Abstract
Aims
The most common BRAF mutation in ovarian low-grade serous neoplasms (LGSNs) involves substitution of valine by glutamic acid at position 600 (V600E). Small studies have demonstrated high specificity of immunohistochemistry with mutation-specific monoclonal antibody VE1. We sought to investigate expression of VE1 protein in LGSNs and its correlation with BRAF mutation-associated histological features and BRAF mutation status.
Methods and results
We reviewed pathology reports and available slides from ovarian serous borderline tumours (SBTs) and low-grade serous carcinomas (LGSCs) diagnosed between 2000-2012. VE1 immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections. Tumours with ≥50% positive cells were considered positive. Statistical analyses were performed using SPSS 24.0. Of 121 LGSNs, there were 73 SBTs, 8 SBTs with micropapillary features (mpSBT), and 40 LGSCs (22 primary, 18 metastatic). VE1 was positive in 52% (38/73) of SBTs and 9% (2/22) of primary LGSCs, and in none of the mpSBTs and metastatic LGSCs (p<0.0001). Of 76 tumours with known mutation status, 42 (55%) harbored mutations, including BRAFV600E (26, 34%), KRASG12D (8, 11%), and KRASG12V (8, 11%). BRAFV600E mutations were present in 48% (25/52) of SBTs and 5% (1/22) of LGSCs (p<0.0001). VE1 was positive in 96% (25/26) of BRAFV600E-mutated tumours and correlated with BRAF mutation-associated histological features (p<0.0001).
Conclusions
BRAFV600E mutations are significantly more common in SBTs than in LGSCs. Immunohistochemical expression of VE1 protein is strongly associated with BRAFV600E mutation and BRAF mutation-associated histological features. VE1 immunohistochemistry is a reliable method for the detection of BRAFV600E mutations.
Keywords: BRAFV600E mutation, low-grade serous carcinoma, serous borderline tumour, ovary, VE1 immunohistochemistry
Introduction
Low-grade serous carcinomas (LGSCs) of the ovary represent slow-growing, genetically stable neoplasms, typically confined to the ovary, and lacking TP53 mutations (1–3). LGSCs may occur de novo or evolve in a stepwise fashion from epithelial inclusions to benign serous cystadenomas or adenofibromas and serous borderline tumours (SBTs) (2, 4, 5). SBTs and LGSCs are commonly characterised by activating somatic mutations in KRAS oncogene and its downstream mediator, BRAF. KRAS and BRAF mutations are usually mutually exclusive in these tumours, with reported mutation frequencies of up to 54% for KRAS and up to 48% for BRAF. BRAF mutations have been reported in 23-48% of SBTs but in only 0-6% of LGSCs (6–11). BRAF encodes a protein from the RAF family of serine/threonine protein kinases, involved in regulating the mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) cell signaling pathway. The most common BRAF mutation involves substitution of valine by glutamic acid at position 600 (V600E) and has been described in various tumours such as melanoma, hairy cell leukaemia, colorectal carcinoma, papillary thyroid carcinoma and non-small cell carcinoma of lung (12, 13).
Immunohistochemistry with anti-BRAFV600E monoclonal antibody VE1 has been shown to be a sensitive and specific method for detection of BRAFV600E mutation in ovarian serous tumours in small studies (14–16). Given that BRAFV600E-mutated SBTs exhibit morphological features that may be misinterpreted as micropapillary SBT (mpSBT; a variant of SBT more frequently associated with advanced stage and LGSC) (17), accurate identification of BRAFV600E-mutated SBTs is clinically important. We sought to investigate expression of VE1 protein in low-grade serous neoplasms of the ovary, and its association with BRAFV600E mutation-associated histological features and BRAFV600E mutation status.
Materials and Methods
This study was performed in accordance with institutional ethics committee guidelines (protocol no. 16-1684; Memorial Sloan Kettering Cancer Center Institutional Review Board; approved 23 December 2016).
Through a search of our institutional database, we identified patients diagnosed with SBTs and LGSCs of the ovary between 2000 and 2012. We reviewed pathology reports and available haematoxylin-eosin stained slides. Briefly, diagnostic criteria used were as follows – SBT: hierarchical branching of irregular papillae with detached cell clusters and single cells (budding), fibromatous to edematous fibrovascular cores, with or without luminal mucin; mpSBT: fibromatous to edematous papillae lined by delicate, filiform papillae lacking fibrovascular cores (5× longer than wide; the minimum size criterion of 5 mm is not used at our institution) or fused papillae imparting a cribriform appearance; and LGSC: destructive stromal invasion with micropapillae, macropapillae or single cells, or solid growth measuring >5 mm. We recorded histological features associated with BRAFV600E mutation, including the presence of epithelial cells with abundant eosinophilic cytoplasm, discrete cell borders, cell budding and detachment, as previously described (18, 19). Tumours exhibiting at least two of these features (eosinophilic cytoplasm and cell budding) were considered positive for BRAFV600E mutation-associated histological features.
Immunohistochemistry was performed on 4-micron thick archival formalin-fixed paraffin-embedded (FFPE) tissue sections using the mouse monoclonal anti-BRAFV600E antibody, clone VE1 (dilution 1:800, catalogue number E19294, Spring Bioscience, Pleasanton, CA, USA) on a Bond III automated staining system (Leica Biosystems, Buffalo Grove, IL, USA) according to the manufacturer’s instructions. Heat-induced antigen retrieval was performed using epitope retrieval solution 2 (ER2, pH 8.0) (Leica Biosystems) for 40 minutes. Primary antibody was incubated for 30 minutes followed by the 3,3′-diaminobenzidine-based Bond Polymer Refine detection system (Leica Biosystems). Cytoplasmic expression of VE1 was scored based on the percentage of positive cells as follows: 0-10%, 25%, 50%, 75% and 90%. Tumours with ≥50% positive tumour cells were considered positive for VE1 expression, as previously described (20).
A subset of tumours was tested for BRAFV600E and KRAS mutations. FFPE tissue samples were macrodissected to enrich for tumour cellularity of at least 80%. DNA extraction was performed using the DNeasy tissue kit according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). BRAF and KRAS hotspot mutations were then detected using a custom iPLEX assay (Sequenom, Inc, San Diego, CA, USA). These variants were manually reviewed and in tumours with sufficient DNA and hotspot mutations mutation status, confirmed with an orthogonal method, as previously described (9).
Statistical analyses were performed using the software package SPSS 24.0. Associations between clinico-pathological covariates and gene mutation or VE1 protein expression status were assessed using contingency tables, and significance of associations was determined using Pearson’s χ2 test or Fisher’s exact test, as appropriate. A significance level of p<0.05 was used for all comparisons.
Results
The study cohort comprised 121 low-grade serous neoplasms, including 73 SBTs, 8 mpSBTs, and 40 LGSCs (22 primary, 18 metastatic). Immunohistochemical studies showed that VE1 was positive in 52% (38/73) of SBTs (Fig. 1A-D, 2A, 2C), 9% (2/22) of primary LGSCs (Fig. 2B, 2D), and in none of the mpSBTs and metastatic LGSCs (p<0.0001). The distribution of the percentage of positive cells in VE1-positive tumours was not significantly different amongst the groups (p>0.05).
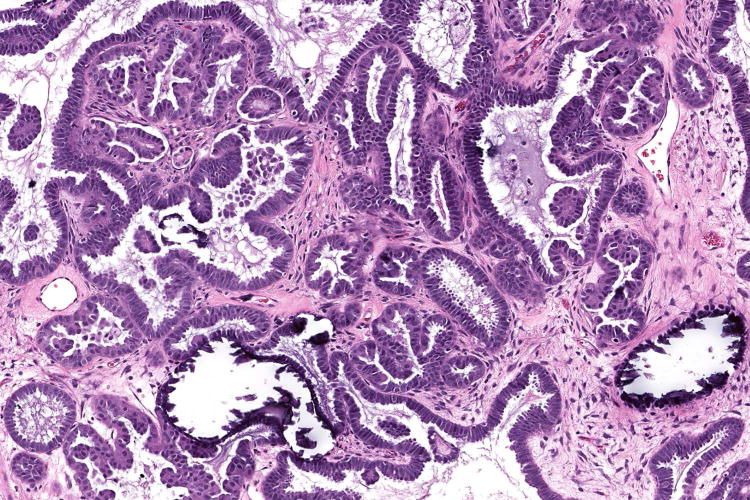
Figure 1.
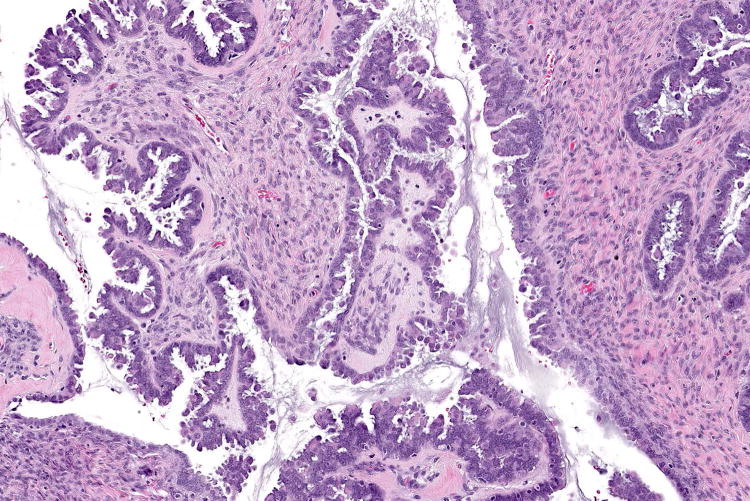
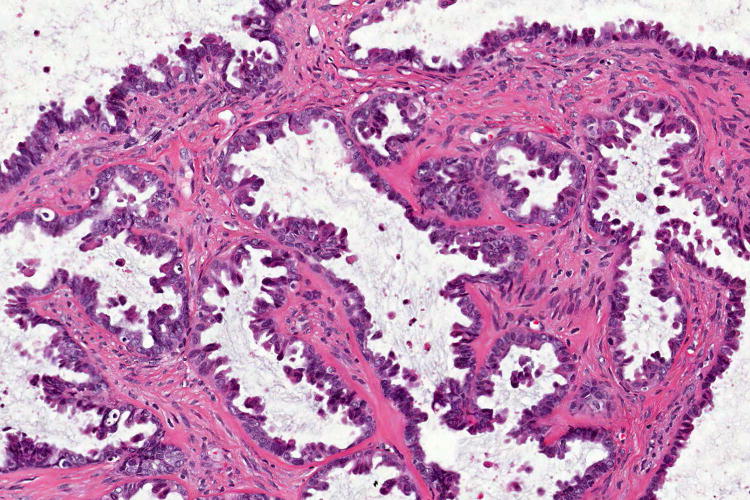
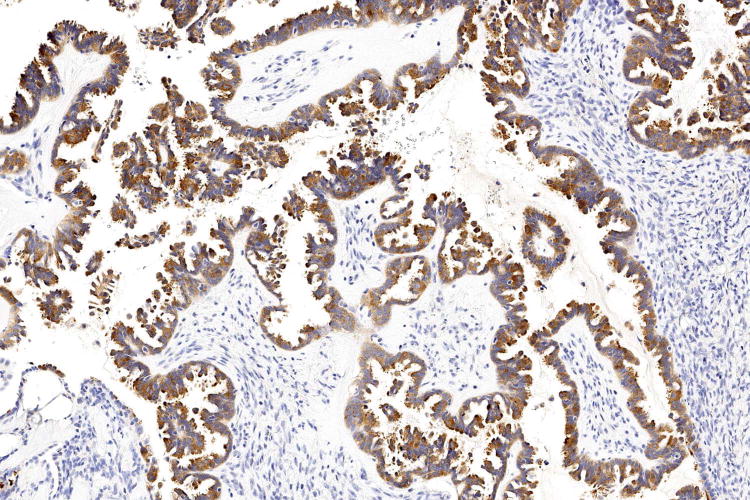
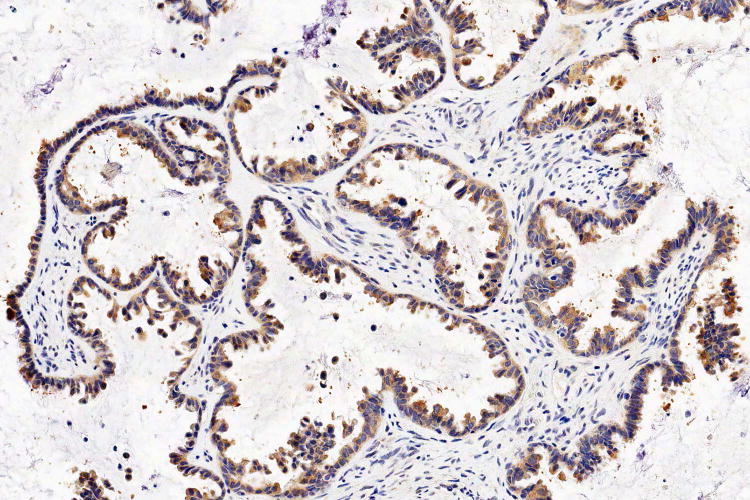
Examples of serous borderline tumour with eosinophilic cells and budding (A, B), with cytoplasmic staining for VE1 (C, D). Haematoxylin-eosin stain (A, B); VE1 immunohistochemical stain (C, D). Original magnification ×100 (A, C); ×200 (B, D).
Figure 2.
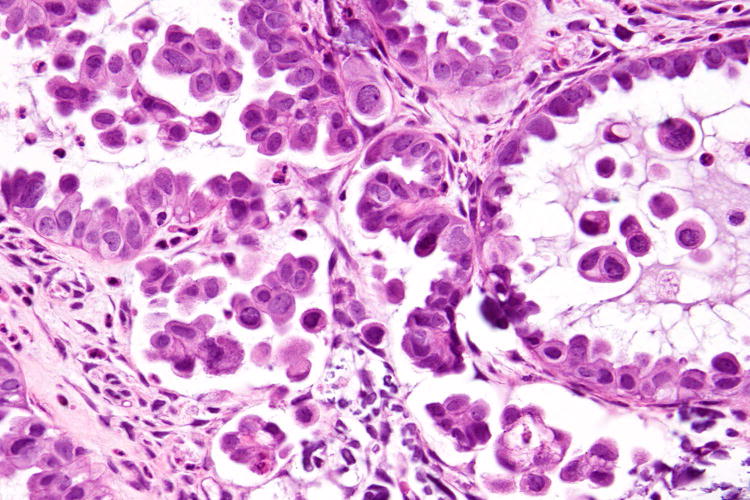
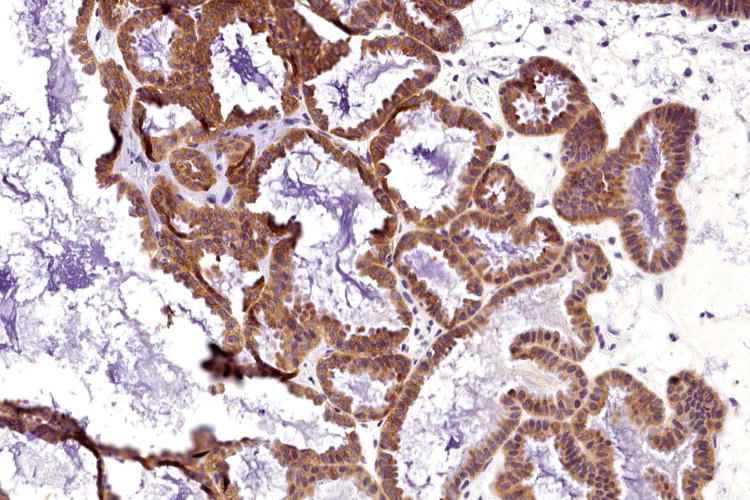
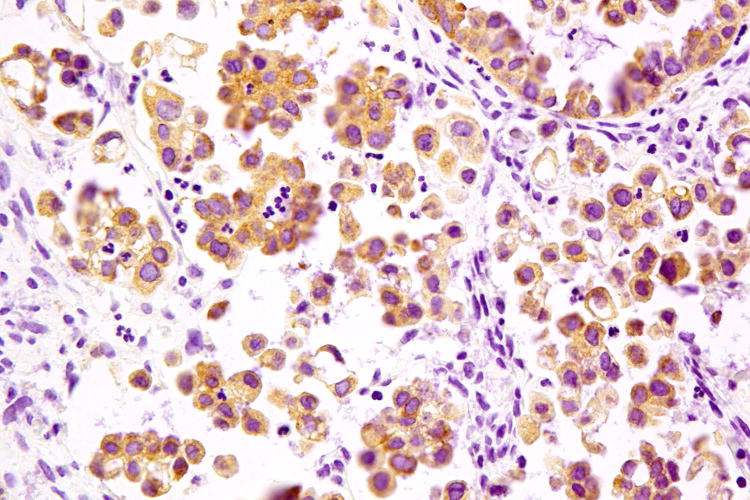
Serous borderline tumour (A) associated with low-grade serous carcinoma (B), both tumours exhibiting cytoplasmic expression of VE1 (C and D, respectively). Haematoxylin-eosin stain (A, B); VE1 immunohistochemical stain (C, D). Original magnification ×100 (A, C); ×400 (B, D).
Mutation status was available in 76 (63%) tumours, including 52 SBTs, 2 mpSBTs, and 22 LGSCs (12 primary, 10 metastatic). Of these 76 tumours, 42 (55%) harbored BRAFV600E or KRAS mutations. Mutations were more common in SBTs compared to other groups (p<0.0001): mutations were identified in 75% (39/52) of SBTs and 14% (3/22) of all LGSCs. Identified mutations were: BRAFV600E in 26 (34%) tumours; KRASG12D in 8 (11%) tumours; and KRASG12V in 8 (11%) tumours.
BRAFV600E mutations were present in 48% (25/52) of SBTs and 5% (1/22) of all LGSCs (p=0.006) (Table 1). Immunohistochemical expression of VE1 was identified in 96% (25/26) of tumours with BRAFV600E mutation, while only 2 of 27 (7%) VE1-positive tumours were negative for BRAFV600E mutation (p<0.0001). The sensitivity, specificity, positive predictive value and negative predictive value for VE1 immunohistochemistry were 96%, 96%, 93% and 98%, respectively.
Table 1.
BRAF mutation-associated histological features, VE1 immunohistochemistry and mutation frequencies in 76 tumours with known mutation status (all p<0.0001)
| N | SBT (n=52) | mpSBT (n=2) | LGSC (n=22) | |
|---|---|---|---|---|
| BRAF mutation-associated histological features | 39 | 38 (73%) | 1 (50%) | 0 |
| Positive VE1 immunohistochemistry | 27 | 26 (50%) | 0 | 1 (5%) |
| KRASG12D or KRASG12V mutation | 16 | 14 (27%) | 0 | 2 (9%) |
| BRAFV600E mutation | 26 | 25 (48%) | 0 | 1 (5%) |
| Overall mutation frequency | 42 | 39 (75%) | 0 | 3 (14%) |
Of the 52 SBTs with known mutation status, BRAF mutation-associated histological features were identified in 38 (73%) tumours. VE1 was positive in 26 (50%) tumours, and 25 (48%) harbored a BRAFV600E mutation (p<0.0001). All of the 25 BRAFV600E-mutated tumours were positive for VE1 by immunohistochemistry. In addition, one of two mpSBTs showed BRAF mutation-associated histological features. However, none of the mpSBTs was positive for VE1 by immunohistochemistry or BRAFV600E mutation. The sensitivity, specificity, positive predictive value and negative predictive value for BRAF mutation-associated histological features were 92%, 70%, 62% and 95%, respectively.
Discussion
SBTs and LGSCs are low-grade serous neoplasms of the ovary that co-exist in approximately 75% of cases. These tumours are typically diagnosed in younger women with a mean age of 42 years for SBTs and 56 years for LGSCs. Although most patients with SBTs have a benign clinical course, recurrences and progression to LGSC have been described in approximately 15% and 5% of patients, respectively (21, 22). Transformation to high-grade serous carcinoma is extremely rare. Predictors for progression include advanced stage, type of extraovarian disease, micropapillary architecture and the presence of microinvasive carcinoma (22). Primary treatment of LGSC includes surgery combined with chemotherapy or hormonal therapy, while patients with recurrent disease generally receive multiple lines of systemic therapy and ultimately die from their disease (23). Median overall survival rates for LGSC range from 82 to 126 months (24, 25). Secondary cytoreduction without gross residual disease has been shown to improve overall survival to 167.5 months versus 88.9 months in patients with gross residual disease (26). Furthermore, patients with microscopic residual disease had a longer median progression-free (33.2 months versus ≤14.7 months) and overall survival (96.9 months versus ≤44.5 months) compared with those with >0.1 cm residual disease (p<0.001). Patients with measurable residual LGSC had a similar adjusted hazard ratio for death (2.12 versus 2.31; p=0.002 and p<0.001, respectively) as patients with high-grade serous carcinoma (27). This is most likely due to the relative resistance of LGSCs to cytotoxic chemotherapy (27, 28).
In genomic profiling studies, LGSCs cluster with SBTs but differ from high-grade serous carcinomas in having higher expression of hormone receptors, frequent KRAS and BRAF mutations, lower rates of TP53 mutations and activation of the MAPK pathway (23, 29). Recurrent disease is generally treated with salvage chemotherapy, hormonal therapy or targeted therapies, such as inhibitors of vascular endothelial growth factor A (e.g. bevacizumab); inhibitors of MAPK enzymes MEK1/2 (e.g. selumetinib, binimetinib, trametinib) and BRAF inhibitors (e.g. vemurafenib), have been explored in clinical trials (23, 29–34).
In our study, V600E BRAFV600E mutations were present in 48% (25/52) of SBTs and 5% (1/22) of all LGSCs. BRAF mutations have been described in up to 48% of SBTs (6–11). Some studies have reported BRAF mutations in 19-33% of LGSCs (6, 35). However, lower mutation frequencies (0-6%) demonstrated by later studies, especially in advanced stage LGSCs (7, 9, 33, 36), may suggest that biologically aggressive LGSCs are more likely to arise from SBTs lacking BRAF mutations (7, 9). KRAS and BRAF mutation status has been shown to be a prognostic factor in LGSC. Patients with KRAS or BRAF mutations show significantly better overall survival than those lacking mutations (21 patients versus 58 patients, 106.7 months (95% CI, 50.6-162.9) versus 66.8 months (95% CI, 43.6.0-90.0)), respectively (p=0.018) (36). Patients with LGSC harboring BRAFV600E mutation also have a better prognosis compared with those without mutation (9). Patients with BRAF-mutated SBTs have been shown to present at earlier stages (37). However, no significant differences were found in overall survival or disease-free survival among BRAF-mutated and non-mutated SBTs or LGSCs in some studies (11, 37).
Several small studies have reported sensitivity and specificity of VE1 immunohistochemistry. One study described VE1 positivity in 13% (4/31) of SBTs and 5% (3/62) of LGSCs, with 1 of 6 SBTs being falsely positive (15). Another study reported positive VE1 in 27% (3/11) of SBTs, with 100% concordance with competitive allele-specific hydrolysis probe polymerase chain reaction (CAST-PCR) and 68% concordance with Sanger sequencing (20). The largest study of VE1 immunohistochemistry reported positive results in 71% (22/31) of SBTs and 14% (1/7) of LGSCs, with 100% concordance with PCR (16). The latest report found VE1 expression in 14% (1/7) of SBTs and 0% (0/35) of LGSCs. In addition, one benign lesion described as “serous superficial papilloma” and one serous cystadenoma with focal proliferation were positive. All three VE1-positive lesions showed BRAFV600E mutation by PCR (38). Our study includes the largest number of SBTs reported to date. In our study, VE1 was positive in 52% (38/73) of all cases of SBTs and 9% (2/22) of primary LGSCs, and in none of the mpSBTs or metastatic LGSCs. Of the 76 tumours with known mutation status, VE1 was positive in 50% (26/52) of SBTs compared with only 5% (1/22) of all LGSCs, and all 25 SBTs with BRAFV600E mutation were positive for VE1.
BRAF-mutated SBTs have been reported to show specific morphological features, including abundant eosinophilic cytoplasm, well-defined cell borders, bland nuclei and cell budding (18). At least two of these features were identified in all 25 SBTs with BRAFV600E mutation, in contrast to only 11% (5/47) of SBTs lacking BRAFV600E mutation. The eosinophilic cells have been reported to show immunohistochemical expression of p16, a senescence-associated marker, associated with a lower Ki-67 proliferation index compared to adjacent non-eosinophilic cells (18). In our study, the presence of BRAFV600E mutation-associated histological features was significantly associated with positive VE1 immunohistochemistry (p<0.0001), and most tumours with BRAFV600E mutation (96%; 25/26) were VE1-positive (p<0.0001). Of note, BRAFV600E mutation associated SBTs can form micropapillae that differ from those of mpSBT but may lead to diagnostic confusion. Given the association of mpSBTs with invasive implants (LGSC) (17), it is very important to distinguish the two entities. Identification of at least two specific histological features and/or VE1 immunohistochemistry can be discriminatory in diagnostically challenging cases.BRAF mutations are thought to mediate progression of serous cystadenomas to SBTs (39). In contrast, in vitro experiments have demonstrated that induction of BRAFV600E expression leads to the development of the aforementioned BRAF mutation-associated histological features in cultured epithelial cells (18). The association of BRAF mutations with this cellular senescence phenotype and upregulation of tumour suppressor genes in SBTs suggest that BRAF mutations may have a protective role against the progression of SBT to LGSC (18, 39). BRAF mutation analysis of SBTs also suggests that peritoneal implants are derived from the primary ovarian tumour with overall concordance of BRAF mutations between ovarian SBTs and peritoneal implants reported to be 95% (59/62) (p<0.00001) (40).
In conclusion, BRAFV600E mutations which are significantly more common in SBTs than in LGSCs. Immunohistochemical expression of VE1 protein is strongly associated with BRAFV600E mutation status and specific histological features. VE1 immunohistochemistry is a reliable method for the detection of BRAFV600E mutations and is faster, less expensive and readily accessible to more laboratories than mutation testing.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
DR KAY PARK (Orcid ID : 0000-0001-8989-2938)
DR RAJMOHAN MURALI (Orcid ID : 0000-0001-6988-4295)
Author contributions: Compilation of Cases and Material for Study Cohort (SC, DFD, KJP, RAS, RM); Data collection (GT, RM, RNG); Pathological Review and Data Analysis (GT, RM); Writing of Manuscript (All Authors)
References
- 1.Vang R, Shih Ie M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16:267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam P. Morphologic, Immunophenotypic, and Molecular Features of Epithelial Ovarian Cancer. Oncology (Williston Park) 2016;30:166–176. [PubMed] [Google Scholar]
- 4.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer G, Oldt R, 3rd, Cohen Y, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 7.Wong KK, Tsang YT, Deavers MT, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vereczkey I, Serester O, Dobos J, et al. Molecular characterization of 103 ovarian serous and mucinous tumors. Pathol Oncol Res. 2011;17:551–559. doi: 10.1007/s12253-010-9345-8. [DOI] [PubMed] [Google Scholar]
- 9.Grisham RN, Iyer G, Garg K, et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119:548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayr D, Hirschmann A, Lohrs U, et al. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–887. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Bi R, Xiao Y, et al. Low frequency of BRAF and KRAS mutations in Chinese patients with low-grade serous carcinoma of the ovary. Diagn Pathol. 2017;12:87. doi: 10.1186/s13000-017-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wooster R, Futreal AP, Stratton MR. Sequencing analysis of BRAF mutations in human cancers. Methods Enzymol. 2006;407:218–224. doi: 10.1016/S0076-6879(05)07018-7. [DOI] [PubMed] [Google Scholar]
- 13.El-Osta H, Falchook G, Tsimberidou A, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS One. 2011;6:e25806. doi: 10.1371/journal.pone.0025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yemelyanova A, Mao TL, Nakayama N, et al. Low-grade serous carcinoma of the ovary displaying a macropapillary pattern of invasion. Am J Surg Pathol. 2008;32:1800–1806. doi: 10.1097/PAS.0b013e318181a7ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preusser M, Capper D, Berghoff AS, et al. Expression of BRAF V600E mutant protein in epithelial ovarian tumors. Appl Immunohistochem Mol Morphol. 2013;21:159–164. doi: 10.1097/PAI.0b013e31825d7402. [DOI] [PubMed] [Google Scholar]
- 16.Bosmuller H, Fischer A, Pham DL, et al. Detection of the BRAF V600E mutation in serous ovarian tumors: a comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum Pathol. 2013;44:329–335. doi: 10.1016/j.humpath.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Prat J, De Nictolis M. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002;26:1111–1128. doi: 10.1097/00000478-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Zeppernick F, Ardighieri L, Hannibal CG, et al. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am J Surg Pathol. 2014;38:1603–1611. doi: 10.1097/PAS.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniar KP, Wang Y, Visvanathan K, et al. Evaluation of microinvasion and lymph node involvement in ovarian serous borderline/atypical proliferative serous tumors: a morphologic and immunohistochemical analysis of 37 cases. Am J Surg Pathol. 2014;38:743–755. doi: 10.1097/PAS.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi Y, Sasaki H, Takeshita S, et al. Usefulness of immunohistochemistry for the detection of the BRAF V600E mutation in ovarian serous borderline tumors. Oncol Rep. 2014;32:1815–1819. doi: 10.3892/or.2014.3442. [DOI] [PubMed] [Google Scholar]
- 21.Vang R, Hannibal CG, Junge J, et al. Long-term Behavior of Serous Borderline Tumors Subdivided Into Atypical Proliferative Tumors and Noninvasive Low-grade Carcinomas: A Population-based Clinicopathologic Study of 942 Cases. Am J Surg Pathol. 2017;41:725–737. doi: 10.1097/PAS.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malpica A, Longacre TA. Prognostic indicators in ovarian serous borderline tumours. Pathology. 2017 doi: 10.1016/j.pathol.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Gershenson DM. The life and times of low-grade serous carcinoma of the ovary. Am Soc Clin Oncol Educ Book. 2013 doi: 10.14694/EdBook_AM.2013.33.e195. [DOI] [PubMed] [Google Scholar]
- 24.Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–368. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 25.Bodurka DC, Deavers MT, Tian C, et al. Reclassification of serous ovarian carcinoma by a 2-tier system: a Gynecologic Oncology Group Study. Cancer. 2012;118:3087–3094. doi: 10.1002/cncr.26618. [DOI] [PubMed] [Google Scholar]
- 26.Crane EK, Sun CC, Ramirez PT, et al. The role of secondary cytoreduction in low-grade serous ovarian cancer or peritoneal cancer. Gynecol Oncol. 2015;136:25–29. doi: 10.1016/j.ygyno.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fader AN, Java J, Ueda S, et al. Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol. 2013;122:225–232. doi: 10.1097/AOG.0b013e31829ce7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114:48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Gershenson DM. Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol. 2016;27(Suppl 1):i45–i49. doi: 10.1093/annonc/mdw085. [DOI] [PubMed] [Google Scholar]
- 30.McLachlan J, Gore M, Banerjee S. Targeting the mitogen-activated protein kinase pathway in low-grade serous carcinoma of the ovary. Pharmacogenomics. 2016;17:1353–1363. doi: 10.2217/pgs.16.24. [DOI] [PubMed] [Google Scholar]
- 31.Grisham RN. Low-Grade Serous Carcinoma of the Ovary. Oncology (Williston Park) 2016;30:650–652. [PubMed] [Google Scholar]
- 32.Grisham RN, Iyer G, Sala E, et al. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer. 2014;24:1010–1014. doi: 10.1097/IGC.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones S, Wang TL, Kurman RJ, et al. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226:413–420. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gershenson DM, Sun CC, Wong KK. Impact of mutational status on survival in low-grade serous carcinoma of the ovary or peritoneum. Br J Cancer. 2015;113:1254–1258. doi: 10.1038/bjc.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbruggen MB, Sieben NL, Roemen GM, et al. v-Raf murine sarcoma viral oncogene mutation status in serous borderline ovarian tumors and the effect on clinical behavior. Int J Gynecol Cancer. 2009;19:1560–1563. doi: 10.1111/IGC.0b013e3181a83119. [DOI] [PubMed] [Google Scholar]
- 38.Sadlecki P, Walentowicz P, Bodnar M, et al. Determination of BRAF V600E (VE1) protein expression and BRAF gene mutation status in codon 600 in borderline and low-grade ovarian cancers. Tumour Biol. 2017;39 doi: 10.1177/1010428317706230. 1010428317706230. [DOI] [PubMed] [Google Scholar]
- 39.Malpica A, Wong KK. The molecular pathology of ovarian serous borderline tumors. Ann Oncol. 2016;27(Suppl 1):i16–i19. doi: 10.1093/annonc/mdw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ardighieri L, Zeppernick F, Hannibal CG, et al. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol. 2014;232:16–22. doi: 10.1002/path.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]


