Abstract
The widespread use of antiretroviral therapy for treatment of human immunodeficiency virus (HIV) infections has dramatically improved the quality and duration of life of HIV positive individuals. Despite this success, HIV persists for the life of an infected person in tissue reservoirs including the nervous system. Thus, whether HIV exacerbates age-related brain disorders such as Parkinson’s disease (PD) is of concern. In support of this idea, HIV infection can be associated with motor and gait abnormalities that parallel late stage manifestations of PD including dopaminergic neuronal loss. With these findings in hand, we investigated whether viral infection could affect nigrostriatal degeneration or exacerbate chemically induced nigral degeneration. We now demonstrate an additive effect of EcoHIV on dopaminergic neuronal loss and neuroinflammation induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication. HIV-1-infected humanized mice failed to recapitulate these EcoHIV results suggesting species-specific neural signaling. The results demonstrate a previously undefined EcoHIV-associated neurodegenerative response that may be used to model pathobiological aspects of PD.
Keywords: Parkinson’s disease; central nervous system; EcoHIV; human immunodeficiency virus; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Introduction
Following the introduction of effective antiretroviral therapy (ART) for the treatment of human immunodeficiency virus type one (HIV-1) infection individuals living with disease live life with limited morbidities (Cardoso et al, 2013). Indeed, numbers of HIV-infected people over the age of 50 increased dramatically in recent years (Wing, 2016). Consequently, as the HIV-infected ART-treated population ages, patients will be prone to age-related neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Added to this likelihood are known similarities between HIV-associated brain disease and AD or PD (Cardoso et al, 2013; Mirsattari et al, 1998). PD is characterized by motor dysfunction linked to the loss of dopaminergic neurons along the nigrostriatal system paralleled with the formation of Lewy bodies and neuroinflammation. The latter results from the accumulation of misfolded or modified alpha-synuclein in affected neurons (Kalia and Lang, 2015). HIV infection can also affect the nigrostriatum and lead to motor impairments (Cardoso, 2002). Thus, a major, yet recurrent question is whether HIV infection affects the development of PD-linked neurodegeneration (Moulignier et al, 2015).
Past studies indicate that the central nervous system is highly susceptible to HIV infection and related disease (Davis et al, 1992; Williams et al, 2012; Williams and Hickey, 2002). Such infection, interestingly, affects dopamine-rich brain subregions that parallel, in measure, those regions affected in PD (Ellis et al, 2009; Kumar et al, 2009). Raising concern for such disease linkages are post-mortem brain tissue examinations. These showed HIV-infected people have increased levels of alpha-synuclein within the substantia nigra pars compacta (Khanlou et al, 2009). HIV-infected brain tissues also show signs of neuroinflammation, lymphocyte brain infiltration, impaired mitochondrial integrity, and increased evidence for oxidative stress (Hong and Banks, 2015; Jones et al, 1998; Moulignier et al, 2015). HIV patients also display similar neurological impairments observed in advanced PD. These include bradykinesia and impaired dexterity, gait, and cognitive functions (Navia et al, 1986; Sheppard et al, 2015) (Rosso et al, 2009). HIV-infected patients have also shown reduced beta oscillations known to affect movement function (Wilson et al, 2013). These reflect a loss in dopaminergic neuronal function in the basal ganglia, substantia nigra and striatum (Berger and Arendt, 2000) linked to loss of dopaminergic neurons and decreased availability of dopamine (Groger et al, 2014). Indeed, HIV-infected patients display up to 53% decreased dopamine availability that changes little following ART (Kumar et al, 2011). Similarly, a decrease in the dopamine metabolite, homovanillic acid (HVA), is common (Kumar et al, 2009; Kumar et al, 2011).
Mutual links between viral infections and PD are known, but can affect disease by divergent mechanisms. First, influenza virus, herpes simplex virus, Epstein-Barr virus, cytomegalovirus, polio virus, HIV, West Nile virus, and Japanese encephalitis B viruses are known to be associated with PD (Jang et al, 2009). Second, each of these viruses is neurotropic, easily enters the brain, results in neural cell death, and elevates local pro-inflammatory cytokine production (Karim et al, 2014). Third, and opposing this view, are disease signs and symptoms which differ between HIV and PD (Boyd et al, 2016; Pakpoor et al, 2017). In attempts to reconcile these views together with the potential that HIV-1 infection could affect neurodegenerative processes, we employed two mouse models of infection in association with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication known to induce nigrostriatal degeneration to assess each influence alone or together on PD-associated neuronal demise. We found that the EcoHIV, a chimeric HIV that can persistently infect conventional mice, enter the brain, and replicate in murine host microglial cells, was selectively neurotoxic to nigral dopaminergic neurons with notable synergy during MPTP exposure (Potash et al, 2005). These results provide credible evidence, for the first time, that in the correct microenvironment, HIV infection affects the onset or progression of PD.
Results
HIV-1 infection does not alter nigrostriatal degeneration in MPTP-intoxicated humanized mice
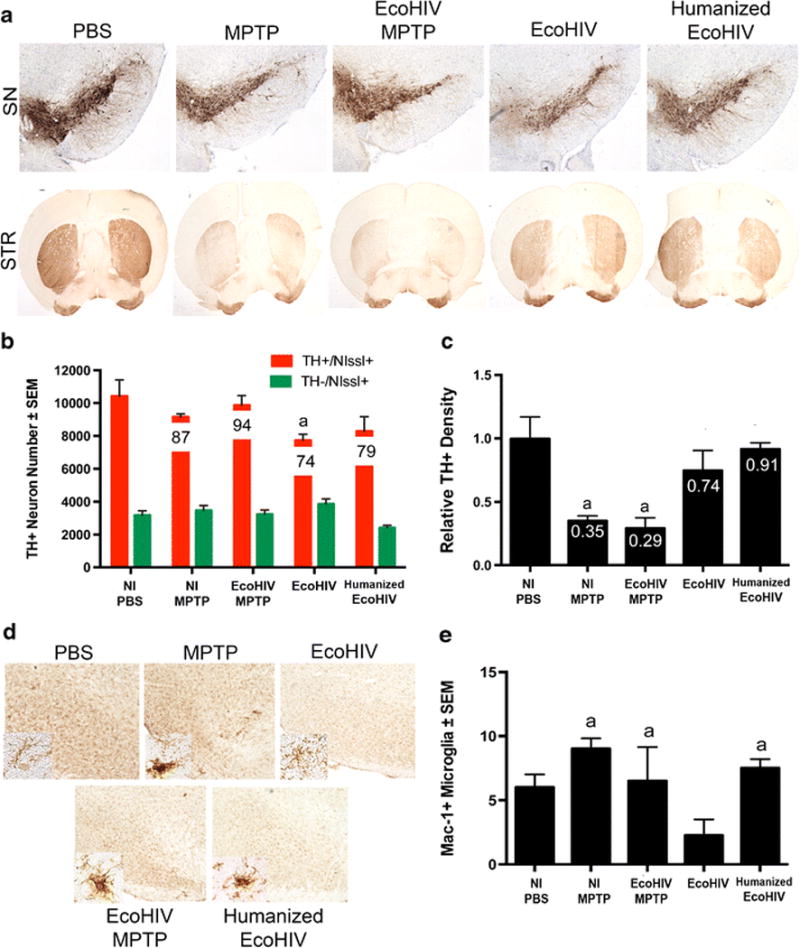
To assess a potential interplay between HIV infection and PD-associated pathologies, humanized NSG mice were used in experimental assays. These mice were either HIV-1 infected or uninfected then MPTP-intoxicated using a low (14 mg/kg) concentration dose so that potential synergy, if present, could be recorded between virus and chemically induced brain tissue damage. Infected mice were sacrificed at indicated times and neuron numbers were assessed by stereological analysis. TH+Nissl+ dopaminergic neuron counts were decreased with MPTP intoxication, as expected from 9012 ± 679 to 6596 ± 580 (Figure 1A and 1B). Combination of HIV infection and MPTP intoxication did not increase the degree of degeneration observed, suggesting no effect on speed or amount of neurodegeneration associated with MPTP intoxication in this animal model. However, with MPTP alone or HIV infection and MPTP, TH density within the striatal termini decreased ~50%, respectively, when compared to PBS controls (Figure 1C). Likewise, upon analysis of microglial reactivity, no significant differences were observed between treatment groups. Mac-1+ counts remained similar between groups (Figure 2A and 2B), indicating that HIV infection did not enhance microglial reactivity above that observed in MPTP-intoxication alone. Taken together, these results show that chronic and active HIV infection did not enhance nigrostriatal degeneration in MPTP-treated humanized mice. These findings indicate either the lack of a synergistic effect causing neuronal loss, a lack of interaction between the reconstituted human lymphocytes and murine microglia within the humanized system, or the inability of HIV to infect mouse microglial cells. The limited loss of dopaminergic neurons is most likely due to the initial neuronal toxicity of the toxic MPTP metabolite, MPP+, rather than the ensuing inflammatory cascade associated with immune activation. Based on these findings, we began to explore the use of EcoHIV infection in conventional mice (He et al, 2014; Potash et al, 2005).
Figure 1. Combination of HIV infection and MPTP intoxication does not affect neurodegeneration in humanized mice.
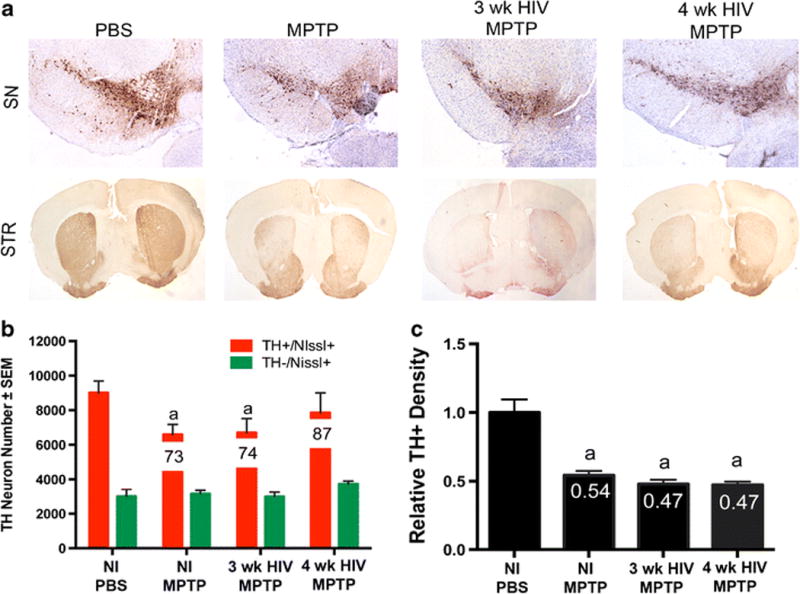
A. Representative images of the substantia nigra (SN) and striatum (STR) of humanized NOD/scid-IL-2Rγc null mice treated with MPTP alone or inoculated with HIV for 3 weeks or 4 weeks followed by MPTP intoxication. B. Total numbers of surviving dopaminergic neurons (TH+Nissl+) and non-dopaminergic neurons (TH-Nissl+) in the SN following MPTP intoxication, or HIV infection followed by MPTP intoxication. Percentages of spared dopaminergic neurons are displayed on the representative bar for each treatment. C. Relative TH+ density within striatal termini of dopaminergic neurons. Differences in means (± SEM, n = 3-6) were determined where P < 0.05 compared to PBS (a).
Figure 2. HIV infection does not increase microglial reactivity after MPTP intoxication in humanized mice.
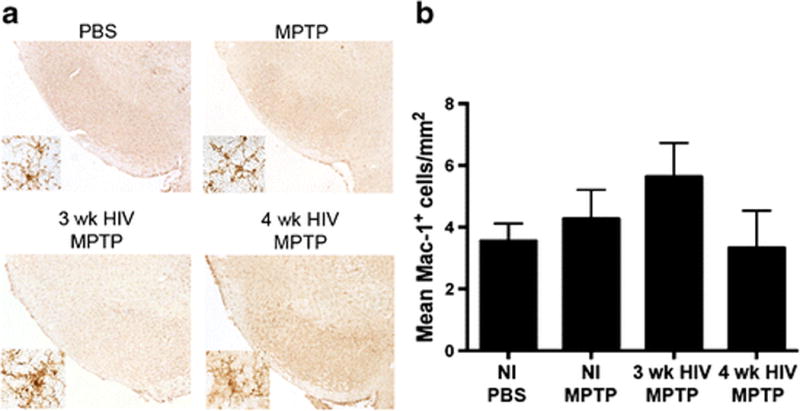
A. Representative images Mac-1+ microglia within the ventral midbrain of humanized NOD/scid/IL-2Rγc null mice treated with MPTP alone or inoculated with HIV for 3 weeks or 4 weeks followed by MPTP intoxication. B. Quantification of reactive microglia cells/mm2 located within the substantia nigra.
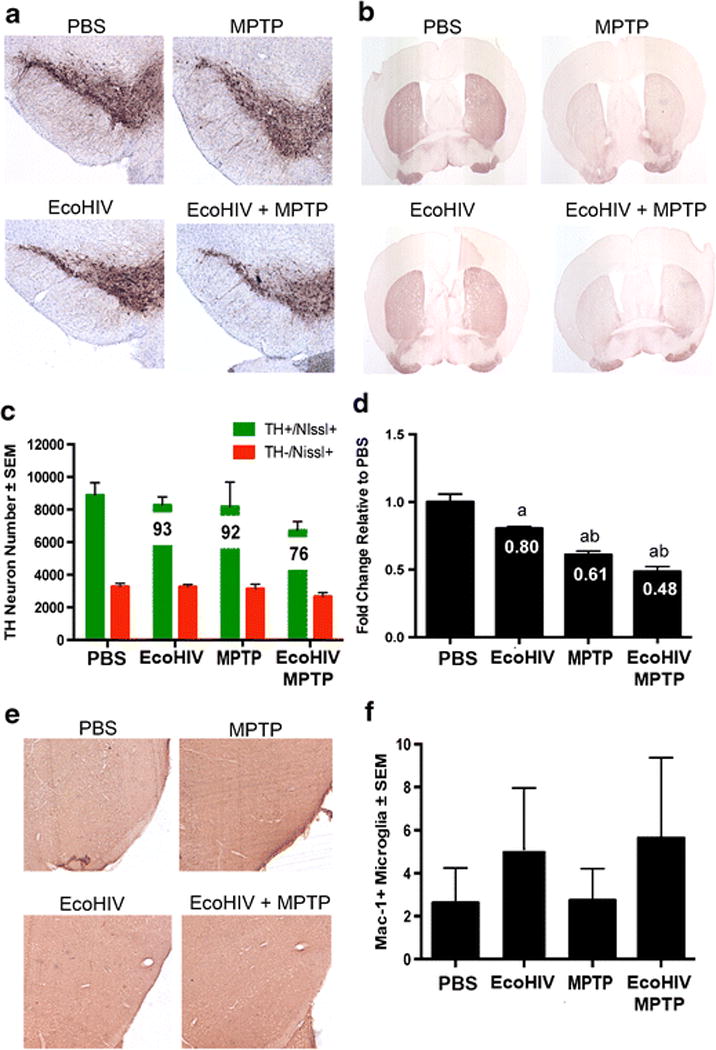
EcoHIV infection and MPTP intoxication affect nigrostriatal degeneration
To determine a time-course evaluation of EcoHIV infection in conventional mice, male, C57BL/6 mice were inoculated with EcoHIV for 2, 3, or 4 weeks, followed by MPTP. Surviving neuron numbers were decreased with all treatments when compared to the control group (Figure 3A-C). EcoHIV infection alone resulted in a significant decrease in neuron counts relative to uninfected controls at all time points, dropping neuron counts from 9556 ± 326 for PBS to 7450 ± 350 at 2 weeks of infection, 7359 ± 364 at 3 weeks of infection, and 6836 ± 999 at 4 weeks of infection, respectively. Addition of MPTP intoxication led to a further decrease in neuronal numbers at all time points, resulting in 5404 ± 671 at 2 weeks of infection + MPTP, 6238 ± 743 at 3 weeks of infection, and 4103 ± 103 at 4 weeks of infection and MPTP. Findings demonstrate that at 4 weeks of infection and MPTP intoxication, numbers of surviving dopaminergic neurons were decreased within the substantia nigra compared to mice treated with PBS, MPTP, or 3 weeks EcoHIV infection and MPTP, indicating an effect occurs with longer periods of viral infection.
Figure 3. EcoHIV co-infection exacerbates MPTP-induced neurodegeneration.
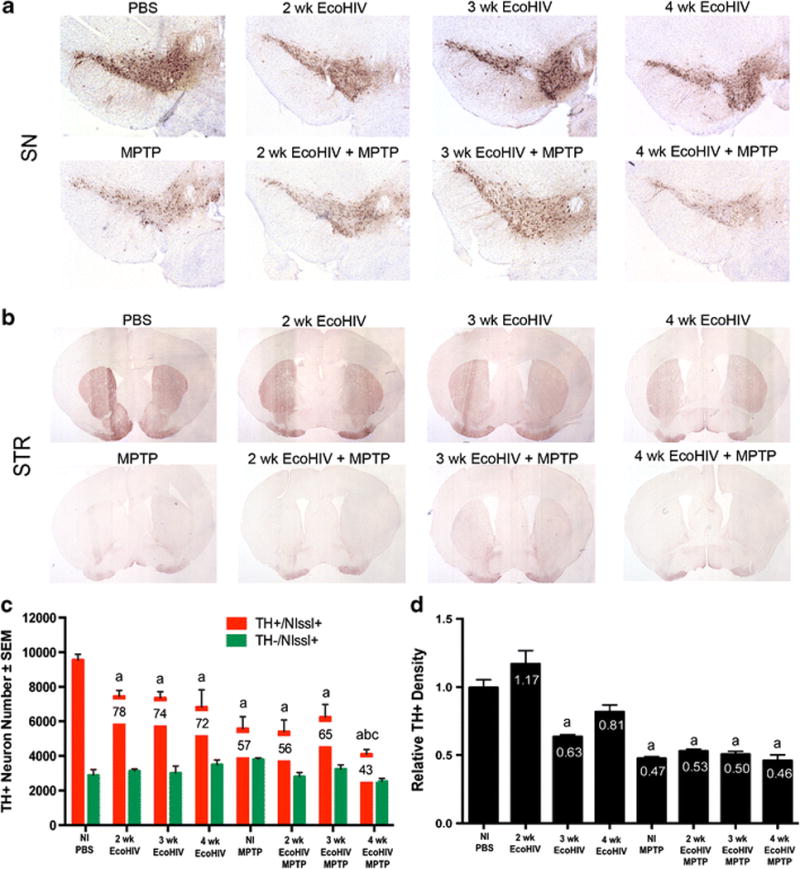
A. Photomicrographs of TH+Nissl+ neurons in the substantia nigra (SN) in C57/Bl6 mice treated with PBS, MPTP, or EcoHIV infection over time. Sections were immunostained with anti-TH and HRP-conjugated secondary antibody, and visualized with DAB. B. Photomicrographs of TH+ striatal termini (STR) following PBS, MPTP or EcoHIV coinfection. C. Total numbers of surviving dopaminergic neurons (TH+Nissl+) and non-dopaminergic neurons (TH-Nissl+) in the SN following MPTP treatment alone, EcoHIV infection alone, or EcoHIV infection followed by MPTP intoxication. Percentages of spared dopaminergic neurons are displayed on the representative bar for each treatment. Differences in means (± SEM, n=8) were determined where P<0.05 compared to PBS (a), MPTP (b), and 3 wk EcoHIV + MPTP (c). D. Relative TH density of striatal dopaminergic termini following infection. Differences in means (± SEM, n = 8) were determined where P < 0.05 compared to PBS (a), 2 wk EcoHIV (b), and 4 wk EcoHIV(c).
Based on these findings, we selected 4 weeks after infection to evaluate potential interactions between HIV infection and MPTP in affecting nigral neurodegeneration. Given that microglial activation and increased reactivity are associated with neuronal cell death in the MPTP mouse model of PD (Kurkowska-Jastrzebska et al, 1999), Mac-1+ reactive microglia were quantified within the ventral midbrain of mice treated with HIV ± MPTP (Figure 4). Numbers of reactive microglia observed were significantly increased from PBS controls in MPTP without infection and at 4 weeks of infection followed by MPTP (Figure 4B). The other experimental groups remained similar to controls. Notably, 4 weeks of infection followed by MPTP intoxication increased reactive microglial counts nearly 10-fold, increasing to 29 ± 4.1 cells/mm2 compared to 3 ± 0.3 cells/mm2 in PBS-treated controls and 3-fold compared to mice treated with MPTP alone. These findings support the notion that EcoHIV infection affects MPTP mediated nigral degeneration and enhances the neuroinflammatory response.
Figure 4. EcoHIV infection leads to increased microglial reactivity.
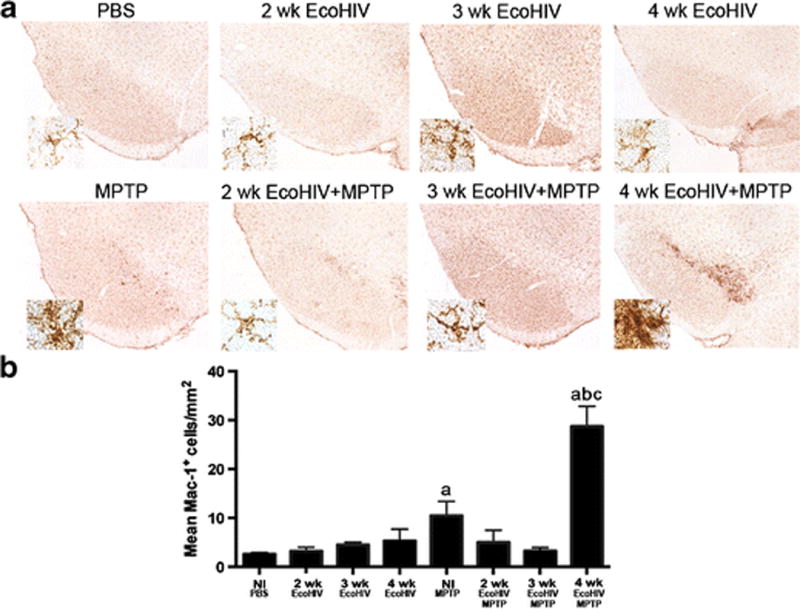
A. Representative photomicrographs of Mac-1+ microglia with the SN of C57/Bl6 mice treated with PBS, MPTP, EcoHIV, or EcoHIV + MPTP over time (4× image, inset = 40× image). B. Quantification of reactive microglia within the SN 7 days post MPTP intoxication. Sections were stained using an anti-Mac-1 antibody, followed by an HRP-conjugated secondary antibody and DAB. Numbers of amoeboid microglia were assessed using stereological analysis. Differences in means (± SEM, n = 8) were determined where P < 0.05 compared with groups treated with PBS (a), MPTP (b), and 4 wk EcoHIV (c).
Studies show that T cells interact with microglial populations to elicit active immune responses (Brochard et al, 2009). To test this possibility in the present system, mice were bled over the indicated time course and peripheral blood collected for analysis of changes in lymphocyte populations (Figure 5). Flow cytometric analysis indicated that CD3, CD4, CD25, and CD8 populations remained relatively unchanged over the course of infection and remained stable after MPTP intoxication, revealing the lack of a shift in number or frequency of T cell populations. These findings confirm that EcoHIV infection does not cause a diminution of peripheral CD4+ T cells, consistent with previous observations (Geraghty et al, 2017) and indicate a potential analogy with HIV positive patients on effective HIV-suppressive cART (Wright et al, 2011). Also, these results indicate that sufficient levels of CD4+ T cells exist to support the necessary immune reactions for successful neurodegeneration following MPTP intoxication.
Figure 5. EcoHIV infection does not affect peripheral T cell populations.
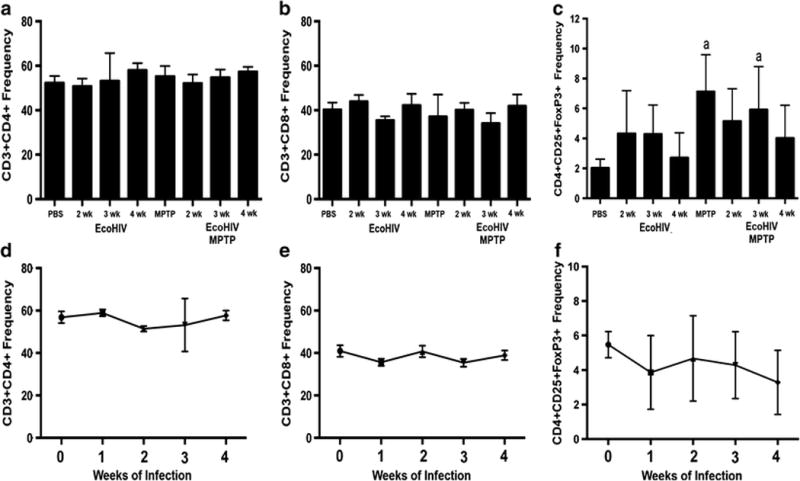
C57/Bl6 mice were inoculated with EcoHIV for 2–4 weeks and then intoxicated with MPTP. Mice were assessed for frequencies of CD3+CD4+ (A), CD3+CD8+ (B), and CD4+CD25+FoxP3+ (C). Differences in means (± SEM, n = 8) were determined where P < 0.05 compared with groups treated with PBS (a). D–F. Changes in T cell populations over time following EcoHIV infection.
Next, we investigated possible mechanisms for increased neuronal loss and microglial reactivity observed at 4 weeks co-insult. Four weeks after EcoHIV infection, we isolated the ventral midbrain of mice following MPTP intoxication alone or at 4 weeks EcoHIV infection alone or at 4 weeks EcoHIV infection followed by MPTP intoxication. Total RNA was isolated to assess changes in inflammatory response gene expression (Figure 6). Although the results did not reach statistical significance, we observed marked trends in gene expression changes suggesting enhanced inflammatory responses upon co-insult. Comparison to EcoHIV alone, co-insult results in increased gene expression levels of the genes encoding for CCR7, CCL2, IFNγ, IL-10, IL-9, and NF-κB and decreased levels of TLR4 and IL-5 (Figure 6A and 6C). When compared to MPTP alone, co-insult leads to upregulation of, CCL2, IFNγ, IL-10, IL-9, NFκB, TLR4, and IL-5 (Figure 6B and 6C). These results suggest a synergistic effect of two insults causing dysregulation in the inflammatory response pathway within the ventral midbrain.
Figure 6. EcoHIV co-infection leads to changes in the inflammatory response pathway.
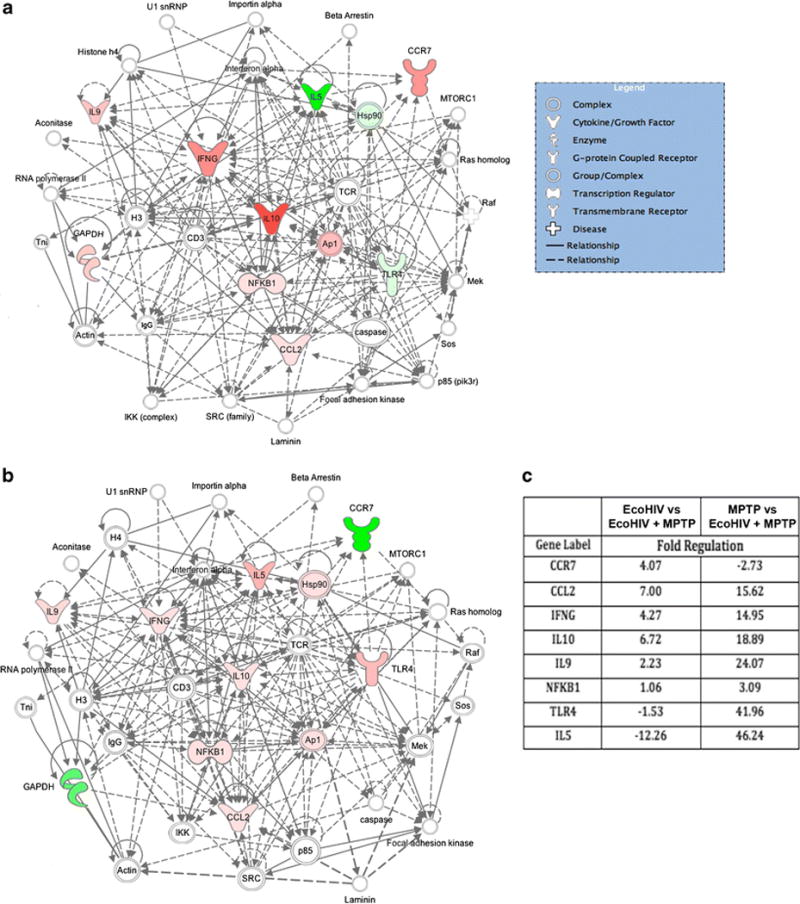
RT-PCR data shows gene expression changes in the ventral midbrains of C57BL/6 mice inoculated with EcoHIV followed by MPTP intoxication (n = 3–4). Fold changes were determined using SABioscience RT2 Profiler PCR Array Data Analysis software, version 3.5. A. Changes in the inflammatory response pathway associated with EcoHIV infection alone vs EcoHIV + MPTP co-treatment. B. Changes in the inflammatory response pathway associated with MPTP intoxication alone vs EcoHIV + MPTP co-treatment. Resulting gene networks from each treatment group were analyzed using Qiagen Ingenuity Pathway Analysis. Pink coloration indicates a modest increase in expression and red indicates a profound increase in expression. Green coloration indicates a decrease in expression. Nodes lacking color indicate a molecule involved in the pathway, but not identified in the PCR data set. C. Fold changes for differentially regulated mRNA levels with EcoHIV + MPTP treatment normalized to either EcoHIV treatment alone or MPTP intoxication alone.
EcoHIV infection does not affect MPTP-induced neuronal degeneration in immunodeficient mice
Next, to evaluate the role of T cell-mediated neurodegeneration in EcoHIV infection, we utilized immunodeficient, non-humanized NSG mice (Figure 7) and C57BL/6 scid mice (Figure 8). Each strain of mice was infected with EcoHIV for 4 weeks, followed by MPTP-intoxication. On day 7 post-MPTP, animals were sacrificed and brains harvested. Neuronal survival and microglial reactivity were assessed and quantified using stereological analysis. MPTP intoxication lead to a 13% and 6% loss in TH+ dopaminergic neurons, respectively, indicating that this strain may not be readily susceptible to MPTP intoxication (Figure 7A and 7B). This is most likely due to the lack of lymphocytes needed for MPTP-induced neurodegeneration susceptibility (Benner et al, 2008; Brochard et al, 2009). However, EcoHIV infection alone significantly decreased neuronal numbers by 26% compared to PBS, suggesting EcoHIV may have a direct effect on dopamine–producing neurons, which is similar to the results observed in the C57BL/6 strain of mice. Likewise, in humanized NSG mice, dopaminergic neuron numbers diminished after infection with EcoHIV alone (Figure 7B), further supporting the idea that EcoHIV may be directly neurotoxic. However, even though the neuronal cell bodies remained intact following MPTP intoxication, striatal termini were not spared (Figure 7C). MPTP intoxication significantly deceased the relative TH+ density within the striatum compared to PBS controls (Figure 7C), but EcoHIV infection alone did not result in a significant loss of termini. These findings support the idea that striatal termini are more susceptible to MPTP-intoxication and degeneration may occur in cell termini prior to the degeneration of neuronal bodies (Burke and O’Malley, 2013). To assess the presence of an inflammatory response, microglial populations were also stained and quantified in the same tissues, as previously performed. Reactive microglial numbers were not significantly increased with MPTP intoxication when compared to PBS controls (Figure 7D and 7E).
Figure 7. EcoHIV and MPTP co-administration does not exacerbate neuronal degeneration in non-humanized NSG mice.
A. Photomicrographs of TH+Nissl+ neurons in the substantia nigra (SN) and striatum (STR) in non-humanized or humanized NOD/scid-IL-2Rγc null mice treated with PBS, MPTP, EcoHIV infection, or EcoHIV + MPTP co-administration. Animals were infected for 3 weeks prior to MPTP intoxication. B. Total numbers of surviving dopaminergic neurons (TH+Nissl+) and non-dopaminergic neurons (TH-Nissl+) in the SN following MPTP treatment alone, EcoHIV infection alone, or EcoHIV infection followed by MPTP intoxication. Percentages of spared dopaminergic neurons are displayed on the representative bar for each treatment. Differences in means (± SEM, n=3–4) were determined where P<0.05 compared to PBS (a). C. Relative TH density of striatal dopaminergic termini following infection and MPTP intoxication. Differences in means (± SEM, n = 3–4) were determined where P < 0.05 compared to PBS (a). D. Representative photomicrographs of Mac-1+ microglia within the midbrain following EcoHIV infection, MPTP intoxication, or EcoHIV infection and MPTP intoxication (4× image, inset = 40× image). E. Quantification of reactive microglia within the SN 7 days post MPTP intoxication. Differences in means (± SEM, n = 3–4) were determined where P < 0.05 compared with groups treated with EcoHIV (a).
Figure 8. Immunodeficient C57BL/6 scid mice are not susceptible to MPTP intoxication and do not exhibit an inflammatory response.
C57BL/6 scid mice were treated with PBS, MPTP, infected with EcoHIV, or infected 3 weeks prior to infection (EcoHIV + MPTP) and tissues examined one week after MPTP. Photomicrographs of (A) TH+Nissl+ neurons in the substantia nigra (SN) and (B) TH+ termini in the striatum (STR). C. Total numbers of surviving dopaminergic neurons (TH+Nissl+) and non-dopaminergic neurons (TH-Nissl+) in the SN following MPTP treatment alone, EcoHIV infection alone, or EcoHIV infection followed by MPTP intoxication (EcoHIV + MPTP). D. Relative TH density of striatal dopaminergic termini following infection and MPTP intoxication. Differences in means (± SEM, n = 5) were determined where P < 0.05 compared to PBS (a) or EcoHIV alone (b). E. Representative midbrain images stained with Mac-1 following PBS, MPTP, EcoHIV + MPTP, or EcoHIV treatment. F. Quantification of Mac-1+ microglial cells/mm2 within the substantia nigra. No significant differences were observed.
In studies using immunodeficient, C57BL/6 scid mice, the results were similar to non-humanized NSG mice. TH+ neuron counts indicate no significant decrease in cell number after MPTP intoxication (Figure 8A and 8C). However, MPTP intoxication still resulted in an 8% and a 24% loss of dopaminergic neurons within the substantia nigra (Figure 8C). EcoHIV infection alone also led to a 7% loss in cell number, further supporting the concept that the virus itself causes dopaminergic cell death. Similarly, striatal termini in all treatment groups were significantly diminished (Figure 8B and 8D). MPTP intoxication alone or EcoHIV infection alone resulted in the 39% and 20% respective loss of striatal termini, while EcoHIV infection plus MPTP-intoxication displayed a 52% loss, thus indicating increased susceptibility of striatal termini (Figure 8D). Microglial reponses remained unreactive (Figure 8E and 8F) as no significant changes in reactive microglia numbers were discernible among treatment groups (Figure 8F). In fact, little to no inflammatory microglial response could be detected.
Discussion
HIV-1 infection is associated with a spectrum of behavioral, motor, and cognitive disorders commonly called HIV-associated neurocognitive disorders (HAND) (Clifford and Ances, 2013; Ungvarski and Trzcianowska, 2000). While disease severity has decreased during the ART era neurocognitive signs and symptoms remain (Clifford and Ances, 2013). A critical question for understanding HAND in the ART era is whether low-level restricted viral infection affects the onset, severity, or progression of neurodegenerative disease (Deleidi and Isacson, 2012). Post-mortem studies in brain tissue from HAND patients demonstrate increased alpha-synuclein and decreased dopamine levels in infected areas. (Kalia and Lang, 2015; Khanlou et al, 2009). However, there are few published reports that investigate the interplay between low-level HIV infection and development of neurodegenerative disease in animal and cellular model systems (Chai et al, 2017; Dickens et al, 2017; Jones et al, 2007; Meeker and Hudson, 2017). Therefore, we investigated the potential interactions between virus and chemically induced neurodegeneration. HIV-1-infected humanized mice were challenged with MPTP. Neither HIV infection alone nor HIV and MPTP showed any virus-specific effect on nigrostriatal degeneration. These results suggested that either interactions between virus and MPTP were lacking or that resident microglia and neurons failed to effectively react with an operative human immune system. The latter possibility reflects our own studies of animals with an operative humanized brain and immune system model of neuroHIV (Li et al, 2017).
A second possibility centers on how neurodegeneration occurs following MPTP intoxication. To initiate a neurodegenerative cascade, MPTP must be metabolized into its neurotoxic form, MPP+. The resulting neurotoxicity accounts for approximately 10% of neuronal cell death (Jackson-Lewis and Przedborski, 2007), and further neuronal loss requires an induced pro-inflammatory microenvironment resulting in reactive microglia. Studies also demonstrate that mice lacking CD4+ T cells are not susceptible to MPTP intoxication (Brochard et al, 2009), (Benner et al, 2008). Thus, we investigated if the lack of neurodegenerative response may be due to insufficient CD4+ T cell and microglial interactions using scid and NSG mice. In these immunocompromised systems, MPTP intoxication resulted in a minor loss in dopaminergic neuron numbers. This loss was likely due to the effect of MPTP metabolism to MPP+ (Jackson-Lewis and Przedborski, 2007). Without functional CD4+ T cells, the secondary inflammatory cascade does not occur. This finding was also observed in the humanized system. Taken together, it appeared prudent to use species-specific substitutes for studies of innate and adaptive immunity. Thus, EcoHIV infection of conventional immunocompetent mice was used to address this question (Hadas et al, 2007; He et al, 2014; Jones et al, 2016; Potash et al, 2005).
EcoHIV/NDK is a chimeric virus on the backbone of HIV-1 clade D, NDK, containing the gp80 envelope gene of murine leukemia virus in place of HIV gp120 (Potash et al, 2005). EcoHIV was shown to infect immunocompetent mice by targeting CD4+ T cells, macrophages, and myeloid cells in the brain, induce anti-viral immune responses, invade the CNS, and increase inflammatory mediators in the brain. EcoHIV infection mimics HIV and offers a testing paradigm for studies of virus-induced neurodegeneration (Hadas et al, 2007; He et al, 2014; Potash et al, 2005). Notably, EcoHIV enters and establishes an infection in the mouse brain as early as 3 weeks after viral exposure and can alter BBB permeability by 2 weeks (Jones et al, 2016). Infected brains express inflammatory factors and mediators such as C3a, IL-1B, STAT-1, and IL-6 indicating an active infection and anti-viral response (Potash et al, 2005). Similarly, we observed a significant decrease in dopaminergic neuron loss by 2 weeks of EcoHIV infection. These findings highlight the strengths and weaknesses of operative neuroHIV model systems for studies of disease pathobiology.
Indeed, with MPTP and EcoHIV co-insult, microglial reactivity was significantly increased 4 weeks after infection, potentially accounting for the increased timed-responses for the neurodegeneration observed. This may occur by induction of microglial inflammation as EcoHIV is known to readily infect macrophages and microglia leading to their activation (He et al, 2014; Potash et al, 2005), a finding confirmed in the current studies. While EcoHIV lacks gp120, virus expression in mouse microglia and the consequent microglial activation (He et al, 2014) likely results in the production of Tat (Jin et al, 2012; Lu et al, 2011; Sheng et al, 2000). Tat exposure induces prolonged microglial activation when compared to PBS-treated controls and lasts for 28 days post-injection, a similar time-course to our studies (Lu et al, 2011). When exposed to Tat, human microglial cells increased production of proinflammatory cytokines and chemokines, TNF-α, IL-1B, IL6, RANTES, and MIP-1α (Sheng et al, 2000). Other possibilities for the observed microglial response include the presence of direct neuronal death, causing resident microglia to become reactive as they sample the microenvironment (Kreutzberg, 1996), or the infiltration of infected peripheral macrophages across the leaky BBB to areas of degeneration (McArthur et al, 2005).
Nonetheless, with significant increases in both neuronal death and microglial activity by 4 weeks infection followed by MPTP, potential changes in the inflammatory response cascade were assessed. Although no significance was observed, these changes may be important in determining a mechanism of action. Changes in gene expression of both pro- and anti-inflammatory mediators were observed with EcoHIV infection followed by MPTP, indicating a general dysregulation of the inflammatory response. Increases in genes that encode pro-inflammatory mediators including CCL2, IFNγ, NFκB, TLR4, and IL-5 were observed as well as the anti-inflammatory mediators, IL-9 and IL-10. The observed increase in Ccl2 here may be linked to BBB permeability. Studies show that CCL2 mediates the transmigration of HIV-infected leukocytes across the BBB, resulting in viral neuroinvasiveness (Eugenin et al, 2006). This may indicate a potential mechanism of CNS invasion with EcoHIV infection. HIV infection also activates NFkB and TLR4, which correlates with increased expression observed in this EcoHIV study (Fiume et al, 2012; Hernandez et al, 2012). Increased expression and activation of the NFkB pathway would ultimately lead to increases in overall pro-inflammatory gene levels (Fiume et al, 2012). Changes in TLR4 levels could affect the pro-inflammatory response by promoting viral replication and disease progression (Hernandez et al, 2012). Overall, changes in protein levels based on these gene changes may give rise to the enhanced microglial reactivity, as well as lead to an enhanced inflammatory microenvironment, further exacerbating neurodegeneration. On the other hand, upregulation of anti-apoptotic and anti-inflammatory interleukins, IL-9 and IL-10, may result from counteracting the neuroinflammatory response in order to maintain a homeostatic balance within the brain (Kwon and Kaufmann, 2010; Renauld et al, 1995).
EcoHIV viral infection alone yielded neuronal death in both immunocompetent and immunodeficient mice, indicating a direct effect on the dopaminergic system that does not require cell-mediated immune responses. To our knowledge, no study has investigated the effect of EcoHIV infection on neurons within the substantia nigra or ventral midbrain, specifically. This brain region exhibits increased susceptibility to neuronal cell death itself, so the presence of virus or viral particles may lead to increased neuronal cell death that has not yet been observed in other regions of the brain (Haddad and Nakamura, 2015; Naoi and Maruyama, 1999). Tat has been implicated in many studies as having a neurotoxic effect on the dopamine transmission system (Gaskill et al, 2017). Tat may directly impact dopaminergic neurotransmission through modulation of the dopamine transporter and dysregulating Ca2+ needed for baseline activity and cell signaling, or by specifically damaging the areas of the brain that are dopamine-rich, including the substantia nigra and striatum (Gaskill et al, 2017; Hu, 2016; Silvers et al, 2007). Tat also inhibits the expression of tyrosine hydroxylase (TH), an enzyme needed for dopamine production, ultimately affecting dopamine transmission (Zauli et al, 2000). Dopaminergic neuron susceptibility to viral factors could lead to the likelihood of developing PD or Parkinson’s-like symptoms later. It is likely that EcoHIV is affecting the dopaminergic system, similarly to HIV infection. Additional studies should be carried out to confirm changes in dopamine synthesis, neurotransmission, and TH gene expression. EcoHIV itself has a cooperative effect in other disease studies as well, including induction of chronic obstructive pulmonary disease (COPD) and increased inflammation within the gut following opioid use (Geraghty et al, 2017; Sindberg et al, 2015). These studies, along with ours, support the idea that EcoHIV infection may reproduce pathological conditions that are necessary for initiating mild, yet chronic diseases commonly observed in immunocompetent HIV-infected individuals.
MPTP treatment generates a neuroinflammatory response, similar to the one observed in human PD (Hirsch et al, 2012; Kurkowska-Jastrzebska et al, 1999). It mimics dopaminergic cell loss associated with PD (Jackson-Lewis and Przedborski, 2007). However, it does not encompass all aspects of PD pathology. Thus, the dual effect observed in this report may not be recapitulated in chronic MPTP intoxication or alpha-synuclein overexpression models (Mochizuki et al, 2006; Munoz-Manchado et al, 2016). Nonetheless, our findings clearly support an effect of EcoHIV infection along the nigrostriatal axis. This observed neurotoxicity is linked to microglial reactivity and changes in the inflammatory microenvironment. If viral infection can elicit neurodegeneration, then it may also affect the onset or progression in PD. Yet, a definitive answer awaits future epidemiological studies.
Materials and Methods
For HIV infection in humanized mouse studies, new-born NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/scid-IL-2Rγcnull or NSG) mice (stock 005557, Jackson Laboratories, Bar Harbor, ME) were transplanted with human CD34+ stem cells obtained from umbilical cord blood, as previously described (Knibbe-Hollinger et al, 2015). Balb/cJ mice (stock 000651, Jackson Laboratories) served as non-humanized controls. After 15 weeks of reconstitution, humanized NSG mice were selected for study based on human CD45+ cells numbers within peripheral blood. Animals with the highest levels of CD45+ populations were selected for infection, whereas those with lower levels were placed into the no infection control groups. The selected animals were infected with HIV-1ADA via intraperitoneal injection at 104 tissue culture infective dose 50 (TCID50)/mouse. After 4 weeks of infection, mice received 4 subcutaneous injections of vehicle (DPBS, 10 ml/kg body weight) or MPTP-HCl (Sigma-Aldrich) at 14 mg MPTP (free base)/kg body weight in DPBS; each dose given at 2 hour intervals. Seven days following MPTP intoxication, mice were sacrificed, brains harvested, and tissues processed for analysis. MPTP safety precautions were followed in accordance with determined safety and handling protocol (Jackson-Lewis and Przedborski, 2007). All animal procedures were also in agreement with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Six- to eight-week old, male C57BL/6J mice (stock 000664) or B6.CB17-Prkdcscid/SzJ (C57BL/6 scid) mice (stock 001913) (The Jackson Laboratory) were used in all EcoHIV studies using previously described protocols (Jones et al, 2016; Potash et al, 2005). Mice were infected by intraperitoneal inoculation of EcoHIV at a dose of 3×106 pg p24/mouse. EcoHIV stock was prepared as previously described (Potash et al, 2005). After 4 weeks of infection, animals were MPTP intoxicated in the same manner as those performed for the HIV studies.
Plasma collection, flow cytometric assessment and T cell Profiling
Peripheral blood was isolated during the course of infection using a maxillary venipuncture. Plasma was collected for analysis of viral load, and whole blood was stained with fluorescently-conjugated monoclonal antibodies to human CD45, CD3, CD4, CD25, CD8, and FoxP3 to quantify T cell frequencies. For intracellular staining, samples were permeabilized using the FoxP3 staining buffer set kit (eBioscience). Fluorescent staining was analyzed using a FACSCalibur flow cytometer.
Perfusions and immunohistochemistry
Following administration of terminal anesthesia (Fatal Plus, pentobarbital), mice were transcardially perfused with DPBS followed by 4% paraformaldehyde/DPBS (Sigma-Aldrich). On day 7 following MPTP intoxication, numbers of dopaminergic neurons in the substantia nigra (SN) and the integrity of termini in the striatum were investigated. Whole brains were isolated and cryosectioned. Frozen midbrain sections (30 μm) were immunostained for tyrosine hydroxylase (TH) (anti-TH, 1:2000, EMD Millipore) and counterstained for Nissl substance, as previously described (Benner et al, 2008). To determine the microglial reactivity, midbrain sections (30 μm) were immunostained for Mac-1 (anti-CD11b, 1:1000, AbD Serotech), as previously described (Kosloski et al, 2013). Total numbers of Mac-1+ cells/mm2, TH+Nissl+ (dopaminergic neurons), and TH-Nissl+ (non-dopaminergic neurons) within the SN were determined by stereological analyses using StereoInvestigator software (MBF Bioscicence). Density of dopaminergic neuron termini in the striatum was calculated by digital densitometry using Image J software (National Institutes of Health).
RNA isolations and polymerase chain reaction
Following 4 weeks of infection and MPTP intoxication, fresh ventral midbrain tissue was isolated, and total RNA was harvested using an RNeasy Mini Kit (Qiagen), using RNAse-free conditions. cDNA was generated from RNA using the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific) and pre-amplification was performed using appropriate primer mixes for RT2 PCR arrays for mouse Inflammatory Response array (Qiagen). Quantitative RT-PCR was performed on an Eppendorf Mastercycler realplex ep as per the manufacturer’s instructions (Eppendorf). Data analysis was performed using RT2 Profiler PCR Array web-based data analysis software, version 3.5 (Qiagen), and gene networks were generated using Ingenuity Pathway Analysis (IPA, Qiagen). The resulting pathway was designed using the Pathway Designer Tool.
Statistical Analysis
All values are expressed as mean ± SEM. Differences in between-group means were analyzed using ANOVA followed by Fisher’s least significant difference (LSD) post hoc test (GraphPad Software, Inc.).
Acknowledgments
We thank the University of Nebraska Medical Center Flow Cytometry Core Research Facility for flow cytometric data acquisition and technical support and Rebecca Wilshusen and Dr. Charles Schutt for their assistance with the mouse studies. We wish to thank Dr. Eran Hadas for the preparation of the EcoHIV used in these studies. This work was supported in part by NIH Grant AG043540, DA028555, NS036126, NS034239, MH064570, NS043985, MH062261, DOD Grant 421-20-09A and the Carol Swarts Emerging Neuroscience Fund to HEG, MH086372, MH083627, DA017618, DA037611, MH104145, DA037611 (DJV), MH104145 (DJV and HEG) and NS094146 (MJP).
Footnotes
Conflict of Interest: No conflict of interest to disclose.
References
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–21. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Boyd JT, Wangensteen KJ, Krawitt EL, Hamill RW, Kao CH, Tsai HH. Hepatitis C virus infection as a risk factor for Parkinson disease: A nationwide cohort study. Neurology. 2016;87:342. doi: 10.1212/01.wnl.0000489939.73359.c3. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–92. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, O’Malley K. Axon degeneration in Parkinson’s disease. Exp Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F. HIV-related movement disorders: epidemiology, pathogenesis and management. CNS Drugs. 2002;16:663–8. doi: 10.2165/00023210-200216100-00002. [DOI] [PubMed] [Google Scholar]
- Cardoso SW, Torres TS, Santini-Oliveira M, Marins LM, Veloso VG, Grinsztejn B. Aging with HIV: a practical review. Braz J Infect Dis. 2013;17:464–79. doi: 10.1016/j.bjid.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Q, Jovasevic V, Malikov V, Sabo Y, Morham S, Walsh D, Naghavi MH. HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nat Commun. 2017;8:1522. doi: 10.1038/s41467-017-01795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–86. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Isacson O. Viral and inflammatory triggers of neurodegenerative diseases. Sci Transl Med. 2012;4:121ps3. doi: 10.1126/scitranslmed.3003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AM, Yoo SW, Chin AC, Xu J, Johnson TP, Trout AL, Hauser KF, Haughey NJ. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci Rep. 2017;7:7748. doi: 10.1038/s41598-017-07570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychol Rev. 2009;19:144–51. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume G, Vecchio E, De Laurentiis A, Trimboli F, Palmieri C, Pisano A, Falcone C, Pontoriero M, Rossi A, Scialdone A, Fasanella Masci F, Scala G, Quinto I. Human immunodeficiency virus-1 Tat activates NF-kappaB via physical interaction with IkappaB-alpha and p65. Nucleic Acids Res. 2012;40:3548–62. doi: 10.1093/nar/gkr1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H. HIV, Tat and dopamine transmission. Neurobiol Dis. 2017;105:51–73. doi: 10.1016/j.nbd.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty P, Hadas E, Kim BH, Dabo AJ, Volsky DJ, Foronjy R. HIV infection model of chronic obstructive pulmonary disease in mice. Am J Physiol Lung Cell Mol Physiol. 2017;312:L500–L509. doi: 10.1152/ajplung.00431.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger A, Kolb R, Schafer R, Klose U. Dopamine reduction in the substantia nigra of Parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS One. 2014;9:e84081. doi: 10.1371/journal.pone.0084081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadas E, Borjabad A, Chao W, Saini M, Ichiyama K, Potash MJ, Volsky DJ. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS. 2007;21:905–9. doi: 10.1097/QAD.0b013e3281574549. [DOI] [PubMed] [Google Scholar]
- Haddad D, Nakamura K. Understanding the susceptibility of dopamine neurons to mitochondrial stressors in Parkinson’s disease. FEBS Lett. 2015;589:3702–13. doi: 10.1016/j.febslet.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Ichiyama K, Do M, Potash MJ, Volsky DJ. Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. J Neuropathol Exp Neurol. 2014;73:59–71. doi: 10.1097/NEN.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JC, Stevenson M, Latz E, Urcuqui-Inchima S. HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. AIDS Res Hum Retroviruses. 2012;28:1313–28. doi: 10.1089/aid.2011.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–2. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT. HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr Drug Targets. 2016;17:4–14. doi: 10.2174/1389450116666150531162212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–51. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta. 2009;1792:714–21. doi: 10.1016/j.bbadis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Lam L, Sadic E, Fernandez F, Tan J, Giunta B. HIV-1 Tat-induced microglial activation and neuronal damage is inhibited via CD45 modulation: A potential new treatment target for HAND. Am J Transl Res. 2012;4:302–15. [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–11. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LD, Jackson JW, Maggirwar SB. Modeling HIV-1 Induced Neuroinflammation in Mice: Role of Platelets in Mediating Blood-Brain Barrier Dysfunction. PLoS One. 2016;11:e0151702. doi: 10.1371/journal.pone.0151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–70. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Karim S, Mirza Z, Kamal MA, Abuzenadah AM, Azhar EI, Al-Qahtani MH, Damanhouri GA, Ahmad F, Gan SH, Sohrab SS. The role of viruses in neurodegenerative and neurobehavioral diseases. CNS Neurol Disord Drug Targets. 2014;13:1213–23. doi: 10.2174/187152731307141015122638. [DOI] [PubMed] [Google Scholar]
- Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, Grant I, Masliah E, Everall IP. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15:131–8. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbe-Hollinger JS, Fields NR, Chaudoin TR, Epstein AA, Makarov E, Akhter SP, Gorantla S, Bonasera SJ, Gendelman HE, Poluektova LY. Influence of age, irradiation and humanization on NSG mouse phenotypes. Biol Open. 2015;4:1243–52. doi: 10.1242/bio.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J Neuroimmunol. 2013;265:1–10. doi: 10.1016/j.jneuroim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15:257–74. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Kwon DS, Kaufmann DE. Protective and detrimental roles of IL-10 in HIV pathogenesis. Eur Cytokine Netw. 2010;21:208–14. doi: 10.1684/ecn.2010.0201. [DOI] [PubMed] [Google Scholar]
- Li W, Gorantla S, Gendelman HE, Poluektova LY. Systemic HIV-1 infection produces a unique glial footprint in humanized mouse brains. Dis Model Mech. 2017 doi: 10.1242/dmm.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Tremblay ME, King IL, Qi J, Reynolds HM, Marker DF, Varrone JJ, Majewska AK, Dewhurst S, Gelbard HA. HIV-1 Tat-induced microgliosis and synaptic damage via interactions between peripheral and central myeloid cells. PLoS One. 2011;6:e23915. doi: 10.1371/journal.pone.0023915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- Meeker RB, Hudson L. Feline Immunodeficiency Virus Neuropathogenesis: A Model for HIV-Induced CNS Inflammation and Neurodegeneration. Vet Sci. 2017;4 doi: 10.3390/vetsci4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsattari SM, Power C, Nath A. Parkinsonism with HIV infection. Mov Disord. 1998;13:684–9. doi: 10.1002/mds.870130413. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Yamada M, Mizuno Y. Alpha-synuclein overexpression model. J Neural Transm Suppl. 2006:281–4. [PubMed] [Google Scholar]
- Moulignier A, Gueguen A, Lescure FX, Ziegler M, Girard PM, Cardon B, Pialoux G, Molina JM, Brandel JP, Lamirel C. Does HIV Infection Alter Parkinson Disease? J Acquir Immune Defic Syndr. 2015;70:129–36. doi: 10.1097/QAI.0000000000000677. [DOI] [PubMed] [Google Scholar]
- Munoz-Manchado AB, Villadiego J, Romo-Madero S, Suarez-Luna N, Bermejo-Navas A, Rodriguez-Gomez JA, Garrido-Gil P, Labandeira-Garcia JL, Echevarria M, Lopez-Barneo J, Toledo-Aral JJ. Chronic and progressive Parkinson’s disease MPTP model in adult and aged mice. J Neurochem. 2016;136:373–87. doi: 10.1111/jnc.13409. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W. Cell death of dopamine neurons in aging and Parkinson’s disease. Mech Ageing Dev. 1999;111:175–88. doi: 10.1016/s0047-6374(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–24. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Pakpoor J, Noyce A, Goldacre R, Selkihova M, Mullin S, Schrag A, Lees A, Goldacre M. Viral hepatitis and Parkinson disease: A national record-linkage study. Neurology. 2017;88:1630–1633. doi: 10.1212/WNL.0000000000003848. [DOI] [PubMed] [Google Scholar]
- Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A. 2005;102:3760–5. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld JC, Vink A, Louahed J, Van Snick J. Interleukin-9 is a major anti-apoptotic factor for thymic lymphomas. Blood. 1995;85:1300–5. [PubMed] [Google Scholar]
- Rosso AL, Mattos JP, Correa RB, Nicaretta DH, Novis SA. Parkinsonism and AIDS: a clinical comparative study before and after HAART. Arq Neuropsiquiatr. 2009;67:827–30. doi: 10.1590/s0004-282x2009000500009. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Hegg CC, Thayer SA, Peterson PK. Activation of human microglial cells by HIV-1 gp41 and Tat proteins. Clin Immunol. 2000;96:243–51. doi: 10.1006/clim.2000.4905. [DOI] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, Gilbert PE, Woods SP. Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol. 2015;21:576–84. doi: 10.1007/s13365-015-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–90. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindberg GM, Sharma U, Banerjee S, Anand V, Dutta R, Gu CJ, Volsky DJ, Roy S. An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J Neuroimmune Pharmacol. 2015;10:74–87. doi: 10.1007/s11481-014-9574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvarski PJ, Trzcianowska H. Neurocognitive disorders seen in HIV disease. Issues Ment Health Nurs. 2000;21:51–70. doi: 10.1080/016128400248266. [DOI] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401–15. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–62. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O’Neill J, Knott NL, Fox HS, Swindells S. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J Neuroimmune Pharmacol. 2013;8:965–74. doi: 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EJ. HIV and aging. Int J Infect Dis. 2016;53:61–68. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Wright ST, Carr A, Woolley I, Giles M, Hoy J, Cooper DA, Law MG. CD4 cell responses to combination antiretroviral therapy in patients starting therapy at high CD4 cell counts. J Acquir Immune Defic Syndr. 2011;58:72–9. doi: 10.1097/QAI.0b013e318225ba62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, Secchiero P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S, Vitale M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–65. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]


