Abstract
The purpose of this clinical study was to assess, in a limited patient population, the potential for a novel advanced wound care treatment based on low intensity, low frequency ultrasound (LFLI-US) to affect wound closure rate in human diabetic foot ulcers (DFU) and to effect changes in the relative expression of pro-inflammatory and anti-inflammatory genes. The ratio of expression of these genes, termed the M1/M2 score because it was inspired by the transition of macrophages from pro-inflammatory (M1) to anti-inflammatory (M2) phenotypes as wound healing progresses, was previously presented as a potential healing indicator for DFUs treated with the standard of care (Nassiri et al. 2015). We have also shown that noncavitational, nonthermal, low frequency (20 kHz), low intensity (spatial peak temporal peak intensity <100mW/cm2; i.e. pressure amplitude of 55 kPa) ultrasound (LFLI-US) delivered with a pulse repetition frequency (PRF) of 25 Hz was effective at improving wound healing in a pilot study of 20 patients with chronic venous ulcers (Samuels et al. 2013). In this study, we assessed the potential for weekly LFLI-US exposures to affect wound healing in patients with diabetic ulcers, and we analyzed temporal changes in the M1/M2 score in debrided diabetic wound tissue. Although this was a limited patient population of only 8 patients, wounds treated with LFLI-US showed a significantly faster reduction in wound size compared to sham-treated patients (p<0.001). In addition, the value of the M1/M2 score decreased for all healing diabetic ulcers and increased for all non-healing diabetic ulcers, suggesting that the M1/M2 score could be useful as an indicator of treatment efficacy for advanced DFU treatments. Such indicator would facilitate clinical decision making, ensuring optimal wound management and thus contributing to reduction of healthcare expenses. Moreover, the results presented may contribute to understanding of the mechanisms underlying ultrasonically assisted chronic wound healing. The knowledge of these mechanisms could lead to personalized or patient-tailored treatment.
Keywords: Diabetic ulcers, Low frequency low intensity ultrasound, inflammation
Introduction
The purpose of this brief clinical study in a limited patient population was to evaluate the potential effects of low frequency, low intensity ultrasound (LFLI-US) on diabetic ulcer healing and the temporal changes in the relative expression of genes associated with pro-inflammatory (M1) and anti-inflammatory/pro-healing (M2) macrophage phenotypes, which may have potential as a healing indicator to aid in clinical decision making to improve wound care.
Chronic ulcers represent a substantial clinical burden; 30.3 million people in the United States alone have diabetes (American Diabetes Association), and it is estimated that 15% of diabetic patients will develop an ulcer at some time during their disease course (Reiber et al. 1998). The standard wound care practice involves debridement of necrotic tissue, application of moist dressings, and offloading (Powers et al. 2013). However, these passive treatments have limited success (Frykberg and Banks 2015), with 5-year amputation rates as high as 29% (Moulik et al. 2003). Compounding the problem is the lack of an effective diagnostic method to determine if the wound is healing or not. The Wound Healing Society recommends using a 40% reduction in wound size as an indicator that a wound is on a healing trajectory, but this method has only 50–70% positive predictive value (Cardinal et al. 2008; Sheehan et al. 2003). It has been estimated that switching to a more effective treatment after 4 weeks of unsuccessful treatment would save approximately $12,000 per patient (Weingarten et al. 2012). At the same time, the most effective advanced wound care treatments have only a ~60% success rate (Johnson et al. 2017). Thus, there is a well-defined need for more effective therapies that actively promote wound healing and for innovative diagnostics that aid in clinical decision making.
We recently reported the results of a pilot clinical study indicating that a novel LFLI-US contact-based applicator actively promoted healing of chronic venous ulcers (Samuels et al. 2013). In that study, patients were exposed to 55 kPa pressure amplitude ultrasound wave corresponding to 100mW/cm2, spatial peak temporal peak intensity (Isptp), for 15 or 45 minutes weekly. The weekly treatment frequency was intentionally chosen to minimize patients’ travel burden associated with clinical visits. All exposures were delivered at 20 kHz or 100 kHz with a pulse repetition frequency (PRF) of 25 Hz. Such field parameters are considered safe since they exclude thermal and cavitation effects, even for prolonged exposure times (>250 min). Wounds that were exposed to 20 kHz ultrasound for 15 minutes per week healed at the fastest rate. The purpose of this study was to determine the potential for the same treatment modality to promote healing of diabetic foot ulcers. In addition, we evaluated the potential of a novel healing indicator based on the behavior of macrophages as healing progresses.
Macrophages, the primary cells of the innate immune system, regulate all stages of the healing process by transitioning from the M1 to the M2 phenotype over time as healing progresses (Mirza and Koh 2011; Spiller et al. 2014). The M1 phenotype is associated with early processes in wound healing, such as the initiation of angiogenesis, while the M2 phenotype is associated with late processes in wound healing, such as blood vessel stabilization (Spiller et al. 2014; Spiller and Koh 2017). The M1-to-M2 transition is known to be defective in chronic wounds, characterized by prolonged M1 activation, leading to impaired healing (Mirza and Koh 2011; Mirza et al. 2013). Previously, we showed that expression of individual genes associated with the M1 and M2 phenotypes did not change appreciably over time in human diabetic ulcers; however, processing the data into a novel ratio of four M1-associated genes (VEGF, CCR7, CD80, and IL1B) to three M2-associated genes (PDGFB, TIMP3, and MRC1) accurately predicted healing or non-healing in all 10 patients in the pilot study. More specifically, the M1/M2 score decreased for healing patients and increased for non-healing patients (Nassiri et al. 2015). By processing the gene expression data into the M1/M2 score, the relative levels of M1 and M2 gene expression were magnified and the data were normalized such that they were not affected by the size of the sample. It is important to note, however, that while the selected genes are associated with macrophage phenotype, they are not specific to macrophages and may be expressed at different levels by other cells present in the wound tissue, especially fibroblasts, keratinocytes, and endothelial cells. Thus, the M1/M2 score can be considered an indicator of wound inflammation, as opposed to macrophage phenotype per se (Nassiri et al. 2015).
In this study, we treated diabetic foot ulcers with the same LFLI-US treatment parameters that were successful in our pilot study of venous ulcers (Samuels et al. 2013). In addition, we analyzed changes in inflammation via the M1/M2 score in tissue samples debrided from LFLI-treated and sham-treated wounds in order to assess the potential of the novel M1/M2 score as a healing indicator when used with advanced wound care treatments such as LFLI-US.
Materials and methods
Patient Enrollment and ethical considerations
The study was conducted in accordance with the ethical guidelines set forth by the 1975 Declaration of Helsinki and in compliance with the study protocol approved by Drexel University Institutional Review Board. All patients were recruited from the Drexel University Wound Healing Center and had at least one diabetic ulcer that had not healed for 2 months at the time of enrollment. Patients who exhibited signs and symptoms of untreated vascular disease or invasive infection such as cellulitis or abscess in the tissue with systemic manifestation were excluded from the study. The study was double blind (neither the patient nor the physician knew whether the patient was being given the treatment or sham, although the assisting researcher did know; an expanded double-blind, NIH-sponsored, randomized controlled study is currently underway, see acknowledgements). After providing informed consent, the patients were randomly assigned to the LFLI-US treatment or sham- treatment groups. The sham group was treated in the exact same way as the ultrasound-treated group, except that the device was not activated. During the study, all participants in both treatment groups underwent standard ulcer care procedures determined by the attending physician, including weekly or biweekly wound debridement, prescribed topical dressings to maintain a moist wound environment, and offloading. Patients whose wounds fully closed within 12 weeks of study enrollment were considered healing, while patients whose wounds did not heal within 12 weeks of study enrollment were considered non-healing (Ashby et al.; Steed et al. 2006). As summarized in Table 1, 8 patients were enrolled in this study.
Table 1.
Baseline characteristics of patients.
| Number of patients | 8 |
| Gender | 4M, 4F |
| Age (Years) | 57.6 ±8.9 |
| BMI | 32.8 ±4.7 |
| Wound size at initial visit (area, cm2) | 0.88 ±0.52 |
Abbreviation: M=males, F=female
Ultrasound Applicator
The wearable ultrasound applicator (Supplementary Figure 1) consisted of three 10mm diameter single element unfocused transducers. The 40mm diameter of the “triangle design” applicator acted as a planar source and generated uniform distribution of field measured in water at a distance of 2.5mm, chosen to mimic the clinical exposure conditions (Sunny et al. 2012). The applicator was intentionally designed as a noncavitational, nonthermal device with pressure amplitude of 55 kPa corresponding to 100 mW/cm2 Isptp at 20 kHz. To further reduce the likelihood for temperature elevation and enhance the battery life-time, the ultrasound energy was delivered using pulse repetition frequency of 25 Hz. These acoustic output parameters were chosen based on the results of the clinical pilot study of patients with venous ulcers (Samuels et al. 2013). The performance of the ultrasound applicator was tested regularly before and after each human exposure (15 minutes, once a week); each test included all relevant acoustic field parameters (pressure amplitude, frequency) and uniformity of the field distribution (Samuels et al. 2013). This testing of the acoustic output of the applicators was important to eliminate the possibility of any malfunctioning of the devices.
Ulcer tissue collection
All patients underwent ulcer debridement as part of standard wound care regimen during each visit. Sharp debridement was conducted using a scalpel or curette after removing the overlying biofilm. Debrided tissue was collected from 6 of the 8 patients (n=3 treated and n=3 non-treated); logistical limitations prevented acquisition of samples from the other two patients. The debrided tissue was immediately stored in RNAlater® Solution (Ambion, Austin, TX, USA) at 4°C overnight before long term storage at −80°C prior to RNA extraction and gene expression analysis.
RNA Extraction, cDNA Synthesis, and qRT-PCR
The collected ulcer samples were thawed at room temperature and total RNA was extracted using chloroform and Trizol method followed by purification with the Qiagen RNeasy kit (Qiagen, Inc., CA, USA) according to the manufacturer’s instructions. DNA was inactivated using DNAse I Amplification Grade (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized by using High-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and samples were stored at −80°C. The analysis of gene expression was performed using quantitative reverse transcription polymerase chain reaction (qRT-PCR) using SYBR green reagents (Thermo Fisher Scientific) and 20ng of cDNA per reaction, as previously described (Nassiri et al. 2015). All primers were custom-designed and synthesized by Thermo Fisher Scientific. The M1/M2 score was calculated by taking the ratio of the sum of gene expression levels (2^-Ct) of M1 genes (VEGF, CCR7, CD80, IL1B) to the sum of gene expression levels of M2 genes (PDGFB, TIMP3, MRC1) following the procedure given in (Nassiri et al. 2015). In the same study, we found that the M1/M2 score could differentiate healing from non-healing wounds as early as 3 weeks from patient enrollment. Similarly, in the present study, we compared M1/M2 scores at the 3rd week of treatment.
Statistical Analysis
All statistical analyses were performed in Graph Pad Prism 6 and all data are shown as mean ± SEM. An unpaired two tailed t-test and one-way ANOVA were used for comparison between two groups and more than two groups, respectively. A p-value of less than 0.05 was considered significant.
Results
Effects of LFLI-US treatment on diabetic ulcer closure
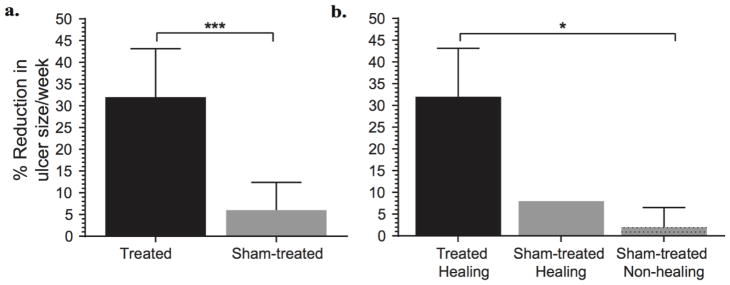
Four of the 8 enrolled patients were treated with LFLI-US and 4 were treated with the sham device (Table 2). All patients also received the standard of care. All 4 treated patients healed within 12 weeks, whereas only 1 of the 4 sham-treated patients healed within the same period. Diabetic ulcers treated with LFLI-US showed a significantly faster closure rate than sham-treated ulcers (Figure 1).
Table 2.
Distribution of diabetic ulcers by treatment and outcome.
| Diabetic Ulcers | Ultrasound Treated | Sham-treated |
|---|---|---|
| Healing | 4 | 1 |
| Non-healing | 0 | 3 |
| Total | 4 | 4 |
Fig 1.
Diabetic ulcer size reduction after treatment and sham treatment. (a) Comparison of ulcer size reduction between treated and sham-treated ulcers (n=4 in each group, Student’s t-test, ***p<0.001). (b) Comparison of ulcer size reduction between treated healing (n=4), sham-treated healing (n=1) and sham-treated non-healing (n=3). Data are shown as mean ± SEM.
Effects of LFLI-US treatment on M1/M2 score in Diabetic Ulcers
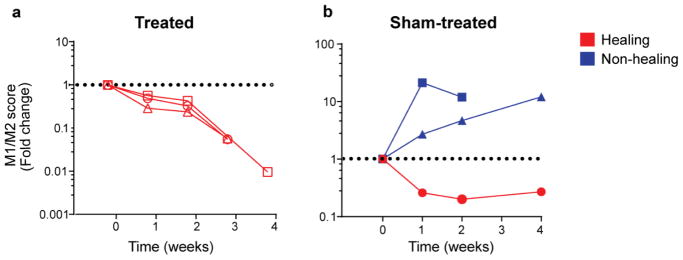
As expected, there were no significant differences in the expression of any of the 7 M1 and M2 genes over time, regardless of treatment or healing status (Supplementary Figure S2). However, in agreement with our previous reports on the utility of the M1/M2 score, combining the values into the M1/M2 score revealed differences between healing and non-healing ulcers regardless of treatment; all healing ulcers showed a decrease in the M1/M2 score over time while all non-healing ulcers showed an increase in the score over time (Figure 2; Supplementary Figure S3).
Fig 2.
Change in combinatorial M1/M2 score over time, represented as fold change compared to the first sample. (a) LFLI-US-treated ulcers; (b) sham-treated ulcers. Each line graph shows data from an individual patient. Healing wounds are shown in red while non-healing wounds are shown in blue.
Discussion
The results of this clinical pilot study suggest that LFLI-US treatment promotes healing of diabetic ulcers. Moreover, ultrasound-induced healing was associated with a reduction in M1/M2 score, which indicates reduced inflammation in treated wounds.
However, it is not possible to determine at present whether the reduction of the M1/M2 score in LFLI-US-treated ulcers was due to direct effects of ultrasound exposure on macrophages. Although macrophages are major regulators of inflammation and wound healing, the genes that comprise the M1/M2 score are not specific to macrophages, and may be expressed at appreciable levels by other cells in the wound, including endothelial cells, fibroblasts, and keratinocytes. In other words, the “M1” genes may also be considered as genes expressed at the early stages of healing by multiple cell types. These genes include those associated with inflammation and the initiation of angiogenesis (CCR7, CD80, IL1B, and VEGF). Likewise, the “M2” genes are associated with the later stages of healing, including the M2 phenotype of macrophages as well as stabilization of angiogenesis (PDGFB, TIMP3, and MRC1). Thus, the relative levels of the early stage factors (CCR7, CD80, IL1B, and VEGF) compared to the late stage factors (PDGFB, TIMP3, MRC1) can be used as a surrogate to track the proper sequence of cellular events required for normal wound healing (Nassiri et al. 2015). These results suggest that once verified with a larger number of subjects, the M1/M2 score could be applicable as an effective healing indicator, permitting clinicians to implement agile modification of the initial treatment options and reduce hospitalization time.
Numerous studies have shown effects of LFLI-US on cells involved in wound healing, including fibroblasts and endothelial cells. For example, the results from in vitro studies performed using identical exposure parameters showed that the ultrasound energy stimulated metabolism and proliferation of murine 3T3 fibroblasts (Samuels et al. 2013). Another study showed that 10min of exposure to 27 kHz continuous wave US at 250 mW/cm2 caused human umbilical vein endothelial cells to increase synthesis of nitric oxide, which could contribute to vasodilation, increased blood flow, and improved healing (Altland et al. 2004). However, at present the mechanisms behind the biophysical effects of ultrasound on cells and tissues are not known. One potential mechanism may be mechanical effects of ultrasound pressure waves on macrophages. For example, low intensity (30 mW/cm2) pulsed (1.5 MHz pulsed at 1 kHz) ultrasound has been shown to activate the RhoA, p38 MAPK, and ERK mechanotransduction pathways in macrophages and to increase their phagocytic capability (Zhou et al. 2008). These characteristics are also associated with M2 macrophage polarization (Leidi et al. 2009; Zhang et al. 2011). In addition, the transforming growth factor-beta-1 (TGFB1) pathway is another mechanotransduction pathway (Hinz 2015) that could be activated in response to ultrasound and would be expected to inhibit M1 polarization of macrophages (Freire-de-Lima et al. 2006).
Another potential mechanism by which ultrasound impacts cells involved in wound healing may be through indirect effects on other cells such as endothelial cells. For example, ultrasound may generate highly localized, transient fluid flow that produces shear stresses. While the effects of shear stress on macrophages have not been thoroughly investigated, it is well known that shear stress impacts endothelial cell behavior, with potentially positive effects on angiogenesis and on regulating macrophage behavior. For example, laminar flow-induced shear stress has been shown to enhance endothelial cell expression of VEGF (Conklin et al. 2002) and TGFB1 (Ohno et al. 1995) and to enhance angiogenesis (Galie et al. 2014). Low intensity (15–30 mW/cm2) low frequency (45 kHz) ultrasound also has been shown to increase VEGF expression by osteoblasts, fibroblasts, and peripheral blood mononuclear cells in vitro (Reher et al. 1999). Both VEGF and TGFB1 are known modulators of macrophage polarization (Eirin et al. 2013; Freire-de-Lima et al. 2006; He et al. 2012), suggesting potential mechanisms by which ultrasound indirectly affects macrophage behavior through effects on other cells. Clearly, further studies are required to narrow down the precise biophysical mechanisms behind ultrasound-induced wound healing.
Finally, other studies have shown that the input of physical energy from other sources affects wound healing. For example, the application of whole body low intensity vibration, which has been shown to increase bone mineral density in patients, accelerated diabetic wound healing in mice, concomitant with increased levels of macrophages and growth factors (Weinheimer-Haus et al. 2014). Electroceutical systems that apply static or dynamic electric fields to the wound have shown promise (Blount et al. 2012; Wahlsten et al. 2016). However, unlike ultrasound, these strategies lack a wide range of control over the energy parameters that can be applied locally to the wound and/or are not suitable for combination with many other wound care treatments such as advanced wound dressings. In addition, the portable, wearable ultrasound applicator design that we describe in this study has the potential for increasing patient compliance because of its convenience and possibility of at-home use.
Although this study suggests that LFLI-US may be effective for diabetic wound healing and that the M1/M2 score may be useful as an indicator of healing, a major limitation of this analysis is the small number of patients. This is a recognized problem in chronic ulcer studies that stems from limited patient compliance in returning for repeat visits as well as limitations in the numbers of eligible patients because of exclusionary criteria, such as vascular insufficiency or extensive osteomyelitis, that are necessary in preliminary mechanistic studies. Another limitation was that only the 7 genes associated with the previously described M1/M2 score (Nassiri et al. 2015) were evaluated. Evaluation of other genes related to inflammation and healing will shed light on the mechanisms behind LFLI-US-induced wound healing. Moreover, gene expression is only a surrogate indicator of cell behavior, and future studies should include analysis of the possible changes on the protein and cellular levels. These limitations notwithstanding, this study presents proof-of-concept indicating that LFLI-US treatment of human chronic diabetic ulcers may assist in the healing process with either direct or indirect effects on inflammation, and that the M1/M2 score might become useful as clinically viable indicator of wound healing treatment.
Conclusions
Although based on limited sample of data, the results of the present study suggest that nonthermal, noncavitational ultrasound may enhance wound healing in diabetic foot ulcers, and that healing progress may be monitored by examining the value of the novel M1/M2 score. The use of the M1/M2 score as a clinically viable biomarker would enhance the diagnostic power of attending physicians and facilitate early decision making of the preferred wound management, thus contributing to reduction of healthcare expenses. The results obtained may also contribute to develop a better understanding of the mechanisms underlying ultrasonically assisted chronic wound healing process. The knowledge of these mechanisms could lead to personalized or patient-tailored treatment.
Supplementary Material
Acknowledgments
This publication was made possible by the Drexel-Coulter Translational Research Partnership and the Drexel Neuroinflammation and Gender Research Program as well as by grants NIBIB 5 R01 EB009670 and NINR 5R01NR015995. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altland OD, Dalecki D, Suchkova VN, Francis CW. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J Thromb Haemost. 2004;2:637–643. doi: 10.1111/j.1538-7836.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- Ashby RL, Gabe R, Ali S, Saramago P, Chuang L-H, Adderley U, Bland JM, Cullum NA, Dumville JC, Iglesias CP. VenUS IV (Venous leg Ulcer Study IV) - compression hosiery compared with compression bandaging in the treatment of venous leg ulcers: a randomised controlled trial, mixed-treatment comparison and decision-analytic model. doi: 10.3310/hta18570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount AL, Foster S, Rapp DA, Wilcox R. The use of bioelectric dressings in skin graft harvest sites: a prospective case series. J Burn Care Res. 2012;33:354–357. doi: 10.1097/BCR.0b013e31823356e4. [DOI] [PubMed] [Google Scholar]
- Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008;16:19–22. doi: 10.1111/j.1524-475X.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res. 2002;102:13–21. doi: 10.1006/jsre.2001.6295. [DOI] [PubMed] [Google Scholar]
- Eirin A, Zhu XY, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, Simari RD, Lerman A, Textor SC, Lerman LO. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol. 2013;33:1006–1013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Advances in wound care. 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Xu J, Warren CM, Duan D, Li X, Wu L, Iruela-Arispe ML. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood. 2012;120:3152–3162. doi: 10.1182/blood-2012-04-422758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Johnson EL, Marshall JT, Michael GM. A comparative outcomes analysis evaluating clinical effectiveness in two different human placental membrane products for wound management. Wound Repair Regen. 2017;25:145–149. doi: 10.1111/wrr.12503. [DOI] [PubMed] [Google Scholar]
- Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256–264. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative Expression of Proinflammatory and Antiinflammatory Genes Reveals Differences between Healing and Nonhealing Human Chronic Diabetic Foot Ulcers. J Invest Dermatol. 2015;135:1700–1703. doi: 10.1038/jid.2015.30. [DOI] [PubMed] [Google Scholar]
- Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JG, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatol Ther. 2013;26:197–206. doi: 10.1111/dth.12055. [DOI] [PubMed] [Google Scholar]
- Reher P, Doan N, Bradnock B, Meghji S, Harris M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine. 1999;11:416–423. doi: 10.1006/cyto.1998.0444. [DOI] [PubMed] [Google Scholar]
- Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg. 1998;176:5S–10S. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- Samuels JA, Weingarten MS, Margolis DJ, Zubkov L, Sunny Y, Bawiec CR, Conover D, Lewin PA. Low-frequency (< 100 kHz), low-intensity (< 100 mW/cm2) ultrasound to treat venous ulcers: A human study and in vitro experiments. The Journal of the Acoustical Society of America. 2013;134:1541–1547. doi: 10.1121/1.4812875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879–1882. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017;122:74–83. doi: 10.1016/j.addr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T. Guidelines for the treatment of diabetic ulcers. Wound Repair and Regeneration. 2006;14:680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Sunny Y, Bawiec CR, Nguyen AT, Samuels JA, Weingarten MS, Zubkov LA, Lewin PA. Optimization of un-tethered, low voltage, 20–100kHz flexural transducers for biomedical ultrasonics applications. Ultrasonics. 2012;52:943–948. doi: 10.1016/j.ultras.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten O, Skiba JB, Makin IRS, Apell SP. Electrical field landscape of two electroceuticals. Journal of Electrical Bioimpedance. 2016;7:13–19. [Google Scholar]
- Weingarten MS, Samuels JA, Neidrauer M, Mao X, Diaz D, McGuire J, McDaniel J, Jenkins L, Zubkov L, Papazoglou ES. Diffuse near-infrared spectroscopy prediction of healing in diabetic foot ulcers: a human study and cost analysis. Wound Repair Regen. 2012;20:911–917. doi: 10.1111/j.1524-475X.2012.00843.x. [DOI] [PubMed] [Google Scholar]
- Weinheimer-Haus EM, Judex S, Ennis WJ, Koh TJ. Low-intensity vibration improves angiogenesis and wound healing in diabetic mice. PLoS One. 2014;9:e91355. doi: 10.1371/journal.pone.0091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xu W, Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. J Immunol. 2011;187:1764–1777. doi: 10.4049/jimmunol.1002315. [DOI] [PubMed] [Google Scholar]
- Zhou S, Bachem MG, Seufferlein T, Li Y, Gross HJ, Schmelz A. Low intensity pulsed ultrasound accelerates macrophage phagocytosis by a pathway that requires actin polymerization, Rho, and Src/MAPKs activity. Cell Signal. 2008;20:695–704. doi: 10.1016/j.cellsig.2007.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.