Abstract
The incidence of acute myelogenous leukemia (AML) increases progressively with age. Favorable genetic mutations are most prevalent in children, and unfavorable profiles increase proportionately in adolescents and young adults (AYA) and into later adulthood. Survival rates of AYA have improved over recent decades to 50–60%, but their accrual to clinical trials remains poor. In contrast to AYA with acute lymphoblastic leukemia, the prognostic benefit for AYA with AML enrolled in pediatric compared with adult trials is minor and only seen when different protocols are used. The distinctive needs of AYA, including intensive psychological services, call for their treatment within specialized centers that offer complex supportive care.
Keywords: acute myelogenous leukemia (AML), adolescents, young adults (AYA), clinical trials, prognosis, survival
INTRODUCTION
Acute myelogenous leukemia (AML) represents 15%–20% of leukemias in children and approximately 33% in adolescents and young adults (AYA). Age is a very strong prognostic factor, with prognosis decreasing with increasing age, independent of other risk factors.1 However, a recent study showed that apart from age, genetic mutations can also be significant prognostic factors.2 Over the last 20 years, overall survival (OS) of children with AML has improved considerably (5-year OS 60%–75%).3–7 However, in AYA, defined as 15–39 years, overall cure rates are only 50%–60%.8–13
A relatively low number of AYA are enrolled in adult cooperative group trials or pediatric trials. Treatment protocols for children and adults often differ, and studies on appropriate therapy specifically for AYA are limited.14,15 In this review, we describe biological features, treatment modalities, and outcomes of AYA with AML.
INCIDENCE
Data from the United States Surveillance, Epidemiology, and End Results (SEER) program for 2000–201416 and the German Childhood Cancer Registry (GCCR 2014)17 suggest that epidemiologic and survival patterns of AYA with cancer vary because of lower patient numbers or differences in race/ethnicity and socioeconomic constructs in different countries.18–20
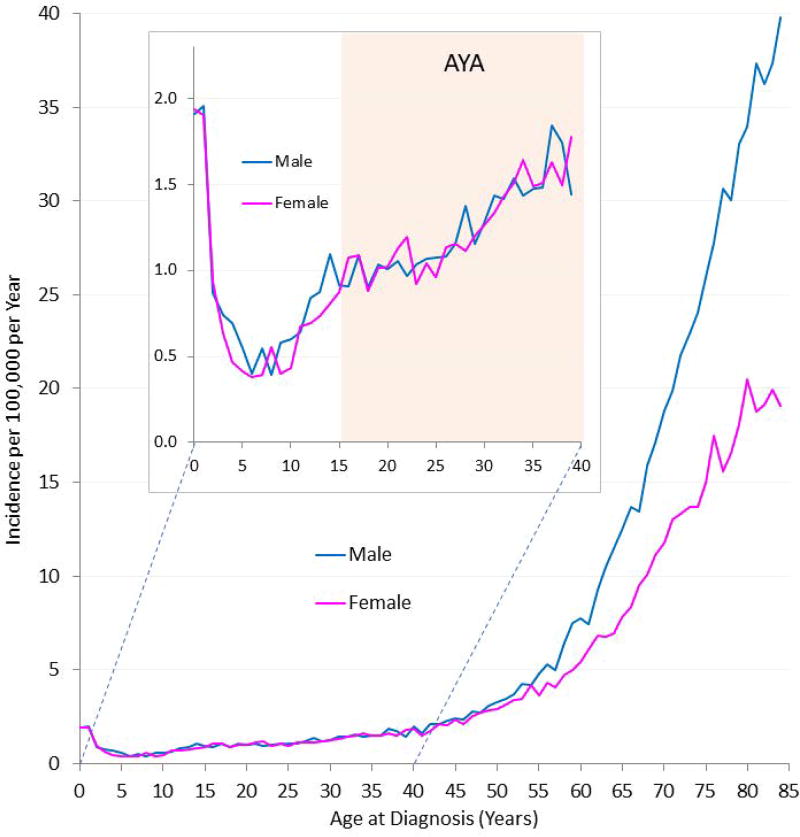
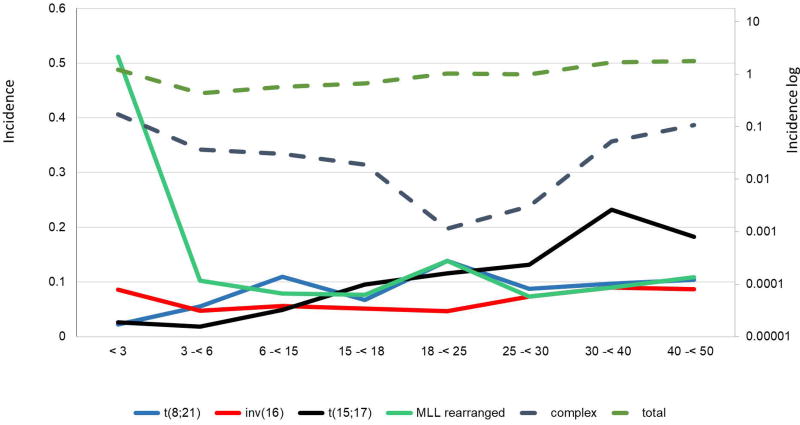
In the US, 5% of all invasive cancers affecting AYA are leukemias, with 40% of them being AML.21 The proportion of AML with respect to all leukemias is higher in AYA than children (16%) or older patients (31%). Fig. 1 shows AML incidence by age and gender in all age groups, and is amplified in the inset for patients younger than 40 years. AML rates are higher in the first years of life and decrease subsequently (nadir at approximately 9 years), followed by a slow increase during adolescence and young adulthood. Fig. 2 gives age-specific, population-based incidences of AML by cytogenetic subtype below 50 years of age, using data extrapolated from 1991 patients diagnosed from 1998 to 2012 in Germany. These incidences are similar to those reported in SEER data for the total patient group (Fig. 1).22 There were little or no differences by gender for patients aged 2–40 years. Therefore, with advancing age, AML incidence increases within the total spectrum of acute leukemias, resulting in inversion of the incidence of acute lymphoblastic leukemia (ALL) and AML in late adolescence. The incidence of AML, unlike that of ALL, is similar in white and black children of all age groups.16
FIGURE 1.
Incidence of AML by gender and single year of age at diagnosis during 2000–2014, U.S. SEER18. Inset depicts age less than 40 years, including AYA range of 15 to 39 years.16
FIGURE 2.
Age-specific population-based incidences of cytogenetic subtypes in AML below 50 years of age. Absolute incidences of various cytogenetic subgroups in AML from a German population are shown. The y-axis shows absolute incidence per 100,000 individuals with the respective cytogenetic subgroups according to age. Different age groups are shown on the x-axis. Dotted lines indicate the logarithmic scale. Modified from Creutzig et al. 201622
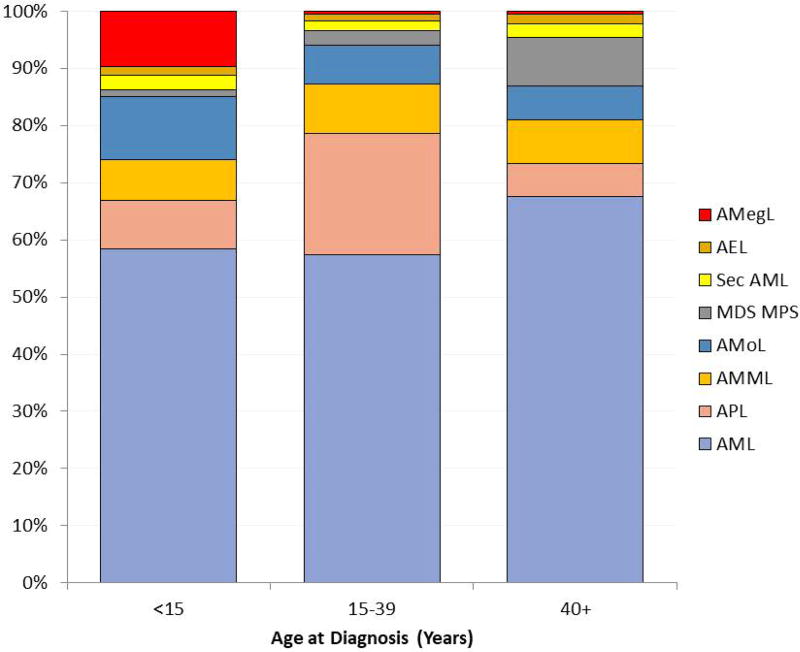
The high incidence of AML in older age groups (especially >60 years) correlates with prolonged exposure to environmental carcinogens and accumulation of mutations from genetic error events in cell division.23,24 The incidence of acute promyelocytic leukemia [APL, t(15;17)/PML-RARA, World Health Organization 2008 classification] and AML with NPM1 mutations appears constant with respect to age after the first decade.25,26 According to SEER data16 the proportion of APL is highest (20%–25%) among AML subtypes in AYA (Fig. 3). In general, APL incidence is higher (20%–24% of AML diagnoses) in certain ethnic populations (e.g., Italian and Latin American) than others (5%–8%). APL incidence is also higher in Asian populations.27 These findings suggest a genetic predisposition for APL and/or exposure to specific environmental factors.28
FIGURE 3.
Proportion of all AML by International Classification of Diseases in Oncology ICD-O3 subtype (SEER 2000–2014).16
AML is often a second malignancy in AYA receiving intensive chemotherapy and radiotherapy. The cumulative incidence of treatment-related AML is approximately 0.6% in children treated for ALL or solid tumors and 3.3%–10% for adults treated for different types of solid tumors.29–31
SURVIVAL TRENDS
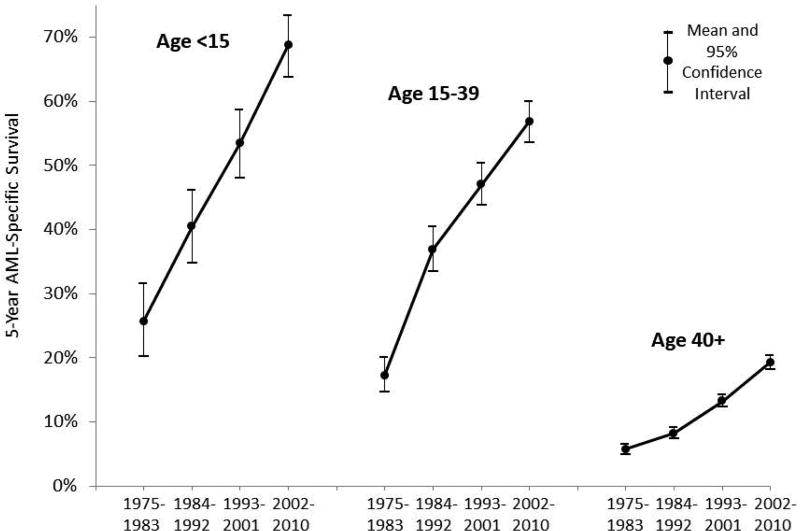
Since the late 1970s, the 5-year AML-specific survival rate has doubled for all age groups. Also, the rate of improvement in the 5-year AML-specific survival rate is similar for children, AYA, and older adults (Fig. 4).16,32
FIGURE 4.
Temporal trends in five-year AML-specific survival, U.S. SEER9, 1975–2010, by 8-year intervals and age.
Population-based estimates of the 5-year survival for AYA in the US increased from 18.6% during 1975–1984 to 56.3% during 2003–2010.16 These estimates were similar to those for AML patients in England: the 5-year relative survival in AYA aged 15–24 years increased from 7% to 53% during 1971–2006.33 Current 5-year survival rates for children and adolescents enrolled in clinical trials (which tend to be higher because trials may exclude patients with unfavorable features or those from small hospitals) are 60%–75%.34 Patients younger than 18 years enrolled in AML Berlin–Frankfurt–Münster (BFM) trials showed progressive improvement in 5-year survival from 49% (AML-BFM 87, 1987–1992) to 60% (1993–1998), 65% (1999–2003), and 75% (2004–2010).4,35 Currently, population-based studies report survival rates of approximately 50% in young adults.10 Outcomes in all age groups have improved over past decades because of intensification of chemotherapy in de novo AML and after relapse as well as better supportive care. With intensive induction chemotherapy, 80%–90% of young patients achieve complete remission (CR).
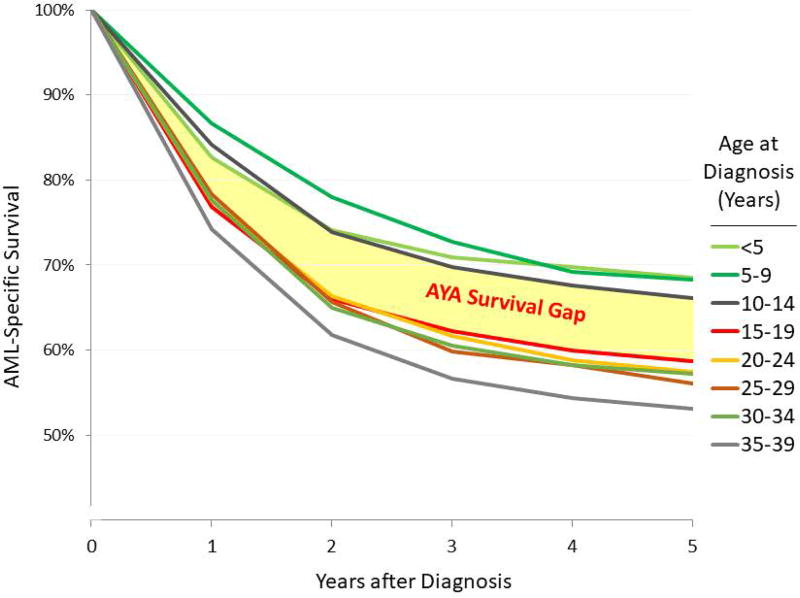
Studies focusing on OS of AYA patients are limited. Population-based data from England and Wales revealed significant improvement in 5-year OS from 36% (1984–1988) to 46% (1989–1994) and 56% (2001–2005) for AML patients aged 15–29 years (although only patients aged 15–24 years were included in 2001–2005).36,37 According to SEER data for 2000–2014, there was a survival gap between children and AYA`s, indicating the decrement in survival from age <15 to the AYA age (Fig. 5).16
FIGURE 5.
AML-specific survival during 2000–2014, U.S. SEER18, Age <40 years by 5-year age Intervals. The survival gap identifies the decrement in survival from age <15 to the AYA age range.16
PREDICTORS OF OUTCOME
According to SEER data for 1973–2012, male patients had a higher risk of mortality, especially in the subgroup of AYA aged 20–24 years, than did female patients.38 Race/ethnicities and socioeconomic variables can also affect the survival of AYA and children.16
Increasing age is an adverse prognostic factor in adults with AML.1,10,39 A comparison of AYA (age 16–29 years) with older patients revealed that the AYA age group was associated independently with improved CR rate and longer CR duration and trended toward longer survival.12 Fig. 5 shows population-based data.16 However, prognostic studies in children and adolescents of different ages treated similarly are rare and often conflicting. The Children’s Cancer Group (CCG) trial CCG-213 [1986–89] revealed no difference in survival.40,41 However in CCG-2891 [1996–2002], patients older than 16 years had event-free survival (EFS) and OS inferior to those for patients <16 years.42
Recently, a cross-study analysis combining data from CCG-2891, CCG-2941, CCG-2961, and Children's Oncology Group (COG) AAML03P1 showed similar survival rates for AYA and younger patients with newly diagnosed AML; however, treatment-related mortality (TRM) was higher in older patients. The OS for AYA (age 16–20 years, n=238) was 49%±7% versus 54%±3% for younger patients (n=1602; P=0.058). Relapse rate was significantly lower (30%±7% vs 41%±3%, P=0.002) but TRM was higher (25%±6% vs 12%±2%, P<0.001) for AYA patients.43 The higher TRM in AYAs was due to high infection rates. Similar outcomes for AYA were observed when data were combined from the most recent COG trials AAML03P1 and AAML0531, in which gemtuzumab ozogamicin (GO) was added to a Medical Research Council (MRC)–based chemotherapy regimen. AYA aged 16–21 years and younger patients (<16 years) had similar 5-year EFS (44.2% vs 50.2%) and 5-year OS (60% vs 64.8%), but TRM rates were higher in AYA than younger patients (13.3% vs 7.3%, P=0.005). Further, AYA patients who received GO or hematopoietic stem cell transplantation (HSCT) had higher TRM rates, which attenuated the survival advantage seen among young patients receiving these interventions.44
Similar findings were reported for TRM rates in patients aged 10–21 years in the St. Jude Children’s Research Hospital AML protocols (1991–2008).45 Survival rates for Japanese patients aged 15–17 years were lower than those for younger patients, mainly due to a higher TRM rate after relapse.46
BIOLOGY AND GENETICS
Biological characteristics across the AYA age spectrum have been rarely reported. The frequency of cytogenetic subgroups of AML is associated with age. Increasing age is associated with a decrease in favorable cytogenetics and an increase in unfavorable cytogenetics (Table 1, Fig. 2).22,29,47,48
TABLE 1.
Frequencies of specific karyotypes classified hierachically downward in patients by age group (<60 years)
| Age (years) | |||||
|---|---|---|---|---|---|
| 0 to <2 | 2 to <12 |
12 to <18 |
18 to <40 |
40 to <60 |
|
| Total (N) | 271 | 477 | 444 | 417 | 1099 |
| % | % | % | % | % | |
| Cytogenetics | |||||
| Normal karyotype | 13.7 | 20.2 | 27.3 | 44.9 | 52.4 |
| t(15;17)(q22;q12)/PML-RARA | 1.9 | 5.9 | 11.7 | 12.4 | 8.1 |
| t(8;21)(q22;q22)/RUNX1-RUNX1T1 | 0.4 | 17.2 | 13.3 | 7.8 | 4.3 |
| inv(16)(p13.1q22)/t(16;16)(p13.1;q22)/CBFB-MYH11 | 6.3 | 10.0 | 8.8 | 5.5 | 4.4 |
| t(9;11)(p22;q23)/MLL-MLLT3 | 19.6 | 8.3 | 4.5 | 2.6 | 1.2 |
| Other 11q23/MLL abnormalities | 25.2 | 10.0 | 7.4 | 5.1 | 2.0 |
| inv(3)/t(3;3)(q21;q26)/GATA2-MECOM | 0 | 0.2 | 0.2 | 1.2 | 0.7 |
| Other 3q26/MECOM-rearranged* | 0 | 0 | 0 | 1.3 | 0.8 |
| t(6;9)(p23;q34)/DEK-NUP214 | 0 | 0 | 0.5 | 1.4 | 0.3 |
| Complex (≥3)** | 13.7 | 7.0 | 4.3 | 2.3 | 7.8 |
| Monosomy 7 | 0.4 | 1.9 | 1.1 | 0.9 | 1.2 |
| 7q abnormalities | 0.7 | 0.6 | 1.4 | 2.1 | 1.3 |
| Chr. 5 abnormalities | 0.4 | 1.3 | 1.4 | 0.5 | 0.8 |
| Trisomy 8 | 1.9 | 2.5 | 4.7 | 3.6 | 4.2 |
| 12p abnormalities | 1.5 | 0.4 | 1.1 | 0.5 | 0.2 |
| 17p abnormalities | 0 | 0 | 0 | 0.2 | 0.3 |
| Other abnormalities | 14.4 | 14.4 | 12.2 | 7.8 | 10.2 |
MECOM was referred to as EVI1 in the past.
Complex karyotype ≥3 aberrations, at least 1 structural aberration, without favorable genetics, and without MLL rearrangements.
Note: Light gray shading indicates favorable and dark gray shading indicates unfavorable cytogenetics.
Note: Modified from Creutzig et al. 201622
Morphological data for children, adolescents, and young adults (age <30 years, excluding APL) in AML-BFM 93/98 (n=869) and AMLCG92 (n=92)1,49 show that, according to the French–American–British (FAB) classification for AML, 68% of children younger than 2 years had FAB subtypes M5 or M7 compared with 18% for older age groups (P<0.0001). However, apart from a trend toward increasing M1 and decreasing M7, there were no differences in FAB types for children (2–12 years) and AYA (13–30 years). Fig. 3 shows the proportion of all AML subtypes according to age by International Classification of Diseases in Oncology ICD-O3 subtype.16
The relationship between age and genetics from birth to 100 years of age was analyzed in 5564 patients with de novo AML diagnosed from 1998 to 2012.22 Frequencies of cytogenetic subgroups were age dependent. In general, favorable subtypes [t(8;21), inv(16)/t(16;16), t(15;17)] decreased from 33% in the pediatric age group (2 years to <18 years) to less than 5% in the oldest group (>70 years, P<0.0001). Interestingly, t(8;21) was less frequent in young adults (21–30 years) than in younger (2–21 years) patients. The frequency of unfavorable cytogenetics [−7/del(7), −5/del(5q) or 5p, inv(3)/t(3;3), t(6;9), complex karyotype, 12p, 17p, and 11q23/MLL aberrations excluding t(9;11)] was 44% in infants (<2 years), 25% in children and young adults, and up to 42% in patients older than 85 years (P=0.01). This difference was even more significant for patients with complex karyotypes (P<0.0001). Interestingly, the frequency of 11q23 abnormalities decreased from infants to older patients (Table 1 and Fig. 2).
COG trials AAML03P1 and AAML0531 compared genetic characteristics of AYA (16–21 years) with children younger than 16 years. AYA patients were more likely to have normal cytogenetics (36.5% vs 20.5%, P<0.001) and the unfavorable genetic finding of FLT3/ITD (20% vs 14.3%, P=0.047) than younger children. AYA patients were also more likely to have favorable prognostic markers such as CEBPA mutations (9.4% vs 4.8%, P=0.012) and NPM1 mutations (12.8% vs 6%, P=0.001).44 The frequency of 11q23/MLL rearrangements was lower in AYA than in younger children (11.5% vs 23.3%, P≤0.001), which is consistent with results from AML-BFM studies.
Comprehensive comparison of AML molecular profiles across groups has shown marked differences in mutated genes, structural variants, and DNA methylation patterns that distinguish AML in infants, children, adolescents, and adults.48 Although mutational burden increased with age, mutation rates of pediatric and young adult AML patients are similar to those of adult AML patients. However, the landscape of somatic variants is markedly different. For example, alterations in RAS, KIT and FLT3, including pediatric-specific activating FLT3 mutations, are more common in children than adults. There is higher prevalence of small sequence variants in older patients, whereas recurrent structural alterations, fusions, and focal copy number aberrations are more common in younger patients. Because of the overlap of genetic abnormalities associated with prognosis among these age-defined groups, testing for these alterations is essential for the management of newly diagnosed patients with AML in any age group.48
TREATMENT AND MANAGEMENT
Treatment regimens for AML are often but not always similar for children, adolescents, and adults. They start generally with intensive induction courses of cytarabine and anthracyclines at dosages adequate to achieve remission, followed by post-remission phases, which have in recent years also included maintenance therapy with novel agents (e.g., tyrosine kinase inhibitors) to destroy residual blasts in the bone marrow or other sites. Induction therapy as well as the duration and optimal strategy for post-remission therapy is under continuous re-assessment as standards change over time. In general, intensive post-remission chemotherapy cycles (consolidation and/or intensification courses) include one or more courses of high-dose cytarabine (depending on the molecular subset of the disease in adults). These courses are co-administered with central nervous system (CNS) prophylaxis and may be followed by a less intensive maintenance chemotherapy or, in treatments under current evaluation, by specific inhibitors. Allogeneic HSCT may be included as another form of intensification, and indications and rates vary by country, study group, and provider (pediatric vs adult).34,50 Majhail et al. analyzed data from the Center for International Blood and Marrow Transplant Research for allogeneic HSCT in patients categorized as children (<15 years, n=900), AYA (15–40 years, N=2708), or older adults (>40 years, N=2728).51 Outcomes for AYA receiving HSCT improved over the 3 studied time periods (1980–1988, 1989–1997, and 1998–2005), and this improvement was similar to that in children and older adults. The OS for AYA was inferior to that for children and superior to that for older adults during each time period. During 1998–2005, the 5-year survival for AYA was 43% compared with 64% for children and 31% for older adults. TRM decreased over the time periods and was a major contributor to improved survival; however, the TRM rate in AYA was twice that in children.
Until recently, except for APL, no definitive specific treatment interventions were used for other AML subgroups. Genotype-specific treatment with the differentiating agent all-trans-retinoic acid (ATRA) for APL patients, which induces cell differentiation and maturation instead of cell destruction,28,52 was proof of concept that non-cytotoxic chemotherapy has a role in AML treatment.28,52 Recently, the combination of ATRA and arsenic trioxide has shown high efficacy and reduced hematologic toxicity in adults with low- or intermediate-risk APL.53 Clinical trials are currently under development in Europe and North America to study this regimen in adolescents and children with APL. The Food and Drug Administration has approved several compounds for specific adult AML subgroups.54,55
Patients require acute management and supportive care during all treatment phases, especially the first few days to weeks of intensive induction therapy. As noted, TRM rates are higher in AYA than in younger children receiving chemotherapy or HSCT, but AYA can usually tolerate more intensive regimens than elderly adults.56 With recent improvements in results of AML treatment, the balance between treatment intensity and toxicity and their interrelation with the disease’s genetic background has become increasingly important, and trials need to use risk- and genotype-adapted therapy.
Compared with children, adolescents have more anticipatory vomiting. Compliance during intensive treatment phases is similar between AYA and children and older patients, as most or all chemotherapy is administered at the hospital. In countries such as Germany that use maintenance therapy for AML, compliance of AYA to maintenance therapy may be lower, similar to lower adherence to oral chemotherapy in adolescents with ALL.57
Psychosocial care is the most difficult aspect of managing adolescents. The needs of adolescents differ from those of young children and are accompanied by typical concerns such as need for autonomy and independence, social development, sexual maturation, education, and employment.58 These problems are similar to those of AYA with other types of cancer. Recently, results of the European Society for Medical Oncology/European Society for Paediatric Oncology survey showed an important neglect and inequity of AYA cancer care across Europe and suggested that education and research be focused on improving cancer care for AYA.59 In the US, young adults are the most uninsured and under-insured age group. Nearly half of all 15- to 19-year-olds lose the healthcare insurance provided by parents and do not acquire adequate coverage thereafter.60
PARTICIPATION IN CLINICAL TRIALS
In the US, participation rates in cancer trials have been strikingly lower in AYA (15–34 years) than younger or older patients.60 This trend remained in recent analyses (1997–2005; up to 45% in 5- to 10-year-old children vs 14% in adolescents, and <5% in older AYA) and was mainly because these patients were not referred to institutions undertaking the clinical trials.61 In Europe, participation rates are higher in AYA with leukemias, as they are generally included in national trials (e.g., >90% in the British MRC trials or German AML trials).26,62,63 As most clinical trials in adults do not report results separately for AYA and older adults, limited data are available for comparisons across age groups.
TREATMENT IN PEDIATRIC VERSUS ADULT TRIALS
Treatment schedules and dosing in the German AML-BFM 93/98 trials for children and adolescents (n=891) and AMLCG92/99 and AMLSG HD93/98A trials for adults were similar during induction and consolidation.64–67 In adult studies 290 patients were 16–30 years old. EFS and OS were comparable in overlapping age groups (>15 to <21 years) in all trials. However, treatment results were most favorable in children. CR rates decreased by age (2–12 years, 89%; 13 to <21 years, 82%; 21–30 years, 72%), and long-term treatment results were most favorable among children aged 2–12 years (5-year EFS 53%±2%), slightly inferior in adolescents (45%±3%, P=0.03), and unfavorable in young adults (28%±3%, P=0.0001). If patients with low-risk cytogenetics [t(8;21), inv16, and t(15;17)] were excluded, results were poorer in adolescents (EFS 39%±5%) and young adults (EFS 25%±6%) than in children aged 2–12 years (EFS 52%±4%).1
Similar to the German studies, a recent study from the Nordic Society of Pediatric Haematology and Oncology found no difference in outcomes for AML patients aged 10–30 years treated during 1993–2009 on pediatric versus adult protocols.68
A retrospective study compared 281 adolescents (16–21 years) in the pediatric cooperative CCG and COG trials (1986–2008) with 149 patients in the same age group in the adult cooperative Cancer and Leukemia Group B (CALGB) and Southwest Oncology Group (SWOG) frontline AML trials (1986–2008). Patient characteristics were similar, but age was a confounding variable (median, COG 17.2 vs CALGB 20.1 and SWOG 19.8 years, P<0.001). The 10-year survival rate for patients treated in CCG or COG trials was 45%±6% versus 34%±7% for those in adult trials (P=0.026).14 Note that intensity of the pediatric schedule was usually higher (including intensively timed induction) than of the adult regimen, but treatment-related mortality was also higher for patients in the COG than in the adult trials.
Several authors support that the prognosis for adolescents with ALL may be improved by introducing pediatric trials that consider prognostic biological features.69 However, differences in outcomes of patients treated in pediatric versus adult trials are more pronounced for adolescent ALL patients than for AML patients14,69,70 even when chemotherapy intensity is generally higher in pediatric AML protocols than adult protocols and HSCT is given more restrictively (risk adapted) than for adults. AYA-specific approaches can be influenced further by referring these patients to centers experienced in managing leukemia or centers participating in clinical trials for children or adults.20,71 Cooperation between pediatric and adult study groups is of utmost importance. AYA patients need to be enrolled in clinical trials, and cross-study analyses comparing strategies for pediatric and adult patients are required.
LATE EFFECTS
Late effects in childhood and adolescent survivors of AML can affect their quality of life significantly. Long-term sequelae of treatment include impaired intellectual and psychomotor functioning, neuroendocrine abnormalities, impaired reproductive capacity, and second malignancies.72 However, most late effects, especially side effects of CNS irradiation (e.g., neurocognitive deficits, growth hormone deficiency, and secondary CNS tumor), affect the younger age group. Anthracycline-related late cardiotoxicity occurs at lower cumulative doses (<300 mg/m2) in patients younger than 18 years than those 18 years or older.73,74
The risk of endocrine dysfunction is relatively low in AML patients receiving standard chemotherapy only (without alkylating agents). However, those receiving HSCT are at increased risk of endocrine dysfunction.72,75 In contrast to growth impairment that can occur in children after busulfan/cyclophosphamide or cyclophosphamide/total body irradiation (TBI) conditioning regimens, gonadal toxicity occurs in all age groups, mainly as gonadal dysfunction, but it is relatively low with modern conventional therapy.72 Gonadal toxicity may lead to abnormal pubertal development, infertility, and sexual dysfunction, and require long-term hormone substitution. In adult women high doses of alkylating agents and TBI increase the risk of ovarian failure, and the probability of restoring ovarian function decreases by a factor of 0.8 per year of age.76 Addition of busulfan to cyclophosphamide causes permanent ovarian failure in nearly all females. In males cytotoxic chemotherapy and TBI can damage the germinal epithelium of the testis, and most males of all age groups can have permanent infertility.76
Studies on second malignancies in AYA after AML treatment are scarce. In AML-BFM patients diagnosed from 1993 to 2010, 10-year cumulative incidence of secondary malignancies was 1.5% (standard error 0.3%). Most of these patients received only chemotherapy. After HSCT the risk of second malignancies is higher for any disease (standard incidence ratio 6.7–11.6 compared with patients given chemotherapy only).77,78
Finally, long-term consequences of treatment in children and adolescents with leukemia and lymphoma include more symptoms of depression and somatic distress than in sibling controls.72 A systematic literature review on health-related quality of life in AML patients aged >18 years identified fatigue as the most problematic symptom domain.79
CONCLUSIONS
AML incidence increases with age, such that the incidence in AYA lies between that of children and older adults. Biological factors vary by age, but biological characteristics of AML in AYA are more similar to those of children than of older adults. Outcomes have improved for all age groups during the last 30 years due to superior treatment strategies for newly diagnosed and relapsed AML as well as improvements in supportive care and prophylaxis of bacterial and fungal infections. However, children tend to have better survival than AYA, which might in part be related to treatment intensity or enrollment in pediatric trials. As AYA show less tolerance to intensive chemotherapy or HSCT than do children, improving supportive care remains an important objective. Chemotherapy could be optimized for AYA by considering the pediatric and adult experience. Further research should be directed toward biologically based and not age-specific trials, which require better cooperation between pediatric and adult study groups and cross-study analyses comparing pediatric and adult strategies.
Acknowledgments
Funding Sources: Supported partly by National Cancer Institute, National Institutes of Health, Cancer Center Support Grant No. CA21765, and by the American Lebanese Syrian Associated Charities.
We thank Vani Shanker, St. Jude Children’s Research Hospital, for scientific editing and Archie Bleyer, Department of Radiation Medicine, Oregon Health and Sciences University, Bend, OR, USA, for generously providing the SEER figures.
Abbreviations list
- ALL
Acute lymphoblastic leukemia
- AML
Acute myelogenous leukemia
- AMLSG
German-Austrian AML study group
- APL
Acute promyelocytic leukemia
- ATRA
All-trans-retinoic acid
- AYA
Adolescents and young adults
- BFM
Berlin–Frankfurt–Munster
- CALGB
Cancer and Leukemia Group B
- CCG
Children’s Cancer Group
- CNS
Central Nervous System
- COG
Children’s Oncology Group
- CR
Complete remission
- EFS
Event-free survival
- FAB
French-American-British
- GCCR
German Childhood Cancer Registry
- GO
Gemtuzumab ozogamicin
- HSCT
Hematopoietic stem cell transplantation
- MRC
Medical Research Council
- OS
Overall survival
- SEER
Surveillance, Epidemiology, and End Results
- SWOG
Southwest Oncology Group
- TBI
Total body irradiation
- TRM
Treatment-related mortality
- US
United States
Footnotes
Conflict of Interest Disclosures: The authors declare no conflict of interest.
Author Contributions: UC, MK, RB, RS and RR designed and performed research and wrote and edited the paper. All the authors reviewed and approved the final version of the manuscript.
References
- 1.Creutzig U, Buchner T, Sauerland MC, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112:562–571. doi: 10.1002/cncr.23220. [DOI] [PubMed] [Google Scholar]
- 2.Kuwatsuka Y, Tomizawa D, Kihara R, et al. Prognostic value of genetic mutations in adolescent and young adults with acute myeloid leukemia. Int J Hematol. 2017 doi: 10.1007/s12185-017-2340-z. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- 4.Creutzig U, Zimmermann M, Bourquin JP, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- 5.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- 8.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125:3878–3885. doi: 10.1182/blood-2015-01-623447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 11.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–1016. doi: 10.1002/cncr.29869. [DOI] [PubMed] [Google Scholar]
- 12.Pemmaraju N, Kantarjian H, Ravandi F, et al. Patient Characteristics and Outcomes in Adolescents and Young Adults (AYA) With Acute Myeloid Leukemia (AML) Clin Lymphoma Myeloma Leuk. 2016;16:213–222. e212. doi: 10.1016/j.clml.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trama A, Botta L, Foschi R, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5. Lancet Oncol. 2016;17:896–906. doi: 10.1016/S1470-2045(16)00162-5. [DOI] [PubMed] [Google Scholar]
- 14.Woods WG, Franklin AR, Alonzo TA, et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer. 2013;119:4170–4179. doi: 10.1002/cncr.28344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson J, Sun CL, Wyatt L, et al. Adolescents and Young Adults with Acute Lymphoblastic Leukemia and Acute Myeloid Leukemia: Impact of Care at Specialized Cancer Centers on Survival Outcome. Cancer Epidemiol Biomarkers Prev. 2017;26:312–320. doi: 10.1158/1055-9965.EPI-16-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SEER NCI. SEER Cancer Statistics Review 1975–2011. 2014 [Google Scholar]
- 17.Kaatsch PSC. German Childhood Cancer Registry - Report 2013/14 (1980–2013): Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz. 2014 [Google Scholar]
- 18.Kahn JM, Keegan TH, Tao L, et al. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122:2723–2730. doi: 10.1002/cncr.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durani U, Go RS. Racial and ethnic disparities in the survival of adolescents and young adults with acute myeloid leukemia: a retrospective study using the US National Cancer Data Base. Leuk Lymphoma. 2017;58:1184–1189. doi: 10.1080/10428194.2016.1231312. [DOI] [PubMed] [Google Scholar]
- 20.Go RS, Bartley AC, Al-Kali A, et al. Effect of the type of treatment facility on the outcome of acute myeloid leukemia in adolescents and young adults. Leukemia. 2016;30:1177–1180. doi: 10.1038/leu.2015.239. [DOI] [PubMed] [Google Scholar]
- 21.Barr RD, Ries LA, Lewis DR, et al. Incidence and incidence trends of the most frequent cancers in adolescent and young adult Americans, including "nonmalignant/noninvasive" tumors. Cancer. 2016;122:1000–1008. doi: 10.1002/cncr.29867. [DOI] [PubMed] [Google Scholar]
- 22.Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122:3821–3830. doi: 10.1002/cncr.30220. [DOI] [PubMed] [Google Scholar]
- 23.Welch JS, Ley TJ, Link DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillis NK, Ball M, Zhang Q, et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol. 2017;18:112–121. doi: 10.1016/S1470-2045(16)30627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers M, Jackson G, Taylor P. The incidence of acute promyelocytic leukemia appears constant over most of a human lifespan, implying only one rate limiting mutation. Leukemia. 2000;14:722–726. doi: 10.1038/sj.leu.2401722. [DOI] [PubMed] [Google Scholar]
- 26.Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO) Ann Hematol. 2017 doi: 10.1007/s00277-017-3150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadam Amare PSJH, Kabre S, et al. Cytogenetic Profile in 7209 Indian Patients with de novo Acute Leukemia: A Single Centre Study from India. Journal of Cancer Therapy. 2016;07:530–544. [Google Scholar]
- 28.Douer D, Preston-Martin S, Chang E, et al. High frequency of acute promyelocytic leukemia among Latinos with acute myeloid leukemia. Blood. 1996;87:308–313. [PubMed] [Google Scholar]
- 29.Leone G, Mele L, Pulsoni A, et al. The incidence of secondary leukemias. Haematologica. 1999;84:937–945. [PubMed] [Google Scholar]
- 30.Loning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95:2770–2775. [PubMed] [Google Scholar]
- 31.Granfeldt Ostgard LS, Medeiros BC, Sengelov H, et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J Clin Oncol. 2015;33:3641–3649. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 32.Nasir SS, Giri S, Nunnery S, et al. Outcome of Adolescents and Young Adults Compared With Pediatric Patients With Acute Myeloid and Promyelocytic Leukemia. Clin Lymphoma Myeloma Leuk. 2017;17:126–132. e121. doi: 10.1016/j.clml.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Shah A, Andersson TM, Rachet B, et al. Survival and cure of acute myeloid leukaemia in England, 1971–2006: a population-based study. Br J Haematol. 2013;162:509–516. doi: 10.1111/bjh.12425. [DOI] [PubMed] [Google Scholar]
- 34.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–3205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 35.Creutzig U, Zimmermann M, Ritter J, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 36.Stiller CA, Benjamin S, Cartwright RA, et al. Patterns of care and survival for adolescents and young adults with acute leukaemia--a population-based study. Br J Cancer. 1999;79:658–665. doi: 10.1038/sj.bjc.6690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cancer Research UK. Teenage and young adult cancer survival statistics. 2015 Dec; http://www.cancerresearchuk.org/cancer-info/cancerstats/teenage-and-young-adult-cancer/survival/
- 38.Hossain MJ, Xie L, Caywood EH. Prognostic factors of childhood and adolescent acute myeloid leukemia (AML) survival: Evidence from four decades of US population data. Cancer Epidemiology. 2015;39:720–726. doi: 10.1016/j.canep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchner T, Heinecke A. The role of prognostic factors in acute myeloid leukemia. Leukemia. 1996;10(Suppl 1):S28–29. [PubMed] [Google Scholar]
- 40.Wells RJ, Arthur DC, Srivastava A, et al. Prognostic variables in newly diagnosed children and adolescents with acute myeloid leukemia: Children's Cancer Group Study 213. Leukemia. 2002;16:601–607. doi: 10.1038/sj.leu.2402390. [DOI] [PubMed] [Google Scholar]
- 41.Wells RJ, Woods WG, Buckley JD, et al. Treatment of newly diagnosed children and adolescents with acute myeloid leukemia: a Childrens Cancer Group study. J Clin Oncol. 1994;12:2367–2377. doi: 10.1200/JCO.1994.12.11.2367. [DOI] [PubMed] [Google Scholar]
- 42.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children's oncology group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canner J, Alonzo TA, Franklin J, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children's Oncology Group. Cancer. 2013;119:4162–4169. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.August KJ, Aplenc R, Sung L, et al. Adolescents and Young Adults (AYA) with Acute Myeloid Leukemia (AML) Have Increased Treatment-Related Mortality with Similar Outcomes -- a Report from the Children's Oncology Group Trials AAML03P1 and AAML0531. Blood. 2014;124:3672–3672. [Google Scholar]
- 45.Rubnitz JE, Pounds S, Cao X, et al. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer. 2012;118:6253–6259. doi: 10.1002/cncr.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomizawa D, Watanabe T, Hanada R, et al. Outcome of adolescent patients with acute myeloid leukemia treated with pediatric protocols. Int J Hematol. 2015;102:318–326. doi: 10.1007/s12185-015-1825-x. [DOI] [PubMed] [Google Scholar]
- 47.Bacher U, Kern W, Schnittger S, et al. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;90:1502–1510. [PubMed] [Google Scholar]
- 48.Bolouri H, Farrar JE, Triche T, Jr, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nature Medicine. 2017;24:103. doi: 10.1038/nm.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creutzig U, Zimmermann M, Bourquin JP, et al. Favorable outcome in infants with AML after intensive first- and second-line treatment: an AML-BFM study group report. Leukemia. 2012;26:654–661. doi: 10.1038/leu.2011.267. [DOI] [PubMed] [Google Scholar]
- 50.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 51.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:861–873. doi: 10.1016/j.bbmt.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guidez F, Ivins S, Zhu J, et al. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–2642. [PubMed] [Google Scholar]
- 53.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 54.Kurtz SE, Eide CA, Kaempf A, et al. Molecularly targeted drug combinations demonstrate selective effectiveness for myeloid- and lymphoid-derived hematologic malignancies. Proc Natl Acad Sci U S A. 2017;114:E7554–e7563. doi: 10.1073/pnas.1703094114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomizawa D, Tanaka S, Kondo T, et al. Allogeneic Hematopoietic Stem Cell Transplantation for Adolescents and Young Adults with Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2017;23:1515–1522. doi: 10.1016/j.bbmt.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children's oncology group. J Clin Oncol. 2012;30:2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penson RT, Rauch PK, McAfee SL, et al. Between parent and child: negotiating cancer treatment in adolescents. Oncologist. 2002;7:154–162. doi: 10.1634/theoncologist.7-2-154. [DOI] [PubMed] [Google Scholar]
- 59.Saloustros E, Stark DP, Michailidou K, et al. The care of adolescents and young adults with cancer: results of the ESMO/SIOPE survey. ESMO Open. 2017;2:e000252. doi: 10.1136/esmoopen-2017-000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107:1645–1655. doi: 10.1002/cncr.22102. [DOI] [PubMed] [Google Scholar]
- 61.Tai E, Beaupin L, Bleyer A. Clinical trial enrollment among adolescents with cancer: supplement overview. Pediatrics. 2014;133(Suppl 3):S85–90. doi: 10.1542/peds.2014-0122B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–3368. doi: 10.1200/JCO.2012.47.4874. [DOI] [PubMed] [Google Scholar]
- 63.Messerer D, Dugas M, Müller T, et al. How many patients with AML were treated in clinical trials in Germany? Rundbrief Kompetenznetz Leukämien. 2003;5:6–7. [Google Scholar]
- 64.Buchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27:61–69. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 65.Buchner T, Hiddemann W, Berdel WE, et al. 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by sequential HAM in adult patients at all ages with de novo acute myeloid leukemia (AML): a randomized trial of the German AML Cooperative Group. J Clin Oncol. 2003;21:4496–4504. doi: 10.1200/JCO.2003.02.133. [DOI] [PubMed] [Google Scholar]
- 66.Schlenk RF, Benner A, Hartmann F, et al. Risk-adapted postremission therapy in acute myeloid leukemia: results of the German multicenter AML HD93 treatment trial. Leukemia. 2003;17:1521–1528. doi: 10.1038/sj.leu.2403009. [DOI] [PubMed] [Google Scholar]
- 67.Schlenk RF, Dohner K, Mack S, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010;28:4642–4648. doi: 10.1200/JCO.2010.28.6856. [DOI] [PubMed] [Google Scholar]
- 68.Wennstrom L, Edslev PW, Abrahamsson J, et al. Acute Myeloid Leukemia in Adolescents and Young Adults Treated in Pediatric and Adult Departments in the Nordic Countries. Pediatr Blood Cancer. 2016;63:83–92. doi: 10.1002/pbc.25713. [DOI] [PubMed] [Google Scholar]
- 69.Schiffer CA. Differences in outcome in adolescents with acute lymphoblastic leukemia: a consequence of better regimens? Better doctors? Both? J Clin Oncol. 2003;21:760–761. doi: 10.1200/JCO.2003.11.116. [DOI] [PubMed] [Google Scholar]
- 70.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ho G, Jonas BA, Li Q, et al. Early mortality and complications in hospitalized adult Californians with acute myeloid leukaemia. Br J Haematol. 2017;177:791–799. doi: 10.1111/bjh.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robison LL, Bhatia S. Late-effects among survivors of leukaemia and lymphoma during childhood and adolescence. Br J Haematol. 2003;122:345–359. doi: 10.1046/j.1365-2141.2003.04499.x. [DOI] [PubMed] [Google Scholar]
- 73.Buzdar AU, Marcus C, Smith TL, et al. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55:2761–2765. doi: 10.1002/1097-0142(19850615)55:12<2761::aid-cncr2820551206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 74.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 75.Baker KS, Bhatia S, Bunin N, et al. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: state of the science, future directions. Biol Blood Marrow Transplant. 2011;17:1424–1427. doi: 10.1016/j.bbmt.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brennan BM, Shalet SM. Endocrine late effects after bone marrow transplant. Br J Haematol. 2002;118:58–66. doi: 10.1046/j.1365-2141.2002.03527.x. [DOI] [PubMed] [Google Scholar]
- 77.Leiper AD. Non-endocrine late complications of bone marrow transplantation in childhood: part II. Br J Haematol. 2002;118:23–43. doi: 10.1046/j.1365-2141.2002.03471.x. [DOI] [PubMed] [Google Scholar]
- 78.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korol EE, Wang S, Johnston K, et al. Health-Related Quality of Life of Patients with Acute Myeloid Leukemia: A Systematic Literature Review. Oncol Ther. 2017;5:1–16. doi: 10.1007/s40487-016-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]