Summary
Smouldering multiple myeloma (SMM) is associated with increased risk of progression to multiple myeloma within 2 years, with no approved treatments. Elotuzumab has been shown to promote natural killer (NK) cell stimulation and antibody‐dependent cellular cytotoxicity (ADCC) in vitro. CD56dim (CD56dim/CD16+/CD3−/CD45+) NK cells represent the primary subset responsible for elotuzumab‐induced ADCC. In this phase II, non‐randomized study (NCT01441973), patients with SMM received elotuzumab 20 mg/kg intravenously (cycle 1: days 1, 8; monthly thereafter) or 10 mg/kg (cycles 1, 2: weekly; every 2 weeks thereafter). The primary endpoint was the relationship between baseline proportion of bone marrow‐derived CD56dim NK cells and maximal M protein reduction; secondary endpoints included overall response rate (ORR) and progression‐free survival (PFS). Fifteen patients received 20 mg/kg and 16 received 10 mg/kg; combined data arepresented. At database lock (DBL, September 2014), no association was found between baseline CD56dim NK cell proportion and maximal M protein reduction. With minimum 28 months' follow‐up (DBL: January 2016), ORR (90% CI) was 10% (2·7–23·2) and 2‐year PFS rate was 69% (52–81%). Upper respiratory tract infections occurred in 18/31 (58%) patients. Four (13%) patients experienced infusion reactions, all grade 1–2. Elotuzumab plus lenalidomide/dexamethasone is under investigation for SMM.
Keywords: smouldering multiple myeloma, multiple myeloma, elotuzumab, monoclonal antibody, natural killer cells
Smouldering multiple myeloma (SMM) is an asymptomatic precursor to active multiple myeloma (MM), first described as an intermediate between monoclonal gammopathy of undetermined significance (MGUS) and MM (Kyle & Greipp, 1980; International Myeloma Working Group, 2003). SMM has since been characterized by the presence of ≥30 g/l serum monoclonal (M) protein or ≥500 mg/24 h urinary M protein and/or 10–60% bone marrow plasma cell (BMPC) infiltration, with no myeloma‐defining event or associated end‐organ damage (Rajkumar et al, 2014).
The standard of care for patients with SMM has been observation until MM develops (Mateos & San Miguel, 2013; Rajkumar et al, 2015). However, with the evolving definition and risk stratification of SMM, the International Myeloma Working Group (IMWG) recommends that patients with high‐risk SMM should be candidates for chemoprevention trials (Rajkumar et al, 2015), as such patients have a median time to progression to active MM of 2 years (Kyle et al, 2007). Despite these IMWG recommendations, there are no currently approved therapies for SMM, representing a significant unmet medical need.
Several agents have been evaluated for the treatment of SMM, but have shown poor safety or efficacy (Martin et al, 2002; Barlogie et al, 2008; D'Arena et al, 2011), or have prolonged time to progression but were associated with a greater number of adverse events (AEs) in the treated population (Mateos et al, 2013). As patients with MM show diminished immune cell function (Dosani et al, 2015), activating the immune system to target myeloma cells via immunotherapy is an important area of ongoing research.
Elotuzumab, a humanized, immunostimulatory immunoglobulin (Ig) G1 monoclonal antibody, targets signalling lymphocytic activation molecule family member 7 (SLAMF7), a glycoprotein expressed on myeloma cells, natural killer (NK) cells and subsets of other haematopoetic lineage cells (Hsi et al, 2008). Elotuzumab has a dual mechanism of action: directly activating NK cells and mediating antibody‐dependent cellular cytotoxicity (ADCC), leading to targeted myeloma cell death (Collins et al, 2013; Balasa et al, 2015; Pazina et al, 2017).
Elotuzumab, combined with lenalidomide and dexamethasone (ELd), has shown durable efficacy with minimal incremental toxicity in patients with relapsed/refractory MM (RRMM) (Lonial et al, 2015; Richardson et al, 2015). ELd is approved for treatment of MM in the US in patients with 1–3 prior therapies, and in Europe for patients with ≥1 prior therapy (Bristol‐Myers Squibb, 2017, http://packageinserts.bms.com/pi/pi_empliciti.pdf; European Medicines Agency, 2016, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003967/WC500206673.pdf). Elotuzumab monotherapy showed minimal activity in heavily treated patients with RRMM (median of 4·5 prior lines of therapy), with a phase I study showing a best overall response (BOR) of stable disease (SD) in 26% of patients (Zonder et al, 2012).
Some studies have shown that patients in earlier stages of MM have better NK cell activity than patients in later stages (Bernal et al, 2009; Dosani et al, 2015); therefore, elotuzumab monotherapy may provide clinical benefit in patients with early‐stage disease. CD56dim (CD56dim/CD16+/CD3−/CD45+) cells are a differentiated subset of NK cells that are the primary effectors of NK‐mediated ADCC in vitro, and are associated with the highest proportions of SLAMF7 expression. In vitro studies have shown that >95% of CD56dim cells express SLAMF7, compared with 50–75% of their precursors, CD56bright NK cells (Balasa et al, 2008). Thus, CD56dim NK cells may serve as a biomarker of response to elotuzumab.
In this study, we examine whether higher baseline proportions of CD56dim bone marrow NK cells are associated with greater reductions in serum M protein following elotuzumab treatment. The efficacy and safety of elotuzumab monotherapy are also explored.
Methods
This was a phase II, non‐randomized, open‐label, multicentre study (NCT01441973) that enrolled patients from eight centres in the USA between February 2012 and September 2013.
Patients
Eligible patients were ≥18 years of age, with an Eastern Cooperative Oncology Group performance status of ≤2 and a confirmed diagnosis of high‐risk SMM according to the 2010 IMWG criteria (Kyle et al, 2010), without evidence of end‐organ damage per elevated calcium, renal insufficiency, anaemia or bone lesions (CRAB features) (International Myeloma Working Group, 2003).
At the time of study initiation, high‐risk SMM was defined as a serum M protein level of ≥30 g/l with ≥10% BMPC; or serum M protein 10–30 g/l, ≥10% BMPC and a free light chain (FLC) ratio of <0·125 or >8·0; or urine M protein levels >200 mg/24 h with ≥10% BMPC and serum FLC ratio of ≤0·125 or ≥8·0 (Kyle et al, 2010). Patients with light chain‐only disease and who met these criteria were also eligible.
Key exclusion criteria included active MM, MGUS, other conditions in which IgM M protein is present in the absence of a clonal plasma cell infiltration with lytic bone lesions, plasma cell leukaemia, significant cardiovascular disease and administration of any systemic therapy for SMM within 6 months prior to day 1 of study treatment cycle 1.
Written informed consent was obtained for all patients, and this study adhered to the ethical principles of the Declaration of Helsinki, including all elements required by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and Good Clinical Practice.
Treatments
Patients were enrolled sequentially to receive elotuzumab 20 or 10 mg/kg in 28‐day cycles. For the 20‐mg/kg cohort, in cycle 1, elotuzumab was administered as an intravenous (IV) infusion on days 1 and 8. In cycle 2 and beyond, elotuzumab was administered as an IV infusion once monthly. For the 10‐mg/kg cohort, in cycles 1 and 2, elotuzumab was administered weekly as an IV infusion and then every 2 weeks starting in cycle 3. No dose reductions were permitted.
Premedication with methylprednisolone (50 mg IV) was administered at least 45 min prior to elotuzumab infusion. In addition, 30–90 min prior to elotuzumab, patients received diphenhydramine (25–50 mg orally or IV) or an equivalent H1 blocker, ranitidine (50 mg IV) or an equivalent H2 blocker and acetaminophen (650–1000 mg orally) or an equivalent analgesic/antipyretic.
Treatment continued until disease progression [includes progressive disease per modified IMWG criteria and progression to active myeloma], unacceptable toxicity or other criteria for discontinuation.
Study endpoints
The primary endpoint was the association between the proportion of baseline CD56dim NK cells in bone marrow and maximal reduction in serum M protein. Secondary endpoints included investigator‐assessed overall response rate (ORR), defined as the proportion of treated patients who achieve a partial response (PR) or better, per modified IMWG criteria; 2‐year progression‐free survival (PFS), defined as the time from first dose until documented progression (per IMWG criteria with development of ≥1 CRAB feature; i.e. time to active myeloma) or death. Safety was an exploratory endpoint. Database lock for the primary endpoint was September 2014, and January 2016 for efficacy and safety endpoints. Minimum follow‐up for efficacy and safety endpoints was 28 months. PFS, as defined above, was also evaluated by baseline proportion of CD56dim cells in an exploratory, post‐hoc analysis which included all treated patients.
Assessments
CD56dim NK cell status at baseline was assessed using cells from bone marrow and peripheral blood samples on day 1 of cycle 1, before elotuzumab or any pre‐medications were administered. CD56dim cells (as a proportion of CD45+ lymphocytes) were assessed by multi‐colour flow cytometry within 72 h of collection.
Serum M and urine protein levels were assessed every 4 weeks from date of first dose until disease progression. Efficacy endpoint assessments (ORR, PFS) were based on serum and urine analysis, tumour scanning and bone marrow assessments, and were conducted with each 4‐week cycle until withdrawal of consent, or disease progression per modified IMWG criteria.
AEs were recorded throughout the study from initiation of study drug and post‐study drug safety assessments were conducted 1 and 2 months after discontinuation of elotuzumab. AEs were categorized using the Medical Dictionary for Regulatory Activities, version 18.1 (https://www.meddra.org/sites/default/files/guidance/file/intguide_18_1_english.pdf). The severity of AEs and other symptoms was graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Serious adverse events (SAEs) were recorded during the screening period and within 60 days after last dose; any SAEs occurring after these periods and deemed to be related to elotuzumab treatment were also reported.
Statistical analyses
Statistical analyses used data from all patients who had received at least 1 dose of elotuzumab.
The primary endpoint was evaluated using a least‐squares linear regression model and a one‐sided α level of 0·10. In this model, reductions in M protein levels are reported as negative numbers. This study required 30 patients to have 91% power with a true linear regression slope of −2, a root mean square of 24 and a standard deviation of baseline proportion CD56dim cells of 6%, based on preliminary analyses of data from a phase I study of ELd in RRMM (Study 1703) (Lonial et al, 2012).
ORR and corresponding 90% exact confidence intervals (CIs) were computed using the Clopper and Pearson method for each cohort separately and for both cohorts pooled together. This analysis was repeated for patients whose best response was a minimal response (MR) or better.
PFS rates were estimated using the Kaplan–Meier method; two‐sided 90% CIs were computed for 1‐ and 2‐year PFS rates using Greenwood's formula, and 95% CIs were calculated for median PFS using the Brookmeyer and Crowley method. Patients who neither progressed nor died were censored on the date of their last tumour assessment.
Results
Patient disposition and baseline characteristics
Thirty‐one patients were treated with elotuzumab (20 mg/kg, n = 15; 10 mg/kg, n = 16). Ten patients (32%) were still on treatment at the time of the January 2016 database lock. The main reason for discontinuation was disease progression (7 patients in the 20 mg/kg cohort and 5 in the 10 mg/kg cohort). Patient disposition is detailed in Fig 1.
Figure 1.
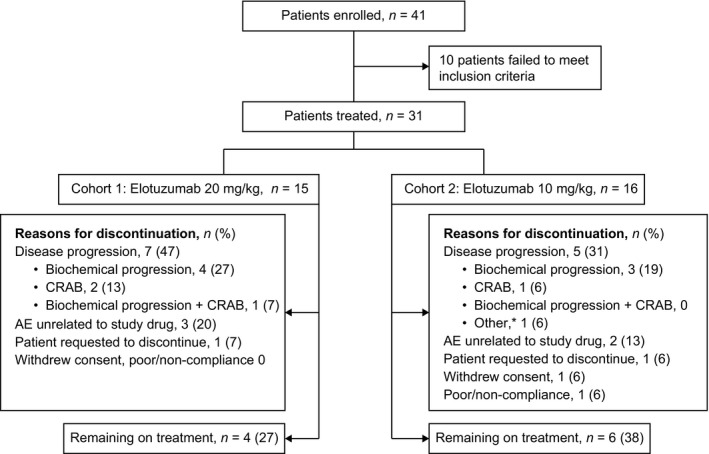
Patient disposition. Database lock: January 2016. *Due to cardiac amyloidosis. AE, adverse event; CRAB, elevated calcium, renal insufficiency, anaemia or bone lesions.
Baseline demographics and disease characteristics are shown in Table 1. Across both cohorts, 32% of patients had serum M protein ≥30 g/l and BMPC ≥10%; 61% had serum M protein 10–30 g/l, BMPC ≥10% and abnormal FLC ratio <0·125 or >8·0; and 13% had urine M protein >200 mg/24 h, BMPC ≥10% and FLC ≤0·125 or ≥8·0.
Table 1.
Baseline demographics and disease characteristics
| Characteristic | Elotuzumab 20 mg/kg (n = 15) | Elotuzumab 10 mg/kg (n = 16) |
|---|---|---|
| Age, years | 58 (45–74) | 61 (39–75) |
| Male | 10 (67) | 7 (44) |
| Myeloma type | ||
| IgG | 15 (100) | 14 (88) |
| IgA | 0 | 1 (6) |
| Light‐chain disease | 0 | 1 (6) |
| ECOG performance status | ||
| 0 | 14 (93) | 13 (81) |
| 1 | 1 (7) | 3 (19) |
| BMPC percentagea | 23 (10–60) | 30 (10–80) |
| Serum β2 microglobulin, mg/l | ||
| <3·5 | 13 (87) | 14 (88) |
| 3·5 to <5·5 | 2 (13) | 2 (13) |
| Chronic kidney disease stage | ||
| 1 | 10 (67) | 7 (44) |
| 2 | 3 (20) | 8 (50) |
| 3 | 2 (13) | 1 (6) |
| Serum M protein, g/l | 23 (10–60) | 18 (0–43) |
| Urine M protein, mg/day | 0 (0–2430) | 16 (0–750) |
| Time since diagnosis, months | 15·3 (0·8–100·1) | 27·2 (1·2–132·7) |
| Satisfied high‐risk SMM criteriab at study entry | ||
| Serum M protein ≥30 g/l, BMPC ≥10% | 5 (33) | 5 (31) |
| Serum M protein 10–30 g/l, BMPC ≥10%, FLC ratio <0·125 or >8·0 | 9 (60) | 10 (63) |
| Urine M protein >200 mg/day, BMPC ≥10%, FLC ratio ≤0·125 or ≥8·0c | 2 (13) | 2 (13) |
At the January 2016 database lock, the median (min–max) duration of treatment was 18 (2–41) months and 22 (2–34) months in the 20‐ and 10‐mg/kg cohorts, respectively. The median (min–max) number of treatment cycles was 18 (3–39) and 24 (3–36) in the 20‐ and 10‐mg/kg cohorts, respectively. Fourteen (93%) patients in the 20‐mg/kg cohort and 15 (94%) patients in the 10‐mg/kg cohort received ≥90% of the planned elotuzumab dose.
Efficacy
No association between baseline proportion of CD56dim NK cells in bone marrow and maximal change in serum M protein was found (Table 2). When examining the association between these two variables, the parameter estimates for the slope of the regression line were directionally different for the 20 mg/kg and 10 mg/kg cohorts.
Table 2.
Least‐squares linear regression of baseline proportion of CD56dim natural killer cells in bone marrow as a predictor of maximal change in serum M protein
| Elotuzumab 20 mg/kg (n = 13) | Elotuzumab 10 mg/kg (n = 12) | Total (n = 25) | |
|---|---|---|---|
| Parameter estimate (95% CI) | −2·56 (−5·44, 0·32) | 2·46 (0·09, 4·84) | 0·27 (−1·72, 2·27) |
| P‐value | 0·08 | 0·04 | 0·78 |
Database lock: September 2014. CI, confidence interval.
ORR (90% CI) was 10% (2·7–23·2%) (n = 3/31). In analysis of BOR, most patients achieved SD or MR, with 29% of patients (n = 9) achieving MR or better (Fig 2).
Figure 2.
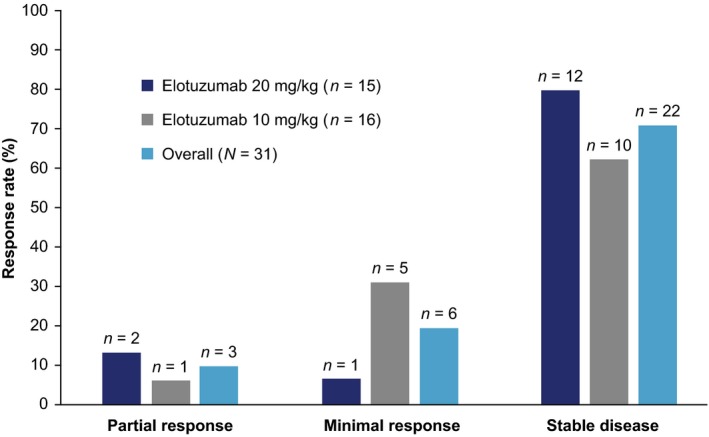
Best overall response. Database lock: January 2016.
At the January 2016 database lock, with a minimum of 28 months' follow‐up, 5 patients in each cohort had documented progression to active MM (including development of CRAB features). There were no marked differences between cohorts in 1‐ or 2‐year PFS rates [per modified IMWG criteria, including CRAB features; Fig 3]. For both cohorts combined, the 1‐year PFS rate (90% CI) was 80% (65–89%), and the PFS rate at 2 years was 69% (52–81%). Median PFS was not reached.
Figure 3.
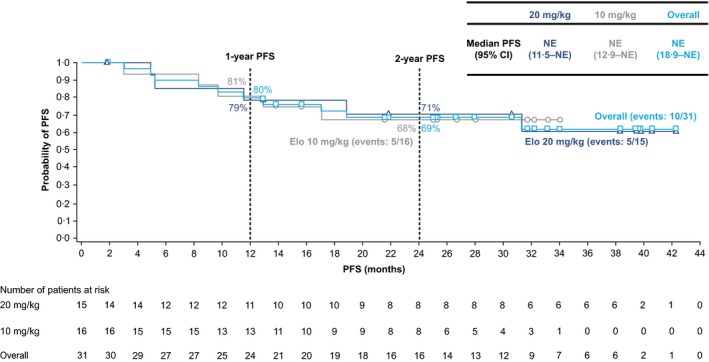
Progression to active MM. Time to progression to active MM based on modified IMWG criteria (Kyle et al, 2010) plus CRAB features. Database lock: January 2016. CI, confidence interval; CRAB, elevated calcium, renal insufficiency, anaemia or bone lesions; Elo, elotuzumab; IMWG, International Myeloma Working Group; PFS, progression‐free survival; MM, multiple myeloma; NE, not evaluable.
Longer PFS was observed in patients with greater than or equal to the median baseline proportion (11·4%) of CD56dim NK cells in bone marrow, compared with patients with less than the median proportion of CD56dim cells (Fig 4), although this was not statistically significant. For patients with greater than or equal to the median baseline proportion of CD56dim cells in bone marrow, the 1‐year PFS rate (90% CI) was 92% (66–98%), and the PFS rate at 2 years was 75% (46–89%); for those with less than the median baseline proportion of CD56dim cells, the 1‐year PFS rate (90% CI) was 64% (35–82%), and the PFS rate at 2 years was 55% (28–75%). Median PFS was not reached for either group of patients.
Figure 4.
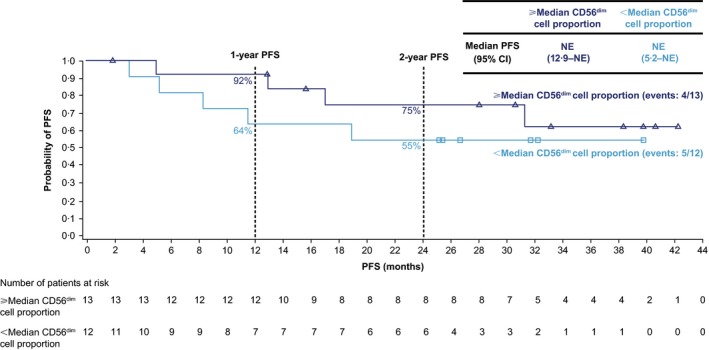
Progression to active MM by baseline proportion of CD56dim cells in the bone marrow. Time to progression to active MM based on modified IMWG criteria (Kyle et al, 2010) plus CRAB features. Database lock: January 2016. CI, confidence interval; CRAB, elevated calcium, renal insufficiency, anaemia or bone lesions; IMWG, International Myeloma Working Group; PFS, progression‐free survival; MM, multiple myeloma; NE, not evaluable.
Safety
Adverse events (any grade) were reported in all patients. AEs reported in ≥20% of patients, with any cause, are shown in Table 3. Grade 3 or 4 AEs were reported in 7 (47%) patients in the 20 mg/kg cohort and 6 (38%) patients in 10 mg/kg cohort. No treatment‐related deaths were reported within 60 days of the last elotuzumab dose.
Table 3.
Adverse events reported in ≥20% of patients
| Adverse event | Elotuzumab 20 mg/kg (n = 15) | Elotuzumab 10 mg/kg (n = 16) | ||
|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Total patients with an adverse event, n (%) | 15 (100) | 7 (47) | 16 (100) | 6 (38) |
| Upper respiratory tract infection | 8 (53) | 1 (7) | 10 (63) | 0 |
| Fatigue | 7 (47) | 0 | 6 (38) | 1 (6) |
| Constipation | 4 (27) | 0 | 3 (19) | 0 |
| Diarrhoea | 4 (27) | 0 | 3 (19) | 1 (6) |
| Arthralgia | 4 (27) | 0 | 5 (31) | 0 |
| Insomnia | 4 (27) | 0 | 6 (38) | 1 (6) |
| Chills | 3 (20) | 0 | 1 (6) | 0 |
| Oral candidiasis | 3 (20) | 0 | 1 (6) | 0 |
Database lock: January 2016.
In total, 7 patients discontinued due to an AE [20 mg/kg, n = 5 (33%), 3 unrelated to treatment; 10 mg/kg, n = 2 (13%), both unrelated to treatment]. SAEs were reported in 15/31 patients [20 mg/kg, n = 8 (53%); 10 mg/kg, n = 7 (44%)]. Second primary malignancies were reported in 1 patient (7%) in the 20 mg/kg cohort (814 days after the first dose) and 3 (19%) in the 10 mg/kg cohort (316–709 days after the first dose). Infusion reactions (IRs) were reported in 3 patients (20%) in the 20 mg/kg cohort and 1 patient (6%) in the 10 mg/kg cohort, and included palpitations, dyspepsia, chills and hypersensitivity (1 event each) in the 20 mg/kg cohort and pyrexia (1 event) in the 10 mg/kg cohort. All IRs were grade 1 or 2 and none led to discontinuation.
Discussion
CD56dim NK cells represent a subpopulation of NK cells with enhanced cytotoxic activity and those that would potentially enhance elotuzumab‐directed ADCC against SLAMF7‐expressing myeloma cells (Balasa et al, 2015).
In this phase II study, we found no association between baseline proportion of CD56dim NK cells in bone marrow and maximal change in serum M protein. The parameter estimate for the slope of the regression line when examining the association between these two variables was negative for the 20 mg/kg cohort, but positive for the 10 mg/kg cohort , indicating a decrease in M protein in the 20 mg/kg cohort but not in the 10 mg/kg cohort. These estimates should, however, be interpreted with caution given their directional difference and the modest changes in M protein levels with elotuzumab monotherapy observed in this study.
Elotuzumab monotherapy demonstrated minimal activity in patients with SMM, with an ORR of 10%. However, 10% achieved a PR, 19% a MR and 71% had SD at the January 2016 database lock, which may be a clinically important outcome in this patient population.
Two‐year PFS rates, as estimated in this analysis, appeared to be a reasonable benchmark for progression from SMM to MM, as this is consistent with a previous analysis showing that patients with high‐risk SMM had a median time to progression to active MM of only 2 years (Rajkumar et al, 2015). Notably, the 2‐year PFS rate in both cohorts was 69%, all patients had been followed for at least 2 years, and the median PFS had not been reached. Post‐hoc analysis suggested a potential PFS benefit in patients with greater than or equal to the median baseline proportion of CD56dim cells in the bone marrow. However, these data should be interpreted with caution, as the study was powered to investigate any association between baseline proportion of CD56dim cells in bone marrow and maximal change in serum M protein, and not the clinical PFS endpoint. The consequent small sample size would not warrant adequate power to assess statistical significance of the observed prolongation of PFS in patients with greater than or equal to the median baseline proportion of CD56dim cells in the bone marrow. PFS reported here included disease progression and development of CRAB features, or death. PFS without development of ≥1 CRAB feature was not reported, in order to keep results as relevant as possible to patients with SMM.
Elotuzumab monotherapy was generally well tolerated, with a safety profile consistent with prior elotuzumab studies (Lonial et al, 2015). Upper respiratory tract infections (URTIs) were the most common AEs, occurring in 18/31 patients (58%), but no cases of URTI were deemed to be related to treatment; one of the 18 cases was grade 3–4, and no patients discontinued elotuzumab due to URTI. All IRs were grade 1 or 2, and no patient discontinued elotuzumab treatment due to an IR. IRs are typically mild to moderate in severity with pre‐treatment, and usually develop during the first dose. Additionally, analysis of this patient population showed no dose‐related effect on corrected QT intervals, and there were no reports of AEs potentially related to proarrythmia where electrocardiogram assessments could be performed (Passey et al, 2016).
This study demonstrated similar safety and efficacy in patients receiving elotuzumab once‐monthly at 20 mg/kg and patients receiving 10 mg/kg every 2 weeks. Future studies may support these results and inform elotuzumab dosing schedules that maximize patient convenience.
The benefits of therapy for SMM must be weighed against the toxicity and lower quality of life that treatment of SMM may bring. An ideal therapy would be expected to demonstrate clinical benefit with minimal side effects in patients with SMM, such as the patient population evaluated herein; however, this was not achieved in this phase II study.
Several other studies have assessed the efficacy of therapeutic agents for SMM. In particular, bisphosphonates have been studied; notably, a prospective, randomized study with a minimum of 5 years, follow‐up evaluated pamidronate versus observation in patients with SMM and found no reduction in the risk of progression from SMM to MM (D'Arena et al, 2011), consistent with results of studies with shorter follow‐ups. A phase III randomized study of thalidomide plus zoledronic acid versus zoledronic acid alone in 68 patients with SMM found no anti‐tumour responses in the zoledronic acid group and an ORR of 37% with the addition of thalidomide (Witzig et al, 2013). PFS was prolonged to 4·1 vs. 3·3 years with the addition of thalidomide, but this difference was not found to be statistically significant (Witzig et al, 2013).
It has been demonstrated that patients with high‐risk SMM can benefit from early treatment. A study of 119 patients with high‐risk SMM randomized to lenalidomide and dexamethasone (Ld) or observation arms showed that early intervention in SMM had significant benefit in terms of time to progression and 3‐year PFS (Mateos et al, 2013). Although Ld delayed the development of active MM, a higher proportion of patients in the Ld arm experienced AEs compared with the observation cohort, including one grade 5 AE (Mateos et al, 2013). A long‐term follow‐up of this study, with a median follow‐up of 75 months, showed that, as of June 2015, the median time to progression was not reached in the Ld group (versus 23 months in the observation group; P < 0·0001) (Mateos et al, 2016).
ELd is also under investigation in patients with high‐risk SMM according to the most recent diagnostic criteria (Ghobrial et al, 2016a). Initial data from a small phase II study showed that patients treated with ELd had a high ORR (74%), including two complete responses, nine very good PR (24%) and 17 PR (45%), with an acceptable safety profile (Ghobrial et al, 2016a). Of patients who received ≥9 cycles of ELd, all showed clinical benefit (MR or better), and 83% had a PR or better (Ghobrial et al, 2016a).
Study limitations
The definition of high‐risk SMM has been updated since this study began (Rajkumar et al, 2015). Notable differences include a FLC ratio of ≥8·0 (versus also including <0·125) and BMPC of 50–60% (vs. ≥10%). In light of this newer guidance, the patients enrolled in this study may be considered intermediate‐to‐high risk. It should also be noted that the IMWG response criteria were designed for patients with active MM; as yet, the precise meaning of response in SMM is incompletely understood.
In total, 31 patients were sequentially enrolled and treated in this phase II study; interpretation of data from this study to fully establish any benefit of elotuzumab monotherapy in patients with SMM is thus limited by the small number of patients, short duration of treatment and lack of observation arm.
Conclusions
No association was found between baseline proportions of CD56dim NK cells in bone marrow and maximal change in serum M protein in response to elotuzumab monotherapy in patients with SMM. Monotherapy with elotuzumab had a low ORR, but was well tolerated with an acceptable safety profile, with an overall 2‐year PFS rate of 69%. Investigations combining elotuzumab with lenalidomide and dexamethasone in patients with high‐risk SMM are ongoing.
Author Contributions
All authors critically reviewed drafts of the manuscript, had approval of the submitted and final versions, attest to the accuracy and integrity of the data, and agree to be accountable for all respective aspects of this work. SJ, JL, EW, KS‐G, CR, MD, and PGR made contributions to study conception and design (led by PGR), the acquisition, analysis/interpretation of data and enrolment of patients. Y‐MJ, ML, MR, SS and KCA made contributions to study conception and design, and analysis/interpretation of data.
Conflict of Interest Statement
SJ has received honoraria from, and has served on an advisory board, for Bristol‐Myers Squibb. JL has received research funding from Novartis, Onyx, Celgene and Millennium. EW has nothing to disclose. KS‐G has served on a speakers bureau for Janssen. CR has received honoraria from Celgene. MD has served on advisory boards for Bristol‐Myers Squibb and Roche. Y‐MJ is an employee of Bristol‐Myers Squibb. ML is an employee of, and owns stocks in, Bristol‐Myers Squibb. MR is an employee of, and owns stocks in, Bristol‐Myers Squibb. SS is an employee of, and owns stocks in, Bristol‐Myers Squibb. KCA has served in a consultancy/advisory role for Bristol‐Myers Squibb, Gilead and Takeda; and is Scientific Founder of Oncopep and C4 Therapeutics. PGR has served in an advisory role for Genmab, and received research funding from Celgene and Millennium.
Acknowledgements
This study was funded by Bristol‐Myers Squibb. Elotuzumab was developed in partnership between AbbVie Biotherapeutics and Bristol‐Myers Squibb. This work was supported by a grant from the National Cancer Institute of the National Institutes of Health, USA (P30CA016359 to Yale Cancer Center). Professional medical writing assistance was provided by Adam Gill at Caudex, funded by Bristol‐Myers Squibb. Editorial assistance was provided by Stephanie Wolfe at Caudex, funded by Bristol‐Myers Squibb.
References
- Balasa, B. , Huseni, M. , Cherukuri, J. , Steinle, R. , Nanisetti, A. , Afar, D.E. , Hsi, E.D. & Vexler, V. (2008) Elotuzumab (HuLuc63) activates CD56dim natural killer cells and monocytes resulting in the release of IP‐10 and MCP‐1. (Abstract). Blood, 112, 108. [Google Scholar]
- Balasa, B. , Yun, R. , Belmar, N.A. , Fox, M. , Chao, D.T. , Robbins, M.D. , Starling, G.C. & Rice, A.G. (2015) Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin‐2 and TNF‐alpha pathways. Cancer Immunology, Immunotherapy, 64, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie, B. , van Rhee, F. , Shaughnessy, J.D. Jr , Epstein, J. , Yaccoby, S. , Pineda‐Roman, M. , Hollmig, K. , AlSayed, Y. , Hoering, A. , Szymonifka, J. , Anaissie, E. , Petty, N. , Kumar, N.S. , Srivastava, G. , Jenkins, B. , Crowley, J. & Zeldis, J.B. (2008) Seven‐year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood, 112, 3122–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal, M. , Garrido, P. , Jimenez, P. , Carretero, R. , Almagro, M. , Lopez, P. , Navarro, P. , Garrido, F. & Ruiz‐Cabello, F. (2009) Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: implications for tumor evasion of T and NK cells. Human Immunology, 70, 854–857. [DOI] [PubMed] [Google Scholar]
- Bristol‐Myers Squibb . (2017). Empliciti™ (elotuzumab) prescribing information. Available at: http://packageinserts.bms.com/pi/pi_empliciti.pdf (Accessed June 7, 2017).
- Collins, S.M. , Bakan, C.E. , Swartzel, G.D. , Hofmeister, C.C. , Efebera, Y.A. , Kwon, H. , Starling, G.C. , Ciarlariello, D. , Bhaskar, S. , Briercheck, E.L. , Hughes, T. , Yu, J. , Rice, A. & Benson, D.M. Jr (2013) Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunology, Immunotherapy, 62, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arena, G. , Gobbi, P.G. , Broglia, C. , Sacchi, S. , Quarta, G. , Baldini, L. , Iannitto, E. , Falcone, A. , Guariglia, R. , Pietrantuono, G. , Villani, O. , Martorelli, M.C. , Mansueto, G. , Sanpaolo, G. , Cascavilla, N. & Musto, P. (2011) Pamidronate versus observation in asymptomatic myeloma: final results with long‐term follow‐up of a randomized study. Leukemia & Lymphoma, 52, 771–775. [DOI] [PubMed] [Google Scholar]
- Dosani, T. , Carlsten, M. , Maric, I. & Landgren, O. (2015) The cellular immune system in myelomagenesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer Journal, 5, e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . (2016). Elotuzumab: Summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003967/WC500206673.pdf (Accessed May 26 2016).
- Ghobrial, I.M. , Badros, A. , Vredenburgh, J.J. , Matous, J. , Caola, A.M. , Savell, A. , Henrick, P. , Paba‐Prada, C.E. , Schlossman, R. , Laubach, J. , Rosenblatt, J. , Yee, A. , Wisch, J.S. , Farber, C.M. , Maegawa, R.O. , Usmani, S. , Cappuccio, J. , Rivotto, B. , Noonan, K. , Reyes, J. , Munshi, N. , Andersen, K.C. & Richardson, P. (2016a) Phase II trial of combination of elotuzumab, lenalidomide, and dexamethasone in high‐risk smoldering multiple myeloma. Blood, 128, 976. [Google Scholar]
- Ghobrial, I.M. , Badros, A. , Vredenburgh, J.J. , Matous, J. , Caola, A.M. , Savell, A. , Henrick, P. , Paba‐Prada, C.E. , Schlossman, R. , Laubach, J. , Rosenblatt, J. , Yee, A. , Wisch, J.S. , Farber, C.M. , Maegawa, R.O. , Usmani, S. , Cappuccio, J. , Rivotto, B. , Noonan, K. , Reyes, J. , Munshi, N. , Andersen, K.C. & Richardson, P. (2016b) Phase II trial of combination of elotuzumab, lenalidomide, and dexamethasone in high‐risk smoldering multiple myeloma [Oral 976]. 58th Annual Meeting & Exposition of American Society of Hematology (ASH); December 3‐5, 2016; San Diego, CA. [Google Scholar]
- Hsi, E.D. , Steinle, R. , Balasa, B. , Szmania, S. , Draksharapu, A. , Shum, B.P. , Huseni, M. , Powers, D. , Nanisetti, A. , Zhang, Y. , Rice, A.G. , van Abbema, A. , Wong, M. , Liu, G. , Zhan, F. , Dillon, M. , Chen, S. , Rhodes, S. , Fuh, F. , Tsurushita, N. , Kumar, S. , Vexler, V. , Shaughnessy, J.D. Jr , Barlogie, B. , van Rhee, F. , Hussein, M. , Afar, D.E. & Williams, M.B. (2008) CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clinical Cancer Research, 14, 2775–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. British Journal of Haematology, 121, 749–757. [PubMed] [Google Scholar]
- Kyle, R.A. & Greipp, P.R. (1980) Smoldering multiple myeloma. New England Journal of Medicine, 302, 1347–1349. [DOI] [PubMed] [Google Scholar]
- Kyle, R.A. , Remstein, E.D. , Therneau, T.M. , Dispenzieri, A. , Kurtin, P.J. , Hodnefield, J.M. , Larson, D.R. , Plevak, M.F. , Jelinek, D.F. , Fonseca, R. , Melton, L.J. III & Rajkumar, S.V. (2007) Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. New England Journal of Medicine, 356, 2582–2590. [DOI] [PubMed] [Google Scholar]
- Kyle, R.A. , Durie, B.G. , Rajkumar, S.V. , Landgren, O. , Blade, J. , Merlini, G. , Kroger, N. , Einsele, H. , Vesole, D.H. , Dimopoulos, M. , San Miguel, J. , Avet‐Loiseau, H. , Hajek, R. , Chen, W.M. , Anderson, K.C. , Ludwig, H. , Sonneveld, P. , Pavlovsky, S. , Palumbo, A. , Richardson, P.G. , Barlogie, B. , Greipp, P. , Vescio, R. , Turesson, I. , Westin, J. & Boccadoro, M. (2010) Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia, 24, 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle, R.A. , Larson, D.R. , Therneau, T.M. , Dispenzieri, A. , Melton, L.J. III , Benson, J.T. , Kumar, S. & Rajkumar, S.V. (2014) Clinical course of light‐chain smouldering multiple myeloma (idiopathic Bence Jones proteinuria): a retrospective cohort study. Lancet Haematology, 1, e28–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonial, S. , Vij, R. , Harousseau, J.L. , Facon, T. , Moreau, P. , Mazumder, A. , Kaufman, J.L. , Leleu, X. , Tsao, L.C. , Westland, C. , Singhal, A.K. & Jagannath, S. (2012) Elotuzumab in combination with lenalidomide and low‐dose dexamethasone in relapsed or refractory multiple myeloma. Journal of Clinical Oncology, 30, 1953–1959. [DOI] [PubMed] [Google Scholar]
- Lonial, S. , Dimopoulos, M. , Palumbo, A. , White, D. , Grosicki, S. , Spicka, I. , Walter‐Croneck, A. , Moreau, P. , Mateos, M.V. , Magen, H. , Belch, A. , Reece, D. , Beksac, M. , Spencer, A. , Oakervee, H. , Orlowski, R.Z. , Taniwaki, M. , Rollig, C. , Einsele, H. , Wu, K.L. , Singhal, A. , San‐Miguel, J. , Matsumoto, M. , Katz, J. , Bleickardt, E. , Poulart, V. , Anderson, K.C. & Richardson, P. (2015) Elotuzumab therapy for relapsed or refractory multiple myeloma. New England Journal of Medicine, 373, 621–631. [DOI] [PubMed] [Google Scholar]
- Martin, A. , Garcia‐Sanz, R. , Hernandez, J. , Blade, J. , Suquia, B. , Fernandez‐Calvo, J. , Gonzalez, M. , Mateo, G. , Orfao, A. & San Miguel, J.F. (2002) Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti‐tumour effect. British Journal of Haematology, 118, 239–242. [DOI] [PubMed] [Google Scholar]
- Mateos, M.V. & San Miguel, J.F. (2013) New approaches to smoldering myeloma. Current Hematologic Malignancy Reports, 8, 270–276. [DOI] [PubMed] [Google Scholar]
- Mateos, M.V. , Hernandez, M.T. , Giraldo, P. , De La Rubia, J. , de Arriba, F. , Lopez, C.L. , Rosinol, L. , Paiva, B. , Palomera, L. , Bargay, J. , Oriol, A. , Prosper, F. , Lopez, J. , Olavarria, E. , Quintana, N. , Garcia, J.L. , Blade, J. , Lahuerta, J.J. & San Miguel, J.F. (2013) Lenalidomide plus dexamethasone for high‐risk smoldering multiple myeloma. New England Journal of Medicine, 369, 438–447. [DOI] [PubMed] [Google Scholar]
- Mateos, M.V. , Hernandez, M.T. , Giraldo, P. , De La Rubia, J. , de Arriba, F. , Corral, L.L. , Rosinol, L. , Paiva, B. , Palomera, L. , Bargay, J. , Oriol, A. , Prosper, F. , Lopez, J. , Arguinano, J.M. , Quintana, N. , Garcia, J.L. , Blade, J. , Lahuerta, J.J. & Miguel, J.F. (2016) Lenalidomide plus dexamethasone versus observation in patients with high‐risk smouldering multiple myeloma (QuiRedex): long‐term follow‐up of a randomised, controlled, phase 3 trial. Lancet Oncology, 17, 1127–1136. [DOI] [PubMed] [Google Scholar]
- Passey, C. , Darbenzio, R. , Jou, Y.‐M. , Lynch, M. & Gupta, M. (2016) Effects of elotuzumab on QT interval and cardiac safety in patients with multiple myeloma. Cancer Chemotherapy and Pharmacology, 78, 1237–1244. [DOI] [PubMed] [Google Scholar]
- Pazina, T. , James, A.M. , MacFarlane, A.W. , Bezman, N.A. , Henning, K.A. , Bee, C. , Graziano, R.F. , Robbins, M.D. , Cohen, A.D. & Campbell, K.S. (2017) The anti‐SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16‐dependent and ‐independent mechanisms. Oncoimmunology, 6, e1339853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar, S.V. , Dimopoulos, M.A. , Palumbo, A. , Blade, J. , Merlini, G. , Mateos, M.V. , Kumar, S. , Hillengass, J. , Kastritis, E. , Richardson, P. , Landgren, O. , Paiva, B. , Dispenzieri, A. , Weiss, B. , Leleu, X. , Zweegman, S. , Lonial, S. , Rosinol, L. , Zamagni, E. , Jagannath, S. , Sezer, O. , Kristinsson, S.Y. , Caers, J. , Usmani, S.Z. , Lahuerta, J.J. , Johnsen, H.E. , Beksac, M. , Cavo, M. , Goldschmidt, H. , Terpos, E. , Kyle, R.A. , Anderson, K.C. , Durie, B.G. & Miguel, J.F. (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology, 15, e538–e548. [DOI] [PubMed] [Google Scholar]
- Rajkumar, S.V. , Landgren, O. & Mateos, M.V. (2015) Smoldering multiple myeloma. Blood, 125, 3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, P.G. , Jagannath, S. , Moreau, P. , Jakubowiak, A.J. , Raab, M.S. , Facon, T. , Vij, R. , White, D. , Reece, D.E. , Benboubker, L. , Zonder, J. , Tsao, L.C. , Andersen, K.C. , Bleickardt, E. , Singhal, A.K. & Lonial, S. ; on behalf of the 1703 study investigators . (2015) Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open‐label, phase 1b‐2 dose‐escalation study. Lancet Haematology, 2, e516–e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig, T.E. , Laumann, K.M. , Lacy, M.Q. , Hayman, S.R. , Dispenzieri, A. , Kumar, S. , Reeder, C.B. , Roy, V. , Lust, J.A. , Gertz, M.A. , Greipp, P.R. , Hassoun, H. , Mandrekar, S.J. & Rajkumar, S.V. (2013) A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia, 27, 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonder, J.A. , Mohrbacher, A.F. , Singhal, S. , van Rhee, F. , Bensinger, W.I. , Ding, H. , Fry, J. , Afar, D.E. & Singhal, A.K. (2012) A phase 1, multicenter, open‐label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood, 120, 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]


