Abstract
To follow up on our prior Part I review, this Part II review summarizes and provides updated literature on novel quinoline and quinazoline alkaloids isolated during the period of 2009–2016, together with the biological activity and the mechanisms of action of these classes of natural products. Over 200 molecules with a broad range of biological activities, including antitumor, antiparasitic and insecticidal, antibacterial and antifungal, cardioprotective, antiviral, anti-inflammatory, hepatoprotective, antioxidant, anti-asthma, antitussive and other activities, are discussed. This survey should provide new clues or possibilities for the discovery of new and better drugs from the original naturally occurring quinoline and quinazoline alkaloids.
Keywords: quinoline alkaloids, quinazoline alkaloids, biological activities, camptothecin, natural products
1. INTRODUCTION
Quinoline and quinazoline alkaloids, two important classes of N-based heterocyclic aromatic compounds, have attracted tremendous attention from researchers worldwide, because of their wide-ranging biological activities and their varied applications over the past 200 years.1 Since quinine was isolated in the 19th century,2 increasing numbers of related natural products have been isolated and identified. Most of these compounds and their derivatives exhibit significant biological activities, such as antitumor, antimalarial, antiparasitic and insecticidal, antibacterial and antifungal, cardioprotective, antiviral, anti-inflammatory, anti-oxidant and other effects.3,4 Many of the derivatives are used today as agricultural products and the pharmaceutical drugs. The prominent applications are antimalarial (Quinine, Quinidine, Chloroquine, Mefloquine, etc), antiviral (Saquinavir), antibacterial (fluoroquinolones such as Ciprofloxacin, Sparfloxacin, etc), antifungal-antiprozoal (Clioquinol), anthelmintic (Oxamniquine), local anesthetic (Dibucaine), antiasthmatic (Montelukast), anticancer (Camptothecin, Irinotecan, Topotecan, Gefitinib, etc), antipsychotic (Aripiprazole, Brexpiprazole, etc), antiglaucoma (Cartiolol) and cardiotonic (Vesnarinone).5
In view of the importance and significant biological activities of quinoline and quinazoline alkaloid natural products, we previously reviewed the developments in this field from the perspective of biological activities (covering the literature up to year 2008). Over the past decade, the marine environment has become an increasingly important resource in natural products research, and more new compounds have been isolated from marine microorganisms and identified as new leads in the discovery of useful chemotherapeutic agents. Meanwhile, research has been continually published on the new marked biological activities and mechanisms of action of quinoline and quinazoline alkaloids.
Consistent with our previous review, this review summarizes and provides updated literature on all novel quinoline and quinazoline alkaloids isolated during the period of 2009–2016 (Table 1), together with the biological activity and the mechanisms of action of these classes of natural products. The authors hope that the two reviews provide new clues or possibilities for the discovery of new and better drugs from naturally occurring quinoline and quinazoline alkaloids.
Table 1.
Quinoline and quinazoline alkaloids isolated during 2009–2016
| No. | Compound | Species | Ref. |
|---|---|---|---|
| 1 | Leucophyllidine | Leuconotis griffithii | 15,16 |
|
| |||
| 2 | Angustifonine A | Bousigonia angustifolia | 17 |
| 3 | Angustifonine B | ||
|
| |||
| 4 | Meloyunine B | Melodinus yunnanensis | 18 |
| 5 | Meloyunine A | ||
| 6 | Meloyunine C | ||
| 7 | 14,15-Dehydromelohenine B | ||
| 8 | Δ14-Vincamenine | ||
|
| |||
| 9 | (7R,8S)-7-Hydroxy-8-methoxyrutaecarpine | Evodia rutaecarpa | 20,21 |
| 10 | (7R,8S)-7-Hydroxy-8-ethoxyrutaecarpine | ||
|
| |||
| 11 | 2-Methoxy-7,8-dehydroruteacarpine | Zanthoxylum poggei | 20,21 |
|
| |||
| 12 | Fumiquinazoline J | Aspergillus sp. | 25,26 |
| 13 | Fumiquinazoline K | ||
| 14 | Fumiquinazoline L | ||
| 15 | Fumiquinazoline M | ||
| 16 | Fumiquinazoline N | ||
| 17 | Fumiquinazoline O | ||
| 18 | Fumiquinazoline P | ||
|
| |||
| 19 | Tryptoquivaline K | Neosartorya siamensis | 27 |
| 20 | Tryptoquivaline | ||
| 21 | Tryptoquivaline F | ||
| 22 | Tryptoquivaline H | ||
| 23 | Tryptoquivaline L | ||
| 24 | Tryptoquivaline O | ||
| 25 | Fiscalin A | ||
| 26 | Fiscalin C | ||
| 27 | Sartorymensin | ||
| 28 | Epi-fiscalin A | ||
| 29 | Epi-fiscalin C | ||
| 30 | Neofiscalin A | ||
| 31 | Epi-neofiscalin A | ||
| 32 | 3′-(4-Oxoquinazolin-3-yl)spiro[1H-indole-3,5′-oxolane]-2,2′-dione | ||
|
| |||
| 33 | 15b-β-Hydroxy-5N-acetylardeemin | Aspergillus terreus | 28 |
| 34 | 16α-Hydroxy-5N-acetylardeemin | ||
| 35 | 5N-Acetylardeemin | ||
|
| |||
| 36 | Fumigatoside B | Aspergillus fumigatus | 29 |
| 37 | Fumigatoside C | ||
| 38 | Fumigatoside D | ||
|
| |||
| 39 | Evollionine B | Evodia rutaecarpa | 30 |
| 40 | Evollionine C | ||
|
| |||
| 41 | 5-Methoxydictamnine | Toddalia asiatica | 33 |
|
| |||
| 42 | 5-Methoxyrobustine | Dictamnus angustifolius | 35 |
| 43 | Isopteleine | ||
|
| |||
| 44 | Aegelbine A | Aegle marmelos | 37 |
| 45 | Aegelbine B | ||
|
| |||
| 46 | 7-Isopentenyloxy-γ-fagarine | Haplophyllum canaliculatum | 38 |
| 47 | Perfamine | ||
|
| |||
| 48 | Anstifoline A | Dictamnus angustifolius | 39 |
| 49 | Anstifoline B | ||
|
| |||
| 50 | 1-Methyl-2-[6-carbonyl-(E)-4-undecenyl]-4(1H)-quinolone | Evodia rutaecarpa | 42 |
| 51 | 1-Methyl-2-[6-carbonyl-(E)-7-tridecenyl]-4(1H)-quinolone | ||
| 52 | 1-Methyl-2-[15-hydroxyl-pentadecenyl]-4(1H)-quinolone | ||
| 53 | 1-Methyl-2-[13-hydroxyl-tridecenyl]-4(1H)-quinolone | ||
|
| |||
| 54 | Sannanine | Streptomyces sannanensis | 56 |
|
| |||
| 55 | (S)-Vasicinone-1-O-β-D-glucopyranoside | Peganum harmala | 57 |
| 56 | (R)-Vasicinone-1-O-β-D-glucopyranoside | ||
|
| |||
| 57 | 4-Methyl-2-quinazolinamine | Streptomyces sp. GS DV232 | 58 |
|
| |||
| 58 | Marcanine A | Polyalthia plagioneura | 59 |
|
| |||
| 59 | Saprosmine A | Saprosma hainanense | 60 |
|
|
|||
| 60 | Saprosmine B | ||
|
|
|||
| 61 | 4-Methoxycarbonyl-5,10-benzogquinolinequinone | ||
|
|
|||
| 62 | Cleistopholine | ||
|
| |||
| 63 | Leucomidine C | Leuconotis griffithii | 61 |
|
| |||
| 64 | Haplophytin A | Haplophyllum acutifolium | 62 |
|
| |||
| 65 | Stauranthine | Stauranthus perforatus | 63 |
| 66 | 3′,6′-Dihydroxy-3′,6′-dihydrostauranthine | ||
| 67 | trans-3′,4′-Dihydroxy-3′,4′-dihydrostauranthine | ||
|
| |||
| 68 | Ammosamide A | Streptomyces variabilis | 65 |
| 69 | Ammosamide B | ||
|
| |||
| 70 | Ammosamide D | Streptomyces variabilis | 67 |
|
| |||
| 71 | Lymphostin | Streptomyces sp. | 64 |
|
| |||
| 72 | Penicinoline E | Penicillium sp. ghq208 | 68,69 |
| 73 | Methyl-penicinoline | ||
|
| |||
| 74 | Penicinoline | ||
|
| |||
| 75 | Eudistidine A | Eudistoma sp. | 70 |
| 76 | Eudistidine B | ||
|
| |||
| 77 | (+)-Spiroreticulatine | Fascaplysinopsis reticulate | 71 |
| 78 | (−)-Spiroreticulatine | ||
|
| |||
| 79 | (+)-Discorhabdin/prianosin A | Latrunculia sp. | 72 |
| 80 | (+)-Discorhabdin B | ||
| 81 | (+)-Discorhabdin G*/I | ||
| 82 | (-)-Discorhabdin W | ||
| 83 | (+)-(6R,8S)-1-Thiomethyldiscorhabdin G*/I | ||
| 84 | 16a,17a-Dehydrodiscorhabdin W | ||
| 85 | (+)-(3S,5R,6S,8S)-3-Dihydrodiscorhabdin A | ||
| 86 | (+)-(3R,5R,6S,8S)-3-Dihydrodiscorhabdin A | ||
|
| |||
| 87 | Melohemsine A | Melodinus hemsleyanus | 73 |
| 88 | Melohemsine B | ||
| 89 | Melohemsine C | ||
| 90 | Melohemsine D | ||
| 91 | Melohemsine E | ||
| 92 | Melohemsine F | ||
| 93 | Melohemsine G | ||
| 94 | Melohemsine H | ||
| 95 | Melohemsine I | ||
| 96 | Meloscine | ||
| 97 | Sandine | ||
| 98 | 10-Hydroxyscandine | ||
| 99 | 10-Hydroxy-14,15-β-epoxysandine | ||
| 100 | 14,15-β-Epoxysandine | ||
| 101 | Meloscine N4-oxide | ||
| 102 | Scandine N4-oxide | ||
| 103 | Melodinine U | ||
|
| |||
| 104 | Cincholenine A | Cinchona ledgeriana | 74 |
| 105 | Cincholenine B | ||
|
| |||
| 106 | Nitidine | Toddalia asiatica | 86 |
|
| |||
| 107 | Evoxine | Teclea gerrardii | 87 |
|
| |||
| 108 | Aplidiopsamine A | Aplidiopsis condluata | 88 |
|
| |||
| 109 | Ascidiathiazone A | Aplidium sp. | 89 |
|
| |||
| 110 | 2-n-Octyl-4-hydroxyquinoline-4-hydroxyquinoline | Pseudomonas aeruginosa BCC76810 | 90 |
| 111 | 2-n-Nonyl-4-hydroxyquinoline | ||
| 112 | 2-n-Undecyl-4-hydroxyquinoline | ||
| 113 | 2-(E)-Non-1′-enyl-4-hydroxyquinoline | ||
| 114 | 2-((Z)-Undec-4′-enyl)-4-hydroxyquinoline | ||
| 115 | 2-(3′-(2′-Hexylcyclopropyl)propyl)-4-hydroxyquinoline | ||
| 116 | n-Heptyl-4-hydroxyquinoline | ||
| 117 | 2-n-Octyl-4-hydroxyquinoline N-oxide | ||
| 118 | 2-n-Nonyl-4-hydroxyquinoline N-oxide | ||
| 119 | 2-((Z)-Undec-4′-enyl)-4-hydroxyquinoline N-oxide | ||
| 120 | 2-n-Heptyl-3-hydroxyquinoline-2,4(1H,3H)-dione | ||
|
| |||
| 121 | Waltherione A | Waltheria indica | 94,95 |
| 122 | Waltherione C | ||
| 123 | Waltherione E | ||
| 124 | Waltherione F | ||
| 125 | Waltherione G | ||
| 126 | Waltherione H | ||
| 127 | Waltherione I | ||
| 128 | Waltherione J | ||
| 129 | Waltherione K | ||
| 130 | Waltherione L | ||
| 131 | 8-Deoxoantidesmone | ||
|
| |||
| 132 | N-Methyl-8-methoxyflindersine | Raputia heptaphylla | 103 |
|
| |||
| 133 | N-Methylswietenidine B | Micromelum falcatum | 104 |
| 134 | Methyl 2-(3-hydroxy-1-methyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)-acetate | ||
| 135 | 3-Hydroxy-1-methyl-3-(2-oxopropyl)-quinoline-2,4(1H,3H)-dione | ||
| 136 | N-Methylflindersine | ||
| 137 | 4-Hydroxy-3-methoxy-1-methyl-2(1H)-quinolinone | ||
|
| |||
| 138 | 6-Deoxyaflaquinolone E | Aspergillus nidulans MA-143 | 105 |
| 139 | Isoaflaquinolone E | ||
| 140 | 14-Hydroxyaflaquinolone F | ||
| 141 | Aniduquinolone A | ||
| 142 | Aniduquinolone B | ||
| 143 | Aniduquinolone C | ||
| 144 | Aflaquinolone A | ||
|
| |||
| 145 | 2-[(S)-Hydroxy(phenyl)methyl]-3-methyl-quinazolin-4(3H)-one | Talaromyces sp. Cf 16 | 106 |
| 146 | 2-[(R)-Hydroxy(phenyl)methyl]-3-methyl-quinazolin-4(3H)-one | ||
|
| |||
| 147 | Aurachin Q | Rhodococcus sp. Acta 2259 | 119 |
| 148 | Aurachin R | ||
|
| |||
| 149 | 4-Oxo-1,4-dihydroquinoline-3-carboxamide | Nocardiopsis terrae YIM 90022 | 120 |
|
| |||
| 150 | 5-Hydroxy-4-(chloromethyl)-5,6,7,8-tetrahydroquinoline | Belize extract | 121 |
|
| |||
| 151 | 5aTHQ-7n | Combined culture of Streptomyces nigrescens HEK616 & Tsukamurella pulmonis TP-B0596 | 122 |
| 152 | 5aTHQ-8i | ||
| 153 | 5aTHQ-8n | ||
| 154 | 5aTHQ-9i | ||
| 155 | 5aTHQ-9n | ||
| 156 | 5aTHQ-10a | ||
| 157 | 5aTHQ-10i | ||
| 158 | 5aTHQ-10n | ||
|
| |||
| 159 | Waltherion M | Waltheria indica | 123 |
| 160 | Waltherion N | ||
| 161 | Waltherion O | ||
| 162 | Waltherion P | ||
| 163 | Waltherion Q | ||
| 164 | 5(R)-Vanessine | ||
|
| |||
| 165 | Shermilamine B | Cystodytes dellechiajei | 124 |
| 166 | N-Deacetylshermilamine B | ||
| 167 | 11-Hydroxyascididemin | ||
| 168 | Cystodimine A | ||
| 169 | Cystodimine B | ||
| 170 | Ascididemin | ||
|
| |||
| 171 | Cleistopholine | Annona salzmannii | 125 |
|
| |||
| 172 | Adhatodine | Justicia adhatoda | 130 |
| 173 | Anisotine | ||
|
| |||
| 174 | Cottoquinazoline A | Aspergillus versicolor | 131 |
| 175 | Cottoquinazoline B | ||
| 176 | Cottoquinazoline C | ||
| 177 | Cottoquinazoline D | ||
|
| |||
| 178 | 8-Hydroxy-9-methyl-furo[2,3-b]quinolin-4(9H)-one | Dictamnus angustifolius | 160 |
|
| |||
| 179 | 8-Hydroxy-4-quinolone | Scolopendra subspinipes mutilans | 161 |
|
| |||
| 180 | Neosartoryadin A | Neosartorya udagawae HDN13-313 | 163 |
| 181 | Neosartoryadin B | ||
|
| |||
| 182 | Oxoglyantrypine | Cladosporium sp. PJX-41 | 164 |
| 183 | Norquinadoline A | ||
| 184 | Deoxynortryptoquivaline | ||
| 185 | Quinadoline B | ||
| 186 | Quinadoline A | ||
| 187 | 3-Hydroglyantrypine | ||
| 188 | Cladoquinazoline | ||
| 189 | epi-Cladoquinazoline | ||
| 190 | Glyantrypine | ||
| 191 | Deoxytrytoquivaline | ||
| 192 | CS-C | ||
| 193 | Prelapatin B | ||
|
| |||
| 194 | 3-(3-Methylbut-2-enyl)-4-(3-methylbut-2-enyloxy)-quinolin-2-ol | Evodia roxburghiana | 165 |
|
| |||
| 195 | Trigonoine B | Trigonostemon lii | 167 |
|
| |||
| 196 | Waltherione B | Melochia odorata | 168 |
| 197 | Waltherione D | ||
|
| |||
| 198 | Pseudane IX | Ruta angustifolia | 169 |
|
| |||
| 199 | 3-[1β-Hydroxy-2-(β-D-glucopyranosyloxy)-ethyl)-4-methoxy-2(1H)-quinolinone | Dictamnus dasycarpus | 171 |
| 200 | Dictangustine A | ||
| 201 | Isomaculosidine | ||
|
| |||
| 202 | 2-[(6Z,9Z)-Pentadeca-6,9-dienyl]quinolin-4(1H)-one | Tetradium ruticarpum | 173 |
|
| |||
| 203 | Clausenaside D | Clausena lansium | 175 |
| 204 | Clausenaside E | ||
| 205 | Clausenaside F | ||
| 206 | 4-Methoxy-8-O-β-D-glucopyranosyloxy-2(1H)-quinolinone | ||
| 207 | Clausenaside G | ||
| 208 | Clausenaside H | ||
| 209 | Integriquinolone | ||
| 210 | Ribalinine | ||
|
| |||
| 211 | Eucophylline | Leuconotis eugenifolius | 176 |
|
| |||
| 212 | Scholarisine II | Alstonia scholaris | 177 |
| 213 | Scholarisine I | ||
|
| |||
| 214 | Leptomerine | Esenbeckia leiocarpa | 184 |
|
| |||
| 215 | Leptanoine A | Evodia lepta | 185,186 |
| 216 | Leptanoine B | ||
| 217 | Leptanoine C | ||
|
| |||
| 218 | Nigellastrine I | Peganum nigellastrum | 191 |
| 219 | Nigellastrine II | ||
|
| |||
| 220 | Peganumine D | Peganum harmala | 192 |
| 221 | S-Peganumine E | ||
| 222 | R-Peganumine E | ||
| 223 | Peganumine F | ||
| 224 | Peganumine G | ||
|
| |||
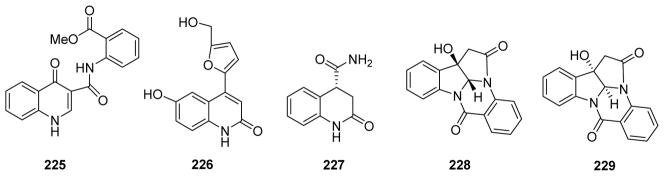
| 225 | Methyl 2-(4-oxo-1,4-dihydroquinoline-3-carboxamido)benzoate | Isatis indigotica | 196 |
| 226 | 6-Hydroxy-4-(5-hydroxymethylfuran-2-yl)-quinolin-2(1H)-one | ||
| 227 | (+)-(R)-2-Oxo-1,2,3,4-tetrahydroquinoline-4-carboxamide | ||
| 228 | (+)-3-Hydroxy-2H-pyrrolo[2,3-b]indolo[5,5a,6-b,a]-quinazoline-9-(8H),7′-dione | ||
| 229 | (−)-3-Hydroxy-2H-pyrrolo[2,3-b]indolo[5,5a,6-b,a]-quinazoline-9-(8H),7′-dione | ||
|
| |||
| 230 | Asperlicin | Aspergillus alliaceus | 199 |
| 231 | Asperlicin B | ||
| 232 | Asperlicin C | ||
| 233 | Asperlicin D | ||
| 234 | Asperlicin E | ||
|
| |||
| 235 | Euodenine A | Evodia asteridula | 206 |
|
| |||
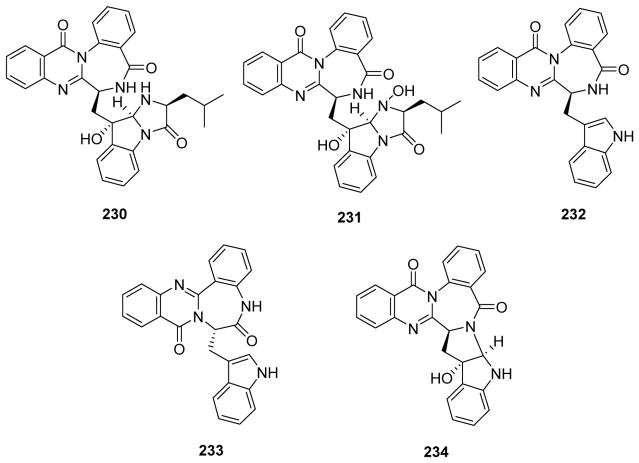
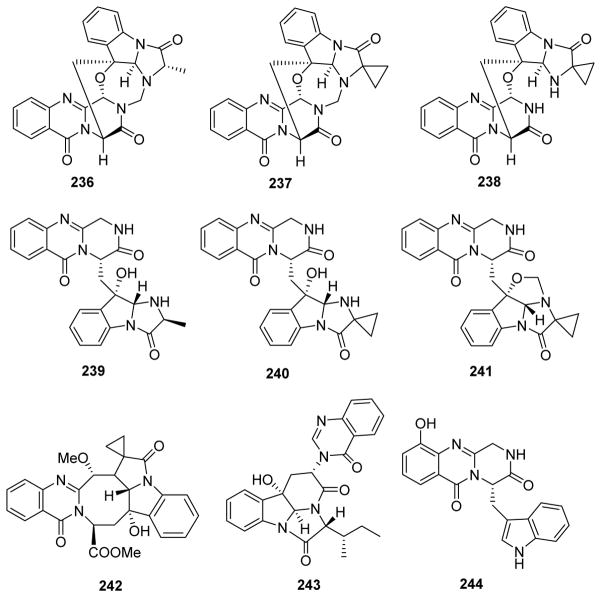
| 236 | Versiquinazoline A | Aspergillus versicolor LZD-14-1 | 208 |
| 237 | Versiquinazoline B | ||
| 238 | Versiquinazoline C | ||
| 239 | Versiquinazoline D | ||
| 240 | Versiquinazoline E | ||
| 241 | Versiquinazoline F | ||
| 242 | Versiquinazoline G | ||
| 243 | Versiquinazoline H | ||
| 244 | Versiquinazoline I | ||
|
| |||
| 245 | Glycosin | Rhizophora apiculata | 209 |
|
| |||
| 246 | Choisyine | Choisya Aztec-Pearl | 211 |
|
| |||
| 247 | Punaravine | Boerhaavia diffusa | 213,214 |
|
| |||
| 248 | Fumiquinazoline S | Aspergillus sp. | 216 |
| 249 | Isochaetominine A | ||
| 250 | Isochaetominine B | ||
| 251 | Isochaetominine C | ||
| 252 | 14-Epi-isochaetominine C | ||
|
| |||
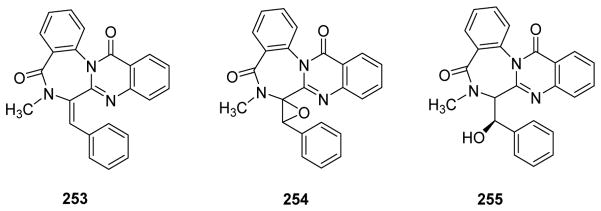
| 253 | Benzomalvin E | Penicillium sp. FN070315 | 225 |
| 254 | Benzomalvin B | ||
| 255 | Benzomalvin C | ||
2. BIOLOGICAL ACTIVITIES OF QUINOLINE AND QUINAZOLINE ALKALOIDS
A. Antitumor Activity
1. Quinoline and quinazoline indole alkaloids
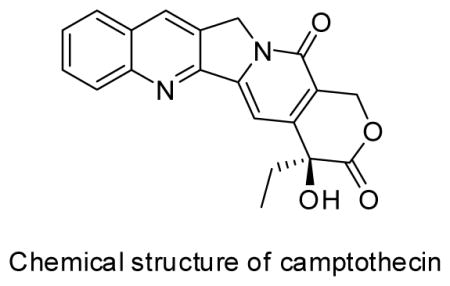
Since 1966, researchers have made significant attention to the important and famous quinoline indole alkaloid camptothecin (CPT), due to its specific and strong inhibitory effects on the DNA-replicating enzyme topoisomerase I.6,7 In the presence of CPT, cells either undergo cell cycle arrest in S-phase or continue progression with subsequent accumulation of DNA damage, ultimately resulting in cell death. Until now, more than 5000 related publications have been published; many focused on the derivatization of CPT. Because excessive harvesting has severely depleted the traditional plant sources. Camptotheca acuminata and Nothapodytes nimmoniana, of CPT over the past two decades,8,9 alternate natural sources of CPT have been urgently sought, leading to the identification of Apodytes dimidiata, Codiocarpus andamanicus, Gomphandra comosa, Gomphandra coriacea, Gomphandra polymorpha, Gomphandra tetrandra, Iodes cirrhosa, Iodes hookeriana, Miquelia dentata, Miquelia kleinii, Natsiatum herpeticum, Nothapodytes foetida, Ophiorrhiza mungos, multiple shoot cultures of Ophiorrhiza rugosa var. decumbens, Pyrenacantha volubilis and Sarcostigma kleinii.10,11,12,13 Meanwhile, methods to obtain CPT from microorganisms have been developed recently. In 2013, CPT, 9-MeO-CPT and 10-OH-CPT also were produced from three fungi, Fomitopsis sp. P. Karst (MTCC 10177), Alternaria alternata (Fr.) Keissl (MTCC 5477) and Phomposis sp. (Sacc.) in mycelial mats in shake flasks containing potato dextrose broth.14
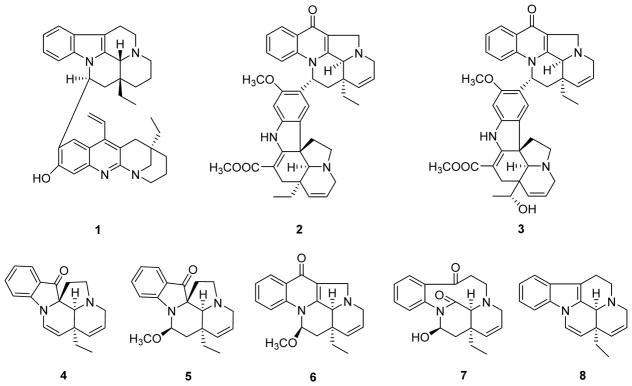
Besides CPT, other cytotoxic quinoline and quinazoline indole alkaloids have been identified. In 2009 and 2014, Kam and coworkers identified three new quinoline bisindole alkaloids.15,16 Leucophyllidine (1) (Fig. 1) from Leuconotis griffithii showed significant and comparable in vitro cytotoxicity toward drug-sensitive and vincristine-resistant (VJ300) human KB cells, with IC50 values of 2.95 and 2.92 μg/mL (5.16 and 5.10 μM), respectively.15 Angustifonines A (2) and B (3) (Fig. 1) from Bousigonia angustifolia exhibited cytotoxicity against HL-60 (leukemia), SMMC-7721 (hepatoma), A549 (lung carcinoma), MCF-7 (breast cancer) and SW480 (colon cancer) (IC50 2.71–16.2 μM).17 Compared with the above compounds, meloyunine B (4) (Fig. 1), a 6/7-seco rearranged spiro-indolone alkaloid from Melodinus yunnanensis, showed weak cytoxicity against the same five human cancer cell lines with IC50 values of 13.3 to 40.0 μM.18 Other new compounds, meloyunine A (5), a monoterpenoid quinoline alkaloid meloyunine C (6), the possible intermediate 14,15-dehydromelohenine B (7) and their precursor Δ14-vincamenine (8) (Fig. 1), were inactive (IC50>40 μM). In comparison, the paclitaxel control exhibited exceptional inhibitory activity (IC50<0.008 μM), and cisplatin displayed IC50 values ranging from 1.05 to 21.9 μM.18
Figure 1.
Chemical structures of compounds 1–8
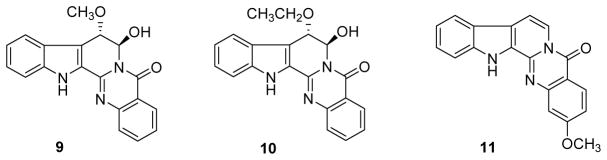
Rutaecarpine, an intriguing indoloquinazoline alkaloid from Evodia rutaecarpa, presents wide-ranging pharmacological activities, such as cardiovascular protective, antitumor, anti-inflammatory and other effects.19 It presented weak cytotoxic effects against A375-S2 (human malignant melanoma), HeLa, MCF7, THP-1 and L929 with the IC50 values ranging from 150 to 239 μM.19 During the last ten years, three new rutaecarpine derivatives, (7R,8S)-7-hydroxy-8-methoxy-rutaecarpine (9), (7R,8S)-7-hydroxy-8-ethoxy-rutaecarpine (10) and 2-methoxy-7,8-dehydroruteacarpine (11) (Fig. 2) were isolated from two plants in the Rutaceae family along with other known alkaloids.20,21 Compounds 9 and 10 displayed IC50 values of 7.82 and 8.31 μM against leukemia HL-60 cells, but only 22.3 and 27.9 μM against gastric carcinoma N-87 cells. Meanwhile, evodiamine presented the best cytotoxic activity among all isolated indolequinazoline alkaloids with significant IC50 values of 5.88 and 7.30 μM against the same two cell lines, respectively.20 The presence of the two substituents at position-7 and -8 of the alkaloid led to decreased potency against N-87. Alkaloid 11 together with 2-hydroxyruteacarpine and 2-methoxyruteacarpine from Zanthoxylum poggei displayed a low level of cytotoxic activity against the prostate cancer PC-3 cell line with IC50 values of 22.1, 17.4 and 19.4 μM, respectively (IC50 value of the positive control doxorubicin was 0.9 μM).21 They also strongly suppressed the phagocytosis response activated with serum opsonized zymosan in in vitro oxidative burst studies using whole blood; the IC50 values ranged from 21.6 to 25.9 μM.21 Rutaecarpine exhibited weak cytotoxic activity against the human monocytic leukemia THP-1 cell line as well as protective effects toward murine liver cancer Hepa-1c1c7 cells against oxidative stress–induced DNA damage by reducing the condensation and fragmentation of nuclei and inhibiting DNA strand breaks and apoptosis.22,23
Figure 2.
Chemical structures of compounds 9–11
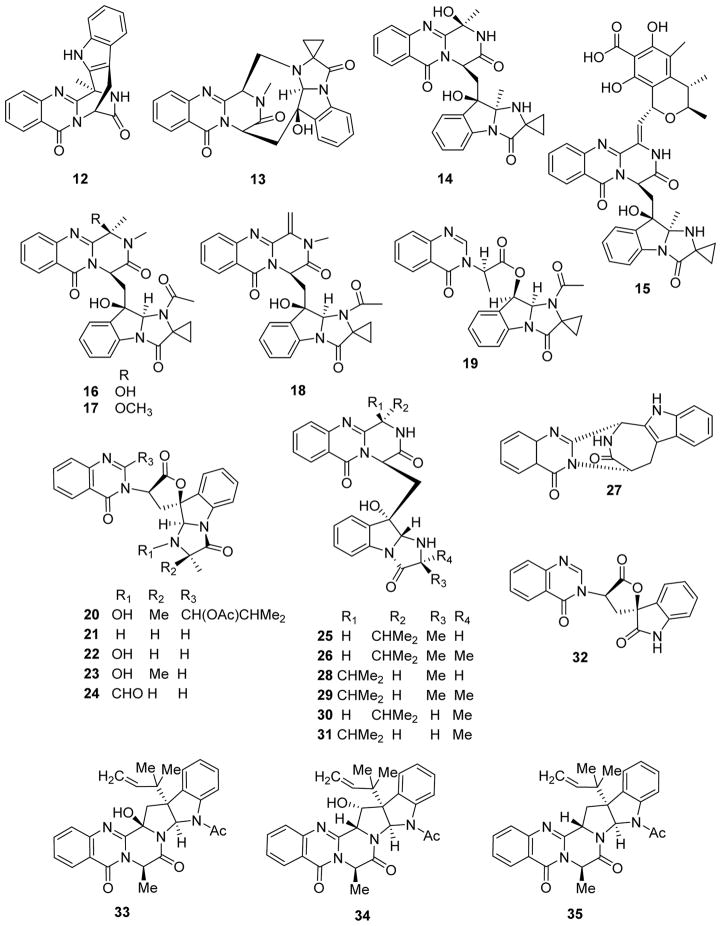
Our previous review reported that fumiquinazolines A–G were first isolated from the fungus Aspergillus fumigatus in 1992 and 1995.24 Subsequently, fumiquinazolines J–P (12–18) and tryptoquivaline K (19) (Fig. 3) were identified from fungus of the same genus.25,26 Fumiquinazoline J exhibited high cytotoxicity against murine lymphoma L5178Y cells with an IC50 value of 3.6 μM, but much lower activity against human ovarian A2780 and leukemia K562 cell lines with IC50 values of 18.5 and 15.0 μM, respectively. The remaining alkaloids were not cytotoxic against these cell lines.25,26 Subsequently, some tryptoquivaline (20) analogues, tryptoquivalines F (21), H (22), L (23) and O (24), fiscalins A (25) and C (26), sartorymensin (27), a quinazolinone-containing indole, epi-fiscalins A (28) and C (29), neofiscalin A (30), epi-neofiscalin A (31) and 3′-(4-oxoquinazolin-3-yl)spiro[1H-indole-3,5′-oxolane]-2,2′-dione (32) (Fig. 3) were isolated from the fungus Neosartorya siamensis (KUFC 6349). Except for alkaloid 27 (IC50 39–73 μM), all of the compounds showed no in vitro growth inhibitory activity (IC50 >100 μM) against U373 (glioblastoma), Hs683a (glioblastoma), A549, MCF-7 and SK-MEL-28 (melanoma) cell lines.27 Meanwhile, 15β-hydroxy-5N-acetylardeemin (33) showed greater cytotoxic activity against human HeLa contaminant KB (IC50 61.4 μM) and rat hepatic HSC-T6 (IC50 88.2 μM) cell lines than its geometric hydroxylated and non-hydroxylated congeners, 16α-hydroxy-5N-acetylardeemin (34) and 5N-acetylardeemin (35) (Fig. 3), from Aspergillus terreus.28
Figure 3.
Chemical structures of compounds 12–35
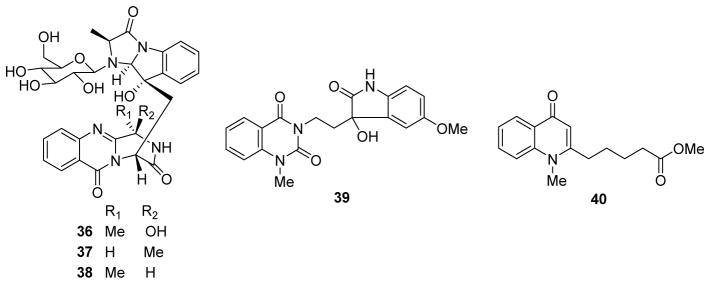
During the past ten years, other new alkaloids without cytotoxic activity also were reported. Examples include three N-glycosylated fumiquinazoline-type alkaloids fumigatosides B–D (36–38) from Aspergillus fumigatus29 and evollionines B and C (39, 40)30 (Fig. 4) from Evodia rutaecarpa.
Figure 4.
Chemical structures of compounds 36–40
2. Furoquinoline alkaloids
The furoquinoline alkaloids are biogenetically derived from 2-substituted oxygenated 4-quinolones after prenylation at C-3. Linear or angular dihydrofuroquinolines result from formation of a ring with the prenyl group at C-2 or C-4, respectively.31,32 To date, more than 30 furoquinoline alkaloids, including some with marked antitumor activity, have been identified. Skimmianine and 5-methoxydictamnine (41) (Fig. 5) demonstrated weak cytotoxic activity in vitro against A549, BGC-823 (gastric cancer), HCT15 (colorectal cancer), HeLa (cervical cancer), HepG2 (liver cancer), MCF-7, SK-MEL-2 and SGC-7901 (gastric cancer) cells with IC50 values between 33.3 and 40.3 μg/mL.33 The former compound also displayed weak cytotoxic activity against THP-1 and HCT116 (colon cancer) cell lines.22,34 Other furoquinoline alkaloids, such as 5-methoxyrobustine (42), dictamnine, robustine, isopteleine (43) (Fig. 5) and γ-fagarine also presented weak cytotoxic activity against MCF-7 cells,35,36 and two new furoquinoline alkaloids, aegelbines A (44) and B (45) (Fig. 5) from Aegle marmelos were weakly active against MCF-7 as well as HepG2 and PC-3 cell lines.37
Figure 5.
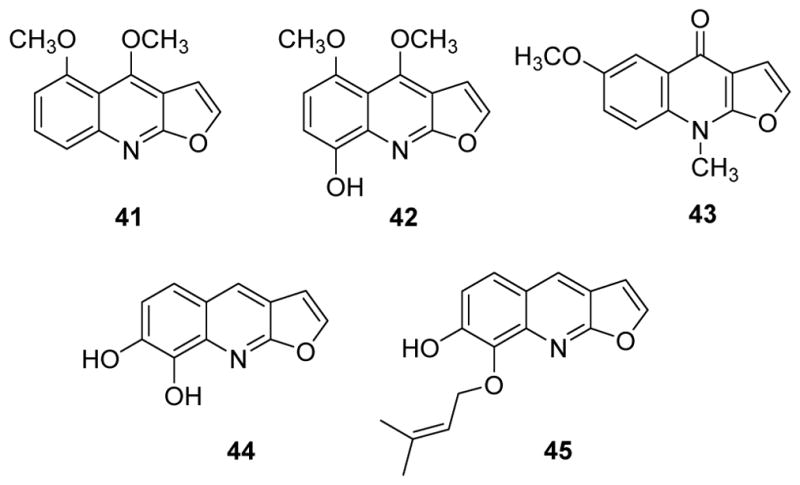
Chemical structures of compounds 41–45
A new γ-fagarine derivative, 7-isopentenyloxy-γ-fagarine (46) (Fig. 6), exhibited significant cytotoxicity against Raji (lymphoma) and Jurkat (leukemia) cell lines with IC50 values of 1.5 and 3.6 μg/mL, respectively, compared with 14.5 and 9.3 μg/mL for atanine and 15.6 and 11.5 μg/mL for skimmianine.38 Compound 46 was also more cytotoxic against the MCF-7 cell line (IC50 15.5 μg/mL), than atanine, skimmianine and perfamine (47) (Fig. 6). Furthermore, all alkaloids displayed moderate to low cytotoxicity against KG-1a (leukemia) and HEp-2 (HeLa contaminant), and higher effects against the multidrug-resistant HL-60/MX1 cell line, compared with the control etoposide (p<0.05)., Flow cytometry analysis on treated Raji and Jurkat cells showed the arrest of cell cycle progression at the sub-G1 phase in a dose-dependent manner, and even at the lowest concentration used (40.9%) compound 46 caused the greatest increase in the number of these cells with sub-G1 DNA content. According to computational analyses, the cytotoxicity toward different cell lines correlated well with certain molecular descriptors. Based on the cytotoxicity relationships with the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), charge transfer may be part of the interaction of these compounds with their target sites. The similar cytotoxic trends against various cell lines suggested that these compounds may act through parallel mechanisms.38
Figure 6.

Chemical structures of compounds 46–49
Anstifolines A (48) and B (49) (Fig. 6), two dimeric furoquinoline alkaloids from Dictamnus angustifolius, exhibited more significant cytotoxicity (IC50 10.6–14.3 μM) against human lung cancers A549 and NCI-H460 than dictamnine and robustine.39 Dictamine also demonstrated cytotoxicity toward HepG2 cells.40
3. 2-Substituted quinoline or quinazoline alkaloids
Evodiamine, a major 2-substituted quinoline alkaloid from Evodia rutaecarpa, displays various biological effects, including antitumor. More specifically, it inhibits cancer cell proliferation, invasion and metastasis, and induces apoptosis many tumor cell lines both in vitro and in vivo,41 including breast cancer, prostate cancer, leukemic T-lymphocyte, melanoma, cervical cancers, colon cancer and lung cancer cells. Moreover, evodiamine shows insignificant toxicity against normal human peripheral blood cells. In addition, this alkaloid sensitizes chemoresistant breast cancer cells to adriamycin, Briefly, evodiamine induces apoptosis by caspase-dependent and caspase-independent pathways as well as the mitochondrial caspase-dependent apoptotic pathway, and could activate Cdc2/cyclin B to induce cell cycle arrest (G2/M phase). It directly inhibits human umbilical vein endothelial cell (HUVEC) tube formation and invasion as well as the release and expression of VEGF, which is related with endothelial cells angiogenesis. Structure-activity relationship studies showed that the functional groups at position 14 and the configuration of hydrogen at position 13b of evodiamine may affect its inhibitory effects on invasion and metastasis of Lewis lung carcinoma, lung metastasis of colon 26-L5 and B16-F10 cells.
In 2015, evodiamine, other known alkaloids and four new quinolone alkaloids, 1-methyl-2-[6-carbonyl-(E)-4-undecenyl]-4(1H)-quinolone (50), 1-methyl-2-[6-carbonyl-(E)-7-tridecenyl]-4(1H)-quinolone (51), 1-methyl-2-[15-hydroxypentadecenyl]-4(1H)-quinolone (52) and 1-methyl-2-[13-hydroxytridecenyl]-4(1H)-quinolone (53) (Fig. 7) were isolated from E. rutaecarpa.42 Among these compounds, evodiamine exhibited the best cytotoxic activity against HL-60 and PC-3 cell lines with GI50 values of 0.51 μM and 14.4 μM, respectively, compared with 1.84 and >80 μM for the positive drug 5-Fu. Other active alkaloids were 1-hydroxyrutaecarpine, evocarpine, 1-methyl-2-undecyl-4(1H)-quinolone, 1-methyl-2-[13-hydroxytridecenyl]-4(1H)-quinolone and dihydroevocarpine with GI50 values between 8.34 and 16.0 μM against HL-60. The potencies of these quinolone alkaloids may relate to the length of the side-chain. An α,β-unsaturated carbonyl in the side-chain decreased the cytotoxicity. Indolopyridoquinazoline alkaloids with a phenolic hydroxyl on ring-E exhibited higher potency than those with an unsubstituted ring-E.42
Figure 7.
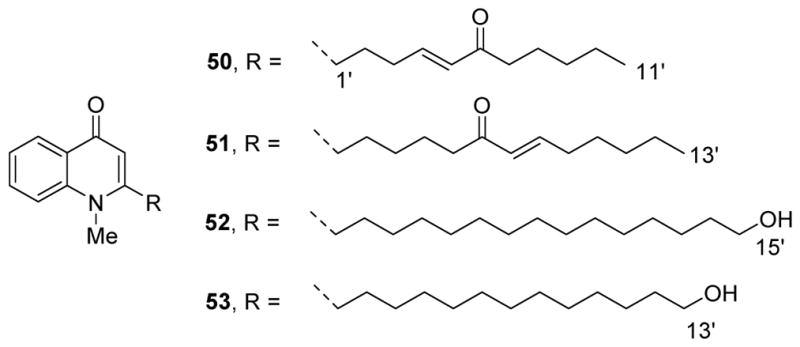
Chemical structures of compounds 50–53
4. Tryptanthrin
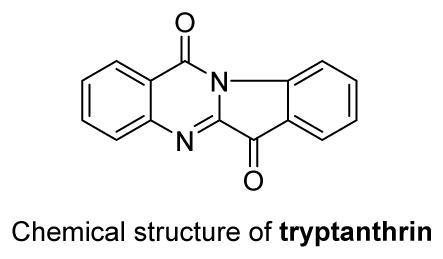
The quinazoline alkaloid tryptanthrin was first isolated from the traditional Chinese medicine (Qingdai) in 1985. Later studies reported its cytotoxic activity against melanoma B16,43 U-937 and HL-60 cells,44 as well as antitumor activity against azoxymethane-induced intestinal tumor.45 At low concentrations (0.5 μg/mL), tryptanthrin induces differentiation of leukemia cells, but at higher concentrations (10 μg/mL), can kill cells through apoptosis, possibly through a caspase-3/Fas antigen pathway.44 In addition, it strongly inhibited HGF production stimulated by various HGF inducers in human dermal fibroblasts, probably through events downstream of MAPK activation.46 More recently, its significant cytotoxic effects against MCF-7, NCI-H460 and SF-268 (glioblastoma) cell lines (IC50 9.4, 8.5 and 22.6 μM) have been reported.47 Tryptanthrin significantly inhibits K562 cell proliferation in a time- and dose-dependent manner, and has proliferation-attenuating and apoptosis-inducing effects on K562 cells. This effect likely results from a reduction in mitochondria membrane potential, mito cyt-c release and pro-caspase-3 activation.48 Flow cytometric analysis showed that tryptanthrin-treated WEHI-3B JCS cells underwent cell cycle arrest at the G0/G1 phase, and had down-regulated expression of cyclin D2, D3, Cdk 2, 4 and 6 genes. Meanwhile, tryptanthrin induced differentiation in the same cells by increasing vacuolation, cellular granularity and NBT-reducing activity. Thus, tryptanthrin exerts an antitumor effect on WEHI-3B JCS (myelomonocytic leukemia) cells by causing cell cycle arrest and triggering cell differentiation in a dose- and time-dependent manner.49
In 2013, studies were conducted to determine tryptanthrin’s effects on the growth and differentiation of LA-N-1 cells, one of the most common extracranial solid cancers in young children.50 Tryptanthrin inhibited the growth of the neuroblastoma cells in a dose- and time-dependent manner and induced cell cycle arrest at the G0/G1 phase. It also significantly reduced N-myc proto-oncogene protein expression. Thus, tryptanthrin suppresses the growth of and induces neuronal differentiation in neuroblastoma cells.50 Subsequent studies showed that tryptanthrin inhibited the proliferation, migration and tube formation of human microvascular endothelial cells (HMEC-1) in a concentration-dependent manner and significantly suppressed angiogenesis in Matrigel plugs in mice in vitro. The in vitro and in vivo anti-angiogenic activities were realized by targeting the vascular endothelial growth factor2 (VEGFR2)-mediated extracellular-regulated kinase 1/2 (ERK1/2) signaling pathway.51 In other studies, tryptanthrin exerted an anti-angiogenic effect by inhibiting cell cycle progression and Akt and FAK signaling in human vascular endothelial cells.52
Furthermore, in addition to the transporter pathway, tryptanthrin down regulated glutathione S-transferase (GST) π expression, reduced GST activity and reversed multi-drug-resistance (MDR) partly by modulating the GST π-related pathway in the MDR response in doxorubicin-resistant MCF-7 cells.53,54 It was well absorbed across Caco-2 monolayers and inhibited P-glycoprotein (P-gp) and multidrug resistance-associated protein 2 (MRP2). These results indicated that tryptanthrin may act as a potential chemoadjuvant agent through multiple targets.
5. Simple quinoline or quinazoline alkaloids
While quinine is a well-known compound for treating malaria, it also exhibited a distinct antiproliferative and pro-apoptotic effect in HeLa and A549 tumor cell lines.55 It inhibited the lipopolysaccharide (LPS)-induced activation of AKT by inhibiting its phosphorylation at Thr-308 and Ser-473. It also reversed LPS-induced proliferation and suppressed the anti-apoptotic protein B-cell lymphoma (BCL)-2, as well as the activation of the pro-apoptotic factor BCL-2-associated X protein. The inhibitory effect on the tumor cell proliferation was attributed to prevention of the tumor necrosis factor receptor-associated factor TRAF6 interaction.55
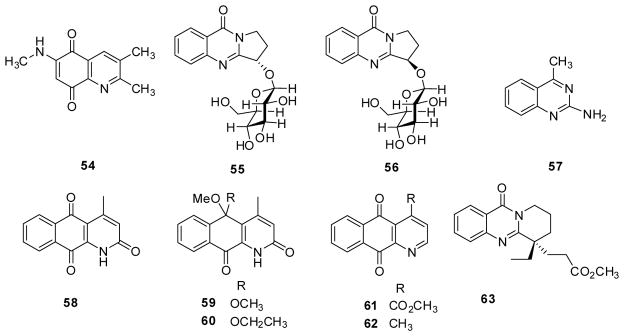
Quinoline-5,8-diones are also interesting potential anticancer agents. One new compound sannanine (54) (Fig. 8) from Streptomyces sannanensis showed cytotoxicity against four human tumor cell lines BGC823 (gastric cancer), PANC1 (pancreatic cancer), HepG2 and NCI-H460, with IC50 values of 6.6, 5.8, 3.1 and 1.8 μM, respectively.56
Figure 8.
Chemical structures of compounds 54–63
Two new quinazoline alkaloids (S)-vasicinone-1-O-β-D-glucopyranoside (55) and (R)-vasicinone-1-O-β-D-glucopyranoside (56), (Fig. 8) together with (S)-vasicinone, vasicine and deoxyvasicinone were isolated from the seeds of Peganum harmala.57 Two (55 and (S)-vasicinone) of the five compounds exhibited only weak inhibitory activity against human gastric cancer MCG-803 cells with IC50 values of 84.1 and 94.5 μM.
In contrast, 4-methyl-2-quinazolinamine (57) (Fig. 8) from Streptomyces sp. GS DV232 presented moderate activity against liver (HepG2), breast (MCF-7) and gastric (AGS, HMO2) cancer cell lines, and good antiproliferative effects on a lung (A549) cancer cell line (IC50 0.154 μM).58 The IC50 values of the positive controls doxorubicin and melphalan were 0.045 and 3.4 μM, respectively.
Moreover, the benzoquinoline alkaloid marcanine A (58) (Fig. 8) from Polyalthia plagioneura displayed growth inhibitory activity against BEL-7402 (liver cancer), K562, SPC-A-1 (lung cancer) and SGC-7409 (gastric cancer) with IC50 values of 9.54, 11.8, 8.69 and 1.53 μM, respectively.59 In contrast, saprosmines A (59) and B (60), with ketal groups rather than an oxo moiety at position-5, did not show cytotoxic activity against the same four cancer cell lines (all IC50 > 50 μM).60 4-Methoxycarbonyl-5,10-benzogquinolinequinone (61) exhibited IC50 values between 11.9 and 29.6 μM against SPCA-1, BEL-7402, SGC-7901 and K-562, while the 4-methylated cleistopholine (62) was inactive.60 The quinazoline leucomidine C (63) (Fig. 8) from Leuconotis griffithii was also inactive against the HL-60 cell line.61
6. Flindersine derivaties
Flindersine, with a more unusual pyranoquinoline core, showed cytotoxicity against Raji cells with an IC50 value of 14.9 μg/mL.38 5-Methoxyflindersine or haplophytin-A (64) (Fig. 9) from Haplophyllum acutifolium induced the classical features of apoptosis and externalization of annexin-V-targeted phosphatidylserine residues in HL-60 cells at a concentration of 50 μM.62 Treatment with compound 64 (50 μM) induced DNA fragmentation, DNA ladder formation and the externalization of annexin-V-targeted phosphatidylserine residues, and also resulted in the activations of caspase-8, -9 and -3, and the cleavage of poly (ADP-ribose) polymerase (PARP). Furthermore, loss of mitochondrial membrane potential (Δψm), release of cytochrome c and Smac/DIABLO to the cytosol, and formation of death-inducing signaling complex (DISC) also were found in the treated HL-60 cells. The alkaloid’s potent apoptotic activity was exerted via both extrinsic and intrinsic pathways, suggesting a good potential to treat leukemia.
Figure 9.

Chemical structures of compounds 64–67
Because some alkaloids function as intercalative topoisomerase poisons, ten quinoline alkaloids were investigated for potential intercalation into DNA using a molecular docking approach.63 Skimmianine, stauranthine (65), 3′,6′-dihydroxy-3′,6′-dihydrostauranthine (66) and trans-3′,4′-dihydroxy-3′,4′-dihydrostauranthine (67) (Fig. 9) were able to dock intercalatively and consistently into DNA, and the docked orientations were also electronically favorable.
7. Pyrrole-containing and other quinoline or quinazoline alkaloids
A growing class of natural products includes pyrrole-containing quinoline alkaloids. Most of them are isolated from marine sources,64 and many exhibit antitumor activity. Ammosamides A and B (68, 69) (Fig. 10) from marine-derived Streptomyces variabilis exhibited activity against colorectal cancer HCT-116 cells via covalent modification of myosin, which are involved in numerous cell processes, including cell cycle regulation, cytokinesis and cell migration.65,66 In studies three years later, the oxidized ring-opened ammosamide D (70) (Fig. 10) exhibited good cytotoxicity (IC50 3.2 μM) against pancreatic cancer MIA PaCa-2 cells, but did not inhibit mTOR up to 20 μM.67 In contrast, the non-chlorinated pyrroloquinoline alkaloid lymphostin (71) (Fig. 10) from Streptomyces sp. KY11783 showed nanomolar inhibition of mTOR and potent cytotoxicity against LNCap (prostate) and MDA-468 (breast) cancer cell lines.64
Figure 10.
Chemical structures of compounds 68–74
Meanwhile, the new 2-pyrrolyl-substituted 4-quinolinone alkaloid penicinoline E (72), together with three known derivatives, methyl-penicinoline (73), penicinoline (74) (Fig. 10) and quinolactacide, were isolated from Penicillium sp. ghq208.68 Only 73 and 74 exhibited moderate cytotoxicity against the HepG2 cell line with IC50 values of 11.3 and 13.2 μM, respectively,68 the latter compound also showed potent in vitro cytotoxicity toward 95-D cells with an IC50 value of 0.57 μg/mL.69 The presence of a carboxyl group at C-3 appears to be essential for the cytotoxicity of this unusual pyrrolyl 4-quinolinone alkaloid type.
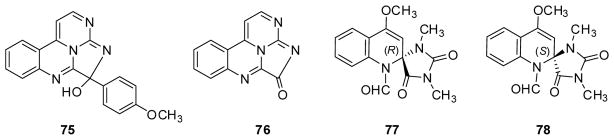
In 2015, novel heterocylic alkaloids eudistidines A (75) and B (76) (Fig. 11) were isolated from Eudistoma sp. in a screen for inhibitors of the binding interaction between HIF-1α and the transcriptional coactivator p300, which plays an important role in the survival of solid tumors in low oxygen environments and involves the C-terminal transactivation domain (C-TAD) and cysteine histidine-rich domain 1 (CH1), respectively, of the two proteins.70 The former compound effectively inhibited CH1/C-TAD binding with an IC50 of 75 μM. The unique scaffold of this natural alkaloid could lead to novel molecular probes to study p300/HIF-1α interactions and the role these proteins play in tumor response to low oxygen conditions or new therapeutic anticancer lead compounds that could disrupt critical protein-protein binding events. During the same year, novel bisheterocyclic quinolineimidazole alkaloids (+)- and (−)-spiroreticulatine (77, 78) (Fig. 11) from Fascaplysinopsis reticulate were inactive against K562, A549, Hela or Jurkat tumor cell lines, but inhibited IL-2 production in a dose-dependent manner.71
Figure 11.
Chemical structures of compounds 75–78
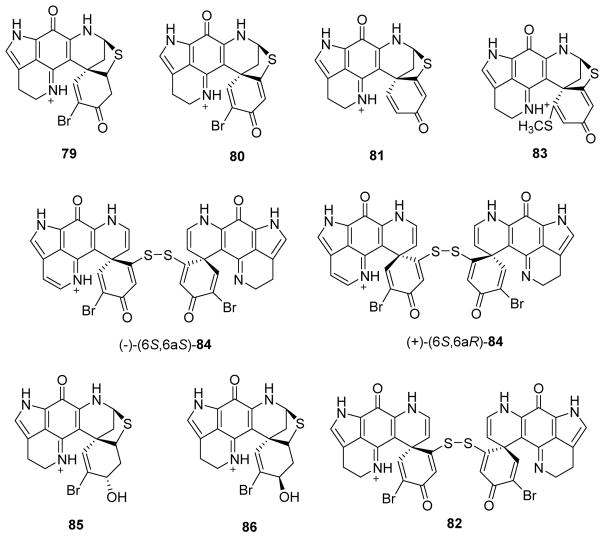
Eight compounds of the discorhabdin A- and B-type, including (+)-discorhabdin/prianosin A (79), (+)-discorhabdin B (80), (+)-discorhabdin G*/I (81), (−)-discorhabdin W (82), (+)-(6R,8S)-1-thiomethyldiscorhabdin G*/I (83), both enantiomers of 16a,17a-dehydrodiscorhabdin W (84) and (+)-3-dihydrodiscorhabdin A [with reassignment of its configuration from (3S,5R,6S,8S)-85 to the C3-epimeric (+)-(3R,5R,6S,8S)-86] (Fig. 12), were isolated from the marine genus Latrunculia.72 In biological screening against the murine leukemia P388 cell line, 83 (IC50 0.28 μM) and both enantiomers of 84 (IC50 0.45 μM) exhibited potent antiproliferative activity comparable to the respective ‘parent’ compounds 81 (IC50 0.6 μM) and discorhabdin W (IC50 0.13 μM). However, 85 (IC50 2.1 μM) and 86 (IC50 1.8 μM) were less potent, reflective of the role played by the C-3 keto group in increasing the antiproliferative potency, as again observed with discorhabdin A (IC50 0.11 μM).72
Figure 12.
Chemical structures of compounds 79–86
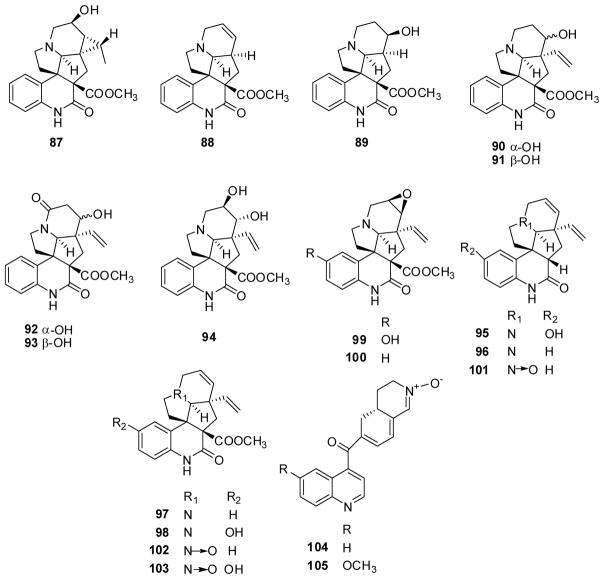
Meanwhile, nine new melodinus-type alkaloids, melohemsines A–I (87–95), meloscine (96), sandine (97), 10-hydroxyscandine (98), 10-hydroxy-14,15-β-epoxysandine (99), 14,15-β-epoxysandine (100), meloscine N4-oxide (101), scandine N4-oxide (102) and melodinine U (103) were isolated from Melodinus hemsleyanus,73 and two rearranged quinuclidine alkaloids, cincholenines A (104) and B (105) (Fig. 13) were found in Cinchona ledgeriana.74 None of the compounds showed IC50 values under 40 μM.
Figure 13.
Chemical structures of compounds 87–105
B. Anti-parasitic and Insecticidal Activities
1. Anti-malarial activity
Malaria is an infectious disease with ravaging effects in many parts of the world. Four protozoan species of the genus Plasmodium (P. falciparum, P. malariae, P. ovale and P. vivax) are responsible for this infection.75 In 2013, this disease led to an estimated 198 million cases and resulted in 584,000 deaths.76 Half of the world’s population is at risk from malaria.77 Isolated quinoline alkaloids have been investigated as possible antimalarial agents since quinine was found to inhibit the endocytosis of P. falciparum78 and quinidine, an erythro diastereoisomer of quinine, was also found to exhibit significant antimalarial activity.79
Recently, increasing numbers of papers have focused on the mechanism of antimalarial drugs. Studies in 2014 showed that quinine significantly inhibited breast cancer resistance protein- (BCRP-) and P-gp-mediated transport at concentrations within the clinically relevant prophylactic and therapeutic range, and inhibited adenosine triphosphate (ATP) binding cassette transporter activity.80 Quinidine and cinchonine, which have identical stereochemistry at carbons 8 and 9, exhibited stronger inhibition of human and Drosophila melanogaster serotonin receptor transporter (hSERT and dSERT) function than their enantiomers, quinine and cinchonidine.81 Furthermore, quinine and cinchonidine bound to the central receptor binding site (S1), whereas quinidine and cinchonine bound to the S2 site in small molecule docking studies. These results indicated that distinct cinchona alkaloids bind to two different sites on SERT, with the most potent antimalarial inhibitors of SERT appearing to preferentially bind to the S2 site. This difference implies separate modes of transporter inhibition.81
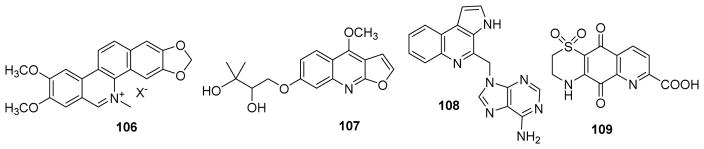
Among quinoline alkaloids reported with antimalarial activity between 2009 to 2016, examples are 6-methoxy-7-hydroxydictamnine,36 4-methoxy-1-methylquinolin-2-one,82 (2′R)-2′,3′-epoxy-N-methylatanine,83 kokusaginine,84 and maculosidine.85 Nitidine (106) (Fig. 14) from Toddalia asiatica exhibited high antiplasmodial activity with an IC50 of 0.045 μg/mL against the K39 strain of P. falciparum in vitro and likely acts by a similar mechanism of that of chloroquine.86 The furoquinoline evoxine (107) (Fig. 14) from Teclea gerrardii displayed the activity against the CQS (chloroquine susceptible) D10 strain of P. falciparum with an IC50 value of 24.5 μM.87
Figure 14.
Chemical structures of compounds 106–109
Aplidiopsamine A (108) (Fig. 15) from Aplidiopsis condluata has a tricyclic 3H-pyrrolo[2,3-c]quinoline substructure conjugated to adenine. It exhibited significant growth inhibition of P. falciparum chloroquine resistant (IC50 1.65 μM for Dd2) and sensitive strains (IC50 1.47 μM for 3D7) and minimal toxicity toward HEK-293 cells.88 Ascidiathiazone A (109) (Fig. 15) was a moderately potent in vitro growth inhibitor of P. falciparum K1 strain (IC50 3.3 μM) and Trypanosoma brucei rhodesiense (IC50 3.1 μM). However, it was effectively inactive towards T. cruzi and Leishmania donovani and exhibited low cytotoxicity against a mammalian cell-line (L6, IC50 167 μM).89
Figure 15.
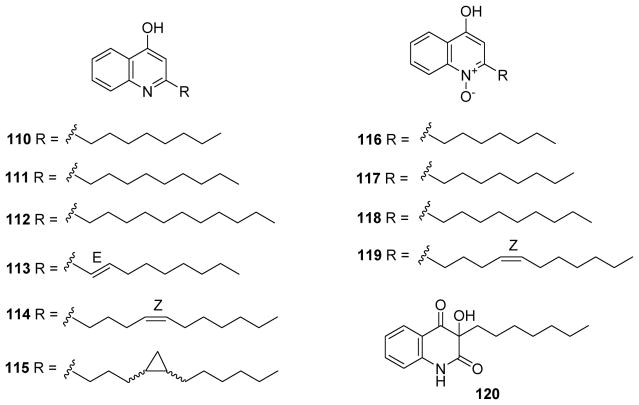
Chemical structures of compounds 110–120
Several 4-hydroxyquinoline alkaloids, including six known [2-n-octyl-4-hydroxyquinoline (110), 2-n-nonyl-4-hydroxyquinoline (111), 2-n-undecyl-4-hydroxyquinoline (112), 2-(E)-non-1′-enyl-4-hydroxyquinoline (113), n-heptyl-4-hydroxyquinoline N-oxide (116), 2-n-nonyl-4-hydroxyquinoline N-oxide (118)] and four new [2-((Z)-undec-4′-enyl)-4-hydroxyquinoline (114), 2-(3′-(2′-hexylcyclopropyl)propyl)-4-hydroxyquinoline (115), 2-n-octyl-4-hydroxyquinoline N-oxide (117) and 2-((Z)-undec-4′-enyl)-4-hydroxyquinoline N-oxide (119)] compounds as well as 3-n-heptyl-3-hydroxyquinoline-2,4(1H,3H)-dione (120) (Fig. 15), from Pseudomonas aeruginosa BCC76810 were evaluated for antimalarial effects. Most of the 11 compounds, including both 4-hydroxyquinolines (110–114) and 4-hydroxyquinoline-N-oxides (116–119), exhibited activity against P. falciparum, K1 strain (IC50 0.25–2.07 μg/mL) with moderate to weak cytotoxicity against cancerous [KB, MCF-7, NCI-H187 (lung cancer)] and non-cancerous (Vero) cells.90
2. Antitrypanosomal and antileishmanial activities
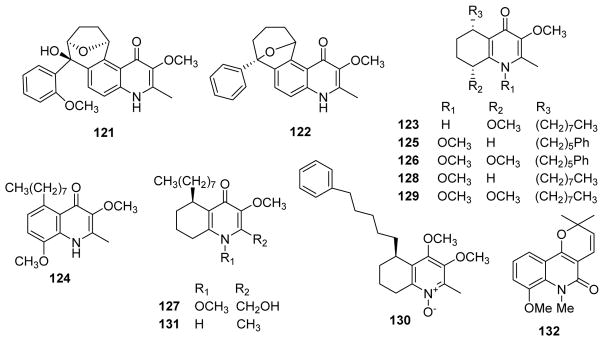
American trypanosomiasis, known as Chagas disease, is a potentially life-threatening illness caused by the protozoan parasite Trypanosoma cruzi. To date, about 7 to 8 million people are estimated to be infected worldwide, mostly in Latin America.91 To avoid the severe side effects induced by the current drugs benznidazole and nifurtimox as well as find enhanced clinical efficacy,92,93 natural products, especially quinoline alkaloids, have been investigated as new antitrypanosomal medicines. Cretton and co-workers isolated waltheriones A (121), C (122) and E–L (123–130), together with antidesmone and 8-deoxoantidesmone (131) (Fig. 16) from Waltheria indica in the years 2014 and 2015.94,95 Compounds 125, 126 and 129 (Fig. 16) showed potent and selective growth inhibition toward Trypanosoma cruzi with IC50 values of 0.02 (125) and 0.04 (126, 129) μM. The remaining compounds were less potent (IC50 0.1–3.1 μM; >50 μM for 121). All compounds exhibited weak activity against T. brucei and T. brucei rhodesiense. However, except for three compounds (121, 122 and antidesmone), the isolated alkaloids also showed significant cytotoxicity against murine skeletal L-6 cells, with 129 being the most cytotoxic (IC50 0.07 μM vs podophyllotoxin 0.02 μM). Thus, the selectivity index (SI: IC50 cytotoxicity/IC50 anti-T. cruzi activity) values of most of the waltheriones ranged from 1.8 to 33.8, making them too toxic to be considered as antichagasic hits.94,95 The sole exception was 122 with a cytotoxicity IC50 of 101 μM and better SI of 52.4.95
Figure 16.
Chemical structures of compounds 121–132
Leishmaniasis, a major health problem worldwide, is a neglected tropical parasitic disease resulting from the infection of macrophages by obligate intracellular parasites of the genus Leishmania.96,97 Due to the prevalence of HIV/VL co-infections, leishmaniasis has recently become a more studied problem,98 and alkaloids are prospective compounds to combat this disease.99–101 Among five investigated furoquinoline alkaloids (dictamine, γ-fagarine, skimmianine, flindersiamine and masculine), γ-fagarine presented significant in vitro activity against L. amazonensis, L. infantum and L. braziliensis with IC50 values of 17.3, 26.5 and 22.2 μM, with comparable inhibition (97%) to that of the reference drug.102 In L. amazonensis infected Balb/c mice treated orally with γ-fagarine at 10 mg/kg daily for 14 days, lesion weight decreased significantly by 90.5% and lesional parasites dropped drastically by 97.4%.102 N-Methyl-8-methoxyflindersine (132) (Fig. 17) also exhibited selective leishmanicidal activity against intracellular promastigotes (EC50: 14.3 μg/mL).103
Figure 17.
Chemical structures of compounds 133–146
3. Acaricidal and insecticidal activity
Luo and co-workers isolated N-methylswietenidine B (133), methyl 2-(3-hydroxy-1-methyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)-acetate (134) and 3-hydroxy-1-methyl-3-(2-oxopropyl)quinoline-2,4(1H,3H)-dione (135), N-methylflindersine (136) and 4-hydroxy-3-methoxy-1-methyl-2(1H)-quinolinone (137) (Fig. 17) from Micromelum falcatum.104 Only 136 showed strong toxicity toward brine shrimp with an LD50 value of 1.39 μg/mL; the LD50 values of the other four compounds were more than 50 μg/mL. Six new 4-phenyl-3,4-dihydroquinolones [6-deoxyaflaquinolone E (138), isoaflaquinolone E (139), 14-hydroxyaflaquinolone F (140), aniduquinolones A (141), B (142) and C (143)] were characterized from the endophytic fungus Aspergillus nidulans.105 The two latter alkaloids as well as the known isolated aflaquinolone A (144) (Fig. 17) had reported LD50 values of 7.1, 4.5 and 5.5 μM, respectively, against brine shrimp (Artemia salina).105 Moreover, two enantiomeric quinazolines, 2-[(S)-hydroxy(phenyl)methyl]-3-methylquinazolin-4(3H)-one (145) and 2-[(R)-hydroxy(phenyl)methyl]-3-methylquinazolin-4(3H)-one (146) (Fig. 17) from the alga-endophytic fungus Talaromyces sp. Cf 16 exhibited weak toxicity toward brine shrimp (LC50 97.8 and 106 μg/mL).106 The latter compound also showed weak inhibition against Staphylococcus aureus.
In addition, penicinoline showed strong insecticidal activity against A. gossypii,65 and quinolactacide showed excellent activity against Myzus persicae.107 8-Hydroxyquinoline was very effective against the growth of Toxoplasma; its IC50 value was 0.213 μM.108 The LD50 values of quinoline-4-carbaldehyde from Ruta chalepensis were 0.084 mg/cm2 and 0.065 mg/cm2 against Sitophilus oryzae by the fumigant and the contact methods, respectively.109 Changing the position of the aldehyde groups in the quinoline skeleton increased the insecticidal activity.
Our group reported that the quinazoline alkaloid vasicine from Peganum harmala presented weak acaricidal activity against Psoroptes cuniculi.110 Its LT50 values were 12.2 h and 9.79 h at 1.25 and 2.5 mg/mL, respectively.
C. Antibacterial and Antifungal Activities
Several antibacterial drugs have been developed from alkaloids. Quinolone antibiotics arose out of the synthesis of quinine, metronidazole resulted from the structural alteration of azomycin, and bedaquiline was produced from studies on the quinoline scaffold.111 Quinolone antibiotics target the bacterial type IIA topoisomerase/gyrase.112,113 The compounds stabilize the enzyme-DNA cleavage complex; two quinolone molecules bind with the enzyme and intercalate into the cleaved DNA. This action stops the free 3′ hydroxyl of the DNA from attacking the phosphotyrosine link in the enzyme-DNA complex and thereby prevents religation of the DNA.114 The decreased level of religation eventually leads to bacterial cell death.
Flindersine alkaloids are a novel class of quinoline alkaloids. Flindersine exhibits both antibacterial and antifungal activities. Its MIC values against bacteria Bacillus subtilis, Staphylococcus aureus, S. epidermidis, Enterococcus faecalis, Pseudomonas aeruginosa, Acinetobacter baumannii and fungi Trichophyton rubrum 57, T. mentagrophytes, T. simii, Epidermophyton floccosum, Magnaporthe grisea and Candida albicans were 31.25, 62.5, 62.5, 31.25, 250, 125, 62.5, 62.5, 62.5, 62.5, 250 and 250 μg/mL.115 The analog 8-methoxyflindersine exhibited similar inhibitory activity against the C. albicans-sensitive mutant strain DSY2621, but was inactive against a wild strain of this yeast.116 Meanwhile, the structurally related veprisine showed moderate activity against S. aureus.117
Furoquinoline alkaloids also exhibit antibacterial and antifungal activities. Skimmianine showed weak antimicrobial and antifungal activities against two Gram-positive bacteria (S. aureus and S. epidermidis), five Gram-negative bacteria (Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, Klebsiella pneumoniae and Shigella dysenteriae), three pathogen fungi (C. albicans, C. tropicalis and C. glabrata) and the oral pathogens S. mutans and S. viridans.33 Skimmianine also exhibited antimycobacterial activity against Mycobacterium tuberculosis H37Ra ATCC 25177 and M. tuberculosis H37Rv ATCC 27294.22 Kokusaginine exhibited moderate antibacterial activity against methicillin-resistant S. aureus SK1with an MIC value of 16 μg/mL.118
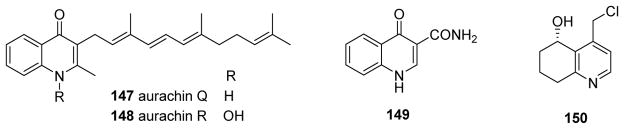
Our prior review summarized the antibacterial effects of four sesquiterpenoid quinoline antibiotics, aurachins A–D. Since then, aurachins C and D together with two new related compounds, aurachins Q (147) and R (148) (Fig. 18), were isolated from Rhodococcus sp. Acta 2259.119 Aurachins C and R exhibited moderate antibacterial activity against Staphylococcus epidermidis DSM 20044, Bacillus subtilis DSM 347 and Propionibacterium acnes DSM 1897, and weak inhibitory activity against glycogen synthase kinase 3β. The two other aurachins were inactive at concentrations below 100 μM, and all four compounds were inactive against Gram-negative bacteria.119 Meanwhile, 4-oxo-1,4-dihydroquinoline-3-carboxamide (149) (Fig. 18) from Nocardiopsis terrae YIM 90022 showed antimicrobial activity against S. aureus, B. subtilis and E. coli with MICs of 64, 64 and 128 μg/mL and antifungal activity against P. oryzae with an MIC of 256 μg/mL.120 4-Hydroxyquinoline N-oxides also displayed anti-Bacillus cereus activity,90 and 5-hydroxy-4-(chloromethyl)-5,6,7,8-tetrahydroquinoline (150) (Fig. 18) from a Belize extract inhibited the growth of pathogenic, saprophytic marine fungi and marine bacteria.121
Figure 18.
Chemical structures of compounds 147–150
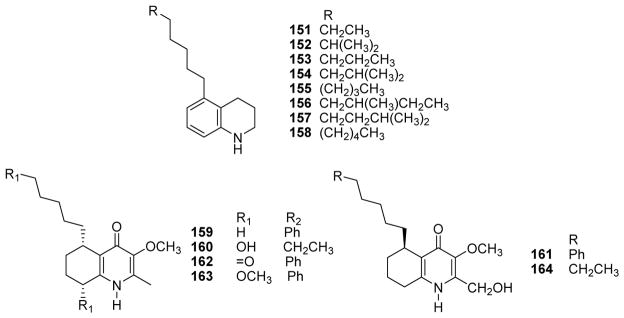
Eight novel 5-alkyl-1,2,3,4-tetrahydroquinolines (5aTHQs, 151–158) (Fig. 19) bearing different side chains were isolated from a combined culture of Streptomyces nigrescens HEK616 and Tsukamurella pulmonis TP-B0596.122 All eight compounds inhibited the growth of yeast cells, most likely by targeting the membrane lipids. 5aTHQ-7n (151) exhibited the most potent growth inhibition with an MIC value of 6.3 μM compared with 0.25 μM for amphotericin B. The length and methylation pattern of the side chain at C-5 had a critical effect on the potency and selectivity toward the growth of yeast cells.122 Among several quinoline alkaloids [waltherions E–L and M–Q (159–163, respectively), 5(R)-vanessine (164) (Fig. 19), 8-deoxoantidesmone and antidesmone] isolated from Waltheria indica, waltheriones E–I, N and Q, 5(R)-vanessine, 8-deoxoantidesmone and antidesmone showed growth inhibitory activity against Candida albicans on both planktonic cells and biofilms (MIC ≤32 μg/mL).123 However, the selectivities were much weaker (SI, 0.3–5) compared with those of fluconazole and caspofungin (>100). All compounds also exhibited in vitro toxicity against HeLa cells with IC50 values ranging between 9.5 and 50 μg/mL.
Figure 19.
Chemical structures of compounds 151–158
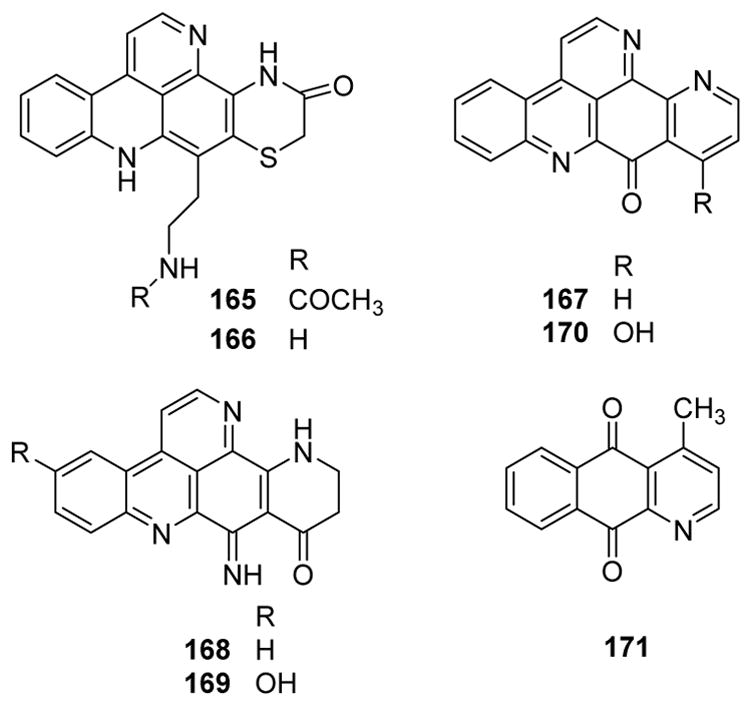
Various new and known pentacyclic alkaloids were isolated from different chromotypes of the western Mediterranean ascidian Cystodytes dellechiajei.124 The purple morph collected in Catalonia contained shermilamine B (165) (known) and N-deacetylshermilamine B (166) (new) (Fig. 20). The green morph collected in the Balearic Islands contained 11-hydroxyascididemin (167) (known) as well as cystodimines A (168) and (169) (new) (Fig. 20). The blue morph collected in Catalonia yielded ascididemin (170) (known) (Fig. 20). Among the tested compounds, 170 exhibited the best activity with MIC values of 0.2 and 0.3 μM against E. coli and E. luteus, respectively, while 167 and 169 showed the weakest activity with MIC values of 2.6 μM against E. coli and 10.5 μM against E. luteus.124 The benzo[g]quinoline-5,10-dione cleistopholine (171) (Fig. 20) from the fruiting tree Annona salzmannii showed significant antibacterial activity (MIC 250 μg/mL) against S. epidermidis, Enterococcus faecalis and Candida dubliniensis.125
Figure 20.
Chemical structures of compounds 165–171
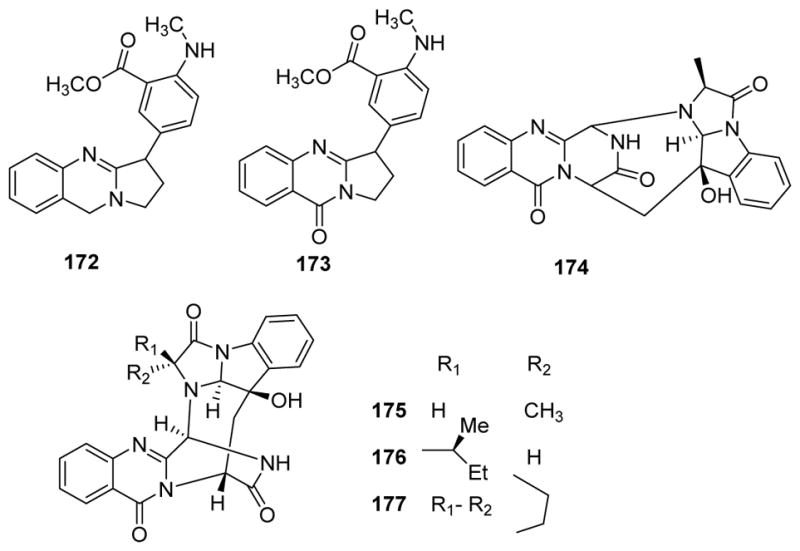
The important quinazoline alkaloid tryptanthrin has a wide range of biological effects, including promising antibacterial activity toward methicillin-resistant S. aureus126 and anti-tubercular activity against Mycobacterium tuberculosis.127 Tryptanthrin could bind the site of InhA with free binding energy of −7.94 kcal/mol and inhibition constant (Ki) of 1.50 microm. Active site residues of InhA interacting with tryptanthrin were Ser13, Thr39, Phe41, Leu63, Asp64, Val65, Ile95, Phe97 and Ile122. So, tryptanthrin has good affinity at the binding site of the enoyl-acyl carrier protein reductase of M. tuberculosis and compounds of this type could be developed as anti-tubercular drugs against this therapeutic target, particularly for combating MDR strains.127 While the quinazoline alkaloid vasicine128 and its acetate129 showed moderate antibacterial activity, vasicine acetate also significantly inhibited M. tuberculosis at 200 and 50 μg/mL for one multi-drug-resistant (MDR) strain and one sensitive strain, respectively. Besides vasicine, additional quinazoline alkaloids vasicoline, vasicolinone, vasicinone, adhatodine (172) and anisotine (173) (Fig. 21) also exhibited anti-tubercular activity.130
Figure 21.
Chemical structures of compounds 172–177
Among the four new cottoquinazolines A–D (174–177) (Fig. 21) from Aspergillus versicolor and A. versicolor LCJ-5-4, only compound 177 showed moderate antifungal activity against Candida albicans.131,132 Rutaecarpine and 7β-hydroxyrutaecarpine also showed antimycobacterial effects.22,133
D. Cardioprotective Activity
As mentioned previously, rutaecarpine exhibits multiple biological effects;134 however, its cardiovascular pharmacological properties, such as inotropic and chronotropic, vasorelaxant, anti-platelet aggregation and anti-inflammatory effects, are undoubtedly among the most important and have attracted much research interest.135,136 It significantly prolonged the latent period of inducing platelet plug formation in mesenteric venules when it was intravenously injected, and prolonged occlusion time by approximately 1.5-fold. Intravenous injection of rutaecarpine prolonged the bleeding time in the severed mesenteric arteries of rats.134 In 2015 studies on endothelial dysfunction related to atherosclerosis, Peng et al. found that pretreatment with rutaecarpine effectively inhibited Cx43 expression, but recovered the expression of Cx37 and Cx40. The alkaloid effectively improved gap junction communication and significantly prevented endothelial dysfunction induced in HUVEC-12 cells by exposure to oxidized low-density lipoprotein (ox-LDL) in vitro, which is related to regulation of connexin expression patterns via TRPV1 activation.137 Rutaecarpine decreased monocyte adhesion by reversing the altered connexin (Cx) expression also induced by ox-LDL.138 In subsequent studies, the same group found that, more specifically, rutaecarpine pretreatment led to recovered Cx37 (artheroprotective) expression and inhibited Cx43 (artherogenic) upregulation, thus, improving adenosine triphosphate-dependent hemichannel activity.138 In earlier studies, Xu et al. found that rutaecarpine promoted reverse cholesterol transport (RCT) in vivo and suppressed atherosclerosis in ApoE/mice by promoting ABCA1 and SR-B1 activities within RCT.139 It triggered promoters of ABCA1 and CLA-1 genes, and increased ABCA1 and SR-BI/CLA-1 expression in vitro related to liver X receptor alpha and liver X receptor beta. In RAW264.7 cells, rutaecarpine could induce cholesterol efflux. Meanwhile, it attenuated macrophages and cholesterol accumulations in atherosclerotic lesions in vivo. The anti-atherosclerotic activity of rutaecarpine, as well as evodiamine, has also been linked to other targets or pathways, such as LIGHT, cAMP-related pathways, etc.140–142 The inhibitory effects of evodiamine and rutaecarpine on LIGHT-induced migration and the activation of CCR1, CCR2, ICAM-1, ERK, and p38 MAPK occur via decreased ROS production and NADPH oxidase activation.140
Moreover, rutaecarpine displays cardioprotective effects against ischaemia-reperfusion injury. Treatment with rutaecarpine led to a hypotensive effect in spontaneously hypertensive rats (SHR) and reversed cardiac susceptibility to reperfusion injury by increasing plasma calcitonin gene-related peptide (CGRP).143 Both cardiac function and vasodilator responses were significantly improved. Stimulation of CGRP release by rutaecarpine also led to reduced hypoxia-induced right ventricular remodeling in rats, and the effects involve the eIF3a/p27 pathway.144 Rutaecarpine down-regulated NOX4 expression and collagen accumulation in rats with pulmonary hypertension induced by monocrotaline.145 In addition, rutaecarpine protected against hypoxia-reoxygenation-induced myocardial cell injury and apoptosis in myocardial H9c2 cells partly due to the inhibition of the NADPH oxidase-ROS pathway,146 increased prolylcarboxypeptidase expression and decreased angiotensin II levels in 2K1C hypertensive rats,147 and inhibited vasoconstriction induced by anaphylaxis in guinea pigs.148
Several studies have reported evodiamine and rutaecarpine alkaloids to be putative selective agonists both in vitro and in vivo for transient receptor potential vanilloid 1 (TRPV1), a nonselective cation channel expressed on either sensory neuronal systems or nonneuronal sites.149–154 In 2016, Wang et al. investigated the inhibitory effects of evodiamine and rutaecarpine against TRPV1 channels, and against capsaicin- or proton-activated TRPV1 activities.155 The results indicated that, compared with capsaicin, the two alkaloids had lower maximum responses for activating TRP1, suggestive of partial agonism. Thus, evodiamine and rutaecarpine may share the capsaicin binding site and act as partial TRPV1agonists (antagonists). In addition, evodiamine desensitized or competitively inhibited the activity of TRPV1. Rutaecarpine could be used as a potential anti-hypertensive lead compound with a novel mechanism.155
Both cardiovascular and cerebrovascular diseases are forms of ischemic vascular disease. Accordingly, Yan and co-workers reported on the neuroprotective effects of rutaecarpine on cerebral ischemia reperfusion injury in 2013.156 The compound enhanced learning and memory ability, ameliorated neurological symptoms, and reduced infarction volume and cerebral water content in mice with cerebral ischemia reperfusion injury.156 Administration of rutaecarpine attenuated hyperglycemia and enhanced insulin sensitivity, significantly decreased obesity, visceral fat accumulation, water consumption, and serum TC, TG and LDL-cholesterol levels in fat-fed, streptozotocin-treated rats. In addition, it increased PI3K p85 subunit levels and Akt/PKB phosphorylation, and decreased IRS-1 phosphorylation in liver tissues. In skeletal muscle cells, it promoted the phosphorylation of AMPK and ACC2, and increased glucose uptake. Results indicated that rutaecarpine improved hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats by regulating the hepatic IRS-1/PI3K/Akt signaling pathway and skeletal muscular AMPK/ACC2 signaling pathway.157
The diastereoisomeric quinoline akaloids quinidine and quinine, which are used to treat arrhythmia and malaria, respectively, block the hERG (human ether-a-go-go-related gene) potassium channel, which is essential for myocardium repolarization.158 Quinine was 14-fold less potent than quinidine. In addition, they distinctly impacted the channel dynamics. The results also indicate stereospecific block effect on the hERG channel, but F656C-hERG reversed this stereoselectivity. The mutation decreased affinity of the two drugs with hERG, and quinine was more potent than quinidine in F656C-hERG blockage.158 Quinine also stimulated adipogenesis through ERK/S6 signaling, acting at least partly via the bitter taste receptor T2R106.159
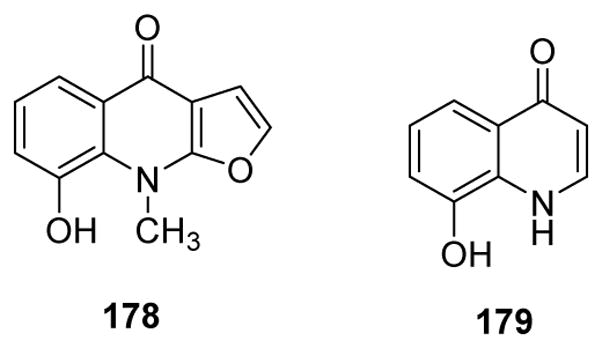
A new quinoline alkaloid, 8-hydroxy-9-methyl-furo[2,3-b]quinolin-4(9H)-one (178) (Fig. 22) together with isopteleine, robustine, γ-fagarine, isodictamnine and skimmianine were isolated from Dictamnus angustifolius.160 All of the compounds showed significant inhibitory effects on platelet aggregation induced by adenosine diphosphate (ADP) at 250 μM. Meanwhile, 8-hydroxy-4-quinolone (179) (Fig. 22), from Scolopendra subspinipes multilans, the Chinese red-headed centipede, exhibited antiplatelet effects.161 It prolonged activated partial thromboplastin and prothrombin times and inhibited the activity and production of thrombin and activated factor X. Furthermore, it inhibited thrombin-catalyzed fibrin polymerization and platelet aggregation, and enhanced antithrombotic effects in an in vivo pulmonary embolism and arterial thrombosis model.
Figure 22.
Chemical structures of compounds 178 and 179
E. Anti-viral Activity
Fumiquinazolines are quinazoline-containing indole alkaloids that possess pyrimido[2,1-b]quinazoline and imidazo[1,2-a]indole moieties linked by a methylene (and in some cases further linked via additional spiro-bridges). The first compounds of this type were isolated from Aspergillus fumigates in 1992.162 During the following decades, about 40 fumiquinazoline analogues were reported from various fungal genera. In 2016, two new fumiquinazoline analogues, neosartoryadins A (180) and B (181) (Fig. 23), were isolated from the endophytic fungus Neosartorya udagawae HDN13-313.163 They displayed anti-influenza virus A (H1N1) effects with IC50 values of 66 and 58 μM, respectively, compared with 94 μM for the positive control ribavirin. Six new indole alkaloids, including five new glyantrypine derivatives and a new pyrazinoquinazoline derivative, together with eight known alkaloids were isolated from a culture of the mangrove-derived fungus Cladosporium sp. PJX-41.164 Oxoglyantrypine (182), norquinadoline A (183), deoxynortryptoquivaline (184) and quinadoline B (185) (Fig. 23) exhibited significant activity against H1N1 virus (IC50 82–89 μM). Trytoquivaline, quinadoline A (186), 3-hydroglyantrypine (187), cladoquinazoline (188), epi-cladoquinazoline (189), glyantrypine (190), deoxytrytoquivaline (191) and CS-C (192) were weakly active (IC50 100–150 μM), and prelapatin B (193) (Fig. 23) was inactive (IC50 >200 μM).164
Figure 23.
Chemical structures of compounds 180–193
Anti-HIV activity is another important biological activity of quinoline and quinazoline alkaloids. In 2010, buchapine and 3-(3-methylbut-2-enyl)-4-(3-methylbut-2-enyloxy)quinolin-2-ol (194) were evaluated for their anti-HIV potential in the human CD4+ T cell line CEM-GFP infected with HIV-1NL4.3 virus.165,166 The IC50 and CC50 values of the former compound were 2.99 and 55.9 μM, respectively, and those of the latter compound were 3.80 and 71.2 μM, respectively, giving them identical therapeutic indices (TIs). In comparison, the IC50 and CC50 values of AZT were 1.04 and 24.0 μM, respectively. From a synthetic study on alkylated quinoline-2,4-diols, an unsubstituted ring B and free 2-OH group are essential for anti-HIV activity, and a prenyl group is best for HIV-1 inhibitory activity.165,166 Furthmore, trigonoine B (195) (Fig. 24) showed weak anti-HIV activity, preventing the cytopathic effects of HIV-1IIIB in C8166 cells with an EC50 value of 17.6 μg/mL and TI value of 4.48, but no anti-HBV activity.167 Meanwhile, waltheriones A (121) and C (122) (Fig. 16) showed significant effects in an in vitro anti-HIV cytoprotection assay at concentrations of 56.2 and 0.84 μM and inhibition of HIV P24 formation of more than 50% at 1.7 and 0.95 μM, respectively, while waltheriones B (196) and D (197) (Fig. 24) were only weakly active or inactive.168
Figure 24.
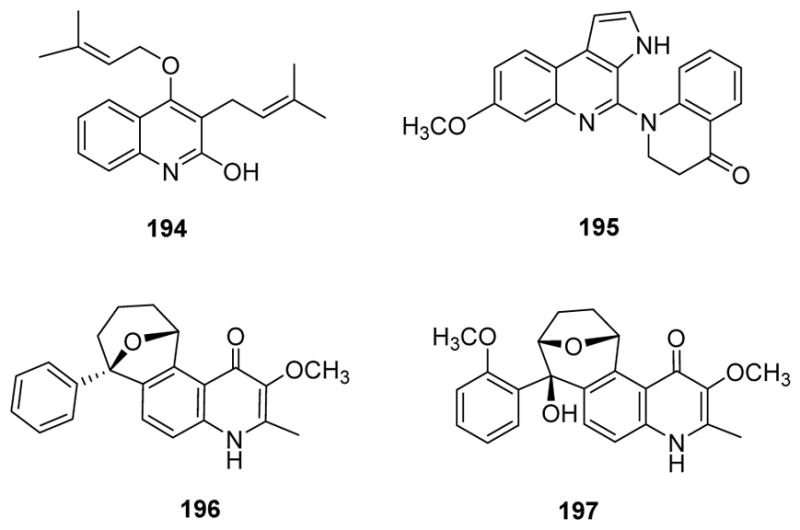
Chemical structures of compounds 194–197
Hepatitis C virus (HCV) infection is highly prevalent among global populations. The 4-quinolone alkaloid pseudane IX (198) (Fig. 25) isolated from Ruta angustifolia possessed strong anti-HCV activity (IC50 1.4 μg/mL) and was more potent than ribavirin (IC50 2.8 μg/mL).169 Also, the furoquinolines γ-fagarine and kokusaginine showed weaker but significant anti-HCV activity (IC50 20.4 and 6.4 μg/mL, respectively).
Figure 25.

Chemical structures of compounds 198
F. Anti-inflammatory Activity
Lipopolysaccharide (LPS) stimulation in cells causes inducible nitric oxide synthase (iNOS) overexpression and subsequently NO synthesis. Such stimulation occurs in response to exposure to inflammatory and immunologic stimuli, and has been implicated in the pathogenesis of numerous diseases, including septic shock, asthma, inflammatory bowel disease, osteoarthritis, and rheumatoid arthritis.170
In 2012, Yoon et al. evaluated the anti-inflammatory activity of alkaloids from Dictamnus dasycarpus by determining NO production in BV2 cells.171 The compounds included the new glycosidic quinoline alkaloid 3-[1β-hydroxy-2-(β-D-glucopyranosyloxy)-ethyl)-4-methoxy-2(1H)-quinolinone (199), preskimmianine, seven known furoquinolines [skimmianine, dictamine, γ-fagarine, iso-γ-fagarine, halopine, dictangustine-A (200), and isomaculosidine (201) (Fig. 26)], and the known pyranoquinolone 8-methoxy-N-methylflindersine. 8-Methoxy-N-methylflindersine and skimmianine showed the most potent inhibition of LPS-induced NO production with IC50 values of 0.4 and 7.0 μM, respectively. The corresponding value for the positive drug NG-nitro-L-arginine was 53.5 μM.171 Other studies showed that, when injected into mice (5.0 mg/kg), skimmianine alleviated carrageenan-induced acute inflammation, decreased the mRNA levels of TNF-α and IL-6, and reduced PGE2 and NO amounts, cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) activities, neutrophil infiltration, lipid peroxidation, and associated oxidative stress in the paw tissue.172 In addition, a new compound, 2-[(6Z,9Z)-pentadeca-6,9-dienyl]quinolin-4(1H)-one (202) from Tetradium ruticarpum and evodol inhibited fMLP/CB-induced elastase release with IC50 values lower than 14.4 μM, while rutaecarpine, evodiamine, and skimmianine inhibited O−2 generation (IC50 ≤20.9 μM) by human neutrophils in response to N-formyl-L-methionyl-L-leucyl-L-phenylalanine/cytochalasin B (fMLP/CB).173 However, skimmianine, haplopine, and glycohaplopine from Zanthoxylum schinifolium did not inhibit NF-κB.174
Figure 26.
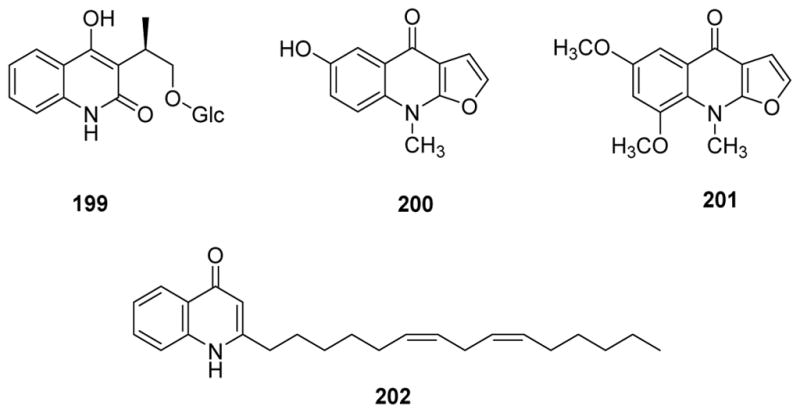
Chemical structures of compounds 199–202
Four new alkaloid glycosides clausenasides D–F (203–205) and 4-methoxy-8-O-β-D-glucopyranosyloxy-2(1H)-quinolinone (206), along with two new quinoline alkaloids, clausenasides G–H (207, 208), as well as other known quinoline alkaloids, including integriquinolone (209), 4-methoxy-2(1H)-quinolone, 4-methoxy-1-methyl-2-quinolone, and ribalinine (210) (Fig. 27), were obtained from Clausena lansium.175 Compounds 203, 205, 206, and 210 showed moderate inhibitory effects on LPS-induced NO production in BV2 cells (IC50 <10 μM), the remaining compounds had IC50 values of over 10 μM. Leucophyllidine and eucophylline (211) (Fig. 27), a new tetracyclic vinylquinoline alkaloid, were isolated from Leuconotis eugenifolius.176 Leucophyllidine caused dose dependent inhibition of NO production stimulated by LPS (IC50 7.1 μM), showed iNOS inhibitory activity, and decreased iNOS protein expression, while eucophylline was inactive. Both compounds showed high cell J774.1 viability at the above concentration.176 In addition, the novel alkaloid scholarisine II (212) selectively inhibited COX-2 and markedly inhibited 5-LOX, while scholarisine I (213) (Fig. 27) with a vinyl rather than hydroxyethyl group did not.177
Figure 27.
Chemical structures of compounds 203–213
The anti-inflammatory activity of tryptanthrin, a well-known indolo[2,1-b]quinazoline alkaloid, has been widely studied and was reported in our previous review. The compound potently inhibited COX-2 and 5-LOX-catalyzed leukotriene synthesis in vitro and in vivo,178–180 as well as iNOS-catalyzed NO production and prostaglandin E2 production by activated macrophages.181 In view of these proven pharmacological effects, in 2016, the pharmacokinetic properties of tryptanthrin and its ability to cross blood-brain barrier (BBB) were evaluated in vivo and in vitro.182 A high BBB permeation potential was found in vivo that correlated well with an immortalized human monoculture BBB model (hBMEC cell line) and corroborated with the in silico prediction of BBB penetration. In addition, tryptanthrin was not subject to active efflux.182
G. Effect on AChE and BChE
The newest drugs to treat Alzheimer’s disease are acetylcholinesterase inhibitors (AChEIs). As a newly discovered AChEI, the furoquinoline alkaloid skimmianine from Zanthoxylum nitidum inhibited 50% of AChE activity at a concentration of 8.6 μg/mL, while the IC50 value of the standard physostigmine was 0.013 μg/mL.183 In the same study, other quinoline alkaloids, inclucing dictamnine, γ-fagarine, (−)-(S)-edulinine, zanthodioline, edulitine and haplopine, were weakly active or inactive. TLC bioautographic assay also supported skimmianine’s inhibitory activity, which was enhanced by the presence of a methoxy group at C-7.183 In other studies, skimmianine, another furoquinoline kokusaginine, and the quinolin-4-one leiokinine A, inhibited AChE inhibition with IC50 values of 1.4 mM, 46 μM, and 0.21 mM, respectively.184 However, leptomerine (214) (Fig. 28), a natural quinolin-4-one from Esenbeckia leiocarpa, showed the highest activity (IC50 2.5 μM), similar to that of the reference compound galanthamine (IC50 1.7 μM). In 2014, Sichaem and co-workers reported that, among three new furoquinoline alkaloids, leptanoines A–C (215–217) (Fig. 28), together with known related alkaloids, including melineurine, skimmianine and 7-hydroxydictamnine, isolated from Evodia lepta,185 melineurine showed the highest inhibitory activity towards butyrylcholinesterase (BChE) with an IC50 value of 47.9 μM, and skimmianine showed the highest inhibitory activity towards AChE with an IC50 value of 69.1 μM.186 Lineweaver-Burk plots indicated that both compounds were mixed mode inhibitors of both ChE enzymes. Meanwhile, the new alkaloid 217 did not inhibit either enzyme (IC50 >200 μM).186 Another known furoquinoline alkaloid evolitrine significantly promoted nerve growth factor-mediated neurite outgrowth in PC12 cells (EC50 3.8 μg/mL), and potentially prevent neuronal degeneration and be useful for the medical treatment of Alzheimer’s disease.187
Figure 28.
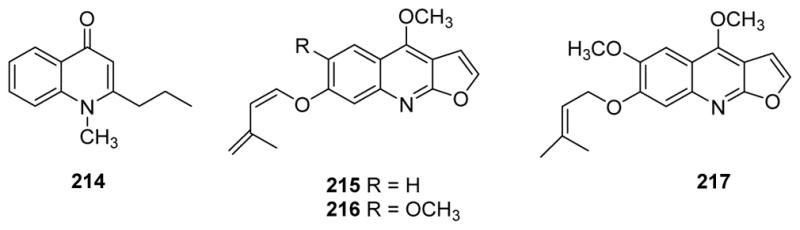
Chemical structures of compounds 214–217
16α-Hydroxy-5N-acetylardeemin (34) (Fig. 3) displayed good inhibitory effect against AChE (IC50, 58.3 μM).28 Waltheriones A (121) (Fig. 16) and B (196) (Fig. 24) also inhibited AChE activity with IC50 values of 134 and 123 μg/mL, respectively, in a dose-dependent manner.188
Deoxyvasicine and vasicine from Peganum harmala showed potent AChE (IC50 2.37 and 0.04 μM, respectively) and BChE (IC50 3.38 and 0.1 μM, respectively) inhibitory activity, but deoxyvasicinone, vasicinone and 2-carboxyl-3,4-dihydroquinazoline were inactive.189 Vasicine undergoes metabolic inactivation in vivo in respect to cholinesterase inhibitory activity, as most of its main metabolites were weaker inhibitors of AChE and BChE.190 In studies on the seeds of P. nigellastrum, two new alkaloids nigellastrine I (218) and nigellastrine II (219) (Fig. 29), along with, vasicinone, vasicine, deoxyvasicinone, deoxyvasicine, showed good inhibitory effects against AChE.191 In 2016, ten new alkaloids were isolated from P. harmala.192 Among the compounds, peganumine D (220), S-peganumine E (221) and R-peganumine E (222) have quinazolone skeletons, and peganumine F (223) and peganumine G (224) (Fig. 29) have both β-carboline and quinazolone skeletons. Compound 220 selectively inhibited AChE (IC50 5.71 μM), while the other compounds were inactive against both AChE and BChE. In addition, compound 224 showed cytotoxicity against a ZR-75-1 (breast cancer) cell line (IC50 6.20 μM).192
Figure 29.
Chemical structures of compounds 218–224
H. Anti-oxidant Activity
The furoquinoline alkaloid haplopine-3,3′-dimethylallyl ether, isolated from Vepris glomerata,, has strong antioxidant effects, similar to and, in some instances, better than those of ascorbic acid.193 This alkaloid together with two additional furoquinoline alkaloids dictamnine and robustine inhibited superoxide anion generation (IC50: 13.0, 17.4, 32.8, 26.1 μM, respectively) and elastase release (IC50: 19.6, 12.1, 29.3, 32.2 μM, respectively).193 In addition, various 4-hydroxyquinoline N-oxides also displayed antioxidant activities, with 2-n-nonyl-4-hydroxyquinoline N-oxide showing the best antagonist activity.90 The nitrogen oxide group was essential for the activity, as well as the length of the alkyl chain. The oxygen radical absorbance capacity (ORAC) value of cleistopholine, a quinoline alkaloid found in Annona salzmannii, was 0.25 relative trolox equivalents.125
In 2015, Popv et al investigated the antioxidant and membranotropic activities of the quinazoline alkaloid tryptantrin (triptantrin from orginal paper) in different model systems.194,195 Tryptanthrin exhibited very weak antioxidant activity, 1000 times lower than that of trolox, a vitamin E water-soluble analog commonly used an anti-oxidative benchmark, and 3000 times lower than that of the bioflavonoid dihydroquercetin. Meanwhile, vasicine, a quinazoline alkaloid isolated from Adhatoda vasica, inhibited the activities of ACh and trypsin by 38.4% and 37.4%, respectively, as well as 2-diphenyl-1-picrylhydrazyl (DPPH) by 70.4% (IC50 212.3 μM).128 Dose-dependent behavior was also seen in a ferric reducing ability of plasma (FRAP) assay.
I. Hepatoprotective Effect
Seventeen new alkaloids and their analogues were isolated from Isatis indigotica in 2012.196 One quinolin-4-one [methyl l2-(4-oxo-1,4-dihydroquinoline-3-carboxamido)benzoate (225)] and two quinolin-2-ones [6-hydroxy-4-(5-hydroxymethylfuran-2-yl)-quinolin-2(1H)-one (226), (+)-(R)-2-oxo-1,2,3,4-tetrahydroquinoline-4-carboxamide (227)] were included. Chiral HPCL was used to separate two enantiomers [(−)- and (+)-3-hydroxy-2H-pyrrolo[2,3-b]indolo[5,5a,6-b,a]quinazoline-9(8H),7′-dione (228, 229)] with a unprecedented pyrrolo[2,3-b]indolo[5,5a,6-b,a]quinazoline skeleton (Fig. 30). Compounds 227–229 reduced DL-galactosamine-induced hepatocyte (WB-F344 cell) damage with 44–55% inhibition at 10 μM, while the positive reference bicyclol resulted in 42% inhibition. However, the isolated alkaloids were inactive at 10 μM against HIV-1, HSV-1, and several human cancer cell lines.196
Figure 30.
Chemical structures of compounds 225–229
In 2014, Moon and co-workers proved that tryptanthrin protected hepatocytes against oxidative stress induced by tert-butyl hydroperoxide (tBHP) by activating the ERK/Nrf2 (nuclear factor erythroid 2-related factor) pathway in HepG2 cells.197 The compound blocked tBHP-induced reactive oxygen species (ROS) production, mitochondrial dysfunction, and cell death, as well as reversed tBHP-induced glutathione (GSH) reduction. Meanwhile, tryptanthrin also brought about nuclear translocation and transactivation of Nrf2, as well as phosphorylation of ERK, a potential upstream kinase of Nrf2. However, rutaecarpine, a related quinazoline alkaloid, decreased the primary rat hepatocyte viability, increased lactate dehydrogenase and reactive oxygen species, reduced JC-1 and caused cell stress and membrane damage. It inhibited the activities of cytochrome P450 enzymes (CYPs) in human liver microsomes, especially CYP1A2, CYP2C9, CYP2C19, CYP2E1 and CYP3A4, which could lead to herb-drug interactions or result in hepatotoxicity.198
Members of the asperlicin family of metabolites, including the major metabolite asperlicin (230) and asperlicins B–E (231–234) (Fig. 31), which are produced by several strains of Aspergillus alliaceus, were discovered in the 1980s as selective antagonists of the cholecystokinin receptor CCKA. Compound 230 was a lead scaffold for subsequent high affinity, selective CCKA ligands. In 2012, Haynes et al. reported the identification of the asperlicin gene cluster and characterization of short, efficient two enzymatic pathways to 234.199
Figure 31.
Chemical structures of compounds 230–234
J. Anti-asthma, Antitussive, Expectorant and Bronchodilating Activities
Quinoline from Ruta chalepensis displayed a stronger relaxant effect (IC50, 0.42 mM) on the contractile activity of guinea pig trachea than five other compounds, including quinoline-3-carboxaldehyde (>0.64 mM), quinoline-4-carboxaldehyde (>0.64 mM), 8-hydroxyquinoline (0.54 mM), quinazoline (0.48 mM) and quinoxaline (0.46 mM).200 The results indicated that quinoline derivatives have anti-asthma activity.
Because of the traditional clinical uses of Peganum harmala, in 2015 Liu and co-worker studied the antitussive, expectorant, and bronchodilating activity of two active quinazoline alkaloids (±)-vasicine and deoxyvasicine, found in this plant.201 In the antitussive studies in animals, the two compounds significantly inhibited coughing frequency and prolonged the cough latency period. The alkaloids also significantly increased phenol red secretion in expectorant testing done in mice. Compared with pretreatment in bronchodilation tests, vasicine and deoxyvasicine prolonged the pre-convulsive time by 28.6% and 29.7% at a dose of 45 mg/kg in guinea pigs, whereas aminophylline prolonged the pre-convulsive time by 47.0%. These results suggested that (±)-vasicine and deoxyvasicine have significant antitussive, expectorant, and bronchodilating properties.201
K. Anti-allergic Activity
Allergic diseases are initiated by the development of allergen-specific T helper type 2 (Th2) cells and amplified by the degranulation of and cytokine release from basophils and mast cells during an effector phase. In 2011, tryptanthrin was studied for its effects on the initiation and effector phase responses of Type I allergy in vitro.202 It suppressed c-Maf mRNA expression in Th2 clone cells, inhibited IgE-mediated degranulation and IL-4 production in RBL-2H3 cells, and reduced differentiation toward the Th2 phenotype, which is a key step in allergic initiation. Thus, tryptanthrin effectively inhibited the effector and exacerbation responses, as well as the initiator responses, of Type I allergy.202 Then in 2016, tryptanthrin was investigated regarding its regulating effects on thymic stromal lymphopoietin (TSLP)-induced mast cell proliferation and proinflammatory cytokine, tumor necrosis factor (TNF)-α production from mast cells.203 It significantly inhibited HMC-1 cell proliferation and suppressed mRNA expression and production of TNF-α induced by TSLP, and inhibited Ki67 mRNA expression, mRNA expression and production of IL-13, as well as the mRNA expression of IL-7 receptor a chain and TSLP receptor in TSLP-promoted HMC-1 cells.203 Moreover, tryptanthrin significantly suppressed the levels of intracellular calcium, IL-4, IFN-α, IL-6, TNF-α, and TARC/CCL17 (thymus and activation-regulated chemokine) as well as the production and mRNA expression of TSLP in activated HMC-1 cells.204 It also inhibited IgE and IL-4 synthesis and promoted the secretion of IFN-γ.205 Cumulatively, these results indicate that tryptanthrin has potential anti-allergic activity and could be used to treat mast cell-mediated allergic diseases or atopic dermatitis-like skin lesions.
The uncommon U-shaped pyranoquinoline alkaloid euodenine A (235) (Fig. 32) from Euodia asteridula induced cytokine response via agonism of the human Toll-like receptor 4 (TLR4).206 Thus, it can likely modulate the Th2 immune response without causing lung damage. Structure-activity relationship studies on the cyclobutane ring led to 10-fold more potent analogs. In cytotoxicity testing toward peripheral blood mononuclear cells (PBMCs), 235 showed a small effect at 33 μM and clear evidence of toxicity at 100 μM.
Figure 32.
Chemical structures of compounds 235
L. Effect on Thioredoxin Reductase
Thioredoxin reductase (TrxR) is a potential target for new treatments for human diseases, such as cancer, AIDS, and autoimmune diseases.207 Since fumiquinazolines A–C were first reported in 1992,24a their structural novelty and potent pharmaceutical effects have attracted much attention from chemists and pharmacologists. In 2016, nine fumiquinazoline-type alkaloids, versiquinazolines A–I (236–244) (Fig. 33), along with cottoquinazolines B–D, were isolated from the gorgonian-derived fungus Aspergillus versicolor LZD-14-1.208 Compounds 236, 237, and 241, bearing a methanediamine or an aminomethanol unit, represent unique fumiquinazoline subtypes found for the first time. Compounds 236, 237, and 242 exhibited inhibitory activity against TrxR (IC50 12–20 μM). All compounds exhibited weak growth inhibitory activity against A549 and A2780 tumor cell lines (IC50 >50 μM).208
Figure 33.
Chemical structures of compounds 236–244
M. Antidiabetic Activity
In 2016, Selvaraj and co-workers isolated glycosin (245) (Fig. 34) from Rhizophora apiculata by bioassay guided fractionation and studied its antidiabetic effects and possible mechanism of action.209 In diabetic rats (induced by streptozotocin and nicotinamide) treated with 245, blood-glucose levels were significantly reduced and hepatic enzyme activities, as well as levels of urea and creatinine, were decreased. However, increases were seen in body weight, hemoglobin, high-density lipoprotein and insulin levels, and hexokinase activity when compared to untreated rats. Compound 245 interacted with dipeptidyl peptidase-IV, insulin receptor, protein tyrosine phosphatase 1B and PPARγ with good affinity and binding energy in a docking simulation. These results indicated that 245 could act as antihyperglycemic agent, associated with antihyperlipidemia, and possibility function as a ligand for proteins that are targets for antidiabetic drugs.209
Figure 34.
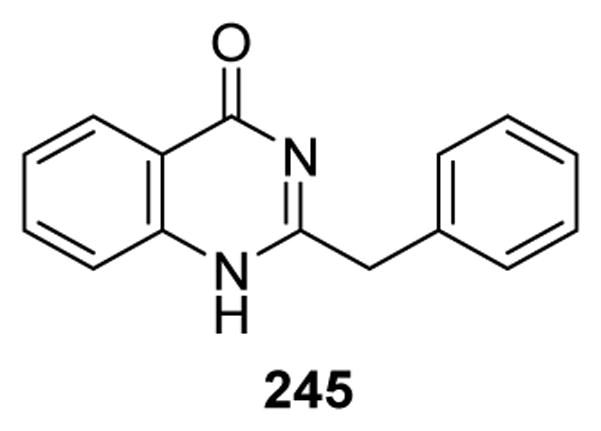
Chemical structures of compounds 245
Rutaecarpine also enhanced the blood lipid profile, mitigated inflammation, and improved kidney, liver, and pancreas pathology status of T2DM rats.210 When incubating 24 hours with this alkaloid (20–180 μmol/L), production of IL-1, IL-6 and TNF-α in cultured IR-PSMC cells induced by palmitic acid significant decreased dose-dependently. However, the alkaloid did not show cytotoxic effect towards the cultured primary skeletal muscle cells at concentrations up to 180 μmol/L. It also promoted glucose consumption and improved insulin resistance, possibly by suppressing inflammatory cytokines in insulin-resistant primary skeletal muscle cells.
N. Antinociceptive Activity
Anhydroevoxine and choisyine (246) (Fig. 35) from Choisya Aztec-Pearl, a hybrid of C. ternata and C. dumosa, were investigated for antinociceptive activity.211 At the doses of 3 and 10 mg/kg, these two furoquinoline alkaloids were two-fold more potent than morphine in treated animals using the hot plate model. Furthermore, mechanistic studies indicated that the opiate and nitric oxide (NO) system may play an important role in the antinociceptive activity. The cholinergic system may also be involved.211
Figure 35.
Chemical structures of compounds 246
O. Other Activities
The prescription of quinine is severely restricted in some countries, although serious adverse effects and fatality are rare. However, in many other countries, quinine is used to treat cramps from all causes. The Grading of Recommendations Assessment, Development and Evaluation system was applied to articles published during the period of 2010–2014 to assess the efficacy and safety of quinine-based treatment of muscle cramps.212 The study found low quality evidence that quinine (200 mg to 500 mg/daily) significantly reduced the total number of cramps and days experiencing cramps, and moderate quality evidence that quinine reduced the intensity of cramps. Moderate quality evidence showed that the use of quinine for up to 60 days did not lead to a significantly greater incidence of serious adverse events than use of placebo in the identified trials. Based on these results, quinine likely can be used safely to treat muscle cramps, but certainly, more evidence should be collected as further proof of safety.212
In 2014, Dhingra and Valecha reported that punaravine (247) (Fig. 36), a quinolin-4-one alkaloid isolated from Boerhaavia diffusa, showed significant antidepressant activity in unstressed and stressed mice in different models.213,214 When compound 247 was administered orally for 14 successive days, immobility periods and sucrose preference were significantly decreased in both stressed and unstressed mice. In addition, the alkaloid also significantly decreased monoamine oxidase activity and malondialdehyde levels, and significantly reversed the stress-induced decreases in reduced glutathione and catalase activity. The stress-induced increases in plasma nitrite and corticosterone levels also were significantly diminished. Evodiamine, a quinazoline alkaloid, also exerted antidepressant effects in rats as determined by reversal of various stress-associated behavior measures (decreased sucrose preference, numbers of crossing in an open-field test, increased immobility time in a forced swimming test) as well as biological measures (decreased 5-HT and Na+ levels).215
Figure 36.
Chemical structures of compounds 247–252
In 2015, known fumiquinazolines F and L (14, Fig. 3) as well as new fumiquinazoline S (248), isochaetominines A–C (249–251), and 14-epi-isochaetominine C (252) (Fig. 36) were isolated from a culture of a marine-derived Aspergillus sp. fungus; they exhibited weak inhibition against Na+/K+-ATPase (IC50 17, 20, 34, 78, 20, 38, and 57 μM, respectively).216 Aspergillus fumigatus Af293 is also a known source of fumiquinazoline alkaloids.217
Indoleamine 2,3-dioxygenase (IDO) is overexpressed in various diseases, including cancer,218 Alzheimer’s disease,219 age-related cataracts,220 and HIV encephalitis.221 Recent studies have suggested that IDO inhibition might enhance the efficacy of cancer treatment and concomitant administration of an IDO inhibitor might improve the efficacy of therapeutic vaccination or chemotherapy, thus, IDO has been highlighted as an attractive chemotherapeutic target.218,222–224 In 2012, three benzodiazepine alkaloids, benzomalvins B (253), C (254), and E (255), (Fig. 37) were isolated from Penicillium sp. FN070315.225 Compound 255 inhibited IDO activity in a dose-dependent manner. While 255 was less potent (IC50 21.4 μM) than mendione, the positive control IDO inhibitor (IC50 3.7 μM), it was more potent than 253 and 254 (IC50 126 and 130 μM, respectively).225
Figure 37.
Chemical structures of compounds 253–255
3. CONCLUSION
Quinoline and quinazoline alkaloids present a promising and expanding platform of active natural compounds and their potential has only been partially developed by both the academic community and the pharmaceutical industry to date. Although the discovery of quinine and camptothecin opened a new area for antimalarial and anticancer drug development, respectively, the identification of additional new compounds or significant biological activities will undoubtedly contribute continually to the development of future new drugs. During the period of 2009 to 2016, more than 200 compounds from these classes were newly found and most of them exhibited marked biological properties. The continued attention and long-lasting research on the isolation and identification of naturally occurring quinoline and quinazoline alkaloids will open the way to targeted pharmacological modelling and synthetic modifications, resulting in new and better drugs based on the original effects of these alkaloids.
Acknowledgments
Support was supplied by NIH grant CA177584 from the National Cancer Institute awarded to K.H. Lee., This work was supported financially by the National Natural Science Foundation of China (31371975, 21672092, 31302136), partial financial support was supplied by the Fundamental Research Funds for the Central Universities (lzujbky-2016-147). Youth Science Foundation of Gansu Province (1506RJYA144).
References
- 1.(a) Prajapati SM, Patel KD, Vekariya RH, Panchal SN, Patel HD. Recent advances in the synthesis of quinolines: a review. RSC Adv. 2014;4:24463–24476. [Google Scholar]; (b) Asif M. Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int J Med Chem. 2014;2014:395637. doi: 10.1155/2014/395637. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rajput R, Mishra AP. A review on biological activity of quinazolinones. Int J Pharm Pharm Sci. 2012;4:66–70. [Google Scholar]
- 2.Wiesner J, Ortmann R, Jomaa H, Schlitzer M. New antimalarial drugs. Angew Chem Int Ed. 2003;42:5274–5293. doi: 10.1002/anie.200200569. [DOI] [PubMed] [Google Scholar]
- 3.Manske RH. The chemistry of quinolines. Chem Rev. 1942;30:113–144. [Google Scholar]
- 4.Anjali PD, Singh D. Quinoline: a diverse therapeutic agent. Int J Pharm Sci Res. 2016;7:1–13. [Google Scholar]
- 5.(a) Afzal O, Kumar S, Haider MR, Ali MR, Kumar R, Jaggi M, Bawa S. A review on anticancer potential of bioactive heterocycle quinoline. Europ J Med Chem. 2015;97:871–910. doi: 10.1016/j.ejmech.2014.07.044. [DOI] [PubMed] [Google Scholar]; (b) Selvan TP, Kumar PV. Quinazoline marketed drugs – a review. Res Pharmacy. 2011;1:1–21. [Google Scholar]; (c) Tiwary BK, Pradhan KP, Nanda AK, Chakraborty R. Implication of quinazoline-4(3H)-ones in medicinal chemistry: a brief review. J Chem Biol Ther. 2015;1 article 104. [Google Scholar]; (d) Patel DB, Vekariyz RH, Patel KD, Projapati NP, Vasava MS, Patel HD. Recent advances in synthesis of quinoline-4-carboxylic acid and their biological evaluation: a review. J Chem Pharm Res. 2017;9:216–230. [Google Scholar]
- 6.Gallo RC, Whang-Peng J, Adamson RH. Studies on the antitumor activity, mechanism of action, and cell cycle effects of camptothecin. Natl J Cancer Inst. 1971;46:789–795. [PubMed] [Google Scholar]
- 7.Herben VM, ten Bokkel Huinink WW, Beijnen JH. Clinical pharmacokinetics of topotecan. Clin Pharmacokinet. 1996;31:85–102. doi: 10.2165/00003088-199631020-00001. [DOI] [PubMed] [Google Scholar]
- 8.Uma Shaanker R, Ramesha BT, Ravikanth G, Rajesh PG, Vsudeva R, Ganeshaiah KN. In: Chemical Profiling of Nothapodytes nimmoniana for Camptothecin, an Important Anticancer Alkaloid: Towards the Development of a Sustainable Production System. Ramawat KG, Merillon JM, editors. Springer-Verlag; Berlin and Heidelberg: 2008. pp. 198–210. [Google Scholar]
- 9.Hombe Gowda HC, Vasudeva R, Georgi PM, Uma Shaanker R, Ganeshaiah KN. Breeding types in Nothapodytes nimmoniana: an important medicinal tree. Curr Sci. 2002;83:1077–1078. [Google Scholar]
- 10.Ramesha BT, Suma HK, Senthilkumar U, Priti V, Ravikanth G, Vasudeva R, Santhosh Kumar TR, Ganeshaiah KN, Uma Shaanker R. New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa. India Phytomedicine. 2013;20:521–527. doi: 10.1016/j.phymed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Shweta S, Bukkambudhi RG, Gudasalamani R, Uma Shaanker R, Manchanahally BS. Endophytic fungi from Miquelia dentata Bedd. produce the anti-cancer alkaloid, camptothecine. Phytomedicine. 2013;20:337–342. doi: 10.1016/j.phymed.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Roja G, Bhavani S. Multiple shoot cultures of Ophiorrhiza rugosa var. decumbens Deb and Mondal-A viable renewable source for the continuous production of bioactive Camptotheca alkaloids apart from stems of the parent plant of Nothapodytes foetida (Wight) Sleumer. Phytomedicine. 2014;21:383–389. doi: 10.1016/j.phymed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Sleumer HO. Nothapodytes foetida (Wight) Sleumer. Notizbl Bot Gart Berlin Dahlem. 1940;15:247. [Google Scholar]
- 14.Shweta S, Hima Bindu J, Raghu J, Suma HK, Manjunatha BL, Mohana Kumara P, Ravikanth G, Nataraja KN, Ganeshaiah KN, Uma Shaanker R. Isolation of endophytic bacteria producing the anti-cancer alkaloid camptothecine from Miquelia dentata Bedd. (Icacinaceae) Phytomedicine. 2013;20:913–917. doi: 10.1016/j.phymed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Gan CY, Robinson WT, Etoh T, Hayashi M, Komiyama K, Kam TS. Leucophyllidine, a cytotoxic bisindole alkaloid constituted from the union of an Eburnan and a new vinylquinoline alkaloid. Org Lett. 2009;11:3962–3965. doi: 10.1021/ol9016172. [DOI] [PubMed] [Google Scholar]
- 16.Kam TS, Lim KH, Yoganathan K, Hayashi M, Komiyama K. Lundurines A-D, cytotoxic indole alkaloids incorporating a cyclopropyl moiety from Kopsia tenuis and revision of the structures of tenuisines A-C. Tetrahedron. 2004;60:10739–10745. [Google Scholar]
- 17.Fu YH, Di YT, He HP, Li SL, Zhang Y, Hao XJ. Angustifonines A and B, cytotoxic bisindole alkaloids from Bousigonia angustifolia. J Nat Prod. 2014;77:57–62. doi: 10.1021/np4005823. [DOI] [PubMed] [Google Scholar]
- 18.Cai XH, Li Y, Su J, Liu YP, Li XN, Luo XD. Novel indole and quinoline alkaloids from Melodinus yunnanensis. Nat Prod Bioprospect. 2011;1:25–28. [Google Scholar]
- 19.a) Jia S, Hu C. Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules. 2010;15:1873–1881. doi: 10.3390/molecules15031873. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang Y, Zhang QH, Wu LJ, Tashiro S, Onodera S, Ikejima T. Atypical apoptosis in L929 cells induced by evodiamine isolated from Evodia rutaecarpa. J Asian Nat Prod Res. 2004;6:19–27. doi: 10.1080/1028602031000119772. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Zhang YB, Yang XW. Indoloquinazoline alkaloids from Euodia rutaecarpa and their cytotoxic activities. J Asian Nat Prod Res. 2011;13:977–983. doi: 10.1080/10286020.2011.602015. [DOI] [PubMed] [Google Scholar]
- 21.Wansi JD, Tcho AT, Toze FAA, Nahar L, Martin C, Sarker SD. Cytotoxic acridone and indoloquinazoline alkaloids from Zanthoxylum poggei. Phytochemistry Lett. 2016;17:293–298. [Google Scholar]
- 22.Luo X, Pires D, Aínsa JA, Gracia B, Duarte N, Mulhovo S, Anes E, Ferreira MU. Zanthoxylum capense constituents with antimycobacterial activity against Mycobacterium tuberculosis in vitro and ex vivo within human macrophages. J Ethnopharmacol. 2013;146:417–422. doi: 10.1016/j.jep.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Ahn H, Nam KW, Kim KH, Mar W. Effects of rutaecarpine on hydrogen peroxide-induced apoptosis in murine Hepa-1c1c7 cells. Biomol Ther. 2012;20:487–491. doi: 10.4062/biomolther.2012.20.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Numata A, Takahashi C, Matsushita T, Miyamoto T, Kawai K, Usami Y, Matsumura E, Inoue M, Ohishi H, Shingu T. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992;33:1621–1624. [Google Scholar]; b) Takahashi C, Matsushita T, Doi M, Minoura K, Shingu T, Kumeda Y, Numata A. Fumiquinazolines A-G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J Chem Soc Perkin Trans 1. 1995:2345–2353. [Google Scholar]
- 25.Zhou Y, Debbab A, Mándi A, Wray V, Schulz B, Müller WEG, Kassack M, Lin WH, Kurtán T, Proksch P, Aly AH. Alkaloids from the sponge-associated fungus Aspergillus sp. Eur J Org Chem. 2013:894–906. [Google Scholar]
- 26.Shao CL, Xu RF, Wei MY, She ZG, Wang CY. Structure and absolute configuration of fumiquinazoline L, an alkaloid from a gorgonian-derived Scopulariopsis sp. fungus. J Nat Prod. 2013;76:779–782. doi: 10.1021/np4002042. [DOI] [PubMed] [Google Scholar]
- 27.Buttachon S, Chandrapatya A, Manoch L, Silva A, Gales L, Bruyère C, Kiss R, Kijjoa A. Sartorymensin, a new indole alkaloid, and new analogues of tryptoquivaline and fiscalins produced by Neosartorya siamensis (KUFC 6349) Tetrahedron. 2012;68:3253–3262. [Google Scholar]
- 28.Ge HM, Peng H, Guo ZK, Cui JT, Song YC, Tan RX. Bioactive alkaloids from the plant endophytic fungus Aspergillus terreus. Planta Med. 2010;76:822–824. doi: 10.1055/s-0029-1240726. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Wei X, Kim EL, Lin X, Yang XW, Zhou X, Yang B, Jung JH, Liu Y. New glucosidated pyrazinoquinazoline indole alkaloids from fungus Aspergillus fumigatus derived of a jellyfish. Tetrahedron. 2015;71:271–275. [Google Scholar]
- 30.Li YH, He J, Li Y, Wu XD, Peng LY, Du RN, Cheng X, Zhao QS, Li RT. Evollionines A-C, three new alkaloids isolated from the fruits of Evodia rutaecarpa. Helvetica Chimica Acta. 2014;97:1481–1486. [Google Scholar]
- 31.Gray AI. Quinoline alkaloids related to anthranilic acid, methods in plant biochemistry. Vol. 8. Academic Press; 1993. pp. 271–308. [Google Scholar]
- 32.Mester I. Structural diversity and distribution of alkaloids in the Rutales. In: Waterman PG, Grundon MF, editors. Chemistry and chemical taxanomy of the Rutales. Academic Press; London: 1983. [Google Scholar]
- 33.Hu J, Shi X, Chen J, Mao X, Zhu L, Yu L, Shi J. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014;148:437–444. doi: 10.1016/j.foodchem.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 34.Mansoor TA, Borralho PM, Luo X, Mulhovo S, Rodrigues CMP, Ferreira MJU. Apoptosis inducing activity of benzophenanthridine-type alkaloids and 2-arylbenzofuran neolignans in HCT116 colon carcinoma cells. Phytomedicine. 2013;20:923–929. doi: 10.1016/j.phymed.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Sun JB, Qu W, Guan FQ, Li LZ, Liang JY. A new quinoline alkaloid from the roots of Dictamnus angustifolius. Chinese J Nat Med. 2014;12:0222–0224. doi: 10.1016/S1875-5364(14)60037-6. [DOI] [PubMed] [Google Scholar]
- 36.Rasamison VE, Brodie PJ, Merino EF, Cassera MB, Ratsimbason MA, Rakotonandrasana S, Rakotondrafara A, Rafidinarivo E, Kingston DGI, Rakotondraibe HL. Furoquinoline alkaloids and methoxyflavones from the stem bark of Melicope madagascariensis (Baker) T.G. Hartley Nat Prod Bioprospect. 2016;6:261–265. doi: 10.1007/s13659-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammed MMD, Ibrahim NA, El-Sakhawy FS, Mohamed KM, Deabes DAH. Two new cytotoxic furoquinoline alkaloids isolated from Aegle marmelos (Linn.) Correa. Nat Prod Res. 2016;30:2559–2566. doi: 10.1080/14786419.2015.1126262. [DOI] [PubMed] [Google Scholar]
- 38.Varamini P, Javidnia K, Soltani M, Mehdipour AR, Ghaderi A. Cytotoxic activity and cell cycle analysis of quinoline alkaloids isolated from Haplophyllum canaliculatum Boiss. Planta Med. 2009;75:1509–1516. doi: 10.1055/s-0029-1185807. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Jiang N, Lv M, Tang B, Wang P, Liang J, Chen L. Anstifolines A and B, two dimeric furoquinoline alkaloids from the root bark of Dictamnus angustifolius. RSC Adv. 2016;6:22550–22554. [Google Scholar]
- 40.Abdel-Sattar E, Zaitoon A, El SAM, Bakhashwain AA. Evaluation of some medicinal plants in controlling Culex pipiens. J Egypt Soc Parasitol. 2014;44:771–778. doi: 10.12816/0007880. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852–1859. doi: 10.3390/molecules14051852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao N, Li ZL, Li DH, Sun YT, Shan DT, Bai J, Pei YH, Jing YK, Hua HM. Quinolone and indole alkaloids from the fruits of Euodia rutaecarpa and their cytotoxicity against two human cancer cell lines. Phytochemistry. 2015;109:133–139. doi: 10.1016/j.phytochem.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Zou J, Huang L. Minor constituents of qing dai, a traditional Chinese medicine. I. Isolation, structure determination, and synthesis of tryptanthrin and qingdainone. Yao Xue Xue Bao. 1985;20:45–51. [PubMed] [Google Scholar]
- 44.Kimoto T, Hino K, Koya-Miyata S, Yamamoto Y, Takeuchi M, Nishizaki Y, Micallef MJ, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Cell differentiation and apoptosis of monocytic and promyelocytic leukemia cells (U-937 and HL-60) by tryptanthrin, an active ingredient of Polygonum tinctorium Lour. Pathol Int. 2001;51:315–325. doi: 10.1046/j.1440-1827.2001.01204.x. [DOI] [PubMed] [Google Scholar]
- 45.Koya-Miyata S, Kimoto T, Micallef MJ, Hino K, Taniguchi M, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Prevention of azoxymethane-induced intestinal tumors by a crude ethyl acetate-extract and tryptanthrin extracted from Polygonum tinctorium Lour. Anticancer Res. 2001;21:3295–3300. [PubMed] [Google Scholar]
- 46.Motoki T, Takami Y, Yagi Y, Tai A, Yamamoto I, Gohda E. Inhibition of hepatocyte growth factor induction in human dermal fibroblasts by tryptanthrin. Biol Pharm Bull. 2005;28:260–266. doi: 10.1248/bpb.28.260. [DOI] [PubMed] [Google Scholar]
- 47.Chang CF, Hsu YL, Lee CY, Wu CH, Wu Y, Chuang TH. Isolation and cytotoxicity evaluation of the chemical constituents from Cephalantheropsis gracilis. Int J Mol Sci. 2015;16:3980–3989. doi: 10.3390/ijms16023980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miao S, Shi X, Zhang H, Wang S, Sun J, Hua W, Miao Q, Zhao Y, Zhang C. Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro. Int J Mol Sci. 2011;12:3831–3845. doi: 10.3390/ijms12063831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan HL, Yip HY, Mak NK, Leung KN. Modulatory effects and action mechanisms of tryptanthrin on murine myeloid leukemia cells. Cell Mol Immunol. 2009;6:335–342. doi: 10.1038/cmi.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao X, Leung KN. Tryptanthrin induces growth inhibition and neuronal differentiation in the human neuroblastoma LA-N-1 cells. Chem Biol Interact. 2013;203:512–521. doi: 10.1016/j.cbi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Liao X, Zhou X, Mak NK, Leung KN. Tryptanthrin inhibits angiogenesis by targeting the VEGFR2-mediated ERK1/2 signalling pathway. PLoS One. 2013;8:e82294. doi: 10.1371/journal.pone.0082294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang HN, Huang ST, Yeh YC, Wang HS, Wang TH, Wu YH, Pang JHS. Indigo naturalis and its component tryptanthrin exert anti-angiogenic effect by arresting cell cycle and inhibiting Akt and FAK signaling in human vascular endothelial cells. J Ethnopharmacol. 2015;174:474–481. doi: 10.1016/j.jep.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Zhang X, Ma G, Yan J, Wang H, Yang Q. Transport characteristics of tryptanthrin and its inhibitory effect on P-gp and MRP2 in Caco-2 cells. J Pharm Pharmaceut Sci. 2011;14:325–335. doi: 10.18433/j3501w. [DOI] [PubMed] [Google Scholar]
- 54.Yu ST, Chen TM, Chern JW, Tseng SY, Chen YH. Downregulation of GST expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through c-jun NH2-terminal kinase-mediated apoptosis. Anti-Cancer Drug. 2009;20:382–388. doi: 10.1097/CAD.0b013e32832a2cd4. [DOI] [PubMed] [Google Scholar]
- 55.Liu W, Qi Y, Liu L, Tang Y, Wei J, Zhou L. Suppression of tumor cell proliferation by quinine via the inhibition of the tumor necrosis factor receptor-associated factor 6-AKT interaction. Mol Med Rep. 2016;14:2171–2179. doi: 10.3892/mmr.2016.5492. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Zheng D, Li J, Han L, Cui X, Lang L, Li M, Wang Z, Zhao J, Huang X. Sannanine, a new cytotoxic alkaloid from Streptomyces sannanensis. J Antibiot. 2009;62:647–648. doi: 10.1038/ja.2009.95. [DOI] [PubMed] [Google Scholar]
- 57.Wang CH, Zeng H, Wang YH, Li C, Cheng J, Ye ZJ, He XJ. Antitumor quinazoline alkaloids from the seeds of Peganum harmala. J Asian Nat Prod Res. 2015;17:595–600. doi: 10.1080/10286020.2015.1042373. [DOI] [PubMed] [Google Scholar]
- 58.Vollmar D, Thorn A, Schuberth I, Grond S. A comprehensive view on 4-methyl-2- quinazolinamine, a new microbial alkaloid from Streptomyces of TCM plant origin. J Antibiot. 2009;62:439–444. doi: 10.1038/ja.2009.68. [DOI] [PubMed] [Google Scholar]
- 59.Liu B, Wang L, Chen G, Han C, Wang J. Isolation and crystal structure of marcanine A from Polyalthia plagioneura. Molecules. 2010;15:6349–6356. doi: 10.3390/molecules15096349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Chen GY, Han CR, Yuan Y, Yang B, Zhang Y, Wang J, Zhong XQ, Huang X. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro. Chem Pharm Bull. 2011;59:338–340. doi: 10.1248/cpb.59.338. [DOI] [PubMed] [Google Scholar]
- 61.Motegi M, Nugroho AE, Hirasawa Y, Arai T, Hadi AHA, Morita H. Leucomidines A-C, novel alkaloids from Leuconotis griffithii. Tetrahedron Lett. 2012;53:1227–1230. [Google Scholar]
- 62.Won KJ, Chung KS, Lee YS, Alia MS, Pervez MK, Fatima S, Choi JH, Lee KT. Haplophytin-A induces caspase-8-mediated apoptosis via the formation of death-inducing signaling complex in human promyelocytic leukemia HL-60 cells. Chem Biol Interact. 2010;188:505–511. doi: 10.1016/j.cbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Byler KG, Wang C, Setzer WN. Quinoline alkaloids as intercalative topoisomerase inhibitors. J Mol Model. 2009;15:1417–1426. doi: 10.1007/s00894-009-0501-6. [DOI] [PubMed] [Google Scholar]
- 64.Miyanaga A, Janso JE, McDonald L, He M, Liu H, Barbieri L, Eustáquio AS, Fielding EN, Carter GT, Jensen PR, Feng X, Leighton M, Koehn FE, Moore BS. Discovery and assembly-line biosynthesis of the lymphostin pyrroloquinoline alkaloid family of mTOR inhibitors in Salinispora bacteria. J Am Chem Soc. 2011;133:13311–13313. doi: 10.1021/ja205655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes CC, MacMillan JB, Gaudencio SP, Jensen PR, Fenical W. The ammosamides: structures of cell cycle modulators from a marine-derived Streptomyces species. Angew Chem Int Ed. 2009;48:725–727. doi: 10.1002/anie.200804890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes CC, MacMillan JB, Gaudencio SP, Fenical W, LaClair JJ. Ammosamides A and B target myosin. Angew Chem Int Ed. 2009;48:728–732. doi: 10.1002/anie.200804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan E, Jamison M, Yousufuddin M, MacMillan JB. Ammosamide D, an oxidatively ring opened ammosamide analog from a marine-derived Streptomyces variabilis. Org Lett. 2012;14:2390–2393. doi: 10.1021/ol300806e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao H, Zhang L, Zhu T, Gu Q, Li D. Unusual pyrrolyl 4-quinolinone alkaloids from the marine-derived fungus Penicillium sp. ghq208. Chem Pharm Bull. 2012;60:1458–1460. doi: 10.1248/cpb.c12-00487. [DOI] [PubMed] [Google Scholar]
- 69.Shao CL, Wang CY, Gu YC, Wei MY, Pan JH, Deng DS, She ZG, Lin YC. Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg Med Chem Lett. 2010;20:3284–3286. doi: 10.1016/j.bmcl.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 70.Chan STS, Patel PR, Ransom TR, Henrich CJ, McKee TC, Goey AKL, Cook KM, Figg WD, McMahon JB, Schnermann MJ, Gustafson KR. Structural elucidation and synthesis of eudistidine A: an unusual polycyclic marine alkaloid that blocks interaction of the protein binding domains of p300 and HIF-1α. J Am Chem Soc. 2015;137:5569–5575. doi: 10.1021/jacs.5b02156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q, Tang X, Luo X, de Voogd NJ, Li P, Li G. (+)- and (−)-Spiroreticulatine, a pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China sea sponge Fascaplysinopsis reticulata. Org Lett. 2015;17:3458–3461. doi: 10.1021/acs.orglett.5b01503. [DOI] [PubMed] [Google Scholar]
- 72.Grkovic T, Copp BR. New natural products in the discorhabdin A- and B-series from New Zealand-sourced Latrunculia spp. sponges. Tetrahedron. 2009;65:6335–6340. [Google Scholar]
- 73.Zhang J, Ding Y, Huang XJ, Jiang RW, Wang Y, Sun PH, Fan RZ, Zhang XQ, Ye WC. Melohemsines A-I, melodinus-type alkaloids from Melodinus hemsleyanus. RSC Adv. 2016;6:92218–92224. [Google Scholar]
- 74.Cheng GG, Zhang Y, Cai XH, Bao MF, Gu J, Li Y, Liu N, Liu YP, Luo XD. Cincholenines A and B, two unprecedented quinoline alkaloids from Cinchona ledgeriana. Tetrahedron Lett. 2013;54:4547–4550. [Google Scholar]
- 75.Nogueira CR, Lopes LMX. Antiplasmodial natural products. Molecules. 2011;16:2146–2190. [Google Scholar]
- 76.World Health Organization. World Malaria Report (WHO) Geneva: 2015. [Google Scholar]
- 77.Vogel G. Infectious disease-New map illustrates risk from the ‘other’ malaria. Science. 2010;329:618–618. doi: 10.1126/science.329.5992.618. [DOI] [PubMed] [Google Scholar]
- 78.Roberts L, Egan TJ, Joiner KA, Hoppe HC. Differential effects of quinoline antimalarials on endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:1840–1842. doi: 10.1128/AAC.01478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bilonda MK, Mammino L. Intramolecular hydrogen bonds in conformers of quinine and quinidine: an HF, MP2 and DFT study. Molecules. 2017;22:E245. doi: 10.3390/molecules22020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rijpma SR, van den Heuvel JJMW, van der Velden M, Sauerwein RW, Russel FGM, Koenderink JB. Atovaquone and quinine anti-malarials inhibit ATP binding cassette transporter activity. Malaria J. 2014;13:359. doi: 10.1186/1475-2875-13-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beckman ML, Pramod AB, Perley DL, Henry K. Stereoselective inhibition of serotonin transporters by antimalarial compounds. Neurochem Int. 2014;73:98–106. doi: 10.1016/j.neuint.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuca Suarez LE, Pattarroyo ME, Lozano JM, Delle Monache F. Biological activity of secondary metabolites from Peltostigma guatemalense. Nat Prod Res. 2009;23:370–374. doi: 10.1080/14786410802228439. [DOI] [PubMed] [Google Scholar]
- 83.Yang X, Feng Y, Duffy S, Avery VM, Camp D, Quinn RJ, Davis RA. A new quinoline epoxide from the Australian plant Drummondita calida. Planta Med. 2011;77:1644–1647. doi: 10.1055/s-0030-1270963. [DOI] [PubMed] [Google Scholar]
- 84.Wansi JD, Hussain H, Tcho AT, Kouam SF, Specht S, Sarite SR, Hoerauf A, Krohn K. Antiplasmodial activities of furoquinoline alkaloids from Teclea afzelii. Phytother Res. 2010;24:775–777. doi: 10.1002/ptr.2894. [DOI] [PubMed] [Google Scholar]
- 85.Onguéné PA, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part 1: a pharmacological evaluation of alkaloids and terpenoids. Malaria J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.a) Gakunju DMN, Mberu EK, Dossaji SF, Gray AI, Waigh RD, Waterman PG, Watkins WM. Potent antimalarial activity of the alkaloid nitidine, isolated from a Kenyan herbal remedy. Antimicrob Agents Chemother. 1995;39:2606–2609. doi: 10.1128/aac.39.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bouquet J, Rivaud M, Chevalley S, Deharo E, Jullian V, Valentin A. Biological activities of nitidine, a potential anti-malarial lead compound. Malaria J. 2012;11:67. doi: 10.1186/1475-2875-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waffo AFK, Coombes PH, Crouch NR, Mulholland DA, El Amin SMM, Smith PJ. Acridone and furoquinoline alkaloids from Teclea gerrardii (Rutaceae: Toddalioideae) of southern Africa. Phytochemistry. 2007;68:663–667. doi: 10.1016/j.phytochem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Carroll AR, Duffy S, Avery VM. Aplidiopsamine A, an antiplasmodial alkaloid from the temperate Australian ascidian, Aplidiopsis confluata. J Org Chem. 2010;75:8291–8294. doi: 10.1021/jo101695v. [DOI] [PubMed] [Google Scholar]
- 89.Lam CFC, Norrie Pearce A, Tan SH, Kaiser M, Copp BR. Discovery and evaluation of thiazinoquinones as anti-protozoal agents. Mar Drug. 2013;11:3472–3499. doi: 10.3390/md11093472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Supong K, Thawai C, Supothina S, Auncharoen P, Pittayakhajonwut P. Antimicrobial and anti-oxidant activities of quinoline alkaloids from Pseudomonas aeruginosa BCC76810. Phytochemistry Lett. 2016;17:100–106. [Google Scholar]
- 91.WHO. Chagas Disease. who.int/topics/chagas_disease/en/(2014.03.03)
- 92.Coura JR, De Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 93.Garcia S, Ramos CO, Senra JFV, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-dos-Santos R, Soares MBP. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother. 2005;49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cretton S, Breant L, Pourrez L, Ambuehl C, Marcourt L, Ebrahimi SN, Hamburger M, Perozzo R, Karimou, Kaiser M, Cuendet M, Christen P. Antitrypanosomal quinoline alkaloids from the roots of Waltheria indica. J Nat Prod. 2014;77:2304–2311. doi: 10.1021/np5006554. [DOI] [PubMed] [Google Scholar]
- 95.Cretton S, Bréant L, Pourrez L, Ambuehl C, Perozzo R, Marcourt L, Kaiser M, Cuendet M, Christen P. Chemical constituents from Waltheria indica exert in vitro activity against Trypanosoma brucei and T. cruzi. Fitoterapia. 2015;105:55–60. doi: 10.1016/j.fitote.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 96.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 97.Jean-Robert I. Natural products for neglected diseases: a review. Curr Org Chem. 2008;12:643. [Google Scholar]
- 98.Cruz I, Nieto J, Moreno J, Canavate C, Desjeux P, Alvar J. Leishmania/HIV co-infections in the second decade. Indian J Med Res. 2006;123:357–88. [PubMed] [Google Scholar]
- 99.Mishra BB, Kale RR, Singh RK, Tiwari VK. Alkaloids: future prospective to combat leishmaniasis. Fitoterapia. 2009;80:81–90. doi: 10.1016/j.fitote.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 100.Hussain H, Al-Harrasi A, Al-Rawahi A, Green IR, Gibbons S. Fruitful decade for antileishmanial compounds from 2002 to Late 2011. Chem Rev. 2014;114:10369–10428. doi: 10.1021/cr400552x. [DOI] [PubMed] [Google Scholar]
- 101.Wright CW, Phillipson JD. Natural products and the development of selective antiprotozoal drugs. Phyto Res. 1990;4:127–139. [Google Scholar]
- 102.Ferreira ME, de Arias AR, Yaluff G, de Bilbao NV, Nakayama H, Torres S, Schinini A, Guy I, Heinzen H, Fournet A. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine. 2010;17:375–378. doi: 10.1016/j.phymed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Coy Barrera CA, Coy Barrera ED, Granados Falla DS, Delgado Murcia G, Luis Cuca Suarez E. seco-Limonoids and quinoline alkaloids from Raputia heptaphylla and their antileishmanial activity. Chem Pharm Bull. 2011;59:855–859. doi: 10.1248/cpb.59.855. [DOI] [PubMed] [Google Scholar]
- 104.Luo XM, Qi SH, Yin H, Gao CH, Zhang S. Alkaloids from the stem bark of Micromelum falcatum. Chem Pharm Bull. 2009;57:600–602. doi: 10.1248/cpb.57.600. [DOI] [PubMed] [Google Scholar]
- 105.An CY, Li XM, Luo H, Li CS, Wang MH, Xu GM, Wang BG. 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus isolated from the mangrove plant Rhizophora stylosa. J Nat Prod. 2013;76:1896–1901. doi: 10.1021/np4004646. [DOI] [PubMed] [Google Scholar]
- 106.Yang H, Li F, Ji N. Alkaloids from an algicolous strain of Talaromyces sp. Chinese J Oceanol Limnol. 2016;34:367–371. [Google Scholar]
- 107.Abe M, Imai T, Ishii N, Usui M, Okuda T, Oki T. Quinolactacidea new quinolone insecticide from Penicillium. Biosci Biotechnol Biochem. 2005;69:1202–1205. doi: 10.1271/bbb.69.1202. [DOI] [PubMed] [Google Scholar]
- 108.Kadri D, Crater AK, Lee H, Solomon VR, Ananvoranich St. The potential of quinoline derivatives for the treatment of Toxoplasma gondii infection. Exp Parasitol. 2014;145:135–144. doi: 10.1016/j.exppara.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Jeon JH, Kim MG, Lee HS. Insecticidal activities of Ruta chalepensis leaves isolated constituent and structure-relationships of its analogues against Sitophilus oryzae. J Korean Soc Appl Biol Chem. 2013;56:591–596. [Google Scholar]
- 110.Shang X, Guo X, Li B, Pan H, Zhang J, Zhang Y, Miao X. Microwave-assisted extraction of three bioactive alkaloids from Peganum harmala L. and their acaricidal activity against Psoroptes cuniculi in vitro. J Ethnopharmacol. 2016;192:350–361. doi: 10.1016/j.jep.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 111.Cushnie TP, Cushnie B, Lamb AJ. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. 2014;44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 112.Mitscher LA. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev. 2005;105:559–592. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- 113.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 114.Laponogov I, Sohi MK, Veselkov DA, Pan XS, Sawhney R, Thompson AW, McAuley KE, Fisher LM, Sanderson MR. Structural insight into the quinolone-DNA cleavage complex of type IIA topoisomerases. Nature Struct Biol. 2009;16:667–669. doi: 10.1038/nsmb.1604. [DOI] [PubMed] [Google Scholar]
- 115.Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J Ethnopharmacol. 2009;123:494–498. doi: 10.1016/j.jep.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 116.Cabral RS, Queiroz EF, Wolfender JL. Targeted isolation of indolopyridoquinazoline alkaloids from Conchocarpus fontanesianus based on molecular networks. J Nat Prod. 2016;79:2270–2278. doi: 10.1021/acs.jnatprod.6b00379. [DOI] [PubMed] [Google Scholar]
- 117.O’Donnell F, Smyth TJP, Ramachandran VN, Smyth WF. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int J Antimicrob Agents. 2010;35:30–38. doi: 10.1016/j.ijantimicag.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 118.Sripisut T, Phakhodee W, Ritthiwigrom T, Cheenpracha S, Prawat U, Deachathai S, Machan T, Laphookhieo S. Alkaloids from Glycosmis cochinchinensis twigs. Phytochemistry Lett. 2013;6:337–339. [Google Scholar]
- 119.Nachtigall J, Schneider K, Nicholson G, Goodfellow M, Zinecker H, Imhoff JF, Süssmuth RD, Fiedler HP. Two new aurachins from Rhodococcus sp. Acta 2259. J Antibiot. 2010;63:567–569. doi: 10.1038/ja.2010.79. [DOI] [PubMed] [Google Scholar]
- 120.Tian S, Yang Y, Liu K, Xiong Z, Xu L, Zhao L. Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Nat Prod Res. 2014;28:344–346. doi: 10.1080/14786419.2013.858341. [DOI] [PubMed] [Google Scholar]
- 121.Soares AR, Engene N, Gunasekera SP, Sneed JM, Paul VJ. Carriebowlinol an antimicrobial tetrahydroquinolinol from an assemblage of marine cyanobacteria containing a novel taxon. J Nat Prod. 2015;78:534–538. doi: 10.1021/np500598x. [DOI] [PubMed] [Google Scholar]
- 122.Sugiyama R, Nishimura S, Ozaki T, Asamizu S, Onaka H, Kakeya H. 5-Alkyl-1,2,3,4-tetrahydroquinolines, new membrane-interacting lipophilic metabolites produced by combined culture of Streptomyces nigrescens and Tsukamurella pulmonis. Org Lett. 2015;17:1918–1921. doi: 10.1021/acs.orglett.5b00607. [DOI] [PubMed] [Google Scholar]
- 123.Cretton S, Dorsaz S, Azzollini A, Favre-Godal Q, Marcourt L, Ebrahimi SN, Voinesco F, Michellod E, Sanglard D, Gindro K, Wolfender JL, Cuendet M, Christen P. Antifungal quinoline alkaloids from Waltheria indica. J Nat Prod. 2016;79:300–307. doi: 10.1021/acs.jnatprod.5b00896. [DOI] [PubMed] [Google Scholar]
- 124.Bontemps N, Bry D, López-Legentil S, Simon-Levert A, Long C, Banaigs B. Structures and antimicrobial activities of pyridoacridine alkaloids isolated from different chromotypes of the ascidian Cystodytes dellechiajei. J Nat Prod. 2010;73:1044–1048. doi: 10.1021/np900751k. [DOI] [PubMed] [Google Scholar]
- 125.Costa EV, da Cruz PEO, de Lourenço CC, de Souza Moraes VR, de Lima Nogueira PC, Salvador MJ. Antioxidant and antimicrobial activities of aporphinoids and other alkaloids from the bark of Annona salzmannii A. DC. (Annonaceae) Nat Prod Res. 2013;27:1002–1006. doi: 10.1080/14786419.2012.688044. [DOI] [PubMed] [Google Scholar]
- 126.Costa DC, Azevedo MM, Silva DO, Romanos MT, Souto-Padrón TC, Alviano CS, Alviano DS. In vitro anti-MRSA activity of Couroupita guianensis extract and its component tryptanthrin. Nat Prod Res. 2017;31:2077–2080. doi: 10.1080/14786419.2016.1272110. [DOI] [PubMed] [Google Scholar]
- 127.Tripathi A, Wadia N, Bindal D, Jana T. Docking studies on novel alkaloid tryptanthrin and its analogues against enoyl-acyl carrier protein reductase (InhA) of Mycobacterium tuberculosis. Indian J Biochem Biophys. 2012;49:435–441. [PubMed] [Google Scholar]
- 128.Shahwar D, Raza MA, Tariq S, Riasat M, Ajaib M. Enzyme inhibition, antioxidant and antibacterial potential of vasicine isolated from Adhatoda vasica Nees. Pak J Pharm Sci. 2012;25(3):651–656. [PubMed] [Google Scholar]
- 129.Ignacimuthu S, Shanmugam N. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness. leaves. J Biosci. 2010;35:565–570. doi: 10.1007/s12038-010-0065-8. [DOI] [PubMed] [Google Scholar]
- 130.Jha DK, Panda L, Lavanya P, Ramaiah S, Anbarasu A. Detection and confirmation of alkaloids in leaves of Justicia adhatoda and bioinformatics approach to elicit its anti-tuberculosis activity. Appl Biochem Biotechnol. 2012;168:980–990. doi: 10.1007/s12010-012-9834-1. [DOI] [PubMed] [Google Scholar]
- 131.Zhuang Y, Teng X, Wang Y, Liu P, Li G, Zhu W. New quinazolinone alkaloids within rare amino acid residue from coral-associated fungus, Aspergillus versicolor LCJ-5–4. Org Lett. 2011;13:1130–1133. doi: 10.1021/ol103164n. [DOI] [PubMed] [Google Scholar]
- 132.Fremlin LJ, Piggott AM, Lacey E, Capon RJ. Cottoquinazoline A and Cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J Nat Prod. 2009;72:666–670. doi: 10.1021/np800777f. [DOI] [PubMed] [Google Scholar]
- 133.Xia X, Luo JG, Liu RH, Yang MH, Kong LY. New alkaloids from the leaves of Evodia rutaecarpa. Nat Prod Res. 2016;30:2154–2159. doi: 10.1080/14786419.2016.1146888. [DOI] [PubMed] [Google Scholar]
- 134.Sheu JR, Hung WC, Wu CH, Lee YM, Yen MH. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br J Haematol. 2000;110:110–115. doi: 10.1046/j.1365-2141.2000.01953.x. [DOI] [PubMed] [Google Scholar]
- 135.Kobayashi Y, Hoshikuma K, Nakano Y, Yokoo Y, Kamiya T. The positive inotropic and chronotropic effects of evodiamine and rutaecarpine, indoloquinazoline alkaloids isolated from the fruits of Evodia rutaecarpa, on the guinea-pig isolated right atria: Possible involvement of vanilloid receptors. Planta Med. 2001;67:244–248. doi: 10.1055/s-2001-12008. [DOI] [PubMed] [Google Scholar]
- 136.Moon TC, Murakami M, Kudo I, Son KH, Kim HP, Kang SS, Chang HW. A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa. Inflamm Res. 1999;48:621–625. doi: 10.1007/s000110050512. [DOI] [PubMed] [Google Scholar]
- 137.Peng WJ, Liu Y, Yu YR, Fu YQ, Zhao Y, Kuang HB, Huang QR, He M, Luo D. Rutaecarpine prevented dysfunction of endothelial gap junction induced by Ox-LDL via activation of TRPV1. Eur J Pharmacol. 2015;756:8–14. doi: 10.1016/j.ejphar.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Fu YQ, Peng WJ, Yu YR, Wu YS, Yan H, Huang QR, He M, Luo D. Rutaecarpine reverses the altered connexin expression pattern induced by oxidized low-density lipoprotein in monocytes. J Cardiovasc Pharmacol. 2016;67:519–525. doi: 10.1097/FJC.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 139.Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X, Zhu N, Liu J, Wu Y, Li Y, Li N, Feng T, Lai F, Zhang M, Hong B, Jiang JD, Si S. Rutaecarpine suppresses atherosclerosis in ApoE/mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res. 2014;55:1634–1647. doi: 10.1194/jlr.M044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heo SK, Yun HJ, Yi HS, Noh EK, Park SD. Evodiamine and rutaecarpine inhibit migration by LIGHT via suppression of NADPH oxidase activation. J Cell Biochem. 2009;107:123–133. doi: 10.1002/jcb.22109. [DOI] [PubMed] [Google Scholar]
- 141.Bobik A, Kalinina N. Tumor necrosis factor receptor and ligand superfamily family members TNFRSF14 and LIGHT: New players in human atherogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1873–1875. [PubMed] [Google Scholar]
- 142.Yu PL, Chao HL, Wang SW, Wang PS. Effects of evodiamine and rutaecarpine on the secretion of corticosterone by zona fasciculata-reticularis cells. J Cell Biochem. 2009;108:469–475. doi: 10.1002/jcb.22276. [DOI] [PubMed] [Google Scholar]
- 143.Li D, Zhang XJ, Chen L, Yang Z, Deng HW, Peng J, Li YJ. Calcitonin gene-related peptide mediates the cardioprotective effects of rutaecarpine against ischaemia-reperfusion injury in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2009;36:662–667. doi: 10.1111/j.1440-1681.2008.05136.x. [DOI] [PubMed] [Google Scholar]
- 144.Li WQ, Li XH, Du J, Zhang W, Li D, Xiong XM, Li YJ. Rutaecarpine attenuates hypoxia-induced right ventricular remodeling in rats. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:757–767. doi: 10.1007/s00210-016-1240-8. [DOI] [PubMed] [Google Scholar]
- 145.Li XW, Wang XM, Li S, Yang JR. Effects of rutaecarpine on right ventricular remodeling in rats with monocrotaline-induced pulmonary hypertension. Zhongguo Yingyong Shengli Xue Zazhi. 2014;30:405–410. [PubMed] [Google Scholar]
- 146.Bao MH, Dai W, Li YJ, Hu CP. Rutaecarpine prevents hypoxia-reoxygenation induced myocardial cell apoptosis via inhibition of NADPH oxidases. Can J Physiol Pharmacol. 2011;89:177–186. doi: 10.1139/Y11-006. [DOI] [PubMed] [Google Scholar]
- 147.Qin XP, Zeng SY, Tian HH, Deng SX, Ren JF, Zheng YB, Li D, Li YJ, Liao DF, Chen SY. Involvement of prolylcarboxypeptidase in the effect of rutaecarpine on the regression of mesenteric artery hypertrophy in renovascular hypertensive rats. Clin Exp Pharmacol Physiol. 2009;36:319–324. doi: 10.1111/j.1440-1681.2008.05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu J, Tan GS, Deng PY, Xu KP, Hu CP, Li YJ. Involvement of CGRP in the inhibitory effect of rutaecarpine on vasoconstriction induced by anaphylaxis in guinea pig. Regul Pept. 2005;125:93–97. doi: 10.1016/j.regpep.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 149.Ivanova B, Spiteller M. Evodiamine and rutaecarpine alkaloids as highly selective transient receptor potential vanilloid 1 agonists. Int J Biol Macromol. 2014;65:314–324. doi: 10.1016/j.ijbiomac.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 150.Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716:61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Pearce LV, Petukhov PA, Szabo T, Kedei N, Bizik F, Kozikowski AP, Blumberg PM. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org Biomol Chem. 2004;2:2281–2286. doi: 10.1039/B404506H. [DOI] [PubMed] [Google Scholar]
- 152.Kobayashi Y, Hoshikuma K, Nakano Y, Yokoo Y, Kamiya T. The positive inotropic and chronotropic effects of evodiamine and rutaecarpine, indoloquinazoline alkaloids isolated from the fruits of Evodia rutaecarpa, on the guinea-pig isolated right atria: possible involvement of vanilloid receptors. Planta Med. 2001;67:244–248. doi: 10.1055/s-2001-12008. [DOI] [PubMed] [Google Scholar]
- 153.Kobayashi Y, Nakano Y, Hoshikuma K, Yokoo Y, Kamiya T. The bronchoconstrictive action of evodiamine, an indoloquinazoline alkaloid isolated from the fruits of Evodia rutaecarpa, on guinea-pig isolated bronchus: possible involvement on vanilloid receptors. Planta Med. 2000;66:526–530. doi: 10.1055/s-2000-8615. [DOI] [PubMed] [Google Scholar]
- 154.Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 2001;67:628–633. doi: 10.1055/s-2001-17353. [DOI] [PubMed] [Google Scholar]
- 155.Wang S, Yamamoto S, Kogure Y, Zhang W, Noguchi K, Dai Y. Partial activation and inhibition of TRPV1 channels by evodiamine and rutaecarpine, two major components of the fruits of Evodia rutaecarpa. J Nat Prod. 2016;79:1225–1230. doi: 10.1021/acs.jnatprod.5b00599. [DOI] [PubMed] [Google Scholar]
- 156.Yan C, Zhang J, Wang S, Xue G, Hou Y. Neuroprotective effects of rutaecarpine on cerebral ischemia reperfusion injury. Neural Regen Res. 2013;8:2030–2038. doi: 10.3969/j.issn.1673-5374.2013.22.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nie XQ, Chen HH, Zhang JY, Zhang YJ, Yang JW, Pan HJ, Song WX, Murad F, He YQ, Bian K. Rutaecarpine ameliorates hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and AMPK/ACC2 signaling pathways. Acta Pharmacol Sin. 2016;37:483–496. doi: 10.1038/aps.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yan M, Fan P, Shi Y, Feng L, Wang J, Zhan G, Li B. Stereoselective blockage of quinidine and quinine in the hERG channel and the effect of their rescue potency on drug-induced hERG trafficking defect. Int J Mol Sci. 2016;17:E1648. doi: 10.3390/ijms17101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ning X, He J, Shi X, Yang G. Regulation of adipogenesis by quinine through the ERK/S6 pathway. Int J Mol Sci. 2016;17:504. doi: 10.3390/ijms17040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun JB, Qu W, Wang P, Wu FH, Wang LY, Liang JY. Degraded limonoids and quinoline alkaloids from Dictamnus angustifolius G. Don ex Sweet. and their anti-platelet aggregation activity. Fitoterapia. 2013;90:209–213. doi: 10.1016/j.fitote.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 161.Lee W, Lee J, Kulkarni R, Kim MA, Hwang JS, Na MK, Bae JS. Antithrombotic and antiplatelet activities of small-molecule alkaloids from Scolopendra subspinipes mutilans. Sci Rep. 2016;6:21956. doi: 10.1038/srep21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Numata A, Takahashi C, Matsushita T, Miyamoto T, Kawai K, Usami Y, Matsumura E, Inoue M, Ohishi H, Shingu T. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992;33:1621–1624. [Google Scholar]
- 163.Yu G, Zhou G, Zhu M, Wang W, Zhu T, Gu Q, Li D. Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Org Lett. 2016;18:244–247. doi: 10.1021/acs.orglett.5b02964. [DOI] [PubMed] [Google Scholar]
- 164.Peng J, Lin T, Wang W, Xin Z, Zhu T, Gu Q, Li D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J Nat Prod. 2013;76:1133–1140. doi: 10.1021/np400200k. [DOI] [PubMed] [Google Scholar]
- 165.McCormick JL, McKee TC, Cardellina JH, II, Boyd MR. HIV inhibitory natural products. 26. Quinoline alkaloids from Euodia roxburghiana. J Nat Prod. 1996;59:469–471. doi: 10.1021/np960250m. [DOI] [PubMed] [Google Scholar]
- 166.Ahmed N, Brahmbhatt KG, Sabde S, Mitra D, Singh IP, Bhutani KK. Synthesis and anti-HIV activity of alkylated quinoline 2,4-diols. Bioorgan Med Chem. 2010;18:2872–2879. doi: 10.1016/j.bmc.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 167.Li SF, Di YT, He HP, Zhang Y, Wang YH, Yin JL, Tan CJ, Li SL, Hao XJ. Trigonoines A and B, two novel alkaloids from the leaves of Trigonostemon lii. Tetrahedron Lett. 2011;52:3186–3188. [Google Scholar]
- 168.Jadulco RC, Pond CD, Van Wagoner RM, Koch M, Gideon OG, Matainaho TK, Piskaut P, Barrows LR. 4-Quinolone alkaloids from Melochia odorata. J Nat Prod. 2014;77:183–187. doi: 10.1021/np400847t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wahyuni TS, Widyawaruyanti A, Lusida MI, Fuad A, Soetjipto, Fuchino H, Kawahara N, Hayashi Y, Aoki C, Hotta H. Inhibition of hepatitis C virus replication by chalepin and pseudane IX isolated from Ruta angustifolia leaves. Fitoterapia. 2014;99:276–283. doi: 10.1016/j.fitote.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 170.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 171.Yoon JS, Jeong EJ, Yang H, Kim SH, Hyun S, Sung YC, Kim YC. Inhibitory alkaloids from Dictamnus dasycarpus root barks on lipopolysaccharide-induced nitric oxide production in BV2 cells. J Enzyme Inhib Med Chem. 2012;27:490–494. doi: 10.3109/14756366.2011.598151. [DOI] [PubMed] [Google Scholar]
- 172.Ratheesh M, Sindhu G, Helen A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm Res. 2013;62:367–376. doi: 10.1007/s00011-013-0588-1. [DOI] [PubMed] [Google Scholar]
- 173.Wang TY, Wu JB, Hwang TL, Kuo YH, Chen JJ. A new quinolone and other constituents from the fruits of Tetradium ruticarpum: effects on neutrophil pro-inflammatory responses. Chem Biodiv. 2010;7:1828–1834. doi: 10.1002/cbdv.200900289. [DOI] [PubMed] [Google Scholar]
- 174.Li W, Yang SY, Yan XT, Sun YN, Song SB, Kang HK, Kim YH. NF-κB inhibitory activities of glycosides and alkaloids from Zanthoxylum schinifolium stems. Chem Pharm Bull. 2014;62:196–202. doi: 10.1248/cpb.c13-00759. [DOI] [PubMed] [Google Scholar]
- 175.Liu J, Li CJ, Ni L, Yang JZ, Li L, Zang CX, Bao XQ, Zhang D, Zhang DM. Anti-inflammatory alkaloid glycoside and quinoline alkaloid derivates from the stems of Clausena lansium. RSC Adv. 2015;5:80553–80560. [Google Scholar]
- 176.Deguchi J, Shoji T, Nugroho AE, Hirasawa Y, Hosoya T, Shirota O, Awang K, Hadi AHA, Morita H. Eucophylline, a tetracyclic vinylquinoline alkaloid from Leuconotis eugenifolius. J Nat Prod. 2010;73:1727–1729. doi: 10.1021/np100458b. [DOI] [PubMed] [Google Scholar]
- 177.Cai XH, Shang JH, Feng T, Luo XD. Novel alkaloids from Alstonia scholaris. Z Naturforsch B Chem Sci. 2010;65:1164–1168. [Google Scholar]
- 178.Danz H, Stoyanova S, Thomet OAR, Simon HU, Dannhardt G, Ulbrich H, Hamburger M. Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Med. 2002;68:875–880. doi: 10.1055/s-2002-34922. [DOI] [PubMed] [Google Scholar]
- 179.Pergola C, Jazzar B, Rossi A, Northoff H, Hamburger M, Sautebin L, Werz O. On the inhibition of 5-lipoxygenase product formation by tryptanthrin: mechanistic studies and efficacy in vivo. Br J Pharmacol. 2012;165:765–776. doi: 10.1111/j.1476-5381.2011.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Danz H, Stoyanova S, Wippich P, Brattström A, Hamburger M. Identification and isolation of the cyclooxygenase-2 inhibitory principle in Isatis tinctoria. Planta Med. 2001;67:411–416. doi: 10.1055/s-2001-15805. [DOI] [PubMed] [Google Scholar]
- 181.Ishihara T, Kohno K, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Tryptanthrin inhibits nitric oxide and prostaglandin E2 synthesis by murine macrophages. Eur J Pharmacol. 2000;407:197–204. doi: 10.1016/s0014-2999(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 182.Jähne EA, Eigenmann DE, Sampath C, Butterweck V, Culot M, Cecchelli R, Gosselet F, Walter FR, Deli MA, Smieško M, Hamburger M, Oufir M. Pharmacokinetics and in vitro blood-brain barrier screening of the plant-derived alkaloid tryptanthrin. Planta Med. 2016;82:1021–1029. doi: 10.1055/s-0042-105295. [DOI] [PubMed] [Google Scholar]
- 183.Yang ZD, Zhang Db, Ren J, Yang MJ. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Med Chem Res. 2012;21:722–725. [Google Scholar]
- 184.Cardoso-Lopes EM, Maier JA, da Silva MR, Regasini LO, Simote SY, Lopes NP, Pirani JR, da Silva BV, Young MCM. Alkaloids from stems of Esenbeckia leiocarpa Engl. (Rutaceae) as potential treatment for alzheimer disease. Molecules. 2010;15:9205–9213. doi: 10.3390/molecules15129205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sichaem J, Jirasirichote A, Sapasuntikul K, Khumkratok S, Sawasdee P, Do TML, Tip-pyang S. New furoquinoline alkaloids from the leaves of Evodia lepta. Fitoterapia. 2014;92:270–273. doi: 10.1016/j.fitote.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 186.Sichaem J, Rojpitikul T, Sawasdee P, Lugsannangarm K, Santi TP. Furoquinoline alkaloids from the leaves of Evodia lepta as potential cholinesterase inhibitors and their molecular docking. Nat Prod Commun. 2015;10:1359–1362. [PubMed] [Google Scholar]
- 187.Xu J, Sun X, Liu X, Peng M, Li S, Jin DQ, Lee D, Bartlam M, Guo Y. Phytochemical constituents from Melicope pteleifolia that promote neurite outgrowth in PC12 cells. J Funct Food. 2016;23:565–572. [Google Scholar]
- 188.Lima MMC, López JA, David JM, Silva EP, Giulietti AM, de Queiroz LP, David JP. Acetylcholinesterase activity of alkaloids from the leaves of Waltheria brachypetala. Planta Med. 2009;75:335–337. doi: 10.1055/s-0028-1112215. [DOI] [PubMed] [Google Scholar]
- 189.Yang Y, Cheng X, Liu W, Chou G, Wang Z, Wang C. Potent AChE and BChE inhibitors isolated from seeds of Peganum harmala Linn by a bioassay-guided fractionation. J Ethnopharmacol. 2015;168:279–286. doi: 10.1016/j.jep.2015.03.070. [DOI] [PubMed] [Google Scholar]
- 190.Liu W, Shi X, Yang Y, Cheng X, Liu Q, Han H, Yang B, He C, Wang Y, Jiang B, Wang Z, Wang Cg. In vitro and in vivo metabolism and inhibitory activities of vasicine, a potent acetylcholinesterase and butyrylcholinesterase inhibitor. PLoS One. 2015;10:e0122366. doi: 10.1371/journal.pone.0122366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zheng XY, Zhang ZJ, Chou GX, Wu T, Cheng XM, Wang CH, Wang ZT. Acetylcholinesterase inhibitive activity-guided isolation of two new alkaloids from seeds of Peganum nigellastrum Bunge by an in vitro TLC-bioautographic assay. Arch Pharm Res. 2009;32:1245–1251. doi: 10.1007/s12272-009-1910-x. [DOI] [PubMed] [Google Scholar]
- 192.Yang YD, Cheng XM, Liu W, Han ZZ, Chou GX, Wang Y, Sun DX, Wang ZT, Wang CH. Peganumines B-I and two enantiomers: new alkaloids from the seeds of Peganum harmala Linn. and their potential cytotoxicity and cholinesterase inhibitory activities. RSC Adv. 2016;6:15976–15987. [Google Scholar]
- 193.Kiplimo JJ, Islam MS, Koorbanally NA. A novel flavonoid and furoquinoline alkaloids from Vepris glomerata and their antioxidant activity. Nat Prod Commun. 2011;6:1847–50. [PubMed] [Google Scholar]
- 194.Popov M, Osipov AN, Korepanova EA, Krivoshapko ON, Shtoda YP, Klimovich AA. Investigation of the antioxidant and membranotropic activity of the quinozaline alkaloid triptantrin in different model systems. Biophysics. 2015;60:574–580. [PubMed] [Google Scholar]
- 195.Popov AM, Osipov AN, Korepanova EA, Krivoshapko ON, Shtoda YP, Klimovich AA. Study of antioxidant and membranotropic activities of quinazoline alkaloid tryptanthrin using different model systems. Biofizika. 2015;60:700–707. [PubMed] [Google Scholar]
- 196.Chen M, Gan L, Lin S, Wang X, Li L, Li Y, Zhu C, Wang Y, Jiang B, Jiang J, Yang Y, Shi J. Alkaloids from the root of Isatis indigotica. J Nat Prod. 2012;75:1167–1176. doi: 10.1021/np3002833. [DOI] [PubMed] [Google Scholar]
- 197.Moon SY, Lee JH, Choi HY, Cho IJ, Kim SC, Kim YW. Tryptanthrin protects hepatocytes against oxidative stress via activation of the extracellular signal-regulated kinase/NF-E2-related factor 2 pathway. Biol Pharm Bull. 2014;37:1633–1640. doi: 10.1248/bpb.b14-00363. [DOI] [PubMed] [Google Scholar]
- 198.Zhang FL, He X, Zhai YR, He LN, Zhang SC, Wang LL, Yang AH, An LJ. Mechanism-based inhibition of CYPs and RMs-induced hepatoxicity by rutaecarpine. Xenobiotica. 2015;45:978–89. doi: 10.3109/00498254.2015.1038742. [DOI] [PubMed] [Google Scholar]
- 199.Haynes SW, Gao X, Tang Y, Walsh CT. Assembly of asperlicin peptidyl alkaloids from anthranilate and tryptophan: a two-enzyme pathway generates heptacyclic scaffold complexity in asperlicin E. J Am Chem Soc. 2012;134:17444–17447. doi: 10.1021/ja308371z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee CH, Lee HS. Relaxant effect of quinoline derivatives on histamine-induced contraction of the isolated guinea pig trachea. J Korean Soc Appl Biol Chem. 2011;54:118–123. [Google Scholar]
- 201.Liu W, Wang Y, He DD, Li SP, Zhu YD, Jiang B, Cheng XM, Wang ZT, Wang CH. Antitussive, expectorant, and bronchodilating effects of quinazoline alkaloids (±)-vasicine, deoxyvasicine, and (±)-vasicinone from aerial parts of Peganum harmala L. Phytomedicine. 2015;22:1088–1095. doi: 10.1016/j.phymed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 202.Iwaki K, Ohashi E, Arai N, Kohno K, Ushio S, Taniguchi M, Fukuda S. Tryptanthrin inhibits Th2 development, and IgE-mediated degranulation and IL-4 production by rat basophilic leukemia RBL-2H3 cells. J Ethnopharmacol. 2011;134:450–459. doi: 10.1016/j.jep.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 203.Han NR, Kim HM, Jeong HJ. Tryptanthrin reduces mast cell proliferation promoted by TSLP through modulation of MDM2 and p53. Biomed Pharmacoth. 2016;79:71–77. doi: 10.1016/j.biopha.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 204.Han NR, Moon PD, Kim HM, Jeong HJ. Tryptanthrin ameliorates atopic dermatitis through down-regulation of TSLP. Arch Biochem Biophys. 2014;542:14–20. doi: 10.1016/j.abb.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 205.Tong M, Guo YN, Zhang GY. Effect and mechanisms of rutaecarpine on treating atopic dermatitis in mice. Sichuan Daxue Xuebao Yixue Ban. 2011;42:234–236. 263. [PubMed] [Google Scholar]
- 206.Neve JE, Wijesekera HP, Duffy S, Jenkins ID, Ripper JA, Teague SJ, Campitelli M, Garavelas A, Nikolakopoulos G, Le PV, de A, Leone P, Pham NB, Shelton P, Fraser N, Carroll AR, Avery VM, McCrae C, Williams N, Quinn RJ. Euodenine A: a small-molecule agonist of human TLR4. J Med Chem. 2014;57:1252–1275. doi: 10.1021/jm401321v. [DOI] [PubMed] [Google Scholar]
- 207.Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- 208.Cheng Z, Lou L, Liu D, Li X, Proksch P, Yin S, Lin W. Versiquinazolines A-K, fumiquinazoline-type alkaloids from the gorgonian-derived fungus Aspergillus versicolor LZD-14-1. J Nat Prod. 2016;79:2941–2952. doi: 10.1021/acs.jnatprod.6b00801. [DOI] [PubMed] [Google Scholar]
- 209.Selvaraj G, Kaliamurthi S, Thirugnasambandan R. Effect of glycosin alkaloid from Rhizophora apiculata in non-insulin dependent diabetic rats and its mechanism of action: in vivo and in silico studies. Phytomedicine. 2016;23:632–640. doi: 10.1016/j.phymed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 210.Yang JW, Nie XQ, Shi HX, Zhang YJ, Zhang JY, Yuan Y, Bian K. Effects of rutaecarpine on inflammatory cytokines in insulin resistant primary skeletal muscle cells. Zhongguo Zhongyao Zazhi. 2014;39:2930–2935. [PubMed] [Google Scholar]
- 211.de Carvalho PR, Ropero DR, Pinheiro MMa, Fernandes PD, Boylan F. Quinoline alkaloids isolated from Choisya Aztec-Pearl and their contribution to the overall antinociceptive activity of this plant. PLoS One. 2016;11:e0164998. doi: 10.1371/journal.pone.0164998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.El-Tawil S, Al Musa T, Valli H, Lunn MP, Brassington R, El-Tawil T, Weber M. Quinine for muscle cramps. Cochrane Database Syst Rev. 2015;5:CD005044. doi: 10.1002/14651858.CD005044.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dhingra D, Valecha R. Punarivine, an alkaloid isolated from ethanolic extract of Boerhaavia diffusa Linn, reverses depression-like behaviour in mice subject to chronic unpredictable mild stress. Indian J Exp Biol. 2014;52:799–807. [PubMed] [Google Scholar]
- 214.Perviz S, Khan H, Pervaiz A. Plant alkaloids as an emerging therapeutic alternative for the treatment of depression. Front Pharmacol. 2016;7:28. doi: 10.3389/fphar.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang ML, Zhang ZX, Li YZ, Wang XH, Yan W, Gong GQ. Antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats. Neurosci Lett. 2015;588:154–158. doi: 10.1016/j.neulet.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 216.Liao L, You M, Chung BK, Oh DC, Oh KB, Shin J. Alkaloidal metabolites from a marine-derived Aspergillus sp. fungus. J Nat Prod. 2015;78:349–354. doi: 10.1021/np500683u. [DOI] [PubMed] [Google Scholar]
- 217.Ames BD, Liu X, Walsh CT. Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochem. 2010;49:8564–8576. doi: 10.1021/bi1012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde B. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 219.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 220.Fujigaki Y, Sakakima M, Sun Y, Goto T, Ohashi N, Fukasawa H, Tsuji T, Yamamoto T, Hishida A. Immunohistochemical study on caveolin-1alpha in regenerating process of tubular cells in gentamicin-induced acute tubular injury in rats. Virchows Arch. 2007;450:671–681. doi: 10.1007/s00428-007-0417-4. [DOI] [PubMed] [Google Scholar]
- 221.Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Ialolawa O, Munn DH, Gendelman HE, Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–329. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 223.Muller AJ, Malachowski WP, Prendergast GC. Indoleamine 2,3-dioxygenase in cancer: targeting pathological immune tolerance with small-molecule inhibitors. Exp Opin Ther Targets. 2005;9:831–849. doi: 10.1517/14728222.9.4.831. [DOI] [PubMed] [Google Scholar]
- 224.Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7:31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- 225.Jang JP, Jang JH, Soung NK, Kim HM, Jeong SJ, Asami Y, Shin KS, Kim MR, Oh H, Kim BY, Ahn JS. Benzomalvin E, an indoleamine 2,3-dioxygenase inhibitor isolated from Penicillium sp. FN070315. J Antibiot. 2012;65:215–217. doi: 10.1038/ja.2011.141. [DOI] [PubMed] [Google Scholar]