Abstract
The neural crest (NC) multipotent progenitor cells form at the neural plate border and migrate to diverse locations in the embryo to differentiate into many cell types. NC is specified by several embryonic pathways, however the role of noncanonical Wnt signaling in this process remains poorly defined. Daam1 is a formin family protein that is present in embryonic ectoderm at the time of NC formation and can mediate non-canonical Wnt signaling. Our interference experiments indicated that Daam1 is required for NC gene activation. To further study the function of Daam1 in NC development we used a transgenic reporter Xenopus line, in which GFP transcription is driven by sox10 upstream regulatory sequences. The activation of the sox10:GFP reporter in a subset of NC cells was suppressed after Daam1 depletion and in embryos expressing N-Daam1, a dominant interfering construct. Moreover, N-Daam1 blocked reporter activation in neutralized ectodermal explants in response to Wnt11, but not Wnt8 or Wnt3a, confirming that the downstream pathways are different. In complementary experiments, a constitutively active Daam1 fragment expanded the NC territory, but this gain-of-function activity was eliminated in a construct with a point mutation in the FH2 domain that is critical for actin polymerization. These observations suggest a new role of Daam1 and actin remodeling in NC specification.
Keywords: Noncanonical, Wnt, Daam1, Xenopus, neural, actin, Sox10, transgenic
Introduction
The neural crest (NC) cells are multipotent progenitors that originate at the neural plate border and give rise to diverse cell types, including facial bones and cartilage, melanocytes, and the peripheral nervous system (Sauka-Spengler & Bronner-Fraser, 2008). As the neural tube closes, NC (neural crest) cells undergo epithelial-mesenchymal transition (EMT) and migrate to different locations in the body. The NC gene regulatory network is established via a crosstalk of BMP, FGF, Wnt and Notch pathways (Milet & Monsoro-Burq, 2012; Sauka-Spengler & Bronner-Fraser, 2008). Among these, Wnt signaling plays a prominent role. Wnt pathways comprise the canonical, β-catenin-dependent branch (Macdonald, Semenov, & He, 2007; van Amerongen & Nusse, 2009) and the β-catenin-independent (or Wnt/PCP) branch, known for the regulation of cell polarity and movements (Angers & Moon, 2009; Komiya & Habas, 2008; Yang & Mlodzik, 2015). Both Wnt signaling branches have been shown to regulate NC specification (Garcia-Castro, Marcelle, & Bronner-Fraser, 2002; Ikeya, Lee, Johnson, McMahon, & Takada, 1997; Ossipova & Sokol, 2011; Saint-Jeannet, He, Varmus, & Dawid, 1997). Whereas the essential function of the Wnt/β-catenin pathway in NC gene activation has been well established, the role of Wnt/PCP signaling in this process remains poorly understood.
The noncanonical Wnt ligands can induce NC marker expression in neuralized ectodermal explants in the absence of β-catenin stabilization (Ossipova & Sokol, 2011). This pathway critically depends on Dishevelled (Dvl), since the mutated Dvl constructs that do not affect β-catenin are still able to modulate NC specification (Ossipova & Sokol, 2011). However, the downstream components of the pathway leading to NC gene activation still need to be identified. One candidate is Dvl-associated activator of morphogenesis 1 (Daam1), a formin protein that can mediate non-canonical Wnt signaling downstream of Dvl (Habas, Kato, & He, 2001). Daam1 has been implicated in morphogenetic processes and epithelial polarity in different models (Ang, Zhao, Lim, & Manser, 2010; Jaiswal et al., 2013; Li et al., 2011; Matusek et al., 2008; Miller et al., 2011; Nishimura et al., 2016). During Xenopus gastrulation, Daam1 was proposed to bind Dvl in response to Wnt signals, leading to RhoA and JNK activation needed for blastopore closure (Liu et al., 2008).
Daam1 transcripts are present in Xenopus embryonic ectoderm during gastrulation and later in NC-derived structures (Nakaya et al., 2004). We, therefore, wanted to examine a possible role of Daam1 downstream of Wnt signaling in NC development. Our loss-of-function studies in wild-type embryos and embryos carrying a sox10:GFP transgene indicate that Daam1 is required for NC-specific gene expression. Our analysis also suggests that Daam1 promotes NC development by modulating actin polymerization. Based on these unexpected findings, we propose a role of actin remodeling in NC-specific gene transcription.
RESULTS
Neural crest specification requires Daam1
To interfere with Daam1 function, we used an established translation-blocking Daam1-specific morpholino oligonucleotide (MO). Mesoderm-targeted microinjection of Daam1 MO into 8-cell embryos caused the formation of shortened and dorsally bent axis, consistent with previous observations (data not shown)(Habas et al., 2001). MO injections were targeted to prospective neural crest territory and their effects on NC markers were examined at early neurula (premigratory) stages. Twist, sox8 and snail2 were all downregulated in embryos injected with Daam1 MO, but not control MO (Fig. 1A–C, E, H, Fig. S1). Daam1 depletion did not change the expression of myod or sox2 (Fig. 1C, H and Fig. S1), suggesting that mesoderm and neuroectoderm are not affected. Occasionally, the sox2 domain was broader on the injected side (Fig. S1D), likely reflecting an expansion of neural progenitors at the expense of NC or a known defect in neural tube closure. The NC defects in Daam1-depelted embryos were partially rescued by the C-Daam1 RNA that lacked the MO target sequence (Fig. S2).
Figure 1. Daam1 is required for premigratory NC development.
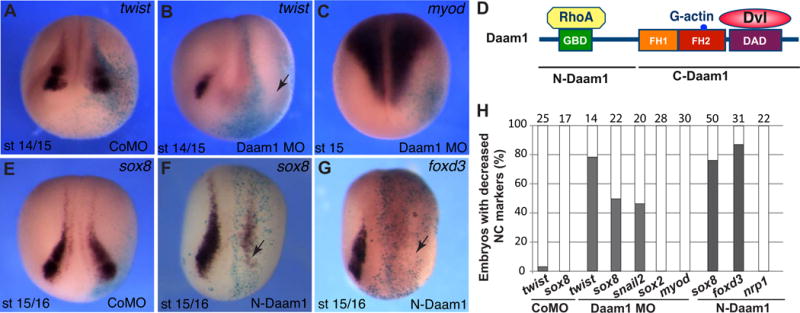
Eight-to-sixteen-cell embryos were unilaterally injected into animal blastomeres with Daam1 MO (20 ng), control MO (CoMO, 20 ng) or N-Daam1 RNA (10-20 pg) as indicated. A-C, E-G, In situ hybridization of stage 14/16 embryos with different antisense RNA probes. The arrows (B, F, G) point to the injected side with inhibited NC marker. Light blue is the lineage tracer (nβGal). Anterior views are shown. NC territory did not change in wild-type (not shown) and CoMO-injected embryos (A, E). D, Daam1 domain structure and interactions are shown. H, Frequencies of embryos with altered NC marker expression. The graphs indicate the percentage of embryos with decreased gene expression. The number of embryos analyzed is shown on top of each bar.
In an independent approach, N-Daam1, a dominant-interfering construct corresponding to the N-terminal domain of Daam1 (Fig. 1D), was expressed in embryonic ectoderm. N-Daam1 was previously reported to inhibit blastopore closure and apical constriction in early embryos (Habas et al., 2001; Ossipova, Chuykin, Chu, & Sokol, 2015). Consistent with the effect of Daam1 MO, N-Daam1 prevented NC formation on the injected side (Fig. 1F–H). Mesodermal (myod) and pan-neural (nrp1 and sox2) markers were not altered (Fig. 1H and data not shown), indicating lack of effect on general mesodermal and neural cell fates. These observations suggest that Daam1 functions in NC specification.
The sox10:GFP transgene is specifically expressed in a subpopulation of neural crest cells
We next characterized the expression of a Xenopus laevis reporter in a line constructed by a random insertion of a 5.1 kb DNA fragment containing upstream regulatory sequences of the Xenopus tropicalis sox10 gene (see Supplementary file 1 and Methods). We refer to this line as Tg(sox10:GFP). The reporter was expressed in approximately 50 % embryos that were obtained from in vitro fertilization of heterozygous eggs with wild-type sperm (Fig. S3). Additionally, a cross of two heterozygotes produced about 70-80 % of GFP-positive embryos (Fig. S3). These results suggest that the transgene has been inserted in a single genetic locus.
The transgene was detected in live embryos starting at stage 19, when two bilaterally expressed domains became visible in the prospective hindbrain area (Fig. 2A). These domains retained their expression at later stages. At stages 23–25, a stream of migratory cranial NC cells became visible in a more ventral position that corresponded to the most posterior migrating NC population (the third stream (Smith, Robinson, Patel, & Wilkinson, 1997) (Fig. 2B, C). Weaker expression of GFP was detectable in the first and the second migratory streams (Fig. 2C). The expression was also detected in the otic vesicle at stage 25 and stage 33/34 (Fig. 2C and D). At stage 33/34, GFP fluorescence remained confined to the area immediately posterior to the otic vesicle adjacent to rhombomeres 5 and 6, with weaker expression in the otic vesicle and the pharyngeal arches 2–4 (Fig. 2D, E). We note that the expression of Tg(sox10:GFP) was not identical to the expression of endogenous sox10 transcripts studied by in situ hybridization (Aoki et al., 2003; Honore, Aybar, & Mayor, 2003). The transgene was not detected before stage 19 and never appeared in the trunk NC. Additionally, the expression in the bright domain in the hindbrain at later stages (Fig. 2E) was not described for endogenous sox10. To establish the identity of GFP-positive cells in transgenic embryos, cryosections at the hindbrain level were double immunostained for GFP and FoxD3, using the available antibodies (Steiner et al., 2006). The majority of GFP-positive cells were co-stained for FoxD3, confirming our assumption that the reporter is activated in the NC lineage (Fig. 3A–C″).
Figure 2. Spatial and temporal domains of the sox10:GFP reporter expression during development.
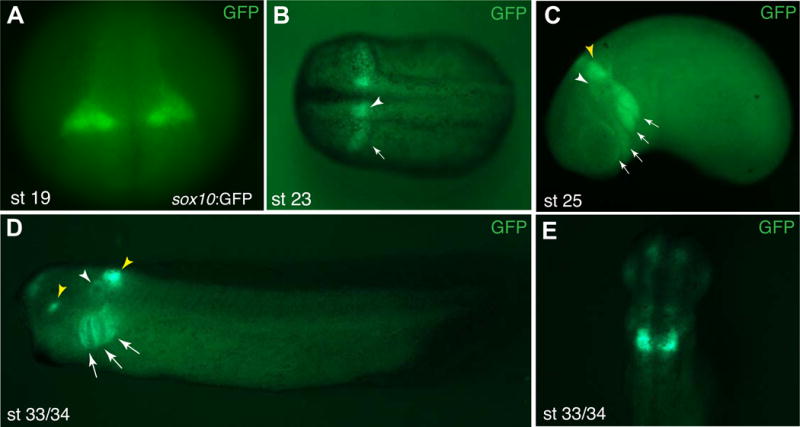
GFP reporter expression in Tg(sox10:GFP) embryos at different stages. A, B, stages 19, 23. White arrowhead in B marks hindbrain expression, the arrow points to migrating NC cells. C, stage 25. Major cranial migratory streams are indicated by arrows, white arrowhead points to the otic vesicle, yellow arrowhead to the expressing hindbrain domain. D, stage 33/34. GFP fluorescence is visible in the otic vesicle (white arrowhead), and three branchial arches (arrows). Yellow arrowheads point to the expression in the eye (a positive control GFP driven by the gamma-crystallin promoter, see Methods) and the hindbrain. (C, D) Lateral view is shown, anterior is to the left. (E) stage 33/34, dorsal view. Strong GFP signal remains in the hindbrain, weaker signals are detected in the forebrain and the otic vesicle.
Figure 3. Characterization of GFP-positive cells in Tg(sox10:GFP) embryos.
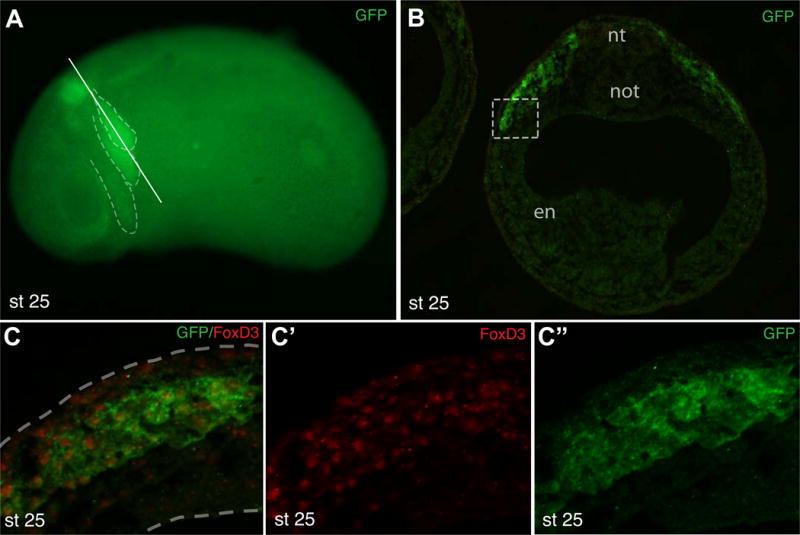
A, Tg(sox10:GFP) embryo (stage 25) with GFP expression in the hindbrain and major migratory streams (dashed lines in A). Approximate position of the section in B is shown by a solid line. B, Cross-section at the hindbrain level of a transgenic embryo double immunostained for GFP and FoxD3. Nt, neural tube; not, notochord; en, endoderm. C, High magnification of the boxed area in B shows that the majority of GFP-expressing cells are FoxD3-positive. Dashed lines indicate tissue boundaries.
Ectoderm explants form atypical epidermis when cultured without additional growth factors, however, they undergo NC differentiation when stimulated with a BMP inhibitor and a Wnt ligand (LaBonne & Bronner-Fraser, 1998; Saint-Jeannet et al., 1997). We used this system to confirm the expression of the sox10:GFP reporter using live imaging and RT-PCR (Fig. 4). Indeed, the reporter was activated by a combined stimulation of ectoderm cells with Chordin and Wnt8, Wnt5a or Wnt11 (Fig. 4A–I), further supporting reporter specificity. No significant GFP fluorescence was detected in control uninjected, chordin, Wnt8- or Wn5a-RNA-expressing ectoderm explants (Fig. 4B, C and data not shown). Together, these results are consistent with our previous findings reporting NC induction by noncanonical Wnt ligands (Ossipova & Sokol, 2011). Most of GFP-containing cells in these ectoderm explants were also positive for FoxD3, consistent with their NC identity (Fig. S4A–D). Notably, some FoxD3+ cells expressed little if any GFP, indicating that the NC population is highly heterogeneous, a conclusion reached by another study for zebrafish embryos (Kwak et al., 2013).
Figure 4. Wnt signaling activates sox10:GFP in neuralized ectoderm.
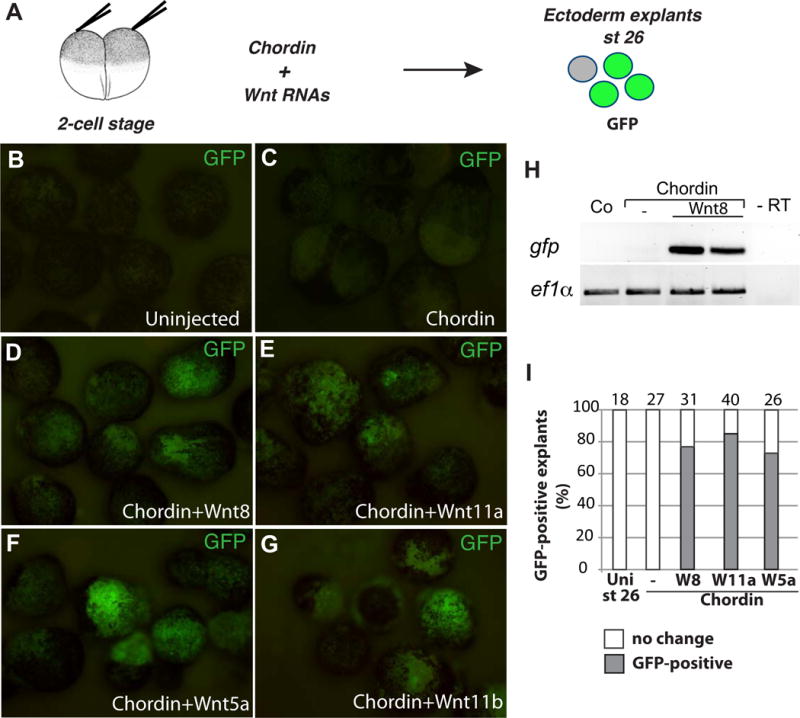
A, Experimental scheme for induction of sox10:GFP in neuralized ectoderm. B-G, Ectoderm explants were prepared at stage 10 from Tg(sox10:GFP) embryos injected with chordin RNA (150 pg, C) alone, or in combination with wnt8 (0.8 ng, D), wnt11a (1 ng, E), wnt5a (1 ng, F), and wnt11b (1 ng, G) RNAs as indicated. The explants were cultured until the equivalent of stage 26 and imaged (B-G) or lysed for RT-PCR (H). H, GFP reporter is activated in transgenic ectoderm explants by chordin and wnt8 RNAs. I, Frequencies of GFP-positive caps (%). Total number of explants is shown above each bar.
Daam1 is essential for sox10:GFP reporter activation by a noncanonical Wnt11 pathway
Our observations indicate that the sox10:GFP reporter is predominantly expressed in a subpopulation of posterior cranial NC cells and can serve as a useful molecular marker of both migratory and non-migratory NC progenitors at late neurula and tailbud stages. We next examined whether Daam1 is needed for sox10:GFP activation. The reporter was inhibited in both knockdown embryos (Fig. 5A–B′, D) and embryos expressing N-Daam1 (Fig. 5C–C′, D). These experiments support the essential function of Daam1 in NC development.
Figure 5. Sox10:GFP activation is prevented by N-Daam1 and Daam1 MO.
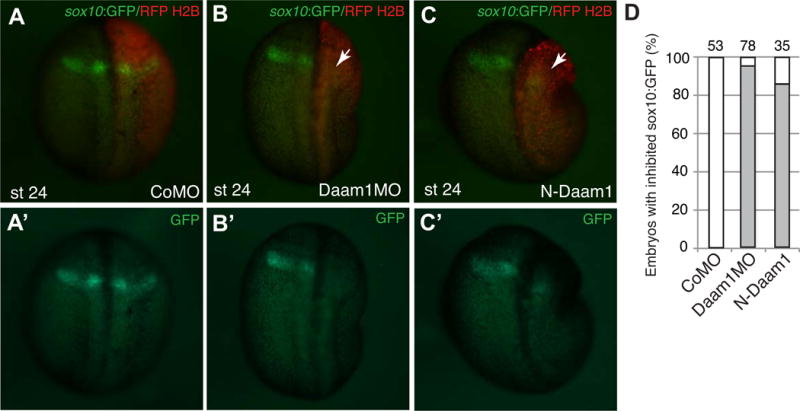
A–C, Embryos were unilaterally injected with control MO (40 ng, A), Daam1 MO (40 ng, B) or with N-Daam1 RNA (3 ng, C) as indicated. RFP-H2B RNA is a lineage tracer (100 pg, red). Dorsal views are shown, anterior is to the top. Arrows in B and C point to lack of reporter activation on the injected side. D, Frequencies of embryos with inhibited sox10:GFP expression. The number of embryos analyzed is indicated on top of each bar.
To understand which Wnt pathway branch involves Daam1, we assessed the involvement of Daam1 in the activation of the sox10: GFP reporter in neutralized ectoderm explants stimulated by Wnt11, Wnt8 and Wnt3a. We found that N-Daam1 inhibited reporter activation by Wnt11a and Wnt11b (Fig. 6). Notably, the responses of the explants to Wnt8 and Wnt3a were not affected in the same experiments, confirming specific participation of Daam1 in noncanonical Wnt signaling. These findings suggest that the induction of NC by different Wnt ligands involves distinct molecular pathways.
Figure 6. Selective inhibition of Wnt signaling by N-Daam1 in neuralized explants.
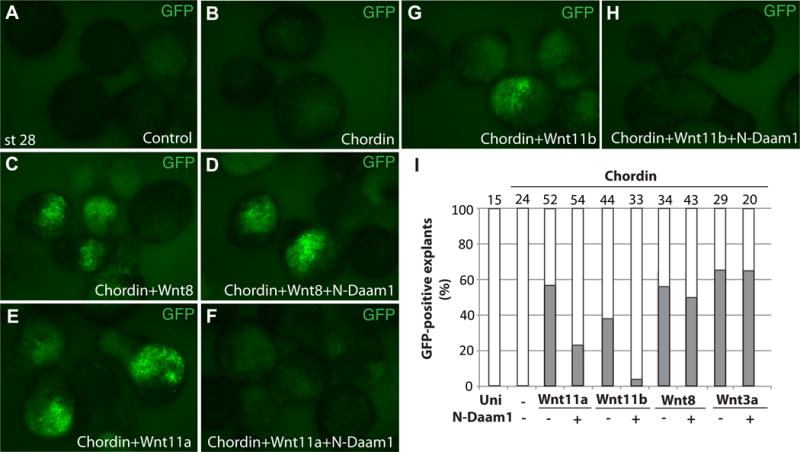
Ectoderm explants were dissected at stage 9-10 from Tg(sox10:GFP) uninjected embryos, and from those injected with indicated RNAs. The explants were cultured until the time when control embryos reached stage 28 and fixed for imaging (A-H). Chordin RNA was used either alone (150 pg, B) or in combination with different wnt RNAs. N-Daam1 (850 pg) inhibits the response to wnt11a (2 ng, E, F), wnt11b (2 ng, G, H), but not to wnt8 (700 pg, C, D) or wnt3a (525 pg, I and data not shown). I, Frequencies of GFP-positive explants (%) are representative of two different experiments. Total number of animal caps is shown above each bar.
Actin polymerization underlies the ability of C-Daam1 to promote neural crest development
Whereas Daam1 is required for NC development, we next asked whether Daam1 can modulate NC specification in a gain-of-function assay. We used C-Daam1, a constitutively active construct that retains the formin-homology domains FH1 and FH2 and the Daam1-autoinhibitory domain (DAD) that interacts with Dvl, triggers actin polymerization and activates RhoA signaling in Xenopus and cultured cells (Habas et al., 2001; Liu et al., 2008). Unilateral injection of C-Daam1 RNA into eight-cell embryos upregulated sox8, foxd3 and pax3 in 40 to 60 % of embryos at premigratory stages (st. 15-16) (Figs. 7A, B, F; Fig. S5A). By contrast, expression of the pan-neural marker nrp1 was not significantly affected (Fig. 7F, Fig. S5B). Confirming the expansion of the NC population in vivo, sox8 and pax3, but not myod were induced by C-Daam1 in ectoderm explants neuralized by chordin RNA (Fig. 7C). These experiments show that activity of Daam1 mutants in NC specification correlates with their function in noncanonical Wnt signaling.
Figure 7. C-Daam1 with an intact FH2 domain promotes NC specification.
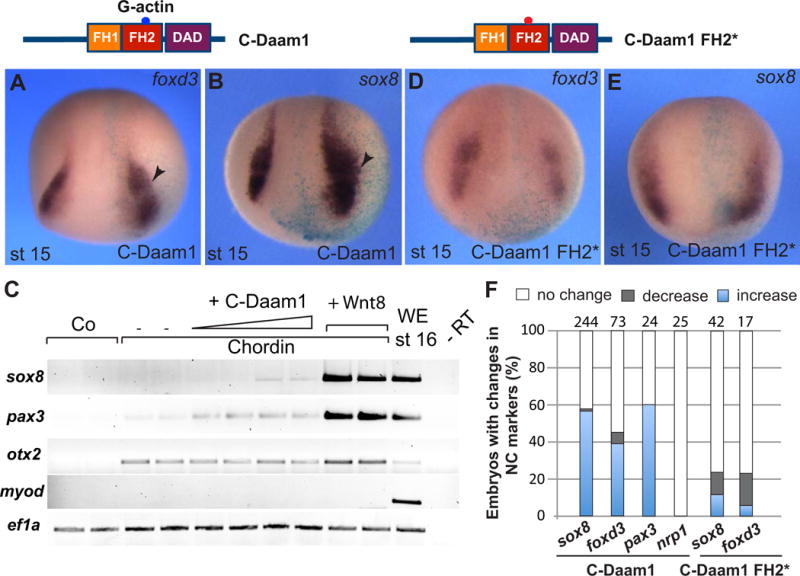
C-Daam1 and C-Daam1 FH2 mutant domain structures are shown on the top. A, B, D, E, in situ hybridization of stage 15/16 embryos with indicated antisense RNA probes. Anterior views are shown. Light blue is the lineage tracer (nβGal). Arrowheads point to expanded foxd3 and sox8 in C-Daam1 RNA-injected embryos (10-20 pg, A, B). C, C-Daam1 upregulates NC gene expression in neuralized ectoderm. Ectoderm explants were prepared from embryos injected with C-Daam1 RNA (10-20 pg) or wnt8 RNA (0.5 ng) and chordin RNA (150 pg) as indicated, cutured to stage 16 and processed for RT-PCR analysis. The explants coexpressing chordin and C-Daam1 upregulate pax-3 and sox8 but do not activate myod. D, E, C-Daam1-FH2* RNA (10-20 pg) does not promote NC gene expression. F, Frequencies of embryos with altered NC marker expression. Total number of explants is shown on top of each bar.
Formin family proteins including Daam1 recruit profilin-associated actin monomers to the elongating ends of filamentous actin (Goode & Eck, 2007), suggesting that Daam1 may affect NC-specific transcription through actin polymerization. To examine this possibility, we compared the effects of C-Daam1 and C-Daam1 with a mutation in the FH2 domain (I698A) that does not polymerize actin (Lu et al., 2007; Xu et al., 2004), but can still activate RhoA in HEK293T cells (Liu et al., 2008). In contrast to C-Daam1, C-Daam1-FH2* construct did not significantly change NC expression domains (Fig. 7D–F). These observations support the idea that actin nucleation by the FH2 domain of Daam1 is critical for its ability to promote NC specification.
Discussion
This study implicates the formin protein Daam1 in NC-specific transcription. The interference with Daam1 function suppressed NC gene activation and inhibited the expression of the sox10:GFP reporter, whereas the constitutively active C-Daam1 construct expanded NC territory in vivo and in isolated neuralized ectoderm explants in vitro. These findings are consistent with our previous results, demonstrating that noncanonical Wnt signaling promotes NC gene expression (Ossipova & Sokol, 2011) and with the proposed function of Daam1 downstream of noncanonical Wnt signaling (Habas et al., 2001).
To demonstrate the role of Daam1 in NC development, we characterized and used a new transgenic Xenopus laevis line, in which GFP expression is driven by sox10 upstream regulatory sequences. This reporter partially recapitulates endogenous sox10 expression (Aoki et al., 2003; Honore et al., 2003), being restricted to a subset of cranial NC cells. Immunostaining revealed that GFP fluorescence is present in FoxD3-expressing NC cells, confirming the utility of the new transgenic line for monitoring NC induction and migration in Xenopus. To our knowledge, this is the first live NC reporter in Xenopus laevis, extending the list of similar reporters described for other vertebrate embryos (Danielian, Muccino, Rowitch, Michael, & McMahon, 1998; Kwak et al., 2013; Stine et al., 2009). Of note, some FoxD3-positive cells in our experiments lacked GFP, reiterating the heterogeneity of the NC population. Using the sox10:GFP reporter, we demonstrated NC induction by both canonical and noncanonical Wnt ligands, reiterating our previous findings (Ossipova & Sokol, 2011) and showed the requirement of Daam1 in NC specification.
Although Daam1 is activated in response to Wnt signaling (Habas et al., 2001), the direct connection between Wnt ligands and the actin cytoskeleton is still largely hypothetical. Whereas Daam1 is generally considered to affect cell polarity and motility, we find that it also regulates NC-specific transcription. In support of this conclusion, another study reported that Daam1 affects kidney development by controlling pronephric epithelial gene expression (Miller et al., 2011). Our data indicate that the ability of Daam1 to modulate NC transcription requires the FH2 domain that is involved in G-actin binding. Nuclear actin and actin-remodeling proteins can directly stimulate gene expression in number of cases, including reprogramming of somatic cells (Miyamoto et al., 2013; Vartiainen, Guettler, Larijani, & Treisman, 2007). One possibility involves inhibition of serum-response factor (SRF) dependent transcription by G-actin binding (Miralles, Posern, Zaromytidou, & Treisman, 2003). Although SRF has been implicated in NC migration at late developmental stages (Vasudevan & Soriano, 2014), the role of this mechanism in NC specification has not been evaluated.
Our experiments indicate the existence of a signaling pathway that involves noncanonical Wnt ligands, receptors and Daam1 to promote NC formation. A similar molecular cascade was previously shown to control early morphogenetic processes including neural tube closure (Liu et al., 2011; Nishimura, Honda, & Takeichi, 2012; Ossipova, Kim, & Sokol, 2015)), suggesting that the physical forces generated during neural tube closure may be required for NC specification (Sokol, 2016). Supporting this possibility, Ror2, a Wnt5a receptor, has been implicated in NC formation (Ossipova & Sokol, 2011; Schille, Bayerlova, Bleckmann, & Schambony, 2016). However, the suppression of Myosin II activity by blebbistatin or Rho-associated kinase inhibitors unexpectedly triggered NC gene expression (Kim, Ossipova, & Sokol, 2015). Additional studies will determine whether other mediators of noncanonical Wnt signaling, such as Profilin or Missing-in-metastasis (MIM)(Liu et al., 2011; Sato et al., 2006), function in both neural tube closure and NC development and should further elucidate the relationship between the two processes.
Methods
Xenopus embryo culture and the Tg(sox10:GFP) transgenic line
In vitro fertilization and culture of Xenopus laevis embryos were carried out as previously described (Dollar, Weber, Mlodzik, & Sokol, 2005). Staging was according to Nieuwkoop and Faber (Nieuwkoop & Faber, 1967). The protocol for working with vertebrate animals has been reviewed and approved by the Mount Sinai Institutional Animal Care and Use Committee.
Upstream 5.1 kb fragment of the sox10 gene has been PCR-amplified from the Xenopus tropicalis DNA with the following primers: forward 5′-AAGTCGACCATGAGCCTGGCCTA-3′ and reverse 5′-AAATGCATTTCCAAGAGCGATGTGATTGG-3′. This PCR fragment was cloned next to the GFP coding sequence using Sal1 and Nsi1 sites in pDPCrtTA-TREG-HS4 (Rankin, Zorn, & Buchholz, 2011), replacing the CMV promoter, rtTA inducer sequence and tetracycline response element (Supplementary file 1). The Tg(sox10:GFP) line was produced by co-injecting the pDPSox10:GFP-HS4 plasmid into Xenopus laevis eggs with the ISce-1 meganuclease enzyme (New England Biolabs) and the corresponding buffer as described (Ogino, McConnell, & Grainger, 2006a, 2006b; Pan, Chen, Loeber, Henningfeld, & Pieler, 2006). The founders were screened for transgene expression based on bi- or uni-lateral GFP expression in the lens through an internal gamma crystalline reporter control. Transient founders were raised to adulthood and bred with non-transgenic animals to verify germline incorporation. Further details are available on request. The Tg(sox10:GFP) line is currently available from the Xenopus National Resource (XNR) at Marine Biological Laboratories in Woods Hole, MA.
Plasmids, mRNA synthesis, morpholinos and embryo microinjections
The plasmids Myc-Daam1-pCS2, Myc-C-Daam1-pCS2, Myc-N-Daam1-pCS2, GFP-C-Daam1-pCS2, GFP-C-Daam1-FH2* (I698A), and nβGal-pCS2 have been described previously (Habas et al., 2001; Liu et al., 2008). Wnt-expressing plasmids have been also described (Ossipova & Sokol, 2011). RFP-H2B-pCS2 was a gift from J. Wallingford. Capped mRNAs were made by in vitro transcription from the T7 or SP6 promoters using mMessage mMachine kit (Ambion). nβGal and RFP-H2B RNAs (100 pg) were lineage tracers.
Morpholino anti-sense oligonucleotides were obtained from GeneTools. The Daam1-specific translation-blocking MO had the following sequence: 5′-GGC-CAT GGC CGC AGG TCT GTC AGT T -3′. The control MO (CO MO) sequence was 5′-GCT TCA GCT AGT GAC ACA TGC AT-3′ (Ossipova, Chu, et al., 2015).
For microinjections, 4-16-cell embryos were transferred into 2 % Ficoll in 0.4 × MMR buffer and 5 nl of mRNAs or MO solution was injected into one or more blastomeres. Amounts of injected mRNA per embryo have been optimized in preliminary dose-response experiments (data not shown) and are indicated in figure legends.
Ectoderm explants, RT-PCR analysis, and in situ hybridization
Ectoderm explants (animal caps) were prepared at stages 9-10, cultured until stage 25/26 for NC and other marker analysis. For RT-PCR, RNA was prepared using RNeasy kit (Qiagen), cDNA was made using the first strand cDNA kit (Invitrogen), and PCR with Taq polymerase was carried out based on standard protocols. Primer sequences used for RT-PCR are published in (Ossipova & Sokol, 2011). GFP primers were: forward 5′-AGG GCT ATG TGC AGG AGA GA-3′ and reverse 5′-CTT GTG GCC GAG AAT GTT TC-3′.
In situ hybridization and XGAL staining were carried out as described (Harland, 1991). Digoxigenin-labeled RNA probes were prepared using the DIG RNA labeling kit (Roche). The antisense probes to NC markers have been described previously (Ossipova & Sokol, 2011).
Immunofluorescence analysis
Primary antibodies were against FoxD3 (1:100, gift of Dan Kessler, (Steiner et al., 2006)), and GFP (1:200, B-2, Santa Cruz, mouse monoclonal or Invitrogen, rabbit polyclonal). Secondary antibodies were against mouse or rabbit IgG conjugated to Alexa Fluor488, Alexa Fluor555 (1:100, Invitrogen) or Cy3 (1:100, Jackson ImmunoResearch). Standard specificity controls were performed to confirm lack of cross-reactivity and no staining without primary antibodies. Immunofluorescence images were captured using the Axioimager fluorescence microscope (Zeiss) and the Axiovision imaging software (Zeiss). Results shown are representative images from two to four independent experiments, each containing 10–15 embryos per group. Cryosectioning and immunostaining was done as described (Ossipova et al., 2014).
Supplementary Material
Acknowledgments
We thank R. Habas and J. Wallingford for plasmids, Dan Kessler for anti-FoxD3 antibodies, K. Itoh for critical reading of the manuscript, and K. Itoh and A. Ioannou for help growing transgenic frogs, S. Sidi for the use of the UV fluorescence stereoscope and members of the Sokol laboratory for discussions. This work has been supported by the National Institutes of Health grant GM122492 to SYS and the American Association of Anatomists to RK.
References
- Ang SF, Zhao ZS, Lim L, Manser E. DAAM1 is a formin required for centrosome re-orientation during cell migration. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. doi: 10.1038/nrm2717. doi:nrm2717 [pii] 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee YH, Credidio C, Saint-Jeannet JP. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev Biol. 2003;259(1):19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8(24):1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]; Dollar GL, Weber U, Mlodzik M, Sokol SY. Regulation of Lethal giant larvae by Dishevelled. Nature. 2005;437(7063):1376–1380. doi: 10.1038/nature04116. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297(5582):848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. In: Kay BK, Peng HB, editors. Methods Cell Biol. Vol. 36. San Diego: Academic Press Inc; 1991. pp. 685–695. [DOI] [PubMed] [Google Scholar]
- Honore SM, Aybar MJ, Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev Biol. 2003;260(1):79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389(6654):966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Jaiswal R, Breitsprecher D, Collins A, Correa IR, Jr, Xu MQ, Goode BL. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Curr Biol. 2013;23(14):1373–1379. doi: 10.1016/j.cub.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Ossipova O, Sokol SY. Neural crest specification by inhibition of the ROCK/Myosin II pathway. Stem Cells. 2015;33(3):674–685. doi: 10.1002/stem.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J, Park OK, Jung YJ, Hwang BJ, Kwon SH, Kee Y. Live image profiling of neural crest lineages in zebrafish transgenic lines. Mol Cells. 2013;35(3):255–260. doi: 10.1007/s10059-013-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125(13):2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Shou W. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138(2):303–315. doi: 10.1242/dev.055566. doi:138/2/303 [pii] 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Komiya Y, Mezzacappa C, Khadka DK, Runnels L, Habas R. MIM regulates vertebrate neural tube closure. Development. 2011;138(10):2035–2047. doi: 10.1242/dev.058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105(1):210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Meng W, Poy F, Maiti S, Goode BL, Eck MJ. Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J Mol Biol. 2007;369(5):1258–1269. doi: 10.1016/j.jmb.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell. 2007;131(6):1204. doi: 10.1016/j.cell.2007.11.036. doi:S0092-8674(07)01539-5 [pii] 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Matusek T, Gombos R, Szecsenyi A, Sanchez-Soriano N, Czibula A, Pataki C, Mihaly J. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28(49):13310–13319. doi: 10.1523/JNEUROSCI.2727-08.2008. doi:28/49/13310 [pii] 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milet C, Monsoro-Burq AH. Neural crest induction at the neural plate border in vertebrates. Dev Biol. 2012;366(1):22–33. doi: 10.1016/j.ydbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol. 2011;22(9):1654–1664. doi: 10.1681/ASN.2010101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113(3):329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Teperek M, Yusa K, Allen GE, Bradshaw CR, Gurdon JB. Nuclear Wave1 is required for reprogramming transcription in oocytes and for normal development. Science. 2013;341(6149):1002–1005. doi: 10.1126/science.1240376. doi:341/6149/1002 [pii] 10.1126/science.1240376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya MA, Habas R, Biris K, Dunty WC, Jr, Kato Y, He X, Yamaguchi TP. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns. 2004;5(1):97–105. doi: 10.1016/j.modgep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North Holland; 1967. [Google Scholar]
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149(5):1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Ito S, Saito H, Hiver S, Shigetomi K, Ikenouchi J, Takeichi M. DAAM1 stabilizes epithelial junctions by restraining WAVE complex-dependent lateral membrane motility. J Cell Biol. 2016;215(4):559–573. doi: 10.1083/jcb.201603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nat Protoc. 2006a;1(4):1703–1710. doi: 10.1038/nprot.2006.208. [DOI] [PubMed] [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006b;123(2):103–113. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Chu CW, Fillatre J, Brott BK, Itoh K, Sokol SY. The involvement of PCP proteins in radial cell intercalations during Xenopus embryonic development. Dev Biol. 2015;408(2):316–327. doi: 10.1016/j.ydbio.2015.06.013. doi:S0012-1606(15)30018-X [pii] 10.1016/j.ydbio.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Chuykin I, Chu CW, Sokol SY. Vangl2 cooperates with Rab11 and Myosin V to regulate apical constriction during vertebrate gastrulation. Development. 2015;142(1):99–107. doi: 10.1242/dev.111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, Lake BB, Itoh K, Ioannou A, Sokol SY. Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nat Commun. 2014;5:3734. doi: 10.1038/ncomms4734. doi:ncomms4734 [pii] 10.1038/ncomms4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Kim K, Sokol SY. Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biol Open. 2015;4(6):722–730. doi: 10.1242/bio.201511676. doi:bio.201511676 [pii] 10.1242/bio.201511676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Sokol SY. Neural crest specification by noncanonical Wnt signaling and PAR-1. Development. 2011;138(24):5441–5450. doi: 10.1242/dev.067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235(1):247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Rankin SA, Zorn AM, Buchholz DR. New doxycycline-inducible transgenic lines in Xenopus. Dev Dyn. 2011;240(6):1467–1474. doi: 10.1002/dvdy.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jeannet JP, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci U S A. 1997;94(25):13713–13718. doi: 10.1073/pnas.94.25.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133(21):4219–4231. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9(7):557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Schille C, Bayerlova M, Bleckmann A, Schambony A. Ror2 signaling is required for local upregulation of GDF6 and activation of BMP signaling at the neural plate border. Development. 2016;143(17):3182–3194. doi: 10.1242/dev.135426. doi:143/17/3182 [pii] 10.1242/dev.135426. [DOI] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol. 1997;7(8):561–570. doi: 10.1016/s0960-9822(06)00255-7. doi:S0960-9822(06)00255-7 [pii] [DOI] [PubMed] [Google Scholar]
- Sokol SY. Mechanotransduction During Vertebrate Neurulation. Curr Top Dev Biol. 2016;117:359–376. doi: 10.1016/bs.ctdb.2015.11.036. [DOI] [PubMed] [Google Scholar]
- Steiner AB, Engleka MJ, Lu Q, Piwarzyk EC, Yaklichkin S, Lefebvre JL, Kessler DS. FoxD3 regulation of Nodal in the Spemann organizer is essential for Xenopus dorsal mesoderm development. Development. 2006;133(24):4827–4838. doi: 10.1242/dev.02663. doi:dev.02663 [pii] 10.1242/dev.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine ZE, Huynh JL, Loftus SK, Gorkin DU, Salmasi AH, Novak T, McCallion AS. Oligodendroglial and pan-neural crest expression of Cre recombinase directed by Sox10 enhancer. Genesis. 2009;47(11):765–770. doi: 10.1002/dvg.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. doi:136/19/3205 [pii] 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316(5832):1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- Vasudevan HN, Soriano P. SRF regulates craniofacial development through selective recruitment of MRTF cofactors by PDGF signaling. Dev Cell. 2014;31(3):332–344. doi: 10.1016/j.devcel.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116(5):711–723. doi: 10.1016/s0092-8674(04)00210-7. doi:S0092867404002107 [pii] [DOI] [PubMed] [Google Scholar]
- Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–646. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


