Abstract
An attenuated Campylobacter jejuni aspartate chemoreceptor ccaA mutant caused gross pathological changes despite reduced colonisation ability in animal models. In chickens, the pathological changes included connective tissue and thickening of the mesenteric fat, as well as the disintegration of the villus tips in the large intestine, whereas in mice, hepatomegaly occurred between 48–72 hours post infection and persisted for the six days of the time course. In addition, there was a significant change in the levels of IL-12p70 in mice infected with the C. jejuni ccaA mutant. CcaA isogenic mutant was hyper-invasive in cell culture and microscopic examination revealed that it had a “run” bias in its “run-and-tumble” chemotactic behaviour. The mutant cells also exhibited lower level of binding to fucosylated and higher binding to sialylated glycan structures in glycan array analysis. This study highlights the importance of investigating phenotypic changes in C. jejuni, as we have shown that specific mutants can cause pathological changes in the host, despite reduction in colonisation potential.
Introduction
Campylobacter infections are one of the top four key global causes of bacterial gastroenteritis world-wide1. Campylobacter jejuni is widely regarded as a commensal of the avian gut, and chickens, specifically, are considered to be the major vector for this zoonotic illness1–3. In humans, however, infection results in severe gastroenteritis in which the pathology presents as severe active inflammation of the intestinal mucosa with an influx of phagocytes4–6.
There are several colonisation factors, which contribute to the infection of the human gastrointestinal tract and colonisation of the avian gut as a commensal. These factors include chemotaxis, motility, capsule formation, two-component regulatory systems, invasion and iron regulation (reviewed in7). Specifically, chemotaxis and motility have been implicated in colonisation and virulence of C. jejuni8,9. During infection in humans, C. jejuni invades and traverses the intestinal epithelium5,10, causing disruption to the epithelium and gaining access to the basal side11. Infection also stimulates the innate immune system with upregulation of the inflammatory cytokines Il-1β, IL-8 and nitric oxide12.
The study of host-pathogen interactions of C. jejuni suffer due to a paucity of suitable animal models which accurately mimic human campylobacteriosis. However, animal models of colonisation and infection, such as chickens13–15 and mice16–18, are commonly used to elucidate interactions of C. jejuni with its hosts. The use of mice however, as a campylobacter infection model has proven problematic, as most wild-type laboratory mouse strains are susceptible only to a short transient infection of the gut with no discernible symptoms. In order to elicit a disease phenotype upon infection, mouse models have subsequently been adapted by employing SIGIRR-deficient (−/−) or IL-10(−/−) mice19–21. However, these mice are immunocompromised and the establishment of infection and disease is unrealistic compared to the immunocompetent response. Despite these limitations, murine models are extensively used to study all aspects of C. jejuni colonisation22,23.
Alternatively, study by McAuley et al.18, showed that 129/SvJ background mice were susceptible to persistent colonisation by C. jejuni which localised in digestive and systemic organs of these mice18. While 129/SvJ mice are useful as a colonisation and not a disease model, similar to that with other murine models, they are immunocompetent and can provide useful information of a mammalian immune response to colonisation with C. jejuni and its isogenic mutants.
One major contrast between the human and avian hosts, is that C. jejuni infection in chickens does not typically lead to the same symptoms and pathological inflammatory response as in humans24. The physiological reasons for this are yet to be elucidated. There is however conflicting evidence as to whether campylobacters can adhere to or invade the chicken gut, and if indeed campylobacters are a commensal2,25. C. jejuni is commonly found in the mucus layer, and especially in the deep crypts of the caecum8, and some evidence suggests that campylobacters have the ability to traverse the intestinal epithelium, as bacteria have been recovered in the liver and lungs of young chicks26. Recent studies have also suggested that C. jejuni infection in the chicken gut initiates an innate immune response27 which has also been shown in avian cell lines with stimulation of pro-inflammatory cytokine response28.
The differences in C. jejuni relationship with its human and animal hosts had been a subject of intense speculation with little evidence to support any of the theories. However, genes involved in flagella, motility and chemotaxis, as either receptors or other elements of chemotaxis machinery, have been shown to be important for colonisation of the gastrointestinal tract of chickens6,15. Additionally, chemotaxis genes were shown to be differentially expressed in C. jejuni cells isolated from a chicken host, as well as the genes involved in electron transport and the central metabolic pathways29. Changes in sensory receptor gene expression have also been described for C. jejuni strains 11168-O, 11168-GS, 81116 and 520 when isolated from different sources, including the intestine of mice and chickens30.
We have previously shown that a mutation of the sensory domain of the aspartate chemosensor CcaA of the original (Skirrow) isolate of C. jejuni, NCTC 11168-O, resulted in a “run” chemotactic motility bias, a reduced ability to colonise the gastrointestinal tract of chickens and increased efficiency in invasion of Caco-2 cells when compared to the 11168-O wild type and ccaA−/+ complemented strains31. Since CcaA appears to play an important role in colonisation of the chicken, the effect of a mutation in ccaA of C. jejuni 11168-O on interaction with both the avian and mammalian hosts needed to be assessed further.
In this study, we describe systematic analysis of the effect of C. jejuni 11168-O CcaA mutation on the interaction of the bacteria with avian and mammalian hosts during different stages of colonisation as compared with the parental wild type strain. Here we report the first observation and analysis of abnormal gross pathology of the liver in a murine model, and thickening of the mesenteric fat around the intestinal connective tissue in the chicken model, following infection with C. jejuni ccaA mutant, but not when infected with wild type or complemented mutant strain, as we described previously31. We further report the of the isogenic mutant’s ability to bind simple and complex glycans as well as the expression profiles of the pro-inflammatory cytokines in the avian and murine model were investigated in response to infection with C. jejuni 11168-O and its isogenic ccaA mutant
Results
Colonisation potential in the murine model
To analyse the colonisation deficiency of the C. jejuni aspartate receptor mutant, the colonisation potential of C. jejuni 11168-O and 11168-OΔccaA::cat was compared in 129/SvJ mice over a 6 day period. The post-mortem analyses of mice intestinal content on day 6 post-inoculation showed that the average bacterial load in the large intestine of mice infected with wild type 11168-O was 1.2 × 104 cfu/g, while mice infected with 11168-OΔccaA::cat had an average bacterial load of 1 × 101 cfu/g. This indicated a 3-log reduction in presence of 11168-OΔccaA::cat cells in the large intestine, when compared to that of 11168-O wild-type (p < 0.001) (Fig. 1). It should be noted that infection with 11168-OΔccaA::cat resulted in a reduction in colonisation for the avian host of 1.5-log, (reported previously31). Selective plating of the viable bacteria recovered from the post-mortem intestinal content from the co-infection of the mice with wild-type and mutant CcaA, showed that >98.8% of bacteria present in the small and large intestines was wild-type 11168-O (p > 0.25) on day 6p.i. The average bacterial load in both the small and large intestine for the 11168-O wild-type as sole inoculum and for co-infection with the ccaA mutant were not statistically different at day 6p.i. (students T-test, p > 0.05).
Figure 1.
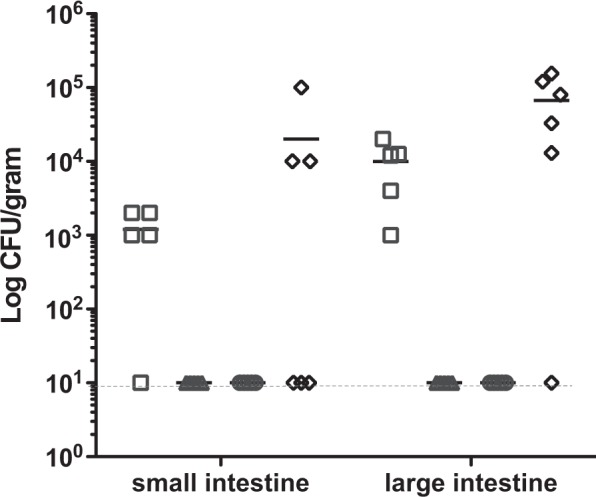
Bacterial load for the small intestine and large intestine at day 6 post-inoculation. Data is displayed as log cfu per gram of intestinal content, n = 5. 11168-O (□), 11168-OΔccaA::cat (Δ), negative non-infected control (○), competition 11168-O WT and ΔccaA::cat (◊). Bar indicates average cfu/gram for each group. <10 cfu/gram of intestinal content was detected for 11168-OΔccaA::cat and mock infected. Broken line indicates level of sensitivity.
Post-mortem analysis of affected murine tissues
Importantly, when mice were sacrificed on day 6p.i., prominent gross pathological differences were observed in the mice infected with 11168-OΔccaA::cat, when compared to those infected with 11168-O wild-type. Despite the reduced bacterial load, the liver of the mice infected with 11168-OΔccaA::cat appeared noticeably larger. The livers of mice infected with 11168-OΔccaA::cat were significantly larger in weight (p < 0.01) when compared to the other groups with mean weight of 1.3 g on day 6p.i., a 60–80% increase in liver weight. Upon dissection of the gastrointestinal tract, the mesenteric lymph nodes were visually more prominent in mice infected with 11168-OΔccaA::cat when compared to 11168-O wild-type. Additionally, the Peyer’s patches were enlarged along the entire length of the intestine, for both 11168-O and 11168-OΔccaA::cat (not shown).
Four-day time course murine infection trial
To ascertain the progression of the pathological changes in the mice, induced by 11168-OΔccaA::cat isogenic mutant, a time course experiment was conducted at time points of 24, 48, 72 and 96 hours post inoculation. (Table S1). The bacterial counts from lungs, liver and spleen for mice singly infected with 11168-O or 11168-OΔccaA::cat were not statistically different (p < 0.05), and there was no difference in bacterial load in the duodenum, small or large intestine (p > 0.05).
Post-mortem analysis of affected murine tissues, 4 day time course trial
Further post-mortem analysis of mice during the time course trial, showed no difference in liver weight (as a percentage of body weight) at 24 h p.i. when comparing the mock-infected control, 11168-O and 11168-OΔccaA::cat inoculated animals (p > 0.18). However, at 48 h post-inoculation, the liver weight for 11168-OΔccaA::cat inoculated group was significantly higher than those inoculated with 11168-O and negative controls (p < 0.02, Fig. 2). For the mice inoculated with 11168-OΔccaA::cat, an average increase of 23% in relative liver size was observed after 48 h (Fig. 2). In order to determine if this phenomenon persisted long-term mice, mice were sacrificed on day 14p.i. There was no statistical difference in the relative liver size for all groups, indicating that this pathology was only present during the acute infection period (data not shown).
Figure 2.
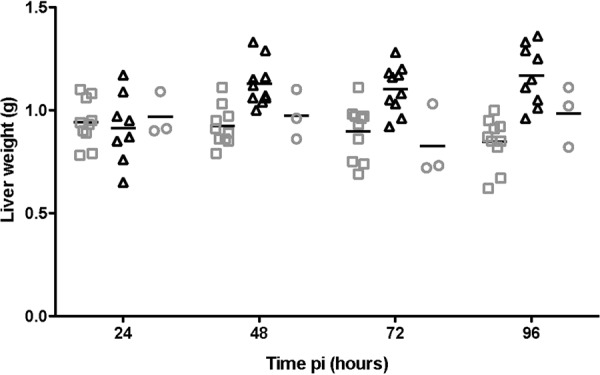
Liver weights in mice. The mean of each group is shown as a bar. 11168-O (□), 11168-OΔccaA::cat (Δ), negative non-infected control (○). Average weight is shown as a bar for each group at each time point. *p < 0.05 ANOVA, 11168-OΔccaA::cat weights are greater than 11168-O and negative groups at 48, 72 and 96 hours post inoculation. N = 3–10 mice.
Histopathological analysis of the systemic and digestive organs of mice infected with C. jejuni 11168-O or 11168-OΔccaA::cat
To further examine the pathological differences seen in the mice infected with isogenic mutant strain of C. jejuni 11168-O, histological samples for the systemic and digestive organs were taken for each mouse at every time point (24–96 h p.i.), and scored blindly according to tissue pathology using haematoxylin and eosin (H&E) staining.
Pathological differences were noted in the severity of inflammatory cells infiltration into the crypts of the mid-colon, which was found to be significantly higher in 11168-OΔccaA::cat infected mice when compared to that of the 11168-O group and non-infected control groups (p < 0.05) as shown in Table S2. Goblet cell loss within the small intestine was observed in all groups, in conjunction with some goblet cell hyperplasia. There was, however, no statistical difference in the extent of goblet cell loss or hyperplasia dependent on inoculum or time point (p > 0.05). Paneth cells were prominent in 11168-OΔccaA::cat-infected mice at 24 h (1/10) and 48 h (4/10), this, however, was not statistically significant. There was also evidence of C. jejuni present within the goblet cells of the mid and distal colon in 11168-OΔccaA::cat-infected mice, however this was not consistently observed within the group (7/40). The liver was scored in terms of nuclei enlargement, blood vessel dilation and granulation. There were no significant differences between the groups. No significant differences were noted for the lungs, liver and spleen or in the tissues of proximal or distal colon obtained from mice infected with 11168-OΔccaA::cat, 11168-O or non-infected controls.
Single-cell tracking microscopic analysis
Unlike other attenuated chemoreceptor mutants previously reported32,33, the mutation of the aspartate receptor CcaA resulted in an increase, rather than the decrease, in both adherence and invasion of cultured Caco-2 cells. This unusual phenotype of the CcaA mutant could be related to a “run” chemotactic behaviour was investigated further. Single-cell tracking of wild-type, mutant and complemented C. jejuni isogenic strains showed that the mutant had increased linear displacement over 1 second in time (Table S3, p < 0.005) confirming a run-biased phenotype, compared to 11168-O and the ccaA−/+ complement which both showed tumbling and running phenotypes. This was further confirmed by a swarm plate assay (Table S3).
Colonisation potential in the avian model
A competitive co-infection assay was used to determine the fitness of the isogenic mutant 11168-OΔccaA::cat. Co-infection of chickens with both C. jejuni 11168-O and 11168-OΔccaA::cat, showed that only the wild type strain could be could be recovered after 24, 48, 72 and 96 h following inoculation (p < 0.01).
It was also noted that when the chickens were sacrificed on day 5 post-inoculation, similar to that seen in the murine host, prominent gross pathological differences were observed in the chickens infected with 11168-OΔccaA::cat, when compared to those infected with 11168-O wild-type or the ccaA−/+ complement. Despite the reduced bacterial load, the intestines were surrounded by fluid and rope-like thickening of the mesenteric fat (Fig. 3).
Figure 3.
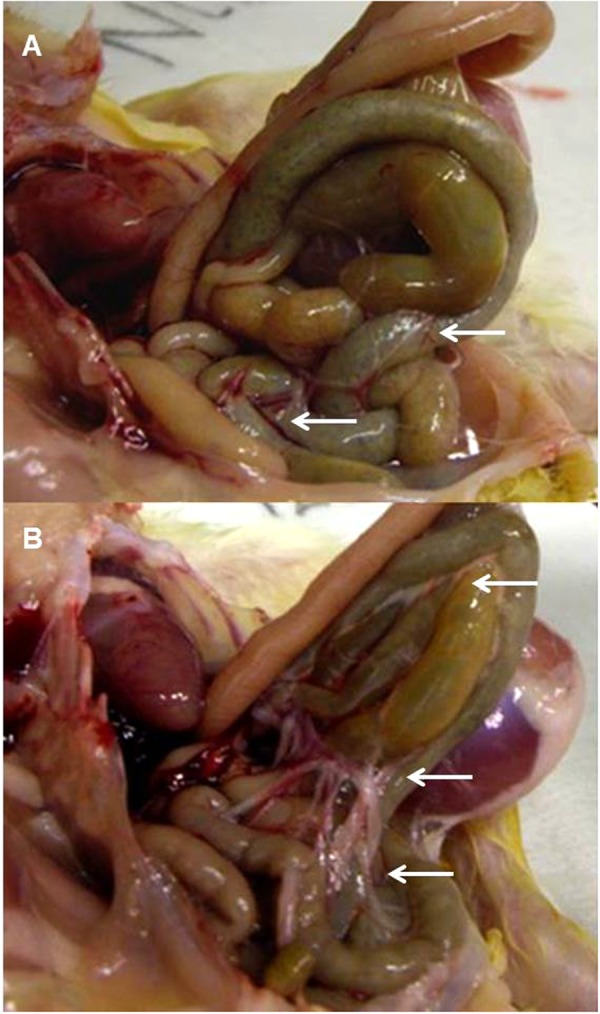
Pathology of the chick gut day 5 post inoculation. (A) Chick infected with C. jejuni 11168-0. (B) Chick infected with C. jejuni 11168-0ΔccaA::cat. Arrows show areas for visual comparison of the mesenteric fat surrounding the intestines.
Four-day time course avian colonisation trial
To ascertain the time of appearance of the pathological changes induced by 11168-OΔccaA::cat isogenic mutant, a time course experiment was then conducted whereby groups of ten day-old chicks, inoculated with C. jejuni mutant and wild type strains, were sacrificed and dissected every 24 h, over a 96 h period. Histological examination revealed thickening of the mesenteric fat at 72 h with increasing evidence at 96 h. Interestingly, no diarrhoea was noted for this group of chickens. In contrast, for the chicks infected with 11168-OΔccaA::cat, stools appeared firm and pellet-like in contrast to typical loose cloacal secretions normally observed for chickens colonised with wild type C. jejuni strains, including 11168-O.
The severity and extent of infection of C. jejuni in systemic organs was assessed by enumeration of bacterial load within each chicken at time points of 24, 48, 72 and 96 h p.i. (Table S4). Despite the changes in gross pathology, there was no statistical difference between the overall bacterial counts in the systemic organs, small or large intestine for animals infected with 11168-O and 11168-OΔccaA::cat isogenic strains at any time point. The average bacterial counts in the liver for chickens infected with 11168-OΔccaA::cat was 2.5 × 102 cfu/gram, when compared to 11168-O which was 1 × 102 cfu/gram. There was no statistical difference in the bacteria present in the for chickens infected with 11168-O or 11168-OΔccaA::cat.
Post-mortem analysis of affected avian tissues
Since gross pathological differences were seen in chickens, the remainder of each tissue collected from the animals during the time course experiment was examined histologically. The H & E stained sections of the tissues were scored blindly according to tissue pathology, Table S5. There was no significant difference in pathology between 11168-OΔccaA::cat, 11168-O and non-infected controls for the lungs, liver, spleen, and small intestines. However, there was a statistical difference in the extent of villus epithelium shedding observed in the large intestine of chickens infected with 11168-OΔccaA::cat which was significantly higher than that for 11168-O or non-infected controls (p < 0.02). Epithelial shedding was characterised by disintegration of the tips of the villi as shown in Fig. 4.
Figure 4.
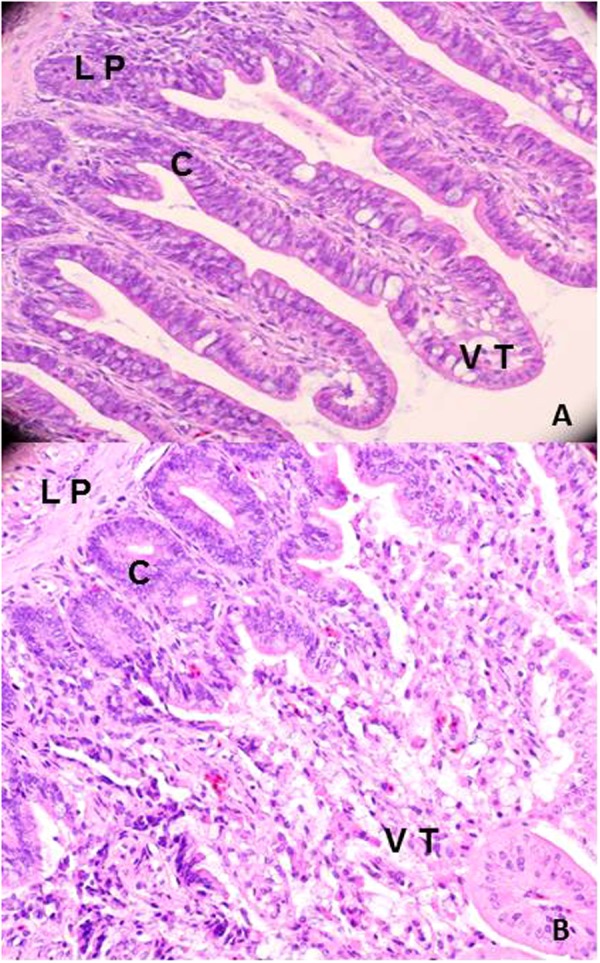
Haematoxylin and eosin (H&E) stained cross sections of the large intestine of chickens infected with 11168-OΔccaA::cat or 11168-O. (A) H&E stained section of large intestine of chicken infected with 11168-O. (B) H&E stained section of large intestine of chicken infected with 11168-OΔccaA::cat. LP: Lamina propria, C: Crypt of villi, VT: Villi tip.
Analysis of adhesion and invasion ability of 11168-O and 11168-OΔccaA::cat mutant in cell culture assays
Since high numbers of C. jejuni were recovered from the chicken lung homogenate, the ability of C. jejuni to adhere and invade a lung cell line was investigated. A549 human lung adenocarcinoma cell line was used, noting that the avian lung differs to the mammalian lung, as it does not have alveoli and some surface glycans on the cells also differ. The results from the in vitro cell culture using A549 cells showed that 11168-O was less adherent (0.22%) when compared to 11168-OΔccaA::cat (1.15%) although the difference was not significant (p = 0.058). There was, however, a statistically significant difference between the invasion ability of 11168-O and 11168-OΔccaA::cat, showing that 11168-OΔccaA::cat was highly invasive, with 2.24% of adhered bacteria invading the cells (p < 0.001), compared to 0.003% for 11168-O wild-type strain, as shown in Fig. 5.
Figure 5.
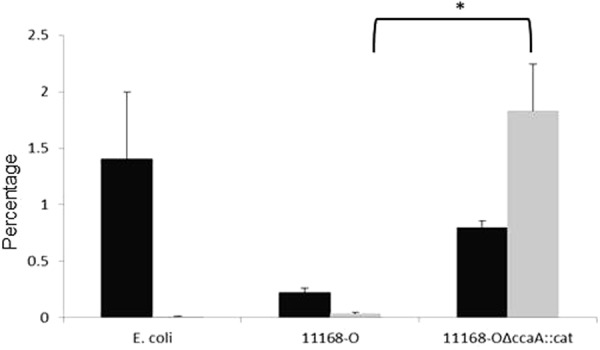
Adherence and Invasion of C. jejuni of in vitro cell culture model using A549 lung epithelial cells. Adherence analysis (black) and invasion (grey) of E. coli DH5α, C. jejuni 11168-0 and 11168-OΔccaA::cat. Results are presented as mean ± SEM adherence or invasion from six to nine wells of a 24-well plate. Two tailed t-test showed no significant increase in adherence of 11168-OΔccaA::cat compared to wild-type, p = 0.058, and a significant increase in invasion of 11168-OΔccaA::cat compared to wild-type (Student’s T-test, p < 0.001).
Since viable C. jejuni 11168-OΔccaA::cat was recovered from mice livers with hepatomegaly, the ability of C. jejuni to adhere to and invade a liver cell line was investigated. The adherence levels of 11168 and 11168-OΔccaA::cat in vitro cell culture of Hep-G2, human hepatocellular liver carcinoma cell line were higher for 11168-OΔccaA::cat at 0.78% when compared to that for 11168-O at 0.1% (p < 0.01, Fig. 6). The isogenic mutant 11168-OΔccaA::cat was also more invasive, with 0.925% of adhered bacteria invading the cells (p < 0.001), compared to 0.0457% for 11168-O.
Figure 6.
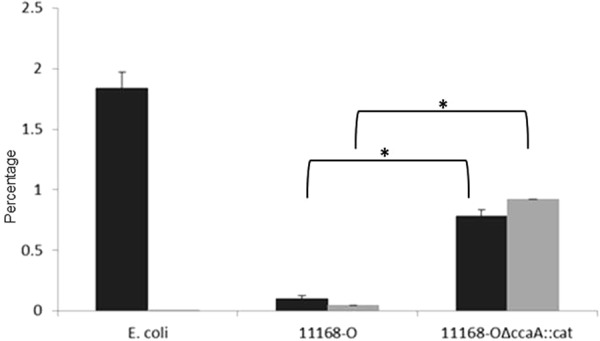
Adherence and Invasion of C. jejuni of in vitro cell culture model using Hep-G2 liver cells. Black) Adherence analysis (black) and invasion (grey) of E. coli DH5α, C. jejuni 11168-0 and 11168-OΔccaA::cat. Results are presented as mean adherence from six to nine wells of a 24-well plate. Standard errors are shown as bars above the mean. Two tailed t-test showed a significant increase in adherence of 11168-OΔccaA::cat compared to wild-type, (p < 0.01) and a significant increase for invasion for the ccaA mutant compared to wild-type (p < 0.001).
Glycan binding profile of C. jejuni isolated from avian hosts
Glycan array analysis of the bacterium isolated from the caecum of chicks was performed in order to determine if the host glycan binding profile has altered with the mutation of the CcaA. Glycan array analysis of whole cell C. jejuni was performed as previously described by Day et al., 2013, whereby glycans (Table S6) were tested for binding by C. jejuni 11168-GS and 11168-O isolated from the caecal content of chicks at day 5p.i. by IMS34. The binding specificities of 11168-O and 11168-OΔccaA::cat were compared and only statistically significant differences (p < 0.05) are described in Table 1. C. jejuni 11168-OΔccaA::cat had reduced ability to bind type II fucosylated structures, including lacto-N-fucopentaose II, Lewisy, blood group H type II trisaccharide and monofucosyllacto-N-hexaose III. The mutant did however display significantly stronger binding to both 3′-sialyllactosamine and 6′-sialyllactosamine compared to the wild type 11168-O.
Table 1.
Glycan binding profile of C. jejuni isolated from avian hosts.
| Compound | Strain | Structure |
|---|---|---|
| β1-6 Galactosyl-N-acetyl glucosamine | WT | ASialo GM1 |
| Lacto-N-neohexaose | WT | Type I |
| Lacto-N-hexaose | WT | Type II |
| GalNAcα1-3Galβ1-4Glc | CcaA | Type I |
| β1-2 N-Acetylglucosamine-mannose | CcaA | N-type glycan |
| α1-3,α1-6-Mannobiose | WT | BiMan |
| α1-3,α1-3α,α1-6-Mannopentaose | CcaA | OligoMan |
| Lacto-N-fucopentaose II | WT | Type II-fucosylated |
| Lewisy | WT | Type II-fucosylated |
| Blood group H type II trisaccharide | WT | Type II-fucosylated |
| Monofucosyllacto-N-hexaose | WT | Type II-fucosylated |
| 3′-Sialyllactosamine | CcaA | Type II-sialylated |
| 6′-Sialyllactosamine | CcaA | Type II-sialylated |
| Colominic acid | WT | Oligo Sialic acid |
| ΔUA-GlucNS-6S | CcaA | Digests of GAGs |
| ΔUA-GalNAc-4S,6S (Delta Di-disE) | CcaA | Digests of GAGs |
WT indicates significantly stronger binding of C. jejuni 11168-O to the specific glycan compared to 11168-OΔccaA::cat. CcaA indicates significantly stronger binding of 11168-OΔccaA::cat to the specific glycan compared to 11168-O.
Immune response in the murine model - Mouse CBA inflammatory cytokine array
In order to analyse the differences in the immune response triggered by the wild type and the aspartate mutant of C. jejuni, inflammatory cytokine levels were examined. A significant change was seen in the levels of IL-12p70 within the C. jejuni 11168-OΔccaA::cat group, with higher levels detected at 48 h and 72 h when compared to the levels at 24 h p.i.(p < 0.05). There appeared to be no other differences in inflammatory cytokine levels between the non-infected control animals and those infected with either the C. jejuni 11168-O or C. jejuni 11168-OΔccaA::cat at 24 h, 48 h or 72 h p.i. (Fig. 7A–C).
Figure 7.
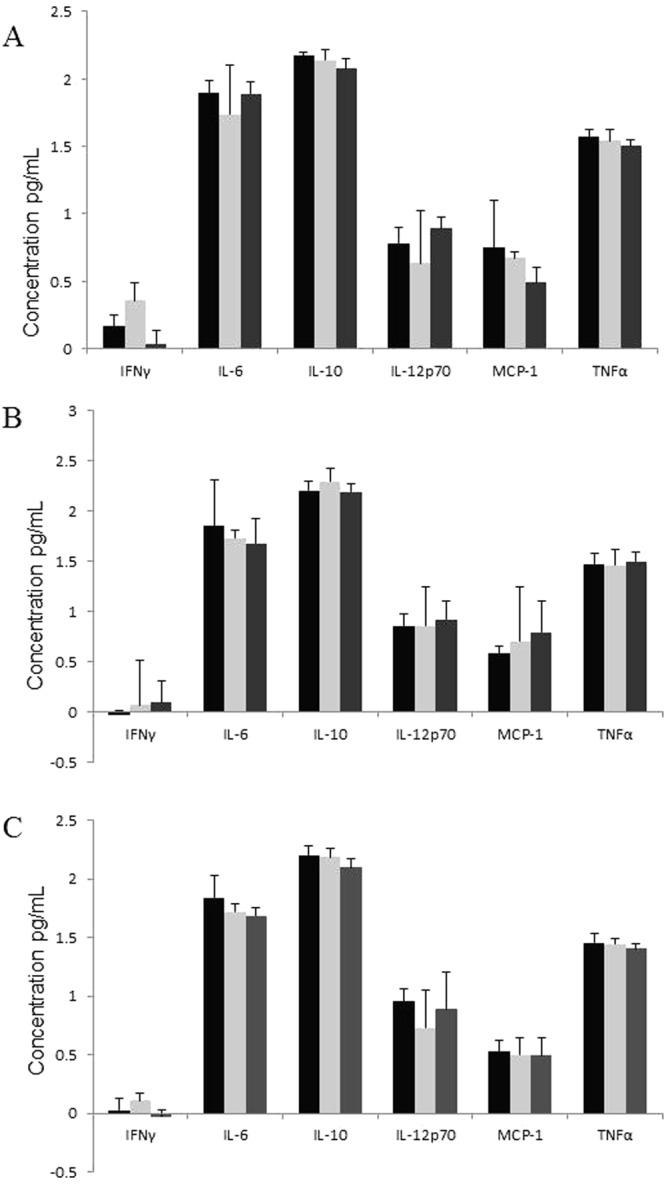
Proinflammatory cytokine concentrations in the small intestine of mice infected with C. jejuni. Proinflammatory cytokine concentrations were determined by CBA array at time points of (A) 24 h, (B) 48 h and (C) 72 h, n = 7. Cytokine levels of non-infected (black), 11168-O wild-type (light grey) and 11168-OΔccaA::cat (mid grey). No statistical differences were noted (ANOVA, p < 0.05).
Quantitation of inflammatory cytokine expression in chicken by qPCR
Similar to the murine host, the inflammatory response of avian host was also examined. RNA extracted from the large intestine of the chickens at 24 h and 96 h time points, cDNA was isolated and qPCR was performed investigating the levels of the inflammatory cytokines IL-8, IL-1β, IL-6, CXCLi1, TNFα and IFNγ. At 24 h p.i., the cytokine levels of IL-8, IL-1β, CXCLi1 and TNFα in chicks infected with both C. jejuni 11168-O and the ccaA mutant showed no significant change in expression. There was however a significant decrease in expression of IFNγ for both WT and mutant infected chicks (Fig. 8A).
Figure 8.
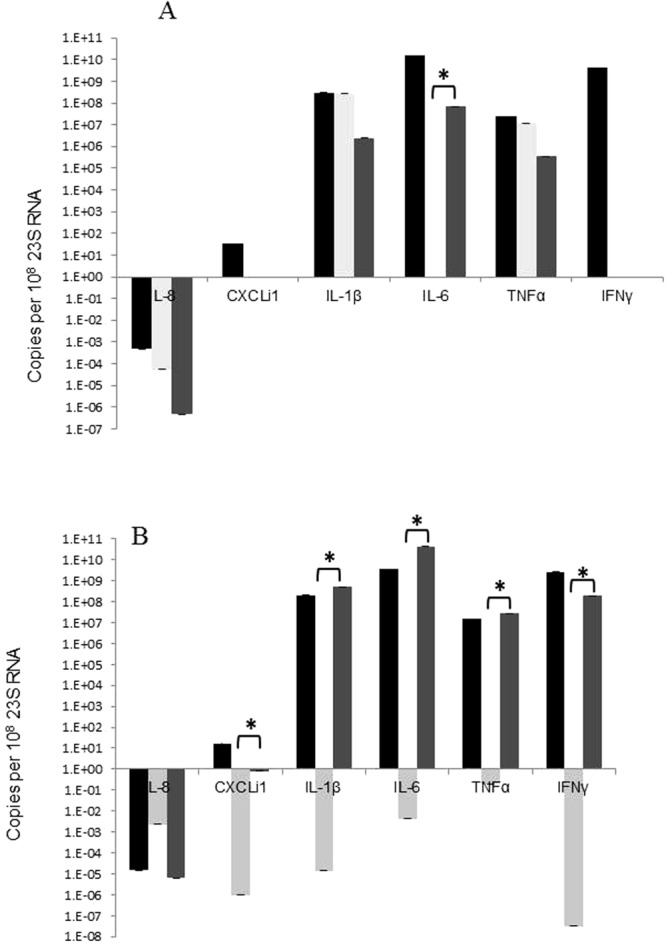
Cytokine levels in chickens determined by qPCR. Relative expression levels of the inflammatory cytokines in chicken large intestines at 24 h (A) or 96 h (B), non-infected control (Black), 11168-O WT (light grey) and 11168- OΔccaA::cat (mid grey), n = 5. Expression is standardised and the scale is shown in log (copies per 108 28S). Statistically significant differences are indicated by *, Student’s T-test (p < 0.01).
At 96 h p.i., the expression of IL-1β, IL-6, TNFα and IFNγ in WT infected chicks was significantly less than the levels determined in the uninfected control group. The expression of these cytokines IL-1β, IL-6, TNFα and IFNγ were all significantly higher for the mutant C. jejuni 11168-OΔccaA::cat group than for the WT control group, and for IL-1β and IFNγ the expression is also statistically greater than the uninfected control levels (Fig. 8B).
At no time point was there any significant change in the expression of IL-8 cytokine or the CXCLi1 chemokine.
Expression of porA, peb1A cdtA, cdtB and cdtC
To ascertain if the “run” phenotype of the aspartate receptor mutant was responsible for the observed pathological changes in the infected animals, the expression of known virulence genes was investigated. The analysis of gene expression profiles of porA, peb1A and cdtABC in C. jejuni cells isolated directly from the chicken caeca and from mouse intestinal tract (by IMS) showed that there was no difference in expression in porA or peb1A for 11168-O when compared to 11168-OΔccaA::cat when grown at 37 °C or 42 °C in vitro (core temperature of mammals and avians respectively). Interestingly, when grown in the chicken host, the expression of porA was not detectable in the wild type strain 11168-O whereas porA was highly expressed in the isogenic mutant 11168-OΔccaA::cat. In the mouse host, however, both were equally expressed. In 11168-OΔccaA::cat, there was no statistical difference in the level of expression of porA and peb1A in vivo when compared to in vitro at 42 °C (Fig. S1).
Expression of cdtA and C, but not B was significantly higher in 11168-OΔccaA::cat when compared to 11168-O when it was culture in vitro at 37 °C (p < 0.05; Fig. S1). The expression of cdtA in both 11168-O and 11168-OΔccaA::cat isolated from chickens was up-regulated. In contrast CdtA and B expression in 11168-O was up-regulated in vivo compared to in vitro culture grown at 42 °C, while cdtB and C were up-regulated in vitro for 11168-OΔccaA::cat, when gene expression levels were compared to those observed for 11168-O at 42 °C (p < 0.05). Most importantly, however, the expression of cdtABC in vivo, in a chicken and mouse host did not show statistical difference in expression (p > 0.05). This indicates that although the overall expressions of the three CDT subunits results showed that gene expression is variable at different temperatures, and expression is regulated in vivo. These genes are unlikely to be involved in the observed gross pathology.
Discussion
Mutational analyses are a classical method for determining gene function and attenuation of virulence, particularly when assessing potential antimicrobial targets. C. jejuni, a commensal in poultry and pathogen in humans, has also been a subject to many such analyses.
Previous avian colonisation studies using various C. jejuni chemosensory pathway mutants, did not report any pathological changes13,32,33. This study revealed that despite attenuation of colonisation in both the murine and avian models, gross pathological changes were observed in both mice and chickens infected with the C. jejuni aspartate chemoreceptor mutant ccaA, including connective tissue and fat roping in chickens and hepatomegaly in mice. The ability of the ccaA mutant to produce pathological changes in animal hosts is a novel and unusual observation.
Specifically, the appearance of intestinal roping was noted at 72 h p.i. along with the disintegration of the tips of the villi in the large intestine of chickens infected with the ccaA mutant, which persisted throughout the entire 96 hour examination period and correlated to the chicken stools appearing unusually firm and pellet-like. This too was unexpected as it has been well established that in humans C. jejuni infection is characterised by diarrhoea whereas in the chickens, C. jejuni colonisation is asymptomatic.
The hardened appearance of the faeces in chickens infected with the ccaA mutant may be due to dis-regulation of CDT toxin production, causing the faeces to become drier and more solid, compared to normal faeces. In humans, the cytolethal distending toxin, CDT, is thought to destroy the mucosal epithelium, cause secretory diarrhoea and necrosis of the colonic epithelium35. Although in chickens, cdt toxin genes are expressed, no symptoms of diarrhoea are usually observed36. The expression of the CDT toxin genes, however, may contribute toward the usually observed appearance of semi-solid chicken faeces. This change in the consistency of the cloacal secretions may contribute to the damage to the villus epithelium in the large intestine as was seen in the histological sections. The cdtA gene expression by ccaA isogenic mutant cells isolated from chickens was up-regulated as was the expression of cdtB in the mutant cells isolated from the mouse. This was in agreement with a previous study showing that in chickens the cdtA/C subunits could be up-regulated34. Therefore, while it may be a contributing pathogenicity factor, it is possible to speculate that they are unlikely to account for the observed pathology.
It is critical to note that ccaA mutant exhibits a “run” bias in its chemotaxis phenotype. It is possible to hypothesise that the up-regulation of some genes in the ccaA isogenic mutant may occur to compensate for changes in chemotactic run-bias phenotype of the ccaA mutant. This lack of ability to tumble may indeed be the main cause of attenuation, as well as the reason for increase in invasiveness in vitro31 and bacterial presence in systemic organs.
In vitro cell culture assays using human cell cultures; Caco-231, HepG2 and A549 cells, have shown that the ccaA mutant is more adherent and highly invasive compared to the wild-type. Although A549 is a human lung cell line, it contains α2,6-sialyl structures similar to those found in the chicken gastrointestinal tract37,38. The hyperinvasive phenotype of the ccaA mutant in A549 cells indicated that this might be similar to the events in the avian gastrointestinal tract.
In addition, the ccaA isogenic mutant was found to have significantly reduced ability to bind type II fucosylated structures, ubiquitous in all eukaryotic tissue types. A significant decrease in binding ability to structures including lacto-N-fucopentaose II, Lewisy, blood group H type II trisaccharide and monofucosyllacto-N-hexaose III was also observed. In contrast, ccaA isogenic mutant had significantly stronger binding to both 3′-sialyllactosamine and 6′-sialyllactosamine. The change in this binding ability, together with the “run” phenotype, may provide some insight into the presence of the mutant in the lung of chickens, as terminal αNeuAc2-3Gal structures are found predominantly in the lung of chickens39. This was evident by increased infection of A549 cells by the ccaA mutant, which could be due to increased expression of this glycan by the A549 cells.
These changes in glycan binding specificity of the ccaA mutant may contribute toward the pathology observed in model animals. The mutant bacteria may be able not only to move unidirectionally, but also be able to bind glycan structures differently in the avian gut, helping the bacteria to transverse the epithelium. In addition, previous studies have shown that binding to 3′- and 6′-sialyllactosamine structures by 11168-O wild-type cells, was significantly stronger at lower temperatures, 25 °C in vitro40. The higher core temperature of the chicken, 42 °C, the temperature at which the wild-type and mutant cells were tested using glycan arrays in this study, may account for the lower level of sialylated glycan binding for the wild-type compared to the ccaA mutant cells.
This study is the first report of gross pathological differences due to infection with an isogenic mutant, whereby mesenteric tissue abnormalities were observed within 72 hours p.i. In humans, a characteristic feature of Crohn’s disease is mesenteric adipose tissue hypertrophy, or fat wrapping (Reviewed in41). Mesenteric adipose tissue is an endocrine system and is able to regulated metabolic function and inflammation41. White adipose tissue is known to synthesise PPAR-γ and TNF-α, and release cytokines including adiponectin and IL-642,43, hence may play a role in inflammatory response in Crohn’s disease. In chickens, IL-6 mRNA expression increases ten-fold at day 4 of colonisation28, and this may be more evident in chickens infected with the ccaA isogenic mutant, as in the mutant, an increase in mesenteric fat may also stimulate release of this cytokine. Although it appears that an inflammatory response is occurring in the chicken, it is unclear as the actual cause of the mesenteric adipose tissue hypertrophy. It is unusual, as in many diseases (including Crohn’s disease) as this manifests over many months, not days. Infectious colitis due to bacterial infection, including Shigella, Salmonella, Yersinia, Campylobacter, Staphylococcus and Chlamydia, have presented symptoms in humans including inflammatory changes in mesenteric fat44. Thus, this suggests that the increase in mesenteric fat may be a clinical sign of this Campylobacter infection.
Gross pathological changes were also observed in the murine model, when mice were infected with the ccaA mutant. The liver weight increased by at least 23% within 48 hours of infection in every mouse infected with the ccaA mutant. In addition, this persisted until at least day 6, even though there was no mutant bacterium present in the gastrointestinal tract at this time point. Similar to the findings of Vučković et al.45 hepatomegaly was observed in BALB/c mice, however there was no spleen enlargement, no yellowish nodes on the liver and no local tissue necrosis of the liver was observed in enlarged livers45. Unlike the study by Vučković45, where mice were infected with C. jejuni via intraperitoneal injection, in our study the mice were infected with C. jejuni by oral inoculation and the method of inoculation may influence the disease pathology observed in the mouse.
It is well established that one of the main roles of the liver is to remove bacteria from the blood stream and to resist infections by producing immune factors46. During inflammatory response to infection, the primary cellular reaction is the stimulation of monocytes and tissue macrophages46. Histological analysis revialed no statistical difference in cellular response in the livers of mice infected with the ccaA mutant compared to uninfected mice. Analyses of cytokine levels in mock-infected, 11168-O and ccaA mutant infected mice also showed no statistical difference in the pro-inflammatory cytokines at 24, 48 or 72 h time points. Since the mediators assessed did not change it is therefore unlikely that they contributed to the effect of gross pathological changes that were observed in the mouse.
Interestingly, the mice infected with the ccaA mutant showed enlarged mesenteric lymph nodes, which is a common feature of Crohn’s disease or ulcerative colitis47. However both groups of mice infected with C. jejuni had hyperplastic Peyer’s patches. The symptom of hyperplastic Peyer’s patches, where they project into the gut lumen as submucosal elevations, along with the enlargement of the liver, which is observed in the mice infected with the ccaA mutant, are typical symptoms of Typhoid or enteric fever48,49. Campylobacter is one of the non-salmonella organisms causes of infection which is clinically indistinguishable from classic enteric fever caused by S. typhi, S. paratyphi and S. choleraesuis50,51. There is little data on the pathological features of the associated enteritis, but similarities may exist between Campylobacter and Salmonella spp, as C. jejuni may access the submucosa via uptake by M cells, however it is unknown if C. jejuni can translocate across the cells in the colon52,53.
This study highlights the importance of an alteration of the phenotype due to irreversibly blocking or altering key colonisation factors, and the reason that they must be fully investigated. These factors are often potential targets for the development of new antimicrobial agents. Although there is a reduction in colonisation potential using both avian and mammalian colonisation models, one must ensure that there are no gross pathological changes occurring in the host due to this infection.
Methods
Bacterial strains and plasmids
The C. jejuni NCTC11168-O original strain was kindly donated by D.G Newell, VLA, London and its isogenic mutant strain 11168-OΔccaA::cat31 were grown on Columbia agar supplemented with 5% defibrinated horse blood (HBA) with Skirrow antibiotic supplement (Oxoid) or Muellar Hinton agar (MHA) (Oxoid) under microaerophilic conditions (5% O2, 15% CO2, 80% N2; BOC gases) for 48 h at 37 °C or 42 °C where appropriate.
Motility assays
Motility assays were performed as described by King et al.54. The motility phenotypes of C. jejuni 11168-O and 11168-OΔccaA::cat were ascertained by calculating the spatial displacement of single cells. Singular cells were tracked via time lapse photography using ImageJ, over a one second time frame.
Ethics Statement
Animal experiments were carried out in strict accordance with the Griffith University Animal Ethics Committee guidelines and assigned approval numbers MSC/04/08/AEC, BDD/01/07 and GLY/02/15/AEC. All procedures involving animals were reviewed and approved by National Health and Medical Research Council Australian code of practice for the care and use of animals for scientific purposes, 7th edition 2004.
Avian and murine models
Unvaccinated Ross breed chickens (Bartters, Rochdale Qld) at one day after hatching were placed into groups of ten, pre-inoculation faecal samples were taken from the cloaca of the chickens and cultured. Chickens were housed in clean barrier cages at 32 °C and supplied sterilised food and water ad libitum for the entire experimental period. 129 × 1/SvJ background male mice (Animal Resource Centre, Western Australia) aged between 6–8 weeks, were housed in groups of 6–10 as specified, supplied ad libitum with food and water.
The animals were inoculated by orally challenging with 30 μL of Brucella broth containing approximately 1 × 108 C. jejuni cells, as previously published31. Post-inoculation cloacal or faecal samples were taken daily and cultured. 24–96 h post-inoculation, as specified, the animals were euthanized by cervical dislocation, and the gastrointestinal tissues including small and large intestine as well as the systemic organs, lungs, liver and spleen, were removed aseptically. Each organ was weighed and apportioned. An appropriate portion was placed in a 5 mL tube with 2 mL of sterile Brucella broth, homogenised and the bacterial load enumerated by viable count. Tissue samples for histology were prepared using a portion of organ (systemic) or half of the longitudinally cut organ (digestive), fixed immediately in formal saline solution for at least 24 h. The samples were prepared for histology as described in Stahl et al.55.
Histopathological scoring of organ tissue
The H & E stained sections were randomly coded for blind scoring, and the pathology of each organ was microscopically examined at 600 x magnification. The lungs, liver and spleen were examined for abnormalities including increase in leukocytes, tissue damage or changes in cellular arrangement. The digestive organs were examined in sections; small intestine, caecum, and proximal, mid and distal colon, for abnormal crypt architecture, crypt length, damage, goblet cell loss or hyperplasia, inflammatory cell infiltrate or the presence of neutrophils in the lamina propria. After scoring of each organ was performed, the samples were decoded and the results analysed.
Direct in vivo Isolation of C. jejuni using M-280 Dyna-Beads
Immunomagnetic separation (IMS) of C. jejuni from mouse intestinal content or chicken caecal content was performed as previously described by King et al.34.
Glycan Array analysis
IMS isolated cells were labelled with CFDA-SE and glycan binding profile was analysed as described in Day et al.56. Full list of glycan structures see Table S6.
Adherence and Invasion Assays
The assays were performed according to22, with the modifications as described in31. The cell lines used in this study were A549 Human lung adenocarcinoma epithelial cell line (ATCC); Hep-G2 Human hepatocellular liver carcinoma cell line (ATCC).
Cytometric bead array for quantification of inflammatory cytokines in mice
Mice were orally inoculated with Brucella broth, C. jejuni 11168-O or C. jejuni 11168-OΔccaA::cat at 5 × 108 CFU. After 24 hours, submandibular punctures were performed to remove approximately 100 µL of blood. Blood was allowed to coagulate for 15 mins, prior to centrifugation to separate serum. The BD CBA inflammatory mouse kit was used according to manufacturer’s instructions. After the beads were washed, data was acquired using a Beckman Coulter Cyan Flow cytometer, using the 488 nm laser with settings for FITC, APC and PE. Data was analysed using FlowJo software. Concentrations of cytokines were extrapolated from standard curves using Microsoft Excel.
qPCR primers and primer design
C. jejuni primers were designed based on the published nucleotide sequence of C. jejuni 1116857. All primers used in this study are listed in Table S7, and source noted. Bacterial 23 s RNA primers or chicken 28 s RNA primers were used for internal controls. All oligonucleotide primers were synthesised by Invitrogen. RNA extraction, cDNA preparation and qPCR were performed as previously described30. A PCR standard curve was generated for each primer set by performing five ten-fold serial dilutions. Quantity values (copies) for gene expression was generated by comparison of the fluorescence generated by each sample with a standard curve of known quantities for each PCR product (Table S8).
Quantification of inflammatory cytokine expression in chickens
Total RNA was extracted from the chicken small intestines from chicks inoculated with Brucella broth (negative), C. jejuni 11168-O or C. jejuni 11168-OΔccaA::cat. The extracted RNA was used as template for the reverse transcription reaction. qPCR was performed using gene specific sense and anti-sense primers for specific chicken cytokines. All qPCR reactions were carried out using the same thermal profile conditions, 94 °C for 5 minutes, then 45 cycles of 94 °C for 30 seconds, 48 °C for 30 seconds then 72 °C for 1 minute, 30 seconds with fluorescence measured during the 72 °C extension phase. Melt curves for each amplification product were measured 80 times over the incremental increases in temperature. Products were visualised by agarose gel electrophoresis.
Statistical analysis
The mean of the groups for bacteria load in each organ (n ≥ 5) were individually compared to that of control groups at the same time point. Significance was determined by un-paired t-tests with an alpha of 0.05. Analysis of variance (ANOVA) was performed to determine significance in relative gene expression in conjunction with an un-paired t-test. Histopathological scores were analysed by chi-square, non-parametric tests.
Electronic supplementary material
Author Contributions
V.K., L.H.T. and C.J.D. conceived the study, all authors contributed to the design of experiments and analysis of data, L.H.T., C.J.D., G.T., E.A.S., Z.K., L.I.C.G. and A.M.B. performed the experiments, L.H.T. and V.K. wrote the paper, all authors reviewed and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30604-5.
References
- 1.WHO. Annual report - Consultation on Campylobacter. (World Health Organization, Copenhagen, Denmark, 2000).
- 2.Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuPont HL. The growing threat of foodborne bacterial enteropathogens of animal origin. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;45:1353–1361. doi: 10.1086/522662. [DOI] [PubMed] [Google Scholar]
- 4.Ketley JM. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143(Pt 1):5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 5.Walker RI, et al. Pathophysiology of Campylobacter enteritis. Microbiological reviews. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassenaar TM, Blaser MJ. Pathophysiology of Campylobacter jejuni infections of humans. Microbes and Infection. 1999;1:1023–1033. doi: 10.1016/S1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]
- 7.Hermans D, et al. Colonization factors of Campylobacter jejuni in the chicken gut. Veterinary Research. 2011;42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young KT, Davis LM, DiRita VJ. Campylobacter jejuni: molecular biology and pathogensis. Nature Reviews of Microbiology. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 9.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. International Journal of Medical Microbiology. 2002;291:605–616. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 10.Wallis MR. The pathogenesis of Campylobacter jejuni. Brititsh. Journal of Biomedical Science. 1994;51:57–64. [PubMed] [Google Scholar]
- 11.Smith CK, et al. Campylobacter jejuni-induced cytokine responses in avian cells. Infection and Immunity. 2005;73:2094–2100. doi: 10.1128/IAI.73.4.2094-2100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enocksson A, Lundberg J, Weitzberg E, Norrby-Teglund A, Svenungsson B. Rectal nitric oxide gas and stool cytokine levels during the course of infectious gastroenteritis. Clinical and diagnostic laboratory immunology. 2004;11:250–254. doi: 10.1128/CDLI.11.2.250-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrixson DR, DiRita VJ. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Molecular Microbiology. 2004;52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 14.Ringoir D, Korolik V. Colonisation phenotype and colonisation potential differences in Campylobacter jejuni strains in chickens before and after passage in vivo. Veterinary microbiology. 2003;92:225–235. doi: 10.1016/S0378-1135(02)00378-4. [DOI] [PubMed] [Google Scholar]
- 15.Jones MA, et al. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infection and Immunity. 2004;72:3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baqar S, et al. Murine intranasal challenge model for the study of Campylobacter pathogensis and immunity. Infection and immunity. 1996;64:4933–4939. doi: 10.1128/iai.64.12.4933-4939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaser MJ, Duncan DJ, Warren GH, Wang W. Experimental Campylobacter jejuni infection of adult mice. Infection and immunity. 1983;39:908–916. doi: 10.1128/iai.39.2.908-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAuley J, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. Journal of Clinical Investigation. 2007;117:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl M, et al. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS pathogens. 2014;10:e1004264. doi: 10.1371/journal.ppat.1004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sham HP, et al. SIGIRR, a negative regulator of TLR/IL-1R signalling promotes Microbiota dependent resistance to colonization by enteric bacterial pathogens. PLoS pathogens. 2013;9:e1003539. doi: 10.1371/journal.ppat.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik A, Sharma D, St Charles J, Dybas LA, Mansfield LS. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunology. 2014;7:802–817. doi: 10.1038/mi.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao RJ, Burr DH, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Molecular Microbiology. 1997;23:1021–1031. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]
- 23.Fox JG, et al. Gastroenteritis in NF-kB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistant colonization with both strains. Infection and immunity. 2004;72:1116–1125. doi: 10.1128/IAI.72.2.1116-1125.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Palacios GM, Escamilla E, Torres N. Experimental Campylobacter diarrhea in chickens. Infection and immunity. 1981;34:250–255. doi: 10.1128/iai.34.1.250-255.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne CM, Clyne M, Bourke B. Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology. 2007;153:561–569. doi: 10.1099/mic.0.2006/000711-0. [DOI] [PubMed] [Google Scholar]
- 26.Young C, Ziprin R, Mume M, Stanker L. Dose response and organ invasion of day-of-hatch Leghorn chicks by different isolates of Campylobacter jejuni. Avian Disease. 1999;43:763–767. doi: 10.2307/1592745. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey S, et al. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio. 2014;5:e01364–01314. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CK, et al. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS immunology and medical microbiology. 2008;54:114–121. doi: 10.1111/j.1574-695X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 29.Woodall CA, et al. Campylobacter jejuni Gene Expression in the Chick Cecum: Evidence for Adaptation to a Low-Oxygen Environment. Infect. Immun. 2005;73:5278–5285. doi: 10.1128/IAI.73.8.5278-5285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day, C. J. et al. Variation of chemosensory receptor content of Campylobacter jejuni strains and modulation of receptor gene expression under different in vivo and in vitro growth conditions. BMC Microbiology12 (2012). [DOI] [PMC free article] [PubMed]
- 31.Hartley-Tassell LE, et al. Identification and characterization of the aspartate chemosensory receptor of Campylobacter jejuni. Molecular Microbiology. 2010;75:710–730. doi: 10.1111/j.1365-2958.2009.07010.x. [DOI] [PubMed] [Google Scholar]
- 32.Rahman H, et al. Characterisation of a multi-ligand binding chemoreceptor CcmL (Tlp3) of Campylobacter jejuni. PLoS pathogens. 2014;10:e1003822. doi: 10.1371/journal.ppat.1003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day CJ, et al. A direct-sensing galactose chemoreceptor recently evolved in invasive strains of Campylobacter jejuni. Nature communications. 2016;7:13206. doi: 10.1038/ncomms13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King RM, et al. Carbohydrate binding and gene expression by in vitro and in vivo propagated Campylobacter jejuni after immunomagnetic separation. Journal of basic microbiology. 2013;53:240–250. doi: 10.1002/jobm.201100466. [DOI] [PubMed] [Google Scholar]
- 35.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect. Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AbuOun M, et al. Cytolethal Distending Toxin (CDT)-Negative Campylobacter jejuni Strains and Anti-CDT Neutralizing Antibodies Are Induced during Human Infection but Not during Colonization in Chickens. Infection and immunity. 2005;73:3053–3062. doi: 10.1128/IAI.73.5.3053-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinya, K. et al. Avian flu: influenza virus receptors in the human airway. Nature440 (2006). [DOI] [PubMed]
- 38.Stevens J, et al. Glycan microarray analysis of the hemagglutinin from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Marinina V, et al. The effect of losing Glycosylation sites near the receptor-binding regions on the receptor phenotype of the Human Influenza Virus H1N1. Molecular Biology. 2003;37:468–472. doi: 10.1023/A:1024207931650. [DOI] [PubMed] [Google Scholar]
- 40.Day, C. et al. Differential carbohydrate recognition by Campylobacter jejuni strain 11168: Influences of temperature and growth conditions. PLos One (2009). [DOI] [PMC free article] [PubMed]
- 41.Maconi G, et al. Prevalence and clinical significance of sonographic evidence of mesenteric fat alterations in Crohn’s Disease. Inflamm Bowel Dis. 2008;14:1555–1561. doi: 10.1002/ibd.20515. [DOI] [PubMed] [Google Scholar]
- 42.Desreumaux P, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/S0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 43.Karagiannides I, et al. Induction of colitis causes inflammatory responses in fat deposits: Evidence for substance P pathways in human mesenteric preadipocytes. PNAS. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horton KM, Corl FM, Fishman EK. CT Evaluation of the Colon: Inflammatory Disease. Radiographics. 2000;20:399–418. doi: 10.1148/radiographics.20.2.g00mc15399. [DOI] [PubMed] [Google Scholar]
- 45.Vuckovic D, Abram M, Doric M. Primary Campylobacter jejuni infection in different mice strains. Microbial pathogenesis. 1998;24:263–268. doi: 10.1006/mpat.1997.0194. [DOI] [PubMed] [Google Scholar]
- 46.Monshouwer M, Hoebe KHN. Hepatic (dys-)function during inflammation. Toxicology in Vitro. 2003;17:681–686. doi: 10.1016/S0887-2333(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 47.Cook MG. The size and histological appearances of mesenteric lymph nodes in Crohn’s disease. Gut. 1972;13:970–972. doi: 10.1136/gut.13.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolf, N. Pathology: Basic and Systemic. (Elsevier Health Sciences, 1998).
- 49.Isaacson, M. & Hale, M. In Tropical Pathology Vol. 8 (eds Dres, H. Doerr, C. & Ashworth, G.) 157–175 (Springer, 1995).
- 50.Pearson, R. & Guerranti, R. In Principles and practice of infectious diseases (eds Mandell, G. L. Bennett, J. E. & Dolin, R.) (Churchill Livingstone, 2000).
- 51.Suwansrinon K, Wilde H, Sitprija V, Hanvesakul R. Enteric fever-like illness caused by infection with Citrobacter amalonaticus. J Med Assoc Thai. 2005;88:837–840. [PubMed] [Google Scholar]
- 52.Walker RI, Schmauder-Chock E, Parker J, Burr DH. Selective association and transport of Campylobacter jejuni through M cells of rabbit Peyer’s patches. Canadian Journal of Microbiology. 1988;34:1142–1147. doi: 10.1139/m88-201. [DOI] [PubMed] [Google Scholar]
- 53.Konkel ME, Monteville M, Rivera-Amill V, Joens L. The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr Issues Intest. Microbiol. 2001;2:55–71. [PubMed] [Google Scholar]
- 54.King RM, Korolik V. Characterization of Ligand-Receptor Interactions: Chemotaxis, Biofilm, Cell Culture Assays, and Animal Model Methodologies. Methods in molecular biology. 2017;1512:149–161. doi: 10.1007/978-1-4939-6536-6_13. [DOI] [PubMed] [Google Scholar]
- 55.Stahl, M., Graef, F. A. & Vallance, B. A. Mouse Models for Campylobacter jejuni Colonization and Infection. Campylobacter jejuni: Methods and Protocols, 171–188 (2017). [DOI] [PubMed]
- 56.Day CJ, Tram G, Hartley-Tassell LE, Tiralongo J, Korolik V. Assessment of glycan interactions of clinical and avian isolates of Campylobacter jejuni. BMC Microbiol. 2013;13:228. doi: 10.1186/1471-2180-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parkhill J, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


