Abstract
Purpose of Review
To review the diagnosis and treatment of prosthetic joint infection (PJI) with a focus on two-stage revision arthroplasty. The text will discuss different spacer constructs in total knee and total hip arthroplasty and will present clinical outcome data for these various options.
Recent Findings
There is no appreciable difference in infection eradication between mobile and static antibiotic spacers. Mobile spacers have shown improved knee range of motion after second-stage re-implantation.
Summary
Two-stage revision arthroplasty is the gold standard treatment for PJI. The first stage involves removal of all components, cement, and compromised soft tissues with placement of an antibiotic-impregnated spacer. Spacer options include both mobile and static spacers. Mobile spacers offer maintenance of ambulation and joint range of motion between staged procedures and have shown to be as effective in eradicating infection as static spacers.
Keywords: Total hip arthroplasty, Total knee arthroplasty, Prosthetic joint infection, Two-stage revision, Antibiotic spacer
Introduction
Adult hip and knee reconstruction offers patients with end-stage degenerative joint disease an improvement in health-related quality of life [1]. Total knee arthroplasty (TKA) and total hip arthroplasty (THA) have been shown to be well-tolerated surgical procedures that serve to reduce pain and improve functional status. This success has led to expanding indications to younger and more active patients. The increase in primary adult arthroplasty and time burden being placed on implants has led to a rise in revision surgery. Kurtz et al. predicted that by 2030, there will be around 96,000 revision total hip arthroplasties and over 260,000 revision total knee arthroplasties conducted in the USA [2]. Revision surgery is associated with higher cost, mortality, and complication rates compared to primary surgery [3, 4]. This chapter will cover prosthetic joint infection (PJI) as a reason for revision, with a focus on two-stage revision for chronic infection.
Diagnosis
Patients presenting with a painful or unstable total hip or knee arthroplasty should undergo a detailed history and physical exam. Pain is the most common complaint leading to revision; therefore, a detailed assessment of the timing and onset, character, severity, location, alleviating and exacerbating factors, and temporal nature of the pain should be documented. Other components of the history that should be elucidated are any history of traumatic events, recent illnesses, or fever indicating infection and any instability events or dislocations. Patients with a painful arthroplasty must always be evaluated for PJI. Laboratory evaluation with white blood cell count (WBC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) should be obtained. Elevated serum inflammatory markers should prompt preoperative aspiration with evaluation of WBC, aerobic, and anaerobic culture. Plain radiographs should be evaluated for component position, evidence of polyethylene wear or osteolysis and, importantly in the case of PJI, signs of component loosening.
Infection and septic failure are a devastating complication following primary THA. There is a 1–2% risk of infection following primary THA and the risk can be as high as 17% following revision THA [5–7]. PJI is divided into either acute (< 90 days from index surgery or onset of symptoms) or chronic (> 90 days) [8•]. The definition of PJI has been divided into major and minor criteria, with the presence of either one major criteria or three minor criteria constituting PJI [8•]. Major criteria are the presence of a sinus tract communicating with the prosthesis or a positive culture from two or more separate tissue or fluid samples [8•, 9]. Minor criteria include elevated ESR and CRP; elevated synovial leukocyte count; elevated leukocyte neutrophil percentage (PMN %); isolation of a microorganism in one culture of tissue or fluid; and > 5 neutrophils per high-power field in 5 high-power fields [8•]. The utility of ESR is limited in acute infection. In chronic infections, a threshold of > 30 mm/h is considered elevated. CRP thresholds differ in the detection of acute (> 100 mg/L) and chronic (> 10 mg/L) PJI [8•, 10].
Proper cell count thresholds as a predictor of acute and chronic infection are controversial. Various authors have recommended threshold values from 1000 to 4000 cells/μL [11–15] and PMN% from 64 to 80% [12, 15] for chronic infection, although published consensus statements and recent data suggest using a threshold of 3000 WBC with 80% segmental neutrophils as criteria suspicious for chronic PJI [8•, 9, 16••]. In the acute post-operative period, a cell count of 27,800 cells/μL provides a positive predictive value of 94% and a negative predictive value of 98%. PMN% less than 89% and a CRP less than 95 mg/L are indicative of a low likelihood of infection [10]. It is important for clinicians to keep in mind that PJI may be present even if these criteria are not met, and in certain cases of low-grade infections (i.e., Propionobacterium Acnes), several of these criteria may not be met. In addition, it has been shown that 2 to 18% of periprosthetic joint infections are culture negative [17].
Treatment Considerations
Accurate and timely diagnosis of PJI is essential for proper management. Once the diagnosis of PJI is made, several factors must be taken into consideration when determining treatment options: the duration of symptoms; patient medical co-morbidities; history of PJI in the current or other joints; status of the wound; functional expectations; and characteristics of the isolated organism [17].
There is a paucity of literature for treatment solely with antibiotics; however, in patients who would be poor surgical candidates because of their state of health and have a low-virulence organism susceptible to antimicrobial agents, antibiotic suppression may be the only option for treatment. Chronic antibiotic suppression is often indicated and has shown to be moderately successful in patients with persistent PJI after proper surgical debridement and/or revision if they cannot tolerate further surgical procedures [17–21].
In patients with acute onset of symptoms (less than 3–4 weeks), a well-fixed and aligned prosthesis and an antibiotic-susceptible organism, irrigation, and debridement with retention of implants are an acceptable surgical option. Successful eradication with this treatment has been reported at a rate of 71% for all organisms [22], although eradication results of methicillin-resistant Staphyloccocus aureus (MRSA) have been shown to be poor [23, 24]. Additionally, failure rate of a two-stage revision following a failed irrigation and debridement has been reported as high as 34% [25].
In patients who do not meet the above criteria, prosthesis resection is required.
This leaves the provider with three basic options: resection arthroplasty, single-stage revision, and two-stage revision. Resection arthroplasty is successful at removing the infection [26–28]; however, functional outcomes are improved with re-implantation of a prosthesis [29]. Below, we review the literature surrounding two-stage revision.
Two-Stage Revision
Two-stage revision is considered the gold standard of treatment. Insall et al. [30] first described the technique in 1983. Garvin and Hanssen [31] conducted a review of 25 studies in 1995 showing an 82% success rate of two-stage revision compared to 58% with one-stage revision. Numerous studies have reported that two-stage revision with use of antibiotic spacers can result in infection eradication rates as high as 95% [32–36], although a recent study by Ford et al. [37•] reported only a 72% success rate in 66 patients. In most circumstances, this involves resection of the prosthesis with or without placement of an antibiotic spacer, antibiotic treatment, following the patient’s response to treatment and re-implantation of a new prosthesis. The first-stage procedure involves thorough debridement of all lingering sources of infection within the joint cavity. This includes all components used in the index procedure, cement, and inflamed tissue within the joint. Antibiotic-impregnated cement is then used to create the spacer of choice to deliver local antibiotics and is used in conjunction with systemic antibiotics to target any isolated organisms.
It is important to think about the type of antibiotic used in a spacer. Desirable characteristics include its availability in powder form, wide antibacterical spectrum, elution from bone cement in high concentrations, thermal stability, and low influence on the mechanical properties of the cement. Typically, vancomycin and aminoglycosides are used as they exhibit these properties. It is recommended that at 3.6 g of antibiotic be present per 40 g batch of cement [32, 38].
It is also important to recognize that many patients may have a culture-negative PJI, where an organism cannot be isolated. This has been reported in up to 18% of cases [17]. It is imperative in these cases to work closely with an infectious disease specialist to determine an appropriate antibiotic plan.
Component-exchange has been widely reported on with or without a spacer construct [39], varying types of spacers [40–42], periods of immobilization and timing of re-implantation. The advocates of spacing devices argue that the soft-tissue envelope is better preserved at the time of re-implantation. Those in favor static spacers argue that joint immobilization complies better with the principle of resting the joint as part of treating the infection. Those that prefer an articulating spacer argue that movement is regained more reliably after re-implantation of the final components [43].
Types of Spacers in Total Knee Arthroplasty
Static Spacers
Common indications for use of a static spacers are in patients with severe uncontrolled infections, ligamentous laxity, extensor mechanism disruption or insufficiency, compromised soft tissue coverage over the joint, or severe bone loss after prosthesis explant [32]. Major advantages of static spacers include providing sufficient rest for very inflamed and congested tissues around the infected joint and significant cost reduction compared to articulating spacers [44]. Advocates of static spacers suggest an improved infection eradication rate due to soft tissue immobilization. Fehring et al. [40] compared 25 patients with static spacers versus 30 patients with articulating spacers. Three patients with static spacers and one patient with an articulating spacer became re-infected. Emerson et al. [45] observed similar results with 2 of 26 patients with static spacers becoming re-infected versus 2 of 22 patients with static spacers (7.7 vs 9.1%; p = 0.8). A systematic review by Voleti et al. [46••] reported on level III and IV studies totaling 1526 patients. They found no significant difference in re-infection rate (12% for static spacers and 8% for articulating spacers; p = 0.2).
However, there are several disadvantages to static spacers that have been suggested. Studies have reported poor limb mobility with static spacers following second-stage re-implantation compared to articulating spacers [33, 38, 44]. Unexpected bone loss attributable to spacer migration has also been reported. Fehring et al. [40] reported that 15 of 25 patients treated with a static spacer had additional bone loss in between procedures, while 0 of 30 patients treated with an articulating spacers had additional bone loss. The use of static spacers can also make exposure difficult during the second-stage procedure due to ligamentous and quadriceps shortening [40].
Articulating Spacers
The main advantage of using articulating spacers is that they allow motion of the affected joint in between procedures. This helps to facilitate recovery of limb function while the infection is being treated. Ambulation in between procedures helps maintain the length and elasticity of the extensor mechanism. This also serves to prevent soft tissue scarring around the affected joint, quadriceps shortening, and capsular stiffening and contracture [32, 44, 47]. This subsequently reduces the extent of surgical exposure required and reduces the overall difficulty of second-stage revision [32, 48, 49].
This maintained motion after first-stage procedure is thought to improve ultimate range of motion (ROM) of the joint following second-stage re-implantation. Emerson et al. [45] reported significantly better ROM after second-stage procedure with articulating spacers over static spacers (average ROM 107.8 vs 93.7 degrees). Voleti et al. [46••] reported similar findings in a systematic review, with significantly greater range of motion after re-implantation (101 vs 91°; p = 0.0002). However, when looking at functional scores, there was no difference between the two types of spacers.
Several different versions of articulating interfaces are available and have been described: cement-on-cement (Fig. 1), cement-on-polyethylene, and metal-on-polyethylene (Fig. 2).
Fig. 1.
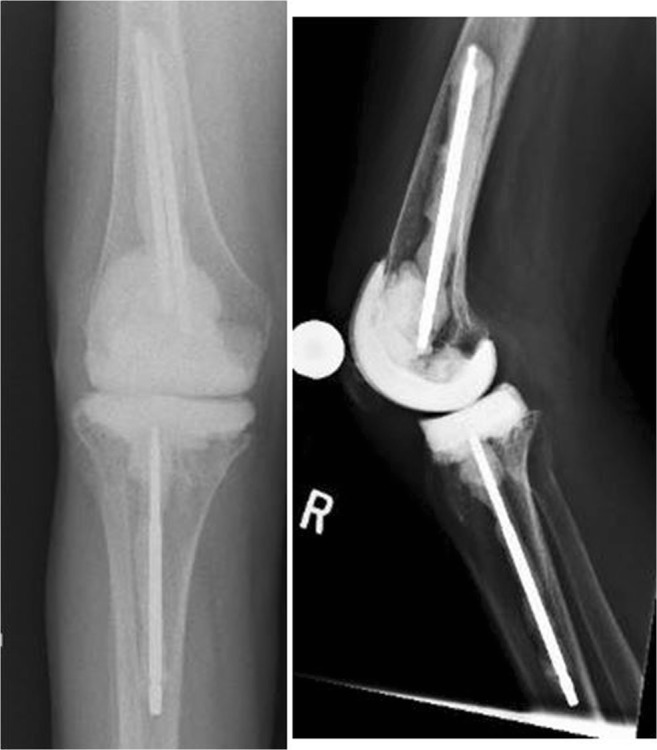
Anteroposterior and lateral radiographs of a cement-on-cement prefabricated articulating spacer (Zimmer Biomet, Warsaw, IN) with metal dowels coated with antibiotic impregnated cement and placed into the femoral and tibial canals
Fig. 2.
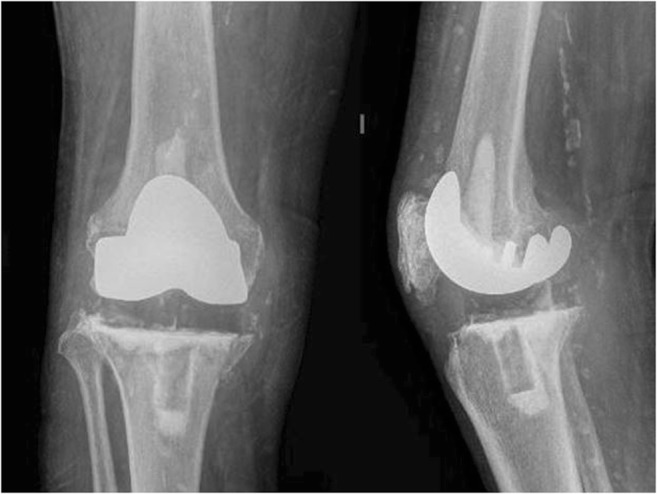
Anteroposterior and lateral radiographs of a metal-on-polyethylene articulating spacer. A metal femoral component is fixed onto the distal femur and a polyethylene component fixed onto the tibia, both with antibiotic impregnated cement
Cement-on-Cement
Mobile spacers with all-cement femoral and tibial components demonstrated ability to eradicate infections similar to that of static spacers [38]. These spacers are usually fabricated by the surgeon, although pre-fabricated models are available. Durbhakula et al. [41] reported on 24 patients with infected knee prostheses at an average follow-up of 33 months. The authors used custom molded femoral and tibial components impregnated with 2.4 g tobramycin and 1.0 g vancomycin. There were no cases of re-infection and ROM at final follow-up with 104°. Ha et al. [50] also reported no cases of re-infection when using articulating spacers formed with molds created from the re-sterilized removed components. ROM at final follow-up was 100°.
There are commercial pre-fabricated spacers available in the USA. The InterSpace Knee temporary knee spacer (Exactech, Gainesville, FL) is available in three sizes with gentamycin doses ranging from 0.8 g to 1.7 g, which is significantly lower than the suggested dose of 3.6 g antibiotic per 40 g of bone cement [32, 38]. The Spacer-K (Tecres, Sommacampagna, Italy) is also available. This spacer comes in four sizes ranging from 60 to 90 mm tibial plateau dimensions. Pitto et al. [51] reported no re-infections in 21 patients when using this device.
Cement-on-Polyethylene
In a study of 28 patients, Evans [52] impregnated each 40 g batch of Palacos R cement (Zimmer, Warsaw, IN) with 4.8 g tobramycin and 4.0 g vancomycin. An all-cement femoral component was either handmade on the back table or was made using a disposable mold. Following placement of the femoral component, a stemmed, all-polyethylene posterior-stabilized tibial component was coated with antibiotic cement and implanted. Two patients became reinfected with the same organism (7%) and five developed new infections (18%). To the author’s knowledge, this is the only report on this type of spacer, and more investigation is needed to determine its efficacy.
Metal-on-Polyethylene
Metal-on-polyethylene interface used in articulating spacers was first described in 1995 by Hoffman et al. [53]. This technique involved sterilization and re-implantation of the original femoral component, fixed with antibiotic cement impregnated with 4.8 g tobramycin per 40 g batch of Simplex P (Stryker, Mahwah, NJ) cement. A new polyethylene component was then cemented onto the tibia. Zero of the 26 patients became re-infected. Average ROM was 106° at a mean follow-up of 30 months. Other authors have reported similar results after re-implanting a sterilized femoral component. PIetsch et al. [54] used a similar technique with 1 g clindamycin and 2 g vancomycin per 40 g Palacos R cement. They reported that 3 of 33 (9%) patients became re-infected. Cuckler [55] reported that 1 of 44 patients became re-infected after using metal-on-polyethylene articulating spacers. Jamsen et al. [56] compared spacers using the Hofmann technique with hand-molded, all-cement articulating spacers. Second-stage procedures were significantly shorter (average 185 vs 247 min; p = 0.008) and had significantly less blood loss (median 425 vs 1500 mL; p = 0.008). A higher proportion of patients treated with the Hofmann method had good or excellent Knee Society Scores. Another technique involves using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) (Depuy, Warsaw, IN). This system includes a stainless steel femoral component that articulates with a posterior-stabilized polyethylene tibial component. Haddad et al. [57] found no evidence of infection in 41 of 44 (91%) patients using this device.
Types of Spacers in Total Hip Arthroplasty
Static Spacers
Technically speaking, there is no true static spacer that prevents motion at the hip. Static spacers often involve placing a large ball or beads of antibiotic cement into the acetabulum and either beads or antibiotic cement-covered rod into the femoral canal [58, 59]. Patients are often allowed to partial weight bear and conduct range of motion exercises with this type of construct. Hsieh et al. [58] reported on 128 patients and compared the use of antibiotic-loaded cement beads with a custom-molded mobile spacer. They found similar rates of infection control; however, the use of a mobile spacer was associated with shorter surgical time and hospital stay for first-stage procedures and reduced blood loss and transfusion requirements during second-stage re-implantation. They also found improved interim hip function when using a mobile spacer, with 49 of 56 patients (88%) able to walk compared to 12 of 63 (19%) in the cement bead group.
Mobile Spacers
Mobile hip spacers are those that create an articulation between the femur and the acetabulum, as opposed to the disconnected nature of static spacers. Handmade, custom-molded, and pre-fabricated spacers have been described [52, 60–66]. This type of construct should not be used in patients with insufficient acetabular bone stock or pelvic discontinuity. Jacobs et al. [38] reported rates of complications after compiling studies of static and mobile spacers. They reported a similar rate of recurrent infection between static (4%) and mobile (6%) spacers. One complication exclusive to mobile spacers is dislocation, with a rate of 7%, while there were no dislocations when using static spacers given their inherent design. There were also no fractures of static spacers, while 2% of mobile spacers suffered a fracture.
Handmade
Significant advantages of handmade spacers are that the surgeon can conform the spacer for each patient and they are relatively low cost. Spacers are shaped like a hemiarthroplasty and can be reinforced with Kirschner wires or rush pins interiorly. This functions to increase fracture resistance and helps with ease of removal during second-stage procedures [63, 67]. This technique places hones upon the surgeon to create a well-balanced construct that will be resistant to failure. Certain aspects of spacer design that can lead to dislocation include a small head-neck ratio and insufficient depth of seating into the acetabulum. Creating spacers that are 2–3 mm smaller than the size of the acetabulum will allow for proper seating and can help reduce dislocation rates [38].
Custom-Molded/Prefabricated
Custom-molded and prefabricated spacers are similar in that they are created to resemble a hemiarthroplasty; however, they contain a metal endoskeleton [60, 62, 65, 66, 68]. These spacer constructs result in more consistent spacer designs and are not as reliant on the surgeon to create the geometry of the spacer. The disadvantage of these spacers is that they are only available in limited sizes, which if mismatched can increase the risk of dislocation [60]. A significant disadvantage of some prefabricated spacers is the relatively low doses of incorporated antibiotics. Magnan et al. [66] reported that 2 of 10 hips treated with the Spacer-G (Exactech) prefabricated spacer were not re-implanted due to unacceptable inflammatory marker levels. Bertazzoni Minelli et al. [61] described drilling holes into prefabricated spacers and placing vancomycin-impregnated cement into these voids in order to increase the dose of antibiotics within the construct. Despite this, 3 of 20 of their patients were not re-implanted due to persistent infection. Of the patients who were re-implanted in these studies, none of them became re-infected.
Metal-on-Polyethylene
Several techniques for constructing mobile spacers with metal-on-polyethylene articulations have been described. Hofman et al. [69] described a technique in which the original femoral stem was sterilized and re-implanted with antibiotic cement. Evans [52] described a similar technique; however, a new femoral implant was used. Evans reported dislocations in four patients who were implanted with a non-constrained liner, while 0 of 19 patients treated with a constrained liner sustained a dislocation. The PROSTALAC system (Fig. 3) features a constrained polyethylene liner with a cemented femoral component [70]. Wentworth et al. [71] reported a success rate of 82.6% in 135 patients treated with the PROSTALAC device, with only 5 reported dislocations or subluxations after first-stage surgery. The author’s preference is to use a constrained liner when implanting a metal-on-polyethylene hip spacer.
Fig. 3.
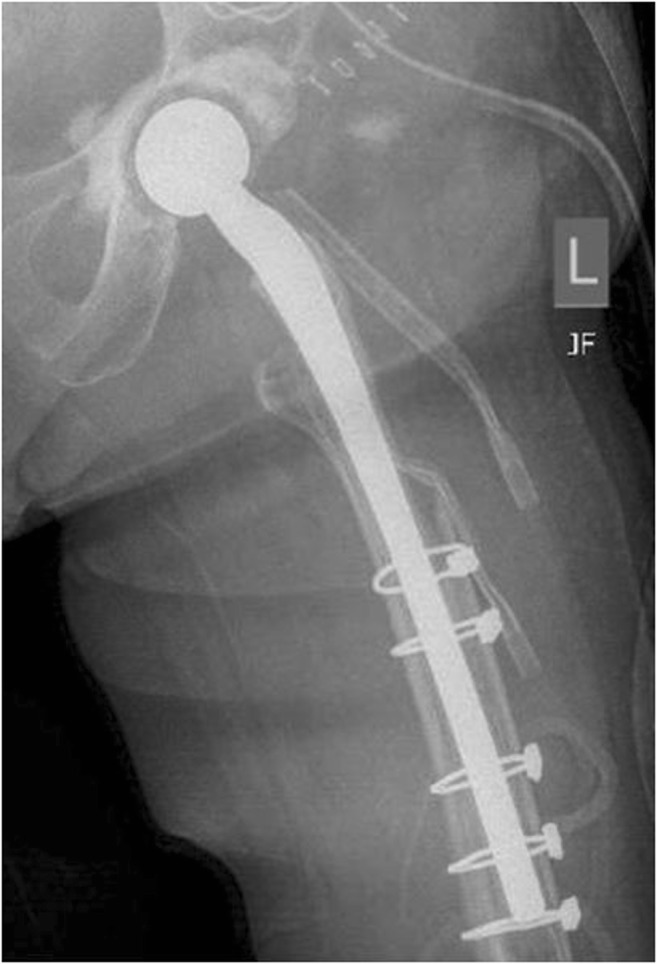
Anteroposterior radiograph of a PROSTALAC (Depuy-Synthes, Warsaw, IN) hip antibiotic spacer. A constrained polyethylene acetabular cup is fixed to the acetabulum with antibiotic impregnated cement and a prefabricated metal femoral component coated with antibiotic cement is fixed into the femur
Interim Management and Second-Stage Re-implantation
Once first-stage explant and placement of the appropriate antibiotic spacer is complete, the patient must be managed with appropriate antibiotics and their clinical course followed in order to properly time second-stage re-implantation. The surgeon must be confident that the infection has been eradicated before considering implanting a new definitive device. Patients are typically followed with serial inflammatory markers, joint fluid analysis, and/or frozen section analysis during second-stage operations.
Systemic Antibiotics
During first-stage explant of the infected prosthesis, several culture samples should be taken in order to isolate a causative organism. Following identification of an organism or organisms in addition to analyzing for antibiotic resistance, systemic antibiotics are given for a period of time to target the causative bacteria. Antibiotics are often given for a period of 6 weeks in between procedures, a protocol first described by Insall et al. [30]. After completion of antibiotic therapy, patients are often given a 2-week “antibiotic holiday” and followed clinically to detect any signs of continued infection [72]. There is, however, no consensus on the proper length of antibiotic therapy or holiday. Some authors question the prolonged use of systemic antibiotics given that the blood supply to periarticular tissue may be attenuated in the setting of a revision surgery and long courses of antibiotics increase the likelihood of systemic toxicity and antibiotic resistance [73, 74].
Hoad-Reddick et al. [75] reported on 38 patients treated with two-stage revision and were given only two doses of systemic antibiotics postoperatively. They used a cement-on-cement articulating antibiotic spacer in addition to antibiotic beads to deliver high local concentrations of antibiotics. At mean follow-up of 56.4 months, the authors reported a success rate of 89%. Stockley et al. [76] gave postoperative systemic antibiotics for just 24 h after first-stage explant in 114 patients and reported infection eradication in 100 patients (87.7%) at 24 months. Hart and Jones [43] treated 48 patients with a 2-week course of systemic antibiotics. Infection was successfully eradicated in 42 patients (88%). When they did subgroup analysis, 96% of patients who were undergoing two-stage revision for a first-time infection were successful, while the success rate was 78% for patients with multiply operated knees. Whittaker [77] also published a series using a 2-week course of antibiotics with a mobile hip spacer and reported successful infection control in 38 of 41 (92.7%) infected hip prostheses. Hsieh et al. [78] compared a 6-week with a 1-week regimen of antibiotic therapy in chronically infected hips. Infection control success rates were similar for both groups as well as hip scores at final follow-up. The prolonged regimen had a higher rate of patients who develop nephrotoxicity compared to the shorter course as well as higher hospital cost and longer hospital stay.
Laboratory Testing and Joint Fluid Analysis
While patients are undergoing systemic antibiotic therapy, they are followed with serial ESR and CRP levels and are monitored for any clinical signs of infection. Declining levels of ESR and CRP and the absence of clinical signs of infection are often a good indication that second-stage re-implantation is safe. However, definitive cutoff numbers for serologic testing and joint fluid analysis have not been established. Ghanem et al. [79] and Kusuma et al. [80] attempted to use receiver operating characteristic (ROC) curves to determine discriminatory values of ESR and CRP in predicting persistent infection. In patients with recurrent infection, mean ESR and CRP levels were similar to those of patients whose infection was successfully treated. Neither study could determine an accurate cut-off level for either serologic measure. Kusuma et al. [80] did report a significant decline in ESR, CRP, and synovial fluid WBC count and differential between stages, regardless of infection eradication or persistence. Shukla et al. [81] also reported a significant decline in ESR, CRP, and synovial fluid WBC counts between stages regardless of infection status, with an ROC cut-off value of 3528 WBC/μL (78% sensitivity and 96% specificity). This value is similar to a commonly accepted value for the diagnosis of chronic PJI [8•].
There is conflicting evidence for arthrocentesis with microbial culture before second-stage surgery. Lonner et al. [82] investigated the role of knee aspiration for re-implantation in 34 patients. They found that knee aspiration had a sensitivity and positive predictive value (PPV) of zero, a negative predictive value (NPV) of 75%, and specificity of 92%. In contrast, Mont et al. [83] reported a sensitivity of 75%, specificity of 100%, a PPV of 100%, and NPV of 97%.
Frozen-section analysis can be performed during second-stage procedures to provide additional information regarding the status of the joint to the surgeon; however, the optimal number of frozen sections and clear cut-off values for number of cells per high-powered fields have yet to be determined. Della Valle et al. [84] used a cut-off of ≥ 10 polymorphonuclear leukocytes (PMNs) per high-powered field in the five most cellular fields as a positive result for persistent infection and found a sensitivity of 25%, specificity of 98%, PPV of 50%, and NPV of 95%. Bori et al. [85] considered a positive value to be ≥ 5 PMNs per higher-powered field in the five most cellular fields and the presence of at least one PMN in 10 cellular fields. They reported a sensitivity of 28.5%, specificity of 100%, PPV of 100%, and NPV of 73.6%. The low sensitivity of these criterion makes it difficult for the surgeon to be confident in ruling out infection in the situation of a negative result.
Summary
Two-stage revision arthroplasty for chronic PJI has been used successfully and is now the gold standard treatment for eradication of infection. The treatment regimen includes a first-stage operation in which all implants, nonviable, and inflamed tissue are removed, cultures are taken and an antibiotic-impregnated cement spacer is placed. The surgeon has many choices of spacer at their disposal including both static and mobile options. Patients are then treated with systemic antibiotics targeted at the isolated organism. The authors’ preference is to use a mobile spacer when the patient’s bone stock and soft tissues are amenable. In terms of cement, the author’s preference is to use PMMA that contains 1 g of Tobramycin (Simplex P, Stryker) and to that 3 g of Vancomycin are added and hand mixed. Classically, a 6-week regimen of systemic antibiotics has been used [30], and this is the author’s preferential length of treatment; however, controversy exists in the literature. There is a paucity of convincing studies to guide an exact cut-off value for ESR, CRP, or synovial WBC count to guide timing of second-stage re-implantation. The author’s preferred method is to obtain serial values of ESR and CRP in between stages, including values 2–4 weeks after the completion of antibiotics. As long as there is a down-trend in values and there are no clinical signs of infection, the patients are then considered for re-implantation. During second-stage surgery, frozen-section analysis is obtained. If frozen section analysis is positive (based on the surgeon’s preferred criteria), then the first-stage surgery is repeated and a new spacer placed, followed by a repeat course of systemic antibiotics in attempt to clear the infection.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Prosthetic Joint Infection
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Martinez-Cano J, Herrera-Escobar J, Arango Gutierrez A, Sanchez Vergel A, Martinez-Rondanelli A. Prospective quality of life assessment after hip and knee arthroplasty: short and mid-term follow up results. Arthroplasty Today. 2016;15:125–130. doi: 10.1016/j.artd.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;4:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Zhan C, Kaczmarek R, Loyo-Berrio N, Sangl J, Bright R. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;3:526–533. doi: 10.2106/JBJS.F.00952. [DOI] [PubMed] [Google Scholar]
- 4.Bozic K, Katz P, Cisternas M, Ono L, Ries M, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. 2005, 3: 570–576. [DOI] [PubMed]
- 5.Salvati E, Gonzalez Della Valle a, Masri B, Duncan C. The infected total hip arthroplasty. Instr Course Lect. 2003;52:223–245. [PubMed] [Google Scholar]
- 6.Spangehl M, Masri B, O'Connell J, Duncan C. Prospective analysis of preoperative and intraoperative. investigations for the diagnosis of infection at the sites of two hundred and. two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81(5):672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Springer B, Cahue S, Etkin C, Lewallen D, McGrory B. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplasty Today. 2017;3(2):137–140. doi: 10.1016/j.artd.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvizi J, Gehrke T. Definition of Periprosthetic joint infection. J Arthroplast. 2014;29:1331. doi: 10.1016/j.arth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Parvizi J, Zmistowski B, Berbari E, Bauer T, Springer B, Della Valle C, Garvin K, Mont M, Wongworawat M, Zalavras C. New definition for Periprosthetic joint infection. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedair H, Ting N, Jacovides C, Saxena a, Moric M, Parvizi J, Della Valle C. The mark Coventry award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34–40. doi: 10.1007/s11999-010-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trampuz A, Hanssen A, Osmon D, Mandrekar J, Steckelberg J, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Medicine. 2004;117(8):556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Schinsky M, Della Valle C, Sporer S, Paprosky W. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90(9):1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 13.Della Valle C, Sporer S, Jacobs J, Berger R, Rosengerg A, Paprosky W. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplast. 2007;22(6 suppl 2):90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Mason J, Fehring T, Odum S, Griffin W, Nussman D. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplast. 2003;18(8):1038–1043. doi: 10.1016/S0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 15.Ghanem E, Parvizi J, Burnett R, Sharkey P, Keshavarzi N, Aggarwal A. Et. A: cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90(8):1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 16.Higuera C, Zmistowski B, Malcom T, Barsoum W, Sporer S, Mommsen P, Kendoff D, Della Valle C, Parvizi J. Synovial fluid cell count for diagnosis of chronic Periprosthetic hip infection. J Bone Joint Surg. 2017;99:753–759. doi: 10.2106/JBJS.16.00123. [DOI] [PubMed] [Google Scholar]
- 17.Parvizi J, Adeli B, Zmistowki B, Restrepo C, Greenwald A. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104. doi: 10.2106/JBJS.K.01417. [DOI] [PubMed] [Google Scholar]
- 18.Segreti J, Nelson J, Trenholme G. Prolonged suppressive antibiotic therapy for infected orthopedic prostheses. Clin Infect Dis. 1998;27(4):711–713. doi: 10.1086/514951. [DOI] [PubMed] [Google Scholar]
- 19.Goulet J, Pellicci P, Brause B, Salvati E. Prolonged suppression of infection in total hip arthroplasty. J Arthroplast. 1988;3(2):109–116. doi: 10.1016/S0883-5403(88)80075-5. [DOI] [PubMed] [Google Scholar]
- 20.Rao N, Crosset L, Sinha R, Le Frock J. Long-term suppression of infection in total joint arthroplasty. Clin Orthop Relat Res. 2003;414:55–60. doi: 10.1097/01.blo.0000087321.60612.cf. [DOI] [PubMed] [Google Scholar]
- 21.Tsukayama D, Wicklund B, Gustilo R. Suppressive antibiotic therapy in chronic prosthetic joint infections. Orthopedics. 1991;14(8):841–844. doi: 10.3928/0147-7447-19910801-07. [DOI] [PubMed] [Google Scholar]
- 22.Odum S, Fehring T, Lombardi A, Zmistowski B, Brown N, Luna J, Fehring K, Hansen E. Periprosthetic infection consortium. Irrigation and debridement for periprosthetic infections: does the organism matter? J Arthroplast. 2011;26(6 suppl):114–118. doi: 10.1016/j.arth.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury T, Fehring T, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum S. The fate of acute methicillin-resistant staphyloccocus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplast. 2009;24(6 suppl):103–107. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Pawasarat I, Azzam K, Joshi A, Hansen E, Bozic K. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467(7):1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherrell J, Fehring T, Odum S, Hansen E, Zmistowki B, Dennos A, et al. The Chitranjan Ranawat award: fate of two-stage reimplantation after failed irrigation and debridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469(1):18–25. doi: 10.1007/s11999-010-1434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canner G, Steinberg M, Heppenstall R, Balderston R. The infected hip after total hip arthroplasty. J Bone Joint Surg Am. 1984;66(9):1393–1399. doi: 10.2106/00004623-198466090-00012. [DOI] [PubMed] [Google Scholar]
- 27.Castellanos J, Flores X, Llusa M, Chiriboga C, Navarro A. The Girdleston pseudarthrosis in the treatment of infected hip replacements. Int Orthop. 1998;22(3):178–181. doi: 10.1007/s002640050236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grauer J, Amstutz H, O'Carroll P, Dorey F. Resection arthroplasty of the hip. J Bone Joint Surg Am. 1989;71(5):669–678. doi: 10.2106/00004623-198971050-00005. [DOI] [PubMed] [Google Scholar]
- 29.Schroder J, Saris D, Besselaar P, Marti R. Comparison of he results of the Girdlestone pseudarthrosis with reimplantation of a total hip replacement. Int Orthop. 1998;22(4):215–218. doi: 10.1007/s002640050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Insall J, Thompson F, Brause B. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. doi: 10.2106/00004623-198365080-00008. [DOI] [PubMed] [Google Scholar]
- 31.Garvin K, Hanssen A. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am. 1995;77:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Han J, Zhang C, Yang Y, Yao Z. Infection after total knee arthroplasty and its gold standard surgical treatment: spacers used in two-stage revision arthroplasty. Intractable Rare Dis Res. 2017;6(4):256–261. doi: 10.5582/irdr.2017.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalore N, Gioe T, Sing J. Diagnosis and management of infected total knee arthroplasty. Open Orthop J. 2011;5:86–91. doi: 10.2174/1874325001105010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Pastor J, Macule-Beneyto F, Suso-Vergara S. Acute infection in total knee arthroplasty: diagnosis and treatment. Open Orthop J. 2013;7:197–204. doi: 10.2174/1874325001307010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun K, Kim K, Chun C. Infection following total knee arthropalsty. Knee Surg Relat Res. 2013;25:93–99. doi: 10.5792/ksrr.2013.25.3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Carvalho L, Tempon E, Badet R. Infection after total knee replacement: diagnosis and treatment. Rev Bras Orthop. 2013;24:389–396. doi: 10.1016/j.rbo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.• Ford A, Holzmeister A, Rees H, Pelich B. Characterization of outcomes of 2-stage exchange arthroplasty in the treatment of prosthetic joint infections. J Arthroplasty. 2018; 10.1016/j.arth.002018.000002.000043. This is a recent review of 80 patients undergoing resection arthroplasty with placement of an antibiotic spacer for PJI at a single institution. They report lower rates of success than previously reported in the literature, including only 66 of the patients undergoing second-stage re-implantation, and 28% of these patients having persistent infection. Additionally, 30% of patients had a serious complication during their treatment course.
- 38.Jacobs C, Christian C, Berend M. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356–358. doi: 10.5435/00124635-200906000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Jhao C, Jiang C. Two-stage reimplantation without cement spacer for septic total joint replacement. J Formos Med Assoc. 2003;102:37–41. [PubMed] [Google Scholar]
- 40.Fehring T, Odum S, Calton T, Mason J. Articulating versus static spacers in revision total knee arthroplasty for sepsis. Clin Orthop. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Durbhakula S, Czajka J, Fuchs M, Uhl R. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768–774. doi: 10.1016/j.arth.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 42.Meek RM, Masri B, Dunlop D. et a: Patient satisfaction and functional status after treatment of infection at teh site of a total knee arthroplasty with the use of the PROSTALAC articulating spacer. J Bone Joint Surg Am. 2003;85A:1888–1892. doi: 10.2106/00004623-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Hart W, Jones R. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. Bone Joint J. 2006;88:1011–1015. doi: 10.2106/JBJS.D.02090. [DOI] [PubMed] [Google Scholar]
- 44.Mazzucchelli L, Rosso F. Marmotti A, bonasia D, Bruzzone M, Rossi R: The use of spacers (static and mobile) in infection knee arthroplasty. Curr Rev. Musculoskelt Med. 2015;8:373–382. doi: 10.1007/s12178-015-9293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emerson R, Muncie M, Tarbox T, Higgins L. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Voleti P, Baldwin K, Lee G. Use of static or articulating spacers for infection following knee arthroplasty: a systematic literature review. J Bone Joint Surg Am. 2013;95(17):1594–1599. doi: 10.2106/JBJS.L.01461. [DOI] [PubMed] [Google Scholar]
- 47.Thabe H, Schill S. Two-stage reimplantation with an application spacer and combined with delivery of antibiotics in the management of prosthetic joint infection. Oper Orthop Traumatol. 2007;19:78–100. doi: 10.1007/s00064-007-1196-4. [DOI] [PubMed] [Google Scholar]
- 48.Hsu Y, Cheng H, Ng T, Chiu K. Antibiotic-loaded cement articulating spacer the 2-stage reimplantation in infected total knee arthropalsty: A simple and economic method. J Arthroplasty. 2007;22:1060–1066. doi: 10.1016/j.arth.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 49.Choi H, Malchau H, Bedar H. Are prosthetic spacers safe to use in 2-stage treatment for infected total knee arthroplasty? J Arthroplasty. 2012;27:1474–1479. doi: 10.1016/j.arth.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Ha C. A technique for intraoperative construction of antibiotic spacers. Clin Orthop Relat Res. 2006;445:204–209. doi: 10.1097/01.blo.0000201161.52196.c5. [DOI] [PubMed] [Google Scholar]
- 51.Pitto R, Castelli C, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;30:257–261. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans R. Successful treatment of total hip and knee infection with articulating antibiotic components: A modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann A, Kane K, Tkach T, Plaster R, Camargo M. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 54.Pietsch M, Hofmann S, Wenisch C. Treatment of deep infection of total knee arthroplasty using a two-stage procedure. Oper Orthop Traumatol. 2006;18:66–87. doi: 10.1007/s00064-006-1163-5. [DOI] [PubMed] [Google Scholar]
- 55.Cuckler J. The infected total knee: Management options. J Arthroplasty. 2005;20(4 suppl 2):33–36. doi: 10.1016/j.arth.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Jamsen E, Sheng P, Halonen P. et a: Spacer prostheses in two-stage revision of infected knee arthroplasty. Int Orthop. 2006;30:257–261. doi: 10.1007/s00264-006-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haddad F, Masri B, Campbell D, McGraw R, Beauchamp C, Duncan C. The PROSTALAC functional spacer in two-stage revision for infected knee replacements: Prosthesis of antibiotic loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh P, Shih C, Chang Y, Lee M, Shih H, Yang W. Two-stage revision hip arthroplasty for infection: Comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am. 2004;1989–1997. [PubMed]
- 59.Haddad F, Muirhead-Allwood S, Manktelow A, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82:689–694. doi: 10.1302/0301-620X.82B5.9668. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto K, MMiyagawa N, Masaoka T, Katori Y, Shishido T, Imakiire A. Clinical effectiveness of antibiotic-impregnated cement spacers for the treatment of infected implants of the hip. J Orthop Sci. 2003;8:823–828. doi: 10.1007/s00776-003-0722-y. [DOI] [PubMed] [Google Scholar]
- 61.Bertazzoni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53:329–334. doi: 10.1093/jac/dkh032. [DOI] [PubMed] [Google Scholar]
- 62.Durbhakula S, Czaijka J, Fuchs M, Uhl R. Spacer endoprosthesis for the treatment of infected total hip arthropalsty. J Arthroplasty. 2004;19:760–774. doi: 10.1016/j.arth.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 63.Barrack R. Rush pin technique for temporary antibiotic-impregnated cement prosthesis for infected total hip arthropalsty. J Arthroplasty. 2002;17:600–603. doi: 10.1054/arth.2002.32698. [DOI] [PubMed] [Google Scholar]
- 64.Koo K, Yang J, Cho S. et a: Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–892. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
- 65.Shoellner C, Fuerderer S, Rompe J, Eckardt A. Individual bone cement spacers (IBCS) for septic hip revision. Arch Orthop Trauma Surg. 2003;123:254–259. doi: 10.1007/s00402-003-0502-3. [DOI] [PubMed] [Google Scholar]
- 66.Magnan B, Regis D, Biscaglia R, Bartolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics: Use of two-stage procedure in 10 patients because of infected hips after total replacement. Acta Orthop Scand. 2001;72:591–594. doi: 10.1080/000164701317269003. [DOI] [PubMed] [Google Scholar]
- 67.Takahira N, Itoman M, HIgashi K, Uchiyama K, Miyabe M, Naruse K. Treatment outcome of two-stage revision total hip arthroplasty using antibiotic-impregnated cement spacer. J Orthop Sci. 2003;8:26–31. doi: 10.1007/s007760300004. [DOI] [PubMed] [Google Scholar]
- 68.Pearle A, Sculco T. Technique for fabrication of an antibiotic-loaded cement hiarthroplasty (ANTILOCH) prosthesis for infected total hip arthroplasty. Am J Orthop. 2002;31:425–427. [PubMed] [Google Scholar]
- 69.Hofmann A, Goldberg T, Tanner A, Cook T. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874–879. doi: 10.1016/j.arth.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 70.Haddad F, Masri B, Garbuz D, Duncan C. The treatment of the infected hip replacement: The complex case. Clin Orthop Relat Res. 1999;369:144–156. doi: 10.1097/00003086-199912000-00015. [DOI] [PubMed] [Google Scholar]
- 71.Wentworth S, Masri B, Duncan C, Southworth C. Hip prosthesis of antibiotic-loaded acrylic cement for the treatment of infections following total hip arthroplasty. J Bone Joint Surg Am. 2002;84(suppl 2):123–128. doi: 10.2106/00004623-200200002-00017. [DOI] [PubMed] [Google Scholar]
- 72.Kuzyk P, Dhotar H, Sternheim A, Gross A, Safir O, Backstein D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: Techniques, Controversies and Outcomes. J Am Acad Orthop Surg. 2014;22:153–164. doi: 10.5435/JAAOS-22-03-153. [DOI] [PubMed] [Google Scholar]
- 73.Masri B, Panagiotopoulos K, Greidanus N, Garbuz D, Duncan C. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22(1):72–78. doi: 10.1016/j.arth.2006.02.156. [DOI] [PubMed] [Google Scholar]
- 74.Smilack J, Flittie W, Williams TJ. Bone concentrations of antimicrobial agents after parenteral administration. Antimicrob Agents Chemother. 1976;9(1):169–171. doi: 10.1128/AAC.9.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoad-Reddick D, Evans C, Norman P, Stockley I. Is there a role for extended antibiotic therapy in a two-stage revision of the infected knee arthroplasty? J Bone Joint Surg Br. 2005;87(2):171–174. doi: 10.1302/0301-620X.87B2.15640. [DOI] [PubMed] [Google Scholar]
- 76.Stockley I, Mockford B, Hoad-Reddick A, Norman P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J Bone Joint Surg Br. 2008;90(2):145–148. doi: 10.1302/0301-620X.90B2.19855. [DOI] [PubMed] [Google Scholar]
- 77.Whittaker J, Warren R, Jones R, Gregson P. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chornic gram-positive infection? J Bone Joint Surg Br. 2009;91(1):44–51. doi: 10.1302/0301-620X.91B1.20930. [DOI] [PubMed] [Google Scholar]
- 78.Hsieh P, Huang K, Lee P, Lee M. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: Retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemoth. 2009;64(2):392–397. doi: 10.1093/jac/dkp177. [DOI] [PubMed] [Google Scholar]
- 79.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: What is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;469(4):1002–1008. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kusuma S, Ward J, Jacofsky M, Sporer S, Della Valle C. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002–1008. doi: 10.1007/s11999-010-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shukla S, Ward J, Jacofsky M, Sporer S, Paprosky W, Della Valle C. Perioperative testing for persistent sepsis following resectino arthroplasty of the hip for periprosthetic infection. J Arthroplasty. 2010;25(6 suppl):87–91. doi: 10.1016/j.arth.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Lonner J, Siliski J, Della Valle C, DiCesare P, Lotke P. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305–309. [PubMed] [Google Scholar]
- 83.Mont M, Waldman B, Hungerford D. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection: A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552–1557. doi: 10.2106/00004623-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Della Valle C, Bogner E, Desai P, et al. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81(5):684–689. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Bori G, Soriano A, Garcia S, Mallofre C, J R, Mensa J. Usefulness of histological analysis for predicting the presence of microorganisms at the time of reimplantation after hip resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 2007;89(6):1232–1237. doi: 10.2106/00004623-200706000-00011. [DOI] [PubMed] [Google Scholar]


