Abstract
The rate at which dietary α-linolenate (ALA) is desaturated and elongated to its longer chain n-3 PUFA in humans is not agreed upon. In this study, we applied a methodology developed in rodents to investigate whole body, presumably hepatic, synthesis-secretion rates of esterified n-3 PUFA from circulating unesterified ALA in two healthy overweight women after ten weeks of low linoleate diet exposure. During continuous iv infusion of d5-ALA, 17 arterial blood samples were collected from each subject at −10, 0, 10, 20, 40, 60, 80, 100, 120, 150, 180, 210 min, and at 4, 5, 6, 7, and 8 h after beginning infusion. Plasma esterified d5-n-3 PUFA concentrations were plotted against infusion time and fit to a sigmoidal curve using nonlinear regression. These curves were used to estimate kinetic parameters using a kinetic analysis developed in rodents. Calculated synthesis-secretion rates of esterified eicosapentaenoate (EPA), n-3 docosapentaenoate, docosahexaenoic acid (DHA), tetracosapentaenate, and tetracosahexaenoate from circulating unesterified ALA were 2.1 and 2.7; 1.7 and 5.3; 0.47 and 0.27; 0.30 and 0.30; 0.32 and 0.27 mg/day for subjects S01 and S02, respectively. This study provides new estimates of whole body synthesis-secretion rates of esterified longer chain n-3 PUFA from circulating unesterified ALA in human subjects. This method now can be extended to study factors that regulate human whole-body PUFA synthesis-secretion in health and disease.
Keywords: kinetics, omega-3 fatty acid, docosapentaenoic acid, tetracosahexaenoic acid, synthesis, liver, low linoleate diet, mass spectrometry
INTRODUCTION
Docosahexaenoic acid (DHA, 22:6n-3) is the most concentrated omega-3 (n-3) PUFA in the human brain, and accounts for 20% of total fatty acids in ethanolamine glycerophosphatides of brain gray matter (O’Brien & Sampson 1965). DHA and its metabolites regulate many important physiological functions in brain and other tissues, and is considered anti-inflammatory (Brenna & Carlson 2014, Salem 1989, Serhan et al. 2015). Mammals cannot synthesize n-3 PUFA de novo, but can produce DHA and other n-3, longer chain, highly unsaturated PUFA (HUFA) by desaturation and elongation of α-linolenic acid (ALA, 18:3n-3), a nutritionally essential C18 n-3 PUFA (Crawford & Sinclair 1972, Tinoco et al. 1979). Here, HUFA is defined as PUFA with 20 or more carbons, and three or more double bonds (Bibus & Lands 2015). Omega-3 HUFA derived by desaturation and elongation of ALA include eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acid (DPAn-3, 22:5n-3), DHA, tetracosapentaenoic acid (TPAn-3, 24:5n-3), and tetracosahexaenoic acid (THA, 24:6n-3). EPA, although not as concentrated as DHA in brain, is taken up from plasma into the brain at similar rates as DHA (Ouellet et al. 2009) and has been reported to be beneficial in treating depression (Hallahan et al. 2016), highlighting the importance of n-3 HUFA for neurological function.
The body’s capacity for desaturation and elongation of dietary ALA to EPA and DHA has previously been reported to be low in humans when studied with stable isotope tracer techniques with single oral administration of deuterated or 13C-U-labeled PUFA (Brenna 2002, Burdge 2006, Brenna et al. 2009, Lin et al. 2010). Studies that orally administered tracers in human subjects found that most adminitered 13C-U-ALA was β-oxidized or lost, and that only a limited amount was converted to longer-chain n-3 PUFA (Vermunt et al. 2000, McCloy et al. 2004, Pawlosky et al. 2001). Following its oral administration in humans, Pawlosky et al. estimated (Pawlosky et al. 2001) that net conversion of d5-ALA was 0.2%, 0.13% and 0.05% of the dose for d5-EPA, d5-DPAn-3, and d5-DHA, respectively. Using compartmental modeling, rates of mass flow for EPA, DPA, and DHA from each immediate precursor were estimated to be 0.021, 0.075, and 0.464 μmol/day, respectively.
However, studies involving oral administration of labeled ALA are limited in their ability to directly measure hepatic synthesis-secretion rates of n-3 HUFA due to the unique absorption pathway of fatty acids (Domenichiello et al. 2015). To overcome this limitation, Igarashi et al. (Igarashi et al. 2006) developed a method and kinetic analysis in unanesthetized rats that involves continuous intravenous infusion of labeled ALA, measuring arterial blood levels and/or liver levels of labeled and unlabeled ALA and its desaturated/elongated n-3 HUFA products, and then applying a non-linear kinetic analysis to calculate whole body (largely liver) PUFA synthesis-secretion rates and other kinetic parameters. Domenichiello and Bazinet et al elaborated and widely applied this approach. Using this method, Domenichiello et al (Domenichiello et al. 2014, Domenichiello et al. 2016, Metherel et al. 2018) estimated whole body synthesis-secretion rates of DHA from circulating ALA to be 1.5 and 0.045 μmol/day in rats consuming a high ALA or a low ALA containing diet, respectively.
Compared to oral administration of stable isotopic ALA, the continuous iv infusion method provides direct rates of hepatic synthesis-secretion of n-3 HUFA, and measures HUFA derived from circulating unesterified ALA thus by-passing the loss of tracer in gastrointestinal tract via oral administration. Considering the advantage of the continuous iv infusion method, and conflict over whether ALA alone can provide sufficient EPA and DHA to maintain optimal organ function in human (Saunders et al. 2013, Brenna 2002, Barcelo-Coblijn & Murphy 2009), the translation of this infusion method developed in unanesthetized rats to the clinic is of great importance. Accordingly, in this pilot study we developed parameters for continuous iv infusion of d5-ALA-albumin in two overweight women consuming a well-defined diet, to quantify hepatic synthesis-secretion rates of EPA, DPAn-3, DHA, TPAn-3, and THA from circulating unesterified ALA. Labeled PUFA appearance vs. infusion time data were fit by sigmoidal curves, and kinetic parameters were estimated as described for the rat (Domenichiello et al. 2014, Domenichiello et al. 2016).
The protocol was approved for twenty-four subjects. However, potential contamination of the infusate that was prepared in the NIH Clinical Center Pharmacy (Bethesda, MD) (McCarthy 2015) allowed us to complete studies on only two subjects on the same diet. Nevertheless, comparable results on the two subjects that presented in this paper show for the first time that PUFA synthesis-secretion rates from circulating unesterified ALA can be measured in humans, and provide initial estimates and procedures for future reference.
MATERIALS & METHODS
Clinical protocol
This is a substudy of an ongoing clinical protocol (NIAAA Protocol #11-AA-0028) that was approved by the Institutional Review Board of the National Institution of Health Addictions. Its National Clinical Trial identifier is NCT01251887 (ClinicalTrial.gov, accessed June 11, 2018) under the study title as “Dietary Essential Fatty Acid regulation of Omega-3 HUFA Metabolism; Satiety and Body Composition”. In the main protocol, healthy overweight premenopausal women volunteers, aged 18–50 yr with body mass index (BMI) of 25–35 kg/m2, were recruited. Two were chosen and placed on the low linoleate diet for 10 weeks. Each was infused intravenously at a constant rate with d5-ALA-albumin over a period of eight hours. The infusion study was unforeseeably discontinued because the NIH Pharmacy, which was charged with preparing sterile infusate, was closed because of unexpected contamination, and the remaining infusate was discarded (McCarthy 2015).
The two subjects who completed the substudy gave written informed consent and were reimbursed for their participation. Subject S01was aged 28 yr, body weight (BW) 98.2 kg, with BMI 29.9; subject S02 was aged 31 yr, BW 75.8 kg, with BMI 26.5. Each consumed a low-linoleate diet (Table 1) containing one percent of energy (en%) of total calories as linoleic acid (LA), 0.5 en% ALA, 0.04 en% from EPA+DHA and 12.3 en% of monounsaturated and 9.9 en% of the saturated fatty acids for at least 10 weeks prior to testing. Participants were admitted to the NIH Clinical Center Metabolic Unit (Bethesda, MD) at 18:00 the day prior to infusion. After overnight fasting, an antecubital intravenous line was inserted in one arm at 06:30 for infusion and maintaining iv fluids. The infusion started at 08:00 and lasted for 8 hours. A clear plexiglas hand-heating box, custom-made by the Department of Chemistry of the University of Vermont (Burlington, VT), was applied to the other arm which was catheterized for removal of arterial blood. Deuterated ALA-albumin infusate was infused into the first arm at a rate of 0.78 mg/kg BW/hr with an Alaris PC 8015 infusion pump (CareFusion, San Diego, CA), equal to 24 mL/min for subject S01 and 18 mL/min for S02. A total of seventeen 3.5 ml blood samples from the arm contralateral to infusion were collected from each subject. One sample was taken 10 min prior to initiation of infusion to verify instrumental baseline. Remaining samples were taken at 0, 10, 20, 40, 60, 80, 100, 120, 150, 180, 210 min, and 4, 5, 6, 7, 8 h after beginning infusion. Both subjects tolerated the infusion without complaint, side effects or an adverse event.
Table 1.
Significant fatty acids in the study diet
| Fatty acids | 18:2n-6 | 18:3n-3 | 20:4n-6 | EPA+DHA 1 | Mono-unsaturated | Saturated |
|---|---|---|---|---|---|---|
| Fatty acids expressed in percent of total daily energy (en%) 2 | ||||||
|
|
||||||
| 1 | 0.5 | 0.04 | 0.04 | 12.3 | 9.9 | |
|
|
||||||
| Fatty acids expressed in weight % of total fatty acids (wt%) 3 | ||||||
|
|
||||||
| 3 | 1.7 | 0.13 | 0.13 | 41 | 33 | |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. Daily intake of EPA+DHA is equivalent to 100 mg.
Total daily fat on the diets was 30 percent of energy consumed.
A 2000 kilocalorie diet was employed for the calculation of fatty acid composition and amount in the diet. Thus, one percent of energy is equivalent to 2.2 g of fatty acids.
Blood samples were drawn into a 6 mL EDTA-tube at each time point. Plasma was separated shortly after collection by centrifugation at 1,750 g × 15 min at 4 °C in an Allegra X-12R Centrifuge (Beckman Coulter, Brea, CA), frozen over dry ice and stored at −80 °C until chemical analysis.
Chemicals and other materials
Human serum albumin (25%, USP) was purchased from Grifols Therapeutics (Research Triangle Park, NC); methanol and chloroform were purchased from Burdick & Jackson (Muskegon, MI); hexane from EMD Chemicals (Gibbstown, NJ); butylated hydroxytoluene (BHT) from Acros (Geel, Belgium); boron trifluoride (BF3) in methanol (14% w/w, 0.9 g/L) was from Sigma Chemicals (St. Louis, MO); 23:0 free fatty acid (23:0 FFA), docosatrienoic acid (22:3n-3 FFA), and the GC reference standard GLC–569B containing fatty acid methyl ester (FAME) were purchased from Nu–Chek Prep (Elysian, MN). All chemicals were of analytical grade and used without further purification.
Stable isotope and preparation of infusate
Deuterium-labeled ALA (d5–17, 17, 18, 18, 18-18:3n-3) potassium salt was custom-prepared by Cambridge Isotope Laboratories (Andover, MA), Lot No. PR-24610. Isotope purity was greater than 98%, chemical purity greater than 95%. The preparation of cGMP terminally sterilized infusate was modified according to the report by Wolfe et al (Wolfe et al. 1980, Wolfe 1992) and was formulated through binding with human serum albumin in the NIH Clinical Center Pharmacy Department. Briefly, a weighed amount of d5-18:3n-3 potassium salt was dissolved in water-for-injection at 50 °C in a water bath prior to gradually mixing with 25% human albumin solution and then passed through a Millipore filter. The final concentration of infusate, d5-ALA-albumin solution, was 10 μmol (equivalent to 3.22 mg) of d5-18:3n-3 per mL of sterile solution (PDS Lot No. 136208). Details are described in Diagram 1.
Diagram 1.
Preparation of D5-linolenic acid albumin solution (3.22 mg/mL)
Chemical analysis
Plasma total lipids were extracted by chloroform: methanol (2:1) according to the method of Folch (Folch et al. 1957). Briefly, 200 μl of human plasma was mixed with 1 mL of methanol containing 50 μg of butylated hydroxytoluene and then extracted twice with 2 mL of chloroform. Two internal standards were added to each sample prior to lipid extraction, 0.05 μg of 22:3n-3 for gas chromatography/ mass spectrometry (GC/MS) assay and 5 μg of 23:0 for gas chromatography/ flame ionization detection (GC/FID) assay, respectively. The total lipid extract was dried and hydrolyzed with 1 mL 5% KOH in methanol and derivatized with pentafluorobenzyl bromide and diisopropylamine in acetonitrile (1:10:1000, v/v/v) to form fatty acid pentafluorobenzyl (PFB) esters. A GC/MS negative chemical ionization (NCI) assay was used to quantify deuterated PUFA and unlabeled minor HUFA in total lipids (Pawlosky et al. 1992, Lin et al. 2005). Fatty acid PFB esters were then transmethylated by 14% of BF3 in methanol for unlabeled fatty acids. Resulting FAME were detected and quantified using GC/FID (Salem et al. 1996, Lin et al. 2012).
Unesterified fatty acids were isolated by thin-layer chromatography (TLC) (Domenichiello et al. 2014) from another portion of total lipids extracted from 300 μl of plasma and prepared into PFB esters for quantification of deuterated ALA as describe previously. The same two internal standards as above were added prior to lipid extraction followed TLC separation.
GC/FID and GC/MS
An Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA) coupled to a flame ionization detector was employed to quantify unlabeled fatty acids. An aliquot of FAME from each sample was injected onto a DB-FFAP fused silica capillary column (Agilent 127-32H2; 15 m × 0.1 mm i.d. × 0.1 μm) through a split/splitless inlet (50:1). The programmed oven temperature and other GC conditions were as previously reported (Lin et al. 2012). GC/MS NCI assay was carried out on an Agilent 6890 GC-5975 inert XL Mass Selective Detector in the negative chemical ionization mode on to a DB-FFAP fused column (30 m × 0.25 mm i.d. × 0.25 μm) and the oven was programmed from 125 °C to 245 °C at 8 °C/min. The M-PFB anion of each analyte was monitored (Lin et al. 2011).
Kinetic analysis
Concentrations of deuterated and unlabeled fatty acids (nmol/mL) in plasma samples were quantified by comparing integrated areas of each fatty acid peak in the gas chromatograms with areas of a known amount of corresponding internal standard added to the samples prior to total lipid extraction.
For this kinetic analysis, the plasma input function (into the liver or any other organ that synthesizes and secretes esterified ALA or its elongated products) is plasma steady-state concentration of unesterified labeled ALA, [d5-ALA]un measured throughout the infusion. Infusing d5-ALA at a constant rate will cause it to achieve a steady-state concentration rapidly, and to remain as such throughout the infusion. Within the liver, [d5-ALA]un passes through multiple compartments in which it is desaturated and/or elongated (Igarashi et al. 2006). HUFA that are synthesized from d5-ALA are released back into blood, esterified within very low density lipoproteins.
To represent these kinetics, appearance data for the total amount of esterified d5-HUFAi,es (Vplasma × [d5-HUFAi]) vs. time were fit to a Boltzmann sigmoidal curve using nonlinear regression (GraphPad Prism 6.07, CA) (Domenichiello et al. 2014, Domenichiello et al. 2016), where total plasma volume (Vplasma) is 3,000 mL per 70 kg BW (Davies & Morris 1993). The esterified concentration data can be represented by Equation 1, where C*i,es is defined as Vplasma*[d5-HUFA]i,es (nmol) for the particular HUFA of interest (i) in the esterified plasma fraction (es), Cmax is the peak value (top), Cmin is the minimum value (bottom) of fitting curve, t is time (min), t50 is time when C is halfway between Cmax and Cmin.
| (Eq. 1) |
This sigmoidal curve describes the appearance of an esterified labeled HUFA i in plasma during unesterified d5-ALA infusion, and includes a delay followed by rapid rise followed by an approach to a steady-state, when the rate of synthesis-secretion approximates the rate of loss from plasma. The first derivative of the curve described by Eq. 1, defined as S (nmol/min) at any time for a HUFA of interest equals the net rate of change of that labeled HUFA in plasma. This rate equals the synthesis-secretion rate of the esterified HUFA from its precursor, minus its rate of loss from plasma,
| (Eq. 2) |
where k1,i (mL/min) is the synthesis-secretion coefficient for esterified HUFAi; [d5-ALA]un (nmol/mL) is the plasma concentration of the infused unesterified d5-ALA; k-1,i (mL/min) is the disappearance coefficient for esterified HUFAi, and [d5-HUFA]i,es (nmol/mL) is the plasma esterified concentration of a d5-labeled HUFAi, that was synthesized from the infused tracer.
The first derivative for the appearance curve (Eq. 2) was determined for each esterified PUFAi as a function of time, using curve fitting. The peak first derivative is at the inflection point of the plot of C*i,es vs. time, and is defined as Smax,i (nmol/min). As Smax,i ignores uptake of synthesized labeled unesterified HUFA from blood at the time, it is less than the true rate of syntheisis, k1,I [d5-ALA]un, as described by the inequality of Eq.3.
| (Eq. 3) |
Rearrangement gives an estimate of k1,i, where the approximation represents the inequality.
| (Eq. 4) |
Multiplying k1,i by the plasma concentration of unesterified unlabeled ALA, [ALA]un, gives the synthesis-secretion rate (Jsyn,est nmol/min) of esterified PUFA i,
| (Eq. 5) |
Plasma turnover (Fi, min−1) and half-life (t1/2,i, min) of esterified HUFA i then can be estimated as follows.
| (Eq. 6) |
| (Eq. 7) |
RESULTS
Plasma fatty acid concentrations in total lipids
Table 2 summarizes plasma unlabeled fatty acid concentration profiles for the two subjects during the infusion period, presented as mean (± SD) nmol per mL of plasma for 17 samples from each subject. The similar plasma concentrations of 18:1n-9 (896 vs. 1,011 nmol/mL) and of 18:2n-6 (1,166 vs 1,340 nmol/mL), respectively, support the fact that both subjects were on the same diet. Only trace amounts of n-3 HUFAs were found in the free fatty acid fraction.
Table 2.
Fatty acid concentrations in human plasma total lipids (nmol/mL)
| Fatty acid | S01 | S02 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| MEAN | SD | MEAN | SD | |||
| 14:0 | 43.9 | ± | 4.7 | 36.8 | ± | 3.5 |
| 16:0 | 1265 | ± | 111 | 1152 | ± | 95 |
| 18:0 | 367 | ± | 36 | 374 | ± | 23 |
| 20:0 | 15.8 | ± | 3.3 | 8.7 | ± | 1.8 |
| 22:0 | 30.5 | ± | 6.8 | 17.4 | ± | 3.7 |
| 24:0 | 21.5 | ± | 4.9 | 15.2 | ± | 3.0 |
|
| ||||||
| 16:1n-7 | 153 | ± | 15 | 91.9 | ± | 6.0 |
| 18:1n-9 | 896 | ± | 93 | 1011 | ± | 106 |
| 18:1n-7 | 98.0 | ± | 7.3 | 104 | ± | 10.4 |
| 20:1n-9 | 7.8 | ± | 0.6 | 9.3 | ± | 1.0 |
| 24:1n-9 | 48.1 | ± | 10.3 | 21.8 | ± | 4.7 |
|
| ||||||
| 18:2n-6 | 1166 | ± | 122 | 1340 | ± | 112 |
| 18:3n-6 | 15.3 | ± | 2.0 | 12.3 | ± | 1.3 |
| 20:2n-6 | 8.6 | ± | 3.1 | 13.6 | ± | 1.1 |
| 20:3n-6 | 98.2 | ± | 3.8 | 79.1 | ± | 2.7 |
| 20:4n-6 | 492 | ± | 33 | 268 | ± | 10.9 |
| 22:4n-6 | 9.6 | ± | 0.5 | 8.5 | ± | 0.5 |
| 22:5n-6 | 16.8 | ± | 4.2 | 8.0 | ± | 0.9 |
|
| ||||||
| 18:3n-3 | 24.3 | ± | 2.1 | 33.5 | ± | 2.4 |
| 20:5n-3 | 16.5 | ± | 1.3 | 14.2 | ± | 0.8 |
| 22:5n-3 | 10.9 | ± | 3.1 | 20.3 | ± | 1.0 |
| 22:6n-3 | 99.0 | ± | 6.0 | 51.2 | ± | 2.6 |
| 24:5n-3 | 2.1 | ± | 0.1 | 2.0 | ± | 0.1 |
| 24:6n-3 | 2.8 | ± | 0.2 | 1.5 | ± | 0.1 |
|
| ||||||
| SUMMARY | ||||||
|
| ||||||
| AA/20:3n-6* | 5.0 | ± | 0.2 | 3.4 | ± | 0.1 |
|
| ||||||
| DPAn-3/EPA* | 0.7 | ± | 0.1 | 1.4 | ± | 0.1 |
|
| ||||||
| DHA/DPAn-3* | 9.4 | ± | 1.2 | 2.5 | ± | 0.1 |
|
| ||||||
| Saturates | 1744 | ± | 154 | 1604 | ± | 123 |
| MUFA | 1203 | ± | 122 | 1238 | ± | 126 |
| n-6 PUFA | 1807 | ± | 159 | 1729 | ± | 126 |
| n-3 PUFA | 617 | ± | 38 | 363 | ± | 13.9 |
| n-6 HUFA | 156 | ± | 11 | 123 | ± | 5.9 |
| n-3 HUFA | 131 | ± | 9.4 | 89 | ± | 4.3 |
Data are mean ± SD for samples collected at 17 time points in each subject.
indicates the ratio of fatty acid concentrations.
Concentrations of deuterated n-3 HUFA
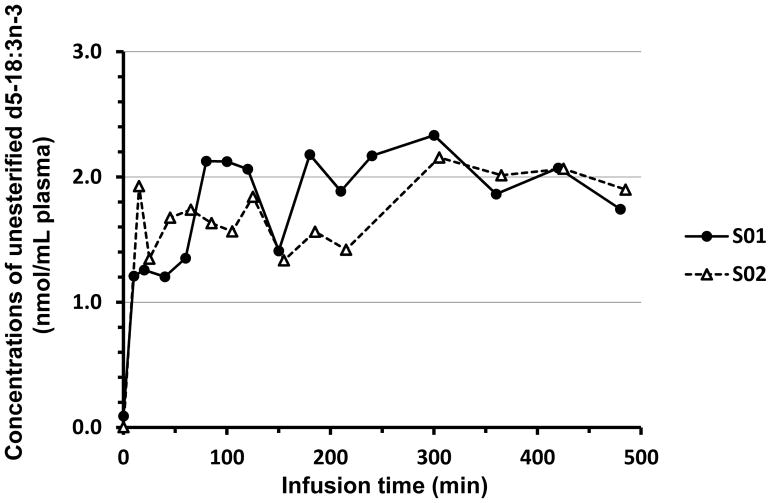
For each subject, plasma concentrations of unesterified d5-18:3n-3 are presented as a function of infusion time in Figure 1. The concentrations reached steady state about 10 min after infusion began, and averaged 1.2 and 1.7 nmol/mL for S01 and S02, respectively. Steady-state levels were maintained until the end of infusion at 8 h.
Figure 1.
Time-courses of experimental plasma concentrations (nmol/mL) of unesterified d5-18:3n-3 as a function of infusion time (min) for two women. The plasma concentration of d5-18:3n-3 of S02 at 6 h was 4.37 nmol/mL but is not presented.
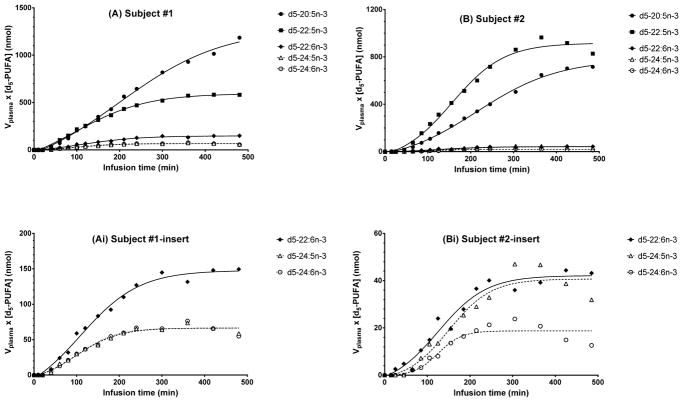
The major labeled esterified desaturation-elongation products in plasma total lipids were d5-18:3n-3, d5-20:5n-3, d5-22:5n-3, d5-22:6n-3, d5-24:5n-3, d5-24:6n-3. The amounts ([d5-n-3 HUFA] × Vplasma) vs. time data (Figure 2) fit well to a sigmoidal curve which plateaued within 300 min after for both subjects (Figs. 2a & 2b). Plasma EPA rose throughout the infusion, reaching an inflection point at approximately 5 h, at 818 nmol for S01 and 504 nmol for S02, then leveling off. The amount of d5-20:5n-3 in S01 and S02 in plasma at the end of infusion was 1185 and 716 nmol, respectively.
Figure 2.
Best-fit curves for the experimentally determined total amount of d5-n-3 PUFAs ([d5-PUFA]*Vplasma, nmol) synthesized from d5-18:3n-3 as a function of infusion time (min). Fit is to nonlinear Boltzmann Sigmoidal equation. Symbols expressed as total amount in plasma (nmol). (A) subject S01 d5-PUFA, (Ai) insert for subject S01 for d5-22:6n-3, d5-24:5n-3, and d5-24:6n-3; (B) subject S02 d5-PUFA, (Bi) insert for subject S02.
Like d5-EPA, d5-22:5n-3 (DPA) and d5-22:6n-3 (DHA) were first detected at 40 min. Both d5-22:5n-3 and d5-22:6n-3 increased for up to 4 h and then leveled off. After 7 h, total esterified d5-22:5n-3 in plasma was 580 nmol for S01, 920 nmol for S02, while the amount of d5-22:6n-3 was 148 nmol for S01 and 44 nmol for S02.
D5-24:5n-3 and d5-24:6n-3 were detected in the first hour of infusion at a similar magnitude compared to d5-22:6n-3 in both subjects. The total amount of d5-24:5n-3 was similar to d5-24:6n-3 in S01 at 7 h, 66.2 nmol vs. 65.6 nmol, while d5-24:5n-3 was greater than d5-24:6n-3, 38.7 vs. 14.9 nmol, respectively.
First derivative of nonlinear fit of deuterated esterified n-3 HUFA concentrations
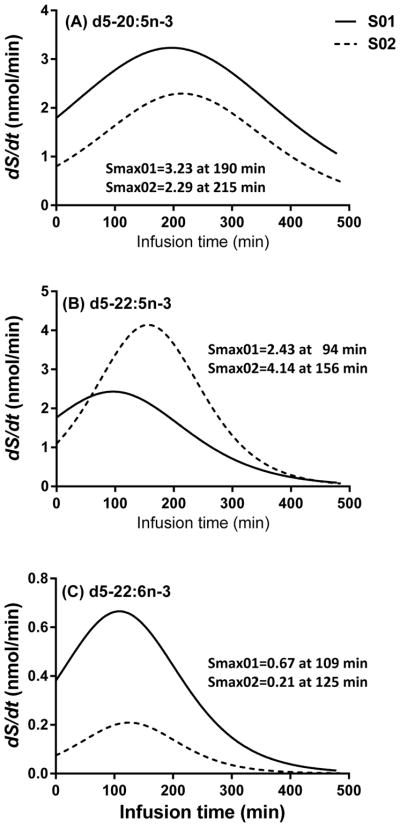
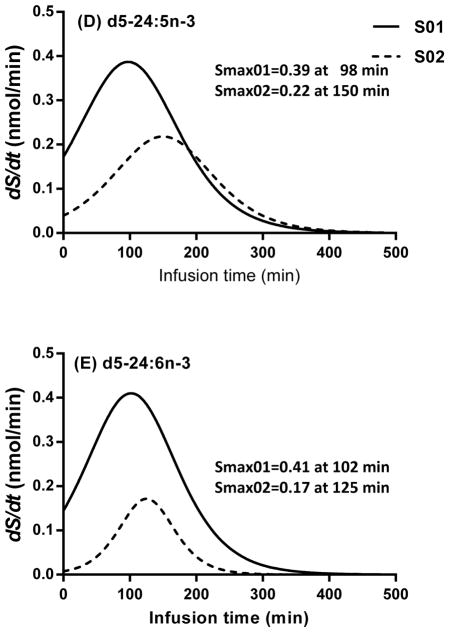
The first derivatives (S, nmol/min) (Eq. 2) of the best-fit sigmoidal curves of esterified amount vs. infusion time are presented in Figure 3. The peaks of these curves (Smax,i) represent the best estimate for synthesis-secretion rate for the deuterated HUFA, i (Eq. 3). For d5-20:5n-3, Smax occurred at about 200 min after the beginning of infusion and was 3.23 nmol/min in S01 and 2.29 nmol/min in S02 (Fig. 3a). For d5-22:5n-3, Smax occurred earlier in S01 (2.43 nmol/min at 94 min) compared to S02 (4.14 nmol/min at 156 min) (Fig. 3b). For d5-22:6n-3 (Fig. 3c), Smax for S01 was higher compared to S02 (0.67 nmol/min vs. 0.21 nmol/min, respectively). Smax for d5-22:6n-3 occurred at a similar time for both subjects, at 120 min of infusion. For both subjects, d5-24:5n-3 (Fig. 3d) and d5-24:6n-3 (Fig. 3e) presented similar magnitudes of Smax at similar times to d5-22:5n-3 and d5-22:6n-3, respectively.
Figure 3.
First derivatives (nmol/min) of nonlinear sigmoidal equations for the d5-n-3 metabolites synthesized from d5-18:3n-3. Values were expressed as the first derivatives multiplied by total plasma volume of each subject as a function of an infusion time over 8 h. (3A) d5-20:5n-3, (3B) d5-22:5n-3, (3C) d5-22:6n-3, (3D) d5-24:5n-3, and (3E) d5-24:6n-3.
Synthesis-secretion coefficients and rates
Smax and synthesis-secretion parameters (see Methods) derived are presented in Table 3. The synthesis-secretion coefficient for EPA, k1,EPA, was 1.91 mL/min for S01 and 1.29 ml/min for S02. k1,DPA was 1.44 mL/min and 2.32 mL/min for S01 and S02, k1,DHA was 0.39 and 0.12 mL/min, k1,TPA was 0.23 and 0.12 mL/min, and k1,THA was 0.24 and 0.10 mL/min for S01 and S02, respectively.
Table 3.
Synthesis-secretion parameters of d5-n-3 PUFAs estimated from circulating unesterified d5-18:3n-3
| n-3 HUFA | Subject | Time at Smax (min) | Smax (nmol/min) | k1 (mL/min) | Jsyn (nmol/min) | Daily Jsyn (μmol/d) | Daily Jsyn (mg/d) | F (min−1) | t1/2 (day) |
|---|---|---|---|---|---|---|---|---|---|
| EPA | S01 | 190 | 3.23 | 1.91 | 4.81 | 6.93 | 2.12 | 7.7E-05 | 6.2 |
| S02 | 215 | 2.29 | 1.29 | 6.09 | 8.77 | 2.68 | 1.5E-04 | 3.3 | |
| DPAn-3 | S01 | 94 | 2.43 | 1.44 | 3.62 | 5.21 | 1.74 | 8.8E-05 | 5.2 |
| S02 | 156 | 4.14 | 2.32 | 11.0 | 15.8 | 5.29 | 1.9E-04 | 2.6 | |
| DHA | S01 | 109 | 0.67 | 0.39 | 0.99 | 1.43 | 0.47 | 2.6E-06 | 182 |
| S02 | 125 | 0.21 | 0.12 | 0.56 | 0.80 | 0.27 | 3.7E-06 | 129 | |
| TPAn-3 | S01 | 98 | 0.39 | 0.23 | 0.58 | 0.83 | 0.30 | 7.4E-05 | 6.5 |
| S02 | 150 | 0.22 | 0.12 | 0.58 | 0.84 | 0.30 | 1.0E-04 | 4.8 | |
| THA | S01 | 102 | 0.41 | 0.24 | 0.61 | 0.88 | 0.32 | 5.8E-05 | 8.2 |
| S02 | 125 | 0.17 | 0.10 | 0.46 | 0.66 | 0.27 | 1.0E-04 | 4.8 |
S01, subject 1; S02, subject 2.
HUFA: long chain, highly unsaturated fatty acids with ≥ 20 carbon chains and ≥ 3 C-C double bonds.
EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; TPAn-3, tetracosapentaenoic acid; THA, tetracosahexaenoic acid
Smax, maximal slope of first derivative of plasma total amount time-course curves of newly synthesized d5-n-3 HUFA.
k1, the synthesis-secretion coefficient of specific d5- n-3 HUFA.
Jsyn, whole body synthesis-secretion rates of n-3 HUFA from circulating unesterified 18:3n-3. Calculated by multiplying k1 by unesterified ALA concentration, 2.52 nmol/mL for S01 and 4.74 nmol/mL for S02.
F, turnover of esterified n-3 HUFA, calculated by dividing Jsyn (nmol/min) by amount of HUFA in plasma total lipids (nmol) (Table 2)
t1/2, half-life of esterified n-3 HUFA derived from unesterified ALA in plasma
Multiplying k1,i by the plasma concentration of unesterified unlabeled ALA, 2.52 nmol/mL for S01 and 4.74 nmol/ml for S02, gave whole-body synthesis-secretion rates Jsyn,i for a given esterified HUFAi (Eq.5). Jsyn,EPA equaled 4.81 nmol/min for S01 and 6.09 nmol/min in S02. Jsyn,DPAn-3 was equal to 3.62 and 11.0 nmol/min in S01 and S02, respectively. In both subjects, Jsyn,EPA and Jsyn,DPAn-3 were higher than Jsyn,DHA, which equaled 0.99 and 0.56 nmol/min for S01 and S02, respectively. Jsyn,THA and Jsyn,TPAn-3 were close in both subjects, 0.5–0.6 nmol/min.
The half-lives of circulating esterified EPA, DPAn-3, TPAn-3, and THA were approximately 3 to 8 days, while the esterified plasma DHA half-life ranged from 129 to 182 days in the two subjects.
DISCUSSION
In this paper, we translated a stable isotope tracer technique and kinetic analysis developed in unanesthetized rats (Domenichiello et al. 2014), to directly estimate whole body synthesis-secretion rates of esterified n-3 HUFA from circulating unesterified ALA in two healthy overweight woment after ten weeks on a low linoleate diet. In each subject, we continuously infused d5-18:3n-3-albumin solution intravenously into one arm for 8 h, to rapidly establish a steady-state plasma concentration, and followed the appearance of esterified desaturated-elongated n-3 d5-HUFA products in plasma as a function of time by repeated sampling of blood from the other heated arm.
Steady-state plasma concentrations of esterified HUFA, including d5-EPA, d5-DPAn-3, d5–DHA, d5-TPAn-3, and d5-THA, were achieved in about 5 hr, while a steady-state concentration of infused unesterified d5-ALA was reached within 15 min after starting the infusion. As in rats, appearance curves for synthesized d5-n-3 HUFA in study subjects were sigmoidal; amounts (concentration × plasma volume) initially rose slowly, then more rapidly, and eventually tended to level off as synthesis approximated loss from plasma. We estimated whole body synthesis-secretion rates as 1.4 μmol (0.47 mg)/day and 0.80 μmol (0.27 mg)/day for DHA, and as 6.9 μmol (2.1 mg)/day and 8.8 μmol (2.7 mg)/day for EPA in subjects S01 and S02, respectively (Table 3).
The infusion technique is minimally invasive and anesthesia free, allowing us to directly estimate esterified HUFA synthesis-secretion rates from plasma unesterified ALA. Compared to rats, the large blood volume and relative ease of implanting catheters in humans allowed for a sufficient amount of blood to be drawn without disturbing in vivo metabolism or hemodynamics. In rat studies, due to possible blood coagulation in narrow catheters, heparin maybe necessary to maintain catheter patency (Domenichiello et al. 2014). Heparin can activate lipoprotein lipase and thus affect in vivo lipid metabolism and lipoprotein secretion (Domenichiello et al. 2014, Domenichiello et al. 2015), but heparin is not necessary during infusion in humans with wider catheters. Appearance concentrations for esterified d5-HUFA that were synthesized and secreted from d5-ALA were fit well to Boltzman sigmoidal curves. The r2 of the goodness of fit ranged from 0.950 to 0.997 for all d5-labeled n-3 HUFAs in each subject, except for d5-THA in subject S02 (0.915), indicating the kinetic analysis appropriately described the data.
The low linoleate diet contained 1 en% of total calories as LA and 0.5 en% ALA, compared to 8.9 en% as LA and 1.1 en% as ALA in dietary intake in United States (Hibbeln et al. 2006). Thus, the estimates of synthesis-secretion rates for circulating unesterified ALA might be expected to be in a higher range because they would be minimally suppressed by endogenous ALA derived from diet. Their calculated synthesis-secretion rates of DHA from circulating ALA were 1.43 and 0.80 μmol per day, respectively. These rates are 200 times greater than rates reported in adult rats, which weigh about 0.5 kg (Domenichiello et al. 2014, Domenichiello et al. 2016). Therefore, per kg weight, the synthesis-secretion rates are of the same order of magnitude, although rat oxidative metabolism is about 2.5 times human oxidative metabolism (Sokoloff 1978). The diet of the two subjects contained EPA and DHA (0.04 en%), while the reported rodent diets were free of the n-3 HUFA. Previous studies have shown that DHA can downregulate enzymes involved in its own synthesis which may lead to a decreased synthesis rate (Lin et al. 2011). Therefore, the synthesis-secretion rates estimated in this report likely underestimate the capacity for humans to synthesize n-3 HUFA, including DHA. Though, this requirement is not agreed upon, estimates for the requirement for human brain DHA range from 2.4–3.8 mg/day (Umhau et al. 2009). Though participants in this study appear to synthesize DHA at a lower rate than required by the brain, the subjects consumed about 100 mg/day as EPA and DHA (~ 60% as DHA). Therefore, synthesis of DHA was likely inhibited by dietary intake of EPA and DHA and did not make a major contribution to maintaining brain DHA. It would be important to use this steady-state infusion method to measure DHA synthesis-secretion in vegans and vegetarians, to determine if DHA synthesis-secretion is increased to meet the body demands with a low or absent EPA/DHA containing diet.
We estimated synthesis-secretion rates of DHA and other n-3 HUFAs from circulating unesterified ALA. It is likely, based on animal studies, that other circulating and tissue pools can be desaturated/elongated to HUFA. Igarashi et al (Igarashi et al. 2007) proposed using a λ factor to account for dilution of the hepatic precursor labeled ALA-CoA pool by unlabeled recycling ALA. λ equals the ratio of steady-state liver labeled ALA-CoA to plasma unesterified labeled ALA, and was estimated as 0.35 in rats fed an n-3 adequate diet (Igarashi et al. 2007). This means that synthesis rates of unesterified DHA from circulating unesterified ALA may be underestimated due to dilution by about 3-fold in rats (Igarashi et al. 2007) or by 10 to 20-fold as estimated by Domenichiello et al (Domenichiello et al. 2014). A similar correction factor λ may operate in humans to increase estimated DHA synthesis rates. Additionally, because Equation 5 is an inequality, synthesis rates are likely underestimated in this paper. Finally, underestimation also may occur because unesterified n-3 PUFAs in the synthesis pathway between ALA and DHA may be reincorporated and elongated to esterified DHA, and we have not taken this into account. In addition to the liver, as other tissues (e.g. skin, (Moore et al. 1995)) can synthesize but not secrete DHA from ALA, which would further increase the estimated whole body synthesis rate of DHA. Therefore, the calculated DHA synthesis rates in this study, 1.4–0.80 μmol (0.47–0.27 mg) per day, is likely underestimated for multiple reasons.
Subject S02 produced more d5-22:5n-3 than d5-20:5n-3. However, despite this greater amount of d5-22:5n-3, subject S02 produced relatively less d5-22:6n-3 compared to S01. This is consistent with the unlabeled, endogenous HUFA concentrations in plasma total lipids (Table 2). The ratio of DPAn-3 to EPA in S01 is 0.66 compared to 1.43 in S02, while the ratio of DHA to DPAn-3 in S01 is 9.4 compared to 2.5 in S02. It is possible that subject S02 had genetic fatty acid desaturase (FADS) polymorphisms that could affect HUFA appearance in the plasma. For instance, it has been reported that single nucleotide polymorphisms (SNP) in the FADS gene cluster results in differences in serum phospholipid PUFAs (Gillingham et al. 2013, Hong et al. 2013). It is possible that subject S01 carries GG group in FADS SNP, while subject S02 T-allele group. Future studies could utilize the steady-state infusion method to measure the effect of genetic variants of FADS and fatty acid elongase (ELOVL) genes on n-3 HUFA synthesis-secretion rates (Steer et al. 2012).
The daily synthesis-secretion rates of n-3 HUFA were estimated from an 8 h infusion started in the morning. If the rates changed throughout the day, or the esterified n-3 HUFA possiblely continues to rise after finishing infusion of d5-ALA-albumin, this would not be noted in our estimates. Additionaly, we measured synthesis-secretion of HUFA and not synthesis of HUFA. Therefore, conditions that impact HUFA synthesizing tissues from secreting these fatty acids will also lead to underestimates of the synthesis rate, such as liver disease. To further consider our method and kinetic analysis, it would be worthwhile to conduct other approaches to interpret these experimental data, such as multi-compartmental modeling analysis (Pawlosky et al. 2001, Wastney et al. 1996). Two or more approaches could provide multi-dimensional understanding of PUFA metabolism. Furthermore, looking into lipid classes and molecular species using liquid chromatography mass spectrometry might further elucidate PUFA metabolism.
In summary, this study establishes a proof-of-concept method and kinetic analysis in human subjects to estimate whole body, presumably hepatic, synthesis-secretion rates of n-3 HUFA from circulating unesterified ALA. These now could be extended for measuring the whole body synthesis-secretion and turnover of other PUFAs directly or from other precursors in a variety of physiological or pathophysiological states, and in relation to different diets.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the NIH Clinical Center Metabolic Unit nursing staff who provided helpful assistance in the organization, scheduling and implementation of the experimental infusion procedures, especially Ms. Irene Rozga. We wish to also acknowledge the NIH Clinical Center Pharmacy Department for designing and preparing the stable isotope labeled infusate preparation, the NIH Clinical Center Metabolic Nutrition staff who worked on providing these patients with the specific diets, and the contribution of Ms. Katherine Ness, for the coordination of the main diet study protocol at the time of this infusion experiment. The authors thank Nancy Terry, NIH Library Writing Center, for manuscript editing assistance. This project was supported by the Intramural Research Programs of the NIAAA, National Institutes of Health. DuPont/Pioneer supported the purchase of the deuterated linolenate in an unrestricted gift to NIAAA but had not any role in the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ALA
α-linolenic acid
- DHA
docosahexaenoic acid
- DPAn-3
n-3 docosapentaenoic acid
- EPA
eicosapentaenoic acid
- ELOVL
fatty acid elongase
- FADS
fatty acid desaturase
- FAME
fatty acid methyl ester
- FFA
free fatty acid
- GC/FID
gas chromatography/flame ionization detection
- GC/MS NCI
gas chromatography/mass spectrometry negative chemical ionization
- HUFA
long chain, highly unsaturated fatty acid (≥ 20 C, ≥ 3 double bonds)
- LA
linoleic acid
- SNP
single nucleotide polymorphism
- THA
tetracosahexaenoic acid
- TLC
thin-layer chromatography
- TPAn-3
tetracosapentaenoic acid
- WFI
water-for-injection
Footnotes
Conflict of Interest: Authors declare no conflict of interest.
Authors’ contributions are as follows (in the alphabetic order):
Clinical trial design, research, and PIs: CER, JRH
Designed and conducted research: ABC, BVM, CER, CTC, JRH, NMS, RPB, SFM, SIR, YHL
Provided essential iv infusion material: HKJ
Data analysis and interpretation: AFD, CTC, RPB, SIR, YHL
Wrote paper: AFD, JRH, RPB, SIR, YHL
Revised manuscript: CER, SFM
References
- Barcelo-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Progress in Lipid Research. 2009;48:355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Bibus D, Lands B. Balancing proportions of competing omega-3 and omega-6 highly unsaturated fatty acids (HUFA) in tissue lipids. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2015;99:19–23. doi: 10.1016/j.plefa.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5:127–132. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Brenna JT, Carlson SE. Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. Journal of Human Evolution. 2014;77:99–106. doi: 10.1016/j.jhevol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC, Lipids ISftSoFAa. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Burdge GC. Metabolism of alpha-linolenic acid in humans. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2006;75:161–168. doi: 10.1016/j.plefa.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Sinclair AJ. The limitations of whole tissue analysis to define linolenic acid deficiency. The Journal of Nutrition. 1972;102:1315–1321. doi: 10.1093/jn/102.10.1315. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharmaceutical Research. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Domenichiello AF, Chen CT, Trepanier MO, Stavro PM, Bazinet RP. Whole body synthesis rates of DHA from alpha-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. Journal of Lipid Research. 2014;55:62–74. doi: 10.1194/jlr.M042275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenichiello AF, Kitson AP, Bazinet RP. Is docosahexaenoic acid synthesis from alpha-linolenic acid sufficient to supply the adult brain? Progress in Lipid Research. 2015;59:54–66. doi: 10.1016/j.plipres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Domenichiello AF, Kitson AP, Chen CT, Trépanier MO, Stavro PM, Bazinet RP. The effect of linoleic acid on the whole body synthesis rates of polyunsaturated fatty acids from α-linolenic acid and linoleic acid in free-living rats. The Journal of Nutritional Biochemistry. 2016;30:167–176. doi: 10.1016/j.jnutbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Gillingham LG, Harding SV, Rideout TC, Yurkova N, Cunnane SC, Eck PK, Jones PJ. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. The American Journal of Clinical Nutrition. 2013;97:195–207. doi: 10.3945/ajcn.112.043117. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE, SanGiovanni JP, Davis JM. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. The British Journal of Psychiatry. 2016;209:192–201. doi: 10.1192/bjp.bp.114.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. The American Journal of Clinical Nutrition. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Hong SH, Kwak JH, Paik JK, Chae JS, Lee JH. Association of polymorphisms in FADS gene with age-related changes in serum phospholipid polyunsaturated fatty acids and oxidative stress markers in middle-aged nonobese men. Clinical Interventions in Aging. 2013;8:585–596. doi: 10.2147/CIA.S42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of alpha-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. Journal of Lipid Research. 2007;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI, DeMar JC., Jr Low liver conversion rate of alpha-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. Journal of Lipid Research. 2006;47:1812–1822. doi: 10.1194/jlr.M600030-JLR200. [DOI] [PubMed] [Google Scholar]
- Lin YH, Llanos A, Mena P, Uauy R, Salem N, Jr, Pawlosky RJ. Compartmental analyses of 2H5-alpha-linolenic acid and C-U-eicosapentaenoic acid toward synthesis of plasma labeled 22:6n-3 in newborn term infants. The American Journal of Clinical Nutrition. 2010;92:284–293. doi: 10.3945/ajcn.2009.28779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Pawlosky RJ, Salem N., Jr Simultaneous quantitative determination of deuterium- and carbon-13-labeled essential fatty acids in rat plasma. Journal of Lipid Research. 2005;46:1974–1982. doi: 10.1194/jlr.M500128-JLR200. [DOI] [PubMed] [Google Scholar]
- Lin YH, Salem N, Jr, Wells EM, Zhou W, Loewke JD, Brown JA, Lands WE, Goldman LR, Hibbeln JR. Automated High-Throughput Fatty Acid Analysis of Umbilical Cord Serum and Application to an Epidemiological Study. Lipids. 2012;47:527–539. doi: 10.1007/s11745-012-3661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Shah S, Salem N., Jr Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. The Journal of Nutritional Biochemistry. 2011;22:758–765. doi: 10.1016/j.jnutbio.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. NIH Clinical Center closes its pharmacy after contamination found. British Medical Journal (Clinical research ed) 2015;350:h3099. doi: 10.1136/bmj.h3099. [DOI] [PubMed] [Google Scholar]
- McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. Journal of Lipid Research. 2004;45:474–485. doi: 10.1194/jlr.M300304-JLR200. [DOI] [PubMed] [Google Scholar]
- Metherel AH, Lacombe RJS, Chouinard-Watkins R, Hopperton KE, Bazinet RP. Complete assessment of whole-body n-3 and n-6 PUFA synthesis-secretion kinetics and DHA turnover in a rodent model. Journal of Lipid Research. 2018;59:357–367. doi: 10.1194/jlr.M081380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Hurt E, Yoder E, Sprecher H, Spector AA. Docosahexaenoic acid synthesis in human skin fibroblasts involves peroxisomal retroconversion of tetracosahexaenoic acid. Journal of Lipid Research. 1995;36:2433–2443. [PubMed] [Google Scholar]
- O’Brien JS, Sampson EL. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. Journal of Lipid Research. 1965;6:545–551. [PubMed] [Google Scholar]
- Ouellet M, Emond V, Chen CT, Julien C, Bourasset F, Oddo S, LaFerla F, Bazinet RP, Calon F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochemistry International. 2009;55:476–482. doi: 10.1016/j.neuint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. Journal of Lipid Research. 2001;42:1257–1265. [PubMed] [Google Scholar]
- Pawlosky RJ, Sprecher HW, Salem N., Jr High sensitivity negative ion GC-MS method for detection of desaturated and chain-elongated products of deuterated linoleic and linolenic acids. Journal of Lipid Research. 1992;33:1711–1717. [PubMed] [Google Scholar]
- Salem N., Jr . Omega-3 fatty acids: molecular and biochemical aspects. In: Spiller GA, Scala J, editors. Current Topics in Nutrition and Disease: New Protective Roles for Selected Nutrients. Vol. 22. Alan R Liss; New York: 1989. pp. 109–228. [Google Scholar]
- Salem N, Jr, Reyzer M, Karanian J. Losses of arachidonic acid in rat liver after alcohol inhalation. Lipids. 1996;31(Suppl):S153–156. doi: 10.1007/BF02637068. [DOI] [PubMed] [Google Scholar]
- Saunders AV, Davis BC, Garg ML. Omega-3 polyunsaturated fatty acids and vegetarian diets. The Medical Journal of Australia. 2013;199:S22–26. doi: 10.5694/mja11.11507. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et Biophysica Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Local cerebral energy metabolism: its relationships to local functional activity and blood flow. Ciba Foundation Symposium. 1978:171–197. doi: 10.1002/9780470720370.ch10. [DOI] [PubMed] [Google Scholar]
- Steer CD, Hibbeln JR, Golding J, Davey Smith G. Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: their associations with two common FADS2 polymorphisms. Human Molecular Genetics. 2012;21:1504–1512. doi: 10.1093/hmg/ddr588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco J, Babcock R, Hincenbergs I, Medwadowski B, Miljanich P, Williams MA. Linolenic acid deficiency. Lipids. 1979;14:166–173. doi: 10.1007/BF02533868. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Zhou W, Carson RE, et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. Journal of Lipid Research. 2009;50:1259–1268. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt SH, Mensink RP, Simonis MM, Hornstra G. Effects of dietary alpha-linolenic acid on the conversion and oxidation of 13C-alpha-linolenic acid. Lipids. 2000;35:137–142. doi: 10.1007/BF02664762. [DOI] [PubMed] [Google Scholar]
- Wastney ME, Angelus P, Barnes RM, Subramanian KN. Zinc kinetics in preterm infants: a compartmental model based on stable isotope data. The American Journal of Physiology. 1996;271:R1452–1459. doi: 10.1152/ajpregu.1996.271.5.R1452. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Wiley-Liss Inc; New York: 1992. [Google Scholar]
- Wolfe RR, Evans JE, Mullany CJ, Burke JF. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biological Mass Spectrometry. 1980;7:168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.