Abstract
Purpose of Review
Although a two-stage exchange revision is reported to have a high success rate, this strategy may fail as a treatment for prosthetic joint infection (PJI). When it does, resection arthroplasty, arthrodesis, amputation, and chronic antibiotic suppression may play a role. The purpose of this review is to determine which are the main risk factors for a two-stage exchange failure and to analyze the indications and results of resection arthroplasty, arthrodesis, amputation, and antibiotic chronic suppression for PJI.
Recent Findings
Recent literature demonstrates that the main risk factors for a two-stage exchange failure are as follows: hemodialysis, obesity, multiple previous procedures, diabetes mellitus, corticosteroid therapy, hypoalbuminemia, immunosuppression, rheumatological conditions, coagulation disorders, and infection due to multidrug-resistant (MDR) bacteria or fungal species. Regarding microorganisms, besides Staphylococcus aureus, Streptococcus spp., Enterobacteriaceae species such as Klebsiella pneumoniae and Enterobacter sp., Pseudomonas aeruginosa, or Acinetobacter baumannii, and fungus including Candida sp. are also considered risk factors for a two-stage exchange failure. Resection arthroplasty, arthrodesis, and amputation have a limited role. Chronic suppression is an option for high-risk patients or unfeasible reconstruction.
Summary
In summary, we report the main risk factors for a two-stage exchange failure and alternative procedures when it occurs. Future research on patient-specific risk factors for a two-stage exchange may aid surgical decision-making and optimization of outcomes.
Keywords: Periprosthetic joint infection (PJI), Two-stage infected exchange failure, Revision hip arthroplasty, Two-stage exchange arthroplasty, Chronic suppression therapy
Introduction
Periprosthetic joint infections (PJIs) are among the most devastating conditions in orthopedic surgery, and it is associated with a significant economic burden for healthcare systems worldwide. The management of PJI is associated with higher healthcare expenditure by health institutions, which was estimated at more than $566 million, and may exceed $1.62 billion by 2020 [1]. PJI patients often present with impaired function, poor quality of life, and lower expectations after having experienced clinical improvements commonly present after primary total hip arthroplasty. Due to the important economic burden of PJI, as well as its significant morbidity, standardized practices were proposed to improve the clinical and surgical management of PJI [2–4]. Several strategies, including those based upon surgical and antimicrobial therapy, have been considered for the management of PJI. Among the operative strategies, debridement, implant retention, and antibiotic therapy (DAIR) were adopted for decades as first-line treatment. In the presence of high failure rates, surgeons have adopted different surgical modalities of treatment, including the exchange of the implant, arthrodesis, or even amputation. Prosthesis exchange is usually performed in a single step, or as a staged two-stage treatment. Administration of intravenous antimicrobial therapy is recommended for both surgical strategies, and it is adjusted according to the cultures and sensitivity results obtained intraoperatively [5–7, 8••].
Predictors and Risk Factors for Failure of a Two-Stage Exchange
In face of the high failure rates after debridement, antibiotics, and implant retention (DAIR), the surgical treatment of PJI is traditionally based on exchange of the implant, which can be performed in one or two stages [15]. The two-stage revision surgery, which is also denominated as staged exchange, has been traditionally adopted worldwide as a successful treatment for PJI, although it is considered more expensive than the one-stage procedure [5–7, 8••]. In addition, patients who had a two-stage revision have lower self-reported health-related quality of life outcomes and it may present inferior health-related quality of life [16]. Even though it has been associated with a prolonged immobilization period, the staged revision is considered to have an acceptable control and eradication of the infection while maintaining joint functionality [5, 7, 17]. The morbidity and mortality of patients undergoing a second-stage revision total hip arthroplasty (THA) for the treatment of PJI is high, which may be due to the necessity of two major surgical procedures [5, 14••]. Removing an infected implant is considered a high-risk surgery and, according to a recent study, this procedure is associated with a 30-day readmissions rate of 11.1% and a 90-day mortality rate of 2.6% [18]. A national investigation reported the reimplantation rates 1 year after the first stage of the revision THA. Only 60% of the patients were reimplanted [12]. The mortality after the first stage was 6.5%. A significant percentage of patients who survived the first stage had either resection arthroplasty (the Girdlestone-type procedure, 5.7%) or repeated debridement procedures (10.8%) [12].
Failure of a two-stage exchange can be defined as the recurrence of clinical infection and radiographic signs of implant loosening, or the need for a subsequent surgery due to infection [19]. Other definitions include persistence of infection with clinical signs and symptoms along with a high C-reactive protein with a subsequent second debridement after the completion of the antibiotic treatment. Additionally, death related to the PJI remains a pertinent outcome [15].
Multiple factors are associated with poor clinical outcomes after a failed staged revision total hip and total knee arthroplasty (Table 1). One of the most important is the persistence of low-grade infection upon which the patient presents with nonspecific signs of infection. Lee et al. evaluated risk factors for treatment failure after PJI in a cohort of 43 patients. Although other factors were considered, including the presence of comorbidities and inadequate treatment with empirical antibiotics, Staphylococcus aureus infection was the only significant risk factor for treatment failure [9]. S. aureus can persist after implant exchange in two-stage revision arthroplasty, increasing the minimum inhibitory concentrations (MIC) of antibiotics commonly used in cement spacers, and posing a risk for infections caused by resistant pathogens [20••]. Persistent infection should be considered as one of the main causes of failure after a two-stage exchange. Even though S. aureus is considered the main pathogen associated with treatment failure, other microorganisms should be thoroughly investigated, including Cutibacterium acnes (former Propionibacterium acnes) and MDR Gram-negative bacilli [21]. A recent study performed by Brown et al. found that, among patients diagnosed with PJI caused by fungal pathogens and treated with a two-stage exchange, reinfection caused by the same fungal organism occurred in two out of three cases [10•]. Streptococcal pathogens are also associated with failure after a two-stage exchange procedure [11]. According to a recent study with 1-year follow-up, mortality in patients undergoing a two-stage exchange revision was significantly associated with male gender, age greater than 85 years old, presence of comorbidities, and hemodialysis [12]. Female gender and depression were associated with a higher risk of not having a reimplantation, while obesity and inflammatory arthritis were associated with repeat debridement without reimplantation [12]. Higher perioperative glucose levels in non-diabetic patients may be a risk factor for PJI [22, 23]. In a recent study investigating two-stage exchange for total knee arthroplasty (TKA), Ma et al. reported a 14.8% failure rate in a cohort of 108 patient who had a two-stage exchange due to PJI. A multivariate analysis revealed body mass index ≥ 30 kg/m, an operative time greater than 4 h, and gout to be the strongest predictors for failure [13•].
Table 1.
Risk factors for failure during two-step exchange for PJI
| Author | Age | Gender | Comorbidities | Pathogen | Duration of surgery | Length of hospital stay | Time to reimplantation |
|---|---|---|---|---|---|---|---|
| Lee et al. [9] | – | – | – | S. aureus | – | – | – |
| Brown et al. [10•] | – | – | – | Fungal pathogens | – | – | – |
| Akgun et al. [11] | – | – | – | Streptococcus | – | – | – |
| Cancienne et al. [12] | ≥ 85 | Males | Diabetes Congestive heart failure Chronic lung disease Liver diseases Kidney diseases Obesity Inflammatory Arthritis Depression |
– | – | – | – |
| Ma et al. [13•] | – | – | BMI ≥ 30 kg/m Gout |
– | ≥ 4 h | – | – |
| Kheir [7] | – | – | – | Enterococcus | – | – | – |
| Aalie Rezai [14••] | – | – | The Charlson comorbidity index | – | – | Longer | Longer |
In light of the increasing proportion of infections caused by non-Staphylococcus pathogens, such as Gram-negative bacilli, the influence of multidrug-resistant pathogens should be continuously investigated [24]. The presence of Enterococcus species has been found to be a strong predictor of failure in two-stage reimplantation according to a recent study [7]. New pathogens can be present even after an aggressive surgical debridement. Infections caused by new microorganisms not causing the index infection are expected to happen in about two thirds of failed staged revision THAs [25]. However, a recent study reported that at 1 year after the two-stage exchange, failure due to reinfection with the same organism occurred in 27% of the failure cases, while 44% were re-infected by a different pathogen [14••]. The authors also reported that the time to reimplantion in a staged procedure was not associated with failure while, risk factors for failure included the presence of comorbidities and a longer length of hospital stay [14••].
The role of patient comorbidities and demographics was recently investigated in a large cohort of patients, which reported younger age, tobacco use, chronic kidney disease, hemodialysis, and depression as risk factors for PJI [12]. The way age influences failure rates in PJI is controversial, and the presence of other independent predictors of infection should be addressed [12, 26]. Other factors reported in a recent systematic review included diabetes mellitus, corticosteroid therapy, hypoalbuminemia, blood transfusion, use of closed suction drainage, wound dehiscence or superficial infection, coexistence of malignancy, and immunosuppression [27]. Rheumatological conditions, coagulation disorders, and morbid obesity were also considered risk factors for PJI [12, 27]. Duration of surgery has also been found to be associated with infection after a joint replacement in a study based on the Norwegian health registers [26].
Smoking has been shown to be a strong independent risk factor for surgical site infections including PJI. The pathophysiological consequences of smoking on surgical outcomes refer to the toxic effects of inhalation leading to local tissue hypoxia by the mechanism of vasoconstriction and inadequate stimulation of fibroblasts under oxidative stress conditions [28]. Delay in wound healing and increased risk of local infection has been associated with decreasing cell migration and inadequate accumulation of connective tissue in the wound [28]. A large number of patients who smoked were retrospectively analyzed for surgical site infection risk factors, and smoking on the day of surgery was independently associated with increased rate of infection following elective surgeries [29]. A recent meta-analysis has shown a significant increase in infection among smokers as identified among 51 studies evaluating surgical procedures distributed across several specialties [30]. Smoking may cause failure after a two-stage exchange after joint replacement.
When to Do Amputation/Arthrodesis
Resection arthroplasty (pseudoarthrosis) has existed as a treatment for hip joint infection since 1928, as described by Girdlestone to treat tuberculosis infection of the hip. This technique was then adapted to treat osteoarthritis decades ago, as described by R. G. Taylor in 1950 [31]. However, the first cases of resection arthroplasty as a treatment for THA infection were reported in 1972 by two groups, Patterson and Brown and as Wilson et al., both as parts of outcome studies for larger sets of THA patients [32, 33]. Wilson et al. reported a high incidence of prosthetic loosening and wound complications in a cohort of 100 patients who had THA using the McKee-Farrar prosthesis and acrylic cement [33]. In the Patterson and Brown study, the same implant was used, and a high incidence of salvage procedures after THA was recorded, of which, the Girdlestone arthroplasty was performed in 26% of the cases [32]. Clegg was the first surgeon to discuss pseudoarthrosis as a treatment for infected THA in a dedicated retrospective study of 26 patients [34]. The author found significant improvements in pain and acceptable functional results, with some limitations including shortening of the operated leg between 4 and 7.5 cm, the need to raise toilet seats, and difficulties with unaided standing and walking [34]. Much of the literature discussing pseudoarthrosis as a treatment for infected THA continued to be written through the mid-1980s, prior to the advent of more modern techniques such as antibiotic spacers and multiple stage revisions [35, 36].
On the other hand, amputation can be utilized as treatment in more complex pathologies. Typically, it is reserved for life-threatening situations in the setting of severe infection or when limb salvage is not possible due to severe bone loss or a vascular injury. A series of 11 patients was described by Fenelon et al. in 1980. The authors found that out of 11 patients, three died, five had good wound healing, three had persistent draining sinuses and improvement in pain, two had phantom pain, four were able to return to work, and seven reported overall happiness with the surgery and were able to move on with their lives outside of the hospital [37]. Bucholz et al. referred to disarticulation as treatment, but minimally addresses indications and subsequent outcomes of this treatment arm, showing that this procedure is performed in the setting of life-threatening sepsis or severe vascular injury [38]. Other studies have shown a disarticulation rate of 0.7 to 1.3% of infected THA cases [39, 40]. As amputation is considered a radical procedure, alternative treatments were also adopted in the setting of PJI. A type of rotationplasty was described by Peterson et al. in 1997, in which the patient’s lower leg can be saved by placing the heel of the calcaneus in the acetabulum and then have the calcaneotalotibial joint to function as the new hip joint [41]. This technique was performed to provide a stump that will facilitate movement and stability of the torso.
Arthrodesis of the infected THA has also been suggested as an alternative treatment, albeit in only rare circumstances. In orthopedic literature, there is a paucity of studies reporting this kind of treatment. In a study performed by Kostuik and Alexander, out of 14 cases where arthrodesis was performed after THA, seven had prior PJI. Only one patient had complications regarding arthrodesis healing [42]. In this case series, the authors show that using a modified AO fusion technique with allograft bone graft, it is possible to successfully fuse an infected THA. As the advent of modern staging techniques have arisen, the functional limitations of a hip arthrodesis may represent a barrier to the procedure, being difficult to advocate fusion as an alternative treatment.
Given the fact that the management of PJI represents a major challenge for surgeons, clinicians, and patients, some authors reported treatment algorithms aiming for better clinical results. Giuleri et al. used different variables for a treatment algorithm for hip PJI, including time of onset of symptoms and clinical aspect of the implant and host soft tissue [43]. The authors reported better clinical outcomes when the algorithm was used. In addition, they dismiss the Girdlestone approach, whereas this lacks in ability to effectively treat infection and moreover, provides a stable, functional hip joint, and, supports the use of suppressive antibiotics or an antibiotic spacer as a bridge to a final THA [43].
On the other side, the decision of converting a resection arthroplasty or arthrodesis to a THA needs to be considered for improving patient’s function and quality of life. Several complications were associated with conversion of hip arthrodesis to THA, including infection, instability, loosening, nerve-related complications, abductor-related complications, and venous-thrombotic events, and up to 12% revision rate according to a recent study [44]. However, when compared to a primary THA, conversion to THA after arthrodesis can have excellent results with similar satisfaction scores and complication rates [45]. Conversion of a Girdlestone procedure to THA, as compared to match controls of patients undergoing THA revision for aseptic loosening, showed similar outcomes after a mean follow-up of 9.3 years [46]. Charlton et al. reviewed the most common complications that occurred after converting an infected hip treated with Girdlestone to THA in 44 patients with an average follow-up of 2 years. Although an improvement in the Harris hip scores was present, complications were notable, including a 11.4% dislocation and 30% of patients with a persistent limp. The authors did not recommend the Girdlestone as a first-line treatment for hip infection as a result of their findings [47].
Clearly, the orthopedic literature does not support either resection arthroplasty, fusion, or hip disarticulation as first-line treatments in the setting of periprosthetic hip infection. However, if necessary, when two-stage revision techniques fail, these are viable options with the possibility to convert back to THA in the future.
Role of Chronic Suppression
Non-operative treatment of PJI is based on chronic suppressive antimicrobial strategies. While remission of infection can be achieved with 6 to 12 weeks of antimicrobial therapy in 69% of the cases, the duration of the chronic suppression is variable, ranging from months to years after surgery [5, 48]. However, most cases of failure are reported on average at 4 months after discontinuation of the antimicrobial therapy [49]. With the potential of being a life-long treatment, several factors may influence the duration of suppressive therapy: virulence of the infecting pathogen, previous antimicrobial therapy and susceptibility profile, availability of the medication at the healthcare facility, and therapy in failure cases which can occur in 25% of the cases [5, 50]. S. aureus is the most common infecting pathogen, and PJIs caused by methicillin-susceptible S. aureus (MSSA) are more prone to respond to antimicrobial suppression [50], while MRSA infections have been shown to be associated with poorer clinical outcomes [48]. A recent review of the antimicrobial agents used in PJI revealed that rifampicin may be used in combination with other drugs such as fluoroquinolones, fusidic acid, trimethoprim-sulfamethoxazole, and minocycline [5, 51–56]. Although rifampicin is an inducer of cytochrome P-450 oxidative enzymes, it is a potent reagent against staphylococcal biofilms, and this antimicrobial agent may decrease the concentrations of other agents such linezolid, clindamycin, and a life-long agent co-trimoxazole needed to treat the infection [5, 56]. In a study of PJI patients who had oral combination therapy and with a minimum follow-up of 2 years, the type of oral antibiotic selected was associated with higher failure rates, while the duration of treatment did not affect failures rates in the setting of PJI [56]. The authors concluded that failure may be due to the antimicrobial selection in PJI and not the duration of treatment [56]. Although still under scientific debate, chronic oral antimicrobial suppression can be administered as a single therapeutic agent or as dual therapy. Albeit associated with therapeutic success, different regimens may cause adverse events. In a study with patients older than 75 years old with a median follow-up of 17.3 months, and that received chronic suppression for PJI treatment, a single-agent treatment composed of either clindamycin, beta-lactams, co-trimoxazole, pristinamycin, or fluoroquinolones was administered in patients with PJI. Combined therapy consisted of fluoroquinolone + rifampicin, fluoroquinolone + clindamycin, co-trimoxazole + fusidic acid, and amoxicillin + clindamycin [57]. Eight out of 21 patients experienced an adverse event including allergy, death from non-septic cause, and systemic progression of sepsis. A new fistula was also observed as a sign of recurrence of infection [57]. The most common agent leading to an adverse event was co-trimoxazole, and most events were caused by one single agent [57]. The authors also reported that indefinite chronic oral antimicrobial suppression was not associated with death or infection, appearing to be an effective strategy for PJI in the elderly [57]. Chronic suppression may be used as palliative therapy to elderly and bedridden patients, whereas high doses of potent antimicrobial agents may aggravate the clinical condition and lead to medication complications in this particularly vulnerable population [48, 57]. Chronic suppression also may have a role in cases where reconstruction is unfeasible (Figs. 1 and 2). Even though most of the drugs are well tolerated in the long-term period, the 2-year survival rate free of complication in the elderly population is about 40% [57]. These results show a controversial benefit of the suppressive therapy in PJI. However, in patients with poor clinical conditions, it may be the only feasible treatment. Given the fact that advanced age is a risk factor for higher glucose levels, elderly patients should be considered to have higher perioperative hyperglycemia, which is associated with increased biofilm formation and increased risk of chronic treatment failure [5, 58, 59]. Diabetic patients are at a higher risk of failure during prolonged oral antimicrobial suppression [59]. In a comparison study regarding the use of chronic suppression, the 5-year infection-free prosthetic survival rate of patients undergoing chronic antimicrobial suppression was 68.5%, a rate significantly higher than the one found in patients who had only irrigation, debridement, and polyethylene exchange as their treatment [60]. Greater benefit was reported to be found in patients who underwent irrigation and debridement with polyethylene exchange and patients in which the infection was caused by S. aureus.
Fig. 1.
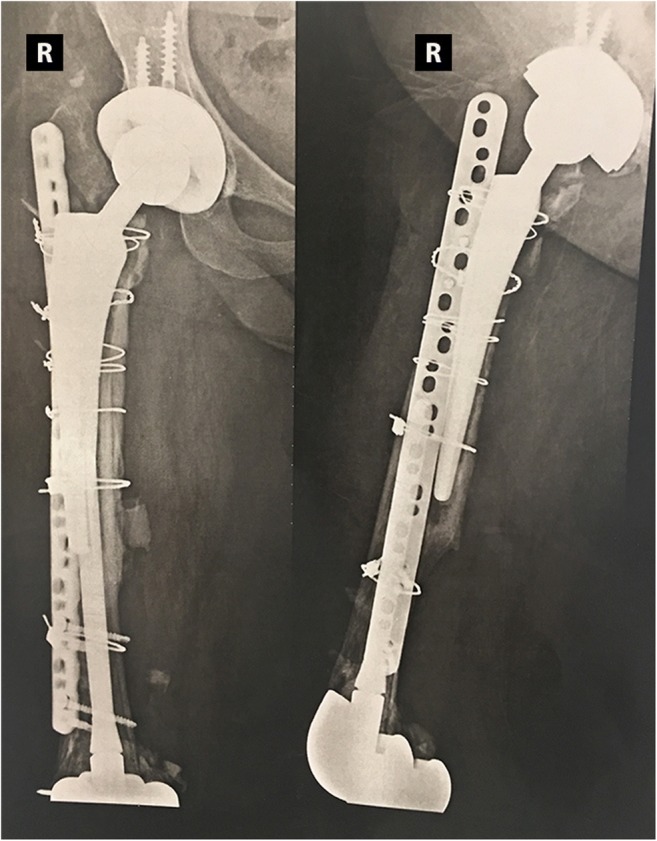
A 57-year-old female patient with rheumatoid arthritis and a right hip infected arthroplasty. The patient had three hip revisions and seven surgical debridement procedures with failure of eradication of infection. This patient had also two knee arthroplasty revisions. In face of a high chance of reconstruction failure and refusal of amputation, this patient was treated with chronic antibiotic suppression therapy
Fig. 2.
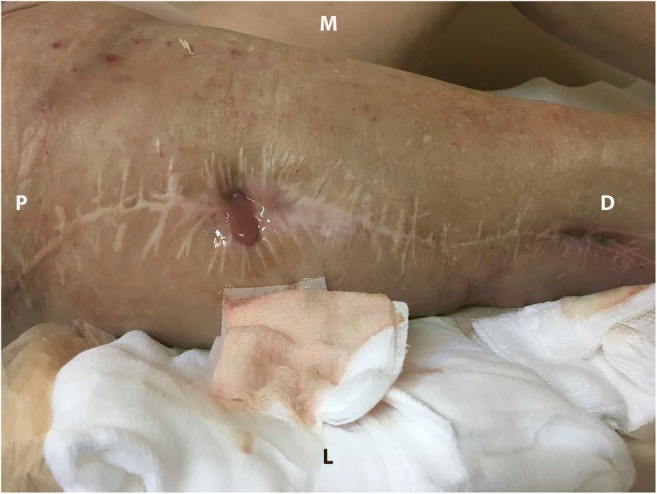
Same patient as in figure one with two active sinuses on the right thigh with purulent effusion. P proximal, M medial, D distal, L lateral
There are some limitations of chronic suppression therapy that involve the type of infectious agent and the type of treatment planned for the PJI. Chronic suppression might not be the ideal treatment for PJI when Gram-negative bacilli treated with fluoroquinolones cause the infection, but in a recent study, the use of fluoroquinolones was associated with a lower failure rate when the PJI was treated with debridement, implant retention, and antibiotics [5, 56]. Differently from PJI treated with DAIR, where rifampin is recommended for PJI caused by S. aureus, in cases of two-stage arthroplasty exchange, where the implant was previously removed, combination therapy with rifampin is not the first choice of treatment due to the fact that there is no retained implant [5, 61]. There is still no consensus to support a combined intravenous therapy with rifampin following implant removal [62]. A common antimicrobial strategy is based on the administration of pathogen-directed intravenous antibiotics for 4 to 6 weeks between the first and second stages [5]. In patients with lower ASA scores or extremely virulent infection pathogens, the chronic suppression may not be the first choice of treatment [26].
Conclusion
In conclusion, recent literature demonstrates that the main risk factors for a two-stage exchange failure are hemodialysis, obesity, multiple previous procedures, diabetes mellitus, corticosteroid therapy, hypoalbuminemia, blood transfusion, immunosuppression, rheumatological conditions, and coagulation disorders. Regarding microorganisms, besides S. aureus, Streptococcus and fungi are also considered risk factors for a two-stage exchange failure. Optimization of the patient’s clinical comorbidities is a key to achieving improved outcomes. Resection arthroplasty, arthrodesis, and amputation have a limited role. Chronic suppression is an option for high-risk patients or unfeasible reconstruction. Future research on patient-specific risk factors for a two-stage exchange may aid surgical decision-making and optimization of outcomes.
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Prosthetic Joint Infection
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
- 1.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplast. 2012;27(8 Suppl):61–5.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parvizi J, Gehrke T. Proceedings of the International Consensus Meeting on periprosthetic joint infection. Rochester: Musculoskeletal Infection Society; 2013. [DOI] [PubMed] [Google Scholar]
- 4.Mühlhofer HM, Pohlig F, Kanz KG, Lenze U, Lenze F, Toepfer A, Kelch S, Harrasser N, von Eisenhart-Rothe R, Schauwecker J. Prosthetic joint infection development of an evidence-based diagnostic algorithm. Eur J Med Res. 2017;22(1):8. doi: 10.1186/s40001-017-0245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res. 2010;96(2):124–132. doi: 10.1016/j.otsr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Kheir MM, Tan TL, Higuera C, George J, Della Valle CJ, Shen M, Parvizi J. Periprosthetic joint infections caused by enterococci have poor outcomes. J Arthroplast. 2017;32(3):933–947. doi: 10.1016/j.arth.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 8.•• Rajgopal A, Panda I, Rao A, Dahiya V, Gupta H. Does prior failed debridement compromise the outcome of subsequent two-stage revision done for periprosthetic joint infection following total knee arthroplasty? J Arthroplast. 2018. 10.1016/j.arth.2018.02.087. This study compared two groups of patients who had a two-stage revision for TKA: a group that had prior DAIR and a group that had a direct two-stage revision. Failure rates were higher in the DAIR group. Although this study was conducted in patients after knee surgery, principles of joint reconstruction and infection control are updated to other joint, including THA.
- 9.Lee J, Kang CI, Lee JH, Joung M, Moon S, Wi YM, Chung DR, Ha CW, Song JH, Peck KR. Risk factors for treatment failure in patients with prosthetic joint infections. J Hosp Infect. 2010;75:273–276. doi: 10.1016/j.jhin.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 10.•Brown TS, Petis SM, Osmon DR, Mabry TM, Berry DJ, Hanssen AD, et al. Periprosthetic joint infection with fungal pathogens. J Arthroplast. 2018. 10.1016/j.arth.2018.03.003. Although rare, fungal infections can pose a major risk for reimplantation failure after a two-stage revision for the management of PJI. This paper showed that, out of three cases of reinfection, two were caused by the same fungal pathogen. [DOI] [PubMed]
- 11.Akgün D, Trampuz A, Perka C, Renz N. High failure rates in treatment of streptococcal periprosthetic joint infection: results from a seven-year retrospective cohort study. Bone Joint J. 2017;99-B(5):653–659. doi: 10.1302/0301-620X.99B5.BJJ-2016-0851.R1. [DOI] [PubMed] [Google Scholar]
- 12.Cancienne JM, Werner BC, Bolarinwa SA, Browne JA. Removal of an infected total hip arthroplasty: risk factors for repeat debridement, long-term spacer retention, and mortality. J Arthroplast. 2017;32(8):2519–2522. doi: 10.1016/j.arth.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 13.•Ma CY, Lu YD, Bell KL, Wang JW, Ko JY, Wang CJ, et al. Predictors of treatment failure after 2-stage reimplantation for infected total knee arthroplasty: a 2- to 10-year follow-up. J Arthroplast. 2018. 10.1016/j.arth.2018.02.007. Patients with a PJI who had a two-stage exchange had a failure rate of 14.8%. Risk factors for failure after reimplantation were BMI ≥ 30, operative time greater than 4 h and gout. [DOI] [PubMed]
- 14.••Aali Rezaie A, Goswami K, Shohat N, Tokarski AT, White AE, Parvizi J. Time to reimplantation: waiting longer confers no added benefit. J Arthroplasty. 2018. 10.1016/j.arth.2018.01.073. This study reported a failure rate of 22.3% for patients who had PJI treated with two-stage exchange arthroplasty. Although time to reimplantation was not significantly associated with failure rates, the authors reported that a late reimplantation (> 26 weeks) was associated with a higher likelihood of failure when compared to those patients who were reimplanted within < 26 weeks. Presence of comorbidities was an important association with failure, and the authors used the Charlson comorbidity index to evaluate that.
- 15.Tornero E, Martínez-Pastor JC, Bori G, García-Ramiro S, Morata L, Bosch J, Mensa J, Soriano A. Risk factors for failure in early prosthetic joint infection treated with debridement. Influence of etiology and antibiotic treatment. J Appl Biomater Funct Mater. 2014;12(3):129–134. doi: 10.5301/jabfm.5000209. [DOI] [PubMed] [Google Scholar]
- 16.Poulsen NR, Mechlenburg I, Søballe K, Lange J. Patient-reported quality of life and hip function after 2-stage revision of chronic periprosthetic hip joint infection: a cross-sectional study. Hip Int. 2017;0. 10.5301/hipint.5000584. [DOI] [PubMed]
- 17.Lange J, Troelsen A, Thomsen RW, Søballe K. Chronic infections in hip arthroplasties: comparing risk of reinfection following one-stage and two-stage revision: a systematic review and meta-analysis. Clin Epidemiol. 2012;4:57–73. doi: 10.2147/CLEP.S29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Browne JA, Cancienne JM, Novicoff WM, Werner BC. Removal of an infected hip arthroplasty is a high-risk surgery: putting morbidity into context with other major nonorthopedic operations. J Arthroplast. 2017;32(9):2834–2841. doi: 10.1016/j.arth.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 19.Sherrell JC, Fehring TK, Odum S, Hansen E, Zmistowski B, Dennos A, Kalore N. Periprosthetic infection consortium. The Chitranjan Ranawat Award: fate of two-stage reimplantation after failed irrigation and débridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469(1):18–25. doi: 10.1007/s11999-010-1434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.••George J, Newman JM, Klika AK, Miller EM, Tan TL, Parvizi J, et al. Changes in antibiotic susceptibility of Staphylococcus aureus between the stages of 2-stage revision arthroplasty. J Arthroplast. 2018. 10.1016/j.arth.2018.01.056. This study highlights the roleS. aureus plays in PJI. The authors investigated the minimum inhibitory concentrations of one of the main drugs used for the clinical management PJI caused byS. aureus: vancomycin. This pathogen can persist after reimplantation, with an associated increase of the MIC. Vancomycin is widely used as a therapeutic intravenous antibiotic, but it is also commonly administrated together with cement spacers. The paper raises concern about the antimicrobial resistance and the role of theS. aureusin PJI. [DOI] [PubMed]
- 21.Boisrenoult P. Cutibacterium acnes prosthetic joint infection: diagnosis and treatment. Orthop Traumatol Surg Res. 2018;104(1S):S19–S24. doi: 10.1016/j.otsr.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Shohat N, Goswami K, Tarabichi M, Sterbis E, Tan TL, Parvizi J. All patients should be screened for diabetes before total joint arthroplasty. J Arthroplast. 2018. 10.1016/j.arth.2018.02.047. [DOI] [PubMed]
- 23.Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- 24.Benito N, Franco M, Ribera A, Soriano A, Rodriguez-Pardo D, Sorlí L, Fresco G, Fernández-Sampedro M, Dolores Del Toro M, Guío L, Sánchez-Rivas E, Bahamonde A, Riera M, Esteban J, Baraia-Etxaburu JM, Martínez-Alvarez J, Jover-Sáenz A, Dueñas C, Ramos A, Sobrino B, Euba G, Morata L, Pigrau C, Coll P, Mur I, Ariza J, REIPI (Spanish Network for Research in Infectious Disease) Group for the Study of Prosthetic Joint Infections Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect. 2016;22(8):732.e1–732.e8. doi: 10.1016/j.cmi.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Zmistowski B, Tetreault MW, Alijanipour P, Chen AF, Della Valle CJ, Parvizi J. Recurrent periprosthetic joint infection: persistent or new infection? J Arthroplast. 2013;28(9):1486–1489. doi: 10.1016/j.arth.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Dale H, Skråmm I, Løwer HL, Eriksen HM, Espehaug B, Furnes O, Skjeldestad FE, Havelin LI, Engesaeter LB. Infection after primary hip arthroplasty: a comparison of 3 Norwegian health registers. Acta Orthop. 2011;82(6):646–654. doi: 10.3109/17453674.2011.636671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Zhang F, Chen W, Liu S, Zhang Q, Zhang Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. J Hosp Infect. 2015;89(2):82–9. doi: 10.1016/j.jhin.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Hawn MT, Houston TK, Campagna EJ, Graham LA, Singh J, Bishop M, Henderson WG. The attributable risk of smoking on surgical complications. Ann Surg. 2011;254:914–920. doi: 10.1097/SLA.0b013e31822d7f81. [DOI] [PubMed] [Google Scholar]
- 29.Nolan MB, Martin DP, Thompson R, Schroeder DR, Hanson AC. Association between smoking status, preoperative exhaled carbon monoxide levels, and postoperative surgical site infection in patients undergoing elective surgery. JAMA Surg. 2017;152:476–483. doi: 10.1001/jamasurg.2016.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255:1069–1079. doi: 10.1097/SLA.0b013e31824f632d. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RG. Pseudoarthrosis of the hip joint. J Bone Joint Surg. 1950;32-B:161–165. doi: 10.1302/0301-620X.32B2.161. [DOI] [PubMed] [Google Scholar]
- 32.Patterson FP, Brown CS. The McKee-Farrar total hip replacement: preliminary results and complications of 368 operations performed in five general hospitals. J Bone Joint Surg. 1972;54-A:257–275. doi: 10.2106/00004623-197254020-00003. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PD, Jr, Amstutz HC, Czerniecki A, Salvati EA, Mendes DG. Total hip replacement with fixation by acrylic cement: a preliminary study of 100 consecutive McKee-Farrar prosthetic replacements. J Bone Joint Surg. 1972;54-A:207–236. doi: 10.2106/00004623-197254020-00001. [DOI] [PubMed] [Google Scholar]
- 34.Clegg J. The results of the pseudarthrosis after removal of an infected total hip prosthesis. J Bone Joint Surg (Br) 1977;59(3):298–301. doi: 10.1302/0301-620X.59B3.893508. [DOI] [PubMed] [Google Scholar]
- 35.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89(4):871–882. doi: 10.2106/JBJS.E.01070. [DOI] [PubMed] [Google Scholar]
- 36.Engesæter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop. 2011;82(5):530–537. doi: 10.3109/17453674.2011.623572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenelon GC, von Foerster G, Engelbrecht E. Disarticulation of the hip as a result of failed arthroplasty. J Bone Joint Surg. 1980;62A:441–446. doi: 10.1302/0301-620X.62B4.7430220. [DOI] [PubMed] [Google Scholar]
- 38.Buchholz HW, Elson RA, Englebrecht E, et al. Management of deep infection of total hip replacement. J Bone Joint Surg. 1981;63B:342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 39.Garvin KL, Hanssen AD. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg. 1995;77A:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 41.Peterson CAII, Koch LD, Wood MB. Tibia-hindfoot osteomusculocutaneous rotationplasty with calcaneopelvic arthrodesis for extensive loss of bone from the proximal part of the femur: a report of two cases. J Bone Joint Surg. 1997;79A:1504–1509. doi: 10.2106/00004623-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Kostuik J, Alexander D. Arthrodesis for failed arthroplasty of the hip. Clin Orthop. 1984;188:173–182. [PubMed] [Google Scholar]
- 43.Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32(4):222–228. doi: 10.1007/s15010-004-4020-1. [DOI] [PubMed] [Google Scholar]
- 44.Jauregui JJ, Kim JK, Shield WP, 3rd, Harb M, Illical EM, Adib F, Maheshwari AV. Hip fusion takedown to a total hip arthroplasty-is it worth it? A systematic review. Int Orthop. 2017;41(8):1535–1542. doi: 10.1007/s00264-017-3436-z. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Fairen M, Murcia-Mazón A, Torres A, Querales V, Murcia A., Jr Is total hip arthroplasty after hip arthrodesis as good as primary arthroplasty? Clin Orthop Relat Res. 2011;469(7):1971–1983. doi: 10.1007/s11999-010-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Rey E, Cruz-Pardos A, Madero R. Clinical outcome following conversion of Girdlestone’s resection arthroplasty to total hip replacement: a retrospective matched case-control study. Bone Joint J. 2014;96-B(11):1478–1484. doi: 10.1302/0301-620X.96B11.33889. [DOI] [PubMed] [Google Scholar]
- 47.Charlton WP, Hozack WJ, Teloken MA, Rao R, Bissett GA. Complications associated with reimplantation after Girdlestone arthroplasty. Clin Orthop Relat Res. 2003;407:119–126. doi: 10.1097/00003086-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Chaussade H, Uçkay I, Vuagnat A, Druon J, Gras G, Rosset P, Lipsky BA, Bernard L. Antibiotic therapy duration for prosthetic joint infections treated by Debridement and Implant Retention (DAIR): similar long-term remission for 6 weeks as compared to 12 weeks. Int J Infect Dis. 2017;63:37–42. doi: 10.1016/j.ijid.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63(6):1264–1271. doi: 10.1093/jac/dkp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodríguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, del Toro MD, Cobo J, Riera M, Ramos A, Jover-Sáenz A, Ariza J, REIPI Group for the Study of Prosthetic Infection A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56(2):182–194. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- 51.Barberán J, Aguilar L, Carroquino G, Giménez MJ, Sánchez B, Martínez D, Prieto J. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med. 2006;119(11):993.e7–993.10. doi: 10.1016/j.amjmed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Vilchez F, Martínez-Pastor JC, García-Ramiro S, Bori G, Maculé F, Sierra J, Font L, Mensa J, Soriano A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439–444. doi: 10.1111/j.1469-0691.2010.03244.x. [DOI] [PubMed] [Google Scholar]
- 53.Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev. 2010;23(1):14–34. doi: 10.1128/CMR.00034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siddiqui MM, Lo NN, Ab Rahman S, Chin PL, Chia SL, Yeo SJ. Two-year outcome of early deep MRSA infections after primary total knee arthroplasty: a joint registry review. J Arthroplast. 2013;28(1):44–48. doi: 10.1016/j.arth.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Aboltins CA, Page MA, Buising KL, Jenney AW, Daffy JR, Choong PF, Stanley PA. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007;13(6):586–591. doi: 10.1111/j.1469-0691.2007.01691.x. [DOI] [PubMed] [Google Scholar]
- 56.Tornero E, Soriano A. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention-authors’ response. J Antimicrob Chemother. 2016;71(12):3627. doi: 10.1093/jac/dkw438. [DOI] [PubMed] [Google Scholar]
- 57.Prendki V, Sergent P, Barrelet A, Oziol E, Beretti E, Berlioz-Thibal M, Bouchand F, Dauchy FA, Forestier E, Gavazzi G, Ronde-Oustau C, Stirnemann J, Dinh A. Efficacy of indefinite chronic oral antimicrobial suppression for prosthetic joint infection in the elderly: a comparative study. Int J Infect Dis. 2017;60:57–60. doi: 10.1016/j.ijid.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Seneviratne CJ, Yip JW, Chang JW, Zhang CF, Samaranayake LP. Effect of culture media and nutrients on biofilm growth kinetics of laboratory and clinical strains of Enterococcus faecalis. Arch Oral Biol. 2013;58(10):1327–1334. doi: 10.1016/j.archoralbio.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 59.Nowak MA, Winner JS, Beilke MA. Prolonged oral antibiotic suppression in osteomyelitis and associated outcomes in a Veterans population. Am J Health Syst Pharm. 2015;72(23 Suppl 3):S150–S155. doi: 10.2146/sp150022. [DOI] [PubMed] [Google Scholar]
- 60.Siqueira MB, Saleh A, Klika AK, O’Rourke C, Schmitt S, Higuera CA, Barsoum WK. Chronic suppression of periprosthetic joint infections with oral antibiotics increases infection-free survivorship. J Bone Joint Surg Am. 2015;97(15):1220–1232. doi: 10.2106/JBJS.N.00999. [DOI] [PubMed] [Google Scholar]
- 61.El Helou OC, Berbari EF, Lahr BD, Eckel-Passow JE, Razonable RR, Sia IG, Virk A, Walker RC, Steckelberg JM, Wilson WR, Hanssen AD, Osmon DR. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis. 2010;29:961–967. doi: 10.1007/s10096-010-0952-9. [DOI] [PubMed] [Google Scholar]
- 62.Restrepo C, Schmitt S, Backstein D, Alexander BT, Babic M, Brause BD, Esterhai JL, Good RP, Jørgensen PH, Lee P, Marculescu C, Mella C, Perka C, Pour AE, Rubash HE, Saito T, Suarez R, Townsend R, Tözün IR, Van den Bekerom MP. Antibiotic treatment and timing of reimplantation. J Arthroplast. 2014;29(2 Suppl):104–107. doi: 10.1016/j.arth.2013.09.047. [DOI] [PubMed] [Google Scholar]


