Abstract
Geriatric nutritional risk index (GNRI) is a novel and useful screening tool for evaluating nutritional status in elderly in-patients. We aimed to investigate whether the preoperative GNRI could be a predictive factor for outcomes in patients over 65 years of age with a diagnosis of hepatocellular carcinoma (HCC). We retrospectively enrolled 261 consecutive HCC patients after hepatectomy and classified them into four risk groups based on the GNRI values: high risk (GNRI, <82), moderate risk (GNRI, 82–92), low risk (GNRI, 92–98), and normal (GNRI, >98). We found that the lower GNRI value was significantly associated with severe postoperative complications (P < 0.001) and liver failure (P < 0.001). By multivariate logistic regression analysis, high risk- and moderate risk GNRI groups were identified as independent risk factors for postoperative serve complications and liver failure. Multivariate Cox regression analysis revealed preoperative GNRI (P < 0.001) adversely affected overall survival. In conclusion, preoperative GNRI could predict severe postoperative complications included liver failure, and the lower GNRI value was associated with worse overall survival after hepatectomy in elderly HCC patients.
Introduction
Liver cancer is the fourth leading cause of cancer-related deaths according to the Global Burden of Disease Study in 20151. The most common type of primary liver cancer is hepatocellular carcinoma, followed by cholangiocarcinoma2. Partial hepatectomy is the preferred curative treatment for patients with HCC. However, malnutrition may contribute to an increase in surgical risk and prolonged hospital stays and was shown to significantly increase postoperative morbidity and mortality in the elderly3,4. In older in-patients, malnutrition is associated with poor clinical outcomes, including impairment in quality of life, performance status, immune function and liver function, as well as decreased survival. Several studies5–7 reported a relationship between preoperative nutritional status and poor prognosis in HCC patients. However, the correlation between preoperative nutritional status and prognosis in elderly patients with HCC remains unclear and deserves investigation.
Geriatric nutritional risk index, a new prognostic nutritional index, has been proposed for evaluation of at risk, in-hospital, elderly patients with malignant tumors such as non-small cell lung cancer8–11, renal cell carcinoma12,13, and esophageal squamous cell carcinoma14,15. The relationship between GNRI and prognosis in patients with HCC after hepatectomy has not yet been reported.
Thus, we conducted a cohort study to investigate the relationship between GNRI and short-, long-term outcomes for elderly HCC patients after hepatectomy.
Materials and Methods
Study population
We included 261 consecutive HCC patients who underwent hepatectomy in West China hospital, Sichuan university between February 2009 and December 2012. Preoperative diagnosis was confirmed based on the current EASL16 or AASLD17 HCC management guidelines. In the present study, the inclusion criteria followed: (1) Pathological diagnosis confirmed hepatocellular carcinoma, (2) receiving radical resection by open operation as the initial treatment, (3) chronic HBV infection history, (4) elderly patients >65 years old. Exclusion criteria included the following: (1) patients with obstructive jaundice, (2) combined with portal vein tumor thrombus, (3) combined with extrahepatic metastasis, (4) inpatients received albumin infusion for preoperative severe hypoproteinemia, (5) liver function of Child-Pugh grade B, (6) loss to postoperative follow-up within 90 days, (7) poor data integrity. We collected the medical records containing patients’ demographics, preoperative laboratory values, imaging examination data and postoperative clinical outcomes from the clinical liver cancer database of the department of Liver Surgery & Liver Transplantation Center, West China Hospital, Sichuan university.
This study was approved by the clinical research ethics committee of the West China Hospital, Sichuan University and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients according to the policies of the committee.
Perioperative management
Careful historical analysis, physical examination and routine preoperative laboratory measurements were performed for all patients. Routine preoperative imaging examination to evaluate the tumor and preoperative cardiopulmonary function evaluation were carried out before surgery as we previously described18. Patients were operated under general anesthesia and intraoperative ultrasonography was used routinely. Hepatic vascular inflow occlusion (hemihepatic or total hepatic blocking) or the Pringle maneuver was used according to the surgeon’s preference in most patients as those previously described19,20. Hepatectomy was performed using the clamp crushing, hooking with ligation or ultrasonic dissector with coagulator. Based on preoperative and intraoperative condition, patients were transferred to the intensive care unit for treatment when necessary.
Parameters definition
The Clavien-Dindo complication classification21 system was used for postoperative complication grading. Postoperative liver failure was defined according to the criterion of International Study Group of Liver Surgery22. Perioperative mortality was defined as any death occurring from the time of surgery up to 90 days after discharge. The GNRI is, an adaptation of the Nutritional Risk Index (NRI) designed by Buzby et al.23, simple nutritional screening tools to estimate nutrition related risk in surgical patients. The formula is as follows: GNRI = 1.487 × serum albumin concentrations (g/L) + 41.7 × preoperative body weight/ideal body weight (kg). Serum albumin levels were measured in a fasting state, blood samples were collected 3–5 days before surgery. Ideal body weight = 22 × squares of height (m), which calculated using Lorentz equations. As in previous reports24,25, the patients were classified as at high risk (GNRI < 82), moderate risk (82–92), low risk (92–98), and normal level (GNRI > 98).
Follow-up
All include patients were received regularly in the out-patient clinic and monitored prospectively by a standard protocol; the follow-up program was same as the previously we used (Follow-up time point: 30 days after the operation, every 90 days thereafter during the first 3 years and then every 180 days in subsequent years)26. HCC recurrence was diagnosed by clinical, laboratory examination and radiological data at each follow up. They were followed up until December 2017 or their death, and their medical records were retrospectively reviewed. Overall survival (OS) time was described as the interval between the operation and death or the last follow up. Recurrence-free survival (RFS) time was described as the interval between the operation and the first incidence of HCC recurrence.
Statistical analysis
Scientific secretaries were trained to take advantage of the collection and analysis responsibilities. Continuous variables were reported as mean (standard deviation [SD]) or median (interquartile range [IQR]), and the comparation using the Student t test for continuous variables with parametric distribution. Mann–Whitney U test or Kruskal–Wallis H test for those with nonparametric distribution. Categorical variables were recorded as numbers and percentages, and compared using Pearson x2 analysis or Fisher exact test. To identify risk factors for postoperative liver failure or severe complications, only significant factors in the univariate analysis were entered into the forward stepwise Logistic regression analysis. Independent risk factors for overall survival were identified using the stepwise forward Cox regression model. Overall survival- and recurrence-free survival curves were plotted using the Kaplan-Meier method and analyzed using the Log-rank test. All statistical analyses were performed using IBM SPSS Statistics software 21.0 and GraphPad Prism 7.00. P < 0.05 was considered as statistically significant.
Results
Patient characteristics
This study comprised a total of 261 patients including 215 (82.4%) males and 46 (17.6%) females. The age of the patients was 69.0 ± 3.4 years, and average total tumor size was 4.9 ± 2.2 cm. In total, 102 (39.1%) patients with HCC met the Milan criteria, whereas the remaining 159 (60.9%) patients exceeded. The preoperative liver function were classified as Child-Pugh A in all included patients. A total of 41 (15.7%) patients developed postoperative liver failure (PLF), 32 (12.2%) patients were classified as PLF grade A and B. Severe postoperative complications occurred in 19 (7.3%) cases included liver failure grade C (9 cases), cardiovascular and pulmonary complications (5 cases), bile leakage (3 cases), intra-abdominal infections (2 cases). no patient dead in all cases. The length of hospital stay after operation was 9.8 ± 3.2 days. Preoperative albumin level was significantly lower in the high-risk group than in the other three groups (P < 0.001). Incidence of severe postoperative complications (5/9, 55.5%) and liver failure (6/9, 66.6%) were significantly higher in the high-risk group (P < 0.001 for both). The details are presented in Table 1.
Table 1.
The characteristics and clinical parameters in the four groups based on the GNRI values.
| Variables | Total | High risk | Moderate risk | Low risk | Normal Level | P value |
|---|---|---|---|---|---|---|
| GNRI < 82 | GNRI 82–92 | GNRI 92–98 | GNRI > 98 | |||
| Patients (n,%) | 261, 100% | 9, 3.4% | 17, 6.5% | 38, 14.6% | 197, 75.5% | |
| Age, median (IQR) | 68 (67–70) | 69 (68–72) | 70 (66–72.5) | 68 (66–70.1) | 68 (67–70) | 0.330 |
| Preoperative TBIL (umol/L), median (IQR) | 14.1 (10.8–18.7) | 16.2 (12.5–27.1) | 17.0 (10.9–19.3) | 15.4 (10.9–19.6) | 13.3 (10.8–18.4) | 0.415 |
| Preoperative ALB (g/L), median (IQR) | 41.4 (38.1–43.8) | 28.1 (24.5–30.1)# | 34.7 (31.0–36.3) | 37.5 (35.9–38.8) | 42.7 (40.2–44.6) | <0.001 |
| Preoperative platelet (109/L), median (IQR) | 132 (91–182) | 133 (86.5–234.5) | 145 (123–173) | 105 (75.0–160.3) | 138 (91–185) | 0.280 |
| Total diameter of tumor (cm), mean (SD) | 4.9 ± 2.2 | 4.7 ± 2.2* | 5.8 ± 2.2 | 5.8 ± 2.6 | 5.3 ± 1.8 | 0.009 |
| Tumor number (single/multiple), median (IQR) | 186/75 | 3/6 | 6/11 | 8/30 | 58/139 | 0.661 |
| Preoperative AFP > 400 ng/mL (Y/N) | 184/77 | 4/5 | 6/11 | 14/24 | 53/144 | 0.413 |
| Positive HBV-DNA load (Y/N) | 70/191 | 4/5 | 5/12 | 12/26 | 49/148 | 0.512 |
| Preoperative GNRI, median (IQR) | 103.4 (98.1–109.7) | 78.4 (76.9–80.0)# | 88.7 (86.3–90.5) | 94.9 (93.5–96.9) | 106.2 (103.4–112.4) | <0.001 |
| Ishak score, median (IQR) | 6 (5–6) | 6 (5–6) | 6 (5–6) | 6 (5–6) | 6 (4–6) | 0.520 |
| Presence of MVI (Y/N) | 29/232 | 0/9 | 2/15 | 6/32 | 21/176 | 0.569 |
| Differentiation | 0.274 | |||||
| Well (n,%) | 28, 10.7% | 1,0.3% | 1,0.3% | 4,1.5% | 22,8.4% | |
| Moderate (n,%) | 218, 83.5% | 7,2.7% | 13,4.9% | 34,13.0% | 164,62.8% | |
| Poor (n,%) | 15, 5.7% | 1,0.3% | 3,1.1% | 0,0% | 11,4.2% | |
| Transfusion (Y/N) | 42/219 | 3/6 | 2/15 | 8/30 | 29/168 | 0.364 |
| Severe complication (Y/N) | 19/261 | 5/9#* | 4/17 | 0/38 | 10/197 | <0.001 |
| Liver failure (Y/N) | 41/261 | 6/9#* | 9/17 | 6/38 | 20/197 | <0.001 |
| Postoperative hospital stay (day), median (IQR) | 9 (8–11) | 10 (8–16) | 12 (8–16) | 9 (8–10) | 9 (7–11) | 0.090 |
GNRI = Geriatric Nutritional Risk Index, AFP = alpha-fetoprotein, TBIL = total bilirubin, ALB = serum albumin, PLT = platelet, MVI = micro-vascular invasion, IQR interquartile range, SD standard deviation.
#P < 0.05, when High risk group vs. Normal level-, Moderate risk- or Low risk-group.
*P < 0.05, when High risk group vs. Moderate risk- or Low risk-group.
Risk factors for severe postoperative complications and liver failure
The GNRI was significantly higher in patients without than those with postoperative complications (Fig. 1). Multivariate logistic regression analysis revealed that preoperative GNRI value (hazard ratio [HR] 0.910, 95% confidence interval [CI] 0.876–0.945, P < 0.001), moderate risk (HR 9.956, 95%CI 3.454–28.699, P < 0.001) and high-risk groups (HR 17.700, 95%CI 4.106–76.291, P < 0.001) were risk factors for postoperative liver failure (Table 2). Moderate-risk (HR 8.726, 95%CI 2.130–35.752, P = 0.003) and high-risk groups (HR 26.336, 95%CI 5.576–124.383, P < 0.001) and transfusion (HR 0.161, 95%CI 0.046–0.560, P = 0.004) were identified as independent risk factors for severe postoperative complications (Table 3).
Figure 1.
Incidence of liver failure (A) and severe complications (B) after hepatectomy according to GNRI. Mean GNRI was 96.29 ± 1.94 in patients who occurred postoperative liver failure (n = 41), mean GNRI was 105.3 ± 0.61 in whose without (n = 220), the significant differences between the two groups. (B) Mean GNRI was 95.76 ± 3.61 in patients (n = 19) who occurred postoperative complication, mean GNRI was 104.6 ± 0.6 in whose without (n = 242), the significant differences between the two groups.
Table 2.
Univariate and multivariate analyses of prognostic factors for postoperative liver failure in elderly patients with HCC.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (year) | 1.001 | 0.892–1.122 | 0.992 | |||
| Child-Pugh score | 1.858 | 0.904–3.819 | 0.092 | |||
| Preoperative TBIL (umol/L) | 0.999 | 0.990–1.008 | 0.827 | |||
| Preoperative ALT (IU/L) | 0.997 | 0.986–1.007 | 0.520 | |||
| Preoperative AST (IU/L) | 1.007 | 0.997–1.017 | 0.193 | |||
| Preoperative ALB (g/L) | 0.979 | 0.880–1.089 | 0.695 | |||
| Preoperative platelet (109/L) | 1.004 | 0.998–1.010 | 0.189 | |||
| Preoperative AFP > 400 ng/mL (Y/N) | 1.317 | 0.572–3.030 | 0.517 | |||
| Positive HBV-DNA load (Y/N) | 1.380 | 0.541–3.517 | 0.500 | |||
| Preoperative GNRI | 0.918 | 0.865–0.975 | 0.005 | 0.910 | 0.876–0.945 | <0.001 |
| Preoperative GNRI grade | ||||||
| Normal Level (GNRI > 98) | 1(Reference) | 1(Reference) | ||||
| Low risk (GNRI 92–98) | 1.547 | 0.476–5.035 | 0.468 | |||
| Moderate risk (GNRI 82–92) | 9.164 | 2.141–39.233 | 0.003 | 9.956 | 3.454–28.699 | <0.001 |
| High risk (GNRI < 82) | 11.07 | 1.289–95.083 | 0.028 | 17.700 | 4.106–76.291 | <0.001 |
| Total diameter of tumor (cm) | 0.894 | 0.737–1.085 | 0.256 | |||
| Tumor number (single/multiple) | 0.486 | 0.186–1.268 | 0.140 | |||
| Transfusion (Y/N) | 0.538 | 0.197–1.467 | 0.226 | |||
GNRI = Geriatric Nutritional Risk Index, AFP = alpha-fetoprotein,TBIL = total bilirubin. AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALB = serum albumin. PLT = platelet.
Table 3.
Univariate and multivariate analyses of prognostic factors for postoperative severe complications in elderly patients with HCC.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (year) | 1.119 | 0.822–1.523 | 0.474 | |||
| Child-Pugh score | 2.532 | 0.970–6.611 | 0.058 | |||
| Preoperative TBIL (umol/L) | 1.000 | 0.981–1.018 | 0.967 | |||
| Preoperative ALT (IU/L) | 1.004 | 0.990–1.019 | 0.573 | |||
| Preoperative AST (IU/L) | 0.998 | 0.985–1.012 | 0.822 | |||
| Preoperative ALB (g/L) | 0.957 | 0.817–1.122 | 0.591 | |||
| Preoperative platelet (109/L) | 0.996 | 0.986–1.006 | 0.431 | |||
| Preoperative AFP > 400 ng/mL (Y/N) | 2.993 | 0.904–9.911 | 0.073 | |||
| Positive HBV-DNA load (Y/N) | 4.040 | 0.839–1.464 | 0.082 | |||
| Preoperative GNRI | 0.919 | 0.849–0.994 | 0.036 | |||
| Preoperative GNRI grade | ||||||
| Normal Level (GNRI > 98) | 1(Reference) | 1(Reference) | ||||
| Low risk (GNRI 92–98) | 0.000 | 0.000 | 0.998 | |||
| Moderate risk (GNRI 82–92) | 5.754 | 1.586–20.874 | 0.008 | 8.726 | 2.130–35.752 | 0.003 |
| High risk (GNRI < 82) | 23.375 | 5.425–100.712 | <0.001 | 26.336 | 5.576–124.383 | <0.001 |
| Ishak score | 1.000 | 0.516–1.938 | 0.999 | |||
| Total diameter of tumor (cm) | 1.105 | 0682–1.792 | 0.684 | |||
| Tumor number (single/multiple) | 0.192 | 0.050–0.745 | 0.017 | |||
| Transfusion (Y/N) | 0.180 | 0.045–0.718 | 0.015 | 0.161 | 0.046–0.560 | 0.004 |
GNRI = Geriatric Nutritional Risk Index, AFP = alpha-fetoprotein,TBIL = total bilirubin.
AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALB = serum albumin.
PLT = platelet.
Long-term outcomes: overall survival and HCC recurrence
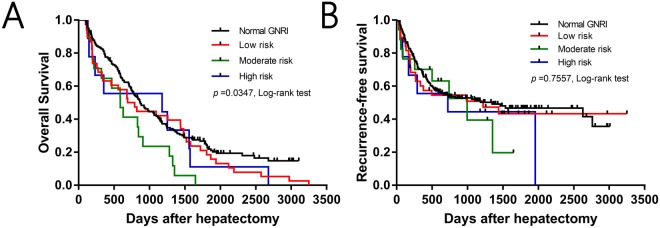
Based on the classification of patients according to GNRI values, 1-, 3-, and 5-year overall survival rates were significantly different among the high-risk (79.6%, 42.4%, and 22.7%, respectively), moderate-risk (63.2%, 42.1%, and 18.4%, respectively), low-risk (64.7%, 23.5%, and 0%, respectively), and normal (55.6%, 55.6%, and 11.1%, respectively) GNRI groups (P = 0.0347, Fig. 2A). However, the 1-, 3-, and 5-year recurrence-free survival rates were not significantly different among the high-risk (67.7%, 52.1%, and 46.7%, respectively), moderate-risk (60.1%, 50.9%, and 43.3%, respectively), low-risk (70.1%, 39.4%, and 19.7%, respectively), and normal (55.6%, 44.4%, and 44.4%, respectively) GNRI groups (P = 0.7557, Fig. 2B). To identify independent risk factors for overall survival, univariate and multivariate analysis was performed, which revealed that preoperative platelet counts (HR 1.003, 95%CI 1.001–1.005, P = 0.001), microvascular invasion (HR 0.634, 95%CI 0.414–0.971, P = 0.036), and preoperative GNRI value (HR 0.977, 95%CI 0.964–0.990, P < 0.001) were independent prognostic factors for overall survival (Table 4).
Figure 2.
Kaplan-Meier curve analysis of Overall survival (A) and Recurrence-free survival (B) in four groups based on GNRI values.
Table 4.
Univariate and multivariate analyses of prognostic factors for overall survival in elderly patients with HCC.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (<70/ ≥ 70) | 1.145 | 0.831–1.578 | 0.408 | |||
| Milan Criteria (within/exceed) | 0.700 | 0.500–0.981 | 0.039 | |||
| Total diameter of tumor (cm) | 1.037 | 0.922–1.167 | 0.541 | |||
| Child-Pugh score | 1.095 | 0.874–1.372 | 0.430 | |||
| Preoperative platelet (109/L) | 1.003 | 1.001–1.005 | 0.003 | 1.003 | 1.001–1.005 | 0.001 |
| Preoperative AFP > 400 ng/mL (Y/N) | 0.762 | 0.540–1.077 | 0.124 | |||
| Positive HBV-DNA load (Y/N) | 0.958 | 0.690–1.331 | 0.799 | |||
| Preoperative GNRI | 0.981 | 0.963–0.999 | 0.039 | 0.977 | 0.964–0.990 | < 0.001 |
| Preoperative GNRI grade | ||||||
| Normal Level (GNRI > 98) | 1(Reference) | |||||
| Low risk (GNRI 92–98) | 1.265 | 0.881–1.816 | 0.202 | |||
| Moderate risk (GNRI 82–92) | 2.003 | 1.208–3.322 | 0.007 | |||
| High risk (GNRI < 82) | 1.313 | 0.669–2.577 | 0.428 | |||
| Ishak score | 1.074 | 0.907–1.273 | 0.408 | |||
| Tumor number (single/multiple) | 1.457 | 1.030–2.062 | 0.034 | |||
| Presence of MVI (Y/N) | 0.623 | 0.398–0.976 | 0.039 | 0.634 | 0.414–0.971 | 0.036 |
| Differentiation | 1.501 | 0.731–3.081 | 0.269 | |||
| Well | 1(Reference) | |||||
| Moderate | 0.703 | 0.461–1.071 | 0.101 | |||
| Poor | 0.679 | 0.341–1.353 | 0.271 | |||
| Transfusion (Y/N) | 0.903 | 0.601–1.356 | 0.623 | |||
GNRI = Geriatric Nutritional Risk Index, AFP = alpha-fetoprotein, TBIL = total bilirubin.
AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALB = serum albumin.
PLT = platelet, MVI = micro-vascular invasion.
Discussion
The postoperative complications of hepatectomy include intractable ascites, bile leakage, intra-abdominal hemorrhage, and liver failure. The incidence of postoperative liver failure, reported to be as high as 5.7%–11%, was reported as a predominant cause of hepatectomy-related mortality with incidence rates as high as 11%22. The complications are widely known to significantly increase the risk of postoperative morbidity and mortality and to have a negative impact on long-term survival.
Malnutrition is associated with worse outcome of partial hepatectomy and appropriate nutritional intervention can improve the outcomes. Previous studies suggested that nutritional supplementation could reduce the postoperative complications and shorten the duration of hospitalization of patients who undergo liver resection for cancer27,28. Hsieh CE et al. reported that postoperative nutritional support could promote the recovery of liver function and shorten length of stay in adult liver donors29.
This is the first study to retrospectively investigate the correlation between preoperative nutritional status using GNRI and prognosis in elderly patients with HCC, which revealed that lower preoperative GNRI value in elderly HCC patients were associated with worse postoperative clinical outcomes, such as liver failure, severe complications, and overall survival rate, but not HCC recurrence-free survival. Although most of the patients appeared to be in good health before the operation, the true malnutrition status and acceptable organ functions were often ignored. The results of the current study demonstrated that preoperative GNRI was an independent predictive factor for prognosis after hepatectomy in elderly patients with HCC.
Changes in physiologic and psychosocial factors resulting in malnutrition were found to increase risk in adults over the age of 65 in a study conducted in the United States4. Malnutrition is associated with an increase in the risk of operation and prolonged hospital stays and markedly contributes to morbidity and mortality in the elderly. Our multivariate logistic regression analysis revealed that GNRI was an independent risk factor for both postoperative liver failure and severe postoperative complications. We also found that platelet count, microvascular invasion, and GNRI were independent risk factors for overall survival analyzed by Cox proportional hazard model. However, for the recurrence-free survival, GNRI was not an independent risk factor in the same analysis. We previously reported that30 postoperative liver failure was significantly associated with low platelet counts. Consistent with previous studies31,32, postoperative liver failure and severe complications affected HCC recurrence and reduced overall survival. The presence of microvascular invasion was shown to be associated with a high incidence of recurrence and worse long-term survival in many studies33–35. Bo et al.14 indicated that the GNRI could predict survival in elderly esophageal cancer patients. Gu et al.13 found that GNRI could be utilized to identify patients with metastatic renal cell carcinoma at risk for poor survival outcomes. Shoji et al.9 reported that elderly patients with non-small cell lung cancer and abnormal preoperative GNRI experienced significantly shorter overall survival. These earlier studies lend further support to our findings in the current study.
Chronic cardiopulmonary disease, hypertension, and glucose and lipid metabolism disorders, which occur at a higher incidence in the elderly population, could affect the BMI values or albumin level in elderly patients. The BMI consists of body weight and height, which is related to malnutrition36. In the study, ascites was found in 62 patients via the preoperative ultrasound examination and no patients complained of abdominal distention. The shifting dullness was negative. The depth of ascetic fluid under ultrasonic examination is not more than 3 cm. In addition, mean (SD) and median (IQR) preoperative albumin levels were 40.79 ± 5.09 and 41.40 (38.05–43.80), respectively. No patients received albumin infusion before operation. Previous studies found that the influence of BMI on the postoperative course and survival have shown controversial results37,38 and albumin could assess the nutritional status and predict long-term mortality of elderly patients39. The authors considered that the GNRI, with the additional information for ideal weight, might predict nutrition-related mortality better than serum albumin or BMI.
The current study has several limitations that should be acknowledged. First, potential information and selection biases cannot be denied in this retrospective, single-center study. Second, the definition of the elderly population is not consistent among studies, and these analyses should also be performed in patients above 70 or even 80 years of age. Third, only GNRI was used as the nutritional screening tool, and GNRI was not compared with other commonly utilized tools to assess nutritional status, which should be addressed in future studies.
In conclusion, this retrospective study revealed that preoperative GNRI could predict severe postoperative complications included liver failure and the lower GNRI score was associated with worse overall survival after hepatectomy in elderly patients with HCC.
Ethical Review
This protocol was approved by the West China Hospital Ethical Committee and written informed consents were obtained from all the patients before their operation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81470037 and 81770653).
Author Contributions
Conception: Yang J.Y. Acquisition of data: Li L., Wang H.Q., and Yang J. Analysis of data: Li L., Wang H.Q. and Yang J. Writing: Li L. and Wang H.Q. Critical revision: all authors. Final approval: all authors.
Availability of data
The data-sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Li and Haiqing Wang contributed equally.
References
- 1.The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol3, 1683–1691 (2017). [DOI] [PMC free article] [PubMed]
- 2.Petrick JL, et al. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139:1534–1545. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CC, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs. 2001;36:131–142. doi: 10.1046/j.1365-2648.2001.01950.x. [DOI] [PubMed] [Google Scholar]
- 4.DiMaria-Ghalili, R. A. & Amella, E. Nutrition in older adults. Am J Nurs105, 40–50; quiz 50–51 (2005). [DOI] [PubMed]
- 5.Harimoto N, et al. Prognostic Significance of Preoperative Controlling Nutritional Status (CONUT) Score in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma. World J Surg. 2017;41:2805–2812. doi: 10.1007/s00268-017-4097-1. [DOI] [PubMed] [Google Scholar]
- 6.Deng Y, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther. 2016;9:5317–5328. doi: 10.2147/OTT.S109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schütte K, Schulz C, Malfertheiner P. Nutrition and Hepatocellular Cancer. Gastrointest Tumors. 2016;2:188–194. doi: 10.1159/000441822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoji F, et al. Relationship Between Preoperative Sarcopenia Status and Immuno-nutritional Parameters in Patients with Early-stage Non-small Cell Lung Cancer. Anticancer Res. 2017;37:6997–7003. doi: 10.21873/anticanres.12168. [DOI] [PubMed] [Google Scholar]
- 9.Shoji F, et al. Preoperative Geriatric Nutritional Risk Index: A predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg Oncol. 2017;26:483–488. doi: 10.1016/j.suronc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Shoji, F. et al. Prognostic significance of immune-nutritional parameters for surgically resected elderly lung cancer patients: a multicentre retrospective study. Interact Cardiovasc Thorac Surg (2017). [DOI] [PubMed]
- 11.Shiroyama T, et al. Carboplatin plus weekly nanoparticle albumin-bound paclitaxel in elderly patients with previously untreated advanced squamous non-small-cell lung cancer selected based on Mini Nutritional Assessment short-form scores: a multicenter phase 2 study. Cancer Chemother Pharmacol. 2017;80:461–467. doi: 10.1007/s00280-017-3385-7. [DOI] [PubMed] [Google Scholar]
- 12.Miyake H, Tei H, Fujisawa M. Geriatric Nutrition Risk Index is an Important Predictor of Cancer-Specific Survival, but not Recurrence-Free Survival, in Patients Undergoing Surgical Resection for Non-Metastatic Renal Cell Carcinoma. Curr Urol. 2017;10:26–31. doi: 10.1159/000447147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu W, et al. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle. 2015;6:222–230. doi: 10.1002/jcsm.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bo Y, et al. The Geriatric Nutritional Risk Index Predicts Survival in Elderly Esophageal Squamous Cell Carcinoma Patients with Radiotherapy. PLoS One. 2016;11:e0155903. doi: 10.1371/journal.pone.0155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamana I, et al. Is the Geriatric Nutritional Risk Index a Significant Predictor of Postoperative Complications in Patients with Esophageal Cancer Undergoing Esophagectomy. Eur Surg Res. 2015;55:35–42. doi: 10.1159/000376610. [DOI] [PubMed] [Google Scholar]
- 16.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol56, 908-943 (2012). [DOI] [PubMed]
- 17.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, H. et al. Immediate postoperative Fibrosis-4 predicts postoperative liver failure for patients with hepatocellular carcinoma undergoing curative surgery. Dig Liver Dis (2017). [DOI] [PubMed]
- 19.Wen T, et al. Continuous normothermic hemihepatic vascular inflow occlusion over 60 min for hepatectomy in patients with cirrhosis caused by hepatitis B virus. Hepatol Res. 2007;37:346–352. doi: 10.1111/j.1872-034X.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Zhang P, Wang H, Yan L, Wang W. Comparing outcomes of two vascular inflow occlusion techniques and treatment without vascular occlusion during major hepatectomy in patients with Hepatitis B-related hepatocellular carcinoma. PLoS One. 2014;9:e107303. doi: 10.1371/journal.pone.0107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahbari NN, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Buzby GP, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988;47:366–381. doi: 10.1093/ajcn/47.2.366. [DOI] [PubMed] [Google Scholar]
- 24.Bouillanne O, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Hai S, Zhou Y, Liu P, Dong BR. The Geriatric Nutritional Risk Index predicts mortality in nonagenarians and centenarians receiving home care. Asia Pac J Clin Nutr. 2018;27:78–83. doi: 10.6133/apjcn.022017.10. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Postoperative Prognostic Nutritional Index Predicts Survival of Patients with Hepatocellular Carcinoma within Milan Criteria and Hypersplenism. J Gastrointest Surg. 2017;21:1626–1634. doi: 10.1007/s11605-017-3414-1. [DOI] [PubMed] [Google Scholar]
- 27.Okabayashi T, et al. Effects of branched-chain amino acids-enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1869–1873. doi: 10.1111/j.1440-1746.2008.05504.x. [DOI] [PubMed] [Google Scholar]
- 28.Vyhnánek F, et al. Postoperative nutritional support in liver surgery. Effects of specialized parenteral nutrition enriched with branched-chain amino acids following liver resections for colorectal carcinoma metastases. Rozhl Chir. 2008;87:21–25. [PubMed] [Google Scholar]
- 29.Hsieh CE, et al. Comparative factor analysis of the effect of postoperative peripheral parenteral nutrition on recovery of right lobe liver donors. Exp Clin Transplant. 2015;13:157–162. [PubMed] [Google Scholar]
- 30.Wang HQ, Yang J, Yang JY, Wang WT, Yan LN. Low immediate postoperative platelet count is associated with hepatic insufficiency after hepatectomy. World J Gastroenterol. 2014;20:11871–11877. doi: 10.3748/wjg.v20.i33.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iguchi K, et al. The impact of posthepatectomy liver failure on the recurrence of hepatocellular carcinoma. World J Surg. 2014;38:150–158. doi: 10.1007/s00268-013-2247-7. [DOI] [PubMed] [Google Scholar]
- 32.Fukushima K, et al. Assessment of ISGLS definition of posthepatectomy liver failure and its effect on outcome in patients with hepatocellular carcinoma. J Gastrointest Surg. 2014;18:729–736. doi: 10.1007/s11605-013-2423-y. [DOI] [PubMed] [Google Scholar]
- 33.Lei Z, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 34.Roayaie S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirokawa F, et al. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44:846–853. doi: 10.1111/hepr.12196. [DOI] [PubMed] [Google Scholar]
- 36.Yılmaz E, Carti E, Karacan E, Bilgiç E, Boylu S. Nutritional condition scanning in gastrointestinal cancer patients. Kocaeli Medical J. 2016;5(2):21–25. [Google Scholar]
- 37.Blom RL, Lagarde SM, Klinkenbijl JH, Busch OR, van Berge Henegouwen MI. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol. 2012;19:766–771. doi: 10.1245/s10434-011-2103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc. 2015;74:405–412. doi: 10.1017/S002966511500169X. [DOI] [PubMed] [Google Scholar]
- 39.Sahyoun NR, Jacques PF, Dallal G, Russell RM. Use of albumin as a predictor of mortality in community dwelling and institutionalized elderly populations. J Clin Epidemiol. 1996;49:981–988. doi: 10.1016/0895-4356(96)00135-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.