, Bondioni S, Peverelli E, Lania AG, Beck-Peccoz P, Spada A. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of albright hereditary osteodystrophy and molecular analysis in 40 patients. J Clin Endocrinol Metab. 2010 Feb;95(2):651–8. doi: 10.1210/jc.2009-0176. [DOI] [PubMed] [Google Scholar]
- 11.Thiele S, Mantovani G, Barlier A, Boldrin V, Bordogna P, De Sanctis L, Elli FM, Freson K, Garin I, Grybek V, Hanna P, Izzi B, Hiort O, Lecumberri B, Pereda A, Saraff V, Silve C, Turan S, Usardi A, Werner R, de Nanclares GP, Linglart A. From pseudohypoparathyroidism to inactivating PTH/PTHrP signalling disorder (iPPSD), a novel classification proposed by the EuroPHP network. Eur J Endocrinol. 2016 Dec;175(6):P1–17. doi: 10.1530/EJE-16-0107. [DOI] [PubMed] [Google Scholar]
- 12.Linglart A, Carel JC, Garabédian M, Lé T, Mallet E, Kottler ML. GNAS1 lesions in pseudohypoparathyroidism Ia and Ic: genotype phenotype relationship and evidence of the maternal transmission of the hormonal resistance. J Clin Endocrinol Metab. 2002 Jan;87(1):189–97. doi: 10.1210/jcem.87.1.8133. [DOI] [PubMed] [Google Scholar]
- 13.Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Galpha(s) in the development of human obesity. J Clin Endocrinol Metab. 2007 Mar;92(3):1073–9. doi: 10.1210/jc.2006-1497. [DOI] [PubMed] [Google Scholar]
- 14.Richard N, Molin A, Coudray N, Rault-Guillaume P, Jüppner H, Kottler M-L. Paternal GNAS mutations lead to severe intrauterine growth retardation (IUGR) and provide evidence for a role of XLαs in fetal development. J Clin Endocrinol Metab. 2013 Sep;98(9):E1549–1556. doi: 10.1210/jc.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bréhin A-C, Colson C, Maupetit-Méhouas S, Grybek V, Richard N, Linglart A, Kottler M-L, Jüppner H. Loss of methylation at GNAS exon A/B is associated with increased intrauterine growth. J Clin Endocrinol Metab. 2015 Apr;100(4):E623–631. doi: 10.1210/jc.2014-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grüters-Kieslich A, Reyes M, Sharma A, Demirci C, DeClue TJ, Lankes E, Tiosano D, Schnabel D, Jüppner H. Early-Onset Obesity: Unrecognized First Evidence for GNAS Mutations and Methylation Changes. J Clin Endocrinol Metab. 2017 Aug 1;102(8):2670–7. doi: 10.1210/jc.2017-00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanet P, Osei L, Netchine I, Pertuit M, Enjalbert A, Reynaud R, Barlier A. Case report of GNAS epigenetic defect revealed by a congenital hypothyroidism. Pediatrics. 2015 Apr;135(4):e1079–1083. doi: 10.1542/peds.2014-2806. [DOI] [PubMed] [Google Scholar]
- 18.WHO. The WHO Child Growth Standards [Internet] WHO; [cited 2017 Oct 26]. Available from: http://www.who.int/childgrowth/standards/en/ [Google Scholar]
- 19.Mamelle N, Cochet V, Claris O. Definition of fetal growth restriction according to constitutional growth potential. Biol Neonate. 2001;80(4):277–85. doi: 10.1159/000047157. [DOI] [PubMed] [Google Scholar]
- 20.Turan S, Thiele S, Tafaj O, Brix B, Atay Z, Abali S, Haliloglu B, Bereket A, Bastepe M. Evidence of hormone resistance in a pseudo-pseudohypoparathyroidism patient with a novel paternal mutation in GNAS. Bone. 2015 Feb;71:53–7. doi: 10.1016/j.bone.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garin I, Mantovani G, Aguirre U, Barlier A, Brix B, Elli FM, Freson K, Grybek V, Izzi B, Linglart A, Perez de Nanclares G, Silve C, Thiele S, Werner R EuroPHP Consortium. European guidance for the molecular diagnosis of pseudohypoparathyroidism not caused by point genetic variants at GNAS: an EQA study. Eur J Hum Genet. 2015 Apr;23(4):438–44. doi: 10.1038/ejhg.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Jüppner H, Kronenberg HM, Chung U. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14794–9. doi: 10.1073/pnas.0405091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006 Apr;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 24.Chagin AS, Vuppalapati KK, Kobayashi T, Guo J, Hirai T, Chen M, Offermanns S, Weinstein LS, Kronenberg HM. G-protein stimulatory subunit alpha and Gq/11α G-proteins are both required to maintain quiescent stem-like chondrocytes. Nat Commun. 2014;5:3673. doi: 10.1038/ncomms4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat Genet. 2004 Aug;36(8):818–26. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Shrestha YB, Podyma B, Cui Z, Naglieri B, Sun H, Ho T, Wilson EA, Li Y-Q, Gavrilova O, Weinstein LS. Gsα deficiency in the dorsomedial hypothalamus underlies obesity associated with Gsα mutations. J Clin Invest. 2017 Feb 1;127(2):500–10. doi: 10.1172/JCI88622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M, Berger A, Kablan A, Zhang J, Gavrilova O, Weinstein LS. Gsα deficiency in the paraventricular nucleus of the hypothalamus partially contributes to obesity associated with Gsα mutations. Endocrinology. 2012 Sep;153(9):4256–65. doi: 10.1210/en.2012-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carel JC, Le Stunff C, Condamine L, Mallet E, Chaussain JL, Adnot P, Garabédian M, Bougnères P. Resistance to the lipolytic action of epinephrine: a new feature of protein Gs deficiency. J Clin Endocrinol Metab. 1999 Nov;84(11):4127–31. doi: 10.1210/jcem.84.11.6145. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker AH, Lomenick JP, Saville BR, Wang W, Buchowski MS, Cone RD. Energy expenditure in obese children with pseudohypoparathyroidism type 1a. Int J Obes 2005. 2013 Aug;37(8):1147–53. doi: 10.1038/ijo.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roizen JD, Danzig J, Groleau V, McCormack S, Casella A, Harrington J, Sochett E, Tershakovec A, Zemel BS, Stallings VA, Levine MA. Resting Energy Expenditure Is Decreased in Pseudohypoparathyroidism Type 1A. J Clin Endocrinol Metab. 2016 Mar;101(3):880–8. doi: 10.1210/jc.2015-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani G, Maghnie M, Weber G, De Menis E, Brunelli V, Cappa M, Loli P, Beck-Peccoz P, Spada A. Growth hormone-releasing hormone resistance in pseudohypoparathyroidism type ia: new evidence for imprinting of the Gs alpha gene. J Clin Endocrinol Metab. 2003 Sep;88(9):4070–4. doi: 10.1210/jc.2002-022028. [DOI] [PubMed] [Google Scholar]
- 32.Bastepe M, Fröhlich LF, Linglart A, Abu-Zahra HS, Tojo K, Ward LM, Jüppner H. Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet. 2005 Jan;37(1):25–7. doi: 10.1038/ng1487. [DOI] [PubMed] [Google Scholar]
- 33.Zazo C, Thiele S, Martín C, Fernandez-Rebollo E, Martinez-Indart L, Werner R, Garin I, Hiort O, Perez de Nanclares G Spanish PHP Group. Gsα activity is reduced in erythrocyte membranes of patients with psedohypoparathyroidism due to epigenetic alterations at the GNAS locus. J Bone Miner Res. 2011 Aug;26(8):1864–70. doi: 10.1002/jbmr.369. [DOI] [PubMed] [Google Scholar]
- 34.Shalitin S, Kiess W. Putative Effects of Obesity on Linear Growth and Puberty. Horm Res Paediatr. 2017;88(1):101–10. doi: 10.1159/000455968. [DOI] [PubMed] [Google Scholar]
- 35.Soellner L, Begemann M, Mackay DJG, Grønskov K, Tümer Z, Maher ER, Temple IK, Monk D, Riccio A, Linglart A, Netchine I, Eggermann T. Recent Advances in Imprinting Disorders. Clin Genet. 2017 Jan;91(1):3–13. doi: 10.1111/cge.12827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.