Abstract
Purpose of Review
Periprosthetic joint infection (PJI) is a devastating complication after total joint replacement. A main source for antibiotic tolerance and treatment failure is bacterial production of biofilm—a resilient barrier against antibiotics, immune system, and mechanical debridement. The purpose of this review is to explore some novel approaches to treat PJI and biofilm-related infections.
Recent Findings
Innovative treatment strategies of bacterial and biofilm infections revolve around (a) augmenting current therapies, such as improving the delivery and efficiency of conventional antibiotics and enhancing the efficacy of antiseptics and (b) administrating completely new therapeutic modalities, such as using immunotherapy, nanoparticles, lytic bacteriophages, photodynamic therapy, novel antibiotics, and antimicrobial peptides.
Summary
Several promising treatment strategies for PJI are available to be tested further. The next requirement for most of the novel treatments is reproducing their effects in clinically representative animal models of PJI against clinical isolates of relevant bacteria.
Keywords: Periprosthetic joint infection, Nanoparticles, Monoclonal antibodies, Antimicrobial peptides, Antibiotic conjugation, Phage therapy
Introduction
Total joint replacement (TJR) of the hip and knee is a common orthopedic procedure that provides significant improvements in patients’ quality of life [1, 2]. Unfortunately, 1% of primary TJR cases develop periprosthetic joint infection (PJI), a devastating complication that requires morbid revision surgery and is associated with high patient dissatisfaction [3, 4]. Although the incidence of PJI is lower compared to other types of surgery, the large volume of TJR procedures performed across industrialized countries have led to the identification of orthopedics as the most common source of surgical-related infections among hospital inpatients [5]. Given that the prevalence of PJI is expected to rise over the next decade [6], and that treating a single case of PJI is over five times that of a primary joint replacement [7], there is tremendous interest from both researchers and health administrators to improve PJI prevention and treatment.
A central aspect to the pathogenesis of PJI involves the adhesion of invading bacteria to the surface of prosthetic implants followed by the formation of “biofilm,” an extracellular polysaccharide glycocalyx which protects the dividing bacteria from the immune system, antibiotics, and even mechanical debridement [8]. Despite recent improvements in understanding how bacteria alter their metabolic processes and communicate while in biofilm, clinical success in eradicating PJI through revision surgery remains poor [9], even in the early postoperative period [10]. Therefore, the purpose of this review is to contrast the limitations of contemporary methods for removing PJI biofilms with novel, innovative strategies that will hopefully become integrated with clinical care.
Why Do Contemporary Treatments Fail?
A commonly cited reason for failure of contemporary PJI treatment is the inability to correctly identify the offending pathogen. Clinical PJI is currently hypothesized to occur either due to perioperative contamination or hematogenous infiltration of the articular space, most commonly by Gram-positive bacteria found on the patient’s skin [11]. Correctly identifying the offending organism through synovial fluid or tissue culture guides selection of both systemic and local antibiotics and has been shown to be an important prognosticator of treatment success [12]. Yet while previous literature focused on managing culture-negative infections, such presentations are rare [13]. Instead, recent studies suggest that standard culture techniques fail to detect all bacteria present within the infected joint space, leading to inadequate antimicrobial therapy [14]. Swearingen and colleagues [15••] utilized 16S ribosomal RNA gene sequencing on components as well as suture material retrieved from three clinical PJI cases. Compared to standard culture, 16S rRNA methods identified at least three distinct bacterial species in each case compared to one alone found in clinical culture. Furthermore, Huang [16] found that culture of sonicated joint fluid and broad-range polymerase chain reaction (PCR) analysis of joint fluid were significantly more sensitive for diagnosing PJI compared to standard tissue culture, with sonicated fluid culture being the best for identifying polymicrobial infections and fungal infections. The improved sensitivity of sonicated joint fluid culture and culture of fluid from sonicated implants has been reported in several other recent publications [17–19], strengthening a growing argument that sonication should become a standard of care for effectively diagnosing PJI [20]. In addition to inadequate diagnostic methods, a lack in evolution of PJI treatment methods is another reason that treatment failures remain common.
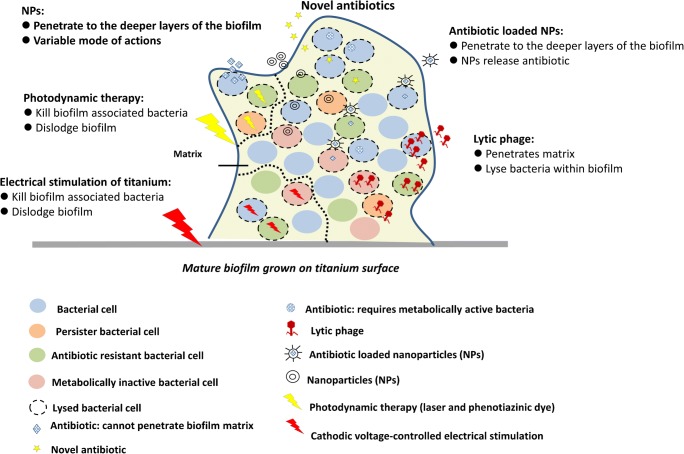
The core approach to the treatment of PJI has not changed in several decades [21], and recent literature confirms that contemporary methods failing to adequately eradicate bacteria from the periprosthetic environment. The “gold standard” treatment for chronic PJI involves removing infected implants and inserting a polymethylmethacrylate cement spacer that elutes high concentrations of antibiotics. Unfortunately, these antibiotics are only eluted in pertinent concentrations for 24–48 h after insertion [22, 23] and have recently been associated with postoperative renal injury [24, 25]. Additionally, bacterial biofilm has been detected on the surface of antibiotic cement spacers at the time of revision surgery [26–28]. Furthermore, since these cement spacers are temporary and purposefully inserted with less structural stability to facilitate later removal, they have been associated with high rates of complications, including joint dislocation, spacer fracture, and fracture of the surrounding bone [29]. The combination of invasive surgery, side effects from antibiotics, and complications from spacer implants culminate in high perioperative morbidity rates with two-stage revision treatments, with rates being reported as high as 15% in a recent series of patients treated in a high volume center [4]. Treatment of early PJI with irrigation and debridement, while less morbid, has been associated with poor cure rates of just over 50% [10, 30]. Such low cure rates are believed to occur in part due to surgeon variability in how surgical debridement is performed, but also due to current topical agents being ineffective in removing biofilm from metal and plastic implant surfaces [30]. Currently, administration of dilute betadine [31], chlorhexidine [32], and vancomycin powder [33] has all been separately advocated to improve irrigation and debridement treatment outcomes, but these effects have only been observed in retrospective case series. The limited quality of evidence and high degree of both morbidity and mortality obviate the need for novel methods of preventing and eradicating biofilm formation around artificial joint implants. The next section will explore emerging treatments (Fig. 1) which can (a) enhance contemporary therapies or (b) represent completely new therapeutic modalities for eradicating PJI.
Fig. 1.
Schematic showing antibiofilm strategies to penetrate, disrupt matrix, and eliminate bacterial cells embedded within the biofilm. These strategies include using lytic phage, nanoparticles (NPs), photodynamic therapy (using laser and phenotiazinic dye), and cathodic voltage-controlled electrical stimulation of titanium
PJI Treatment—Enhancing Contemporary Methods
Antibiotic Combination Therapy
Combining synergistic antibiotic medications is an established approach in infectious disease medicine that results in a superior antimicrobial effect against biofilm-based infections and lower resistance rates compared to individual antibiotic use. An antibiotic used in PJI management that significantly increases in efficacy when used in combination therapy is rifampin. Rifampin is an inhibitor of DNA-dependent RNA synthesis that is particularly effective against Staphylococcus aureus. However, due to the rapid development of S. aureus resistance, rifampin is usually combined clinically with flucloxacillin (a penicillin-based antibiotic that inhibits cell wall synthesis) [34, 35]. An alternative to flucloxacillin is moxifloxacin, a fluoroquinolone that targets DNA synthesis and had been reported to have a broad spectrum of activity, high efficiency against Staphylococcus infections, rapid tissue penetration, long half-life, and comparable bio-viability whether administered orally or intravenously [36, 37]. Greime et al. explored possible synergistic activities of rifampin combined with moxifloxacin using a mouse model of PJI and demonstrated that the rifampin-moxifloxacin combination exhibited superior bactericidal effects compared to the monotherapy, effectively clearing bone and tissue of bacteria after 14 days of treatment. Combination therapy also significantly reduced viable bacteria counts within the biofilm formed on the stainless-steel implant. Furthermore, while some bacterial resistance was observed in the rifampin-alone group, no resistance was reported for the rifampin-moxifloxacin combination [38]. This combination was comparable in bactericidal activities to a rifampin-flucloxacillin combination, suggesting that moxifloxacin is a suitable alternative for clinical use [38]. This effort is a good example of why continuous exploration of synergistic interactions between antibiotics is necessary, especially when considering that the increasing number of resistant PJI organisms encountered clinically.
Antibiotic Conjugation
Intracellular colonization is an increasingly understood method bacteria utilize to evade host immune detection and antibiotic binding. S. aureus has demonstrated this capability, with both in vivo and in vitro studies demonstrating its ability to colonize phagocytic and non-phagocytic cells [39–41]. Novel antibiotic development methods have focused on uptake of antibiotics by the infected host cell, leading to intracellular antibiotic-bacterial interaction and intracellular elimination of the intracellular bacteria. Surewaard et al. demonstrated intracellular colonization of hepatic Kupffer cells (KC) by methicillin-resistant Staphylococcus aureus (MRSA) in a mouse-model. While conventional vancomycin failed to eradicate the infection and resulted in a 50% mouse mortality, treatment with vancomycin-loaded liposomes (vancosomes) produced intracellular uptake of vancomycin, death of intracellular MRSA and 100% mouse survival [41]. Another proposed method involves conjugating an antibiotic to an antibacterial antibody, producing an antibody-antibiotic conjugate (AAC). Lehar conjugated an anti-S. aureus antibody to rifalogue, a rifamycin derivative [40]. While this AAC had no bactericidal activity against free-floating extracellular bacterial cells and was not able to diffuse into the mammalian cells due to its size, it was capable of attaching to extracellular MRSA cells. These opsonized bacterial cells were then phagocytosed by human macrophages, endothelial and epithelial cells, and intracellular proteases cleaved the antibody-antibiotic link, producing antibiotic-mediated bacterial death. Antibiotic conjugation to kill intracellular bacterial cells is a novel and very promising approach to prevent antibiotic treatment failure. It is yet to be tested in an in vivo PJI model.
Nanoparticles for Antibiotic Delivery
Nanoparticles (NPs) are microscopic materials ranging from 10 to 100 nm. NP matrix can be made of organic (liposome, d solid lipid NPs) or inorganic (silver, copper, gold NPs) material [42••]. Various NPs have been investigated for delivering antibiotics, including solid lipid NPs (SLNs, a lipid based colloidal carrier, 50–100 nm in size), liposomes (phospholipid vesicles, 20–100 nm in size), and polymeric micelles (block amphiphilic copolymer micelles, 10–1000 nm in size) [42••]. NPs are biostable, biocompatible, and capable of carrying hydrophilic and hydrophobic drugs, making them attractive, localized antibiotic delivery systems that could produce fewer toxic side effects compared to antibiotic boluses [42••]. In addition, some NPs have been documented to enhance antibiotic penetration within bacterial biofilm. Liu et al. demonstrated significant improvement of the anti-S. aureus biofilm and bactericidal activities of triclosan in vitro when it was loaded into NPs of engineered mixed shell polymeric micelles (MSPMs) [43]. The MSPMs penetrated the S. aureus biofilm matrix at different depths and bound to the bacterial cells. This interaction led to bacterial production of lipase enzymes that degraded the NPs core, and resulted in triclosan release causing bacterial cell death within the biofilm. Another group reported success with augmenting in vitro efficacy of rifampin loaded SLN in comparison to using rifampin alone against Staphylococcus epidermidis biofilm biomass and viable bacterial cells [44]. Chetoni [45] documented that using tobramycin-SNL resulted in a reduction in tobramycin MIC for Pseudomonas aeruginosa to 2 μg/mL compared to using tobramycin alone (4 μg/mL). Moreover, antibacterial activity of polymorphonuclear granulocytes (PMNs) against phagocytosed P. aeruginosa was significantly increased when tobramycin-SNL was administrated, possible due to tobramycin-SNL penetrating both PMNs and bacterial cells [45]. NP modalities show promise in eliminating resistant infections, with in vivo testing using a representative PJI model being the next required step.
Nonthermal Plasma
Plasmas are ionized gases (partially or completely). Nonthermal (low temperature) plasma uses noble gases (e.g., helium) and chemically active gases (e.g., oxygen) [46]. Nonthermal (cold) plasma possesses antibacterial properties due to its ability to cause oxidative stress and DNA damage [47, 48]. It is used in several medical and biomedical areas such as sterilization of heat sensitive surfaces, superficial wound and skin disinfection, and surface alteration [49]. Cold plasma antibiofilm activities have been reported against Gram-negative, and to a lesser extent against Gram-positive bacteria in vitro [50]. Chlorhexidine digluconate (CHG) is an antiseptic that is used as an irrigation solution during PJI treatment process. However, reports have indicated Staphylococcus biofilm tolerance and incomplete biofilm elimination after 2% CHG treatment [51, 52]. Recently, published data has reported that combining physical and chemical treatment of nonthermal plasma with 1% CHG. At clinically relevant doses, this combination had antibiofilm activities on orthopedic surfaces. Specifically, it resulted in a significant sterilization of mature P. aeruginosa biofilm grown on titanium coupons compared to using each agent separately, for 5, 10, or 15 min [53]. Interestingly, this high efficiency is achieved when plasma treatment preceded chlorhexidine application. It is thought that treatment order effect is the result of the plasma breaking and dislodging the biofilm, which resulted in more accessible, easily eliminated bacterial cells by CHG. The combination of cold plasma and CHG seems like a promising method to de-contaminate biofilms that are attached to titanium surfaces. More in vitro work is needed to test the efficiency of this treatment method against PJI relevant bacteria (such as Staphylococcus). Furthermore, in vivo experiments will be required to confirm the ease, suitability, efficiency, and any cytotoxic or deleterious effects on the surrounding tissue due to the usage of cold plasma and CHG treatment.
PJI Treatments—Novel Agents
Antibiotics
Oritavancin and dalbavancinare are newly FDA approved antibiotics that have specific antimicrobial activity against skin infections caused by Gram-positive bacteria [54]. Oritavancin is a semisynthetic lipoglycopeptide with a long half-life that acts by disrupting bacterial cell membrane and inhibiting transglycosylation and transpeptidation [55]. Oritavancin has been reported to have bactericidal activities against methicillin-sensitive Staphylococcus aureus (MSSA), MRSA, vancomycin-resistant S. aureus, and methicillin-resistant coagulase-negative Staphylococci. Lehoux et al. investigated in vivo bone penetration and concentration of oritavancin after being administrated intravenously. The group showed that oritavancin was rapidly absorbed by rabbit bones with stable concentrations for more than 165 h [56]. Similarly, dalbavancin is a semisynthetic lipoglycopeptide antibiotic that has a long half-life and acts by inhibiting bacterial cell wall synthesis [57]. It is capable of penetrating and spreading into bone and articular tissue [58]. Dalbavancin was shown to retain antibiofilm activities in vitro against PJI clinical isolates of S. epidermidis and S. aureus [59]. Its efficiency to treat MRSA rat sternal osteomyelitis demonstrated significant bactericidal properties after 14 days of treatment and it was as effective as vancomycin. Moreover, dalbavancin was able to control the systematic spread of MRSA [60]. Currently, oritavancin and dalbavancin are only approved for the treatment of skin and skin structure infections; however, there is a great potential for their usage in treating PJIs especially since they possess the ability to penetrate bone and articular tissue. While the high cost of these antibiotics might be a limitation, future work should focus on testing these two antibiotics in vivo in a clinically representative PJI model.
Immunotherapy (Monoclonal Antibodies)
S. aureus surface protein A (SasA) is a microbial surface component recognizing adhesive matrix molecules (MSCRAMMs) and has been identified as a target for monoclonal antibodies (mAbs). Yang et al. generated anti-SasA mAb (2H7), which binds to a widely conserved region of SasA, and evaluated it against MRSA in a mouse of model of sepsis [61]. 2H7 was capable of protecting 40% of mice from death when administrated 1 h following intravenous injection with MRSA [61]. 514G3 is another promising mAb that targets S. aureus cell wall moiety Protein A (SpA), and has shown successful in vitro opsonization of both MRSA and MSSA facilitating their clearance by immune cells [62]. In vivo intravenous administration of 514G3 rescued mice suffering from MRSA bacteremia when used alone or in combination with vancomycin [62]. These examples of mAb therapy are promising and should be appraised in in vivo PJI models.
Nanoparticles with Bactericidal Effects
Several NPs have been identified to have passive antibacterial properties. Silver, copper, zinc oxide, and quantum dots (their core is made of semiconductor materials like cadmium or zinc) are just few examples of designed NPs with bactericidal activities [42••]. NP lyse bacterial cells by several mechanisms. They act by creating and increasing the concentrations of reactive oxygen species, which results in the loss of cell membrane integrity. Also, NPs interact with bacterial cell wall which affects proteins participating in cell metabolism and affects electron transport chain and physiological process in bacterial cells [42••]. Due to some NPs multiple bactericidal modes of actions, it is more challenging for bacterial cells to develop resistance against them [42••]. Several in vitro reports documented the ability of NPs, such as silver-NPs, graphene oxide-silver nanocomposites, and spermidine-carbon quantum dots (Spd-CQDs), to effectively lyse a broad range of Gram-positive (S. aureus, Streptococcus mutans) and Gram-negative bacteria (Klebsiella pneumoniae, E. coli, Enterobacter cloacae, P. aeruginosa), with minimal cytotoxic effects and high biocompatibility [63–65]. In vivo work using a MRSA-infected wound healing model indicated high efficiency in eliminating MRSA-wound infection in rats when the wound was covered with Spd-CQDs treated gauze. The treatment also showed faster wound healing and formation of collagen fibers [63]. NPs provide a very promising approach to treat multidrug resistance related infections. The technology of NPs is yet to be tested in an in vivo PJI model.
Antimicrobial Peptides
Antimicrobial peptides (AMPs) are small oligopeptides made of 5–100 amino acids. AMPs have been reported to possess efficiency against bacteria, viruses, and parasites [66]. The peptide’s length, net charge, hydrophobicity, and structure play a role in determining its antimicrobial activities [66]. According to the LAMP Database, there are thousands of AMPs of natural (e.g., human, animal, bacteria) or synthetic origins [67]. Antibacterial AMPs are capable of rapidly killing bacteria by creating pores in the cell membrane or disrupting DNA, RNA, and proteins biosynthesis [66]. The low possibility of resistance development against AMPs, low cytotoxicity, and immunogenicity among AMPs make them ideal agents to treat biofilm infections [66]. Nisin and modified medusin-PT are promising peptides, originally secreted by tarsier leaf frog skin; they have shown efficiency in vitro against multi species biofilm (including various Streptococcus species) and MRSA biofilms, respectively [68, 69]. Zapotoczna et al. (2017) reported the therapeutic potential of the peptide D- Bac8c2,5Leu. The data demonstrated that this AMP was efficient in eradicating viable bacteria in a 5-day-old biofilms of MRSA and MSSA in vitro. It showed anti-biofilm MRSA activities in vivo as well (reduction of 9 logs- CFU/mL) when used as a catheter lock solution in a rat central venous catheter infection model. Moreover, D- Bac8c2,5Leu cytotoxicity and hemolytic activities were at higher concentrations than its biofilm eradiation ability [70]. Another promising peptide is DJK-5, a cationic synthetic peptide. It was reported to synergistically interact with conventional antibiotics (such as ciprofloxacin and tobramycin) in vitro and significantly eliminate preformed MRSA and P. aeruginosa biofilms in a cutaneous abscess mouse model [71, 72]. It was also capable of inhibiting lesion formation [72].
In a recently published article, de Breij and colleagues had succeeded in modifying the human peptide LL-37 to produce a superior version, namely, SAAP-148 [73]. SAAP-148 possesses enhanced bactericidal abilities against multidrug resistance (MDR) bacteria (Enterococcus faecium, S. aureus, K. pneumoniae, Acinetobacter baumannii, P. aeruginosa, Enterobacter species) and persister cells. It also had better biofilm eradication powers compared to LL-37 when tested in vitro in 50% pooled human plasma. Moreover, ex vivo wounded human skin infection model and in vivo mouse skin models showed complete elimination of established biofilm-associated infections of MRSA and MDR A. baumannii after 4 h of treatment with SAAP-148 ointment. Notably, no induced resistance was reported [73].
AMPs have a lot of potential to treat biofilm related infections. It would be beneficial to test the efficiency of some of the previously mentioned AMPs in an in vivo PJI model and monitor the effects of AMPs activities on the joint microenvironment. The high cost of AMPs synthesis and possibility of degradation by host proteases could be limiting factors for their usage.
Phage Therapy
Bacteriophages (phages) are naturally occurring viruses that specifically attack and kill bacteria. Phages have very high specificity towards bacterial species without affecting the human microbiota [74]. This makes them great cell-specific killing machinery against bacterial infections. Lytic phages kill bacteria by recognizing certain bacterial surface proteins, adsorbing, and injecting their genomic material into the bacterial cell. The lytic phage then hijacks the replication and translation machinery of the bacteria to make more phages. The new phage particles are released which results in bacterial lysis and death [74]. Their efficiency against multidrug resistance bacteria and even biofilm infections has been documented. Lytic phages are used in food industry for the prevention of bacterial contamination and growth (such as E. coli and Salmonella) in dairy products. In fact, several commercial phage preparations used for biocontrol of bacterial pathogens are approved by the FDA and the US Department of Agriculture [75••]. Moreover, a number of studies have reported no adverse reactions of using lytic phages as a treatment strategy for bacterial infections in humans [76, 77]. Phage therapy for clinical usage is not yet approved by the FDA; however, in a recent case report, and after getting FDA special emergency approval, intravenous administration of phage cocktails successfully saved the life of a male who had septic shock secondary to disseminated multidrug resistant Gram-negative A. baumannii [76]. Phage efficiency has been reported against mature biofilms as well. Alves showed that a phage cocktail (made of six P. aeruginosa specific phages) in vitro was capable of significantly reducing the biomass and viable cell count (> 4 logs) of P. aeruginosa in a mature biofilm after 48 h of treatment [78]. Others have shown that applying phages to mature biofilm cultures followed by antibiotics such as vancomycin or ciprofloxacin against S. aureus or P. aeruginosa, respectively, synergistically reduced bacterial burden [79, 80]. In vivo, a S. aureus specific phage cocktail was used to treat MRSA osteomyelitis in rabbits. Rabbits recovered from the infection, and wound swab cultures were negative after 2 weeks of intralesional injections of phage cocktail in the infected soft tissue [81]. Another group reported the efficiency of using phage cocktails to significantly reduce S. aureus load in a mastitis mouse model [82]. Unfortunately, no representative PJI animal model has been used yet to demonstrate the efficiency of phage treatment [77]. The usage of lytic phages to treat biofilm related infections is a very promising therapeutic strategy and its safety had been documented. It is widely used in Eastern European medicine; however, it has not yet been approved in clinical settings in Western countries.
Photodynamic Therapy
Photodynamic therapy (PDT) is another innovative avenue to treat biofilm infections. PDT involves using light and the proper non-toxic phenotiazinic dyes (such as methylene blue—MB) which get absorbed by the bacteria. In the presence of oxygen and upon exposure to light, at a certain wavelength, the absorbed dye gets activated resulting in the production of reactive oxygen species leading to bacterial plasma membrane and DNA damage with eventual cell death [83]. Briggs and Giannelli reported that PDT (laser and MB) treatment in vitro eliminated MRSA, MSSA, S. epidermidis, and P. aeruginosa mature biofilm grown on polished titanium alloy (Ti-6Al-4V) discs or moderately rough titanium surface [84••, 85]. Briggs observed improved PDT efficiency compared to using MB or laser alone [84••]. The low possibility of bacterial resistance, and the fast minute-based bactericidal action, makes PDT an attractive PJI treatment strategy specifically during revision surgery to sterilize the infected area after removing implants [84••]. Nevertheless, further investigation is required in vivo to assess PDT efficacy and the histologic effects on surrounding healthy tissue.
Electrical Stimulation of Titanium
Cathodic voltage-controlled electrical stimulation (CVCES) of titanium had been reported to effectively eliminate bacteria on titanium surfaces. This stimulation method is composed of three electrodes that provide titanium with constant cathodic voltage [86]. CVCES causes an alteration to the electrochemical properties of the titanium implant. While the exact mechanism of action of the electrical stimulation is not clear, it is thought to be linked to the changes in titanium voltage-dependent electrochemical properties [86]. In vitro experiments demonstrated significant bacterial elimination of MRSA formed on coupons of commercially pure titanium (cpTi), as well as in the surrounding solution following treatment with cathodic voltage-controlled electrical stimulation [86]. A similar pattern of MRSA elimination was seen using a rat model of an infected shoulder titanium implant. In another study, using the same in vivo rat model, subcutaneous injections of vancomycin (150 mg/kg), and cathodic voltage-controlled electrical stimulation (− 1.8 V for 1 h) of the titanium implant, resulted in 99.8% reduction in MRSA biofilm load on the implant and in the synovial fluid [87••]. This combination therapeutic strategy was better than using vancomycin or cathodic voltage-controlled electrical stimulation separately. The proposed mechanism of elimination was linked to possible disruption of the biofilm biomass from the implant surface resulting in bacteria being released in the surrounding environment, which may make it more susceptible to vancomycin treatment. Histological evaluation of the treated area surrounding the implant indicated no negative effect of the stimulation [87••]. While CVCES shows promise, further optimization of the treatment protocol and parameters is needed using in vivo models.
Conclusion
PJI remains a significant cause of morbidity and mortality for joint replacement patients, with current treatments being limited by their invasive nature and inability to consistently eradicate bacterial biofilm. Several promising treatment modalities for PJI have been presented in this review (Fig. 1), and the growing number of publications related to PJI annually suggests interest for innovations in treatment will continue for years to come. The next requirement for most of the presented novel treatments is reproducing their effects in clinically representative animal models of PJI against clinical isolates of offending organisms. In addition to measuring bactericidal effects, monitoring for adverse physiological effects and preservation of joint locomotion will be important before moving forward to clinical trials.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Prosthetic Joint Infection
References
Papers of particular interest, published recently, have been highlighted as: •• Of major Importance
- 1.Norman-Taylor FH, Palmer CR, Villar RN. Quality-of-life improvement compared after hip and knee replacement. J Bone Joint Surg Br Vol. 1996;78(1):74. doi: 10.1302/0301-620X.78B1.0780074. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Cano JP, Herrera-Escobar JP, Arango Gutierrez AS, Sanchez Vergel A, Martinez-Rondanelli A. Prospective quality of life assessment after hip and knee arthroplasty: short- and mid-term follow-up results. Arthroplast Today. 2017;3(2):125. doi: 10.1016/j.artd.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet (Lond Engl) 2016;387(10016):386. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 4.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471(2):510. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamagni T. Epidemiology and burden of prosthetic joint infections. J Antimicrob Chemother. 2014;69(Suppl 1):i5. doi: 10.1093/jac/dku247. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplast. 2012;27(8 Suppl):61. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Alp E, Cevahir F, Ersoy S, Guney A. Incidence and economic burden of prosthetic joint infections in a university hospital: a report from a middle-income country. J Infect Public Health. 2016;9(4):494. doi: 10.1016/j.jiph.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Ciofu O, Rojo-Molinero E, Macia MD, Oliver A. Antibiotic treatment of biofilm infections. APMIS: Acta Pathol Microbiol Immunol Scand. 2017;125(4):304. doi: 10.1111/apm.12673. [DOI] [PubMed] [Google Scholar]
- 9.Marculescu C, Berbari E, Hanssen AD, Steckelberg J, Harmsen S, Mandrekar J, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42(4):471. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 10.Urish KL, Bullock AG, Kreger AM, Shah NB, Jeong K, Rothenberger SD. A multicenter study of irrigation and debridement in total knee arthroplasty periprosthetic joint infection: treatment failure is high. J Arthroplast. 2018;33(4):1154. doi: 10.1016/j.arth.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klement MR, Siddiqi A, Rock JM, Chen AF, Bolognesi MP, Seyler TM. Positive blood cultures in periprosthetic joint infection decrease rate of treatment success. J Arthroplast. 2018;33(1):200. doi: 10.1016/j.arth.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Yoon HK, Cho SH, Lee DY, Kang BH, Lee SH, Moon DG, Kim DH, Nam DC, Hwang SCA. Review of the literature on culture-negative periprosthetic joint infection: epidemiology, diagnosis and treatment. Knee Surg Relat Res. 2017;29(3):155. doi: 10.5792/ksrr.16.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wang Q, Shen H, Zhang X. Comparable outcome of culture-negative and culture-positive periprosthetic hip joint infection for patients undergoing two-stage revision. Int Orthop. 2018;42(3):469. doi: 10.1007/s00264-018-3783-4. [DOI] [PubMed] [Google Scholar]
- 14.Dibartola AC, Swearingen MC, Granger JF, Stoodley P, Dusane DH. Biofilms in orthopedic infections: a review of laboratory methods. APMI: Acta Pathol Microbiol Immunol Scand. 2017;125(4):418. doi: 10.1111/apm.12671. [DOI] [PubMed] [Google Scholar]
- 15.•• Swearingen MC, DiBartola AC, Dusane D, Granger J, Stoodley P. 16S rRNA analysis provides evidence of biofilms on all components of three infected periprosthetic knees including permanent braided suture. Pathog Dis 2016;74(7). This paper compared conventional bacterial culture techniques to 16S rRNA analysis of samples taken from patients undergoing revision knee replacement for infection. 16S sequencing identified multiple bacteria types on implant and suture material surfaces, demonstrating that the prevalence of polymicrobial infection is underestimated and that biofilm can form on all foreign materials inside the patient. [DOI] [PMC free article] [PubMed]
- 16.Huang Z, Wu Q, Fang X, Li W, Zhang C, Zeng H, Wang Q, Lin J, Zhang W. Comparison of culture and broad-range polymerase chain reaction methods for diagnosing periprosthetic joint infection: analysis of joint fluid, periprosthetic tissue, and sonicated fluid. Int Orthop. 2018. [DOI] [PubMed]
- 17.Rothenberg AC, Wilson AE, Hayes JP, O'Malley MJ, Klatt BA. Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res. 1827;475(7):2017. doi: 10.1007/s11999-017-5315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tani S, Lepetsos P, Stylianakis A, Vlamis J, Birbas K, Kaklamanos I. Superiority of the sonication method against conventional periprosthetic tissue cultures for diagnosis of prosthetic joint infections. Eur J Orthop Surg Traumatol. 2018;28(1):51. doi: 10.1007/s00590-017-2012-y. [DOI] [PubMed] [Google Scholar]
- 19.Janz V, Trampuz A, Perka CF, Wassilew GI. Reduced culture time and improved isolation rate through culture of sonicate fluid in blood culture bottles. Technol Health Care. 2017;25(4):635. doi: 10.3233/THC-160660. [DOI] [PubMed] [Google Scholar]
- 20.Clauss M. CORR insights((R)): sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res. 1837;475(7):2017. doi: 10.1007/s11999-017-5350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson PD, Jr, Salvati EA, Blumenfeld EL. The problem of infection in total prosthetic arthroplasty of the hip. Surg Clin North Am. 1975;55(6):1431. doi: 10.1016/S0039-6109(16)40803-0. [DOI] [PubMed] [Google Scholar]
- 22.Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80(2):193. doi: 10.3109/17453670902884700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertazzoni Minelli E, Benini A, Samaila E, Bondi M, Magnan B. Antimicrobial activity of gentamicin and vancomycin combination in joint fluids after antibiotic-loaded cement spacer implantation in two-stage revision surgery. J Chemother (Florence, Italy) 2015;27(1):17. doi: 10.1179/1973947813Y.0000000157. [DOI] [PubMed] [Google Scholar]
- 24.Edelstein AI, Okroj KT, Rogers T, Della Valle CJ, Sporer SM. Nephrotoxicity after the treatment of periprosthetic joint infection with antibiotic-loaded cement spacers. J Arthroplasty. 2018. [DOI] [PubMed]
- 25.Berliner ZP, Mo AZ, Porter DA, Grossman JM, Hepinstall MS, Cooper HJ, et al. In-hospital acute kidney injury after TKA revision with placement of an antibiotic cement spacer. J Arthroplast. 2017; [DOI] [PubMed]
- 26.Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. 2014;472(7):2208. doi: 10.1007/s11999-014-3571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmolders J, Hischebeth GT, Friedrich MJ, Randau TM, Wimmer MD, Kohlhof H, Molitor E, Gravius S. Evidence of MRSE on a gentamicin and vancomycin impregnated polymethyl-methacrylate (PMMA) bone cement spacer after two-stage exchange arthroplasty due to periprosthetic joint infection of the knee. BMC Infect Dis. 2014;14:144. doi: 10.1186/1471-2334-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anagnostakos K, Furst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: current status. Acta Orthop. 2006;77(4):628. doi: 10.1080/17453670610012719. [DOI] [PubMed] [Google Scholar]
- 29.Erivan R, Lecointe T, Villatte G, Mulliez A, Descamps S, Boisgard S. Complications with cement spacers in 2-stage treatment of periprosthetic joint infection on total hip replacement. Orthop Traumatol Surg Res: OTSR. 2017. [DOI] [PubMed]
- 30.Armstrong MD, Carli AV, Abdelbary H, Poitras S, Lapner P, Beaule PE. Tertiary care centre adherence to unified guidelines for management of periprosthetic joint infections: a gap analysis. Can J Surg Journal canadien de chirurgie. 2018;61(1):34. doi: 10.1503/cjs.008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruder JA, Springer BD. Treatment of periprosthetic joint infection using antimicrobials: dilute povidone-iodine lavage. J Bone Joint Infect. 2017;2(1):10. doi: 10.7150/jbji.16448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frisch NB, Kadri OM, Tenbrunsel T, Abdul-Hak A, Qatu M, Davis JJ. Intraoperative chlorhexidine irrigation to prevent infection in total hip and knee arthroplasty. Arthroplast Today. 2017;3(4):294. doi: 10.1016/j.artd.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riesgo AM, Park BK, Herrero CP, Yu S, Schwarzkopf R, Iorio R. Vancomycin povidone-iodine protocol improves survivorship of periprosthetic joint infection treated with irrigation and debridement. J Arthroplast. 2018;33(3):847. doi: 10.1016/j.arth.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant–related staphylococcal infections: a randomized controlled trial. JAMA. 1998;279(19):1537. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly T, Kunz S, Sande E, Zak O, Sande M, Täuber MG. Relationship between antibiotic concentration in bone and efficacy of treatment of staphylococcal osteomyelitis in rats: azithromycin compared with clindamycin and rifampin. Antimicrob Agents Chemother. 1992;36(12):2693. doi: 10.1128/AAC.36.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keating GM, Scott LJ. Moxifloxacin. Drugs. 2004;64(20):2347. doi: 10.2165/00003495-200464200-00006. [DOI] [PubMed] [Google Scholar]
- 37.Joukhadar C, Stass H, Müller-Zellenberg U, Lackner E, Kovar F, Minar E, Müller M. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob Agents Chemother. 2003;47(10):3099. doi: 10.1128/AAC.47.10.3099-3103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greimel F, Scheuerer C, Gessner A, Simon M, Kalteis T, Grifka J, Benditz A, Springorum H-R, Schaumburger J. Efficacy of antibiotic treatment of implant-associated Staphylococcus aureus infections with moxifloxacin, flucloxacillin, rifampin, and combination therapy: an animal study. Drug Des Devel Ther. 2017;11:1729. doi: 10.2147/DDDT.S138888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koziel J, Maciag-Gudowska A, Mikolajczyk T, Bzowska M, Sturdevant DE, Whitney AR, Shaw LN, DeLeo FR, Potempa J. Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors. PLoS One. 2009;4(4):e5210. doi: 10.1371/journal.pone.0005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Morisaki JH. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527(7578):323. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 41.Surewaard BG, Deniset JF, Zemp FJ, Amrein M, Otto M, Conly J, Omri A, Yates RM, Kubes P. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med : JEM. 2016 20160334. [DOI] [PMC free article] [PubMed]
- 42.Zaidi S, Misba L, Khan AU. Nano-therapeutics: a revolution in infection control in post antibiotic era. Nanomedicine. 2017;13(7):2281. doi: 10.1016/j.nano.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Busscher HJ, Zhao B, Li Y, Zhang Z, van der Mei HC, Ren Y, Shi L. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10(4):4779. doi: 10.1021/acsnano.6b01370. [DOI] [PubMed] [Google Scholar]
- 44.Bazzaz BSF, Khameneh B, Zarei H, Golmohammadzadeh S. Antibacterial efficacy of rifampin loaded solid lipid nanoparticles against Staphylococcus epidermidis biofilm. Microb Pathog. 2016;93:137. doi: 10.1016/j.micpath.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Chetoni P, Burgalassi S, Monti D, Tampucci S, Tullio V, Cuffini AM, Muntoni E, Spagnolo R, Zara GP, Cavalli R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: pharmacokinetic studies on rabbits. Eur J Pharm Biopharm. 2016;109:214. doi: 10.1016/j.ejpb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Hippler R, Kersten H, Schmidt M, Schoenbach KH. Low temperature plasmas: fundamentals, technologies and techniques: Wiley-VCH. 2008.
- 47.Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226(1–2):1. doi: 10.1016/S0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- 48.Moreau M, Orange N, Feuilloley M. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008;26(6):610. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Laroussi M, Lu X, Keidar M. Perspective: the physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J Appl Phys. 2017;122(2):020901. doi: 10.1063/1.4993710. [DOI] [Google Scholar]
- 50.Ermolaeva SA, Varfolomeev AF, Chernukha MY, Yurov DS, Vasiliev MM, Kaminskaya AA, Moisenovich MM, Romanova JM, Murashev AN, Selezneva ST, II, Sysolyatina EV, Shaginyan IA, Petrov OF. Mayevsky EI, Fortov VE, Morfill GE, Naroditsky BS, Gintsburg AL. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J Med Microbiol. 2011;60(Pt 1):75. doi: 10.1099/jmm.0.020263-0. [DOI] [PubMed] [Google Scholar]
- 51.Taha M, Kalab M, Yi QL, Landry C, Greco-Stewart V, Brassinga AK, Sifri CD, Ramirez-Arcos S. Biofilm-forming skin microflora bacteria are resistant to the bactericidal action of disinfectants used during blood donation. Transfusion. 2014;54(11):2974. doi: 10.1111/trf.12728. [DOI] [PubMed] [Google Scholar]
- 52.Horner C, Mawer D, Wilcox M. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother. 2012;67(11):2547. doi: 10.1093/jac/dks284. [DOI] [PubMed] [Google Scholar]
- 53.Gupta TT, Karki SB, Matson JS, Gehling DJ, Ayan H. Sterilization of biofilm on a titanium surface using a combination of nonthermal plasma and chlorhexidine digluconate. Biomed Res Int. 2017;2017:6085741. doi: 10.1155/2017/6085741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brade KD, Rybak JM, Rybak MJ. Oritavancin: a new lipoglycopeptide antibiotic in the treatment of Gram-positive infections. Infect Dis Ther. 2016;5(1):1. doi: 10.1007/s40121-016-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhanel GG, Schweizer F, Karlowsky JA. Oritavancin: mechanism of action. Clin Infect Dis. 2012;54(suppl_3):S214. doi: 10.1093/cid/cir920. [DOI] [PubMed] [Google Scholar]
- 56.Lehoux D, Ostiguy V, Cadieux C, Malouin M, Belanger O, Far AR, Parr TR. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501. doi: 10.1128/AAC.00981-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crotty MP, Krekel T, Burnham C-AD, Ritchie DJ. New gram-positive agents: the next generation of oxazolidinones and lipoglycopeptides. J Clin Microbiol 54(9): 2225, 2016. [DOI] [PMC free article] [PubMed]
- 58.Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 59(4): 1849, 2015. [DOI] [PMC free article] [PubMed]
- 59.Fernández J, Greenwood-Quaintance KE, Patel R. In vitro activity of dalbavancin against biofilms of staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis. 2016;85(4):449. doi: 10.1016/j.diagmicrobio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Barnea Y, Lerner A, Aizic A, Navon-Venezia S, Rachi E, Dunne MW, Puttagunta S, Carmeli Y. Efficacy of dalbavancin in the treatment of MRSA rat sternal osteomyelitis with mediastinitis. J Antimicrob Chemother. 2015;71(2):460. doi: 10.1093/jac/dkv357. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Qian M, Yi S, Liu S, Li B, Yu R, Guo Q, Zhang X, Yu C, Li J. Monoclonal antibody targeting staphylococcus aureus surface protein A (SasA) protect against staphylococcus aureus sepsis and peritonitis in mice. PLoS One. 2016;11(2):e0149460. doi: 10.1371/journal.pone.0149460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varshney AK, Kuzmicheva GA, Lin J, Sunley KM, Bowling RA, Jr, Kwan T-Y, Mays HR, Rambhadran A, Zhang Y, Martin RL. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS One. 2018;13(1):e0190537. doi: 10.1371/journal.pone.0190537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li YJ, Harroun SG, Su YC, Huang CF, Unnikrishnan B, Lin HJ, Lin CH, Huang CC. Synthesis of self-assembled spermidine-carbon quantum dots effective against multidrug-resistant bacteria. Adv Healthcare Mater. 2016;5(19):2545. doi: 10.1002/adhm.201600297. [DOI] [PubMed] [Google Scholar]
- 64.Qayyum S, Khan AU. Biofabrication of broad range antibacterial and antibiofilm silver nanoparticles. IET Nanobiotechnol. 2016;10(5):349. doi: 10.1049/iet-nbt.2015.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulshrestha S, Qayyum S, Khan AU. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: a comparative study on inhibition of gram-positive and gram-negative biofilms. Microb Pathog. 2017;103:167. doi: 10.1016/j.micpath.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 66.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. 2013;6(12):1543. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, Wu H, Lu H, Li G, Huang QLAMP, A Database Linking antimicrobial peptides. PLoS One. 2013;8(6):e66557. doi: 10.1371/journal.pone.0066557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao Y, Wu D, Wang L, Lin C, Ma C, Xi X, Zhou M, Duan J, Bininda-Emonds ORP, Chen T, Shaw C. Targeted modification of a novel amphibian antimicrobial peptide from Phyllomedusa tarsius to enhance its activity against MRSA and microbial biofilm. Front Microbiol. 2017;8:628. doi: 10.3389/fmicb.2017.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin JM, Ateia I, Paulus JR, Liu H, Fenno JC, Rickard AH, Kapila YL. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front Microbiol. 2015;6:617. doi: 10.3389/fmicb.2015.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zapotoczna M, Forde É, Hogan S, Humphreys H, O’gara JP, Fitzgerald-Hughes D, Devocelle M, O’neill E. Eradication of Staphylococcus aureus biofilm infections using synthetic antimicrobial peptides. J Infect Dis 215(6): 975, 2017. [DOI] [PubMed]
- 71.de la Fuente-Núñez C, Reffuveille F, Mansour SC, Reckseidler-Zenteno SL, Hernández D, Brackman G, Coenye T, Hancock RE. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 2015;22(2):196. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansour SC, Pletzer D, de la Fuente-Nunez C, Kim P, GYC C, Joo HS, Otto M, REW H. Bacterial abscess formation is controlled by the stringent stress response and can be targeted therapeutically. EBioMedicine. 2016;12:219. doi: 10.1016/j.ebiom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Breij A, Riool M, Cordfunke RA, Malanovic N, de Boer L, Koning RI, Ravensbergen E, Franken M, van der Heijde T, Boekema BK. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci Transl Med. 2018;10(423):eaan4044. doi: 10.1126/scitranslmed.aan4044. [DOI] [PubMed] [Google Scholar]
- 74.Salmond GP, Fineran PC. A century of the phage: past, present and future. Nat Rev Microbiol. 2015;13(12):777. doi: 10.1038/nrmicro3564. [DOI] [PubMed] [Google Scholar]
- 75.Gutiérrez D, Rodríguez-Rubio L, Martínez B, Rodríguez A, García P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front Microbiol. 2016;7:825. doi: 10.3389/fmicb.2016.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61(10):e00954. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akanda ZZ, Taha M, Abdelbary H. Current review—the rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J Orthop Res, 2017. [DOI] [PubMed]
- 78.Alves DR, Perez-Esteban P, Kot W, Bean J, Arnot T, Hansen LH, Enright MC, Jenkins ATA. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Microb Biotechnol 9(1): 61, 2016. [DOI] [PMC free article] [PubMed]
- 79.Kumaran D, Taha M, Yi Q, Ramirez S, Diallo J-S, Carli A, Abdelbary H. Does treatment order matter? Investigating the ability of bacteriophage to augment antibiotic activity against Staphylococcus aureus biofilms. Front Microbiol. 2018;9:127. doi: 10.3389/fmicb.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS One. 2017;12(1):e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kishor C, Mishra RR, Saraf SK, Kumar M, Srivastav AK, Nath G. Phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Indian J Med Res. 2016;143(1):87. doi: 10.4103/0971-5916.178615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breyne K, Honaker RW, Hobbs Z, Richter M, Żaczek M, Spangler T, et al. Safety of a bovine-associated Staphylococcus aureus phage cocktail in a murine model of mastitis. Front Microbiol. 2017;8 [DOI] [PMC free article] [PubMed]
- 83.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagn Photodyn Ther. 2005;2(1):1. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.•• Briggs T, Blunn G, Hislop S, Ramalhete R, Bagley C, McKenna D, Coathup M. Antimicrobial photodynamic therapy—a promising treatment for prosthetic joint infections. Lasers Med Sci. 2017;1. This paper involved a thorough evaluation of the photodynamic therapy (PDT) on the biofilms of four distinct clinical isolates of bacteria found in clinical periprosthetic joint infection. The study found that staphylococcus bacteria could be effectively removed using PDT in tested conditions/.
- 85.Giannelli M, Landini G, Materassi F, Chellini F, Antonelli A, Tani A, Nosi D, Zecchi-Orlandini S, Rossolini GM, Bani D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: in vitro study. Lasers Med Sci. 2017;32(4):857. doi: 10.1007/s10103-017-2185-y. [DOI] [PubMed] [Google Scholar]
- 86.Ehrensberger MT, Tobias ME, Nodzo SR, Hansen LA, Luke-Marshall NR, Cole RF, Wild LM, Campagnari AA. Cathodic voltage-controlled electrical stimulation of titanium implants as treatment for methicillin-resistant Staphylococcus aureus periprosthetic infections. Biomaterials. 2015;41:97. doi: 10.1016/j.biomaterials.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 87.Nodzo SR, Tobias M, Ahn R, Hansen L, Luke-Marshall NR, Howard C, Wild L, Campagnari AA, Ehrensberger MT. Cathodic voltage-controlled electrical stimulation plus prolonged vancomycin reduce bacterial burden of a titanium implant-associated infection in a rodent model. Clin Orthop Relat Res. 2016;474(7):1668. doi: 10.1007/s11999-016-4705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]