Abstract
Objectives/Hypothesis
Reconstruction of craniofacial cartilagenous defects are among the most challenging surgical procedures in facial plastic surgery. Bioengineered craniofacial cartilage holds immense potential to surpass current reconstructive options but limitations to clinical translation exist. We endeavored to determine the viability of utilizing adipose-derived stem cell-chondrocyte co-culture and three-dimensional (3D) printing to produce 3D bioscaffolds for cartilage tissue engineering. We describe a feasibility study revealing a novel approach for cartilage tissue engineering with in vitro and in vivo animal data.
Methods
Porcine adipose-derived stem cells and chondrocytes were isolated and co-seeded at 1:1, 2:1, 5:1, 10:1, and 0:1 experimental ratios in a hyaluronic acid/collagen hydrogel in the pores of 3D-printed polycaprolactone scaffolds to form 3D bioscaffolds for cartilage tissue engineering. Bioscaffolds were cultured in vitro without growth factors for 4 weeks then implanted into the subcutaneous tissue of athymic rats for an additional 4 weeks before sacrifice. Bioscaffolds were subjected to histologic, immunohistochemical, and biochemical analysis.
Results
Successful production of cartilage was achieved using a co-culture model of adipose-derived stem cells and chondrocytes, without the use of exogenous growth factors. Histology demonstrated cartilage growth for all experimental ratios at the post-in vivo time point confirmed with type II collagen immunohistochemistry. There was no difference in sulfated-glycosaminoglycan production between experimental groups.
Conclusion
Tissue engineered cartilage was successfully produced on 3D-printed bioresorbable scaffolds using an adipose-derived stem cell and chondrocyte co-culture technique. This potentiates co-culture as a solution for several key barriers to a clinically-translatable cartilage tissue engineering process.
Keywords: auricular reconstruction, microtia, anotia, nasal reconstruction, computer-aided design, computer-aided manufacturing, CAD/CAM, three-dimensional printing, tissue engineering, craniofacial reconstruction
INTRODUCTION
Reconstruction of craniofacial cartilagenous defects, performed for an unsalvageable auricular or nasal framework in the setting of trauma, oncologic resection, or congenital malformation, are among the most technically challenging surgical procedures in facial plastic surgery1. A select number of surgeons have mastered the art of using autologous rib cartilage, the current gold standard, to carve a neo-framework resulting in aesthetically and functionally acceptable results2–3. Alternately, some surgeons have elected to use synthetic constructs fashioned in the shape of an auricle or nose4–5.
The field of cartilage tissue engineering (CTE) holds immense potential for creation of bioengineered craniofacial cartilage that both surpasses current reconstructive options and decreases patient morbidity. Several promising studies have demonstrated the use of chondrocytes or chondrogenically pulsed mesenchymal stem cells (MSCs) to produce neocartilage that is histologically and mechanically comparable to native auricular cartilage6–9. These techniques can be combined with patient-specific computer-aided design (CAD) and three-dimensional (3D) printing to produce high-fidelity auricular and nasal tissue engineering scaffolds10. However, with the many positive prospects in CTE, significant challenges remain, most notably the need for a large (107) number of cells.
Co-culture, where chondrocytes and MSCs are simultaneously seeded onto tissue engineering scaffolds, is a new technique that holds promise for circumventing some of the current limitations of utilizing chondrocytes or MSCs alone. Here we report the use of a co-culture model using adipose-derived stem cells (ASCs) and chondrocytes for CTE. We hypothesize that co-culture technique can be adapted for craniofacial cartilage applications using hydrogels combined with 3D-printed bioresorbable scaffolds and that a variety of ratios of ASCs-to-chondrocytes may be utilized. This approach affords the potential for patient-specific CTE using computer-aided design (CAD) while mitigating the limitations of cell availability and need for prolonged in vitro cell culture or exogenous growth factor exposure of traditional CTE approaches. These represent key barriers to the eventual goal of creating a clinically translatable patient-specific craniofacial CTE methodology. Our objective is to assess the feasibility of this approach and the effect of ASC-to-chondrocyte ratio on cartilage production using histologic, immunohistochemical, and biochemical analysis.
MATERIALS AND METHODS
Protocol approval was obtained by the University of Michigan Institutional Animal Care & Use Committee and the University of Illinois Institutional Animal Care & Use Committee (University of Michigan #3857, University of Illinois #10114).
Scaffold Design and Manufacturing via 3D Printing
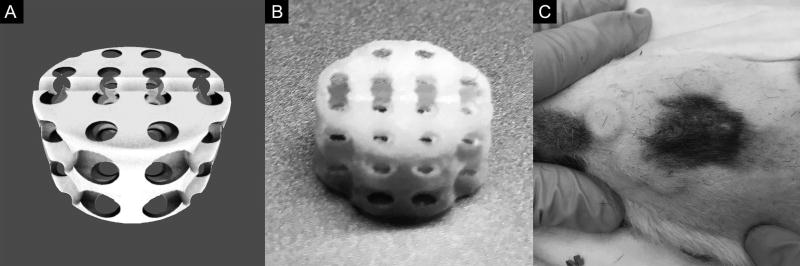
Scaffolds were created using previously described image-based hierarchical design methods developed by Hollister and colleagues11–17. This process can be used to create patient-specific tissue engineering scaffolds of any geometry10. A standard 10 mm × 5 mm cylindrical disc scaffold macroarchitecture with a 2.7 mm spherical pore internal microarchitecture was chosen for this study to produce consistency of constructs for tissue analysis (Figure 1A). This yielded an overall scaffold porosity of 68.3% with an available volume per scaffold of 268 µL. A midline groove was incorporated into the scaffold to facilitate bifurcation during analysis. The final scaffold design was then 3D printed using an EOS P100 laser sintering system (EOS North America, Novi, Michigan) adapted to laser sinter L-polycaprolactone (PCL) powder (PCL Source: Polysciences, Warrington, Pennsylvania; PCL Preparation: Jet Pulverizer, Moorsetown, New Jersey)18. The laser sintering process can accurately reproduce feature sizes on the order of 700 µm and produce over 500 representative scaffolds with a single print cycle (Figure 1B). Scaffolds were cleaned of residual excess powder via sonication in 70% sterile ethanol then sterilized in a 24 hour 70% sterile ethanol soak prior to use.
Figure 1. Computer-Aided Design and Three-Dimensional Printing Process for Production of Porous Bioresorbable Tissue Engineering Scaffolds.
(A) Rendering of stereolithography (.STL) file for cylindrical tissue engineering scaffold with 2.7 mm spherical pore internal microarchitecture. (B) Final tissue engineering scaffold manufacturing via selective laser sintering three-dimensional printing technique. Scaffold features down to 70 µm can be successfully reproduced with this approach. (C) Final tissue engineering scaffolds after cell seeding in a hyaluronic acid/collagen hydrogel implanted into four randomized quadrants in a subcutaneous pocket on the dorsum of an athymic rat.
Cell Harvest and Culture
Porcine ASCs derived from subcutaneous back fat and chondrocytes derived from auricular and tracheal cartilage were harvested from adolescent Yorkshire pigs using the methods previously developed by Wheeler and colleagues19–20. Porcine cells were utilized for experiments due to the investigators’ experience with these cells and availability of the necessary cell types. Primary (P0) ASC and chondrocyte cells were spun down and frozen prior to cell seeding experiments. At the time of preparation for scaffold seeding, cells were thawed and expanded in growth media consisting of high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco) with 10% fetal bovine serum (FBS) (Gibco), 1% pen/strep, and 0.2% Fungizone in a 37°C, 5% CO2 incubator. ASCs were expanded to passage 2 (P2) and chondrocytes expanded to passage 1 (P1) to provide sufficient cells for seeding. Cells were passaged at 90% confluence.
Creation of Experimental Ratios and Scaffold Seeding
Adipose-derived stem cells and chondrocytes were rinsed with Hank’s buffered saline solution (HBSS) (Gibco), trypsinized (0.25% trypsin) (Gibco), and aliquoted into experimental ratios of 1:1, 2:1, 5:1, 10:1, and 0:1 ASC-to-chondrocytes. Cell counting was performed prior to cell mixing using an automated cell counter (Moxi Z, Orflo Technologies). Given that the cells were harvested from several animals, each cell type was pooled prior to creation of experimental ratios. Each experimental group was then re-suspended in a type I collagen:hyaluronic acid hydrogel solution and seeded into a pre-wetted cylindrical PCL scaffold21. The hydrogel consisted of type I collagen at a concentration of 6 mg/mL in acetic acid (Discovery Labware) and hyaluronic acid at a concentration of 3 mg/mL (LifeCore Biomedical). Prior to seeding, the PCL scaffolds were placed in custom-fabricated silicone (Sylgard, Dow Corning) molds to prevent extravasation of the seeding solution prior to gelation. A 0.05N NaOH in NaCO3 solution was used to induce gelation and scaffolds were subsequently transferred to 24-well low attachment plates (Fischer Scientific) for culture. The cell seeding density was 2×106 cells/cm3 and total of 12 scaffolds per experimental group were seeded.
In Vitro Incubation
Seeded constructs were placed individually within a 24-well low attachment plate with 1 scaffold per well and placed in a sterile CO2 incubator. Scaffolds were incubated at 37°C with 5% CO2 on an orbital shaker within the incubator for 4 weeks. Culture media was changed every 2–3 days by aspirating old media from each culture well and replacing with 1.25 mL of fresh media warmed to 37°C within a sterile culture hood. Culture media consisted of low-glucose DMEM with 10% FBS, 1% pen/strep, and 0.2% Fungizone. After 4 weeks, six scaffolds from each experimental group were extracted for post-in vitro biochemical analysis, while the remaining six were reserved for in vivo implantation.
In Vivo Implantation
Seven athymic rats underwent implantation with tissue engineering scaffolds under general anesthesia with isoflurane delivered by mask. All scaffolds were rinsed with HBSS prior to implantation. All animals were male with each weighing between 250 and 305 g. Each animal was shaved, prepped with iodine solution after induction of anesthesia and a vertical incision was sharply made on the dorsum of the animal. A total of 4 scaffolds per animal were implanted in a subcutaneous pocket into randomized quadrants on the back of the animal. The incision was then closed with surgical staples, which were removed on post-operative day seven. After 4 weeks, the animals were euthanized and the scaffolds were harvested for post-in vivo analysis.
Biochemical Analysis
Post-in vitro and post-in vivo specimens were split along the midline groove to double the number of constructs for analysis. One-half of each construct was weighed wet, lyophilized, reweighed dry, and digested in 1 mg/mL Papain stock solution (Fischer Scientific) at 65°C for 16 hours. PicoGreen assay (Invitrogen, Colecular Probes) was used to quantify the DNA content of the constructs with Lambda phage DNA (0–1 mg/mL) as a standard. The sulfated-glycosaminoglycan (s-GAG) content was measured using the Blyscan Glycosaminoglycan Assay (Accurate Chemical & Scientific Corp).
Histology and Immunhistochemistry
Remaining post-in vivo constructs were fixed in 4% formalin for 24 hours, embedded in paraffin (TissuePrep, Fischer Scientific), and processed using standard histologic procedures with a slice thickness of 10 µm. Stains included hematoxylin and eosin, Safrinin-O, and toluidine blue. Type II collagen immunohistochemical staining was performed using 5 µg/mL primary mouse anti-type II collagen monoclonal antibodies (Developmental Studies Hybridoma Bank, University of Iowa).
Statistical Analysis
Data for biochemical analysis (DNA and s-GAG expression) were collected from six samples per co-culture experimental group after 4 weeks of in vitro cell culture and 4 weeks of in vivo growth. Data was expressed as mean ± standard error of the mean (SEM). Results were analyzed using Student’s t-test using SPSS 17.0 (SPSS Inc., Chicago, IL) and statistical significance was set to 5% (α=0.05) in all analyses.
RESULTS
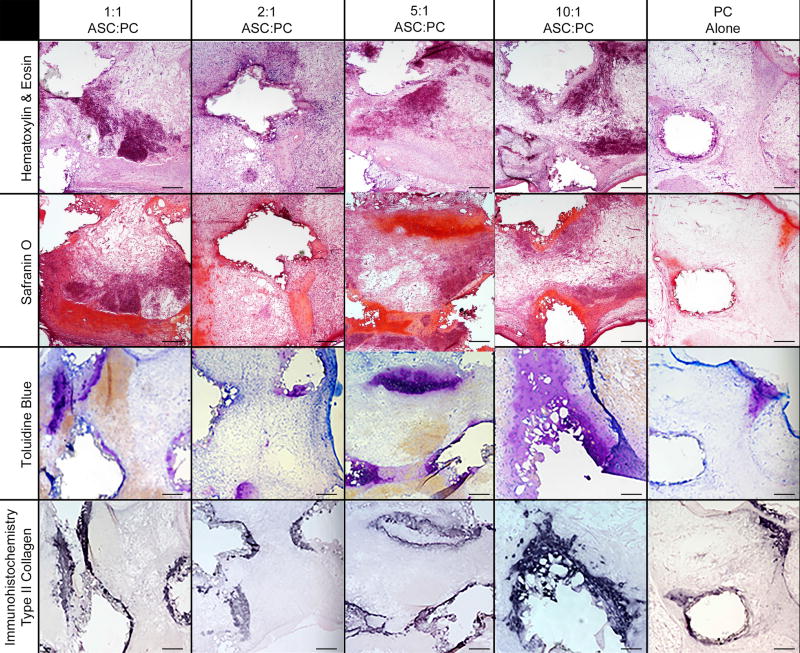
In vitro co-culture of porcine ASCs and chondrocytes in 3D-printed PCL cylindrical discs with an internal spherical porous architecture resulted in growth and maintenance of cartilage-like tissue after 8 weeks (4 weeks in vitro + 4 weeks in vivo). Surgical implantation was straightforward and the scaffolds were well tolerated by the animals with no minor or major complications. There was good maintenance of structural support by the PCL scaffolds after 4 weeks growth in a subcutaneous pocket, as shown in Figure 1C. Histologically normal appearing cartilage growth was noted in all experimental co-culture groups after 1 month of in vivo culture, which was confirmed with type II collagen immunohistochemistry (Figure 2). Notably, the 5:1 ASC-to-chondrocyte ratio demonstrated well delineated hyaline cartilage architecture in histology, with dense collagen deposition and lacunae surrounding the chondrocytes and differentiated ASCs within the microspheres of the scaffold (Figure 3–4)
Figure 2. 40× histologic and immunohistochemical results of co-culture experimental groups at differing ratios of ASCs-to-chondrocytes after 4 weeks of in vitro followed by 4 weeks of in vivo culture.
Representative slices are shown of each experimental group through the inner sections of the tissue engineering scaffolds at 40× magnification. ASC = Adipose-derived stem cells. Black scale bar in all panels = 300 µm.
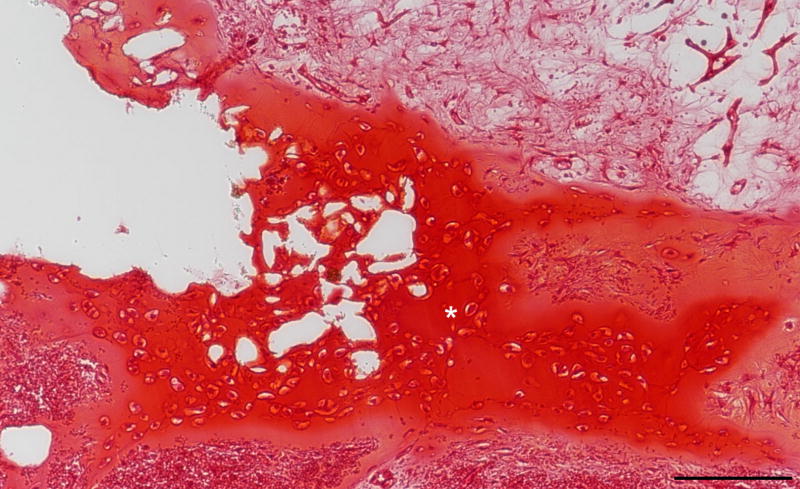
Figure 3. 100× Safranin O stain of 5:1 ASC:chondrocyte co-culture scaffold after 4 weeks in vivo growth representative of a longitudinal inner section of a construct.
White asterisk denotes well defined lacuna around chondrocytes within cartilage matrix. ASC = Adipose-derived stem cells. Black scale bar = 100 µm.
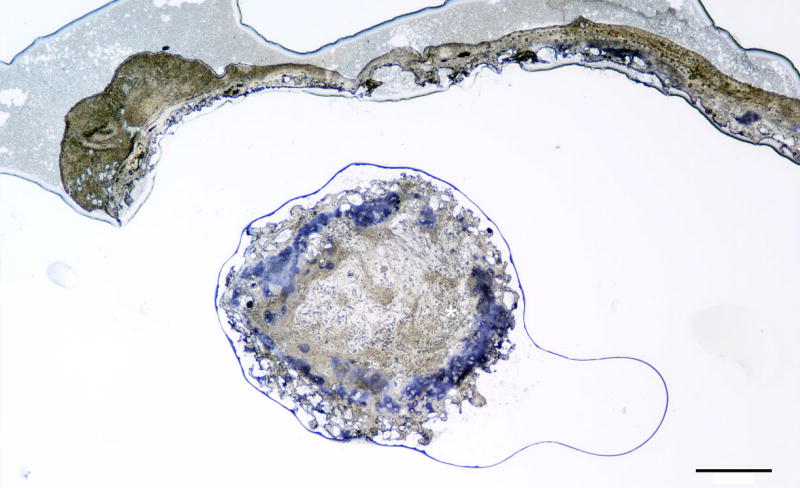
Figure 4. 40× type II collagen immunohistochemical stain of 5:1 ASC:chondrocyte co-culture scaffold after 4 weeks in vivo growth representative of a transverse outer section of a construct.
ASC = Adipose-derived stem cells. Black scale bar = 1 mm.
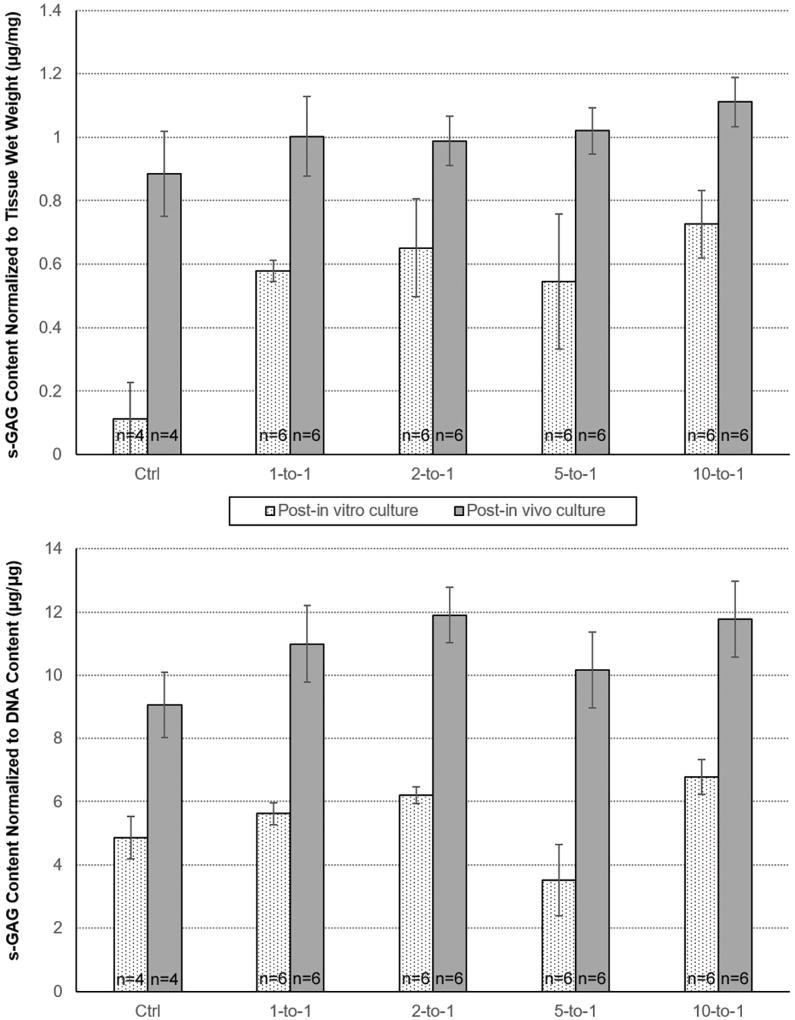
Biochemical analysis results are summarized in Figure 5. There was no statistically significant difference in s-GAG content normalized to scaffold wet weight (µg/mg) or DNA content (µg/mg) between the co-culture experimental groups at the post-in vitro or post-in vivo time points. There was a statistically significant higher s-GAG content normalized to scaffold wet weight (µg/mg) and DNA content (µg/mg) in all co-culture groups compared to the chondrocyte-alone control group (p<0.05 for all analyses) at the post-in vitro timepoint, however this difference disappeared at the post-in vivo timepoint.
Figure 5. Biochemical characterization for all experimental groups.
All values expressed as mean values. All ratios expressed as adipose-derived stem cells-to-chrondrocytes. Error bars represent standard error of the mean. s-GAG = sulfated glycosaminoglycan. DNA = dioxyribonuclease.
DISCUSSION
Reconstruction of the auricular and external nasal frameworks, whether performed in the setting of trauma, oncologic resection, or congenital malformation, are some of the most demanding procedures in facial reconstructive surgery. Few surgeons have mastered the art of using autogenous rib cartilage, the current gold standard, to carve a neo-auricular framework resulting in aesthetically pleasing results2,3. Moreover, this approach typically requires multiple revision procedures and carries risks of donor site morbidity, pneumothorax, undesired scarring, and graft failure or infection. Alternately, some surgeons elect to use synthetic porous high-density polyethylene (MedPor, Styker Corp., Kalamazoo, Michigan) for reconstruction4. This rigid, synthetic material can be fashioned into a neo-auricular framework for sub-cutaneous implantation. However, rates of framework extrusion through and necrosis of the overlying skin while poorly reported, occur at a rate of at least 3–5%4.
Tissue engineering holds several ubiquitous advantages, including the ability to create a patient-specific living construct using the patient’s own cells. Several promising studies have demonstrated the use of chondrocytes or MSCs cultured in pro-chondrogenic growth factors (GFs) as being able to develop neocartilage that is histologically and mechanically similar to native ear cartilage6–9. In these models, chondrocytes or MSCs are harvested, passaged and expanded, then seeded onto a scaffold and cultured in vitro prior to sub-cutaneous implantation. A variety of scaffold materials, including polyglycolic acid, polycaprolactone, chitosan, and silk, have been used successfully with varying properties that are favorable for different conditions6–9. However, with the many positive prospects of producing a tissue engineered auricle, new challenges have also surfaced.
The primary limitation of utilizing solely chondrocytes for CTE is the large number of cells (up to 5×107) needed to seed human-sized craniofacial frameworks9,22–26. The number of chondrocytes available from autologous cartilage is limited and passaging chondrocytes induces dedifferentiation with loss of type II collagen and sulfated glycosaminoglycan (s-GAG) production27. Mesenchymal stem cells, of which ample cell quantities are available, have been posited as a solution to seeding requirements. Prior experiments have shown a variety of MSC types, including ASCs and bone-marrow stromal cells, to be capable of chondrogenic differentiation28–29. However, chondrogenic commitment of MSCs requires exogenous delivery of GFs for weeks and cells can demonstrate a propensity for ossification30–31. Additionally, neovascularization of 3D tissue engineered cartilagenous constructs has proven to be a challenge to the long-term stability of these constructs, particularly with fragility of ASCs in hypoxic tissue environments32.
Co-culturing of chondrocytes and MSCs is a new technique that holds promise for circumventing some limitations of utilizing chrondrocytes or MSCs alone. In a co-culture model, chondrocytes and MSCs are simultaneously seeded onto a tissue engineering scaffold. Chondrocytes have been found to induce chondrogenic differentiation of the MSCs via production of exogenous GFs such as cytokine-like protein 1 (Cytl1), bone morphogenic protein-2 (BMP-2), parathyroid hormone-related peptide (PTHrP), and transforming growth factor-beta (TGF-β) as well as paracrine, juxtacrine, and gap-junction signaling pathways33–36. In this way, chondrocytes maintain the chondrogenic niche required for commitment of MSCs to the chondrogenic phenotype, circumventing the need for exogenous GF delivery. Additionally, chondrocytes provide matrix for MSC migration and prevent ossification of MSC-derived chondrocytes37.
Our group has previously developed a process utilizing image-based design and 3D printing to produce high-fidelity patient-specific tissue engineering scaffolds using PCL, a bioresorbable polymer10. Utilization of a bioresorbable material allows for eventual replacement of the scaffold with chondrocyte extracellular matrix, thus best emulating natural craniofacial cartilage. We have previously seeded these scaffolds with primary chondrocytes to produce tissue engineered auricular and nasal constructions10. One advantage of a scaffold-specific CAD approach utilizing 3D-printed biomaterials is the ability to generate an intra-scaffold environment more conducive to cell survival and neovascularization. Our group has previously performed studies demonstrating that a spherical pore microarchitecture results in improved cell survival and cartilage deposition for CTE38. This process affords the ability to rapidly produce high-fidelity anatomic scaffolds while also allowing meticulous control of the pore microarchitecture. However, our prior investigations have been limited by many of the previously described constraints, including the limited number of chondrocytes available for harvest, the need to passage chondrocytes in cell culture, and the need for prolonged exogenous GF exposure.
Although other studies have utilized ASC/chondrocyte co-culture for CTE39, this report is the first to our knowledge describing successful use of ASC co-culture on 3D-printed tissue engineering scaffolds for successful CTE. The use of ASCs with a co-culture technique is particularly advantageous for a clinically-translatable approach, given the low morbidity to harvest these cells vis-à-vis to bone-marrow derived stromal cells. The described process can readily be adapted for tissue engineering constructs of any shape, including patient-specific auricular and nasal constructs using DICOM data (Figure 6)10.
Figure 6. Computer-Aided Design and Three-Dimensional Printing Methodology for Patient-Specific Cartilage Tissue Engineering.
DICOM data of the anatomic structure of interest is acquired and used the generate a three-dimensional model of the structure. The model is then converted into a porous structure using negative Boolean operations and manufactured from polycaprolactone using a selective laser sintering three-dimensional printer. The bioresorbable scaffold is then seeded with cells suspended in a hyaluronic acid/collagen hydrogel prior to implantation. (Adapted from Otolaryngology-Head & Neck Surgery, Volume 152, Issue 1, DA Zopf, AG Mitsak, CL Flanagan, et al. Computer-aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction, 57–62, Copyright (2015), with permission from SAGE Publishing).
Our results demonstrate that all the experimental ratios of ASC-to-chondrocytes in our study resulted in type II collagen and cartilage production on short term in vivo analysis. Notably, this was achieved without the use of exogenous GFs during scaffold incubation. Using a cell count goal of 5×107 as the number of cells needed for a typical human-sized auricle, ratios of 10:1 and 5:1 ASC:PC yield cell number requirements which are clinically achievable from a cell harvest without the need for prolonged passaging of cells in the laboratory setting40. These represent important barriers to a clinically-translatable process for craniofacial CTE which appear to be overcome with a co-culture technique.
Interestingly, the co-culture scaffold groups all appeared to perform similarly, despite different ratios of ASC-to-chondrocytes. Additionally, the co-culture groups appeared to outperform the chondrocyte-alone scaffolds in amount of histologic cartilage deposition, despite identical cell seeding densities. This may represent an inherent superior viability of co-cultured cells in this methodology or synergistic interaction of co-cultured cells to promote chondrogenesis. However, this could also be an artifact of decreased viability of chondrocytes in cell culture. Given that no analysis of cell viability or gene expression was performed in this study, these ideas remain speculative.
Our feasibility study is limited by a short in vivo incubation period and small number of constructs. As such, statistical differences of the biochemical characteristics of the experimental groups may have not been captured, as well as differences in the trajectory of tissue deposition with more prolonged in vivo growth. Inferences regarding human translatability of a tissue engineering model utilizing porcine cells should be tempered until similar results can be demonstrated using human-derived PCs and ASCs. Our PCL constructs are anticipated to maintain construct fidelity for 2 to 3 years prior to resorption. More prolonged in vivo study will be necessary to determine whether the tissue engineered construct contracts or resorbs after scaffold dissolution. Additional study of the microscopic orientation and mechanical characteristics of ASC:PC co-cultured constructs will help delineate whether co-cultured cartilage creates primarily elastic cartilage or fibrocartilage, although this is of less importance in a structure such as an auricle. Further cell viability and gene expression analysis studies would also help characterize the chondrogenic commitment of ASCs and ability of ASCs and PCs to tolerate co-culture on 3D-printed bioscaffolds. We chose an incubation period of 4 weeks for our scaffolds after cell seeding in concordance with previously described CTE techniques. However, given the lack of a need for exogenous GF delivery with a co-culture approach, we are currently investigating a “seed and go” approach where scaffolds are implanted immediately after cell seeding. Given that our MSCs were not immunofluorescently labeled, direct demonstration of chondrogenic differentiation of the MSCs was not possible, nor was it possible to assess migration of host MSCs into the constructs. However, we feel that histologic markers are a sufficient surrogate in the scope of this experiment given prior work which has demonstrated the successful chondrogenic commitment of MSCs using this technique.
CONCLUSION
We describe the successful use of an ASC-chondrocyte co-culture technique and CAD-designed 3D-printed tissue engineering scaffolds for CTE in an animal model. This co-culture model produced formal cartilage production on short term in vivo follow-up in all experimental groups, including 5:1 and 10:1 ASC:chondrocyte ratios. The clinical availability of ASCs and lack of a need for prolonged exogenous GF exposure suggest this approach mitigates many of the limitations of traditional CTE approaches. These represent key barriers to the eventual goal of creating a clinically translatable patient-specific craniofacial CTE methodology that may be overcome using co-culture and 3D printing.
Acknowledgments
Funding: This work was funded through the AAO-HNSF Centralized Otolaryngology Research Efforts (CORE) program by the American Society of Pediatric Otolaryngology (ASPO) Research Grant. Dr. Morrison is supported by NIH grant T32 DC005356-12. Dr. Zopf and Dr. Hollister have filed provisional patents (2115-007095-US-PS1) for the 3D-printed auricular and nasal scaffolds discussed.
Following Conclusion of manuscript.
We thank Rhima Coleman, PhD for her assistance with methods development and Jane Modes for her assistance with histology (University of Michigan). Further, we thank Heather Lopez-Lake, M.S. for the isolation and culture of the porcine chondrocytes and adipose-derived stem cells (University of Illinois).
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Presentation: Abstract was presented in the “Best of Orals” section at the 2016 American Academy of Otolaryngology-Head & Neck Surgery Foundation Annual Meeting and OTO EXPO; September 18–21, 2016; San Diego, California.
Author Contributions: Dr. Morrison had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of data analysis. Study concept and design: Morrison, Zopf, Milner, Flanagan, Wheeler, Green, Hollister. Acquisition of data/research methods: Morrison, Nasser, Kashlan, Flanagan, Hollister. Analysis and interpretation of data: Morrison, Nasser, Kashlan, Zopf, Milner, Flanagan, Wheeler, Green, Hollister. Drafting of the manuscript: Morrison, Nasser, Kashlan. Critical revision of the manuscript: Morrison, Nasser, Kashlan, Zopf, Milner, Flanagan, Wheeler, Green, Hollister. All named authors reviewed and revised the manuscript and approved the manuscript as submitted.
Institutional Review Board Approval: Protocol approval was obtained by the University of Michigan Institutional Animal Care & Use Committee and the University of Illinois Institutional Animal Care & Use Committee (University of Michigan #3857, University of Illinois #10114).
Level of Evidence: NA
References
- 1.Bichara DA, O'Sullivan NA, Pomerantseva I, et al. The tissue-engineered auricle: Past, present, and future. Tissue Eng Part B Rev. 2012;18(1):51–61. doi: 10.1089/ten.TEB.2011.0326. [DOI] [PubMed] [Google Scholar]
- 2.Bauer B. Reconstruction of Microtia. Plast Reconstr Surg. 2009;124(1):14e–26e. doi: 10.1097/PRS.0b013e3181aa0e79. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes GH, Wong J, Guilfoyle R. Microtia reconstruction. Plast Reconstr Surg. 2014;134(3):464–479. doi: 10.1097/PRS.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 4.Romo T, 3rd, Presti PM, Yalamanchili HR. Medpor alternative for microtia repair. Facial Plast Surg Clin North Am. 2006;14(2):129–36. vi. doi: 10.1016/j.fsc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Wellisz T. Clinical experience with the Medpor porous polyethylene implant. Aesthetic Plast Surg. 1993;17(4):339–44. doi: 10.1007/BF00437109. [DOI] [PubMed] [Google Scholar]
- 6.Isogai N, Kusuhara H, Ikada Y, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12(4):691–703. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 7.van Osch GJ, van der Veen SW, Verwoerd-Verhoef HL. In vitro redifferentiation of culture-expanded rabbit and human auricular chondrocytes for cartilage reconstruction. Plast Reconstr Surg. 2001;107(2):433–440. doi: 10.1097/00006534-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Vacanti CA, Cima LG, Ratkowski D, Upton J, Vacanti JP. Tissue engineered growth of new cartilage in the shape of a human ear using synthetic-polymers seeded with chondrocytes. 1992;252:374. [Google Scholar]
- 9.Shieh SJ, Terada S, Vacanti JP. Tissue engineering auricular reconstruction: In vitro and in vivo studies. Biomaterials. 2004;25(9):1545–1557. doi: 10.1016/s0142-9612(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 10.Zopf DA, Mitsak AG, Flanagan CL, Wheeler MB, Green GE, Hollister SJ. Computer Aided-Designed, 3-Dimensionally Printed Porous Tissue Bioscaffolds for Craniofacial Soft Tissue Reconstruction. Otolaryngol Head Neck Surg. 2015;152(1):57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollister SJ, Levy RA, Chu TMJ, et al. An image based approach to design and manufacture craniofacial scaffolds. Int J Oral Maxillofac Surg. 2000;29:67–71. doi: 10.1034/j.1399-0020.2000.290115.x. [DOI] [PubMed] [Google Scholar]
- 12.Hollister SJ, Maddox RD, Taboas JM, et al. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–4103. doi: 10.1016/s0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 13.Lin CY, Kikuchi N, Hollister SJ. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J Biomech. 2004;37:623–636. doi: 10.1016/j.jbiomech.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Hollister SJ, Lin CY, Saito E, et al. Engineering craniofacial scaffolds. Orthod Craniofac Res. 2002;8:162–173. doi: 10.1111/j.1601-6343.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 15.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 16.Hollister SJ, Lin CY. Computational design of tissue engineering scaffolds. Comput Methods Appl Mech Eng. 2005;196:2991–2998. [Google Scholar]
- 17.Kang H, Lin CY, Hollister SJ. Topology optimization of three dimensional tissue engineering scaffold architectures for prescribed bulk modulus and diffusivity. Struct Multidiscipl Optim. 2010;42:633–644. doi: 10.1007/s00158-010-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partee B, Hollister SJ, Das S. Selective laser sintering process optimization for layered manufacturing of CAPA 6501 polycaprolactone bone tissue engineering scaffolds. J Manuf Sci Eng. 2006;128:531–540. [Google Scholar]
- 19.Kim D, Monaco E, Maki A, et al. Morphologic and transcriptomic comparison of adipose- and bone-marrow-derived porcine stem cells cultured in alginate hydrogels. Cell Tissue Res. 2010;341(3):359–70. doi: 10.1007/s00441-010-1015-3. [DOI] [PubMed] [Google Scholar]
- 20.Herzog KK, Milner DJ, Johnson SJ, Wheeler MB. Chondrogenic potential of porcine adipose-derived stem cells, chondrocytes, periosteal cells, and fibroblasts in a pellet culture system. Reproduction Fertility and Development. 2014;27(1):253. [Google Scholar]
- 21.Liao E, Yaszemski M, Krebsbach PH, Hollister SJ. Tissue engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene) fumarate scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LI, Pomerantseva EK, Bassett CM, et al. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A. 2011;17(11–12):1573–1581. doi: 10.1089/ten.TEA.2010.0627. [DOI] [PubMed] [Google Scholar]
- 23.Xue JB, Feng R, Zheng Y, et al. Engineering earshaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34(11):2624–2631. doi: 10.1016/j.biomaterials.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ruszymah BH, Chua KH, Mazlyzam AL, Aminuddin BS. Formation of tissue engineered composite construct of cartilage and skin using high density polyethylene as inner scaffold in the shape of human helix. Int J Pediatr Otorhinolaryngol. 2011;75(6):805–810. doi: 10.1016/j.ijporl.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Yanaga H, Imai K, Fujimoto T, Yanaga K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast Reconstr Surg. 2009;124(3):817–825. doi: 10.1097/PRS.0b013e3181b17c0e. [DOI] [PubMed] [Google Scholar]
- 26.Kusuhara H, Isogai N, Enjo M, et al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17(1):136–146. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 27.Von Der Mark K, Gauss V, Von Der Mark H, Mueller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsumi S, Shimazu A, Miyazaki K, et al. Retentio of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochemical and Biophysical Research Communications. 2011;288(2):413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 29.Martin I, Shastri VP, Padera RF, et al. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. Journal of Biomedical Materials Research. 2001;55(2):229–235. doi: 10.1002/1097-4636(200105)55:2<229::aid-jbm1009>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Hwang NS, Elisseeff J. Application of stem cells for articular cartilage regeneration. Journal of Kne Surgery. 2009;22:60. doi: 10.1055/s-0030-1247728. [DOI] [PubMed] [Google Scholar]
- 31.Hickok NJ, Haas AR, Tuan RS. Regulation of chondrocyte differentiation and maturation. Microsc Res Tech. 1998;43:174–90. doi: 10.1002/(SICI)1097-0029(19981015)43:2<174::AID-JEMT9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Merceron C, Vinatier C, Portron S, et al. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010 Feb;298(2):C355–64. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Sun H, Yan D, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31(36):9406–9414. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 34.Keller B, Yang T, Munivez E, et al. Interaction of TGFbeta and BMP signaling pathways during chondrogenesis. PLoS One. 2011;6:316421. doi: 10.1371/journal.pone.0016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JS, Ryoo ZY, Chun JS. Cytokine-like (Cytl1) regulates the chondrogenesis of mesenchymal cells. J Biol Chem. 2007;282:29359–67. doi: 10.1074/jbc.M700965200. [DOI] [PubMed] [Google Scholar]
- 36.Choi YS, Lim SM, Shin HC, et al. Chondrogenesis of human periostium-derived progenitor cells in atelocollagen. Biotechnol Lett. 2007;29:323–9. doi: 10.1007/s10529-006-9240-2. [DOI] [PubMed] [Google Scholar]
- 37.Mo XT, Guo SC, Xie HQ, et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45(1):42–51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 38.Zopf DA, Morrison RJ, Flanagan CL, Mitsak AG, Green GE, Hollister SJ. Surface Area Effects on Chondrogenic Potential of 3-Dimensionally Printed Porous Tissue Bioscaffolds for Auricular Reconstruction. Paper presented at: Combined Otolaryngology Spring Meetings, The Triologic Society, American Society of Pediatric Otolaryngology Section. 2014 May 16; [Google Scholar]
- 39.Goh BS, Che Omar SN, Ubaidah MA, Saim L, Sulaiman S, Chua KH. Chondrogenesis of human adipose derived stem cells for future microtia repair using co-culture technique. Acta Otolaryngol. 2016 Nov 30;:1–10. doi: 10.1080/00016489.2016.1257151. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]