Abstract
Background
Thymol is a Transient Receptor Potential Ankyrin Subtype 1 channel, (TRPA1) agonist found in thyme and oregano. Thymol has antioxidant, anti-inflammatory, and antimicrobial properties; thus thymol is added to many commercially available products including Listerine mouthwash. Thymol is also cytotoxic to HL-60 (acute promyelotic leukemia) cells in vitro. Therefore, we evaluated the effects of thymol against oral squamous cell carcinoma (OSCC) and its anticancer mechanism-of-action.
Methods
The antiproliferative effects of thymol in OSCC Cal27 cells were determined by MTS assays. Antitumor effects were evaluated in Cal27- and HeLa-derived mouse xenografts. Calcium imaging, mitochondrial transmembrane potential (ΔΨm) studies, and western blot analysis of cleaved PARP (c-PARP) evaluated thymol’s mechanism-of-action.
Results
Thymol had significant, long-lasting antiproliferative effects in vitro. In vivo, thymol displayed significant antitumor effects in Cal27-derived tumors. Thymol’s anticancer effects were confirmed in HeLa-derived xenografts demonstrating that thymol effects are not tumor-type specific. Calcium imaging verified calcium influx in Cal27 cells that was reversed with the TRPA1 antagonist, HC030031. However, no calcium influx was seen in HeLa cells indicating that TRP channels do not regulate thymol cytotoxicity. This was confirmed using cell viability assays in which pre-treatment with HC030031 had no effect on thymol cytotoxicity. Instead, ΔΨm studies revealed that thymol induces significant ΔΨm depolarization and apoptosis.
Conclusion
Our findings provide the first evidence of thymol’s novel antitumor effects against OSCC in vivo, which do not rely on TRPA1 activity. Instead we show that thymol induces mitochondrial dysfunction and apoptosis and may be efficacious against multiple cancers.
Keywords: Thymol, Oral Squamous Cell Carcinoma, TRPA1, Apoptosis, Mitochondrial Dysfunction
Introduction
Head and neck squamous cell carcinomas (HNSCC) are the sixth most common cancer diagnosed in the United States and 50% of patients will succumb to these cancers(1). This is due primarily to the late-stage at which many of these cancers are diagnosed that are refractive to conventional therapies including surgical resection, platinum based chemotherapy and/or radiation therapy(1). Therefore there is a significant need to develop new drugs to effectively treat this deadly disease. Thymol is a phenolic compound found in the essential oil of many plants including thyme, oregano, rosemary, bay laurel leaves, mandarin oranges, and cranberries(2–6). Thymol has antimicrobial, antifungal, anti-inflammatory, and antioxidant properties and is added to a variety of products including cosmetics, pharmaceutical preparations and it is listed as an active ingredient in Listerine mouthwash(6–10). Given that thymol is already used in over-the-counter oral rinses, we evaluated its antiproliferative and antitumor effects against HNSCC using cell based assays and mouse xenograft models.
Thymol is a known TRP channel agonist that activates TRPA1, TRPV3 (Vanilloid subtype 3), and weakly, TRPM8 (Melastatin subtype 8) channels allowing the influx of cations, preferentially calcium, into the cell(11–13). TRP channel expression is differentially expressed in numerous tumor types, however their role in tumorigenesis remains largely unknown(12, 14–18). It is hypothesized that activation of TRP channels in cancers will result in a large influx of Ca2+ and subsequent Ca2+ mediated apoptosis thereby making these channels potential therapeutic targets. Indeed, studies evaluating thymol’s effect on calcium homeostasis and viability of human glioblastoma cells demonstrated an increase in Ca2+ levels that coincided with thymol-induced cytotoxicity(19). However this cytotoxicity was not abolished by Ca2+ chelation implicating another mechanism-of-action. Our laboratory reported expression of TRPV1 in human HNSCC cell lines and tumors. We also demonstrated that the significant antitumor effects of vanilloids are due to a TRPV1-independent mechanism(14). Furthermore, we showed that vanilloids induce reactive oxygen species (ROS) and subsequent apoptosis in the absence of TRPV1 activity. Similarly, studies evaluating thymol cytotoxic effects in HL-60 (acute promyelotic leukemia) cells demonstrate induction of ROS and depolarization of the mitochondrial membrane potential (ΔΨm) with subsequent apoptosis(20). However, TRP channel expression and activation were not examined in HL-60 cells. Therefore, in the present study we evaluated if thymol cytotoxicity involves TRP channel activation in HNSCC or if it is due to an alternate mechanism involving mitochondrial dysfunction leading to apoptosis.
Materials and Methods
Cell Lines
OSCC cell lines derived from primary tongue tumors, Cal27, SCC4, and SCC9 were obtained from ATCC (Manassas, VA, USA). Additional cancer cell lines including HeLa (cervical cancer), H460, (non-small cell lung cancer), MDA-231(breast cancer), and PC3 (prostate cancer) were also obtained from ATCC. Cal27, SCC4, HeLa, and PC3 cells were maintained in DMEM (Gibco, Carlsbad, CA, USA). SCC9 cells were maintained in 50/50 mixtures of DMEM and F12 media (Gibco) and supplemented with 400 ng/ml hydrocortisone. H460 and MDA-231 cells were maintained in RPMI 1640 media (Gibco). All media contained 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin and cells were maintained at 37°C in 5% CO2. CHO cells overexpressing TRPA1 and TRPV1 were provided by Dr. Ardem Patapoutian and cultured as described(21).
Reagents
Thymol was purchased from Sigma and 2M stock solutions made up in 100% DMSO for all cell-based assays and all animal studies. Variations in the final DMSO concentrations between experiments are due to utilization of different working stocks; however all final DMSO concentrations were maintained at or below 0.5% so as not to affect the results. In addition, vehicle controls contained the same concentration of DMSO as treated groups.
MTS Cell Viability Assays
Cytotoxicity was assessed using the Cell Titer 96 ® Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) according to manufacturer’s protocol. Cells were plated and treated for 48 h with DMEM containing thymol at the indicated concentrations as previously described(22). Final DMSO concentrations were maintained at 0.1%. Absorbance values of test groups were compared to vehicle-treated controls (n=4).
In Vitro Clonogenic Assays
Cells (300 cells per plate) were allowed to adhere in 60 mm3 tissue culture dishes and subsequently treated with vehicle (final concentration of 0.5% DMSO) or thymol (4.3mM) for 2 min. Cells were washed with sterile phosphate buffered saline (PBS), and fresh growth medium was added and cells were allowed to grow and form colonies for 9 days. Following the incubation period, cells were fixed and stained with 0.5% crystal violet in 10% methanol, washed with PBS and quantified using Genetools Software.
Antitumor Evaluations
All studies were approved by our Institutional Animal Care and Use Committee and conform to ARRIVE guidelines. Six week-old female athymic nude mice (Harlan, Indianapolis, IN, USA) were used in a laminar air-flow cabinet under pathogen-free conditions. Mice were provided with a 12 h light/dark schedule at controlled temperature and humidity with food and water ad libitum. Mice were acclimated for one week prior to study initiation.
Mice were injected subcutaneously in the right flank with 3 × 106 Cal27 or HeLa cells in 0.1 ml of sterile PBS. Two to four weeks post-inoculation, tumors grew to an average volume of 90 mm3 (Cal27) and 150 mm3 (HeLa). Mice were stratified into two experimental groups (Cal27 n=6 per group; HeLa n=8 per group) which received the following treatments via intra-tumor injection: group A, vehicle control (50 μl of 0.25% DMSO diluted in sterile saline); and group B, 4.3 mM thymol (32 μg diluted in 50 μl sterile saline with a final concentration of 0.25% DMSO). Treatments were repeated every other day during the time indicated. Mice were monitored daily for tumor growth (using digital calipers) and cachexia. Body weight was monitored weekly. Tumor volumes were calculated by the elliptical formula: 1/2(length × width2)(23). At experimental conclusion, tumors were fixed and processed for histological analysis. Hematoxylin and eosin (H&E) staining, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), and Ki67 staining (Ventana Medical Systems Inc., Tucson, AZ, USA; catalog no. 790-4286) were performed to detect apoptotic figures and proliferating cells by the South Texas Reference Laboratory Histopathological Core facility using standard methods.
Calcium Imaging
Calcium imaging was performed using Oregon Green 488 BAPTA-1, AM (Cat#06807, Life Technologies, Hercules, CA, USA) according to manufacturer’s protocol. Cells were incubated with 2μM Oregon Green at 37°C for 25 min then treated with thymol (200μM, 400μM, or 1mM); n=4 per group. As a positive control, cells were treated with 3μM ionomycin, a non-selective cation channel activator. To evaluate specific TRP channel activity, cells were pretreated with the TRPA1 inhibitor HC030031 (10μM), the TRPM8 inhibitor RQ00203078 (10nM), and/or the TRPV1 inhibitor capsazepine (CZP; 10μM) followed by thymol. Images were acquired on Sweptfield confocal with a Nikon Ti inverted microscope and a 40× oil immersion/NA1.30 objective for 5 min recordings. Loading and imaging were carried out in recording media (12.78 g/liter DMEM, 5% FBS, 25μM HEPES, pH 7.2, no phenol red) at 37°C. Images were analyzed with MetaMorph software (Sunnyvale, CA, USA). Background corrected fluorescence was normalized prior to treatments. Photo-bleaching effect was corrected against vehicle only images that were collected at the exact settings and focus and maintained by the system’s Perfect Focus Device.
Mitochondrial Membrane Potential
Cells were plated at 5 × 105 in 60 mm dishes, grown overnight, and treated for 2 h with thymol diluted at the indicated concentrations. Cells were then stained with 1μM JC-1 dye (ThermoFisher Scientific, Waltham, MA, USA) for 15 min at 37°C, washed, and analyzed by FACS analysis.
Western Blot Analysis
Cells were treated for 48 h with vehicle control, 200μM, 400μM, 600μM, or 800μM thymol (final DMSO concentration of 0.1%) and then harvested and lysed in 1% Triton-PBS. Cell lysate concentration was determined by A280 reading using Nanodrop 3300 (Thermo, Scientific, Waltham, MA). Cell lysates containing equal concentration of protein (100units/A280) were separated using electrophoresis in 10% SDS-PAGE. SDS-PAGE separated proteins were transferred to PVDF membrane and membrane blocked in 5% milk solution. Anti-cPARP rabbit polyclonal antibody (Cell Signaling, Cat #5625S, Danvers, MA) was diluted 1:1250 and rabbit monoclonal anti-α/β-tubulin antibody (Cell Signaling, Cat #CS2148) was diluted 1:1000 in a total of 5ml diluent (1% milk in PBS-0.1% Tw-20) and incubated overnight at 4°C. The membrane was thrice washed with PBS-Tw-20 and incubated with ECL Plus detection solution (GE Healthcare, South San Francisco, CA) for 1min. Signal was detected by exposure to radiograph film for 30 seconds.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism4 (San Diego, CA, USA). Cell viability and mitochondrial membrane potential results were analyzed by one-way ANOVA and Bonferroni’s post-hoc test (n=4 and n=3 per group respectively). Statistical analyses of tumor growth and body weight were made using two-way analysis of variance with repeated measures with Bonferroni’s post-hoc test. Data is presented as mean ± SD. Ki67 staining and TUNEL assays were analyzed by a Student’s T-test; data is presented as mean ± SEM. A p value less than 0.05 was considered statistically significant.
Results
Thymol has antiproliferative effects in multiple cancer cell types
Given that thymol is found in numerous commercial products including Listerine, we questioned whether thymol is cytotoxic to oral cancer cells. Thus we initially performed cell viability assays using OSCC cell lines Cal27, SCC4, and SCC9. Thymol induced a concentration-dependent reduction in cell viability in all OSCC cell lines tested at concentrations that are dramatically lower than the concentration found in Listerine (4.3 mM) with GC50s ranging from 300 μM to 550 μM (Fig. 1A). We then tested thymol’s antiproliferative effects against a panel of cancer cell lines, which included cervical cancer (HeLa), non-small cell lung cancer (H460), breast cancer (MDA-231) and prostate cancer (PC3) cell lines (Fig. 1B). Similarly, thymol induced a concentration-dependent reduction in cell viability in all cancer cell lines tested with GC50s ranging from 350 μM to 500 μM indicating that thymol’s cytotoxic effects are not specific to oral cancers.
Fig. 1. Thymol inhibits cancer cell proliferation in vitro.
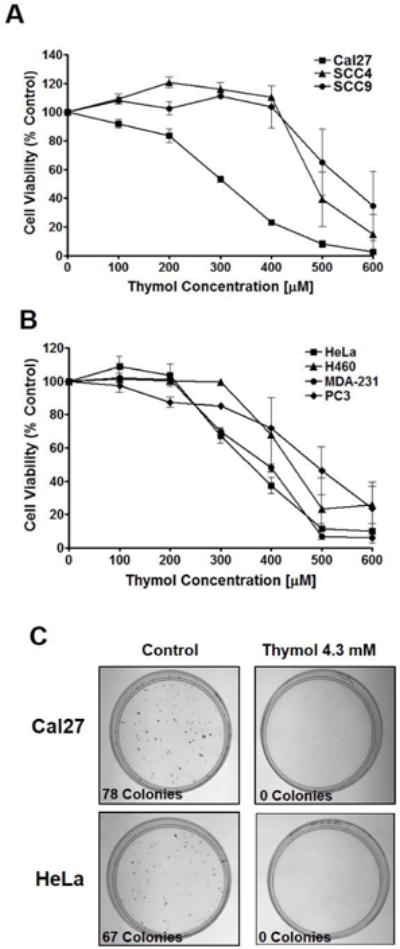
A) MTS cell viability assay of OSCC cell lines, Cal27, SCC4, and SCC9 treated with thymol for 48 h revealed GC50 values of 300 μM, 500 μM, and 550 μM respectively (n=4 per group). B) MTS cell viability assay of a panel of cancer cell lines (HeLa, H460, MDA-231, and PC3) treated with thymol for 48 h revealed GC50 values ranging from 350 μM, to 500 μM (n=4 per group). C) Clonogenic assays of Cal27 and HeLa cells 9 days following a 2 min exposure to thymol (4.3 mM; right panels) or vehicle control (left panels).
Thymol has long-lasting cytotoxic effects
Clonogenic assays were performed to determine if a short-term exposure to thymol at the concentration found in commercial products such as Listerine (4.3 mM) has long-lasting anti-proliferative effects. Cal27 and HeLa cells treated for 2 minutes with thymol (4.3 mM), washed, and placed in fresh media, revealed a long-lasting cytotoxic effect in both cell lines with no colony formation following 9 days of incubation in thymol-free complete media (Fig. 1C, right panels). Cal27 cells treated with vehicle control generated 78 colonies during the 9 day incubation period (Fig. 1C, left panels). Similarly, control-treated HeLa cells generated 67 colonies (Fig. 1C, left panels).
Thymol significantly inhibits tumor growth in vivo
To determine if thymol has antitumor effects against OSCC we generated Cal27-derived xenografts in athymic nude mice. Once tumors reached 90 mm3, mice were treated every other day with thymol or vehicle control. Inhibition of Cal27-derived tumor growth was evident by day 8. By day 16, there was a significant reduction in tumor volumes that was maintained throughout the remainder of the study (26 days) with mean tumor volumes of 423 mm3 for control tumors and 96 mm3 for thymol-treated tumors (Figs. 2A & 2B; p<0.001). Similarly, HeLa-derived xenografts displayed significant reductions in tumor volumes of thymol-treated tumors by day 18 compared to control tumors (Figs. 2D & 2E). In contrast to Cal27 OSCC tumors, thymol did not fully inhibit tumor growth of HeLa xenografts although a significant reduction in tumor growth rate (p<0.001) was demonstrated with mean tumor volumes of 903 mm3 for control tumors and 537 mm3 for thymol-treated tumors. Of note, no adverse reactions were observed immediately after treatments or at any point during the study. Adjacent non-cancerous tissues appeared healthy with no erythema, swelling, or ulceration and no nocifencive behaviors or weight-loss was noted. Rather, the animals’ body weight slightly increased as the animals grew older with no significant differences between thymol-treated mice and control animals (Figs. 2C & 2F).
Fig. 2. Thymol inhibits Cal27- and HeLa-derived tumor growth in vivo.
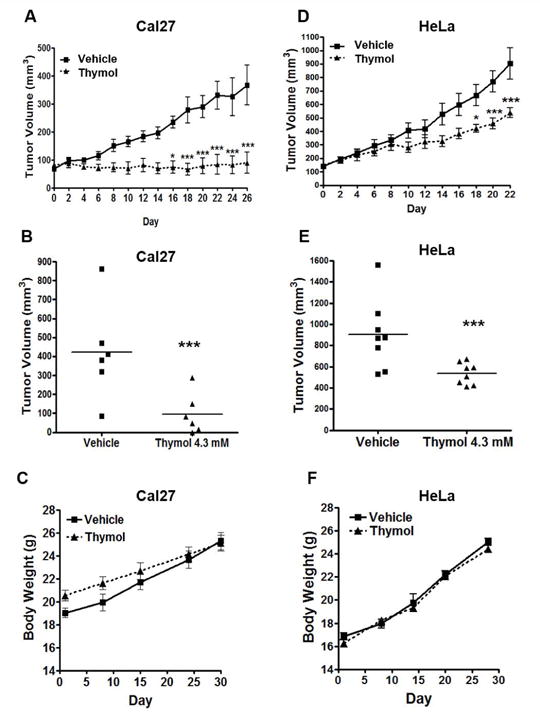
A & D) Tumor volumes of Cal27 (n=6 per group) and HeLa (n=8 per group) xenografts treated with thymol (4.3 mM) every other day for 26 and 22 days respectively. Significant reduction in Cal27-derived tumor volumes is seen by day 16 (* p<0.05) and in HeLa-derived tumor volumes by day 18 (*p<0.05). B & E) Scatter plot of Cal27 and HeLa tumor volumes respectively. Mean tumor volume for Cal27 control tumors is 423 mm3 whereas mean tumor volume for thymol treated Cal27 tumors is 96 mm3. Mean tumor volume for HeLa control tumors is 903 mm3 whereas mean tumor volume for thymol treated HeLa tumors is 537 mm3. C & F) Changes in body weight over the course of the study is shown for tumor-bearing mice with Cal27-derived xenografts (C) and HeLa-derived xenografts (F).
Thymol-treated Cal27-derived xenografts displayed significantly fewer proliferating cells and significantly greater apoptotic cells
Histopathological analysis of H&E stained Cal27-derived tumors from mice treated with thymol revealed large necrotic cores with numerous inflammatory infiltrates and viable cells (Fig. 3A) along the tumor margins. Ki67 staining and TUNEL assays were performed and quantified within the growing tumor front to assess changes in cell proliferation and apoptosis respectively (Fig. 3B). Thymol significantly reduced the number of proliferating cells (p<0.001; Fig. 3B). A significant increase in the number of apoptotic cells was also noted (p<0.01; Fig. 3B).
Fig. 3. Histopathological analyses of Cal27-derived tumors treated thymol.
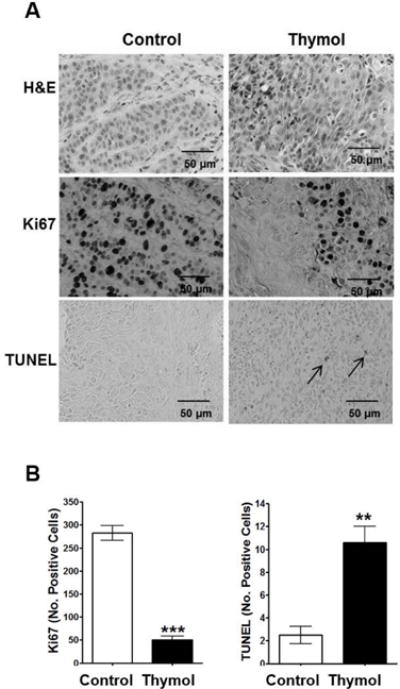
A) Representative photomicrographs of Cal-27 derived tumors (40 × magnification) stained with H&E or Ki67, and TUNEL assays are shown; apoptotic cells are illustrated by arrows; scale bars = 50 μm. B) Quantification of Ki67 positive cells and apoptotic cells in treated tumors (mean ± SEM; n=3 per group; 3 fields per specimen; **p<0.01, ***p<0.001).
Thymol OSCC cytotoxicity is through a unique mechanism that does not involve TRP channel activation
Thymol is a known TRPA1 agonist and is also reported to activate TRPV3 and weakly TRPM8 channels. To determine if thymol cytotoxicity is mediated by TRP channel activation in OSCC, we performed calcium imaging studies using Cal27 and HeLa cells in addition to CHO cells that over-express TRPA1 (CHO-TRPA1; positive control) and TRPV1 (CHO-TRPV1; negative control). While lower thymol concentrations (< 400 μM) failed to activate CHO-TRPA1 channels (data not shown), 400 μM thymol successfully induced calcium influx in CHO-TRPA1 cells with no effect on calcium influx in CHO-TRPV1 cells (Figs. 4A & 4B respectively). Likewise, 400 μM thymol induced calcium influx in Cal27 cells with no effect on HeLa cells which do not express TRPA1 channels (data not shown; Figs. 4C and 4D respectively). Treatment with the non-selective cation channel agonist Ionomycin (3 μM) confirmed calcium influx of both cell lines in response to cation channel activation, with HeLa cells being much less responsive. We confirmed that the calcium influx in Cal27 cells is due primarily to TRPA1 activation, which was reversed when the cells were pre-treated with the TRPA1 inhibitor, HC030031 (Fig. 5A). Pre-treatment with the TRPM8 inhibitor, RQ00203078, muted the response to thymol but did not abolish it (Fig. 5B). There are no commercially available TRPV3 inhibitors, thus we did not assess TRPV3 activity in Cal27 cells. Taken together, this data suggests that the calcium influx in Cal27 cells is primarily due to TRPA1 activation. Notably, the antiproliferative mechanism-of-action most likely does not involve TRP channel activity given the significant antiproliferative effects seen in HeLa cells, which do not exhibit thymol induced calcium influx. Furthermore, while TRP channel activation occurs at concentrations similar to the GC50 (350 μM), cell viability assays reveal that TRPA1 and TRPM8 inhibition does not reverse thymol’s cytotoxicity in vitro (Fig. 5C), confirming that TRP channel activation is not the mechanism-of-action for thymol’s antitumor effects.
Fig. 4. Calcium imaging of cell lines treated with thymol (400 μM) or ionomycin positive control (3 μM) show Cal27 cells have TRP channel activity while HeLa cells do not.
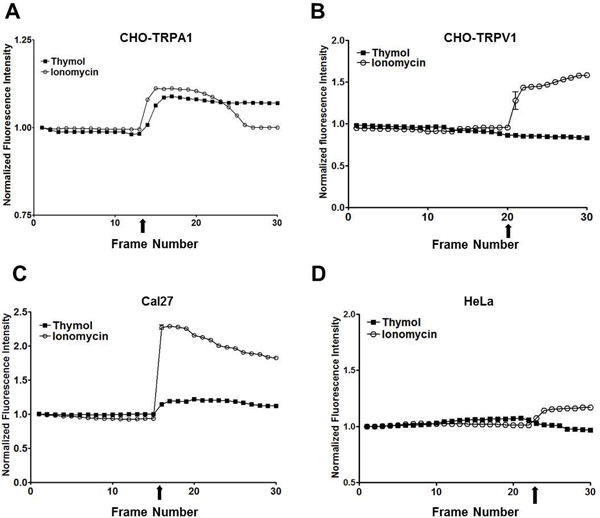
A) CHO-TRPA1 cells show a thymol-induced calcium influx. B) CHO-TRPV1 cells do not show an influx of calcium in response to thymol. C) Cal27 cells show a thymol-induced calcium influx. D) HeLa cells do not show an influx of calcium in response to thymol.
Fig. 5. TRPA1 inhibition reduces calcium influx in Cal27 cells whereas the TRPM8 inhibitor does not.
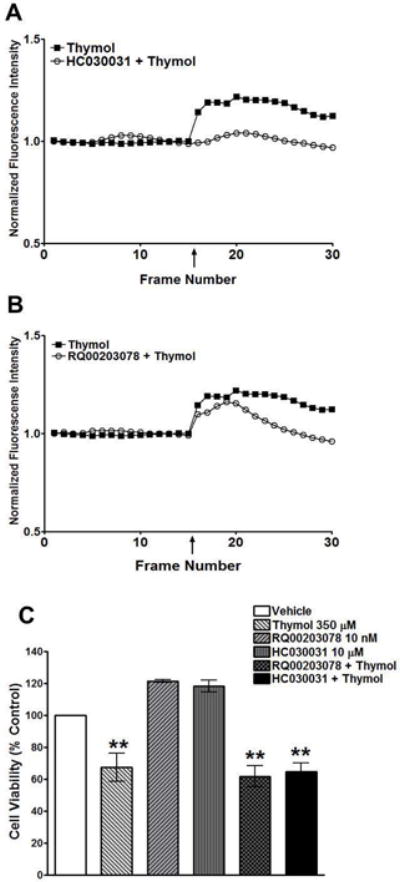
A) Calcium imaging of Cal27 cells pre-treated with the TRPA1 antagonist, HC030031 (10 μM), followed by thymol (400 μM) compared to thymol alone (400 μM) show a dramatic reduction in calcium influx. B) Calcium imaging of Cal27 cells pre-treated with the TRPM8 antagonist, RQ00203078 (10 μM), followed by thymol (400 μM) compared to thymol alone (400 μM) have a muted calcium influx even in the presence of TRPM8 inhibition. C) MTS cell viability assay of Cal27 cells treated with thymol (350 μM), HC030031 (10 μM; TRPA1 antagonist), RQ00203078 (10 nM; TRPM8 antagonist), or pre-treated with the TRPA1 or TRPM8 inhibitors followed by thymol (350 μM).
Thymol causes changes in mitochondrial membrane potential and apoptosis
A concentration-dependent induction of cleaved PARP (c-PARP), indicative of apoptosis, was seen in both Cal27 and HeLa cells treated for 48 h with increasing concentrations of thymol (Fig. 6A). Evaluation of mitochondrial membrane potential (ΔΨm), as measured by JC-1 aggregate to monomer ratio, demonstrated a concentration-dependent depolarization of the mitochondria in both Cal27 and HeLa cells resulting in cell death (Figs. 6B & 6C). Notably, thymol’s antiproliferative effects were completely reversed with the addition of the antioxidant N-acetyl-cysteine (NAC) providing additional evidence that thymol’s anticancer effect is through mitochondrial dysfunction and subsequent apoptosis (Figs. 6D & 6E; p<0.001).
Fig. 6. Thymol inhibits mitochondrial membrane potential and induces apoptosis.
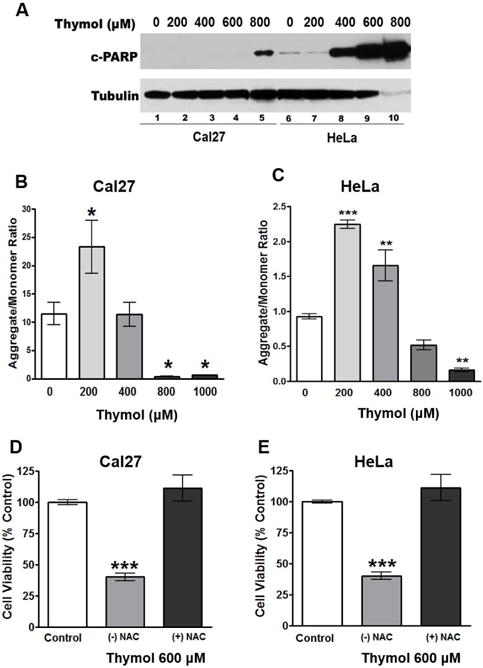
A) Western blot of c-PARP in Cal27 and HeLa cells treated with increasing concentrations of thymol for 48 h show a dose dependent induction of apoptosis. B & C) Cal27 and HeLa cells, respectively, treated with increasing concentrations of thymol for 2 h show significant reduction in mitochondrial membrane potential as measured by JC-1 aggregate to monomer ratio. D & E) MTS cell viability assays of Cal27 and HeLa cells, respectively, following 48 h treatment demonstrate that 10 nM NAC reverses the anti-proliferative effects of thymol.
Discussion
Thymol is a known agonist for TRPA1, TRPV3, and weakly activates TRPM8 and thus is deemed a potential therapy for cancers that over-express these channels. We demonstrate for the first time, the therapeutic potential of thymol against OSCC and confirm anticancer effects in the absence of TRP channel activation. Studies evaluating thymol-induced calcium influx in MG63 osteosarcoma cells and DBTRG-05MG glioblastoma cells confirmed a dose-dependent calcium influx that correlated with cytotoxic concentrations(8, 19). While these studies did not evaluate TRP channels specifically, the primary source of calcium was found to be from extracellular Ca2+ in the media and a minor portion from depleting intracellular Ca2+ stores. Similarly, treatment of MG63 cells and DBRTG-05MG cells with the calcium chelator, BAPTA/AM, inhibited calcium influx but failed to inhibit cytotoxicity of thymol indicating that calcium overload is not the cause of cell death. Likewise, we show that TRPA1 and TRPM8 inhibitors inhibit calcium influx in Cal27 cells, but do not impede the cytotoxic effect of thymol. Furthermore, HeLa cells show no calcium influx in response to thymol yet their proliferation is significantly reduced when treated with thymol.
Thymol is a phenolic compound that reportedly has antioxidant activity at low concentrations and pro-oxidant activities at high concentrations(24). Our studies found similar GC50 values in Cal27 and HeLa cell lines regardless of TRP channel activation levels indicating a mechanism-of-action with no selectivity based upon TRP channel expression. Previously, we described Vanilloid induction of free radicals in OSCC and subsequent apoptosis most likely through inhibition of electron transport(14). This study shows ΔΨm hyperpolarization when treated with 200 μM thymol followed by a dramatic dose-dependent ΔΨm depolarization in both Cal27 and HeLa cells; indicating that thymol is acting on the mitochondria and may play a role in mitochondrial dysfunction in cancer cells. The apparent ΔΨm hyperpolarization at lower thymol concentrations is most likely due to endoplasmic reticulum (ER) stress and subsequent release of Ca2+ into the cytosol, which interferes with the accuracy of ΔΨm dyes that serve as indirect measures of the mitochondrial pH values(25). Nevertheless, as thymol concentrations increased mitochondrial membrane potential decreased and apoptosis ensued.
The hydrophobic nature of thymol allows it to interact with lipids found in the cell and in mitochondrial membranes. In addition, thymol, is a redox inhibitor that interferes with electron transport similar to Vanilloids thereby inducing apoptosis(26). Accordingly, studies of thymol in B16 melanoma cells show that thymol cytotoxicity is reversible with the addition of Vitamin C or Vitamin E(27). Likewise we show that thymol cytotoxicity in OSCC is reversed by the addition of the antioxidant NAC providing further evidence that the anticancer mechanism-of-action of thymol involves mitochondrial dysfunction, generation of free radicals, and subsequent apoptosis. Importantly, we show for the first time that thymol has significant antitumor effects in Cal27 and HeLa xenografts with no adverse effects on adjacent non-malignant tissues at concentrations that are FDA-approved for commercial products. Thus thymol may also have chemopreventive effects against oral cancers and, more importantly, can be further developed to treat OSCC.
Acknowledgments
This work was supported by the University of Texas Health Science Center of San Antonio (UTHSCSA), Cancer Therapy and Research Center (CTRC). Calcium imaging was performed at the CTRC Core Imaging Facility supported by a National Cancer Institute Cancer Center Support Grant (P30 CA054174). The authors would also like to thank Rachel M. Hartley, Brigette Lee, and Susan L. Mooberry for assistance with clonogenic assays.
Abbreviations
- OSCC
Oral Squamous Cell Carcinoma
- TRP
Transient Receptor Potential Channel
- TRPA1
Transient Receptor Potential Channel Ankyrin Subtype 1
- TRPM8
Transient Receptor Potential Channel Melastatin Subtype 8
- TRPV3
Transient Receptor Potential Channel Vanilloid Subtype 3
Footnotes
DR CARA B GONZALES (Orcid ID: 0000-0002-1739-4574)
Conflict of Interest Statement
The authors have no conflict of interest.
References
- 1.Cancer Facts and Figures: Vol.: American Cancer Society. 2015 https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html Accessed on 11-13-17.
- 2.CONFORTI F, STATTI G, UZUNOV D, et al. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp piperitum (Ucria) coutinho seeds. Biol Pharm Bull. 2006;29:2056–64. doi: 10.1248/bpb.29.2056. [DOI] [PubMed] [Google Scholar]
- 3.EL BABILI F, BOUAJILA J, SOUCHARD JP, et al. Oregano: chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J Food Sci. 2011;76:C512–8. doi: 10.1111/j.1750-3841.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 4.MIYAZAWA N, FUJITA A, KUBOTA K. Aroma character impact compounds in Kinokuni mandarin orange (Citrus kinokuni) compared with Satsuma mandarin orange (Citrus unshiu) Biosci Biotechnol Biochem. 2010;74:835–42. doi: 10.1271/bbb.90937. [DOI] [PubMed] [Google Scholar]
- 5.OZCAN MM, CHALCHAT JC. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int J Food Sci Nutr. 2008;59:691–8. doi: 10.1080/09637480701777944. [DOI] [PubMed] [Google Scholar]
- 6.PANDEY SK, UPADHYAY S, TRIPATHI AK. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi. Parasitol Res. 2009;105:507–12. doi: 10.1007/s00436-009-1429-6. [DOI] [PubMed] [Google Scholar]
- 7.AHMAD A, KHAN A, AKHTAR F, et al. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis. 2010;30:41–50. doi: 10.1007/s10096-010-1050-8. [DOI] [PubMed] [Google Scholar]
- 8.CHANG HT, HSU SS, CHOU CT, et al. Effect of thymol on Ca2+ homeostasis and viability in MG63 human osteosarcoma cells. Pharmacology. 2011;88:201–12. doi: 10.1159/000331864. [DOI] [PubMed] [Google Scholar]
- 9.COSENTINO S, TUBEROSO CI, PISANO B, et al. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett Appl Microbiol. 1999;29:130–5. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 10.MOHAMMED MJ, AL-BAYATI FA. Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine. 2009;16:632–7. doi: 10.1016/j.phymed.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 11.BARALDI PG, PRETI D, MATERAZZI S, et al. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem. 2010;53:5085–107. doi: 10.1021/jm100062h. [DOI] [PubMed] [Google Scholar]
- 12.LEE SP, BUBER MT, YANG Q, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153:1739–49. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ORTAR G, MORERA L, MORIELLO AS, et al. Modulation of thermo-transient receptor potential (thermo-TRP) channels by thymol-based compounds. Bioorg Med Chem Lett. 2012;22:3535–9. doi: 10.1016/j.bmcl.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 14.GONZALES CB, KIRMA NB, DE LA, CHAPA JJ, et al. Vanilloids induce oral cancer apoptosis independent of TRPV1. Oral Oncol. 2014;50:437–47. doi: 10.1016/j.oraloncology.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MERGLER S, STROWSKI MZ, KAISER S, et al. Transient receptor potential channel TRPM8 agonists stimulate calcium influx and neurotensin secretion in neuroendocrine tumor cells. Neuroendocrinology. 2007;85:81–92. doi: 10.1159/000101693. [DOI] [PubMed] [Google Scholar]
- 16.PREVARSKAYA N, ZHANG L, BARRITT G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–46. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.SANCHEZ MG, SANCHEZ AM, COLLADO B, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–7. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 18.WISSENBACH U, NIEMEYER B, HIMMERKUS N, et al. TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Commun. 2004;322:1359–63. doi: 10.1016/j.bbrc.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 19.HSU SS, LIN KL, CHOU CT, et al. Effect of thymol on Ca2+ homeostasis and viability in human glioblastoma cells. Eur J Pharmacol. 2011;670:85–91. doi: 10.1016/j.ejphar.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 20.DEB DD, PARIMALA G, SARAVANA DEVI S, et al. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 21.ROONEY L, VIDAL A, D’SOUZA AM, et al. Discovery, optimization, and biological evaluation of 5-(2-(trifluoromethyl)phenyl)indazoles as a novel class of transient receptor potential A1 (TRPA1) antagonists. J Med Chem. 2014;57:5129–40. doi: 10.1021/jm401986p. [DOI] [PubMed] [Google Scholar]
- 22.GONZALES CB, DE LA CHAPA JJ, SAIKUMAR P, et al. Co-targeting ALK and EGFR parallel signaling in oral squamous cell carcinoma. Oral Oncol. 2016;59:12–9. doi: 10.1016/j.oraloncology.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JENSEN MM, JORGENSEN JT, BINDERUP T, et al. Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med Imaging. 2008;8:16. doi: 10.1186/1471-2342-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.OZKAN A, ERDOGAN A. A comparative study of the antioxidant/prooxidant effects of carvacrol and thymol at various concentrations on membrane and DNA of parental and drug resistant H1299 cells. Nat Prod Commun. 2013;7:1557–60. [PubMed] [Google Scholar]
- 25.PERRY SW, NORMAN JP, BARBIERI J, et al. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.KIM JH, CHAN KL, MAHONEY N, et al. Antifungal activity of redox-active benzaldehydes that target cellular antioxidation. Ann Clin Microbiol Antimicrob. 2011;10:23. doi: 10.1186/1476-0711-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SATOOKA H, KUBO I. Effects of thymol on B16-F10 melanoma cells. J Agric Food Chem. 2012;60:2746–52. doi: 10.1021/jf204525b. [DOI] [PubMed] [Google Scholar]


