Abstract
Galectin-3 (Gal-3), a β-galactoside-binding lectin that is expressed in mammalian cells, is known to modulate several biological functions such as cell-cell adhesion, macrophage activation, angiogenesis, metastasis, and fibrosis. The goal of this study was to evaluate the ability of Gal-3 depletion apheresis using an adsorption column with immobilized anti-Gal-3-antibody to reduce inflammation induced by Complete Freund’s Adjuvant injection in a skin inflammation porcine model. Here we report that plasma perfusion by apheresis through a Gal-3 binding immuno-affinity column reduces plasma Gal-3 levels to below limits of quantitative detection, and results in significant decrease in skin inflammation, including degree and duration of inflammatory lesions. Human plasma was tested ex vivo and found to be efficiently depleted using the anti-Gal-3 affinity column. This study demonstrates the potential of Gal-3 depletion apheresis as a therapeutic method for inflammation-mediated disease, supporting continued research in this area for clinical application.
Keywords: Therapeutic-apheresis, plasmapheresis, complete Freund’s adjuvant, lesion induration
INTRODUCTION
Galectin-3 (Gal-3), a β-galactoside-binding lectin, is established as a multifunctional and multi-locational chimeric galectin. It can be localized in the nucleus, the cytoplasm, or secreted to the extracellular milieu, and can also be found in the systemic circulation. Gal-3 has been identified as instigator and biomarker for a range of diseases including allergies, autoimmune, atherosclerosis, cancer, cardiovascular, fibrosis of many different tissues, and chronic inflammation.1–4 The genetic disruption studies link Gal-3 to an array of pro-inflammatory and pro-fibrotic responses,5,6 and its overexpression is associated with tumor growth, angiogenesis, and metastasis as well as with the fibrotic remodeling of kidney, heart, lung, and liver tissues.7–13 Gal-3 gained an increased interest as a novel biomarker in patients with heart failure where elevated Gal-3 levels are associated with left ventricular dysfunction and poor prognosis.8,14–18 More recent evidence supports the causality of Gal-3 involvement in the pathology of myocardial fibrosis, suggesting that Gal-3 actively contributes to the development of heart failure.1,9 These characteristics and roles make Gal-3 a novel target for the creation of methods that try to inhibit, diminish, or remove Gal-3 to achieve therapeutic benefit in multiple medical conditions.20
Given its capacity to bind β-galactosides, we previously demonstrated that it is possible to remove Gal-3 in vitro from plasma and serum samples using affinity chromatography mini-columns with different substrates with greater selective affinity to Gal-3.21 Sepharose coupled with a monoclonal antibody that binds Gal-3 could remove Gal-3 from porcine sera ex vivo. Here, we describe an apheresis column perfusion system using affinity chromatography with Sepharose-bound to the Gal-3 monoclonal antibody and demonstrate its use in a porcine model of skin inflammation using Complete Freund’s Adjuvant (CFA) injection. CFA is composed of heat-inactivated Mycobacterium tuberculosis emulsified in non-metabolizable oils and used as an immune stimulating antigen which creates an intense inflammatory reaction at the site of deposition. The goal was to assess the effects of circulating Gal-3 depletion by apheresis column adsorption as a therapeutic method for reducing induced inflammation.
MATERIALS AND METHODS
Animals
Massachusetts General Hospital - Major Histocompatibility Complex (MHC)-defined miniature swine ranging in weight from 40-50 kg were used for these studies. The characteristics of this herd have been described previously.22,23 All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of Massachusetts General Hospital.
Complete Freund’s Adjuvant injections and evaluation
Animals in the apheresis control group (apheresis alone without column perfusion) (n=3) and experimental group (apheresis with column perfusion) (n=3) underwent dual central line insertion between day -6 and day -3 followed on day 0 by injection of CFA. After shaving the back neck of the swine, a 2 by 2-inch square was drawn, with four inner squares 1 by 1 inch, on both the right and left side. Then 0.5 mL CFA [Each mL contains 1 mg of Mycobacterium tuberculosis (H37Ra, ATCC 25177), heat killed and dried, 0.85 mL paraffin oil and 0.15 mL mannide monooleate (Sigma-Aldrich Corp. St. Louis, MO; product F5881)] was injected in the middle of each inner square. Each square was labeled 1 to 4. The right side was used to assess the induration and macroscopic appearance, whereas the left side was reserved for biopsies (Figure 1A). An additional naïve control animal without catheter insertion was injected in 4 separate sites on the back of the neck in an equivalent manner. A schematic representation of Gal-3 depletion by plasmapheresis, with representation of Gal-3, its pentamers, and lattice structures is shown in Figure 1B.
Figure 1.

Schematic representation of the model. A. Schematic representation of the CFA inflammatory skin injection. B1-B8 indicate biopsy sites around CFA injection. Pigs were injected with CFA on the left (L) and right (R) side of the neck region behind the ears using 2 X 2-inch templates, separated by at least 2 inches along the dorsal line. CFA was injected into the center of each 1-inch square. B. Schematic representation Gal-3 depletion by plasmapheresis, with representation of Gal-3, its pentamers, and lattice structures in right box.
The degree of inflammation was assessed through induration measurements and biopsies at defined time-points until end of study, 37 days post-CFA injection. The diameter of induration surrounding each injection site was recorded and graphed. In cases where the area of induration merged between injection sites, the greatest distance of induration from the center of each injection site to the outer edge was measured and then doubled to represent diameter. All CFA injections were done with the needle positioned at right angle flush to the skin surface using a 15.8 mm long 25-gauge needle with the intent to ensure consistent depth of injection in the subcutaneous space well below the dermal layer. Post-CFA injection, the needle was held in place for 60 seconds and then slowly removed over an additional 60 seconds to avoid exposure of CFA on the skin surface.
For each animal, the area of injection was marked, and the diameter of induration recorded pre-apheresis until day 37 post-CFA injection. Animals were anesthetized pre-CFA injection, 12 hours post-CFA injection, pre-apheresis, and then again on days 3, 9, 16, 23, 30, and 37 to allow for accurate induration measurements of the right-side injection sites. These time points were chosen to track the early and late inflammation response, including the post-apheresis period.
At each of the assessment time-points for the experimental and apheresis control animals, two 3 mm deep tissue punch biopsies were performed on the left side in a rotating pattern starting on the diagonal adjacent to the center of each injection site to monitor for inflammation histologically. Animals were monitored for signs of inflammation and toxicity to plasmapheresis with complete blood count (CBC) before every procedure and blood chemistry collection every 2 weeks {White blood cells [porcine normal range (PNR);11-22 billion/L], red blood cells (PNR; 4.6-7.3 trillion/L), lymphocytes (LY) (PNR; 4.5-13 K/mm3), monocytes (MO) (PNR; 0.2-2 K/mm3), granulocytes (Gran) (PNR; 3.2-13.2 K/mm3), % LY, % MO, % Gran, hematocrit (PNR; 32-50%), hemoglobin (PNR; 8.7-15.2 g/dL), platelets (PNR; 259-734 billion/L), glucose (PNR; 50-100 mg/dL), blood urea nitrogen (PNR; 7-25 mg/dL), creatinine (PNR; 0.4-1.2 mg/dL), albumin (PNR; 2.8-4.4 g/dL), alanine aminotransferase (PNR; 20-120 units/L), aspartate aminotransferase (PNR; 23-94 units/L), alkaline phosphatase (PNR; 73-210 units/L), gamma-glutamyl transferase (PNR; not established), total bilirubin (PNR; not established), amylase (PNR;149-500), lipase (PNR; not established), lactate dehydrogenase (PNR; 578-1800 units/L)}. The CBC analysis was performed on Heska HemaTrue Analyzer (Heska Corporation, Loveland, CO) and the blood chemistry using an Idexx Catalyst Dx Chemistry Analyzer (Idexx Laboratories, Westbrook ME). Additionally, blood was drawn through the catheter pre-CFA injection on all days of apheresis plus weekly thereafter for serum collection.
Experimental apheresis
Experimental and apheresis control animals underwent a total of at least 400 minutes of apheresis, or 3 plasma volumes, during each apheresis procedure. In the experimental group (n=3), animals underwent apheresis with Gal-3 immuno-affinity column perfusion for 400 minutes after 12 hours, 4, 9, 15, 18, and 22 days post-CFA injection. These specific days were chosen to allow removal of Gal-3 immediately after the CFA injection, and on a regular basis for the first three weeks, in order to observe changes in the inflammation response during that time, and for two additional weeks after the discontinuation of apheresis. In the apheresis control group (n=3), animals also underwent apheresis on the same days for 400 minutes, but without column perfusion.
Apheresis column preparation and perfusion
The affinity columns contained LEAF™ purified (BioLegend, San Diego, CA) monoclonal antibody M3/38 rat anti-mouse Gal-3 monoclonal antibody from hybridoma M3/38.1.2.8 HL.2 (ATCC® TIB-166™),24,25 covalently bound to hydrated cyanogen-bromide activated Sepharose 4B (GE Healthcare Life Sciences, Pittsburgh, PA) as previously described.21 For column perfusion, 60 mL matrix volume was packed into a GE XK50/20 column (GE Healthcare Life Sciences, Pittsburgh, PA) with a diameter of 50mm and bed height of ~30mm. Therapeutic apheresis was performed using a COBE Spectra 4.0 (Terumo BCT, Lakewood, CO), per an established therapeutic plasma exchange protocol (TPE) recommended by Terumo BCT using anticoagulant citrate dextrose (ACD) solution (Terumo BCT, Lakewood, CO) in a ratio of 14: 01 (parts of blood: ACD) and a fluid balance set at 115% for each animal. During apheresis, the inlet flow rate typically ranged between 12-14 mL/minute resulting in a column retention time of ~4-5 minutes, calculated based on the column having an internal diameter of 50 mm and the Sepharose matrix bed height being approximately 3 cm. To assess the binding efficiency of porcine Gal-3 to the M3/38 antibody, plasma samples from treated animals were taken pre- and post-passage through the affinity column during apheresis, and Gal-3 concentrations quantified by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described.21 The Gal-3 specific ELISA has a linear range from 0.3 to 20 ng/ml with a lower limit of detection of 300 pg/ml. Gal-3 levels in sera from systemic blood drawn before and after the apheresis procedure were also measured.
Induration measurements
To assess the relative therapeutic benefits of the method, our primary outcome measure was to compare the appearance of the injected area of skin of experimental animals that underwent apheresis and column perfusion through the Gal-3 depletion column. The apheresis control animals that underwent apheresis alone without column perfusion and a naïve control animal that was injected with CFA in an equivalent manner but did not undergo apheresis or column perfusion were used to complete the comparison. Induration was assessed by measuring the diameter of palpable lesions. Secondary assessment endpoints were Gal-3 levels present in plasma as measured by ELISA, histological assessment of skin biopsies, and CBC and blood chemistry profiles for signs of liver toxicity and side effects.
Ex vivo depletion of Gal-3 in human plasma samples
For the ex vivo assessment of human Gal-3 affinity, 2 separate mini-columns of 1 mL with either Sepharose alone (control group) or Sepharose-bound to 2 mg/mL of M3/38 antibody (Sepharose+M3/38 antibody treatment group) were prepared using Poly-Prep chromatography columns (Bio-Rad Laboratories, Hercules, CA). Human plasma samples (1.5 mL) from three volunteers (n=3) were prepared in EDTA tubes, centrifuged, and frozen before being passed through the column. The volunteers provided signed written informed consent for their participation. Undiluted fractions from the effluent were used in ELISA to quantify the amount of Gal-3 removed. Sample concentrations were measured using a human Gal-3 ELISA kit (BG Medicine, Waltham, MA).
Statistical analysis
The data we collected represent paired samples and repeated measures and were analyzed usingtwo tail, unequal variance t-test, repeated measures ANOVA, and linear mixed models were all performed with GraphPad Prism 7 software. A p-value < 0.05 was considered to be of statistical significance. For the ANOVA and mixed models, we included subject (ELISA data) or animal (inflammation data) as a random variable to account for the potential for serial dependence. Repeated measures ANOVA was used to determine the statistical significance of the treatment effect in the later phase (>3 weeks post-CFA injection). The treatment effect from 3 weeks to the end of the study was tested by means of time of treatment interaction term.
RESULTS
In situ monitoring of Gal-3 removal by apheresis columns
The data shown are from plasma samples drawn directly from pre- and post-column during the apheresis procedure. Figure 2A shows the ability of Gal-3 therapeutic apheresis to deplete Gal-3 from porcine blood. During apheresis on days 1, 4, 9, 15, 18, and 22 plasma samples were collected pre- and post-Gal-3 depletion-apheresis column. Gal-3 levels in plasma pre-apheresis ranged from 2–12 ng/mL. Levels varied between different animals and at different time points.
Figure 2.

Gal-3 plasma levels in samples collected immediately pre- and post-column for experimental animal 23147 (p-value < 0.0001). A. Gal-3 levels (ng/mL) in plasma samples from a representative animal that were collected early (early) or at the end (late) of the apheresis procedure, pre- (Above) and post- (Below) Gal-3 depletion-apheresis column. * Below limit of detection. B. Comparison of Gal-3 levels (ng/mL) in porcine plasma with known concentrations (5 or 2.5 ng/mL), with or without dilution with post-apheresis plasma.
Regardless of Gal-3 levels pre-apheresis, the levels following passage through the Gal-3 apheresis column dropped to below the limit of detection. This observation was consistent regardless of whether plasma samples were collected within 30 minutes of column perfusion (early) or at the end (late) of the apheresis procedure (p-value < 0.0001).
As demonstrated in Figure 2B we confirmed that the undetectable levels of Gal-3 presented in Figure 2A were not due to an interference from shedding of IgG-Gal 3 antibodies from the column into the plasma. Known concentrations of porcine Gal-3 (5 or 2.5 ng/mL) were diluted with post-apheresis column plasma and Gal-3 levels measured by ELISA were compared with reference Gal-3. The measured Gal-3 levels in the analyzed samples showed only minimal deviation from the expected values.
Gal-3 depletion effect on CFA-induced skin inflammation
The difference in induration was assessed among animals undergoing column perfusion with the anti-Gal-3 antibody, apheresis control animals that underwent apheresis alone without column perfusion, and a naïve control animal that did not undergo apheresis or column perfusion (Figure 3). Induration measurements are shown only for those animals with confirmed injection into the subcutaneous space based on skin depth measurements at autopsy. The time course of skin induration following CFA injection is shown in Figure 3A (Experimental n=2; Control n=3).
Figure 3.

Comparison of area of induration surrounding each CFA injection site over time (Experimental n=2; Control n=3). A. The time course of skin induration following CFA injection treatment in experimental (red line) and control (blue line) animals. B. Individual experimental animals (23171 and 23147) compared with control animals (22877, 22681, 23166) at days 0.5 (12 hours), 3, and 37. Induration measurements are shown only for those animals with confirmed injection into the subcutaneous space based on skin depth measurements at autopsy.
Pre-Gal-3 apheresis, the experimental group had greater early inflammatory skin responses that manifested as greater skin induration at day 0.5 (12 hours post-injection). By contrast, the apheresis control group had a gradual, protracted inflammatory skin response that developed between baseline and day 3. By day 9 control and experimental animals developed pronounced cutaneous inflammation as assessed by histology (not shown), indicating a severe inflammatory reaction to CFA, and by day 16 both groups had similar induration measurements. Between days 16 and 37, however, the average diameter size of induration decreased rapidly and significantly in the experimental group compared with the control group. In fact, the diameter size of induration of the control group increased on average over the course of the experiment (p-value < 0.05). Thus, albeit the considerable variability around the early inflammatory skin response, with greater early inflammatory response in the experimental group, the Gal-3 depletion apheresis group showed a significant trend of rapid decrease in diameter size of induration while the diameters in the apheresis control group remained (Figure 3A). Due to the complications with the functioning of indwelling catheters, the apheresis procedure for control animal 22877 was aborted on day 9 and the apheresis and column perfusion procedures were discontinued after day 9 for one of the experimental animal 23171. A summary of the animals in each group and the time-points of apheresis and column perfusion for each individual animal is provided in Table 1.
TABLE 1.
Detailed summary of the treated and the control animals.
| Age | Weight | Apheresis +/− Column Perfusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Animal | sex | (months) | (kg) | 12hrs | Day 4 | Day 9 | Day 15 | Day 18 | Day 22 |
| Experimental | 22663 | M | 9.5 | 45.6 | + | + | + | + | + | + |
| 23147 | M | 6.1 | 43 | + | + | + | + | + | + | |
| 23171 | F | 6.8 | 49 | + | + | + | * | * | * | |
| Apheresis Control | 22876 | M | 8.5 | 37 | − | − | − | − | − | − |
| 22681 | F | 9.4 | 47.3 | − | − | − | − | − | − | |
| 22877 | M | 9.4 | 47 | − | − | * | − | − | − | |
| Naïve Control | 23166 | F | 7.6 | 41 | * | * | * | * | * | * |
Indicates no apheresis performed
Induration from animals in each group plotted at 0.5, 3, 9, 16, 23, and 37 days post-CFA injection (experimental animals: 23171, 23147) vs (control animals: 22877, 22681, 23166), shows a significantly decreased induration area by day 37 for animals undergoing column perfusion with M3/38 antibody compared with control animals undergoing apheresis alone or no treatment (Figure 3B). Apheresis control animal 22876 was found to have been inadvertently injected into the muscle due to an unusually thin subcutaneous tissue layer. Although induration was not able to be measured by surface palpation, this animal showed large granulomatous tissue formation in the muscle at end of the study confirming unresolved inflammatory response to CFA injection (data not included). Since we did not examine skin depth at autopsy in experimental animal 22663 to confirm subcutaneous injection, we removed this animal from the induration analysis as well. Both animals 22876 and 22663 showed no measurable induration at end of study (day 37).
We observed a trend towards reduction in systemic Gal-3 levels in the experimental group between days 15 and 18, following the completion of 3-5 succeeding treatments, and at later time point between days 20-37 (data not shown).
Depletion of Gal-3 in human plasma samples ex vivo
The M3/38 antibody that effectively captures porcine Gal-3 is directed against mouse Gal-3 and has known cross-reactivity to human Gal-3. We used plasma samples from three volunteers to confirm that the anti-Gal-3 affinity columns could remove human Gal-3 from plasma. Samples were halved: one-half was passed through a 1 mL column of Sepharose resin and the other through Sepharose resin bound with 2 mg of Gal-3 monoclonal antibody. Each sample was processed individually. Gal-3 concentrations of original plasma and the effluent samples were similar for the Sepharose-only columns. By contrast, samples that passed through the M3/38 Sepharose column contained significantly lower Gal-3 levels compared with original plasma samples (Figure 4). This result confirms that Gal-3 depletion apheresis could potentially be used for clinical applications.
Figure 4. Ex vivo.
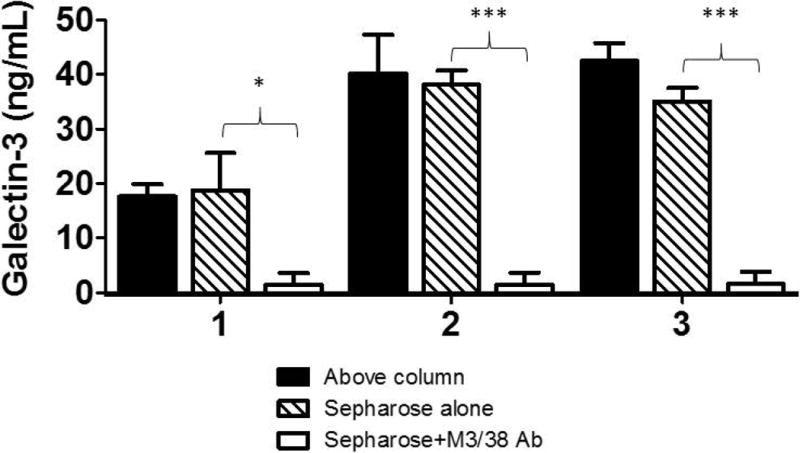
depletion of Gal-3 from human plasma (n=3). Gal-3 levels (ng/mL) in 3 individual samples, pre- (Above column), post-Gal-3 depletion column (Sepharose +M3/38 Ab) and post-control column (Sepharose-alone). The plasma samples were obtained from volunteers who signed informed consent forms to use their blood anonymously. * p-value < 0.05; *** p-value < 0.0001.
Toxicity
Both the experimental and control animals tolerated the apheresis procedure well, with no apparent side effects or adverse events. Complete blood counts and comprehensive metabolic panels remained normal throughout the study without any significant change from pre-treatment baselines and confirmed the lack of toxicity due to plasmapheresis (data not shown).
DISCUSSION
The study described herein was designed for proof of concept evaluation of Gal-3 depletion apheresis. We selected a model of skin inflammation for feasibility, given the key role Gal-3 is recognized to play in pro-inflammatory and pro-fibrotic responses.26,27 Gal-3 is postulated to act as a regulatory molecule operating at various stages along the continuum of inflammation, from acute to chronic inflammation and consecutive tissue fibrosis.4 For example, while wild-type aldosterone treated mice exhibit enhanced aortic Gal-3 expression, inflammation, and enhanced collagen type I, Gal-3 genetically deficient (knock out) mice were resistant to the inflammatory and fibrotic effects triggered by aldosterone.4 In a similar manner, Gal-3 ablation protected mice from diet-induced nonalcoholic steatohepatitis, by decreasing hepatic triggered advanced lipoxidation end products accumulation, with attenuation of inflammation, hepatocyte injury, and fibrosis.28 In the context of myocardial fibrogenesis, genetic disruption of Gal-3 was shown to attenuate angiotensin II triggered cardiac fibrosis, left ventricle dysfunction, and subsequent heart failure.5
The present report, utilizing a CFA-induced inflammatory skin disease model in miniature swine, demonstrates the ability of a selective anti-Gal-3 affinity apheresis system to efficiently deplete Gal-3. The Gal-3 depletion led to a statistically significant reduction in the inflammatory response in the apheresis experimental group as compared with the apheresis control group as measured by induration at the site of CFA injection.
We observed a trend towards reduction in systemic Gal-3 levels in the experimental group at the later time points. The variability in the levels of Gal-3 in the circulation of the experimental animals can possibly be due to a release of Gal-3 from inflamed tissues. It may also be related to the shift in the balance between monomers and pentamers in the circulation.29,30 We plan to perform additional analysis in the future to better address this point. Results will be presented in a future report upon completion.
Blood samples are being tested for inflammatory and profibrotic cytokines using Luminex cytokine profiling. Histology samples collected are undergoing further immunochemistry staining for Gal-3 and downstream inflammatory and profibrotic biomarkers. Results will be presented in a future report upon completion.
Furthermore, we demonstrate that the Gal-3 depletion column, utilized during the present experiment, which has an established reactivity to human Gal-3, has successfully lowered the levels of Gal-3 from ex vivo plasma of consenting volunteers. Notably, two of the 3 subjects had significantly elevated plasma Gal-3 levels (26.0 and 31.5 ng/mL vs. median “normal” level of 10.7 [8.9-12.7] ng/mL,31 which dropped to the lowest detection limit, post-Gal-3 depletion column. This evidence, along with the effectiveness of the matrix applied within an apheresis column perfusion system, further substantiates the potential to use Gal-3 depletion apheresis in clinical applications.
CONCLUSIONS
This is the first time that an apheresis system was designed to specifically deplete Gal-3 from the circulation. The removal of Gal-3 from the plasma reduced the inflammation at the injection sites. The reduction in skin lesions diameters instigated by the selective reduction of Gal-3 in plasma during apheresis treatment is encouraging for further translational research to clinical application of this therapy.
Acknowledgments
The authors would like to thank Mr. Barry Wilk and Ms. Elaine Weil for their excellent contribution with project management, logistics, and planning; Dr. Michael Fuller for his assistance with the statistical analysis; Dr. Ayala Hezi-Yamit for assistance with data analysis; Ms. Shannon Pratts, Dr. Aarti Patil, Dr. George Abraham, Mr. Denis Valyuk and Ms. Aurore Pruneveille for excellent technical, animal care, apheresis assistance; and Dr. Ivy Rosales for histology assistance.
We acknowledge support from CO6RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine. This work was conducted with statistical support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers, and from Eliaz Therapeutics, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
References
- 1.Liu FT, Rabinovich GA. Galectins as modulators of tumor progression. Nat Rev Cancer. 2005;5(1):29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 2.Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–82. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 3.Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi F, De Francesco GP, Carlo Bellotti C, Salehi LB, Ricci A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int J Mol Sci. 2018 Jan 26;19(2) doi: 10.3390/ijms19020379. pii: E379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230(1):160–71. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Silljé HH, de Boer RA. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6(1):107–17. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Sharma BB, Yu L, Zuberi R, Weng IC, Kawakami Y, Kawakami T, Hsu DK, Liu FT. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J Immunol. 2006;177(8):4991–7. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- 7.Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863(3):427–37. doi: 10.1016/j.bbamcr.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Chen SC, Kuo PL. The role of galectin-3 in the kidneys. Int J Mol Sci. 2016;17(4):565. doi: 10.3390/ijms17040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Sun Y, Zhao Y, Zhang W, Yang Z, Gao Y, Cai H, Li Y, Wang Q, Bian B, Nie J. Prognostic value of plasma galectin-3 levels in patients with coronary heart disease and chronic heart failure. Int Heart J. 2015;56(3):314–8. doi: 10.1536/ihj.14-304. [DOI] [PubMed] [Google Scholar]
- 10.O’Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol. 2013;24(9):1470–7. doi: 10.1681/ASN.2012090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, Shirai K. Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56(1):57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 12.Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156(10):3939–44. [PubMed] [Google Scholar]
- 13.Ge XN, Ha SG, Liu FT, Rao SP, Sriramarao P. Eosinophil-expressed galectin-3 regulates cell trafficking and migration. Front Pharmacol. 2013;4:37. doi: 10.3389/fphar.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudowska M, Gruszewska E, Cylwik B, Panasiuk A, Rogalska M, Flisiak R, Szmitkowski M, Chrostek L. Galectin-3 concentration in liver diseases. Ann Clin Lab Sci. 2015;45(6):669–73. [PubMed] [Google Scholar]
- 15.Maiolino G, Rossitto G, Pedon L, Cesari M, Frigo AC, Azzolini M, Plebani M, Rossi GP. Galectin-3 predicts long-term cardiovascular death in high-risk patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2015;35(3):725–32. doi: 10.1161/ATVBAHA.114.304964. [DOI] [PubMed] [Google Scholar]
- 16.Grupper A, Nativi-Nicolau J, Maleszewski JJ, Geske JR, Kremers WK, Edwards BS, Kushwaha SS, Pereira NL. Circulating galectin-3 levels are persistently elevated after heart transplantation and are associated with renal dysfunction. JACC Heart Fail. 2016;4(11):847–856. doi: 10.1016/j.jchf.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Fenster BE, Lasalvia L, Schroeder JD, Smyser J, Silveira LJ, Buckner JK, Brown KK. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels. 2016;31(6):939–46. doi: 10.1007/s00380-015-0691-z. [DOI] [PubMed] [Google Scholar]
- 18.AbouEzzeddine OF, Haines P, Stevens S, Nativi-Nicolau J, Felker GM, Borlaug BA, Chen HH, Tracy RP, Braunwald E, Redfield MM. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure) JACC Heart Fail. 2015;3(3):245–52. doi: 10.1016/j.jchf.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takemoto Y, Ramirez RJ, Yokokawa M, Kaur K, Ponce-Balbuena D, Sinno MC, Willis BC, Ghanbari H, Ennis SR, Guerrero-Serna G, Henzi BC, Latchamsetty R, Ramos-Mondragon R, Musa H, Martins RP, Pandit SV, Noujaim SF, Crawford T, Jongnarangsin K, Pelosi F, Bogun F, Chugh A, Berenfeld O, Morady F, Oral H, Jalife J. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC Basic Transl Sci. 2016;1(3):143–54. doi: 10.1016/j.jacbts.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliaz I, Weil E, Dutton JA, McCalley AE, Nolte B, Moriarty PM. Lipoprotein apheresis reduces circulating galectin-3 in humans. J Clin Apher. 2016 Aug;31(4):388–92. doi: 10.1002/jca.21413. [DOI] [PubMed] [Google Scholar]
- 21.Eliaz I, Patil A, Navarro-Alvarez N, Wang Z, Eliaz A, Weil E, Wilk B, Sachs DH, Huang CA. Methods for the detection and serum depletion of porcine galectin-3. J Clin Apher. 2017;32(5):335–41. doi: 10.1002/jca.21521. [DOI] [PubMed] [Google Scholar]
- 22.Sachs DH. MHC Homozygous Miniature Swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as models in biomedical research. 1st. Ames: Iowa State University Press; 1992. pp. 3–15. [Google Scholar]
- 23.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–67. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ho MK, Springer TA. MAC-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982;128:1221–8. [PubMed] [Google Scholar]
- 25.Springer TA. Monoclonal antibody analysis of complex biological systems. Combination of cell hybridization and immunoadsorbents in a novel cascade procedure and its application to the macrophage cell surface. J Biol Chem. 1981;256:3833–9. [PubMed] [Google Scholar]
- 26.Larsen L, Chen HY, Saegusa J, Liu FT. Galectin-3 and the skin. J Dermatol Sci. 2011;64(2):85–91. doi: 10.1016/j.jdermsci.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saegusa J, Hsu DK, Chen HY, Yu L, Fermin A, Fung MA, Liu FT. Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am J Pathol. 2009;174(3):922–31. doi: 10.2353/ajpath.2009.080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, Delucchi F, Serino M, Federici M, Pricci F, Pugliese G. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54(5):975–83. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad N, Gabius HJ, André S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279(12):10841–7. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 30.Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci. 2015;128(13):2213–9. doi: 10.1242/jcs.151159. [DOI] [PubMed] [Google Scholar]
- 31.van der Velde AR, Meijers WC, Ho JE, Brouwers FP, Rienstra M, Bakker SJ, Muller Kobold AC, van Veldhuisen DJ, van Gilst WH, van der Harst P, de Boer RA. Serial galectin-3 and future cardiovascular disease in the general population. Heart. 2016;102(14):1134–41. doi: 10.1136/heartjnl-2015-308975. [DOI] [PMC free article] [PubMed] [Google Scholar]


