Abstract
Viral infection of the central nervous system can result in encephalitis. About 20% of individuals who develop viral encephalitis go on to develop epilepsy. We have established an experimental model where virus infection of mice with Theiler’s murine encephalomyelitis virus (TMEV) leads to acute seizures, followed by a latent period (no seizures/epileptogenesis phase) and then spontaneous recurrent seizures – epilepsy. Infiltrating macrophages (CD11b+CD45hi) present in the brain at day 3 post infection are an important source of interleukin-6, which contributes to the development of acute seizures in the TMEV-induced seizure model. Time course analysis of viral infection and inflammatory [CD11b+CD45hiLy-6Chi] and patrolling [CD11b+CD45hiLy-6Clow] monocyte and T-cell infiltration into the brains of TMEV-infected C57BL/6J mice over the entire course of the acute viral infection was performed to elucidate the role of virus and the immune response to virus in seizures and viral clearance. The infiltrating inflammatory macrophages were present early following infection but declined over the course of acute viral infection, supporting a role in seizure development, while the lymphocyte infiltration increased rapidly and plateaued, advocating that they play a role in viral clearance. In addition, we showed for the first time that, while TMEV infection of RAG1−/− mice did not alter the number of mice experiencing acute seizures, TMEV infection of C57BL/6J mice depleted of macrophages resulted in a significant decrease in the number of mice experiencing seizures, again supporting a role for infiltrating macrophages in the development of acute seizures in the TMEV-induced seizure model.
Keywords: Theiler’s murine encephalomyelitis virus, Seizures, Viral encephalitis, Picornavirus, Immune response, Monocytes
Introduction
The Theiler’s murine encephalomyelitis virus (TMEV)-induced seizure model, the first viral infection-driven animal model of epilepsy, is a powerful tool that we have been using to elucidate mechanisms of viral pathogenesis and the development of acute seizures [reviewed in (Libbey and Fujinami 2011)]. An important feature of this model is that induction of seizures is through a natural trigger, the naturally occurring enteric pathogen of the mouse, TMEV. This is similar to what is seen in humans in which numerous neurotropic viruses cause encephalitis, and seizures are common in patients during acute viral infection of the central nervous system (CNS). Another important feature of this model is that acute seizures develop in approximately 50–80% of the infected C57BL/6J mice, allowing for a comparison of genetically identical animals that received identical treatments, but experienced different outcomes (seizures, no seizures) following infection [reviewed in (Libbey and Fujinami 2011)]. We have shown that the percentage of mice experiencing seizures can be adjusted by changing the titer of TMEV in the inoculum dose (Libbey et al. 2011b). In this model, seizures are observed in a percentage of mice starting on day 3 post infection (p.i.), with the percentage of mice having seizures increasing daily until day 6 p.i. [reviewed in (Libbey and Fujinami 2011)]. By day 10 p.i., the seizures cease. The severity of seizures is scored using the Racine scale, and the majority of seizures are scored as ≥ 3. Following the cessation of the seizures (day 10 p.i.) and clearance of infectious virus (day 14 p.i.), mice enter a latent period of several weeks in which they do not experience seizures. Following the latent period, the mice develop spontaneous recurrent seizures: epilepsy [reviewed in (Libbey and Fujinami 2011)].
Monocytes are a population of myeloid-lineage cells originated in the bone marrow from hematopoietic stem cells and are found in the spleen, bone marrow and blood stream. They are part of the innate immune response and migrate, in response to inflammation and pathogen challenge or in steady state conditions, into tissues where they become infiltrating macrophages [reviewed in (Ashhurst et al. 2014; Italiani and Boraschi 2014)]. Infiltration of blood-derived monocytes into the CNS only occurs under pathological conditions and is associated with differentiation of these cells into immune effector cells: the classically activated macrophage, also referred to as M1 or ‘inflammatory’, and defined henceforth as CD11b+ CD45hi Ly-6Chi, and the alternatively activated macrophage, also referred to as M2 or anti-inflammatory or ‘patrolling’, and defined henceforth as CD11b+ CD45hi Ly-6Clow [reviewed in (Ashhurst et al. 2014; Italiani and Boraschi 2014; Katsumoto et al. 2014)]. While inflammatory macrophages are involved in promoting inflammation by secreting pro-inflammatory molecules such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6 and inducible nitric oxide synthase (iNOS), patrolling macrophages are important to resolve inflammation and restore homeostasis by producing immunosuppressive cytokines such as IL-10 [reviewed in (Mosser and Edwards 2008; Sica et al. 2014). These infiltrating macrophages, originating from the bone marrow, are distinct from the resident tissue macrophages of the CNS, the microglia, which originate from the yolk sac during embryogenesis [reviewed in (Epelman et al. 2014; Gutknecht and Bouton 2014; Jenkins and Hume 2014; Swirski et al. 2014)].
Previous studies, using such techniques as gene expression analysis, the generation of chimeric mice, flow cytometry and anti-inflammatory drug treatments, have implicated macrophage infiltration of the brains of C57BL/6J mice in the development of acute seizures following intracerebral (i.c.) infection with TMEV (Cusick et al. 2013; Kirkman et al. 2010; Libbey et al. 2011a). First, C57BL/6J mice experiencing acute seizures following TMEV infection had greatly elevated expression of both CCL2 (453.8-fold increase at day 6 p.i.), which is produced by CNS resident cells, as well as its receptor CCR2 (26.3-fold increase at day 6 p.i.) (Libbey et al. 2011a), which is found on inflammatory monocytes/macrophages, both of which together effect the migration of monocytes/macrophages from the circulation into the CNS. Second, by distinguishing microglia (CD11b+ CD45lo/int) from infiltrating macrophages (CD11b+ CD45hi) via flow cytometry, it was found that the number of infiltrating macrophages was significantly higher in the brains of TMEV-infected mice at day 3 p.i., compared to mock-infected mice, while the number of microglia was not significantly changed (Cusick et al. 2013). Third, the CA1 and CA2 regions of the pyramidal neuron layer of the hippocampus are damaged in C57BL/6J mice experiencing seizures following TMEV infection (Kirkman et al. 2010; Libbey et al. 2008; Libbey et al. 2010; Stewart et al. 2010). Howe and colleagues demonstrated that inflammatory macrophages (CD11b+ CD45hi), found concentrated in the hippocampus at 12 and 18 hours p.i., are the critical mediators of hippocampal injury during acute TMEV infection of the brain (Buenz et al. 2009; Howe et al. 2012a; Howe et al. 2012b). Depletion of inflammatory macrophages preserved the hippocampal neurons in C57BL/6J mice which are normally susceptible to hippocampal injury following TMEV infection (Howe et al. 2012a). Alternatively, immune reconstitution, via adoptive transfer, of SJL/J mice (H-2s haplotype recipient mice which are normally resistant to hippocampal injury and have minimal inflammatory macrophage infiltration into the brain following TMEV infection) with bone marrow from C57BL/10SnSg (B10.S-H2s/SgMcdJ) mice [H-2s haplotype donor mice which have a robust inflammatory macrophage response and hippocampal injury comparable to C57BL/6J mice (H-2b haplotype)] resulted in extensive hippocampal damage at day 7 p.i. and robust infiltration of donor-derived inflammatory macrophages at 18 hours p.i. with TMEV (Howe et al. 2012b). Additionally, our group demonstrated that infiltrating macrophages expressing the green fluorescence protein (CD11b+ CD45hi GFP+) clustered near the CA1–CA2 region of the hippocampus on days 5 and 14 p.i. in mice experiencing seizures following TMEV infection of chimeric C57BL/6J mice (Cusick et al. 2013). Fourth, treatment with drugs, such as minocycline (Libbey et al. 2011a) and wogonin (Cusick et al. 2013), shown to decrease infiltration of immune cells such as macrophages and lymphocytes and decrease activation within the CNS during viral infection, resulted in a significant reduction in the number of TMEV-infected mice experiencing seizures. Finally, the level of the pro-inflammatory cytokine IL-6, which has previously been shown to be important in acute seizure development in this model (Kirkman et al. 2010; Libbey et al. 2011a), was observed, via direct intracellular cytokine staining and flow cytometry, to be higher in infiltrating macrophages (CD11b+ CD45hi GFP+), compared to microglia (CD11b+ CD45lo/int GFP−), on day 3 p.i., in the brain following TMEV infection of C57BL/6J mice (Cusick et al. 2013). Taken together, these data support the hypothesis that inflammatory macrophages, and the IL-6 which they produce, play a role in the development of seizures following viral infection of the brain.
In this study, we observed that high titers of infectious virus were present for a few days immediately following infection after which the titer decreased rapidly until infectious virus became undetectable. Macrophages infiltrated the infected brains as early as day 3 p.i., when acute seizures begin, and the peak of macrophage infiltration correlated with the peak of seizure development (day 7 p.i.). This initial robust infiltration was greatly reduced over time (day 10 p.i.). Importantly, TMEV-infected mice experiencing seizures had a more robust inflammatory macrophage infiltration than TMEV-infected mice that did not experience seizures, supporting a role for inflammatory macrophages in seizure development. Conversely, lymphocytes were elevated in the brains of infected mice irrespective of the presence of seizures and the levels increased rapidly and plateaued, implicating a role in viral clearance. We also showed for the first time that depletion of macrophages, but not T cells, resulted in a significant reduction in the percentage of mice experiencing seizures. Conversely, adoptive transfer of bone marrow-derived monocytes directly into the brains of TMEV-infected mice resulted in an increase in the percentage of mice experiencing seizures. Therefore, we were able to demonstrate that macrophages are not only important but also essential for the development of acute seizures and that manipulation of these cells and their secreted cytokines may present a potential pharmacological approach to controlling the development of acute seizures.
Results
The peak of acute seizures does not correlate with the peak of viral replication
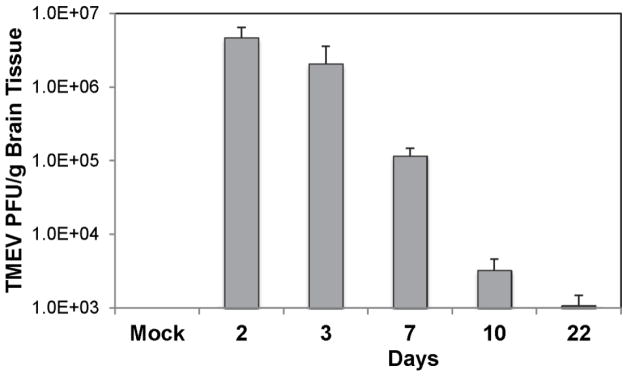
In our experimental mouse model of virus-induced seizures and epilepsy, TMEV-infected mice develop acute seizures that start at day 3 p.i., peak at day 7 p.i. and have ceased by day 10 p.i. [reviewed in (DePaula-Silva et al. 2017)]. Previously, immunohistochemistry was used to show the presence of TMEV antigen in hippocampal neurons on days 6 and 7 p.i. (peak of disease), but the viral antigen was cleared by day 14 p.i. (Kirkman et al. 2010; Libbey et al. 2011b; Libbey et al. 2010). In order to determine whether the peak of acute seizures corresponded with the peak of viral replication, we examined the brains of TMEV-infected mice for the presence of infectious virus over the entire course of the acute infection via plaque assay. Large quantities of infectious virus, normalized as PFU/g brain tissue, were present on days 2 and 3 p.i. (Fig. 1). Calculating for the quantity of infectious virus expected to be present within the whole brain, the day 2 p.i. brains contained almost 7-fold more virus than the initial viral inoculum (3 × 105 PFU/mouse) and the day 3 p.i. brains contained almost 3-fold more virus than the initial viral inoculum. The amount of infectious virus decreased by one log unit between day 3 and day 7 p.i. (Fig. 1), which is 6-fold less virus than the initial viral inoculum, and decreased again by another 2 log units between day 7 and day 10 p.i. (Fig. 1), which is 210-fold less virus than the initial viral inoculum. Very little infectious virus was still present in the brains by day 22 p.i. (Fig. 1), 670-fold less virus than the initial viral inoculum. Therefore, TMEV titer within brains peaks early during acute viral infection, on day 1 or 2 p.i., which does not correspond with the peak of acute seizures (day 7 p.i.), suggesting that the development of acute seizures likely occurs due to the recruitment of immune cells into the CNS but not due to viral replication.
Fig. 1.
Time course of viral infection in the brains of TMEV-infected mice. Half brains were harvested from mock-infected mice and TMEV-infected mice on days 2, 3, 7, 10 and 22 post infection. The titer, in PFU, of infectious virus per gram (g) of brain tissue was determined using plaque assays. Data are presented as the mean + SEM with 4–8 mice per group
For days 3, 7, 10 and 22 p.i., the percent of mice that had seizures at these time points was 40%, 40%, 25% and 0%, respectively. Day 2 is before seizures are observable, so this determination cannot be made. Days 3 and 7 p.i. both presented with 40% seizures even though the amount of infectious virus within the brains decreased by one log unit between day 3 and day 7 p.i., suggesting that it is the titer of the initial viral inoculum that is important in the development of seizures and the seizures occur independent of viral replication. By day 22 p.i., viral antigen has been cleared from the brain, so very little infectious virus is expected to be found at this time point whether the mice experience seizures or not.
IL-6 production in the brains of TMEV-infected mice is high leading up to seizures
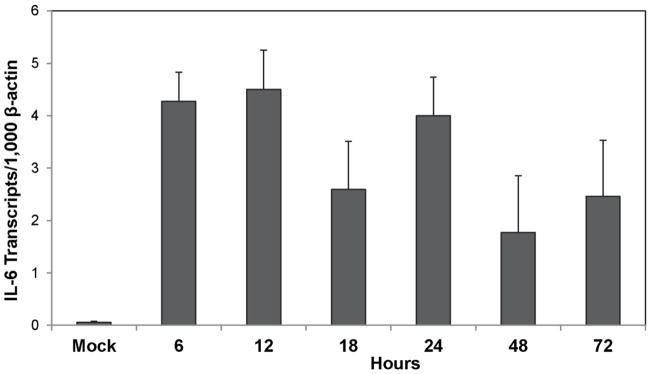
We have previously demonstrated that IL-6 is important for the development of seizures in the TMEV model (Cusick et al. 2013; Kirkman et al. 2010; Libbey et al. 2011a). To determine whether the expression level of IL-6 increased immediately following infection as a means of setting the stage for the development of acute seizures at day 3 p.i., we examined the brains of TMEV-infected mice for the expression of IL-6 over the first three days of the acute infection via TaqMan PCR. Although the level of IL-6 expression in the brain varied somewhat over the early times examined, expression was markedly increased over mock infection as early as 6, 12 and 24 hours p.i., and seemed to be on the rise again at 72 hours p.i. (Fig. 2), when seizures are first observed, confirming the involvement of IL-6 in the development of acute seizures after TMEV infection.
Fig. 2.
Time course of IL-6 gene expression in the brains of TMEV-infected mice. Brains were harvested at 6, 12, 18, 24, 48 and 72 hours post infection from TMEV-infected mice and at 72 hours post injection from mock-infected mice. Real time PCR for the detection of IL-6 was performed using TaqMan PCR as described in the Methods. Data are presented as the mean + SEM with 3 mice per group
Cellular infiltration of inflammatory macrophages, but not lymphocytes, correlates with the peak of seizures
Previous work from our laboratory has demonstrated that immune cells infiltrating into the brain by day 3 following TMEV infection are involved in the development of acute seizures (Cusick et al. 2013; Libbey et al. 2011a). To further characterize the role that infiltrating cells are playing throughout the entire time course of the acute seizures, we obtained brains from mock-infected mice and TMEV-infected mice on days 3, 7 and 10 p.i. that either were or were not experiencing seizures and examined the phenotype of the cells present in the brains using flow cytometry. The cell surface markers CD45 and CD11b were used to visualize microglia (CD45low/int CD11b+), macrophages (CD45hi CD11b+) and lymphoid cells (CD45+ CD11b−). As demonstrated in Fig. 3a, cellular infiltration began as earlier as day 3 p.i., peaked at day 7 p.i., which is also when seizures are greatest, and was trending downwards at day 10 p.i., when seizures are no longer observed. Quantification of total CD45+ cells in the brains of infected mice demonstrated that although an increased number of cells were observed at day 3 p.i., the highest infiltration occurred at day 7 p.i. (Fig. 3b). Analysis of the percentage of microglia in the brains of infected mice that experienced seizures was significant lower than in mice that did not have seizures (Fig. 3c). However, no significant difference was observed in the total number of microglia (Fig. 3d). While lymphocyte infiltration increased rapidly and plateaued (Fig. 3g,h), macrophage infiltration was significantly higher in mice that experienced seizures at day 7 p.i. compared to mice that did not experienced seizures (Fig. 3e,f) and this difference disappeared at day 10 p.i.
Fig. 3.
Time course of cell infiltration in the brains of TMEV-infected mice. a) Representative flow cytometry plots of microglia (CD45lo/int CD11b+), macrophages (CD45hi CD11b+) and lymphocytes (CD45hi CD11b−) isolated from brains harvested from mock-infected mice (PBS), TMEV-infected mice not experiencing seizures on days 3, 7 and 10 post infection and TMEV-infected mice experiencing seizures on days 7 and 10 post infection. Gates were set according to FMO, as described in the Methods. b) Quantification of cellular infiltration (total CD45hi cells) in the brains of TMEV-infected mice. Data is presented as the mean +SEM with 10 mice per group. Percentage (c) and total number (d) of microglia (CD45lo/int CD11b+) in the brains of TMEV-infected mice. Percentage (e) and total number (f) of macrophages (CD45hi CD11b+) in the brains of TMEV-infected mice. Percentage (g) and total number (h) of lymphocytes (CD45hi CD11b−) in the brains of TMEV-infected mice. Data (c–h) is presented as the mean + SEM with 5 mice per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, Student’s t test
The macrophage population (CD45hi CD11b+) was further examined, via flow cytometry, to determine if these cells were inflammatory or patrolling (anti-inflammatory) based on the expression of Ly-6C, as shown in Fig. 4a. The Ly-6C monocyte marker distinguishes between patrolling monocytes (Ly-6C− cells) and inflammatory monocytes (Ly-6C+ cells). Quantification of the flow cytometry data represented in Fig. 4a demonstrates that the total number of patrolling macrophages is significantly increased as seizures stop (day 10 p.i.) compared to days 3 and 7 p.i. (Fig. 4b). Interestingly, the highest number of inflammatory macrophages was observed at day 7 p.i., which corresponds to the peak of seizure activity, and decreased at day 10 p.i. when seizures are no longer present (Fig. 4c). We also found that at day 7 p.i., mice experiencing seizures had elevated numbers of inflammatory macrophages in the brain compared to those mice that did not develop seizures (Fig. 4d).
Fig. 4.
Characterization of the CD45hi CD11b+ macrophage population in the brains of TMEV-infected mice. a) Representative flow cytometry plots of cells isolated from brains harvested from TMEV-infected mice not experiencing seizures on days 3, 7 and 10 post infection and TMEV-infected mice experiencing seizures on days 7 and 10 post infection. Gates were set according to FMO. b–c) Quantification of the total number of b) patrolling macrophages (CD45hi CD11b+ Ly6C−) and c) inflammatory macrophages (CD45hi CD11b+ Ly6C+) in the brains of TMEV-infected mice (10 mice per group). d) Quantification of the total number of inflammatory macrophages (CD45hi CD11b+ Ly6C+) in the brains of TMEV-infected mice at day 7 post infection, comparing mice experiencing seizures to mice not experiencing seizure (5 mice per group). Data is presented as the mean +SEM. *p<0.05, **p<0.01, Student’s t test
T cells are not required for acute seizure development
TMEV-infected mice had elevated numbers of lymphocytes in the brains, compared to mock-infected mice, whether the mice were experiencing seizures or not (Fig. 3g,h). To gain a better insight into the role of lymphocytes in the development of acute seizures following TMEV infection, we infected RAG1−/− mice, which are deficient in mature T and B cells, and compared them to C57BL/6J mice infected with TMEV. Seizures were monitored daily after day 3 p.i. and mice were euthanized at day 7 p.i. As shown in Fig. 5a, both CD4+ and CD8+ T cells were absent from the brains of RAG1−/− mice compared to C57BL/6J mice, as expected. Interestingly, the number of RAG1−/− mice experiencing seizures was similar to control mice (Fig. 5b), suggesting that lymphocytes are not playing a role in the development of acute seizures following TMEV infection.
Fig. 5.
T cells are not required for the development of acute seizures. a) Representative flow cytometry plots of microglia (CD45low/int CD11b+), macrophages (CD45hi CD11b+) and lymphocytes (CD45hi CD11b−) in the brains of TMEV-infected mice 7 days post infection. CD4+ T cells and CD8+ T cells were detected within the lymphocyte population (CD45hi CD11b−). b) Percent mice with seizures (Racine scale, stages 3 to 5) in C57BL/6J wild-type (N=57) and RAG1−/− (N=63) mice. The percentage of mice with seizures was calculated as follows: (number of mice with seizures/total number of mice infected) × 100. p=0.4782 (ns, not significant), chi-square test
Macrophage depletion reduces acute seizure development
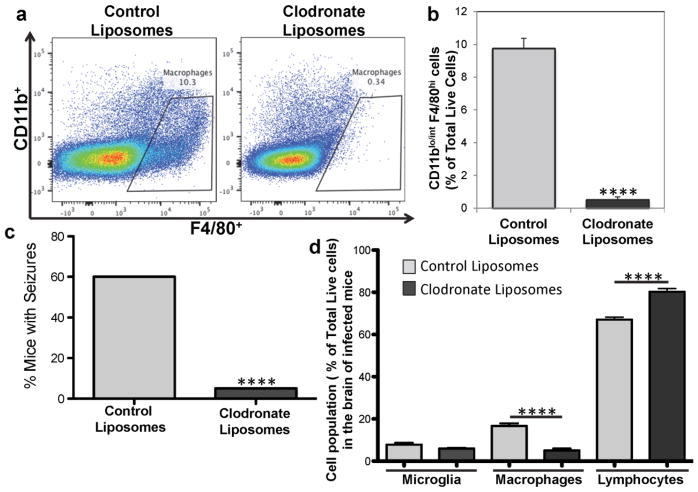
Macrophages infiltrate as early as day 3 p.i. and the peak of seizure activity correlates with the peak of inflammatory macrophage infiltration (day 7 p.i.). In order to determine the requirement of macrophages in the development of acute seizures after TMEV infection, monocytes/macrophages were depleted in vivo through intraperitoneal injection of clodronate-containing liposomes. Daily injections started two days prior to infection and injections continued every two days after infection. Control liposomes were injected in parallel for comparison. Monocyte/macrophage depletion was confirmed via flow cytometry of splenocytes by analyzing the expression of CD11b+ and F4/80+ on these cells (Fig. 6a); monocytes/macrophages were found to be significantly depleted (p<0.0001, Student’s t test) on day 3 p.i. (Fig. 6b). These monocyte/macrophage-depleted mice were monitored over a period of 7 days p.i. for seizures. Interestingly, the number of mice experiencing seizures following monocyte/macrophage depletion was significantly reduced compared to mice receiving control liposomal injections (p<0.0001, chi-square) (Fig. 6c), which is in agreement with work done by the Löscher group (Waltl et al. 2018). Also, there was no difference in the amount of TMEV antigen-positive cells in the brains of clodronate liposome-treated or control liposome-treated mice (data not shown), again in agreement with what was seen by the Löscher group (Waltl et al. 2018), which shows that the difference in the percentage of mice experiencing seizures is not due to lower viral infection in the clodronate liposome-treated group.
Fig. 6.
Monocyte/Macrophage depletion decreases the development of acute seizures after TMEV infection. TMEV-infected C57BL/6J mice were treated with clodronate-containing liposomes and control liposomes, as described in the Methods. a) Representative flow cytometry plots of monocytes/macrophages (CD11blo/int F4/80hi) from spleens at 3 days post infection. b) Quantification of flow cytometry data represented in (a). Data is presented as the mean + SEM with a total of 10 mice per group. ****p<0.0001, Student’s t test. c) Percent mice with seizures (Racine scale, stages 3 to 5) in liposome-treated mice (20 mice per group). The percentage of mice with seizures was calculated as follows: (number of mice with seizures/total number of mice infected) × 100. ****p<0.0001, chi-square test. d) Quantification of microglia (CD45low/int CD11b+), macrophages (CD45hi CD11b+) and lymphocytes (CD45hi CD11b−) from the brains of liposome-treated mice. Data from two independent experiments is presented as the mean + SEM with a total of 20 mice per group. ****p<0.0001, Student’s t test
The various cell populations present in the brains of these monocyte/macrophage-depleted mice were examined via flow cytometry (as represented in Fig. 3a) on day 7 p.i. While there was no difference in the microglial (CD45lo/int CD11b+) cell population, the macrophage cell population (CD45hi CD11b+) was significantly reduced (p<0.0001, Student’s t test) and the lymphoid cell population (CD45hi CD11b−) was significantly increased (p<0.0001, Student’s t test) in the clodronate-containing liposome-treated mice compared to the control liposome-treated mice (Fig. 6d). Additionally, the population of patrolling macrophages (Ly-6C− cells) was significantly increased (p<0.01, Student’s t test) while the population of inflammatory macrophages (Ly-6C+ cells) was significantly reduced (p<0.001, Student’s t test) in the clodronate-containing liposome-treated mice compared to the control liposome-treated mice (data not shown). Therefore, the infiltration of inflammatory macrophages into the CNS of TMEV-infected mice is necessary for the development of acute seizures.
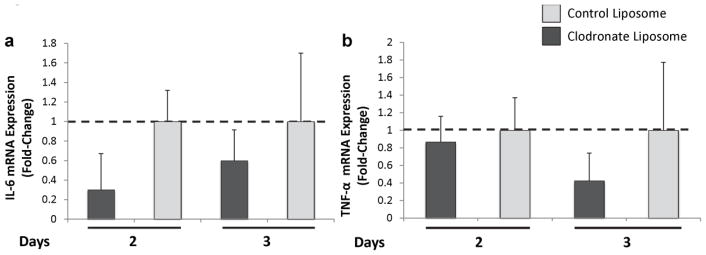
The pro-inflammatory cytokines IL-6 and TNF-α were previously shown to be important for the development of acute seizure, since TMEV infection of mice lacking IL-6 or TNF-α resulted in fewer mice having seizures. Also, infiltrating macrophages were shown to be enriched for IL-6 (Cusick et al. 2013; Kirkman et al. 2010; Libbey et al. 2011a). In contrast, TNF-α was shown to be enriched in microglial cells in the brains of TMEV-infected mice (Cusick et al. 2013). Transcriptional analyses for both IL-6 and TNF-α were performed on the hippocampus of TMEV-infected, control liposome-treated or clodronate-containing liposome-treated mice at days 2 and 3 p.i. We found that clodronate-containing liposome-treated mice tended to have lower expression of IL-6, as expected with a decrease in macrophages, at days 2 and 3 p.i. compared to control liposome-treated mice (Fig. 7a). We also found that expression of TNF-α tended to be lower in clodronate-containing liposome-treated mice at day 3 p.i. (Fig. 7b), possibly due to a lack of activation of microglia in the absence of macrophages, IL-6 and seizures. Therefore, the levels of IL-6 and TNF-α present in the hippocampus of TMEV-infected mice tended to decrease concomitant with the depletion of monocytes/macrophages. The Löscher group’s attempts to measure IL-6 by either cytokine array or immunohistochemistry failed due to technical difficulties (Waltl et al. 2018).
Fig. 7.
Gene expression analysis of IL-6 (a) and TNF-α (b) in the hippocampi of TMEV-infected mice treated with control liposomes or clodronate-containing liposomes. Mice were treated with control or clodronate-containing liposomes as described in the Methods and hippocampi were harvested at days 2 and 3 post infection (6 mice per group). Real time PCR for the detection of IL-6 (a) and TNF-α (b) was performed using TaqMan PCR as described in the Methods. Fold changes were calculated using the ΔΔCT method, in which fold change data are represented as 2−ΔΔCT. Error bars depict the SEM ΔCT values
Adoptively transferred monocytes, but not T cells, increase the percent of mice experiencing seizures
Having determined that the number of TMEV-infected mice experiencing seizures could be reduced by depleting monocytes (Fig. 6b), but not when T cell were lacking (Fig. 5b), we set out to determine whether we could increase the number of TMEV-infected mice experiencing seizures through the adoptive transfer of bone marrow-derived monocytes or spleen-derived T cells directly into the brains of TMEV-infected mice. Mice were infected with a substandard dose of 3 × 103 PFU of TMEV so that any increase in the number of mice experiencing seizures could be readily observed. Cells were injected on day 2 p.i. and animals were observed for seizures through day 7 p.i. The number of mice experiencing seizures following monocyte adoptive transfer was significantly increased over control mice receiving separation buffer alone (p<0.01, chi-square) (Fig. 8). TMEV-infected mice receiving T cells via adoptive transfer also had an increased number of mice experiencing seizures, compared to control mice; however this difference did not reach significance (Fig. 8). Therefore macrophage infiltration of the CNS is sufficient to drive seizure development in mice infected with a substandard dose of TMEV.
Fig. 8.
Percent mice with seizures (Racine scale, stages 3 to 5) in mice receiving monocytes and T cells via adoptive transfer. TMEV-infected C57BL/6J mice were injected with monocytes and T cells, as described in the Methods, and monitored for seizures through day 7 post infection. There were 38 mice in the control group receiving separation buffer alone, 36 mice in the group receiving monocytes and 19 mice in the group receiving T cells. The percentage of mice with seizures was calculated as follows: (number of mice with seizures/total number of mice infected) × 100. **p<0.01, chi-square test
Discussion
Epilepsy is the third most common neurological disorder affecting 70 million people worldwide (Ngugi et al. 2010). Although treatments, such as anticonvulsants, are available, they are only designed to suppress symptoms. Additionally, 30% of patients are refractory to established treatments [reviewed in (Laxer et al. 2014)]. Epilepsy is characterized by recurrent seizures, which happen in response to imbalances between excitatory and inhibitory impulses within the CNS. Glutamate is the most common excitatory neurotransmitter and excess glutamate is removed from the synaptic cleft by astrocytes in order to prevent hyperexcitation and excitotoxicity (Hu et al. 2000; Nadler 2012; Tilleux and Hermans 2008). It has been demonstrated that pro-inflammatory cytokines can prevent glutamate uptake by astrocytes (Hu et al. 2000), implying a mechanistic association between inflammation and the development of acute seizures.
While 50% of seizures are idiopathic (cause unknown), 50% can develop in response to CNS injuries, such as brain tumor, traumatic brain injury (TBI) or CNS infection (Annegers et al. 1988; Delorenzo et al. 2005). A common outcome for CNS injury is encephalitis (brain inflammation), which seems to be a major factor in the development of acute seizures (Misra et al. 2008). There are more than 100 neurotropic viruses that can infect humans, and patients who develop viral encephalitis have a 20% increased chance of developing seizures and are 16 times more likely to develop epilepsy (Bale 2015; Getts et al. 2008; Griffin 2003; Libbey and Fujinami 2011; Vezzani et al. 2016).
In order to study the role of inflammation in the development of acute seizures, we have used our mouse model of viral-induced seizures/epilepsy [reviewed in (DePaula-Silva et al. 2017)]. CNS infection of C57BL/6J mice with the Daniels (DA) strain of TMEV causes an acute viral infection followed by clearance of the virus (Lipton and Dal Canto 1979). Approximately 50% of these TMEV-infected mice experience acute behavioral seizures during this time (Libbey et al. 2008). While the acute seizures are known to occur between days 3 and 10 p.i. with the peak of seizure activity occurring around day 7 p.i., it was not known whether the peak of viral replication coincides with the peak of seizure activity. Therefore, the brains of TMEV-infected mice, sacrificed at various time points throughout the course of the acute viral infection, were examined for the presence of infectious virus via plaque assay. It was found that the viral titer peaks early, at day 1 or 2 p.i. (Fig. 1), and thus does not coincide with the peak of seizure activity. The fact that the peak of viral replication occurs prior to the onset of seizures suggests that it is the immune response to the viral infection and not the actual presence of the virus that causes seizures in this model. This supports previous data demonstrating that seizures cease even if virus persists (Kirkman et al. 2010).
As IL-6 has been shown to be important for the development of seizures in the TMEV model (Cusick et al. 2013; Kirkman et al. 2010; Libbey et al. 2011a), the brains of TMEV-infected mice, sacrificed at various time points over the first three days of acute viral infection, underwent transcriptional analysis for IL-6. The levels of IL-6 expression were high at 6 and 12 hours p.i., likely due to the viral infection, and elevated again at 24 hours p.i., possibly due to the start of cellular infiltration into the brain, and elevated again at 72 hours, coinciding with the first occurrence of seizures. The up-and-down course of IL-6 expression presented in Fig. 2 is supported by data obtained using a 32×3 Custom RT2 Profiler PCR array (Qiagen) (Libbey et al. 2017). Because seizures start as early as day 3 p.i., independent of the peak of viral replication but dependent on IL-6 (Cusick et al. 2017; Libbey et al. 2011a), it suggests that seizures are occurring due to activation of the innate immune response.
In order to better understand the role of immune cell infiltration in the development of acute seizures, examination, via flow cytometry, of the immune response to the viral infection was performed (Fig. 3). We found that cellular infiltration (CD45+ cells) occurred as early as day 3 p.i., when seizures start, and peaked at day 7 p.i., which is also the peak of seizure activity. Also, macrophage infiltration was highest at day 7 p.i. in mice that experienced seizures compared to mice that did not, confirming the involvement of this innate immune cell in acute seizure development.
The macrophage population (CD45hi CD11b+) of infiltrating cells was examined for the presence of both patrolling macrophages (Ly-6C− cells) and inflammatory macrophages (Ly-6C+ cells). The significantly higher level of inflammatory macrophages in mice experiencing seizures, compare to mice not experiencing seizures, on day 7 p.i., when seizure activity is at its peak, supports a role for inflammatory monocytes in the induction of seizures in this model (Fig. 4). The significant decrease of inflammatory macrophages by day 10 p.i., the day by which seizures resolve, suggests that acute seizures cannot be sustained in the absence of these cells. Furthermore, at day 10 p.i. there are significant more patrolling macrophages than inflammatory macrophages in the brains of infected mice, suggesting a role for these patrolling macrophages in resolving the inflammation.
The individual time course data, as discussed above, for seizure activity, infectious virus and the various immune responses examined can be compiled into an idealized graph, such as is shown in Fig. 9. The days from 1 to 10 p.i. (x-axis) span the course of the acute disease; the intensity of each response on the y-axis is arbitrary and not to scale. As can be seen from the graph, early viral replication induces an immune response in the form of infiltrating inflammatory macrophages and lymphocytes (CD4+ and CD8+ T cells). The rapid response by the inflammatory macrophages may serve to stem the replication of the virus as well as to induce and/or sustain seizures. The building and sustained response by the lymphocytes likely results in viral clearance. The late response by the patrolling macrophages likely serves to resolve the inflammation and support the repair of damaged tissue.
Fig. 9.
Time course of acute disease. Virus titer, seizure activity and cell infiltration of inflammatory and patrolling macrophages and lymphocytes are plotted for days 1 to 10 post infection. Note that the intensity of each response on the y-axis is arbitrary and not to scale
In continuing the examination of the role of the immune response in the development of acute seizures, manipulation of the immune response was undertaken to determine its effect on seizures. We manipulated the immune response through both depletion/deletion and supplementation. Monocytes/macrophages were depleted through administration of clodronate-containing liposomes and T cells were deleted through the use of RAG1−/− mice. Both monocytes and T cells were supplemented through adoptive transfer. We found that deletion of T cells did not affect the development of acute seizures (Fig. 5b). Although we observed an increase in natural killer (NK) cell infiltration in the brains of infected mice (data not shown), depletion of these cells using anti-NK1.1 antibody in C57BL/6J mice infected with TMEV did not significantly affect the number of mice experiencing acute seizures (Libbey et al. 2011a). In comparison, depletion of macrophages decreased significantly the number of mice experiencing seizures (Fig. 6c). Recently another group demonstrated that depletion of macrophages, via the same method as was used here, resulted in a significant reduction in the number of mice experiencing seizures without affecting the extent of hippocampal damage, which we did not examine (Waltl et al. 2018). Additionally, monocyte supplementation significantly increased the number of mice experiencing seizures (Fig. 8), supporting the suggestion that macrophage infiltration into the CNS is sufficient for the development of seizures in the TMEV model.
Neuroinflammation after viral infection is important in order to mount an efficient immune response against the infection and to prevent/repair tissue damage. Although macrophage infiltration is generally associated with inflammatory responses, macrophages may also play a role in resolving inflammation and repairing tissue. Due to their high plasticity, macrophages can respond and polarize into inflammatory or patrolling macrophages depending on the environmental stimuli. Inflammatory macrophages are known to secrete pro-inflammatory cytokines such as IL-6 and TNF-α. mRNA level of IL-6 and TNF-α were shown to be increased after TMEV infection (Kirkman et al. 2010; Theil et al. 2000). Additionally, IL-6, TNF-α and IL-1β were also found to be elevated in the serum and cerebrospinal fluid from patients with epilepsy (Baranzini et al. 2002; Ishikawa et al. 2015; Theil et al. 2000) and TBI, a condition that is associated with seizures (Yang et al. 2013). In agreement with these findings, TNF-α−/− mice and IL-6 knockout mice have significant fewer mice experiencing seizures following infection with TMEV than wild-type mice (Kirkman et al. 2010; Patel et al. 2017), supporting a role for these pro-inflammatory cytokines in the development of acute seizures. Furthermore, transgenic mice overexpressing IL-6 under the control of the astrocyte promoter glial fibrillary acidic protein (GFAP) within the CNS have been shown to develop spontaneous seizures (Campbell et al. 1993). Studies suggest that these pro-inflammatory cytokines may induce hyperexcitation and seizures by modulating glutamate signaling (Hu et al. 2000). However, although a clear relationship exists between neuroinflammation and the development of acute seizures, the mechanism of how seizures are triggered under this condition remains unknown. Identifying and understanding these mechanisms at the molecular level will allow for the development of new immunomodulatory epilepsy treatments.
Materials and Methods
Animal experiments
Four week old, male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). RAG1−/− mice, on a C57BL/6J background, were obtained from Dr. Matthew Williams, University of Utah, and bred in-house. All animal experiments were reviewed and approved by the University of Utah Institutional Animal Care and Use Committee (Protocols #12-09006 and #15-08004) and conducted in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council. All efforts were made to minimize suffering. Mice were euthanized through an overdose of isoflurane.
TMEV infection
C57BL/6J and RAG1−/− mice were anesthetized with isoflurane by inhalation and infected i.c. with 3 × 105 PFU (unless stated otherwise) of the DA strain of TMEV at a final volume of 20 μl per mouse. Mice were mock-infected with 20 μl phosphate-buffered saline (PBS) as a control. The DA strain of TMEV was propagated as previously described (Tsunoda et al. 1997).
Plaque assay
C57BL/6J mice infected with TMEV were euthanized on days 2, 3, 7, 10 and 22 p.i. Mock-infected mice were euthanized as a control. The mice were perfused with PBS, brains were harvested, divided in half, snap frozen in liquid nitrogen and stored at −80°C until use. Each half brain per mouse was weighed and homogenized in 0.5 ml PBS. The homogenate was centrifuged and the supernatant was examined for the presence of infectious virus via plaque assay (Zurbriggen and Fujinami 1989). The lower limit of detection for the plaque assay was 48 PFU/g brain tissue.
Seizure scoring
C57BL/6J and RAG1−/− mice infected with TMEV were weighed and monitored daily for seizures through day 7 p.i. The monitoring of seizure activity was performed as previously described (Libbey et al. 2011a). Briefly, mice were observed for 2 hours each day. Seizure activity was graded using the Racine scale: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling (Benkovic et al. 2004; Racine 1972).
Flow cytometry
Mice were euthanized on the indicated days and perfused with PBS. Subsequently, cells were mechanically isolated from both the brains and the spleens and suspended in RPMI-1640 media (Mediatech, Herndon, VA) supplemented with 1% L-glutamine (Mediatech), 1% antibiotics (Mediatech), 50 μM 2-mercaptoethanol (Sigma, St. Louis, MO) and 10% Cosmic calf serum (CCS) (Hyclone, Logan, UT). Cells from the brains were further purified with Histopaque-1083 (Sigma), while cells from the spleens were further purified by incubation in ACK red blood cell lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, pH 7.3). Cells were treated with Fc blocker (BioLegend, San Diego, CA), stained with the indicated anti-mouse antibodies for 30 min at 4°C [Brilliant Violet 650 anti-mouse CD11b (eBioscience), anti-mouse CD45 V500 (BD Bioscience), Brilliant Violet 421 anti-mouse F4/80, Pe-Cy7 anti-mouse Ly-6C, Brilliant Violet 785 anti-mouse CD4, Pe anti-mouse CD8a (all from Biolegend)], and analyzed by flow cytometry. Brain-derived cells were stained and analyzed individually for each mouse. Gating was determined by fluorescence-minus-one (FMO). More specifically, FMO controls contained each antibody conjugate used in the experiment except one, with the addition of the appropriate isotype control for the excluded fluorochrome. This was performed for each fluorochrome using TMEV-infected brain samples. Live cells were determined by forward and side scatter fluorescence on a BD LSRFortessa X-20 Cell Analyzer (BD Bioscience, San Jose, CA). Flow cytometry data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR).
Monocyte/macrophage depletion
Monocytes/macrophages were depleted in vivo through the induction of apoptosis by the administration of clodronate-containing liposomes, as per the manufacturer’s recommendations (ClodronateLiposomes.com, The Netherlands). Briefly, clodronate-containing liposomes and control liposomes (0.2 ml per injection) were administered to mice intraperitoneally on days −2, −1, 0, 2, 4 and 6 p.i. with TMEV.
IL-6 and TNF-α gene expression analysis
TMEV-infected mice were euthanized at 6, 12, 18, 24, 48 and 72 hours p.i. and perfused with PBS and mock-infected mice were euthanized at 72 hours p.i. and perfused with PBS. Clodronate-containing liposome-treated and control liposome-treated mice were euthanized on days 2, and 3 p.i. and perfused with PBS. Hippocampi were harvested, snap frozen in liquid nitrogen and stored at −80°C until use. RNA was isolated from frozen hippocampi using RNeasy Plus Mini (Qiagen, Chatsworth, CA). The RNA was quantified and stored at −80°C until use. First-strand cDNA was synthesized from 2 μg RNA per sample using a 50 μl reaction mixture containing the M-MLV Reverse Transcriptase (200U), random primers (6 μg), DTT (0.01 M) and dNTPs (0.2 mM) (all from Life Technologies). The first strand cDNAs were then diluted 1:20 and stored at −20°C until use. Real time PCR for the detection of IL-6 and TNF-α was performed using TaqMan Gene Expression Assays (Life Technologies) and TaqMan Gene Expression Master Mix (Life Technologies) in a 20 μl reaction volume containing 1 μl of cDNA template. The PCR reactions were assayed on a LC480 Light Cycler (Roche, Indianapolis, IN) 96-well block, using the following cycling parameters: denaturation at 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec, 60°C for 1 min. Relative expression levels were determined using ΔΔCt.
Monocyte adoptive transfer
Donor bone marrow cells were obtained from naive euthanized mice that were at least 8 weeks old. The bone marrow cells were isolated from the tibias and femurs of the donor mice by injecting PBS containing 5% CCS. Red blood cells were lysed with ACK buffer [0.15 M NH4Cl, 10 mM KHCO3 (pH 7.3)] for 5 min and the remaining cells were washed in PBS and counted. Monocytes were isolated from this cell suspension using the Monocyte Isolation kit (BM), mouse (Miltenyi Biotec, Auburn, CA) and LS Columns (Miltenyi Biotec), according to the manufacturer’s recommendation. Briefly, FcR blocking reagent was added to the cell suspension, followed by the mouse monocyte biotin-antibody cocktail. Cells were incubated for 5 min at 4°C and washed. Anti-Biotin Microbeads were added to the resuspended cells, the mixture was incubated for 10 min at 4°C and magnetic separation over a LS column was performed. The enriched monocytes were then counted. For the adoptive transfer, 1 × 106 monocytes were injected i.c. in a 20 μl volume on day 2 p.i. into C57BL/6J mice infected i.c. with 3 × 103 PFU of DA. Control mice were injected with separation buffer alone.
T cell adoptive transfer
Spleens were obtained from the same mice from which bone marrow was isolated. Red blood cells were lysed with ACK buffer for 5 min and the remaining cells were washed in PBS and counted. T cells were isolated from this cell suspension using the CD90.2 Microbeads, mouse kit (Miltenyi Biotec) and LS Columns, according to the manufacturer’s recommendation. Briefly, cells were washed, resuspended in buffer and incubated for 15 min at 4°C with CD90.2 microbeads. The cells were washed again and resuspended in buffer and magnetic separation over a LS column was performed. The enriched T cells were then counted. For the adoptive transfer, 1 × 106 T cells were injected i.c. in a 20 μl volume on day 2 p.i. into C57BL/6J mice infected i.c. with 3 × 103 PFU of DA. Control mice were injected with separation buffer alone.
Statistical analysis
The programs StatView (SAS Institute Inc., Cary, NC) and Prism (GraphPad Software, La Jolla, CA) were used for most statistical analyses performed. The Student’s t test was performed for pairwise comparisons. The chi-square test was utilized for nominal data (seizures: yes or no).
Acknowledgments
We would like to thank Jordan T. Sim, BA, Mitchell A. Wilson, Kelley M. Ingram and Samantha P. Duzy, for excellent technical assistance, Tyler Hanak, BS, John Sanchez, BS and Matthew F. Cusick, PhD for many helpful discussions. This work was supported by the National Institute of Health 5R01NS065714 and T32A1055434 (A.B.D-S.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Reference List
- Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407–1410. doi: 10.1212/wnl.38.9.1407. [DOI] [PubMed] [Google Scholar]
- Ashhurst TM, van Vreden C, Niewold P, King NJ. The plasticity of inflammatory monocyte responses to the inflamed central nervous system. Cell Immunol. 2014;291:49–57. doi: 10.1016/j.cellimm.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale JF., Jr Virus and Immune-Mediated Encephalitides: Epidemiology, Diagnosis, Treatment, and Prevention. Pediatr Neurol. 2015;53:3–12. doi: 10.1016/j.pediatrneurol.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, Laxer K, Bollen A, Oksenberg JR. Gene expression analysis reveals altered brain transcription of glutamate receptors and inflammatory genes in a patient with chronic focal (Rasmussen’s) encephalitis. J Neuroimmunol. 2002;128:9–15. doi: 10.1016/s0165-5728(02)00109-1. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O’Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Buenz EJ, Sauer BM, Lafrance-Corey RG, Deb C, Denic A, German CL, Howe CL. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol. 2009;175:668–684. doi: 10.2353/ajpath.2009.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Doty DJ, DePaula-Silva AB, Fujinami RS. The role of peripheral interleukin-6 in the development of acute seizures following virus encephalitis. J Neurovirol. 2017;23:696–703. doi: 10.1007/s13365-017-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaula-Silva AB, Hanak TJ, Libbey JE, Fujinami RS. Theiler’s murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: Models for multiple sclerosis and epilepsy. J Neuroimmunol. 2017;308:30–42. doi: 10.1016/j.jneuroim.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Balcar VJ, Matsumoto I, Muller M, King NJ. Viruses and the immune system: their roles in seizure cascade development. J Neurochem. 2008;104:1167–1176. doi: 10.1111/j.1471-4159.2007.05171.x. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht MF, Bouton AH. Functional significance of mononuclear phagocyte populations generated through adult hematopoiesis. J Leukoc Biol. 2014;96:969–980. doi: 10.1189/jlb.1RI0414-195R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Lafrance-Corey RG, Sundsbak RS, Lafrance SJ. Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. J Neuroinflammation. 2012a;9:50. doi: 10.1186/1742-2094-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Lafrance-Corey RG, Sundsbak RS, Sauer BM, Lafrance SJ, Buenz EJ, Schmalstieg WF. Hippocampal protection in mice with an attenuated inflammatory monocyte response to acute CNS picornavirus infection. Sci Rep. 2012b;2:545. doi: 10.1038/srep00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Kobayashi Y, Fujii Y, Kobayashi M. Increased interleukin-6 and high-sensitivity C-reactive protein levels in pediatric epilepsy patients with frequent, refractory generalized motor seizures. Seizure. 2015;25:136–140. doi: 10.1016/j.seizure.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol. 2014;35:358–367. doi: 10.1016/j.it.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193:2615–2621. doi: 10.4049/jimmunol.1400716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, Resnick T, Benbadis SR. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70. doi: 10.1016/j.yebeh.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Cusick MF, Doty DJ, Fujinami RS. Complement Components Are Expressed by Infiltrating Macrophages/Activated Microglia Early Following Viral Infection. Viral Immunol. 2017;30:304–314. doi: 10.1089/vim.2016.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler’s murine encephalomyelitis virus. Future Virol. 2011;6:1339–1350. doi: 10.2217/fvl.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011a;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Lack of correlation of central nervous system inflammation and neuropathology with the development of seizures following acute virus infection. J Virol. 2011b;85:8149–8157. doi: 10.1128/JVI.00730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Wilcox KS, White HS, Fujinami RS. Role for complement in the development of seizures following acute viral infection. J Virol. 2010;84:6452–6460. doi: 10.1128/JVI.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect Immun. 1979;26:369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49(Suppl 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JV. Plasticity of Glutamate Synaptic Mechanisms. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information; Bethesda, MD: 2012. Bookshelf ID: NBK98204. [PubMed] [Google Scholar]
- Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DC, Wallis G, Dahle EJ, McElroy PB, Thomson KE, Tesi RJ, Szymkowski DE, West PJ, Smeal RM, Patel M, Fujinami RS, White HS, Wilcox KS. Hippocampal TNFalpha Signaling Contributes to Seizure Generation in an Infection-Induced Mouse Model of Limbic Epilepsy. eNeuro. 2017;4 doi: 10.1523/ENEURO.0105-17.2017. e0105-0117.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia. 2010;51:1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Hilgendorf I, Robbins CS. From proliferation to proliferation: monocyte lineage comes full circle. Semin Immunopathol. 2014;36:137–148. doi: 10.1007/s00281-013-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil DJ, Tsunoda I, Libbey JE, Derfuss TJ, Fujinami RS. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler’s virus infections. J Neuroimmunol. 2000;104:22–30. doi: 10.1016/s0165-5728(99)00251-9. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Down-regulation of astrocytic GLAST by microglia-related inflammation is abrogated in dibutyryl cAMP-differentiated cultures. J Neurochem. 2008;105:2224–2236. doi: 10.1111/j.1471-4159.2008.05305.x. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, McCright IJ, Kuang LQ, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler’s murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Fujinami RS, White HS, Preux PM, Blumcke I, Sander JW, Loscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131:211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltl I, Kaufer C, Broer S, Chhatbar C, Ghita L, Gerhauser I, Anjum M, Kalinke U, Loscher W. Macrophage depletion by liposome-encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis. Neurobiol Dis. 2018;110:192–205. doi: 10.1016/j.nbd.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Yang SH, Gustafson J, Gangidine M, Stepien D, Schuster R, Pritts TA, Goodman MD, Remick DG, Lentsch AB. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J Surg Res. 2013;184:981–988. doi: 10.1016/j.jss.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A, Fujinami RS. A neutralization-resistant Theiler’s virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989;63:1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]