Abstract
Purpose of Review
Despite significant progress in recent years, the diagnosis of periprosthetic joint infection (PJI) remains a challenge and no gold standard test exists. A combination of serological, synovial, microbiological, histological, and radiological investigations is performed that are expensive, often invasive, and imperfect. Novel biomarkers and molecular methods have shown promise in recent years. The purpose of this review is to provide an update about the diagnostic recommendations for PJI and cover a selection of emerging diagnostic tools.
Recent Findings
Recent literature highlights a new evidence-based definition for diagnosing hip and knee PJI that shows excellent performance on formal external multi-institutional validation. There is also increasing evidence to support the measurement of selected biomarkers in serum and synovial fluid, such as alpha-defensin, D-dimer, and interleukin-6. Finally, the emerging utility of next-generation sequencing for pathogen identification is discussed.
Summary
In summary, we describe current recommendations and emerging tests for the diagnosis of PJI. Residual limitations and directions for future research are also discussed.
Keywords: Periprosthetic joint infection, Diagnosis, Alpha-defensin, Leucocyte esterase, IL-6, Next-generation sequencing
Introduction
While significant improvements in the prevention of periprosthetic joint infection (PJI) have limited this often-feared complication, infection remains a significant challenge and common mode of failure following total joint arthroplasty. PJI is the most common cause of revision for failed total knee arthroplasty (16.8 to 25.2%) and the third leading cause for revision total hip arthroplasty (14.8%) [1, 2]. The raw number of infected arthroplasties has more than doubled since 2001. Nearly 18,000 PJI were reported in 2011, at a cost of $771 million, and this is expected to increase to over 42,000 cases by 2020, at a cost of $1.1 billion [1, 3, 4].
In addition to the financial implications and treatment costs, which are four times higher than for a typical primary TJA, there is considerable morbidity and mortality associated with PJI. Multiple surgical interventions, prolonged hospitalization, and higher complication rates typically ensue [5–8]. Furthermore, a fivefold increase in mortality has been reported for patients treated for PJI in comparison with their aseptic revision counterparts [9].
Although prevention is the most effective strategy, obtaining a clear and timely PJI diagnosis remains critical for success and guiding definitive treatment [10]. Over the last decade, several workgroups have convened to generate a standardized definition and diagnostic approach to the patient with a suspected PJI. In 2010, the American Academy of Orthopaedic Surgeons Clinical Practice Guidelines on Diagnosis of Periprosthetic Joint Infection were published [11]. Soon after, the Musculoskeletal Infection Society (MSIS) and the Infectious Disease Society of America (IDSA) devised criteria to standardize the definition of PJI in 2011 [12, 13]. The International Consensus Meeting (ICM) for PJI in 2013 then endorsed the MSIS definition and modified it slightly [14•]. These definitions have now become widely established among orthopedic surgeons worldwide and their use has significantly improved clinical decision-making, as well as diagnostic research, by allowing for consistency between studies and enhancing the potential for collaboration. Most recently, a new 2018 evidence-based PJI definition has been published which demonstrates improved performance for diagnosing hip and knee PJI on formal external validation [15•].
Many serum and synovial markers have also been investigated in recent years and become clinically available [16–18], including serum D-dimer [19•], synovial leukocyte esterase (LE) [20–22], synovial fluid alpha-defensin [23–25], and molecular diagnostic techniques such as next-generation sequencing (NGS) [26•, 27, 28, 29•]. Emerging data suggests that these tests may help reach a diagnosis of PJI when conventional tests are equivocal. However, no test is 100% sensitive or specific and publications in the recent years have shown different diagnostic performance for the various aforementioned emerging tests [18, 30].
This review article will address the current recommendations for diagnosis of PJI, highlight evidence regarding a selection of established diagnostic tests, and summarize recent advances that highlight an increasingly important role of biomarkers and molecular diagnostics in this setting.
Current Diagnostic Criteria for Acute and Chronic PJI
With an emphasis on avoiding a delay in diagnosis, any painful joint prosthesis should be considered a possible infection until proven otherwise. Evaluation of PJI should begin with a focused history and physical examination along with review of appropriate radiographs [31]. The existence of any secondary symptoms of infection, such as fever or chills, accompanied by the location, quality, and onset of pain should all be closely considered [32]. The joint should be examined for any signs of a draining sinus, effusion, or overlying cellulitis. Additional physical examination findings concerning for infection include erythema, joint effusion, decreased range of motion, and an inability to bear weight.
Current MSIS- and ICM-modified recommendations for diagnosis of PJI
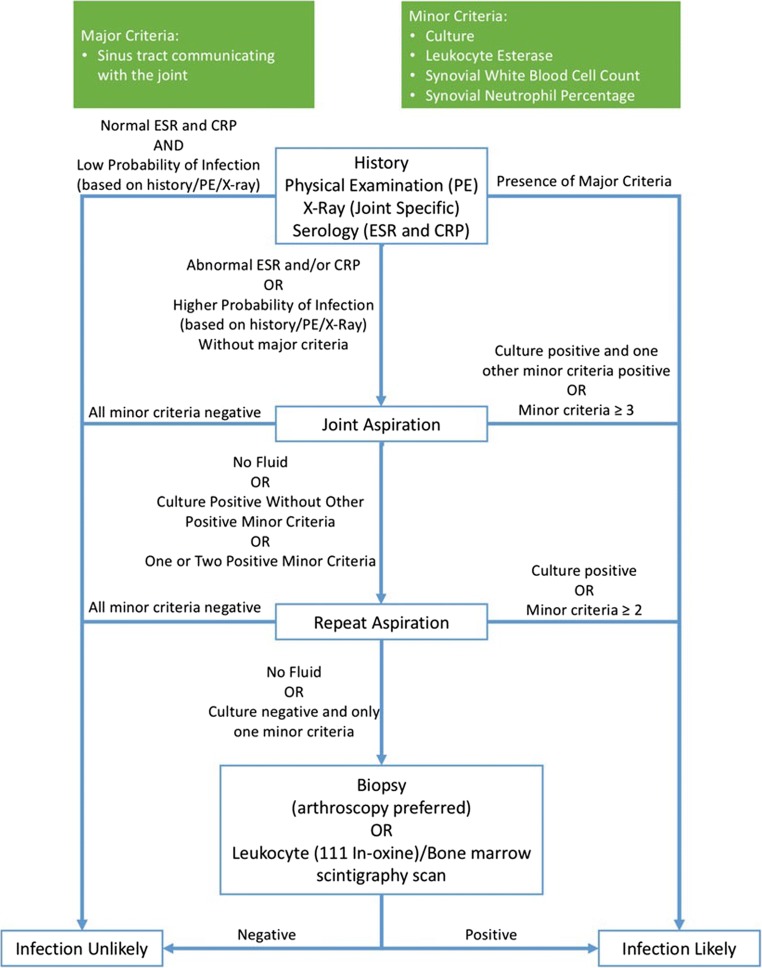
In addition to a detailed clinical assessment, physicians have to utilize a panel of serological, microbiological, histological, and radiological tests in order to diagnose PJI. In 2011, the diagnostic definition for PJI was standardized by the MSIS and at the ICM 2013, this was modified slightly [12, 33]. The latter is now in widespread usage globally. These definitions can be found in Table 1 and Table 2 [12, 34], respectively. Based on this ICM-modified definition, a PJI should be diagnosed when one of two “Major criteria,” or three of five “Minor criteria” are met (Table 2). In addition to defining PJI, the ICM also recommended an algorithm for diagnosing PJI using tests available in clinical practice (Fig. 1).
Table 1.
The Musculoskeletal Society 2011 definition of PJI [12] (Reprinted with permission from Definition of Periprosthetic Joint Infection. Javad Parvizi and Thorsten Gehrke. The Journal of Arthroplasty. Elsevier; 2014. License number 4332751327806)
| MSIS definition of PJI—PJI exists when: | |
|---|---|
| 1 | There is a sinus tract communicating with the prosthesis; or |
| 2 | A pathogen is isolated by culture from two or more separate tissue or fluid samples obtained from the affected prosthetic joint; or |
| 3 | When 4 of the following 6 criteria exist: a. Elevated serum erythrocyte sedimentation rate and serum C-reactive protein (CRP) concentration b. Elevated synovial white blood cell count c. Elevated synovial polymorphonuclear percentage (PMN %) d. Presence of purulence in the affected joint e. Isolation of a microorganism in one culture of periprosthetic tissue or fluid, or f. Greater than 5 neutrophils per high-power field in 5 high-power fields observed from histologic analysis of periprosthetic tissue at × 400 magnification |
Table 2.
The International Consensus Meeting (ICM) definition of PJI [14] (Reprinted with permission from Definition of Periprosthetic Joint Infection. Javad Parvizi and Thorsten Gehrke. The Journal of Arthroplasty. Elsevier; 2014. License number 4332751327806)
| ICM definition of PJI | |||
|---|---|---|---|
| PJI is present if one of two major criteria or three of five minor criteria exists: | |||
| Major criteria | 1. There is a sinus tract communicating with the prosthesis; or | ||
| Major criteria | 1. Two positive periprosthetic cultures with phenotypically identical organisms; or | ||
| Minor criteria | Having three of the following minor criteria: | Acute PJI (< 90 days) | Chronic PJI (> 90 days) |
| 1.1. Elevated ESR or CRP | ESR: no threshold | ESR: > 30 mm/h | |
| CRP > 100 mg/L | CRP > 10 mg/L | ||
| 2. Elevated SF WBC count or |
10,000 cells/μL | 3000 cells/μL | |
| Changes in leukocyte esterase strip | + or ++ | + or ++ | |
| 3. Elevated SF PMN % | 90% | 80% | |
| 4. Positive histologic analysis of the periprosthetic tissue | > 5 neutrophils per high-power field in 5 high-power fields (× 400) | > 5 neutrophils per high-power field in 5 high-power fields (× 400) | |
| 5. A single positive culture | |||
CRP C-reactive protein, ESR sedimentation rate, SF WBC synovial fluid white blood cell, SF PMN synovial fluid neutrophil differential
Fig. 1.
ICM algorithm for diagnosing PJI (Reprinted with permission from “Diagnosis of Periprosthetic Joint Infection.” Journal of Orthopaedic Research. John Wiley and Sons Publishing Company; 2014, p. 78. License number 4332751175802)
The New 2018 Definition for Diagnosis of PJI
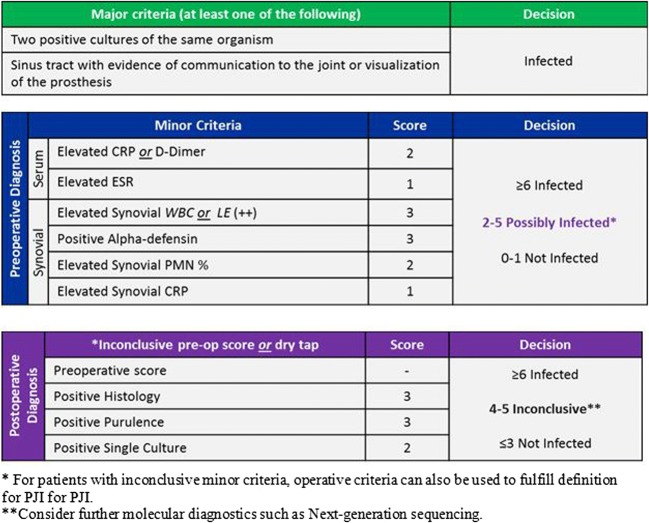
While the prior MSIS and ICM definitions were ground-breaking with respect to standardizing PJI diagnosis for patients, as well as facilitating collaborative research, these recommendations were generated largely through expert opinion, rather than being evidence-based or properly validated. Earlier this year, a multi-institutional effort using a stepwise approach with random forest statistical analysis and multivariate regression culminated in a new PJI diagnostic scoring system (Fig. 2) which shows improved diagnostic performance on formal external validation versus the current ICM and MSIS definitions [15•]. The new criteria demonstrate a higher sensitivity of 97.7% compared to the MSIS (79.3%) and International Consensus Meeting definition (86.9%), with a similar specificity of 99.5% (Table 3). This updated schema also incorporates newer diagnostic biomarkers and molecular tests, many of which are discussed later in this review, that were not considered in the prior definition published five years prior.
Fig. 2.
The 2018 validated and score-based ICM definition for PJI [15] (Reprinted with permission from “The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria.” The Journal of Arthroplasty. Elsevier; 2018; License Number 4332741333898)
Table 3.
Performance of the new PJI definition versus the current MSIS and ICM criteria [15] (Adapted with permission from “The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria.” The Journal of Arthroplasty. Elsevier; 2018; License Number 4332741333898)
| Criteria | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| MSIS (2011) | 79.3% (73.4–84.4) | 99.5% (97.3–99.99) |
| ICM (2013) | 86.9% (81.8–91.1) | 99.5% (97.3–99.99) |
| New PJI definition (2018) | 97.7% (94.7–99.3) | 99.5% (97.2–99.99) |
While the individual test thresholds remain similar to the established chronic PJI thresholds, an aggregate score is generated based on differential positive weighing of each test (Fig. 2). Patients with an aggregate preoperative score of ≥ 6 are deemed to have PJI, while a score between 2 and 5 requires further testing for intraoperative findings to confirm or refute the diagnosis. In addition, a final total combined postoperative score (incorporating the preoperative score) of ≥ 6 is considered infected, a score between 4 and 5 is inconclusive, and a score of 3 or less is deemed not infected.
Despite best efforts, the proposed criteria may still be confounded in the setting of adverse local soft tissue reactions, crystalline deposition disease, rheumatological flares, or infection with indolent organisms. A high index of suspicion should thus be maintained in these scenarios, as diagnostic tests may generate misleading results. However, this caveat applies to the current definitions too, and it seems apparent on formal validation that the new 2018 definition performs significantly better at diagnosing PJI (Table 3).
Established Diagnostic Tests
Synovial Fluid White Cell Counts and Neutrophil Percentage
Joint fluid aspiration for the assessment of synovial white blood cell (WBC) count and neutrophil differential (PMN %) is invaluable for the diagnosis of acute and chronic PJI with good evidence to support testing [35–39]. Both are important parameters specified as minor criteria within the current ICM definition, but with variable diagnostic thresholds in the acute and chronic postoperative period [40].
For the acute period (within less than 6 weeks postoperatively), a threshold of > 10,000 cells per microliter for the synovial WBC count as well as a threshold of > 90% for synovial PMN % is recommended to diagnose PJI [34]. For the chronic postoperative period (greater than 6 weeks from index TJA), a threshold of > 3000 cells per microliter for synovial WBC count and a threshold of > 80% for synovial PMN % is recommended to aid in the diagnosis of chronic PJI [34]. Using these threshold recommendations, a sensitivity of 85.8% (95% CI 82.5–89.1) and specificity of 83.0% (95% CI 79.3–86.7) have been reported for synovial WBC count [30]. Moreover, sensitivity of 85.8% (95% CI 82.5–89.2) and specificity of 80.8% (95% CI 76.9–84.8) have been reported for synovial PMN % for diagnosis of PJI [30].
Using a statistical calculation known as the diagnostic odds ratio (DOR), defined as the ratio of the odds of positivity in patients with a disease compared with the odds of positivity in patients without disease [DOR = (True positive/False negative)/(False positive/True negative)], Shahi et al. established that the synovial fluid WBC count had the second highest diagnostic odds ratio (DOR 29.4, 95% CI 20.2–42.8) out of a panel of conventional tests [41]. This same study found PMN % to have an even greater DOR of 25.5 (95% CI 17.5–37.0) [30].
In certain clinical scenarios, synovial fluid leukocyte-related results should be interpreted with particular caution [42–44]. Published data suggests that an automated synovial fluid WBC count and PMN differential may be unreliable in the setting of a failed metal-on-metal bearing or corrosion reaction [42]. A manual synovial fluid WBC count is recommended in this setting as monocytes with phagocytosed metal particles can interfere with certain laboratory instruments, leading to false-positive interpretations [43]. Traumatic aspirations should also be corrected to determine the true level of synovial cells in bloody joint fluid by using a validated formula that adjusts for synovial RBC, serum RBC, and serum WBC counts [44]. Also, as is the case with other routine tests, the diagnostic utility of synovial WBC count and PMN % can be negatively impacted by previous antibiotic use [45, 46]. In contrast, a concurrent diagnosis of inflammatory arthropathy does not impact the thresholds or utility of synovial WBC counts and PMN % [47].
Leukocyte Esterase
Leukocyte esterase is an enzyme produced by activated neutrophils at the site of infection and has traditionally been used to help diagnose urinary tract infections in the inpatient and outpatient setting [48]. Part of the reason for its widespread usage and inclusion within the standard PJI diagnostic algorithm by the ICM is that it can be measured quickly and easily with a colorimetric strip (urinalysis dipstick) [22]. Furthermore, synovial fluid LE testing is simple and inexpensive with an estimated cost of $0.17 per test [20]. Another advantage is the capacity to provide almost immediate test results, which can prove invaluable in the operative setting.
Of note, one conceivable disadvantage is the possibility for blood within the synovial fluid to interfere with the color change of the urinalysis strip [25, 49]. To prevent this from happening, clinicians should ensure removal of blood contamination from a synovial fluid sample with the use of a centrifuge for at least 2 min [50]. When this protocol is followed, LE has shown to be an effective diagnostic tool for PJI.
A meta-analysis by Wyatt et al. showed a pooled sensitivity of 81% coupled with a specificity of 97% using a (++) reading as a threshold for PJI [20]. Another recent study compared the performance of LE using (+) and (++) as threshold along with standard diagnostic tests (including serum ESR, serum CRP, synovial fluid WBC, and PMN %) and found LE to have the highest test performance for diagnosing PJI (OR 30.06, 95% CI 17.8–50.7) [30].
Intraoperative Cultures—Duration of Incubation and Number of Samples
Current consensus recommendations suggest that at least three but not more than six intraoperative samples should be sent for culture [51–54]. In order to achieve optimal yield from traditional cultures, these should be incubated for a minimum of 5 to 14 days, with a longer duration > 14 days considered in cases of suspected culture-negative PJI or where indolent, slow growing, and fastidious organisms such as Propionibacterium acnes are suspected [55].
Whether to hold perioperative prophylactic antibiotics prior to sampling for culture in the operating room remains contentious. Current guidance suggests that prophylactic antibiotics need only be withheld in cases with a high suspicion for PJI in which the infecting organism has not been identified. Two prospective studies, of which one was randomized, have demonstrated that prophylactic preoperative antibiotics do not impair the sensitivity of traditional intraoperative cultures [56, 57]. It is therefore recommended that mandatory withholding of antibiotic prophylaxis at the start of operative procedures is not justified in cases where a pathogen has already been identified.
The existence of two positive cultures is considered to be diagnostic for PJI [34], whereas a single positive culture may occur from a contaminating organism and should thus be considered in conjunction with other markers of infection. Culture results are not only helpful for diagnosis of PJI but also for selection of appropriate antimicrobials that will be effective for treating the infecting organism. However, while cultures have historically been used as a standard reference for pathogen identification in PJI, there are several limitations to their use [58] and it has been reported that up to 30% of PJI patients are culture negative [55, 59, 60].
Emerging Diagnostic Tests
Interleukin-6
Interleukin-6 (IL-6) is a cytokine produced by activated monocytes and macrophages as part of the inflammatory response [61–64]. Serum levels have been shown to rise to 30–340 pg/ml in infection, trauma, and the postoperative setting [64, 65]. Since this cytokine lies upstream of other markers, such as CRP, in the acute phase inflammatory cascade [66], it may be a faster and more sensitive marker for the detection of PJI [67]. Furthermore, IL-6 serum levels return to normal (< 1 pg/ml) relatively rapidly postoperatively, within approximately 2 to 3 days of uncomplicated arthroplasty [65, 68], which also increases its utility as a potential marker of PJI.
Two meta-analyses have highlighted the diagnostic potential of IL-6 in the context of PJI [63, 67]. Berbari et al. showed that IL-6 had a higher diagnostic odds ratio, which is indicative of better overall test performance, than CRP (314.7 versus 13.1) and a pooled sensitivity of 0.97 (95% CI 0.93–0.99) and specificity of 0.91 (95% CI 0.93–0.99) for diagnosis of PJI [63]. In a more recent meta-analysis of 17 studies, Xie et al. found that the pooled sensitivity and specificity of serum IL-6 was 0.72 (95% CI 0.63–0.80) and 0.89 (95% CI 0.77–0.95), respectively [67]. Synovial fluid IL-6 had a higher diagnostic value for PJI than the serum test, with a collated sensitivity and specificity of 0.91 (95% CI 0.82–0.96) and 0.90 (95% CI 0.84–0.95), respectively.
While IL-6 shows significant promise for the early detection of PJI, it is currently not widely used in the clinical setting or part of current diagnostic guidelines due to variability and lack of consistency in the results. For instance, Randau et al. performed a prospective review of 120 revision knee and hip cases, and reported sensitivities and specificities of IL-6 ranging from 49 to 79% and 58 to 88%, respectively [69]. Further investigation is thus required to validate routine use and the costs associated with IL-6 testing.
Alpha-Defensin
Alpha-defensin is a promising synovial biomarker for the diagnosis of PJI that is widely available for clinical use. Alpha-defensin is a naturally occurring antimicrobial peptide released from activated neutrophils as part of the innate immune response to pathogens [70, 71]. Its endogenous mechanism of antimicrobial activity is via permeabilization of microbial membranes [72]. It has shown to rise in response to low-virulence organisms and is unaffected by prior antibiotic administration [17, 23, 71, 73, 74]. Unlike LE, alpha-defensin is quite expensive with each test costing approximately $760; however, as a diagnostic tool, it has proven to be highly accurate for early detection and diagnosis of PJI [75].
A meta-analysis by Wyatt et al. demonstrated excellent pooled sensitivity of 100% with specificity at 96% in diagnosis of PJI [20]. Interestingly, there are two different methods to utilize alpha-defensin. Namely, these are laboratory-based α-defensin immunoassay, which gives a quantitative readout within 24 h, and the rapid lateral flow test, which gives a qualitative binary readout result within around 20 min. Both methods have their pros and cons. Laboratory testing may be cost-prohibitive for certain departments. Furthermore, it requires that samples be shipped to a centralized Synovasure lab, thereby adding processing time before results are available. In contrast, the lateral flow test may be performed as a point-of-care test locally; however, there are reports of results being user-dependent and not as accurate as the formal laboratory Synovasure method [24, 76, 77].
In a recent systematic review, Eriksson et al. showed the lateral flow test has lower overall diagnostic value compared with the laboratory immunoassay (area under the curve, 0.75 versus 0.98) but retains its specificity (90 versus 96%; p = 0.06) and therefore it may be useful as a rapid test to “rule in” infection perioperatively [78]. Similarly, a meta-analysis of ten studies by Suen et al. reported the pooled sensitivity and specificity of the lateral flow test (0.77 and 0.91) as lower than those of the α-defensin lab-based immunoassay (0.95 and 0.97) [77].
Of note, Plate et al. compared the lateral flow test results versus the current ICM definition in a series of 109 cases of suspected PJI. They found that the lateral flow test may be falsely positive in conjunction with an underlying non-infectious inflammatory disease or crystal deposition disease [79], and therefore advocated its use only in addition to the current ICM criteria and assessment of crystals deposition in synovial fluid aspirates.
In a similar fashion to the synovial fluid WBC count, adverse local tissue reactions secondary to a failed metal-and-metal bearing or corrosion at the head neck junction may confound interpretation of α-defensin results. Okroj et al. examined a multicenter cohort of patients with ALTR who underwent α-defensin testing. They reported that 31% of these cases had a falsely positive α-defensin, but were otherwise negative per the MSIS criteria for PJI diagnosis [80].
Serum D-dimer
D-dimer is a fibrin degradation product released into the blood released following the fibrin clot breakdown by plasmin. While D-dimer is a nonspecific serum marker that aids in the screening algorithm for venous thromboembolism, it has recently shown promise as a biomarker for the diagnosis of PJI, as well as timing of reimplantation [19]. In a prospective study of 245 primary and revision arthroplasty patients, serum D-dimer outperformed both ESR and serum CRP, with a sensitivity of 89.5% and specificity of 92.8%. A threshold of 850 ng/ml was calculated in this study as the optimal cut-off value for serum D-dimer for PJI diagnosis [19].
Further investigation by Lee et al. showed that D-dimer has a more rapid rise and fall in the early postoperative period after joint arthroplasty than ESR and CRP [18]. In contrast to serum ESR and CRP, which remained elevated until postoperative day 5 and day 3, D-dimer levels generally decreased to baseline levels at postoperative day 2, before reaching a second peak at postoperative week 2 [18].
Measurement of serum D-dimer is a widely available and accessible test that may be effective screening tool for the early detection of PJI. However, further validation work is required to reproduce these findings and confirm the relative test performance of D-dimer versus other more established serum markers [81].
Next-generation Sequencing
While microbiological culture remains the mainstay for pathogen identification in the context of PJI, molecular technologies such as next-generation sequencing (NGS) have started to become clinically feasible and demonstrate utility in this setting (Table 4). In a recent report from the American Academy of Microbiology [82, 83], NGS was cited as having the potential to dramatically revolutionize the clinical microbiology laboratory by “replacing current time-consuming and labor-intensive techniques with a single, all-inclusive diagnostic test.”
Table 4.
Emerging molecular diagnostic tools
| Methodology | Description |
|---|---|
| MALDI-TOF mass spectrometry | ○ Analyzes the patterns of protein biomolecules produced by microbes, which is both cost effective and rapid, but requires a proteomic reference database. |
| Next-generation sequencing (NGS) | ○ A collection of non-Sanger-based high-throughput DNA sequencing methods that produce data in vastly larger amounts, at lower cost, in shorter time, than previous methods. ○ Two broadly available types include 16S-amplicon targeted NGS and shotgun metagenomics. |
| 16S-amplicon targeted NGS | ○ Targeted NGS following amplification of the 16S gene via a PCR reaction (16S chosen as it avoids the issue of host eukaryotic DNA extraction) ○ Amplified DNA is then pooled and loaded onto beads for emulsion PCR, generating high sample levels for further NGS sequencing ○ Sequencing is performed on a platform, such as the PGM Ion Torrent or Illumina MiSeq, which produces a signal change with every nucleotide incorporated into DNA. Reads are then de-noised and filtered to remove interference ○ Lastly, there is comparison of sequence reads against the curated database, such as the National Institutes of Health GenBank library of all known microbes, which generates genus- and species-level distinction. |
| Shotgun metagenomics | ○ Application of whole genome sequencing to a mixture of microbial genomes. ○ DNA is extracted, producing a mixture of genomes, which are randomly fragmented to produce a collection of random DNA sequences which are aligned and compared against bioinformatic sequence databases. |
NGS refers to a collection of non-Sanger-based high-throughput DNA sequencing methods that can produce data in vastly larger amounts, at greatly lower cost, in shorter time, and with less manual intervention than previous methods [82]. Unlike methods based upon polymerase chain reaction (PCR), NGS can be used in “open” mode which does not rely on a set of parameters or a panel of PCR primer targets. It is therefore capable of characterizing all microbial DNA present within a given clinical sample, and providing a complete picture of the microbial profile without the need for preconceived ideas of the possible responsible pathogen. NGS searches all known microbial databases for a match—including bacteria, viruses, yeast, fungi, and parasites—without the need for additional individual testing. NGS also has the potential to suggest antimicrobial resistance through identification of known resistance genes [84].
Two broad methodological approaches to implement NGS for PJI diagnosis are currently being explored within the literature: (1) 16S amplicon targeted NGS and (2) shotgun metagenomic sequencing.
The 16S amplicon targeting method has demonstrated particular utility for pathogen detection, for instance with the detection of Streptococcus canis in a previously presumed culture-negative PJI [28]. In a recently published report, NGS was a useful adjunct for pathogen detection in 81.8% of culture-negative PJI where intraoperative tissue samples were analyzed [26•]. Furthermore, in a series of 86 synovial fluid samples, high concordance with microbiological culture was seen with NGS of synovial fluid alone [27].
Shotgun metagenomic sequencing has also shown recent promise in sonicate fluid samples with a 43.9% detection rate of potential pathogens in culture-negative PJI [85] and species-level sensitivity of 88% [29•] However, there remain significant issues with host DNA contamination and the overall cost of this method is approximately tenfold greater than 16S-amplicon targeted sequencing. That said, further work into microbial DNA enrichment methods and bioinformatics may help address these issues.
Ultimately, multicenter clinical studies and eventually clinical trials examining patient treatment outcomes will be necessary to validate and reinforce the benefits as well as cost savings of using NGS-based tests for the diagnosis of PJI and targeting antimicrobial treatment.
Conclusion
In conclusion, we present an overview of the literature, as well as the newly published validated, evidence-based criteria for diagnosing PJI after hip and knee arthroplasty. Despite extensive research, particularly over the last decade, the diagnosis of PJI remains uncertain in some cases. These patients may benefit from the use of emerging biomarkers or novel techniques such as next-generation sequencing. Further research is needed to correlate the signal of novel tests with patient treatment outcomes, to ultimately justify their clinical utility and expense.
Conflict of Interest
Dr. Parvizi reports grants, royalties, stocks, and/or consultancy with the following entities: Alphaeon, Ceramtec, Ceribell, ConvaTec, Corentec, Cross Current Business, Datatrace, Elsevier, Ethicon, Heron, Hip Innovation Technology, Intellijoint, Invisible Sentinel, JayPee, Joint Purification Systems, MDValuate, MicrogenDx, Parvizi Surgical Innovations, Physician Recommended Nutriceuticals, PRN-Veterinary, SLACK, Tenor, Tissue Gene, Wolters Kluwer, and Zimmer, outside of the submitted work.
Dr. Courtney is a consultant for Hip Innovation Technology, outside of the submitted work.
Dr. Goswami has no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Prosthetic Joint Infection
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Jt Surg. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Hackett DJ, Rothenberg AC, Chen AF, Gutowski C, Jaekel D, Tomek IM, Parsley BS, Ducheyne P, Manner PA. The economic significance of orthopaedic infections. J Am Acad Orthop Surg. 2015;23:Suppl:S1–Suppl:S7. doi: 10.5435/JAAOS-D-14-00394. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplast. 2012;27:61–65.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33:659. doi: 10.3928/01477447-20100722-42. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 8.Lavernia C, Lee DJ, Hernandez VH. The increasing financial burden of knee revision surgery in the United States. Clin Orthop. 2006;446:221–226. doi: 10.1097/01.blo.0000214424.67453.9a. [DOI] [PubMed] [Google Scholar]
- 9.Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95:2177–2184. doi: 10.2106/JBJS.L.00789. [DOI] [PubMed] [Google Scholar]
- 10.Gehrke T, Alijanipour P, Parvizi J. The management of an infected total knee arthroplasty. Bone Jt J. 2015;97-B:20–29. doi: 10.1302/0301-620X.97B10.36475. [DOI] [PubMed] [Google Scholar]
- 11.Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K, American Academy of Orthopaedic Surgeons American Academy of Orthopaedic surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93:1355–1357. doi: 10.2106/JBJS.9314EBO. [DOI] [PubMed] [Google Scholar]
- 12.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Valle CJD, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469:2992–4. 10.1007/s11999-011-2102-9 [doi]. [DOI] [PMC free article] [PubMed]
- 13.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;56:1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 14.•.Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt J. 2013;95-B:1450–1452. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 15.•.Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 Definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplast 2018. doi:10.1016/j.arth.2018.02.078. Newly published 2018 definition for PJI that is evidence-based and formally validated on an external hold sample. New scoring system has improved diagnostic performance versus the current ICM and MSIS definitions, as summarized in Table 3.
- 16.Patel R, Alijanipour P, Parvizi J. Advancements in diagnosing periprosthetic joint infections after total hip and knee arthroplasty. Open Orthop J. 2016;10:654–661. doi: 10.2174/1874325001610010654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop. 2014;472:3254–3262. doi: 10.1007/s11999-014-3543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YS, Koo K-H, Kim HJ, Tian S, Kim T-Y, Maltenfort MG, Chen AF. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99:2077–2084. doi: 10.2106/JBJS.17.00123. [DOI] [PubMed] [Google Scholar]
- 19.•.Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am. 2017;99:1419–1427. doi: 10.2106/JBJS.16.01395. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2016;98:992–1000. doi: 10.2106/JBJS.15.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for musculoskeletal infection society criteria. J Bone Joint Surg Am. 2014;96:1917–1920. doi: 10.2106/JBJS.M.01591. [DOI] [PubMed] [Google Scholar]
- 22.Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93:2242–2248. doi: 10.2106/JBJS.J.01413. [DOI] [PubMed] [Google Scholar]
- 23.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439–1445. doi: 10.2106/JBJS.M.01316. [DOI] [PubMed] [Google Scholar]
- 24.Sigmund IK, Holinka J, Gamper J, Staats K, Böhler C, Kubista B, Windhager R. Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Jt J. 2017;99-B:66–72. doi: 10.1302/0301-620X.99B1.BJJ-2016-0295.R1. [DOI] [PubMed] [Google Scholar]
- 25.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE, Parvizi J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop. 2015;473:198–203. doi: 10.1007/s11999-014-3722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.•.Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am. 2018;100:147–154. doi: 10.2106/JBJS.17.00434. [DOI] [PubMed] [Google Scholar]
- 27.Tarabichi M, Shohat N, Goswami K, Parvizi J. Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Jt J. 2018;100-B:127–133. doi: 10.1302/0301-620X.100B2.BJJ-2017-0531.R2. [DOI] [PubMed] [Google Scholar]
- 28.Tarabichi M, Alvand A, Shohat N, Goswami K, Parvizi J. Diagnosis of Streptococcus canis periprosthetic joint infection: the utility of next-generation sequencing. Arthroplasty Today. 2018;2017 10.1016/j.artd.2017.08.005. [DOI] [PMC free article] [PubMed]
- 29.•.Street TL, Sanderson ND, Atkins BL, Brent AJ, Cole K, Foster D, McNally MA, Oakley S, Peto L, Taylor A, Peto TEA, Crook DW, Eyre DW. Molecular diagnosis of orthopaedic device infection direct from sonication fluid by metagenomic sequencing. J Clin Microbiol. 2017;55:2334–2347. doi: 10.1128/JCM.00462-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahi A, Tan TL, Kheir MM, Tan DD, Parvizi J. Diagnosing periprosthetic joint infection: and the winner is? J Arthroplast. 2017;32:S232–S235. doi: 10.1016/j.arth.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Nodzo SR, Bauer T, Pottinger PS, Garrigues GE, Bedair H, Deirmengian CA, Segreti J, Blount KJ, Omar IM, Parvizi J. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23(Suppl):S18–S25. doi: 10.5435/JAAOS-D-14-00385. [DOI] [PubMed] [Google Scholar]
- 32.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Lorenzo D, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E. Diagnosis of periprosthetic joint infection. J Arthroplast. 2014;29:77–83. doi: 10.1016/j.arth.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 34.Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection. Foreword. J Orthop Res Off Publ Orthop Res Soc 2014;32 Suppl 1:2. doi:10.1002/jor.22543 [DOI] [PubMed]
- 35.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplast. 2003;18:1038–1043. doi: 10.1016/S0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 36.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Bedair H, Ting N, Jacovides C, Saxena A, Moric M, Parvizi J, Della Valle CJ. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop. 2011;469:34–40. doi: 10.1007/s11999-010-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27:1589–1593. doi: 10.1016/j.arth.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 39.Dinneen A, Guyot A, Clements J, Bradley N. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Jt J. 2013;95-B:554–557. doi: 10.1302/0301-620X.95B4.30388. [DOI] [PubMed] [Google Scholar]
- 40.Parvizi J, Gehrke T. International consensus group on periprosthetic joint infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. doi: 10.1016/j.arth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/S0895-4356(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 42.Kwon Y-M, Antoci V, Leone WA, Tsai T-Y, Dimitriou D, Liow MHL. Utility of serum inflammatory and synovial fluid counts in the diagnosis of infection in taper corrosion of dual taper modular stems. J Arthroplast. 2016;31:1997–2003. doi: 10.1016/j.arth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Yi PH, Cross MB, Moric M, Levine BR, Sporer SM, Paprosky WG, Jacobs JJ, Della Valle CJ. Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clin Orthop. 2015;473:498–505. doi: 10.1007/s11999-014-3902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghanem E, Houssock C, Pulido L, Han S, Jaberi FM, Parvizi J. Determining “true” leukocytosis in bloody joint aspiration. J Arthroplast. 2008;23:182–187. doi: 10.1016/j.arth.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Shahi A, Deirmengian C, Higuera C, Chen A, Restrepo C, Zmistowski B, Parvizi J. Premature therapeutic antimicrobial treatments can compromise the diagnosis of late periprosthetic joint infection. Clin Orthop. 2015;473:2244–2249. doi: 10.1007/s11999-015-4142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahi A, Parvizi J, Kazarian GS, Higuera C, Frangiamore S, Bingham J, Beauchamp C, Valle CD, Deirmengian C. The alpha-defensin test for periprosthetic joint infections is not affected by prior antibiotic administration. Clin Orthop. 2016;474:1610–1615. doi: 10.1007/s11999-016-4726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipriano CA, Brown NM, Michael AM, Moric M, Sporer SM, Della Valle CJ. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J Bone Joint Surg Am. 2012;94:594–600. doi: 10.2106/JBJS.J.01318. [DOI] [PubMed] [Google Scholar]
- 48.Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin N Am. 2014;28:75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplast. 2012;27:8–11. doi: 10.1016/j.arth.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal VK, Tischler E, Ghanem E, Parvizi J. Leukocyte esterase from synovial fluid aspirate: a technical note. J Arthroplasty. 2013;28:193–195. doi: 10.1016/j.arth.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Mikkelsen DB, Pedersen C, Højbjerg T, Schønheyder HC. Culture of multiple peroperative biopsies and diagnosis of infected knee arthroplasties. APMIS Acta Pathol Microbiol Immunol Scand. 2006;114:449–452. doi: 10.1111/j.1600-0463.2006.apm_428.x. [DOI] [PubMed] [Google Scholar]
- 52.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 53.Kamme C, Lindberg L. Aerobic and anaerobic bacteria in deep infections after total hip arthroplasty: differential diagnosis between infectious and non-infectious loosening. Clin Orthop. 1981:201–7. [PubMed]
- 54.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group J Clin Microbiol. 1998;36:2932–2939. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parvizi J, Erkocak OF, Della Valle CJ. Culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:430–436. doi: 10.2106/JBJS.L.01793. [DOI] [PubMed] [Google Scholar]
- 56.Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop. 2014;472:3997–4003. doi: 10.1007/s11999-014-3828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burnett RSJ, Aggarwal A, Givens SA, McClure JT, Morgan PM, Barrack RL. Prophylactic antibiotics do not affect cultures in the treatment of an infected TKA: a prospective trial. Clin Orthop. 2010;468:127–134. doi: 10.1007/s11999-009-1014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120:570–574. doi: 10.1007/s004020000174. [DOI] [PubMed] [Google Scholar]
- 59.McLawhorn AS, Nawabi DH, Ranawat AS. Management of resistant, atypical and culture-negative periprosthetic joint infections after hip and knee arthroplasty. Open Orthop J. 2016;10:615–632. doi: 10.2174/1874325001610010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aggarwal VK, Bakhshi H, Ecker NU, Parvizi J, Gehrke T, Kendoff D. Organism profile in periprosthetic joint infection: pathogens differ at two arthroplasty infection referral centers in Europe and in the United States. J Knee Surg. 2014;27:399–406. doi: 10.1055/s-0033-1364102. [DOI] [PubMed] [Google Scholar]
- 61.Cesare PED, Chang E, Preston CF, Liu CJ. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Jt SurgeryAm. 2005;87:1921–1927. doi: 10.2106/JBJS.D.01803. [DOI] [PubMed] [Google Scholar]
- 62.Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Gotze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Jt SurgeryBritish. 2007;89:94–99. doi: 10.1302/0301-620X.89B1.17485. [DOI] [PubMed] [Google Scholar]
- 63.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Jt SurgeryAm. 2010;92:2102–2109. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- 64.Song M, Kellum JA. Interleukin-6. Crit Care Med. 2005;33:S463–S465. doi: 10.1097/01.CCM.0000186784.62662.A1. [DOI] [PubMed] [Google Scholar]
- 65.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–196. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Köhl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000;28:2793–2798. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 67.Xie K, Dai K, Qu X, Yan M. Serum and synovial fluid interleukin-6 for the diagnosis of periprosthetic joint infection. Sci Rep. 2017;7:1496. doi: 10.1038/s41598-017-01713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ettinger M, Calliess T, Kielstein JT, Sibai J, Bruckner T, Lichtinghagen R, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61:332–341. doi: 10.1093/cid/civ286. [DOI] [PubMed] [Google Scholar]
- 69.Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, Limmer A, Wirtz DC, Gravius S. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9:e89045. doi: 10.1371/journal.pone.0089045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 71.Lehrer RI, Ganz T. Defensins: endogenous antibiotic peptides from human leukocytes. CIBA Found Symp. 1992;171:276–290. doi: 10.1002/9780470514344.ch16. [DOI] [PubMed] [Google Scholar]
- 72.Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop. 2015;473:2229–2235. doi: 10.1007/s11999-015-4152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahi A, Parvizi J. Serum D-dimer levels may be a reliable test for diagnosis of periprosthetic joint infection. Orthop today 2016.
- 75.Alvand A, Rezapoor M, Parvizi J. The role of biomarkers for the diagnosis of implant-related infections in orthopaedics and trauma. Adv Exp Med Biol. 2017;971:69–79. 10.1007/5584_2017_11 [doi]. [DOI] [PubMed]
- 76.Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M. Intraoperative diagnosis of periprosthetic joint infection using a novel alpha-defensin lateral flow assay. J Arthroplast. 2016;31:2871–2874. doi: 10.1016/j.arth.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 77.Suen K, Keeka M, Ailabouni R, Tran P. Synovasure “quick test” is not as accurate as the laboratory-based α-defensin immunoassay: a systematic review and meta-analysis. Bone Jt J. 2018;100-B:66–72. doi: 10.1302/0301-620X.100B1.BJJ-2017-0630.R1. [DOI] [PubMed] [Google Scholar]
- 78.Eriksson HK, Nordström J, Gabrysch K, Hailer NP, Lazarinis S. Does the alpha-defensin immunoassay or the lateral flow test have better diagnostic value for Periprosthetic joint infection? A systematic review. Clin Orthop. 2018;476:1065–1072. doi: 10.1007/s11999.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plate A, Stadler L, Sutter R, Anagnostopoulos A, Frustaci D, Zbinden R, Fucentese SF, Zinkernagel AS, Zingg PO, Achermann Y Inflammatory disorders mimicking periprosthetic joint infections may result in false positive α-defensin. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2018. doi:10.1016/j.cmi.2018.02.019. [DOI] [PubMed]
- 80.Okroj KT, Calkins TE, Kayupov E, Kheir MM, Bingham JS, Beauchamp CP, Parvizi J, Della Valle CJ. The alpha-defensin test for diagnosing periprosthetic joint infection in the setting of an adverse local tissue reaction secondary to a failed metal-on-metal bearing or corrosion at the head-neck junction. J Arthroplast. 2018;33:1896–1898. doi: 10.1016/j.arth.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Saleh A, George J, Faour M, Klika AK, Higuera CA. Serum biomarkers in periprosthetic joint infections. Bone Jt Res. 2018;7:85–93. doi: 10.1302/2046-3758.71.BJR-2017-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. MBio. 2015;6:e01888–e01815. doi: 10.1128/mBio.01888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Applications of clinical microbial next-generation sequencing, February 2016 n.d. https://www.asm.org/index.php/colloquium-reports/item/4462-applications-of-clinical-microbial-next-generation-sequencing (accessed April 19, 2018).
- 84.Dunne WM, Westblade LF, Ford B. Next-generation and whole-genome sequencing in the diagnostic clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2012;31:1719–1726. doi: 10.1007/s10096-012-1641-7. [DOI] [PubMed] [Google Scholar]
- 85.Thoendel M, Jeraldo P, Greenwood-Quaintance KE, Chia N, Abdel MP, Steckelberg JM, et al. A possible novel prosthetic joint infection pathogen, mycoplasma salivarium, identified by metagenomic shotgun sequencing. Clin Infect Dis Off Publ Infect Dis Soc Am 2017. doi:10.1093/cid/cix296. [DOI] [PMC free article] [PubMed]