Abstract
Transient receptor potential mucolipin (TRPML) channels are the most recently identified subfamily of TRP channels and have seen a surge of new reports revealing both structural and functional insight. In 2017, several groups published multiple conformations of TRPML channels using cryo-EM. Similar to other TRP channels, the ML subfamily consists of six transmembrane helices (S1–S6), and a pore region including S5, S6, and two pore helices (PH1 and PH2). However, these reports also reveal distinct structural characteristics of the ML subfamily. Asp residues within the luminal pore may function to control calcium/pH regulation. A synthetic agonist, ML-SA1, can bind to the pore region of TRPMLs to force a direct dilation of the lower gate. Finally, biophysical and electrophysiological characterizations reveal another natural agonist binding site in the unique domain of TRPMLs, presumably regulating the conformation of the S4–S5 linker to open the channel. This work elucidates the molecular architecture and provides insights into how multiple ligands regulate TRPMLs.
Keywords: cryo-EM, ML-SA1, pH regulation, PIP2, TRPML
Introduction
The Transient Receptor Potential (TRP) superfamily is a diverse group of ion channels, responsive to a wide range of stimuli [1]. This ability to respond to a variety of environmental stimuli contributes to the central role of TRP channels in sensory physiology including hearing, vision, olfaction, thermo- and mechano-sensation, and nociception. Based on sequence and topology, the approximately 28 cation channel genes within this superfamily are divided into seven subfamilies within two main groups [2]. Group 1 TRPs, which include the TRPAs, TRPCs, TRPMs, TRPNs, and TRPVs, are related to the classical TRPC channels, first isolated by Cosens and Manning in 1969 from a spontaneous mutation in the trp gene leading to abnormal vision in Drosophila [3]. Group 2 TRPs include the TRPPs and TRPMLs, both of which share transmembrane sequence homology and a large luminal loop separating S1 and S2, which was determined in the beginning of 2017 [4]. The founding members of the group 2 TRPPs and TRPMLs were discovered as the mutated gene products (PKD2, MCOLN) in autosomal dominant polycystic kidney disease (ADPKD) and mucolipidosis type IV (MLIV), respectively. In mammals, the TRPML, or mucolipin subfamily, consists of three channels: TRPML1, TRPML2, and TRPML3.
With advances in cryo-electron microscopy and novel electrophysiological approaches, much of what we have learned about group 2, and in particular the mucolipin subfamily, has only recently been published. In the beginning of 2017, the crystal structure of the luminal domain between S1 and S2 was obtained confirming the physiologically significant Ca2+ and H+ interactions for TRPML1 [4]. These interactions were initially described in 2006, where the Muallem group first proposed that TRPML1 serves as a lysosomal proton leak channel helping to maintain the low pH necessary for lysosomal function [5]. However, in 2007, Xu et al. reported no detectible proton permeation for TRPML1. Instead, they claimed that TRPML1 behaves more like the classical TRP inward-rectifying cation and calcium channels [6]. Subsequent work further detailed regulation and permeation by Ca2+ and the mobilization of Fe2+, Zn2+, and Mn2+ within the lysosomes [2,7–9] TRPML1 is also involved in many endolysosome-dependent cellular processes, such as cholesterol accumulation, lipid trafficking, exocytosis, and autophagy [10,11]. Mutations on TRPML1 can cause a lysosomal storage disease named mucolipidosis type IV (MLIV) that leads to retinal degeneration and corneal opacity [12]. Compared with TRPML1, there is a profound knowledge gap as to the physiological functions of TRPML2 and TRPML3, although these three proteins have high sequence homology. TRPML2 has been proposed to play an important role in immune cells [13], whereas TRPML3 is critical in the endocytic pathway. Dysfunction of TRPML3 is associated with early deafness, pigmentation abnormalities, and perinatal lethality in mice [6,14]. TRPML channels are activated by various small-molecule synthetic compounds [15,16] (e.g., ML-SA1 and MK6-83) as well as endogenously by PI(3,5) P2 [17,18] and are repressed by sphingomyelins and PI (4,5)P2 [11].
Cryo-EM Structures of TRPMLs
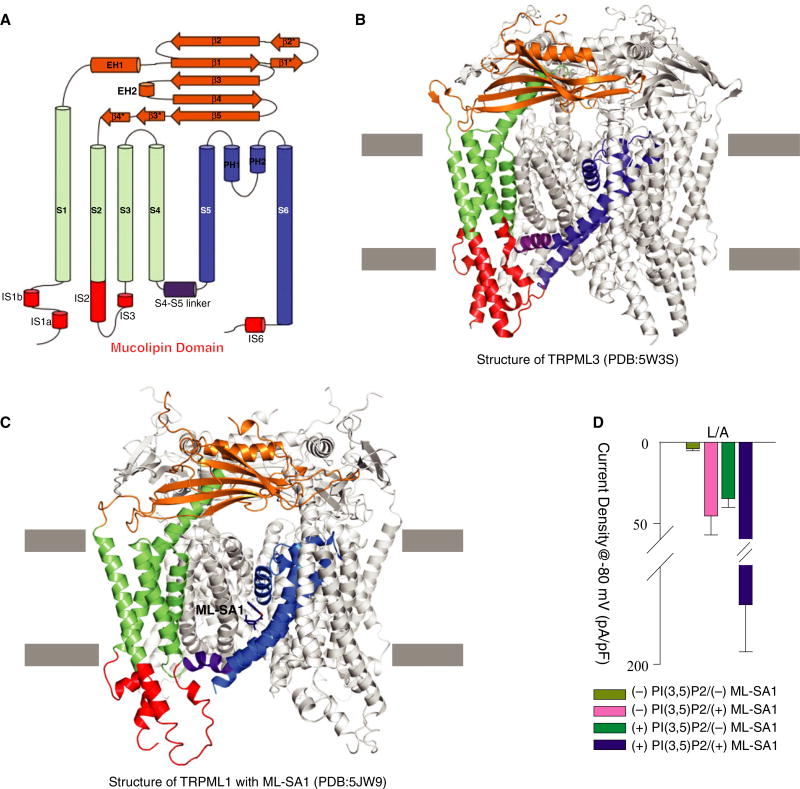
Recently, five groups reported the cryo-EM structures of TRPMLs [19–23], which substantially enhance our knowledge of TRPMLs and the TRP family. Like other TRP channels, TRPMLs have six transmembrane helices (S1–S6), and a pore region consisting of S5, S6, and two pore helices (PH1 and PH2) (Fig. 1A). Structural comparison of the group 2 TRP channels shows that TRPMLs have a TRPP1/PKD2-like topology [24] in their transmembrane and extracellular domains. However, TRPMLs contain three unique features (collectively called the mucolipin domain in this review): (a) the S2 and S3 helices are longer than those in PKD2 and protrude from the membrane bilayer into the cytosol, (b) the N-terminal S1 helix has an extension (IS1a and IS1b) enriched in many basic amino acids that presumably bind to lipids in the membrane bilayer, and (c) a small helix (IS6) extends from the C-terminus of S6 into the cytosol (only observed in TRPML3) (Fig. 1B,C).
Fig. 1.
(A) The secondary structure of TRPMLs. (B) The structure of C. jacchus TRPML3 (PDB code: 5W3S). (C) The structure of human TRPML1 with its agonist ML-SA1 (PDB code: 5JW9). (D) Whole-cell current density at −80 mV recorded at pH 4.6 for cells transfected with pEGFP-C1 vector-coding human TRPML1 L/A. Values are mean ± SEM, this panel reproduced from [18].
TRPML1 is ubiquitously expressed with elevated levels detected in brain, kidney, and liver [25]. However, the strict cellular localization to the late endosome and lysosome (LEL) for TRPML1 channels makes standard whole-cell patch-clamp recordings more complicated than for surface expressing voltage-sensitive TRP channels like TRPV1. By pretreating cells with vacuolin-1, Dong et al. were able to isolate enlarged lysosomes for characterization of TRPML1 ion permeation [8]. Using this technique, they established that TRPML1 might play a role in iron homeostasis in the mature LEL. An alternative technique involved mutating the lysosomal targeting motifs at the N- and C-termini of TRPML1. The two di-leucine motifs facilitate trafficking to the lysosome and regulation of endocytic efficiency, respectively [26]. When all four Leu residues are mutated to Ala (L/A), TRPML1 localizes to the cell surface allowing for standard whole-cell patch-clamp and electrophysiological characterization. In this configuration, the lysosomal luminal side of TRPML1 is exposed to the extracellular environment providing a rapid means to test different lysosomal conditions on TRPML1 channel activity.
Similar to results from isolated lysosomes, Figure 1D shows how TRPML1 is activated by the anionic lipid PI(3,5)P2 and the synthetic agonist ML-SA1. While the activation of TRPML1 occurs separately with each agonist, the presence of both PI(3,5)P2 and ML-SA1 stimulated TRPML1 considerably more than either ligand alone, as previously reported (Fig. 1D). Interestingly, other inositides and even sphingomyelin may function to inhibit this channel [15]. PI(4,5)P2 was shown to block PI(3,5)P2-activated channels as well as inhibit channels activated by application of another synthetic agonist, SF-51, even in the absence of PI(3,5) P2 [17]. While it is likely that lipid ligands and synthetic agonists are binding to distinct sites, these reports indicate the possibility of allosteric interactions and a structurally important bridge between the two sites warranting further investigation. The distinctly different responses that arise from application of PI (3,5)P2 and PI(4,5)P2 likely reveal a form of lipid based channel regulation as proposed by Hilgemann et al. [27]. In this situation, TRPML1 channels are held inactive within maturing endosomes that are more enriched in PI(4,5)P2. As endosomes mature into LELs, PI(4,5)P2 is displaced with increasing levels of PI(3,5)P2, enhancing activation of TRPML1 only within the fully matured target organelle.
pH regulatory mechanism of TRPML1
Another interesting aspect of TRPML1 physiology is how pH can control channel activity. Due to the proton-pumping V-type ATPase, the lumen of the LEL is highly acidic (~ 4.5). Previously proposed as a proton leak pathway helping to stabilize this pH in coordination with the proton pumps, TRPML1 has recently been implicated as a key component of calcium and heavy metal mobility out of the lysosome where these ions accumulate. Regardless, electrophysiological data have revealed a clear increase in channel activity when either PI(3,5)P2 or ML-SA1 is applied at lower pH. After resolving the 213-residue luminal domain of human TRPML1, Li, M et al. proposed that a series of Asp residues within the luminal pore controls calcium/pH regulation [4]. Furthermore, Chen et al. showed that mutation of Asp472 (the Ca2+ blockage site) to Asn causes a diminished luminal Ca2+ block [21]. At neutral pH, the Asp residues are negatively charged, increasing their affinity to calcium, and facilitating a calcium pore blockade of the channel. In the acidic pH environment typically seen in LELs, the Asp residues are uncharged allowing calcium access through the pore and therefore conductance across the membrane. In this manner, pH regulation of ion transport through TRPML1 is not mediated by significant changes in structure. Indeed, electrophysiological evidence for this theory exists from current recordings under divalent-free conditions.
Like many TRP channels, TRPML1 will produce a large monovalent conductance in the absence of divalent ions like calcium. In this condition, reports of potentiation in divalent-free conditions are relatively independent of pH. We have routinely observed that ML-SA1-and PI(3,5)P2-stimulated channel activity is potentiated by nominally divalent-free conditions at pH 7.4. In the absence of calcium, the charged Asp residues have no significant impact on the conductance of monovalent cations. However, this theory remains speculative, as there is a lack of structural data showing an effect of pH and this mode of transport could represent a different conformation from more physiological calcium-containing states. Adding to the complexity of TRPML regulation, TRPML3 currents display a unique inhibition by H+ and Na+ [22]. Interestingly, pH regulation may represent an additional layer of organelle regulation similar to how different inositides control localized activation of the various TRPML channels. As the lysosomal concentration of both H+ and Na+ is significantly higher than the surrounding cytoplasmic concentration, TRPML3 would be inhibited and function primarily at the cell surface, while TRPML1 activity is predominantly lysosomal [22].
Molecular details of agonist-mediated TRPMLs activation
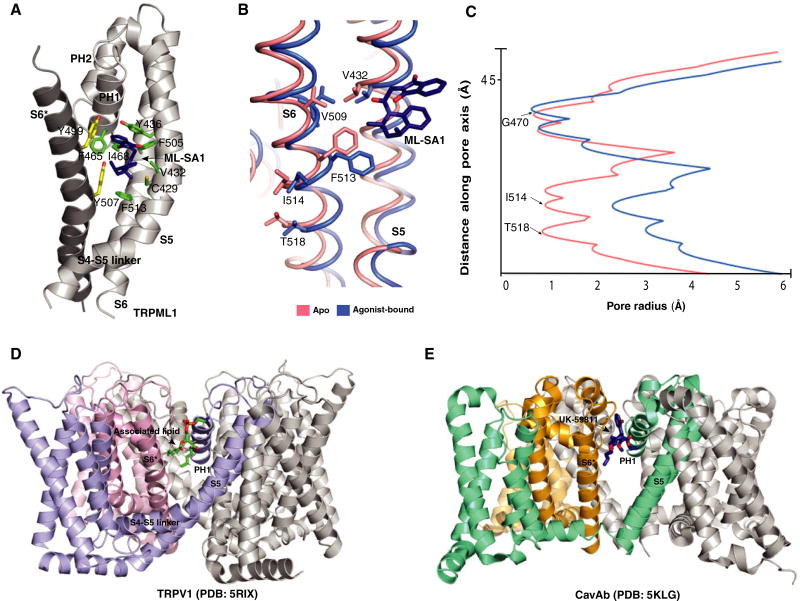
According to the structures of TRPML1–ML-SA1 and TRPML3–ML-SA1, the ML-SA1 binds to a hydrophobic cavity created by the aromatic and hydrophobic residues of pore helix 1 (PH1), S5, S6, and S6 in the neighboring subunit (Fig. 2A). Intriguingly, some of these residues (Y436, F465, and Y499 in TRPML1), when mutated, lead to a dead channel and cause mucolipidosis type IV. Unlike TRPV1, the dilation of the selectivity filter of TRPML1 does not substantially increase after binding ML-SA1 due to the tight packing of PH1 and PH2. However, the lower gate undergoes a significant dilation with ML-SA1 compared to the closed apo-form (Fig. 2B,C). This dilation is consistent with the lower gate opening in agonist-bound TRPV1. The comparable ML-SA1–binding site in TRPV1 [28] and CaVAb [29] could accommodate other ligands such as the CaVAb antagonist UK-59811 or endogenous lipids suggesting this site could allosterically regulate numerous ion channels and open the possibility of novel targets for ligand-mediated channel regulation. (Fig. 2D,E).
Fig. 2.
(A) detail of the interaction of TRPML1 with ML-SA1. Residues are presented as sticks in green (from the same subunit) and yellow (from the neighboring subunit). (B) Molecular mechanism of V432-mediated TRPML1 regulation. (C) Comparison of pore radius for ML-SA1-bound (blue) and apo TRPML1 structures (red). (D) Structure of resiniferatoxin-bound TRPV1 (PDB code: 5IRX). (E) Structure of UK-59811-bound CaVAb (PDB code: 5KLG). This figure reproduced from [18].
Structural comparison of apo and ML-SA1 bound TRPMLs suggests a molecular mechanism for the proline substitution at V432 (TRPML1Va), which causes a gain-of-function phenotype that activates TRPML1 [14,30] (Fig. 2B). In the apo state, the Cα distances between V432-V509 and V432-F513 are 3.9 Å and 4.7 Å, respectively, maintaining strong van der Waals force and keeping the position of S6 in the closed state. The V432P mutation disrupts these hydrophobic interactions, unleashing the S6 from S5; this unleashing may allow S6 to swing open akin to the action of ML-SL1 triggering the lower gate to open.
Molecular insights into PI(3,5)P2-mediated TRPMLs activation
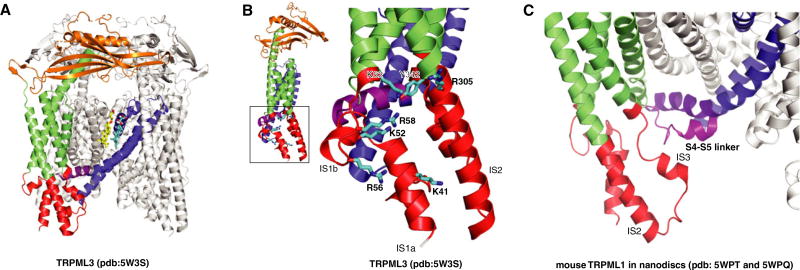
Beyond the insight gained from the structures of human TRPML1, work on other orthologs and TRPML3 has also provided substantial advances in our understanding of the mucolipin subfamily. Recently, the structure of the marmoset TRPML3 determined at 2.9 Å resolution reveals several lipidbinding sites in the transmembrane domain [20]. One of these sites is consistent with the ML-SA1–binding area and one of the associated lipid-binding sites in TRPV1 (Fig. 3A). The electrophysiological and biophysical characterizations of TRPML3 suggest that the mucolipin domain, including several basic amino acids, might be responsible for PI(3,5)P2 binding and channel activation (Fig. 3B).
Fig. 3.
(A) Endogenous lipid-binding sites in TRPML3 structure (PDB code: 5W3S). (B) The putative PI(3,5)P2-binding site in TRPML3. (C) The structural comparison of the conformations of S4–S5 linker of mouse TRPML1 in the two closed states (5WPQ and 5WPT).
Notably, mutation of the putative PI(3,5)P2-binding site could decrease PI(3,5)P2 binding in TRPML3 and almost abolish the PI(3,5)P2 stimulation in TRPML1. However, when A419P (that is V432P in TRPML1) was introduced to the TRPML3 R305A mutant, the channel did not respond to PI(3,5)P2 but was in an open state suggesting that the activation mechanism of A419P mutation is distinct from PI (3,5)P2-mediated activation. Hirschi et al. proposed a gating pulley model where the signals from PI(3,5)P2 binding to the mucolipin domain are transmitted to the S6 gate, opening the channel pore [20].
Deleting the positive charge-rich N-terminal region of the mouse TRPML1 mucolipin domain abolished PI(3,5)P2 binding and regulation[21]. The structures of mouse TRPML1 in nanodiscs reveal two distinct conformations of the S4–S5 linker close to the mucolipin domain in the closed form, implying a gating mechanism of PI(3,5)P2 by the S4–S5 linker (Fig. 3C).
Perspective
The development of synthetic compounds similar to ML-SA1 and its analogs may be used for the treatment of related lysosomal storage diseases as they can significantly stimulate the activity of TRPMLs. The structure of TRPML1 with ML-SA1 could provide atomic details between these two molecules, enabling the design of more potent small compounds for related diseases. Further TRPML1 structural studies may also lead to a new class of compounds that utilize the S4–S5 linker for pore regulation, similar to PI(3,5)P2. This linker has been widely studied as the mechanism for capsaicin activation in TRPV1 and may play an important role in many TRP channels [31]. However, as with many genetic diseases, it is likely that some MLIV-causing mutations in TRPML1 will lead to a dead channel, which cannot be activated by either ML-SA1 or PI(3,5)P2 [19]; therefore, other approaches must be used to treat those mutations.
Although these studies present atomic models of TRPML channels and suggest the mechanism of synthetic agonist binding, the molecular detail of lipid-mediated activation or inhibition remains poorly understood. In particular, the fact that both PI(3,5)P2 and ML-SA1 together can stimulate TRPML1 more considerably than either ligand alone presents an interesting avenue of further research. The knowledge of the possible cooperation of these two ligands in TRPML1 might present a novel active conformation among the TRP channels. Future studies on structural investigations and electrophysiological characterizations will help facilitate the understanding of TRPML1 regulation and other TRP channel family members.
Acknowledgments
We thank Elias Coutavas for help in manuscript preparation. This work was supported by the Endowed Scholars Program in Medical Science of UT Southwestern Medical Center and O’Donnell Junior Faculty Funds (to XL); XL is Rita C. and William P. Clements, Jr. Scholar in Biomedical Research of UT Southwestern.
Abbreviations
- ADPKD
autosomal dominant polycystic kidney disease
- Cryo-EM
cryo-electron microscopy
- LEL
late endosome and lysosome
- MLIV
mucolipidosis type IV
- ML-SA1
Mucolipin Synthetic Agonist 1
- PH
pore helix
- PI(3,5)P2
phosphatidylinositol 3,5-bisphosphate
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- TRPML
transient receptor potential mucolipin
Footnotes
Authors contributions
XL supervised the writing process; all the authors analyzed the data and wrote the paper.
References
- 1.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 2.Zeevi DA, Frumkin A, Bach G. TRPML and lysosomal function. Biochem Biophys Acta. 2007;1772:851–858. doi: 10.1016/j.bbadis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Zhang WK, Benvin NM, Zhou X, Su D, Li H, Wang S, Michailidis IE, Tong L, Li X, et al. Structural basis of dual Ca(2 +)/pH regulation of the endolysosomal TRPML1 channel. Nat Struct Mol Biol. 2017;24:205–213. doi: 10.1038/nsmb.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Delling M, Li L, Dong X, Clapham DE. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci USA. 2007;104:18321–18326. doi: 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichelsdoerfer JL, Evans JA, Slaugenhaupt SA, Cuajungco MP. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J Biol Chem. 2010;285:34304–34308. doi: 10.1074/jbc.C110.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatachalam K, Wong CO, Zhu MX. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium. 2015;58:48–56. doi: 10.1016/j.ceca.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Zhang X, Gao Q, Xu H. TRPML1: an ion channel in the lysosome. Handb Exp Pharmacol. 2014;222:631–645. doi: 10.1007/978-3-642-54215-2_24. [DOI] [PubMed] [Google Scholar]
- 12.Altarescu G, Sun M, Moore DF, Smith JA, Wiggs EA, Solomon BI, Patronas NJ, Frei KP, Gupta S, Kaneski CR, et al. The neurogenetics of mucolipidosis type IV. Neurology. 2002;59:306–313. doi: 10.1212/wnl.59.3.306. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Anoveros J, Wiwatpanit T. TRPML2 and mucolipin evolution. Handb Exp Pharmacol. 2014;222:647–658. doi: 10.1007/978-3-642-54215-2_25. [DOI] [PubMed] [Google Scholar]
- 14.Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci USA. 2002;99:14994–14999. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CC, Keller M, Hess M, Schiffmann R, Urban N, Wolfgardt A, Schaefer M, Bracher F, Biel M, Wahl-Schott C, et al. A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat Commun. 2014;5:4681. doi: 10.1038/ncomms5681. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Li X, Xu H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci USA. 2012;109:11384–11389. doi: 10.1073/pnas.1202194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2 +) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmiege P, Fine M, Blobel G, Li X. Human TRPML1 channel structures in open and closed conformations. Nature. 2017;550:366–370. doi: 10.1038/nature24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschi M, Herzik MA, Jr, Wie J, Suo Y, Borschel WF, Ren D, Lander GC, Lee SY. Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature. 2017;550:411–414. doi: 10.1038/nature24055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, She J, Zeng W, Guo J, Xu H, Bai XC, Jiang Y. Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature. 2017;550:415–418. doi: 10.1038/nature24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Li M, Su D, Jia Q, Li H, Li X, Yang J. Cryo-EM structures of the human endolysosomal TRPML3 channel in three distinct states. Nat Struct Mol Biol. 2017;24:1146–1154. doi: 10.1038/nsmb.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Li N, Zeng W, Gao N, Yang M. Cryo-EM structures of the mammalian endo-lysosomal TRPML1 channel elucidate the combined regulation mechanism. Protein Cell. 2017;8:834–847. doi: 10.1007/s13238-017-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen PS, Yang X, DeCaen PG, Liu X, Bulkley D, Clapham DE, Cao E. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell. 2016;167:763–773. e11. doi: 10.1016/j.cell.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 26.Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534:347–351. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang L, Gamal El-Din TM, Swanson TM, Pryde DC, Scheuer T, Zheng N, Catterall WA. Structural basis for inhibition of a voltage-gated Ca2 + channel by Ca2 + antagonist drugs. Nature. 2016;537:117–121. doi: 10.1038/nature19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong XP, Wang X, Shen D, Chen S, Liu M, Wang Y, Mills E, Cheng X, Delling M, Xu H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J Biol Chem. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang F, Xiao X, Cheng W, Yang W, Yu P, Song Z, Yarov-Yarovoy V, Zheng J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat Chem Biol. 2015;11:518–524. doi: 10.1038/nchembio.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]