Abstract
Purpose of Review
The purpose of this manuscript is to provide a critical review of peer-reviewed literature over the last 5 years related to low virulent organisms associated with periprosthetic joint infection (PJI). We evaluated the most common organisms, the diagnostic challenges, and the novel tools available in the perioperative workup of PJI as well as the current understanding of how biofilm potentiates the indolent clinical presentation and explore a possible shift in the surgical management of these patients.
Recent Findings
Biofilm actively prevents macrophage phagocytosis by suppressing proinflammatory activity through the recruitment of myeloid-derived suppressor cells. Given the appropriate host and organism conditions, increased utilization of one-stage exchange arthroplasty in the surgical treatment of these low virulent infections may be on the rise.
Summary
Biomarkers and molecular techniques offer encouraging results to diagnose low virulent organisms and future research focused on the disruption of biofilm may ultimately give rise to improved treatment strategies.
Keywords: Periprosthetic infection, Low-virulent organisms, Biofilm
Introduction
Infection after total joint arthroplasty (TJA) is a devastating and unfortunate complication that brings significant challenges to the patient, treating surgeon, and healthcare system. Periprosthetic joint infection (PJI) is associated with technically difficult revision procedures, extensive therapeutic treatments including long-term antibiotics, often unpredictable or poor patient outcomes and substantial economic burden. A multidisciplinary team approach is required for optimizing the success of these complex treatment regimens for PJI as well as minimizing patient morbidity. PJI is the most common reason for total knee arthroplasty (TKA) revisions and is a major reason for total hip, total shoulder, total elbow, and total ankle arthroplasty revisions [1, 2••, 3–5, 6•]. In 2010, the estimated annual incidence was over one million TJAs performed in the USA with a cost of $18.75 billion [7]. Our aging population desires to remain physically active and improve their quality of life, ensuring that there will be an exponential increase in the numbers of total joint arthroplasty in the USA. The demand for total hip and total knee arthroplasties is projected to increase by 174 and 673% respectively by the year 2030, with an estimated 572,000 total hip arthroplasties (THA) and 3.48 million TKAs being performed annually by 2030 [8]. The growth rates of upper extremity arthroplasty procedures have been reported to be between 7 and 13% annually, with total shoulder arthroplasty rates projected to increase by more than 150% by the year 2020 [9]. With these projections, there is growing concern for an exponential increase in the number of PJI cases with significant fiscal implications to the patient, hospital, and healthcare system.
The rates of PJI after primary TJA have been reported between 1.55 and 2.5%, with hospital costs previously estimated at $566 million annually [10–13]. The cost of treating a case of PJI in the hip and knee is on average 2.8–4.8 times higher than a primary TJA, with a projected rise in annual costs to $1.6 billion by 2020 [11, 14]. The cost disparity noted between primary arthroplasty and management of PJI is largely driven by the complex algorithms associated with successful treatment of PJI including longer operative times with multiple procedures, extended lengths of stay (LOS), higher complication rates, subsequent hospitalizations with higher inpatient charges, administration of long-term antibiotics, and more outpatient visits to various providers [15]. Management of PJI employs significant morbidity on patients and may even increase the risk of mortality [16]. Various definitions of success have been proposed, but there remains no uniform definition for success or failure of treatment in the PJI literature. Success in PJI treatment is multifactorial and includes infection control, functional outcomes, patient satisfaction, cost effectiveness, and minimization of complications. There is continued debate in the orthopedic community regarding the ideal management for PJI, as failure rates, defined as reinfection by the same or different organism(s), continue to range from 0 to 24% [17, 18, 19••, 20••].
Identification of the causative organism is critical for timely, targeted, and appropriate treatment. This may be especially challenging in the case of low virulent organisms, such as Propionibacterium acnes (P. acnes), as the clinical presentations and utility of classic diagnostic tools may not be as efficacious in establishing the presence of PJI. Previously, some of these low virulent organisms were thought to be culture contaminants when isolated, largely due to their presumed indolent nature, as well as presence on normal skin flora and role in maintenance of the human microbiome. These low virulent bacteria are now recognized as true pathogens and infecting organisms of PJI. The two most common organisms isolated from intraoperative cultures from upper extremity arthroplasty procedures are P. acnes and coagulase-negative staphylococci (CoNS), both of which are low virulent organisms [21, 22]. For lower extremity arthroplasty procedures, the most common isolated organisms are CoNS and methicillin-susceptible Staphylococcus aureus [23, 24•]. Cultures fail to isolate an infecting organism in up to 50% of PJI cases [25–27, 28•]. Culture-negative patients that meet the Musculoskeletal Infection Society (MSIS) Criteria for PJI (Table 1) are difficult to treat and have been associated with 4.5 times increased risk of reinfection when compared to those patients where an organism was identified by culture [29, 30]. In a recent study, culture-negative patients who met criteria for MSIS infection underwent next-generation sequencing and an organism was identified in 81.8% of samples with the majority being low-virulent organisms [31••]. Low-virulent organisms pose a unique challenge to successful diagnosis and treatment of PJI and due to their widespread nature as causative organisms. It is important to continue to try and understand the behavior patterns, tailor management protocols, and closely observe patient outcomes.
Table 1.
Musculoskeletal Infection Society (MSIS) Diagnostic Criteria: 2013–2018 [15]
| Major criteria (1 required) | Minor criteria (3 of 5 required) |
|---|---|
| Sinus tract communicating with the affected joint | Elevated ESR and CRP |
| Elevated synovial fluid white blood cell count | |
| Two positive periprosthetic cultures with phenotypically identical organisms | Elevated synovial polymorphonuclear neutrophil percentage (PMN%) or ++ change on leukocyte esterase test strip |
| Positive histological analysis of periprosthetic tissue | |
| A single positive culture |
One major criterion and/or 3 of 5 minor criteria must be present for the diagnosis of PJI according to the MSIS Diagnostic Criteria
ESR, erythrocyte sedimentation rate; CRP, C-reactive protein
Clinical Presentation: Low-Virulent Organisms and Diagnostic Tools
There are many challenges surrounding the management of patients who present clinically with concern for PJI. Perhaps one of the most difficult aspects is promptly and accurately establishing a definitive diagnosis of PJI with identification of the causative organism(s). Low-virulence organisms, such as P. acnes, pose a unique challenge to successful diagnosis of PJI. The clinical presentation is often indolent without the characteristic signs of infection and can lead to delayed treatment and increased associated costs.
Inflammatory Markers and Preoperative Aspiration
Classic nonspecific inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), as well as preoperative synovial fluid aspirations used in many diagnostic algorithms are often unreliable [32, 33, 34•]. Synovial fluid aspirations often produce scant amounts of fluid where cell counts may not be elevated and cultures may be negative, especially in the shoulder [35•]. Histological findings at time of surgery are often negative, especially with certain low-virulent organisms and in the setting of chronic infection [36•]. Identifying PJI in the setting of low-virulence organisms largely depends on intraoperative findings and tissue culture results [37]. Some organisms have extended culture growth times, including P. acnes, which can take up to 14–21 days to grow on certain media [38]. There are concerns with holding cultures for two to three times longer than standard institutional protocols, including an increased risk of contamination [38]. In some instances, cultures remain negative for a multitude of reasons, including prior antibiotic use and laboratory protocols. A major risk factor for culture-negative PJI is postoperative wound drainage and the use of vancomycin and cephalosporins [39••]. Due to these diagnostic challenges, enhanced effort has been placed on various techniques for rapid and accurate detection of PJI and identification of an infecting organism for determination of appropriate treatment, predicting prognosis and cost containment.
Biomarkers
With this growing need for improved diagnostic methods for PJI, synovial fluid biomarkers have demonstrated potential and may be a valuable addition to the diagnostic armamentarium. Previous work has shown synovial fluid biomarkers to have a high accuracy in establishing the presence of PJI, even in the setting of patients with systemic inflammatory disease and ongoing antibiotic treatment. Many synovial fluid biomarkers have previously been assessed with a recent meta-analysis evaluating the diagnostic accuracy and highest diagnostic odds ratio of eight index tests: leukocyte count, percent polymorphonuclear leukocytes (PMN%), CRP, alpha-defensin, leukocyte esterase, IL-6, IL-8, and culture. The overall sensitivity of the eight markers was 85%, and each marker showed sensitivity greater than 80%, except culture. All markers showed a specificity of greater than 90% with alpha-defensin demonstrating the highest log diagnostic odds ratio [40••]. Alpha-defensin has also shown to respond to a wide spectrum of organisms having consistent results for all organism types, Gram type, species, or virulence of the organism [41]. The rapid and accurate diagnosis of PJI may be established with the assistance of synovial fluid biomarkers, but it is critical to promptly identify the organism and tailor treatment options.
Sonication
Sonication of components that have been explanted in the setting of PJI with culture of the sonication fluid is utilized to enhance diagnostic sensitivity [42, 43]. A clinically significant percentage of patients undergoing a presumed aseptic revision have been shown to have positive sonicate cultures, with the most common isolated organisms being CoNS and P. acnes [24•]. Culturing fluid from sonicated implants is an adjunctive tool when diagnosing PJI, especially when considering the presence of biofilm. Sonication has been shown to mechanically disrupt biofilm from implants, increasing the number of bacteria available for culture. Certain organisms are harbored and protected within the complex structure and matrix of biofilms, and the use of sonication can theoretically allow for identification of species otherwise not detected on standard culture. The use of sonication continues to evolve with multiple studies demonstrating varying results. A recent study showed that regular use of implant sonication culture for presumed aseptic revisions may improve the accuracy of diagnosing PJI by detecting low-virulence organisms [24•]. Whereas, another report concluded that implant sonication of fluid culture in revision shoulder arthroplasty showed no significant benefits over standard intraoperative cultures in diagnostic utility for PJI [44•].
Molecular Techniques for Diagnosing PJI
Multiplex polymerase chain reaction (PCR) has been used previously to isolate organisms to aid in the diagnosis of PJI. However, PCR raised concerns as this particular technique revealed a false-positive rate up to 88% in prior literature [45, 46]. The overall performance of PCR has been shown to be comparable to culture, although a recent study demonstrated that PCR was superior for detection of low-virulent bacteria, such as Cutibacterium species and CoNS, when compared to synovial fluid culture [47••]. Multiplex PCR can produce results within 5 h, whereas cultures can take several days for growth, identification, and speciation. Broad-range PCR has not proved to be advantageous over traditional culture and has limitations including the inability to detect multiple organisms at one time and lower sensitivities ranging from 67.1 to 73.3% [26, 48].
Next-generation sequencing (NGS), also known as high-throughput sequencing, is a term used to describe multiple modern techniques used for rapidly sequencing all DNA and RNA present in a sample, thus allowing for a more complete microbial profile. A recent study investigated the utility of next-generation sequencing in PJI and found that next-generation sequencing was able to identify an organism in almost 90% of patients with PJI as defined by the MSIS criteria, with a large percent classified as polymicrobial. NGS was found to be more sensitive, but less specific than culture. This study also discovered that this technology detected a potential pathogen in about 80% of culture-negative PJIs and had an 88.2% correlation with culture. When reviewing the patients who underwent aseptic revisions, next-generation sequencing found microbial DNA in 25% of cases, with the most predominant organism being P. acnes [31••].
Low-Virulent Organisms Associated with PJI
Though most microbes are capable of establishing PJI, in clinical practice only, a select few are frequently identified. While virulent organisms, such as Staphylococcus aureus and gram-negative aerobes, are frequently seen as an acute presentation of PJI often occurring in early-onset following total joint arthroplasty, low-virulent organisms such as CoNS, P. acnes, enterococcus, and actinomyces should be considered with indolent and delayed onset PJI. Many low-virulent organisms are gram-positive in nature and are able to form biofilm of abiotic surfaces [49, 50]. The microbiological features, many virulence factors, some biofilm properties, and commonly used antibiotics are outlined in Table 2 [33, 51, 52, 54].
Table 2.
Low-virulent organism profiles
| Organism | Characteristics | Virulence properties | Biofilm properties | Antibiotics commonly used* |
|---|---|---|---|---|
| Coagulase-negative Staphylococci [51] S. epidermidis S. lugdunensis S. saprophyticus S. haemolyticus S. capitis S. caprea |
Gram-positive cocci, aerobe, or facultative anaerobe; common on normal skin flora and mucous membranes | Biofilm production, immune system avoidance, antimicrobial tolerance, adherence to host proteins or biomaterials, toxin production—injury to host cells, exoenzymes—skin and wound colonization and destruction of host tissue | PIA—polysaccharide intracellular adhesion; Aap—accumulation of biofilm cells; Bhp—accumulation; DNA—structure of biofilm/nutrient/ gene transfer; ica—locus important for biofilm creation | Beta-lactams (oxacillin), vancomycin |
| Propionibacterium acnes [33] | Gram-positive rod, facultative anaerobe, nonsporulating; common on normal skin flora | Biofilm production, bacterial seeding, modification and manipulation of host immune response, host tissue-degrading enzymes, cell adhesion, inflammation, cohemolytic activity—cytotoxic to macrophages, beta-hemolytic activity—lysis RBCs | Slime/capsular polysaccharide, PIA—polysaccharide intracellular adhesion; lipoglycan—adherence to skin tissue/biofilm, increased lipase activity—neutrophil lysis, ExoA, glycosyltransferases | Beta-lactams, penicillin, doxycycline, rifampin |
| Enterococcus [52] E. faecalis E. faecium |
Gram-positive; inhabits oral cavity and gastrointestinal flora | Strong biofilm production, antibiotic multi-resistance, gelatinase, cytolysin, extracellular superoxide | Esp—enterococcal surface protein, hydrophobicity, aggregation substance, fsrB gene (locus) | Ampicillin, vancomycin, daptomycin |
| Actinomyces [53] | Gram-positive rod, filamentous, branching, microaerophilic or facultative anaerobe, nonsporulating | Capable of crossing tissue planes with no respect to anatomical boundaries or divisions. | EPS—exopolysaccharides, polysaccharide deacetylase, Spo0J containing ParB-like nuclease domain, TransRDD family protein | Penicillin, doxycycline |
*Commonly used antibiotics are representative of methicillin-sensitive CONS
RBCs, red blood cells
Coagulase-Negative Staphylococcus
There are currently over 40 species of CoNS identified and these species are closely related to Staphylococcus aureus, but are differentiated by their inability to produce free coagulase [51]. CoNS are members of native skin flora and mucous membranes. Historically, these bacteria have rarely caused disease and were frequently considered contaminants in microbiological cultures. CoNS have recently been recognized as true pathogens and an important cause for PJI due to ability to colonize prosthetic material as well as produce biofilm. CoNS demonstrate many virulent properties, the most important of which is the ability to produce a highly structured, tenacious biofilm [55]. Though a common cause for early-onset PJI, these organisms are also one of the most common causes for delayed or late-onset PJI largely due to the formation of a robust biofilm causing antibiotic tolerance and host defense evasion [51, 56]. Staphylococcus epidermidis is the most frequently identified PJI-associated coagulase-negative staphylococcus, though other staphylococci have been reported as the inciting organism in PJI to include Staphylococcus saprophyticus, Staphylococcus haemolyticus, and Staphylococcus lugdunensis. Of these, Staphylococcus lugdunensis may be the most virulent with multiple cases documenting acute and aggressive infection mimicking that of S. aureus [57]. Although, it has been our clinical experience that a more indolent presentation may occur as well.
Propionibacterium acnes
P. acnes may be the most quintessential low-virulence organism to cause PJI and is the most common isolated organisms during revision shoulder arthroplasty [4]. There is joint specificity with regard to how patients present with P. acnes PJI. A recent study found that inflammatory markers, including ESR, CRP, and synovial fluid white blood cell count, were significantly higher in the knee and hip groups compared to the shoulder group [58••, 59•]. Patients typically have a slowly evolving clinical course with pain likely being the only manifestation of infection [38, 60]. Extended culture incubation up to 21 days is utilized to enhance detection of P. acnes and may be indicated for identification of indolent PJI. There is a need for long hold anaerobic culture protocols up to a minimum of 14 days to be able to accurately identify P. acnes as the causative organism, especially in the setting of TKA [59•]. Given the ubiquity of P. acnes as common skin flora, differentiating infection from contamination may be difficult to determine. Genomic studies have isolated distinct phylotypes of P. acnes with type IB being frequently associated with orthopedic infections [61]. There are various adaptive virulence properties throughout each of the phylotypes that have been shown to contribute to the pathogenic potential of the bacterium. These virulence properties include the ability to degrade and invade host cells, produce an enhanced host inflammatory response, demonstrate antibiotic resistance, and form biofilms [33, 62, 63]. Christie-Atkins-Munch-Petersen (CAMP) factor serves as a toxin to host cells and is found in the P. acnes genome [62]. CAMP may be associated with virulence properties such as beta-hemolytic activity. Certain strains of P. acnes that have been clinically correlated with true infection have demonstrated beta-hemolytic activity through their phenotypic expression of hemolysis [64] (Figs. 1 and 2). Hemolytic strains of P. acnes have also been associated with increased antibiotic resistance patterns, especially to clindamycin [65••] (Fig. 3).
Fig. 1.
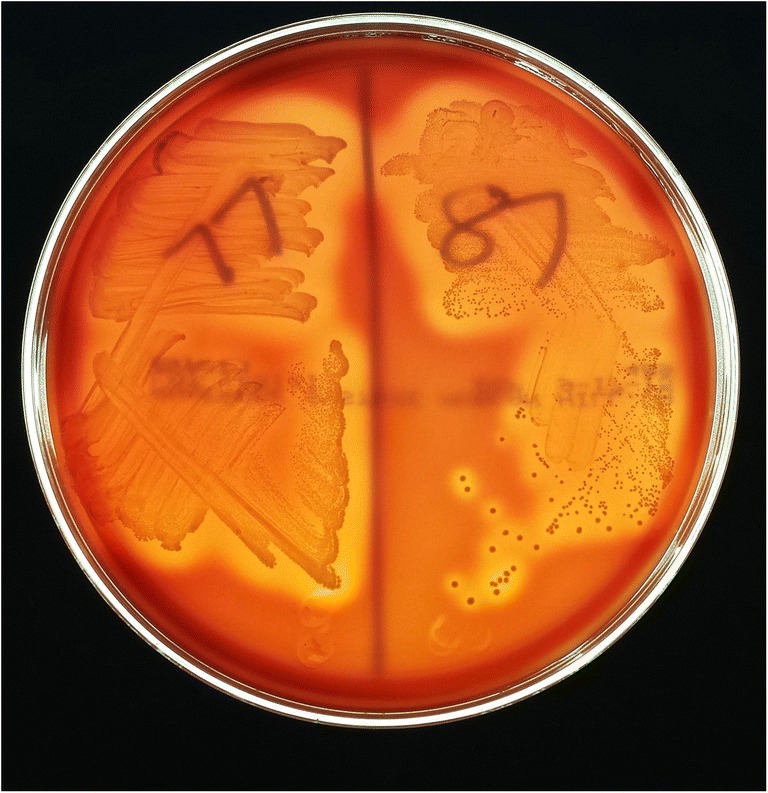
P. acnes strains 77 and 87 on Brucella Blood Agar plates demonstrating the hemolytic phenotype as defined by greater than 2 mm of clearance surrounding the bacteria
Fig. 2.
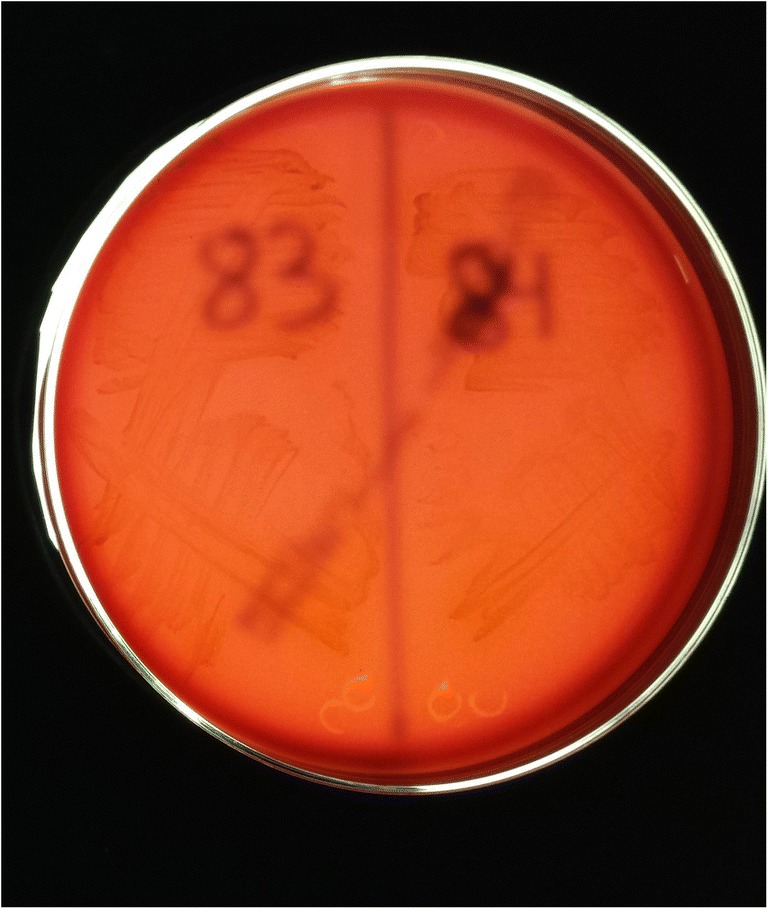
P acnes strains 83 and 84 on Brucella Blood Agar plates not demonstrating the hemolytic phenotype as shown by no evidence of clearance around the bacteria
Fig. 3.
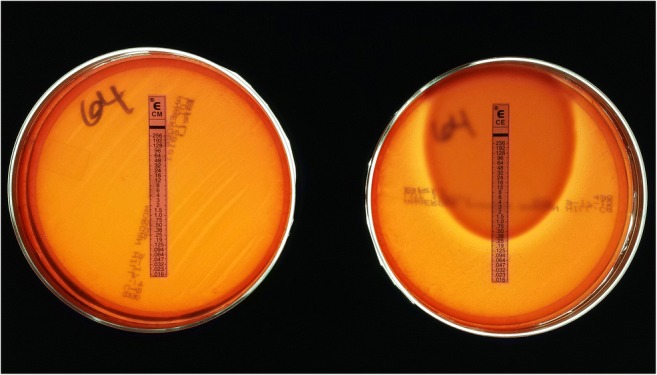
P. acnes strain 64 on the left demonstrating resistance to clindamycin by showing the bacteria growing up to the e test strip at the highest minimum inhibitory concentration (MIC). P. acnes strain 64 on the right showing an MIC of 0.25 for cephalothin (first-generation cephalosporin), which is considered susceptible based on EUCAST (European Committee on Antimicrobial Susceptibility Testing) standards
Enterococcus
Though seen in 17% of patients with early-onset PJI, enterococcus typically presents as part of a polymicrobial infection, where a delayed presentation can be seen with monomicrobial PJI [66]. In a review of 50 cases of enterococcal PJI, the median length of time between joint implantation and diagnosis of PJI was 36 months with median duration of symptoms before PJI diagnosis of 164 days [53]. Clinical presentation was consistent with a low-virulence organism, where patients presented with pain and loosening of the prosthesis with minimal systemic features.
Actinomyces
Actinomyces are a rare cause of monomicrobial anaerobic PJI with a typical indolent presentation and histology showing signs of chronic infection typical of a low-virulence organism [67]. Actinomyces remains a rare cause of PJI with dental work, IV drug use, and intrauterine device use believed to increase risk for infection. This genus of bacteria is capable of crossing tissue planes with no adherence to anatomical boundaries and has the ability to form a robust mesh-like biofilm structure, especially in the oral cavity setting [68].
Biofilm Considerations
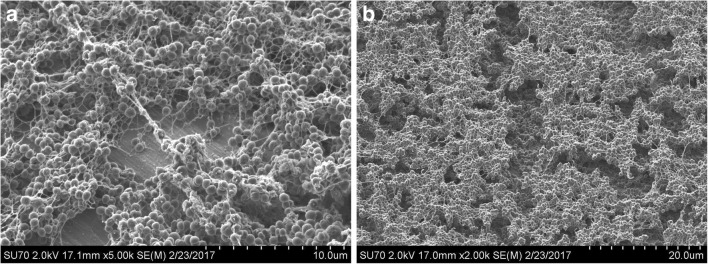
Biofilm is a complex three-dimensional structure that adheres to surfaces and describes monomicrobial or polymicrobial bacterial organisms residing in a self-produced matrix comprised of proteins, polysaccharides, and extracellular DNA [56, 69, 70]. Research has demonstrated Staphylococcal biofilms establish chronic infections by actively preventing macrophage phagocytosis and by a penchant for suppressing proinflammatory activity through the recruitment of myeloid-derived suppressor cells [71•, 72]. S. epidermidis, in particular, produces a thick biofilm matrix with extensive polysaccharide network to assist in host immune avoidance in addition to minimal toxin production as compared with more virulent organisms such as S. aureus [71, 73]. Figures 4 and 5 demonstrate the differences in biofilm architecture between S. epidermidis and methicillin-resistant Staphylococcus aureus. Biofilm allows bacteria to thrive by providing a potent defense against both host immune system responses and antimicrobial pharmacologic agents. Multiple mechanisms for antibiotic recalcitrance have been described depending on the organism and type of antibiotic administered for treatment [74, 75]. Research continues to better understand the complex pathogenesis of biofilm and the unique characteristics offending organisms elicit. Future strategies will be aimed toward optimizing current treatment strategies and developing innovative techniques for managing biofilm associated PJI.
Fig. 4.
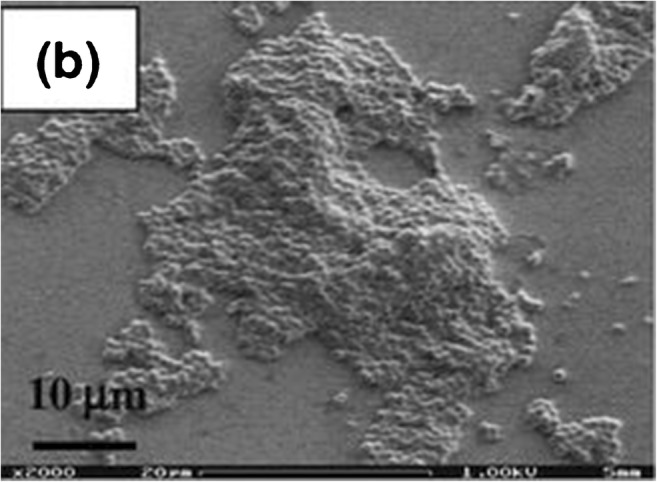
S. epidermidis biofilm formation when evaluating microfluidic devices for studying growth and detachment of S. epidermidis biofilms. Reprinted with permission from Lee, JH., Kaplan, J.B., and Lee, W.Y. Biomed Microdevices (2008) 10: 489. 10.1007/s10544-007-9157-0. Please note that this photo originally appeared in a multi-paneled figure. The “(b)” on the image is not relevant to this review
Fig. 5.
a, b MRSA (clinical isolate NRS70) biofilm image by scanning electron microscope (SEM) grown on titanium in vitro. Courtesy of Caelen Clark, PhD University at Buffalo
Treatment of PJI Caused by Low-Virulent Organisms
The goals of PJI treatment are to eradicate the infection, reconstruct the joint to restore pain-free motion, improve the quality of life, and minimize the treatment-related morbidity and mortality for the patient. Surgical treatment options for PJI include open debridement without complete removal of the prosthesis, the so-called debridement antibiotic implant retention (DAIR) with an exchange of modular components, one-stage exchange arthroplasty, two-stage exchange arthroplasty, arthrodesis, and amputation. Medical managements with antimicrobial-targeted treatment based on organism sensitivity or long-term suppression therapy with or without surgery are possible strategies to treat PJI. While two-stage exchange arthroplasty is the most accepted treatment for PJI in the USA, centers in Europe have reported results of single-stage revision for hip and knee PJI that are nearly similar to results of two-stage revision [76–78]. There has been a renewed interest in one-stage exchange arthroplasty, particularly for treating PJI associated with low-virulent offending organisms driven by decreased patient morbidity and increased function with decreased cost compared to two-stage exchange [79, 80, 81••]. Prior to one-stage exchange, we recommend considering the conclusions drawn by the international consensus group of arthroplasty surgeons and researchers from the meeting held in July 2018. Patient comorbidities that predispose to infection such as diabetes, obesity, immunosuppression, renal disease, smoking, severe cardiac disease, and metastatic carcinoma or compromised soft tissue envelope are likely to negatively impact the results of one-stage revision [15]. Further, as recently summarized by Jiranek et al., and based on the work from a 2013 international consensus group, we suggest a patient may be considered for one-stage exchange arthroplasty if they do not have generalized sepsis, the offending low-virulent organism without antibiotic resistance is identified in the preoperative setting, and the host is medically optimized without sinus tract or severe soft tissue compromise over the joint [81••, 82].
Conclusion
PJI is a devastating complication that requires sound clinical judgment and decision-making, as well as an algorithmic approach to preoperative workup including the use of biomarkers and molecular testing. The primary goal in the early stage of diagnosing PJI is to identify the offending organism and antibiotic susceptibility. A co-management approach between surgeons, infectious disease specialists, and ancillary professional staff to successfully eradicate infection and restore maximal functional and satisfaction of the patient is recommended. Coupled with a burst of novel peer-reviewed research focused on PJI in general over the last several years, there has been a particular recognition and acceptance of low virulent organisms as a cause for failure in total joint arthroplasty. Research continues to provide incremental answers on how to best define and manage this problem while sparking new questions as we learn more about the complex nature of low virulent organisms and the dynamic role between host and pathogen.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Prosthetic Joint Infection
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current epidemiology of revision Total knee arthroplasty in the United States. J Arthroplast. 2017;32(9):2663–2668. doi: 10.1016/j.arth.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 3.Gwam CU, Mistry JB, Mohamed NS, Thomas M, Bigart KC, Mont MA, Delanois RE. Current epidemiology of revision total hip arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J Arthroplast. 2017;32(7):2088–2092. doi: 10.1016/j.arth.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elb Surg. 2012;21(11):1534–1541. doi: 10.1016/j.jse.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patton D, Kiewiet N, Brage M. Infected total ankle arthroplasty: risk factors and treatment options. Foot Ankle Int. 2015;36(6):626–634. doi: 10.1177/1071100714568869. [DOI] [PubMed] [Google Scholar]
- 6.Krukhaug Y, Hallan G, Dybvik E, Lie SA, Furnes ON. A survivorship study of 838 total elbow replacements: a report from the Norwegian Arthroplasty Register 1994–2016. J Shoulder Elb Surg. 2018;27(2):260–269. doi: 10.1016/j.jse.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Cost of hospital discharges with common hospital operating room procedures in nonfederal community hospitals, by age and selected principle procedure: United States, selected years 2000–2012.
- 8.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplast. 2012;27(8 Suppl):61–5 e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 12.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Al-Mulhim FA, Baragbah MA, Sadat-Ali M, Alomran AS, Azam MQ. Prevalence of surgical site infection in orthopedic surgery: a 5-year analysis. Int Surg. 2014;99(3):264–268. doi: 10.9738/INTSURG-D-13-00251.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87(8):1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 15.Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint J. 2013;95-B(11):1450–1452. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- 16.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471(2):510–518. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherrell JC, Fehring TK, Odum S, Hansen E, Zmistowski B, Dennos A, et al. The Chitranjan Ranawat Award: fate of two-stage reimplantation after failed irrigation and debridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469(1):18–25. doi: 10.1007/s11999-010-1434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehring TK, Odum SM, Berend KR, Jiranek WA, Parvizi J, Bozic KJ, Della Valle CJ, Gioe TJ. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin Orthop Relat Res. 2013;471(1):250–257. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan AJ, Abdel MP, Sanders TL, Fitzgerald SF, Hanssen AD, Berry DJ. Irrigation and debridement with component retention for acute infection after hip arthroplasty: improved results with contemporary management. J Bone Joint Surg Am. 2017;99(23):2011–2018. doi: 10.2106/JBJS.16.01103. [DOI] [PubMed] [Google Scholar]
- 20.Nodzo SR, Boyle KK, Nocon AA, Henry MW, Mayman DJ, Westrich GH. The influence of a failed irrigation and debridement on the outcomes of a subsequent 2-stage revision knee arthroplasty. J Arthroplast. 2017;32(8):2508–2512. doi: 10.1016/j.arth.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Grosso MJ, Sabesan VJ, Ho JC, Ricchetti ET, Iannotti JP. Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elb Surg. 2012;21(6):754–758. doi: 10.1016/j.jse.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher N, Sofianos D, Berkes MB, Obremskey WT. Prevention of perioperative infection. J Bone Joint Surg Am. 2007;89(7):1605–1618. doi: 10.2106/JBJS.F.00901. [DOI] [PubMed] [Google Scholar]
- 23.Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34(4):505–510. doi: 10.1007/s00264-009-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg AC, Wilson AE, Hayes JP, O'Malley MJ, Klatt BA. Sonication of arthroplasty implants improves accuracy of Periprosthetic joint infection cultures. Clin Orthop Relat Res. 2017;475(7):1827–1836. doi: 10.1007/s11999-017-5315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanmugasundaram S, Ricciardi BF, Briggs TW, Sussmann PS, Bostrom MP. Evaluation and management of periprosthetic joint infection—an international, multicenter study. HSS J. 2014;10(1):36–44. doi: 10.1007/s11420-013-9366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50(11):3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bare J, MacDonald SJ, Bourne RB. Preoperative evaluations in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:40–44. doi: 10.1097/01.blo.0000218727.14097.d5. [DOI] [PubMed] [Google Scholar]
- 28.Stone GP, Clark RE, O’Brien KC, Vaccaro L, Simon P, Lorenzetti AJ, Stephens BC, Frankle MA. Surgical management of periprosthetic shoulder infections. J Shoulder Elb Surg. 2017;26(7):1222–1229. doi: 10.1016/j.jse.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 29.Mortazavi SM, Vegari D, Ho A, Zmistowski B, Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res. 2011;469(11):3049–3054. doi: 10.1007/s11999-011-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parvizi J, Erkocak OF, Della Valle CJ. Culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(5):430–436. doi: 10.2106/JBJS.L.01793. [DOI] [PubMed] [Google Scholar]
- 31.Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am. 2018;100(2):147–154. doi: 10.2106/JBJS.17.00434. [DOI] [PubMed] [Google Scholar]
- 32.Piper KE, Fernandez-Sampedro M, Steckelberg KE, Mandrekar JN, Karau MJ, Steckelberg JM, Berbari EF, Osmon DR, Hanssen AD, Lewallen DG, Cofield RH, Sperling JW, Sanchez-Sotelo J, Huddleston PM, Dekutoski MB, Yaszemski M, Currier B, Patel R. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5(2):e9358. doi: 10.1371/journal.pone.0009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Prieto D, Portillo ME, Puig-Verdie L, Alier A, Martinez S, Sorli L, et al. C-reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections. Int Orthop. 2017;41(7):1315–1319. doi: 10.1007/s00264-017-3430-5. [DOI] [PubMed] [Google Scholar]
- 35.Sethi PM, Sabetta JR, Stuek SJ, Horine SV, Vadasdi KB, Greene RT, Cunningham JG, Miller SR. Presence of Propionibacterium acnes in primary shoulder arthroscopy: results of aspiration and tissue cultures. J Shoulder Elb Surg. 2015;24(5):796–803. doi: 10.1016/j.jse.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 36.Figa R, Muneton D, Gomez L, Matamala A, Lung M, Cuchi E, et al. Periprosthetic joint infection by propionibacterium acnes: clinical differences between monomicrobial versus polymicrobial infection. Anaerobe. 2017;44:143–149. doi: 10.1016/j.anaerobe.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Dodson CC, Craig EV, Cordasco FA, Dines DM, Dines JS, Dicarlo E, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elb Surg. 2010;19(2):303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 38.Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA, 3rd, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490–2495. doi: 10.1128/JCM.00450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon HK, Cho SH, Lee DY, Kang BH, Lee SH, Moon DG, Kim DH, Nam DC, Hwang SC. A review of the literature on culture-negative periprosthetic joint infection: epidemiology, diagnosis and treatment. Knee Surg Relat Res. 2017;29(3):155–164. doi: 10.5792/ksrr.16.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, Chen AF. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99(24):2077–2084. doi: 10.2106/JBJS.17.00123. [DOI] [PubMed] [Google Scholar]
- 41.Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE., Jr The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229–2235. doi: 10.1007/s11999-015-4152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29(4):617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 43.Puig-Verdie L, Alentorn-Geli E, Gonzalez-Cuevas A, Sorli L, Salvado M, Alier A, et al. Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Joint J. 2013;95-B(2):244–249. doi: 10.1302/0301-620X.95B2.30486. [DOI] [PubMed] [Google Scholar]
- 44.Grosso MJ, Frangiamore SJ, Yakubek G, Bauer TW, Iannotti JP, Ricchetti ET. Performance of implant sonication culture for the diagnosis of periprosthetic shoulder infection. J Shoulder Elb Surg. 2018;27(2):211–216. doi: 10.1016/j.jse.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247–2254. doi: 10.2106/JBJS.L.00210. [DOI] [PubMed] [Google Scholar]
- 46.Melendez DP, Uhl JR, Greenwood-Quaintance KE, Hanssen AD, Sampath R, Patel R. Detection of prosthetic joint infection by use of PCR-electrospray ionization mass spectrometry applied to synovial fluid. J Clin Microbiol. 2014;52(6):2202–2205. doi: 10.1128/JCM.00570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgenstern C, Cabric S, Perka C, Trampuz A, Renz N. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018;90(2):115–119. doi: 10.1016/j.diagmicrobio.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Marin M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcala L, Gimeno M, Cercenado E, Sanchez-Somolinos M, Radice C, Bouza E. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583–589. doi: 10.1128/JCM.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joyanes P, Pascual A, Martinez-Martinez L, Hevia A, Perea EJ. In vitro adherence of enterococcus faecalis and enterococcus faecium to urinary catheters. Eur J Clin Microbiol Infect Dis. 2000;19(2):124–127. doi: 10.1007/s100960050443. [DOI] [PubMed] [Google Scholar]
- 50.Veenstra GJ, Cremers FF, van Dijk H, Fleer A. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J Bacteriol. 1996;178(2):537–541. doi: 10.1128/jb.178.2.537-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers KL, Fey PD, Rupp ME. Coagulase-negative staphylococcal infections. Infect Dis Clin N Am. 2009;23(1):73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Arciola CR, Baldassarri L, Campoccia D, Creti R, Pirini V, Huebner J, Montanaro L. Strong biofilm production, antibiotic multi-resistance and high gelE expression in epidemic clones of enterococcus faecalis from orthopaedic implant infections. Biomaterials. 2008;29(5):580–586. doi: 10.1016/j.biomaterials.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 53.El Helou OC, Berbari EF, Marculescu CE, El Atrouni WI, Razonable RR, Steckelberg JM, et al. Outcome of enterococcal prosthetic joint infection: is combination systemic therapy superior to monotherapy? Clin Infect Dis. 2008;47(7):903–909. doi: 10.1086/591536. [DOI] [PubMed] [Google Scholar]
- 54.Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24(19):3221–3227. doi: 10.1016/S0142-9612(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 55.Mack D, Rohde H, Harris LG, Davies AP, Horstkotte MA, Knobloch JK. Biofilm formation in medical device-related infection. Int J Artif Organs. 2006;29(4):343–359. doi: 10.1177/039139880602900404. [DOI] [PubMed] [Google Scholar]
- 56.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li A, Gow N, Atkins BL, Taylor A, Peto T, McNally MA, et al. Metalware-associated orthopaedic infections caused by Staphylococcus lugdunensis: an emerging pathogen. J Inf Secur. 2017;75(4):368–370. doi: 10.1016/j.jinf.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nodzo SR, Boyle KK, Bhimani S, Duquin TR, Miller AO, Westrich GH. Propionibacterium acnes host inflammatory response during periprosthetic infection is joint specific. HSS J. 2017;13(2):159–164. doi: 10.1007/s11420-016-9528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nodzo SR, Westrich GH, Henry MW, Miller AO. Clinical analysis of propionibacterium acnes infection after total knee arthroplasty. J Arthroplast. 2016;31(9):1986–1989. doi: 10.1016/j.arth.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47(6):1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sampedro MF, Piper KE, McDowell A, Patrick S, Mandrekar JN, Rouse MS, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009;64(2):138–145. doi: 10.1016/j.diagmicrobio.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 62.Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011;6(4):e14797. doi: 10.1371/journal.pone.0014797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gristina AG, Naylor P, Myrvik Q. Infections from biomaterials and implants: a race for the surface. Med Prog Technol. 1988;14(3–4):205–224. [PubMed] [Google Scholar]
- 64.Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for propionibacterium acnes orthopedic infection. Am J Orthop (Belle Mead NJ) 2014;43(5):E93–E97. [PubMed] [Google Scholar]
- 65.Wright TE, Boyle KK, Duquin TR, Crane JK. Propionibacterium acnes susceptibility and correlation with hemolytic phenotype. Infect Dis (Auckl) 2016;9:39–44. doi: 10.4137/IDRT.S40539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cobo J, Miguel LG, Euba G, Rodriguez D, Garcia-Lechuz JM, Riera M, et al. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin Microbiol Infect. 2011;17(11):1632–1637. doi: 10.1111/j.1469-0691.2010.03333.x. [DOI] [PubMed] [Google Scholar]
- 67.Hedke J, Skripitz R, Ellenrieder M, Frickmann H, Koller T, Podbielski A, Mittelmeier W. Low-grade infection after a total knee arthroplasty caused by Actinomyces naeslundii. J Med Microbiol. 2012;61(Pt 8):1162–1164. doi: 10.1099/jmm.0.030395-0. [DOI] [PubMed] [Google Scholar]
- 68.Mashimo C, Kamitani H, Nambu T, Yamane K, Yamanaka T, Sugimori-Shinozuka C, Tatami T, Inoue J, Kamei M, Morita S, Leung KP, Fukushima H. Identification of the genes involved in the biofilm-like structures on Actinomyces oris K20, a clinical isolate from an apical lesion. J Endod. 2013;39(1):44–48. doi: 10.1016/j.joen.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74(2):470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gries CM, Staphylococcal Biofilms KT. Immune polarization during prosthetic joint infection. J Am Acad Orthop Surg. 2017;25(Suppl 1):S20–SS4. doi: 10.5435/JAAOS-D-16-00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol. 2014;192(8):3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes Infect. 2002;4(4):481–489. doi: 10.1016/S1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 74.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 75.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis. 2015;211(4):641–650. doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gehrke T, Kendoff D. Peri-prosthetic hip infections: in favour of one-stage. Hip Int. 2012;22(Suppl 8):S40–S45. doi: 10.5301/HIP.2012.9569. [DOI] [PubMed] [Google Scholar]
- 77.Wolf CF, Gu NY, Doctor JN, Manner PA, Leopold SS. Comparison of one and two-stage revision of total hip arthroplasty complicated by infection: a Markov expected-utility decision analysis. J Bone Joint Surg Am. 2011;93(7):631–639. doi: 10.2106/JBJS.I.01256. [DOI] [PubMed] [Google Scholar]
- 78.Gulhane S, Vanhegan IS, Haddad FS. Single stage revision: regaining momentum. J Bone Joint Surg (Br) 2012;94(11 Suppl A):120–122. doi: 10.1302/0301-620X.94B11.30746. [DOI] [PubMed] [Google Scholar]
- 79.Ure KJ, Amstutz HC, Nasser S, Schmalzried TP. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. J Bone Joint Surg Am. 1998;80(7):961–968. doi: 10.2106/00004623-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 80.De Man FH, Sendi P, Zimmerli W, Maurer TB, Ochsner PE, Ilchmann T. Infectiological, functional, and radiographic outcome after revision for prosthetic hip infection according to a strict algorithm. Acta Orthop. 2011;82(1):27–34. doi: 10.3109/17453674.2010.548025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiranek WA, Waligora AC, Hess SR, Golladay GL. Surgical treatment of prosthetic joint infections of the hip and knee: changing paradigms? J Arthroplast. 2015;30(6):912–918. doi: 10.1016/j.arth.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 82.Lichstein P, Gehrke T, Lombardi A, Romano C, Stockley I, Babis G, et al. One-stage versus two-stage exchange. J Orthop Res. 2014;32(Suppl 1):S141–S146. doi: 10.1002/jor.22558. [DOI] [PubMed] [Google Scholar]