Abstract
Melatonin and its derivatives [N1-acetyl-N2-formyl-5-methoxykynurenine (AFMK) and N-acetyl serotonin (NAS)] have broad spectrum protective effects against photocarcinogenesis, including both direct and indirect antioxidative actions, regulation of apoptosis and DNA damage repair; these data were primarily derived from in vitro models. This study evaluates possible beneficial effects of melatonin and its active derivatives against UVB-induced harm to human and porcine skin ex vivo and to cultured HaCaT cells. The topical application of melatonin, AFMK or NAS protected epidermal cells against UVB-induced 8-OHdG formation and apoptosis with a further increase in p53ser15 expression especially after application of melatonin or AFMK but not after NAS use. The photoprotective action was observed in pre- and post-UVB treatment in both human and porcine models. Melatonin along with its derivatives up-regulated also the expression of antioxidative enzymes after UVB radiation of HaCaT cells. The exogenous application of melatonin or its derivatives represent a potent and promising tool for preventing UVB-induced oxidative stress and DNA damage. This protection results in improved genomic, cellular and tissue integrity against UVB-induced carcinogenesis, especially when applied prior to UV exposure. In addition, our ex vivo experiments provide fundamental justification for further testing the clinical utility of melatonin and metabolites as protectors again UVB in human subjects. These ex vivo experiments constitute the link between laboratory and clinical translation and warrant further experiments related to the clinical utility of melatonin in skin homeostasis.
Keywords: skin, epidermis, UVB, photoprotection, melatonin
INTRODUCTION
Highly energetic ultraviolet B (UVB) radiation (280–320 nm) penetrates skin to the depth of the papillary dermis and inflicts significant epidermal damage including direct DNA destruction [as cyclobutane pyrimidine dimers (CPD), pyrimidine (6-4) pyrimidone photoproducts (6-4PP), double-strand breaks (DSB) and others]. Moreover, it generates an oxidative environment, thus leading to mutagenic and carcinogenic effects [reviewed in [1, 2]]. Skin, as the outermost layer of the body that is exposed to noxious environmental insults including ultraviolet radiation, has developed precise mechanisms that protect or restore local (cutaneous) or impact whole body homeostasis [reviewed in [3, 4]]. UVB radiation, while inducing DNA damage activates p53 tumor suppressor, which synchronizes cellular responses and induces cell-cycle arrest, necessary for DNA repair or apoptosis; this allows for organized elimination of potentially cancerogenic cells [5–8]. In addition, in response to UVB-induced damage, the histone 2AX (H2AX) is phosphorylated (designated as γH2AX) by kinases and rapidly accumulated in the nucleus. γH2AX is considered a highly sensitive biomarker of cellular response to double strand breaks and DNA damage [9].
Cells also possess several protective mechanisms against oxidative stress, including free radical scavengers and antioxidative enzymes [2, 5]. One of them, the transcription nuclear E2-related factor 2 (Nrf2), plays a major, control role and, when activated, translocates to the nucleus where it heterodimerizes and binds to the antioxidant/electrophile response elements of phase-2 detoxification enzyme genes, including NADPH: glutathione S-transferase (GST), quinone oxidoreductase (NQO1) and heme oxygenase 1 (HO-1) [10].
Melatonin (N-acetyl-5-methoxytrypamine) is highly evolutionary conserved molecule with multifunctional activities, including regulation of circadian rhythms and cellular responses necessary for cell survival and restoration of homeostasis [reviewed in [11–16]]. Melatonin is produced by the pineal gland under the control of the suprachiasmatic nucleus as well as in other peripheral organs [11–14]. Similarly, melatonin is also produced and metabolized by skin [17–19]. There are three major metabolic pathways of melatonin in the skin: classical, indolic, and kynuric [17], of which the products 6-hydroxymelatonin, 5-methoxytryptamine (5MTT), N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), accumulate in the human epidermis, with 6-hydroxymelatonin being the most abundant metabolite [17, 20–23]. These metabolites are biologically active and may play roles in photoprotection, as defined by experiments with cultured keratinocytes and melanocytes [21, 24–31].
Melatonin, protects against oxidative stress in both direct and indirect manners, acting as an electron donor and interacting with reactive oxygen species, or activating an antioxidative enzymes and regulating their gene expression [31–36]. Melatonin protects skin cells against harmful effects of UV radiation, including oxidative DNA damage, depletion of mitochondrial membrane potential and apoptosis [24–26, 28, 30, 37].
To further define the photoprotective properties of melatonin and its metabolites, herein, we tested their effects using human and porcine skin ex vivo. Metabolites, i.e., AFMK and N-acetylserotonin (NAS) were selected based on our previous data showing their beneficial activity, similar to that of melatonin [25, 26].
Porcine skin is an excellent model for biomedical research because of its anatomical, histological [38], immunological [39], immunohistochemical [40] and genomic [41] similarities to human skin. We have also previously shown that human and porcine skin susceptibility to UV radiation is comparable [42, 43]. This study was designed to evaluate the possible beneficial effects of topical application of melatonin and its active derivatives against the UVB-induced damage as the crucial step before clinical testing their efficacy in human subjects.
Materials and Methods
Ex-vivo experiments
Tissue collection
Porcine (Large White cross Piétrain, male, 4-month-old, n=3) skin was collected from the Animal Resources Program upon approval of the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham (UAB). Human skin (from three male Caucasian donors, 40–69 years old, upon approval of the Institutional Review Board of the UAB) was collected from the leg after lower extremity amputations (Department of Pathology, UAB). The subcutaneous fat was removed, skin shaved, disinfected briefly in 70% ethanol, cut onto 1 cm2 pieces (in triplicate for each condition) and rinsed in PBS.
Pre-treatment experiment
Skin scraps (triplicate) were treated (applied topically exclusively on the epidermis) with either active compound, i.e., melatonin, AFMK, or NAS, diluted in ethanol up to the final concentration of 5×10−4 M or vehicle (ethanol as control) with the exact amount of 300 µl per each skin piece. Next, samples were histocultured (in triplicates) dermis down onto 12-well-plate filled partially air-medium interface in DMEM medium supplemented with 1% antibiotic/antimycotic solution (Mediatech, Inc. Manassas, VA) and insulin (5 µg/mL) in 37°C, 5% CO2 for 4 h. Thereafter, skin was placed on Waltham paper coated 60-mm-dishes submerged in PBS and irradiated with 400 mJ/cm2 of UVB and maintained in 12-well-plate culture with fresh media for 24 h in the above described conditions. Based on a preliminary UVB dose-response experiments, 400 mJ/cm2 was the most effective dose for oxidative stress and stress-response activation [44, 45] without necrotic signs observed in human and porcine skin (Fig. 1).
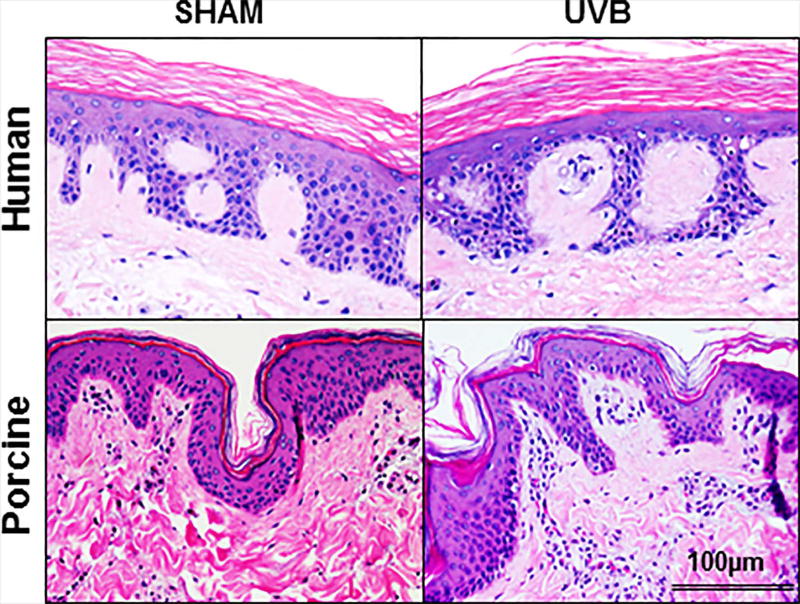
Figure 1.
A single (400 mJ/cm2) dose of ultraviolet B (UVB) does not lead to a visible damage of human and porcine skin. There is lack of detectable epidermal necrosis, keratinocyte apoptosis or acute inflammatory responses in UVB-treated and sham-irradiated skin. H&E staining. Scale bar 100µm.
Post-treatment experiment
Skin scraps (triplicates) were placed dermis down on Waltham paper coated 60-mm-petri-dish filled with PBS and irradiated with 400 mJ/cm2 of UVB or sham treated. Soon thereafter, treatment with either active compound (melatonin, AFMK or NAS dissolved in ethanol as vehicle, final concentration of 5×10−4 M) or ethanol used as control was performed. Next, skin scraps were cultured under the same conditions as described above (pre-treatment) for 24 h. After incubation, skin samples were frozen (−80° C) for RNA isolation with further qRT-PCR or protein isolation for quantitative Western Blot analyses. The third skin scrap from each condition was fixed in 4% buffered (pH=7.4) paraformaldehyde overnight for quantitative immunohistochemistry (IHC) or TUNEL assay.
Irradiation protocol
Skin was irradiated with 400 mJ/cm2 of UVB light (290–320 nm) filtered with Kodacell filter (cuts off any wavelengths shorter than 290 nm) using lamp (USHIO G15T8E, USHIO America, Inc., USA), as described previously [44, 45]. The dose of UVB irradiation was chosen based on a preliminary studies (the most effective and not harmful) and time was calculated based on the formula: Time [s] = Dose/Intensity [J × cm−2/W × cm−2]. The dosimetry (calculation of bulb intensity and wavelength specificity) was performed by The Rapid Precision Testing Laboratories (Cordova, TN) from the same distance as experimental irradiation (2.5 inches) as described previously [46].
RNA isolation and qRT-PCR
The epidermis was dissected from the frozen dermis and immediately homogenized in TRIzol reagents (Invitrogen, Carlsbad, CA) to isolate RNA according to the Chomczynski’s method as described earlier in [44]. The RNA concentration was quantified, and 1 µg was reversely transcribed with SuperScript First-Strand Synthesis System (Applied Biosystems, Foster City, CA). Primers used for PCR amplification are listed in Table 1a,b and were designed using the Universal Probe Library (Roche, https://www.roche-applied-science.com) and synthesized by Integrated DNA Technologies (Coralville, IA). The PCR reaction was performed in triplicate with SYBR Green I Master Mix (Roche, Manheim, Germany). The data were collected on a Light Cycler 480 (Roche). The amount of amplified product for each gene was compared with housekeeping genes, i.e., β-actin, cyclophilin B or GAPDH utilizing a comparative ΔΔCT method and presented as the fold change ± SD.
Table 1.
List of primer sequences used for qRT-PCR reaction in human (a) and porcine skin (b).
| a) | |||
|---|---|---|---|
| Target | Gene symbol |
Forward sequence 5’-3’ |
Reverse sequence 5’-3’ |
| Transcription factor NF-E2-related factor 2 | NFE2L2 | TTCTGTTGCTCAGGTAGCCCC | TCAGTTTGGCTTCTGGACTTGG |
| Glutamylcysteine synthetase (GCS) | GCLC | TTGCAGGAAGGCATTGATCA | GCATCATCCAGGTGTATTTTCTCTT |
| Catalase | CAT | CGTGCTGAATGAGGAACAGA | AGTCAGGGTGGACCTCAGTG |
| Glutathione-S-transferase (GSTP1) | GSTP1 | CCTGGTGGACATGGTGAATGA | CCTGGTGCAGATGCTCACATAGT |
| Manganese-dependent superoxide dismutase | SOD2 | GCTCATGCTTGAGACCCAAT | CACCCGATCTCGACTGATTT |
| Glutathione peroxidase GPx | GPX1 | ACGATGTTGCCTGGAACTTT | GATGTCAGGCTCGATGTCAA |
| Cyclophilin B | PPIB | TGTGGTGTTTGGCAAAGTTC | GTTTATCCCGGCTGTCTGTC |
| β-actin | ACTB | TCA AGG AGA AGC TGT GCT ACG | TCC AGG GAG GAGGAG GAC |
| b) | |||
|---|---|---|---|
| Target | Gene symbol |
Forward sequence 5’-3’ |
Reverse sequence 5’-3’ |
| Manganese-dependent superoxide dismutase | SOD2 | GGA ATT CCA GCT GCA CCA CAG CGA GC | GGA ATT CGA TCC CCA GCA GCG GAA CC |
| β-actin | ACTB | TCA AGG AGA AGC TGT GCT ACG | TCC AGG GAG GAG GAG GAC |
Immunohistochemistry
Paraformaldehyde fixed skin was paraffin-embedded and cut in 10 µm thick sections and mounted on silanized slides (Dako, Carpinteria, CA). Following deparafinization and rehydration, sections were rinsed in PBS, and antigen retrieval was performed by boiling in citrate buffer (pH=6.0) for 20 min. After rinsing in PBS, the non-specific blocking and permeabilization (5% donkey serum, 0.1% BSA, 0.2% Triton X-100 in PBS) was performed for 1 h at room temperature (RT). Next, the primary antibody (listed in Table 2a) diluted in blocking solution were applied overnight at 4° C. After thorough rinsing in PBS, the species-specific secondary antibody-HRP conjugated were applied for 1 h at RT. The 3,3'Diaminobenzidine (DAB) was used for chromogenic reaction which was stopped with water, slides were dried, preserved with mounting medium, and covered with a cover glass. The positive signals inside the epidermis layer were calculated using ImageJ software (National Institutes of Health) and statistically compared between conditions to the appropriate controls and presented as number of positive cells per 100 consecutive epidermal cells. Six areas per section, separated by at least 100 µm, were investigated. The negative controls consisted of tissues incubated without primary antibody (omission) or with non-immune rabbit or mouse serum (replacement).
Table 2.
| A. List of primary antibodies used for IHC. | |||
|---|---|---|---|
| Antigen | Host | Titer | Vendor |
| 8OHdG | mouse | 1:200 | JaICA, Japan (MOG-020P) |
| γH2AX | rabbit | 1:500 | Bethyl Laboratories, Inc., USA (A300-081A-M) |
| p53ser15 | rabbit | 1:1,000 | Cell Signaling, USA (9284) |
| Nrf2 | rabbit | 1:4,000 | Santa Cruz Biotechnology, USA (sc-722) |
| B. List of primary antibodies used for WB. | |||
|---|---|---|---|
| Antigen | Host | Titer | Vendor |
| Nrf2 | rabbit | 1:1,000 | Santa Cruz Biotechnology, USA(sc-722) |
| Phospho-p53 (Ser15) | rabbit | 1:1,000 | Cell Signaling, USA (9284) |
| Catalase | goat | 1:1,000 | Santa Cruz Biotechnology, USA(sc-34285) |
| CuSOD | goat | 1:1,000 | Santa Cruz Biotechnology, USA(Sc-8637) |
| MnSoD | goat | 1:1,000 | Merck Millipore, USA (06-984) |
| β-actin-HRP | mouse | 1:10,000 | Sigma-Aldrich, USA (A3854) |
| Lamin A | rabbit | 1:10,000 | Santa Cruz Biotechnology, USA(sc-20680) |
TUNEL assay
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labeling (TUNEL) assay, using TdT-catalyzed polymerization of nucleotides to free 3’-OH DNA ends in a template-independent manner, was used to label DNA strand breaks and identify apoptotic cells. The TUNEL assay was performed using the DeadEnd Fluorometric TUNEL system according to the manufacturer’s protocol (Promega, Madison, WI). Briefly, sections were deparaffinized, rehydrated and permeabilized with proteinase K. After labeling DNA strand breaks by recombinant TdT (rTdT) enzyme with fluorescent nucleotides (fluorescein-12-dUTPs) sections were mounted with medium containing DAPI. In the negative controls, rTdT enzyme was omitted.
In vitro experiments
Cell culture
Immortalized human epidermal keratinocytes (HaCaT) were cultured in Dulbecco’s minimal essential media (DMEM) supplemented with 5% charcoal stripped fetal bovine serum (Serum Source International, Inc. Charlotte, NC) and 1% Antibiotic-Antimycotic Solution (Mediatech, Inc. Manassas, VA).
UVB irradiation
UVB irradiation was performed using a Biorad UV transluminator 2000 (Bio-RAD Laboratories, Hercules, CA, USA) as previously described [37, 47, 48]. Cells were covered with a thin layer of phosphate buffered saline (PBS). The culture dishes were exposed to achieve a single dose of 400 mJ/cm2.
Western blot analysis
HaCaT cells were plated at a density of 5×105 cells/dish in 10 cm tissue culture dishes for 24 h. For a pre-treatment experiment, melatonin, AFMK or NAS were applied for 30 min at the concentration of 5×10−5 M or with vehicle control (0.1% ethanol) and replaced with PBS and irradiated with UVB 400 mJ/cm2. For post-treatment, cells were irradiated with UVB 400 mJ/cm2 and further treated with melatonin, AFMK or NAS, for 30 min at the concentration 5×10−5 M or with vehicle control (0.1% ethanol). Immediately after treatment, the cells were harvested. Nuclear and cytoplasmic fractions of total proteins were isolated separately using a Nuclear Extract Kit according to the manufacturer's instructions (Active Motif, Carlsbad, CA, USA). Western blot analyses were performed to detect phosphorylated p53, Nrf2, catalase (CAT), CuSOD (superoxide dismutase) and MnSOD protein expression as previously described [49]. The proteins were resolved on the 8–16% (w/v) SDS-PAGE gel and transferred to the PVDF membrane. The membranes were blocked in 5% (w/v) skim milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h and incubated at 4 °C overnight with the primary antibody (listed in Table 2b) diluted in 5% skim milk. The membranes were washed with PBS and incubated for 1 h at RT with the HRP-conjugated secondary antibodies (goat anti-mouse IgG, donkey anti-rabbit IgG and donkey anti-rabbit IgG from Santa Cruz Biotechnology; cat no: sc-2005, sc-2077, and sc-2020; respectively) at the titration 1:5,000 in Tris-buffered saline containing 0.1% Tween 20. For chemiluminescent detection, the membranes were incubated with SuperSignal WestPico Substrate (Thermo Scientific) for 5 min and the membranes were subsequently exposed to an autoradiography film (MidSci, St. Louis, MO, USA) and developed with film processor (SRX-101A; Konica, Taiwan) as previously described [50]. The protein expressions were normalized to the expression of loading controls: anti-β-actin-peroxidase (A3854, Sigma) (1:10,000) for cytosolic fraction or rabbit-anti-Lamin A (sc-20680, Santa Cruz Biotechnology) (1:1,000) for nuclear fraction.
Statistics
Data are presented as means ± SD where the level of differences between two groups was assessed with t-test with the significance level set up at P<.05, P<.01, P<.001, P<.0001 and presented as *, **, ***, ****, respectively. Statistical analysis was performed using GraphPad Prism, version 4.00 (GraphPad Software, Inc. CA, USA).
Results
Skin morphology
Figure 1 shows that a single dose of UBV (400 mJ/cm2) has morphologically no detectable effect on the viability of either human and porcine skin during the experimental observation.
Effects of melatonin, AFMK and NAS on UVB-induced oxidative stress and DNA damage in human and porcine skin
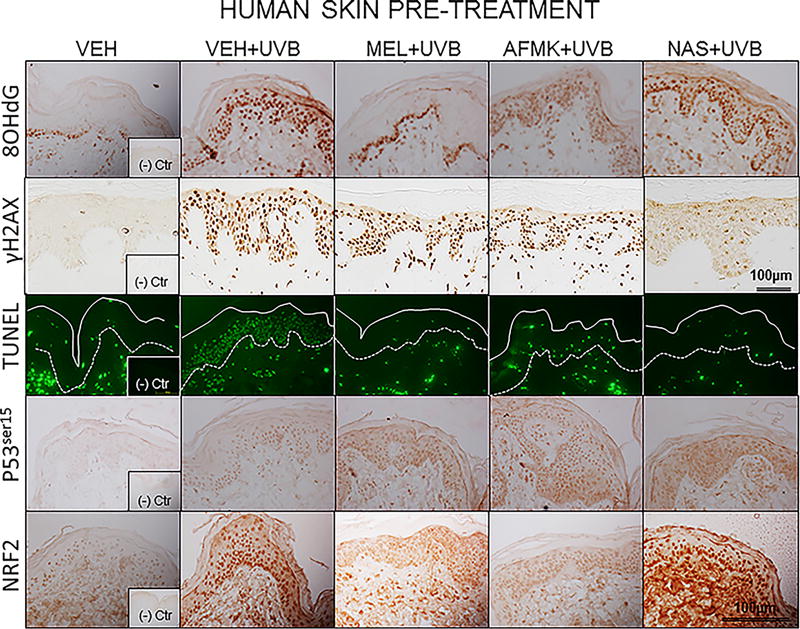
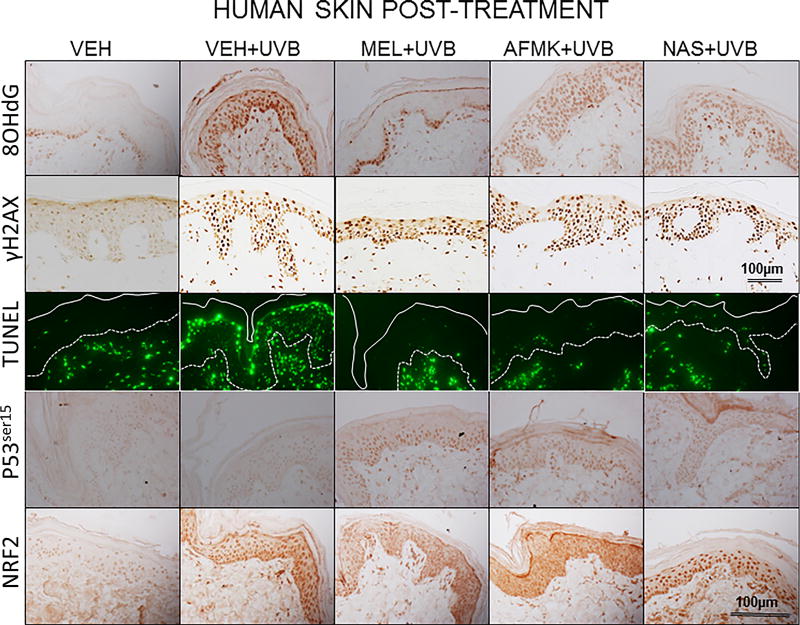
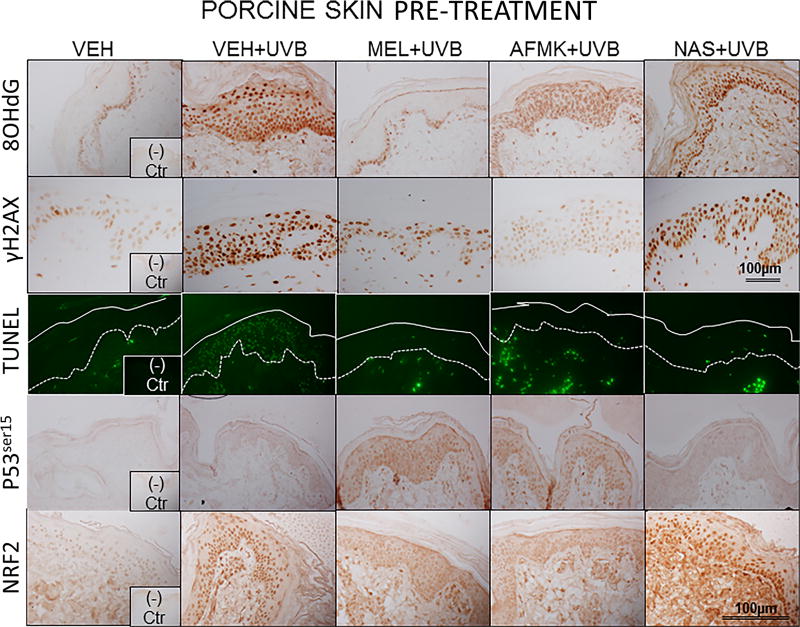
The representative images of 8-OHdG formation, expression of γH2AX, p53ser15 and Nrf2 and apoptotic cells, as assessed by TUNEL method are depicted on Figure 2A, B in human and Figure 3A, B in porcine skin treated with melatonin, AFMK or NAS prior to (Fig. 2A, 3A) or after (Fig. 2B, 3B) UVB exposure and sham-irradiated controls.
Figure 2.
The representative images of the indicators of oxidative stress (8OHdG), DNA damage (γH2AX), apoptosis (TUNEL), and DNA-repair and antioxidative mechanisms (p53ser115 and Nrf2) after a single dose of 400 mJ/cm2 of UVB applied to human skin ex vivo. Skin was topically treated with melatonin, AFMK or NAS 4 h prior to (A) or immediately after irradiation (B).
Figure 3.
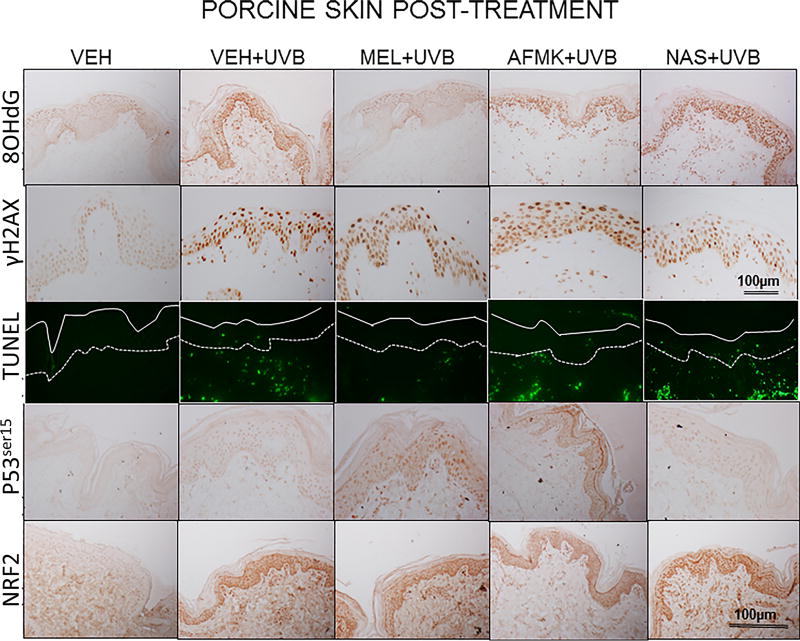
The representative images of the indicators of oxidative stress (8OHdG), DNA damage (γH2AX), apoptosis (TUNEL), DNA-repair and antioxidative mechanisms (p53ser115 and Nrf2) after a single dose of 400 mJ/cm2 of UVB applied to porcine skin ex vivo. Skin was topically treated with melatonin, AFMK or NAS 4 h prior to (A) or immediately after irradiation (B).
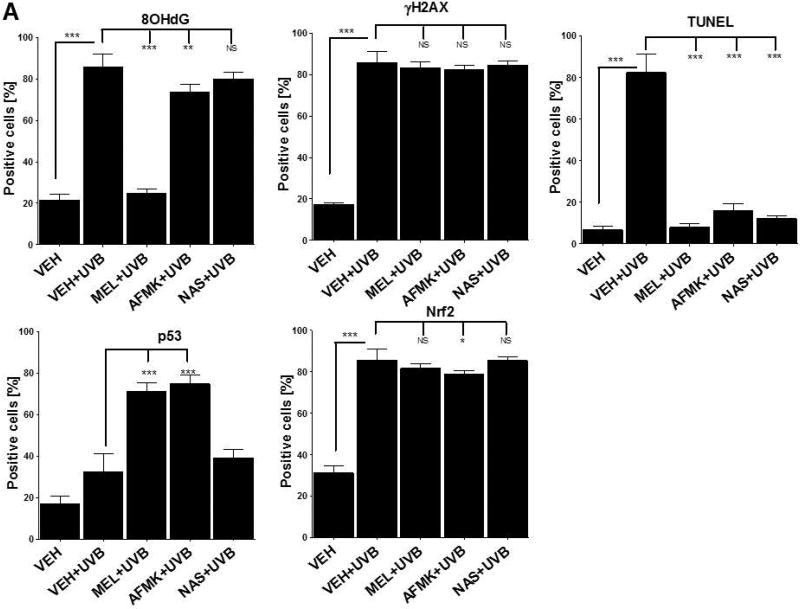
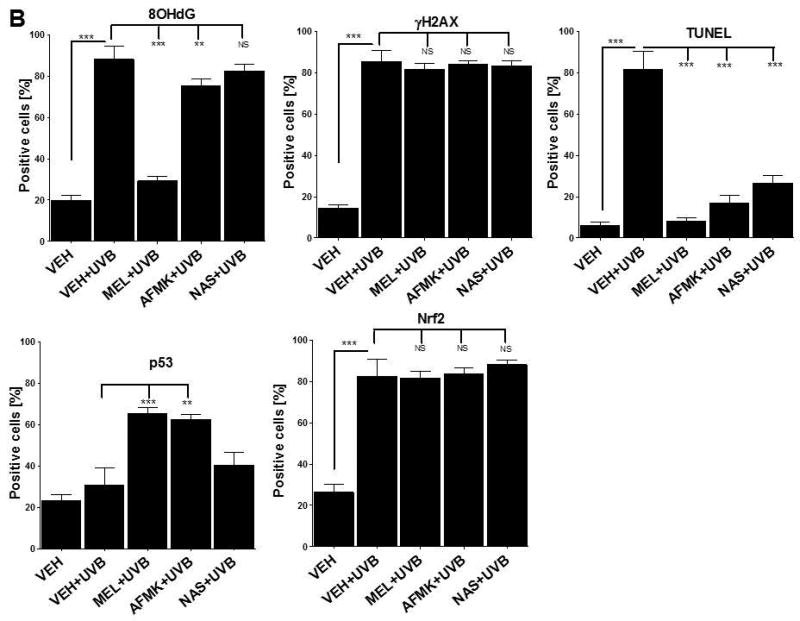
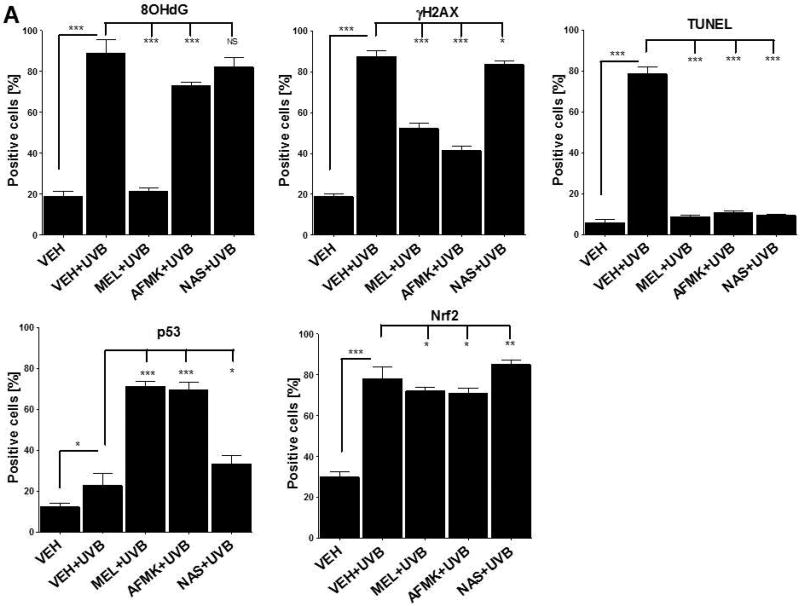
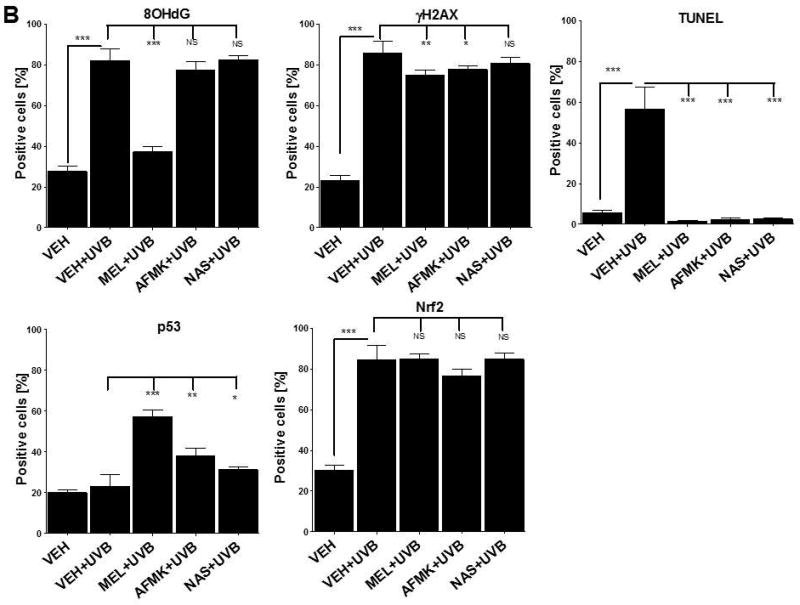
UVB exposure markedly stimulated the formation of 8-OHdG in human and porcine epidermal cells in comparison to a control (sham-irradiated skin) in which the formation of this oxidative stress biomarker was very low (Figs. 4, 5). Both pre-treatment (Fig. 4A, 5A) or post-treatment (4B, 5B) with melatonin or AFMK to human and porcine skin, respectively, led to a significant decrease in the 8-OHdG formation exposed beforehand to UVB, with the strongest photoprotective effects observed for melatonin. Treatment of human or porcine skin with NAS before or after UVB exposure showed no significant differences in 8-OHdG expression and the quantified levels of 8-OHdG-positive cells were similar to the control (vehicle)-treated tissues (Figs. 4, 5).
Figure 4.
The quantitative analysis of the modulatory effects of topical treatment with melatonin, AFMK or NAS on 8OHdG, γH2AX, TUNEL, p53ser115 and Nrf2 expression in human skin. The compounds were topically applied 4 h prior to (A) or immediately after (B) UVB exposure. Data are presented as the percentage of positively-stained cells per 100 consecutive epidermal cells ± SD; the statistical significance of the differences was evaluated by Student’s t test; where *P < 0.05, **P < 0.01, ***P < 0.0001, and NS – lack of significance.
Figure 5.
The quantitative analysis of the inhibitory effects of topical treatment with melatonin, AFMK or NAS on 8OHdG, γH2AX, TUNEL, p53ser115 and Nrf2 expression in porcine skin. The compounds were topically applied 4 h prior to (A) and immediately after (B) UVB radiation. Data are presented as the percentage of positively-stained cells per 100 consecutive epidermal cells ± SD; the statistical significance of the differences was evaluated by Student’s t test; where *P < 0.05, **P < 0.01, ***P < 0.0001, and NS – lack of statistical significance.
UVB radiation resulted also in significant increases of γH2AX expression in human and porcine epidermis when compared to a very low expression in sham-irradiated samples (Figs. 4, 5). The topical pre- or post-application of melatonin, AFMK or NAS did not affect the expression of γH2AX compared to ethanol (vehicle) application alone (Figs. 4A, 4B) in human skin. On the contrary, when active compounds were applied on porcine skin the reduction in the number of γH2AX-positive cells was observed especially for melatonin and AFMK and in a lesser extend found only for pretreatment with NAS (Fig. 5A, 5B).
Similary to 8-OHdG and γH2AX, the exposure of human and porcine skin to UVB radiation significantly increased the number of apoptotic cells as compared to sham-irradiated control skin samples, as assessed by the TUNEL method (Fig. 4, 5). In addition, both the pre- and post-treatment with melatonin, AFMK or NAS significantly protected the epidermal cells of human and porcine skin from apoptosis (Fig. 4A, 4B, 5A, 5B). The strongest protective effects were observed with melatonin or AFMK followed by NAS. In parallel these changes were accompanied by gene expression of human skin where the down-regulation of anti-apoptotic Bcl2 gene in melatonin- compared to the control (vehicle)-treated tissues was observed (Table 3).
Table 3.
Effect of pre- and post-treatment with melatonin, AFMK and NAS on expression of apoptosis and oxidative stress genes in human skin. The expression is presented as a fold change in relation to the expression in control (ethanol-treated samples).
| Gene | Human skin | |
|---|---|---|
|
|
||
| Pre-treatment | Post-treatment | |
| BCL2 | ||
| melatonin | 0.09±0.19** | 0.01±0.22** |
| AFMK | 0.01±0.38* | 0.02±0.33* |
| NAS | 0.02±0.35* | 0.04±0.36* |
| NFE2L2 | ||
| melatonin | 0.81 ± 0.39 | 2.18 ± 0.34** |
| AFMK | 1.83 ± 0.36* | 2.81 ± 0.21*** |
| NAS | 1.64 ± 0.32* | 3.15 ± 0.38*** |
| GCLC | ||
| melatonin | 2.76±0.34*** | 34.94±0.30**** |
| AFMK | 4.91±0.25**** | 32.93±0.28**** |
| NAS | 3.49±0.34*** | 82.48±0.33**** |
| CAT | ||
| melatonin | 7.07±0.36**** | 2.40±0.32** |
| AFMK | 3.28±0.36*** | 1.94±0.40* |
| NAS | 3.38±0.430*** | 2.05±0.21** |
| GSTP1 | ||
| melatonin | 2.30±0.47** | 18.48±0.29**** |
| AFMK | 3.42±0.44*** | 8.29±0.29**** |
| NAS | 1.73±0.39* | 34.39±0.26**** |
| SOD2 | ||
| melatonin | 5.19±0.47**** | 2.54±0.25** |
| AFMK | 4.80±0.21**** | 4.80±0.37*** |
| NAS | 3.14±0.31*** | 3.55±0.21*** |
| GPX1 | ||
| melatonin | 15.78±0.32**** | 2.83±0.20*** |
| AFMK | 21.54±0.18**** | 2.61±0.38** |
| NAS | 4.63±0.34**** | 4.37±0.34**** |
t-test:
p<0.05,
p<0.01,
p<0.001,
p<0.0001
Interestingly, UVB irradiation did not stimulate the expression of p53ser15 in control samples (Fig. 4, 5). While UVB exposure combined with pre- and post-treatment with melatonin or AFMK but not with NAS significantly increased p53ser15 expression in human skin (Fig. 4A, 4B). While, in porcine skin, also the treatment with NAS, in addition to melatonin and AFMK led to a statistical increase in p53ser15 expression (Fig. 5A, 5B).
Not surprisingly, UVB irradiation significantly stimulated Nrf2 expression in human (Fig 4) and porcine skin (Fig. 5) in comparison to sham-irradiated controls in which this antigen expression was very low. Only the pre-treatment with AFMK for human and AFMK or melatonin for porcine skin resulted in slight decrease of Nrf2-positive cells when compared to control UVB-treated samples (Fig. 4A, 4B, 5A, 5B). However, the treatment of porcine skin with NAS before UVB irradiation resulted in significant rise of Nrf2 expression (Fig. 5A).
Gene expression analysis revealed that UVB irradiation significantly simulated the expression of antioxidant defense system in human skin (Table 3). AFMK and NAS applied before or after UVB exposure on human skin samples resulted in up-regulation of Nrf2 expression. Melatonin led to a significant up-regulation of Nrf2 only when applied after irradiation. UVB exposure significantly stimulated also the expression of glutamylcysteine synthetase (GCS), glutathione S-tranferase (GSTP1), glutathione peroxidase (GPX1) and MnSOD in human skin treated with melatonin, AFMK or NAS before or after irradiation (Table 3). In porcine skin, UVB exposure resulted in up-regulation of MnSOD expression, as assessed with qRT-PCR, especially when melatonin, AFMK or NAS were applied on skin before or after irradiation (data not shown).
Attenuating effects of melatonin, AFMK and NAS on UVB-damage in HaCaT keratinocytes
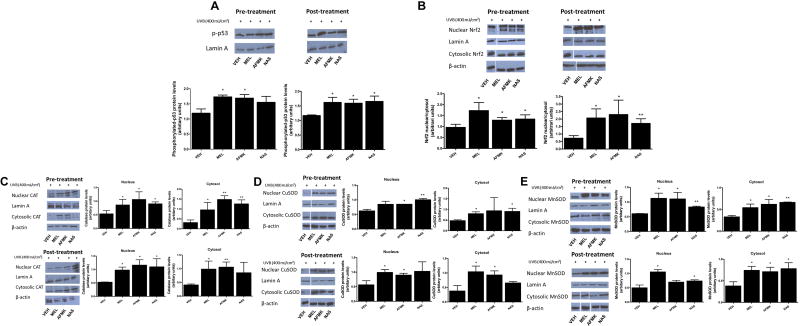
Application of melatonin, AFMK or NAS prior to or after UVB-irradiation on HaCaT cells affected the expression of proteins involved in DNA repair and antioxidative responses (Fig. 6). melatonin, AFMK or NAS applied after UVB irradiation and melatonin and AFMK applied before UVB exposure resulted in significantly increased phosphorylated p53 (p-p53) expression as compared to the cells treated with vehicle (Fig. 6A). The expression of Nrf2 was elevated after UVB irradiation when HaCaT cells were treated with melatonin, AFMK or NAS both before or after irradiation and quantitative analysis showed significant rises in the nuclear-to-cytosolic ratio in treated cells in comparison to the control cells (Fig. 6B). Similarly, both cytoplasmic and nuclear expression of antioxidative enzymes were affected after UVB exposure when cells were treated with melatonin, AFMK or NAS. CAT expression was increased (both cytoplasmic and nuclear fraction) when melatonin, AFMK or NAS were applied prior to UVB irradiation. When cells were treated with melatonin, AFMK or NAS after UVB the expression of CAT was also increased, except the cytoplasmic fraction for NAS (Fig. 6C). Similarly, the expression of CuSOD was stimulated when melatonin, AFMK or NAS were applied before UVB irradiation, except for the nuclear fraction for melatonin and cytoplasmic fraction for AFMK. Application of melatonin and AFMK after UVB exposure resulted in rises in CuSOD, while NAS showed no effect on CuSOD expression when compared to the control (Fig. 6D). MnSOD expression was significantly elevated when melatonin, AFMK or NAS were applied before exposure to UVB, while application of each compound after UVB irradiation resulted in increases in cytoplasmic MnSOD and nuclear MnSOD only for melatonin and NAS treatment (Fig. 6E).
Figure 6.
Treatment with melatonin, AFMK or NAS increased the UVB-induced phosphorylation of p53 in HaCaT cells (A), nuclear translocation of Nrf2 (B), catalase (C), CuSOD (D), and MnSOD (E) protein expression. In pre-treatment experiment, cells were treated with melatonin, AFMK or NAS, at the concentration 5 ×10−5 M or 0.1% EtOH (vehicle control) for 30 min and subsequently, under PBS, irradiated with UVB 400 mJ/cm2. For post-treatment experiment, cells were irradiated with UVB 400 mJ/cm2 and then treated with melatonin, AFMK or NAS at the concentration 5 ×10−5 M or 0.1% EtOH (vehicle control) for 30 min. Cells were harvested immediately after treatment and western blotting was performed to evaluate the phosphorylation rate of p53, nuclear translocation of Nrf2, catalase, CuSOD, and MnSOD protein expression. Phosphorylated p53 was detected at 53 kDa, Nrf2 at 61 kDa, catalase at 64 kDa, CuSOD at 23 kDa, MnSOD at 24 kDa, lamin A (a loading control for nuclear protein) at 69 kDa, and β-actin (a loading control for cytosol protein) at 42 kDa. Data was expressed as means ± SD. The statistical significance of differences was evaluated by Student’s t test. *P < 0.05; **P < 0.01 versus EtOH (vehicle control).
Discussion
The present ex vivo studies provide evidence that melatonin and its active derivatives including AFMK and NAS applied both prior to or after UVB irradiation protect epidermal keratinocytes from DNA damage, apoptosis and oxidative stress by enhancing the expression of repair, anti-apoptotic proteins and antioxidant enzymes (p53, Nrf2, GCS, GSTP1, GPX1, CAT, CuSOD and MnSOD). The strongest photoprotective effects were observed for melatonin and AFMK, while NAS exhibited weaker efficacy limited to only few analyzed genes and markers of cellular damage. This protection was strongly pronounced when skin was treated with melatonin or its metabolites prior UVB exposure. It should be noted that only slight to moderate effects of melatonin or its derivatives on Nrf2 and γH2AX expressions were observed in ex vivo experiment. Previously it was demonstrated that melatonin and/or its metabolites protect skin cells, including keratinocytes [37], melanocytes [26], fibroblasts [51, 52], and leukocytes [29, 30] from UV-induced DNA damage. Melatonin in cultured cells exposed to UV radiation also protected against the mitochondrial membrane potential reduction and membrane lipid peroxidation [52, 53]. In addition, photoprotective properties of melatonin are related to the suppression of the activation of mitochondrial pathway-related initiator caspase 9 and down-regulation of effector caspases as caspase 3 and caspase 7, resulting in reduction or prevention of apoptosis of keratinocytes [37, 53]. In present study, we found both a significantly reduced number of apoptotic epidermal cells, as assessed by TUNEL method, and a lower expression of anti-apoptotic Bcl-2 gene in skin samples treated with melatonin, AFMK and NAS, confirming the protective properties of melatonin and its active derivatives against apoptosis in ex vivo skin models.
Melatonin and its metabolites exhibit broad spectrum of photoprotection properties, including direct and indirect antioxidative actions, represent optimal protectors against UV-induced oxidative damages [12, 36]. Moreover, melatonin and AMK act as free radical scavengers [54, 55]. In a cell culture-based study, melatonin and its derivatives reduced the UVB-induced reactive oxygen species generation in keratinocytes and melanocytes [25, 26] and in an ex vivo human skin model, melatonin applied on skin before irradiation protected against 8-OHdG formation by ultraviolet irradiation [28]. Similarly, in present study, in ex vivo human and porcine skin treated with melatonin or AFMK, but not NAS, the number of epidermal cells exhibiting oxidative DNA damage, i.e., 8OHdG-positive cells, was significantly reduced. In addition, this photoprotective action of melatonin and AFMK was apparent in samples treated both before and after UVB irradiation. Melatonin also prevents the depletion or increases the expression of antioxidative enzymes [28, 56]. Fischer et al [28] found that pre-incubation of skin samples with melatonin prevented the depletion of CAT, GPx, Cu/ZnSOD, MnSOD gene expression after UVB irradiation. In our ex vivo models, we found that treatment of skin samples with melatonin and its metabolites not only prior to UVB irradiation, but also after exposure to UVB, resulted in up-regulation of the expression of antioxidative enzymes, including GCS, CAT, GSTP1, MnSOD and GPX1; in the cell-based experiments we found increased expression of CuSOD, MnSOD and CAT. The current studies using ex vivo models are thus in agreement with other reports showing the antioxidative properties of melatonin and its metabolites and indicate that the protective effects are observed also when those compounds are applied after UVB exposure.
Exposure of keratinocytes to UV rays induced phosphorylation of H2AX and, under certain conditions, this process is a dose-dependent [9]. Similarly, our study shows that treatment of porcine skin with melatonin or AFMK, especially prior to UVB exposure, protected cells from UV-induced genotoxic effects, as assessed by the number of γH2AX-positive epidermal cells; there were also slight protection effects, limited to NAS, when applied after UVB irradiation. These results document the protective effects of melatonin and AFMK against DNA damage, and show that these molecules are most protective when applied before UVB irradiation.
Melatonin induces the translocation of Nrf2 from the cytosol into the nucleus after UV irradiation of keratinocytes, resulting in phase-2 antioxidative enzymes gene expression enhancement; this include γ-glutamylcysteine synthetase, HO-1, NQO1, thereby providing protection of irradiated cells [57]. Also in melanocytes, melatonin and its derivatives increased the expression of Nrf2, and showed the protection of the cells from UVB-induced DNA damage and oxidative stress; these actions occurred in membrane bound melatonin receptor–independent manner [26]. In the present study in HaCaT cells, we also observed the nuclear translocation and significant increase of Nrf2 expression after UVB exposure in samples treated with melatonin, AFMK or NAS. In ex vivo experiments the application of melatonin, AFMK or NAS on human or porcine skin had a minimal or no effect on the number of Nrf2-positive keratinocytes. This could be secondary to already strong induction of Nrf2 in the epidermis and spatial interactions among keratinocytes at different level of differentiation. However, stimulatory effects of test compounds on NFE2L2 (gene coding Nrf2 protein) were observed (Table 3). Thus, while cell culture studies indicate that Nrf2 is downstream to melatonin’s protective action [26, 57], additional mechanism-oriented in vivo studies are required to better understand these processes in the epidermal compartment of the skin.
Different stressors, including UV radiation, induce DNA damage and p53 modulation initiate cell-cycle arrest and apoptosis [5, 6]. Activation of p53 requires its phosphorylation with translocation to the nucleus. Also, melatonin increases p53 expression [8] and phosphorylation at Ser-15, and prevents DNA damage accumulation [58]. Phosphorylation-related activation of p53 is mediated by melatonin receptors MT1 and MT2 [59]. In current study, we found an elevated number of p53ser15-positive cells when ex vivo skin samples were treated with melatonin or AFMK, suggesting DNA repair and/or cell-cycle arrest in the epidermis. In HaCaT keratinocytes, p53 expression was also elevated after treatment with melatonin and its active derivatives, which further substantiated the in situ data obtained from histocultured skin.
Studies on healthy volunteers revealed that melatonin applied topically on the skin before UV irradiation protected against erythema induced by UV, with no protective affects when applied after UV irradiation [60, 61]. Also Dreher et al [62] in their human study observed photoprotective properties of melatonin against erythema when applied before UV irradiation. Melatonin also enhanced the protective features of ascorbic acid and α-tocopherol [62]. Finally, melatonin at high doses protected against erythema induced by exposure to sun radiation with UV index 9 [63]; these doses of melatonin had no undesirable effects [64]. In our study, in addition to melatonin, also AFMK and NAS showed protective properties against UV-induced noxious effects not only when they were applied topically prior to UVB exposure, but also after UVB irradiation. In contrast to studies of Bangha et al [60, 61] and Dreher et al [62] who assessed UV-induced erythema, our evaluation was based on the UVB-induced changes at the epidermal/cellular levels. Therefore, we believe that the beneficial effects of melatonin application after UV exposure are real, but again would require future clinical trials.
In summary, melatonin or its active metabolites, including AFMK and NAS, applied before or after UVB irradiation, inhibit DNA damage, apoptosis and oxidative stress in human and porcine skin ex vivo. Thus, exogenous, topically-applied melatonin and its derivatives represent potential protective agents for preventing oxidative stress, and DNA damage. Furthermore, topically applied melatonin and its derivatives can also trigger DNA repair and/or cell cycle arrest and prevent UVB-induced skin cancerogenesis. Melatonin and its derivatives show the strongest protective properties against UV-induced genotoxic effects when applied to the skin before UV exposure. This protection results in genomic, cellular changes and in the maintenance of tissue integrity, which aid in preventing UVB-induced carcinogenesis. Finally, our ex vivo data are fundamental for further testing of clinical utility of melatonin and metabolites as protectors again UVB-induced pathology including its capability to attenuate the already induced pathological process.
Acknowledgments
The studies were supported by NIH grants 1R01AR056666-01A2 and 1R01AR071189–01A1 to AS. Partial support of Thailand Research Fund [Grant no. RSA5980066] to UP and the Royal Golden Jubilee Ph.D Scholarship Program (Grant number PHD/0181/2554) to SJ are also acknowledged.
Footnotes
Author contribution
C.S. designed and carried out the experiments, prepared figures and wrote the manuscript
A.A.B. interpreted the results, analyzed IHC and RT- PCR data and wrote the manuscript
Z.J. designed and conducted the RT- PCR experiments
Y.S. performed cell-culture experiments and WB experiments
A.S.W.O. performed TUNEL assays
U. P. analyzed and interpreted the results and contributed to writing the manuscript
T.-K.K. participated in tissue extraction, application of compounds and cell culture experiments
R.J.R. analyzed and interpreted the results and contributed to writing the manuscript
A.T.S. designed the experiments, provided access to human skin, performed histopathological analyses, analyzed interpreted the data, contributed to writing and approved the final version of the manuscript
References
- 1.Brozyna A, Zbytek B, Granese J, et al. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev Dermatol. 2007;2:451–69. doi: 10.1586/17469872.2.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadet J, Douki T, RAVANAT JL. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem Photobiol. 2015;91:140–55. doi: 10.1111/php.12368. [DOI] [PubMed] [Google Scholar]
- 3.Slominski AT, Zmijewski MA, Skobowiat C, et al. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v–vii. 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 5.Geyer RK, Nagasawa H, Little JB, et al. Role and regulation of p53 during an ultraviolet radiation-induced G1 cell cycle arrest. Cell Growth Differ. 2000;11:149–56. [PubMed] [Google Scholar]
- 6.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 7.Loughery J, Cox M, Smith LM, et al. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res. 2014;42:7666–80. doi: 10.1093/nar/gku501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Melatonin increases p53 and p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci. 1999;65:415–20. doi: 10.1016/s0024-3205(99)00262-3. [DOI] [PubMed] [Google Scholar]
- 9.Barnes L, Dumas M, Juan M, et al. GammaH2AX, an accurate marker that analyzes UV genotoxic effects on human keratinocytes and on human skin. Photochem Photobiol. 2010;86:933–41. doi: 10.1111/j.1751-1097.2010.00744.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–9. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter RJ, Rosales-Corral S, Tan DX, et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cellular and Molecular Life Sciences. 2017;74:3863–81. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter RJ, Mayo JC, Tan DX, et al. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–78. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 13.Acuna-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardeland R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res. 2017;62:12311. doi: 10.1111/jpi.12377. [DOI] [PubMed] [Google Scholar]
- 15.Cardinali DP, Hardeland R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology. 2017;104:382–97. doi: 10.1159/000446543. [DOI] [PubMed] [Google Scholar]
- 16.Slominski AT, Zmijewski MA, Semak I, et al. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74:3913–25. doi: 10.1007/s00018-017-2617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski AT, Semak I, Fischer TW, et al. Metabolism of melatonin in the skin: Why is it important? Exp Dermatol. 2017;26:563–8. doi: 10.1111/exd.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski A, Tobin DJ, Zmijewski MA, et al. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Fischer TW, Zmijewski MA, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137–48. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TK, Lin Z, Tidwell WJ, et al. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. doi: 10.1016/j.mce.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27:2742–55. doi: 10.1096/fj.12-224691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer TW, Sweatman TW, Semak I, et al. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–6. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 23.Kim TK, Lin Z, Li W, et al. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology. 2015;156:1630–6. doi: 10.1210/en.2014-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski AT, Kleszczynski K, Semak I, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15:17705–32. doi: 10.3390/ijms151017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janjetovic Z, Nahmias ZP, Hanna S, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57:90–102. doi: 10.1111/jpi.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janjetovic Z, Jarrett SG, Lee EF, et al. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 2017;7:1274. doi: 10.1038/s41598-017-01305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer TW, Elsner P. The antioxidative potential of melatonin in the skin. Curr Probl Dermatol. 2001;29:165–74. doi: 10.1159/000060665. [DOI] [PubMed] [Google Scholar]
- 28.Fischer TW, Kleszczynski K, Hardkop LH, et al. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2'-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303–12. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- 29.Fischer TW, Scholz G, Knoll B, et al. Melatonin suppresses reactive oxygen species in UV-irradiated leukocytes more than vitamin C and trolox. Skin Pharmacol Appl Skin Physiol. 2002;15:367–73. doi: 10.1159/000064543. [DOI] [PubMed] [Google Scholar]
- 30.Fischer TW, Scholz G, Knoll B, et al. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J Pineal Res. 2004;37:107–12. doi: 10.1111/j.1600-079X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 31.Fischer TW, Slominski A, Zmijewski MA, et al. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17:713–30. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 32.Fischer TW, Kleszczynski K, Hardkop LH, et al. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2'-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2012;54:303–12. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- 33.Tan DX, Manchester LC, Esteban-Zubero E, et al. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20:18886–906. doi: 10.3390/molecules201018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan DX, Reiter RJ, Manchester LC, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 35.Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29:325–33. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 36.Reiter RJ, Rosales-Corral S, Tan DX, et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer TW, Zbytek B, Sayre RM, et al. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J Pineal Res. 2006;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 38.Montagna W, Yun JS. The Skin of the domestic pig. J Invest Dermatol. 1964;42:11–21. [PubMed] [Google Scholar]
- 39.Summerfield A, Meurens F, Ricklin ME. The immunology of the porcine skin and its value as a model for human skin. Mol Immunol. 2015;66:14–21. doi: 10.1016/j.molimm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Debeer S, Le Luduec JB, Kaiserlian D, et al. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol. 2013;23:456–66. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 41.Hart EA, Caccamo M, Harrow JL, et al. Lessons learned from the initial sequencing of the pig genome: comparative analysis of an 8 Mb region of pig chromosome 17. Genome Biol. 2007;8:R168. doi: 10.1186/gb-2007-8-8-r168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brozyna A, Chwirot BW. Porcine skin as a model system for studies of ultraviolet a effects in human skin. J Toxicol Environ Health A. 2006;69:1155–65. doi: 10.1080/15287390500360323. [DOI] [PubMed] [Google Scholar]
- 43.Brozyna A, Wasilewska K, Wesierska K, et al. Porcine skin as a model system for studies of adverse effects of narrow-band UVB pulses on human skin. J Toxicol Environ Health A. 2009;72:789–95. doi: 10.1080/15287390902800363. [DOI] [PubMed] [Google Scholar]
- 44.Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J Invest Dermatol. 2015;135:1638–48. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skobowiat C, Dowdy JC, Sayre RM, et al. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–93. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skobowiat C, Sayre RM, Dowdy JC, et al. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 48.Slominski AT, Janjetovic Z, Kim TK, et al. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeayeng S, Wongkajornsilp A, Slominski AT, et al. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic Biol Med. 2017;108:918–28. doi: 10.1016/j.freeradbiomed.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slominski AT, Kim TK, Takeda Y, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–89. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rezzani R, Rodella LF, Favero G, et al. Attenuation of ultraviolet A-induced alterations in NIH3T3 dermal fibroblasts by melatonin. Br J Dermatol. 2014;170:382–91. doi: 10.1111/bjd.12622. [DOI] [PubMed] [Google Scholar]
- 52.Ryoo YW, Suh SI, Mun KC, et al. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J Dermatol Sci. 2001;27:162–9. doi: 10.1016/s0923-1811(01)00133-5. [DOI] [PubMed] [Google Scholar]
- 53.Fischer TW, Zmijewski MA, Wortsman J, et al. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44:397–407. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaefer M, Hardeland R. The melatonin metabolite N-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J Pineal Res. 2009;46:49–52. doi: 10.1111/j.1600-079X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 55.Kleszczynski K, Tukaj S, Kruse N, et al. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J Pineal Res. 2013;54:89–99. doi: 10.1111/j.1600-079X.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- 56.Antolin I, Rodriguez C, Sainz RM, et al. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996;10:882–90. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- 57.Kleszczynski K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (gamma-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK) J Pineal Res. 2016;61:187–97. doi: 10.1111/jpi.12338. [DOI] [PubMed] [Google Scholar]
- 58.Santoro R, Marani M, Blandino G, et al. Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene. 2012;31:2931–42. doi: 10.1038/onc.2011.469. [DOI] [PubMed] [Google Scholar]
- 59.Santoro R, Mori F, Marani M, et al. Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis. 2013;34:1051–61. doi: 10.1093/carcin/bgt025. [DOI] [PubMed] [Google Scholar]
- 60.Bangha E, Elsner P, Kistler GS. Suppression of UV-induced erythema by topical treatment with melatonin (N-acetyl-5-methoxytryptamine). A dose response study. Arch Dermatol Res. 1996;288:522–6. doi: 10.1007/BF02505248. [DOI] [PubMed] [Google Scholar]
- 61.Bangha E, Elsner P, Kistler GS. Suppression of UV-induced erythema by topical treatment with melatonin (N-acetyl-5-methoxytryptamine). Influence of the application time point. Dermatology. 1997;195:248–52. doi: 10.1159/000245953. [DOI] [PubMed] [Google Scholar]
- 62.Dreher F, Gabard B, Schwindt DA, et al. Topical melatonin in combination with vitamins E and C protects skin from ultraviolet-induced erythema: a human study in vivo. Br J Dermatol. 1998;139:332–9. doi: 10.1046/j.1365-2133.1998.02447.x. [DOI] [PubMed] [Google Scholar]
- 63.Scheuer C, Pommergaard HC, Rosenberg J, et al. Dose dependent sun protective effect of topical melatonin: A randomized, placebo-controlled, double-blind study. J Dermatol Sci. 2016;84:178–85. doi: 10.1016/j.jdermsci.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Scheuer C, Pommergaard HC, Rosenberg J, et al. Effect of topical application of melatonin cream 12.5% on cognitive parameters: A randomized, placebo-controlled, double-blind crossover study in healthy volunteers. J Dermatolog Treat. 2016;27:488–94. doi: 10.3109/09546634.2016.1161154. [DOI] [PubMed] [Google Scholar]