Abstract
Naps in early childhood support declarative memory consolidation. However, emotional memories are unique in the neural basis of encoding as well as the sleep physiology underlying consolidation. Specifically, while consolidation of declarative memories has been associated with slow wave sleep, a prevailing theory suggests that REM sleep is necessary for consolidation of memories with emotional valence. Thus, we presented children (34–64 months) with faces paired with mean or nice descriptions. There were no significant main effects of emotional valence on recognition memory. Change in memory accuracy also did not differ when probed after a nap compared to the change in memory accuracy after an interval awake. However, when memory was probed again following overnight sleep, the change in memory accuracy was greater if the child napped the previous day. Greater nap slow wave activity was associated with greater memory decay during the nap. Yet nap slow wave activity also predicted greater overnight improvement in memory. These results suggest that sleep bouts can interact to benefit memory in early childhood.
Introduction
Early childhood is a critical time for emotional development as language skills and the ability to regulate thoughts and behaviors emerge1. To capitalize on this developmental window, socio-emotional curriculum is now recommended in early education settings. Socio-emotional curriculum has been shown to increase prosocial behavior and improve long-term academic success. Thus, enhancing emotional learning at this young age has wide-reaching and long-lasting impact.
In adults, emotion processing and emotional memory are enhanced over sleep. Anecdotal evidence from caregivers and experimental evidence in toddlers suggests that naps support emotional regulation in early childhood. For example, children exhibit more frustration when faced with an unsolvable puzzle when nap deprived compared to rested. Moreover, emotional attention biases are reduced following a nap compared to an equal interval awake.
Yet, it is unknown whether emotionally valenced memories are also consolidated over naps in early development. Declarative memories have been shown to be consolidated over sleep in early childhood, thus naps may likewise benefit emotional memories. However, the REM Sleep Hypothesis of Emotional Processing holds that emotional memory consolidation requires REM sleep2 consistent with studies in adults3–5. Naps in early childhood lack this sleep stage6. Thus, naps may not be sufficient for emotional memory consolidation. If such were the case, REM in subsequent overnight sleep may support delayed emotional memory processing.
In spite of overwhelming support of REM’s role in emotional memory consolidation, recent studies suggest slow wave sleep (SWS) – specifically slow wave activity (SWA; spectral power in the delta band) in SWS – may also play a role. For example, selective memory for negative aspects of complex scenes is positively correlated with both SWS and SWA during a nap, although correlated with nocturnal REM sleep7. Likewise, negative memories selectively cued during SWS are better remembered than uncued items8. Such effects have been specifically associated with noradrenergic activity in SWS8. As such, emotional memories may benefit from SWS presence in the naps, which accounts for 42% of naps at this age.
To address whether naps contribute to memory consolidation for items with emotional valence in early childhood, we examined performance on an emotional memory task following a nap and an equivalent interval spent awake in young children using a within-subjects design. We also considered that REM in overnight sleep may provide a delayed benefit and, thus, assessed memory again the following day (Fig. 1). To assess memory for emotional information, we adopted a task used by Kinzler and Shutts9. This task was selected for three reasons: (1) it is age appropriate (as opposed to IAPS stimuli); (2) an emotional distinction is evident in behavior at this age (as evidenced by greater memory for negative compared to positive items at encoding9); and (3) enough items could be encoded to probe at three recall stages: baseline (immediately after encoding), following the nap/wake interval, and again the following day.
Figure 1.
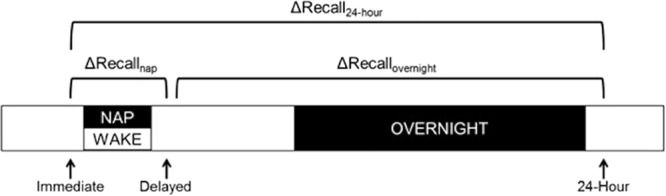
Encoding was followed by an immediate recognition memory probe. Recognition memory was probed again following a nap/wake interval (Delayed) and again the following morning (24-Hour).
Polysomnography (PSG) was recorded in both nap and overnight sleep in a subset of participants to clarify the role of REM and SWS. We hypothesized that both positive and negative items would be remembered better following an interval with a nap relative to an equivalent interval spent awake. This hypothesis is based on: (1) children are observed to be both grumpy and giddy when nap-deprived suggesting naps may contribute to processing of both positive and negative emotions; and (2) naps reduce both positive and negative emotional attention biases in children at this age10. However, an alternative hypothesis is that negative memories would not benefit from the nap while positive memories would be greater following this mid-day sleep bout. Supporting this alternative: (1) REM sleep has been associated with negative memory consolidation4,5 and may not be involved in positive memory processing. Thus, the absence of REM from the nap would leave negative memories unchanged while positive memories, like neutral memories6, may benefit from non-REM sleep spindles; (2) a preliminary study in young adults suggested that sleep deprivation impairs positive and not negative memories11. As such, positive memories may be better remembered following a nap compared to wake (i.e., a nap benefit) while negative memories may be impervious to the effects of nap deprivation (the wake condition), particularly for children who nap habitually. Although9 neutral items were not included, consistent with Kinzler and Shutts9, this paradigm was sufficient to address these alternatives.
Results
Of the 64 children (43 female; M = 51.55 months, SD = 7.23, range = 34–64 months) tested, 1 was unable to be wake promoted, 3 were unable to be nap promoted, and 3 were absent on one testing day, and were therefore excluded from analyses. In addition, as we would not expect sleep to yield performance benefits if the nap was of insufficient length, we further excluded children (n = 8) whose nap length was less than one standard deviation (SD = 22.99 min) below the mean (M = 71.92 min). This left a remaining sample of 49 children (30 female; n = 29 classroom, n = 20 laboratory; M = 51.55 months, SD = 7.16, range = 34–64.30 months) in the following analyses. Note that 2 children were absent for the 24-hr recognition phase but are included in all other analyses. Children tested in the laboratory compared to those tested in the classroom did not differ in age (t(47) = −0.688, p = 0.495), nap length (t(47) = 0.370, p = 0.713), or accuracy across any of the recognition phases (all p’s greater than 0.200); as such, behavioral data was collapsed to increase power.
Children’s self-report of both sleepiness and mood in session 1 (following immediate recognition) did not differ across conditions (p’s > 0.209). Experimenter ratings of mood also did not differ across conditions. However, experimenters did rate children as significantly more sleepy in the nap condition after both immediate recognition (t(18) = 2.348, p = 0.031; 95% CI: [0.039, 0.698]) and delayed recognition (t(19) = 3.943, p = 0.001; 95% CI: [0.281, 0.919]). It should be noted that average sleepiness was still rated as low in the nap condition for both the immediate (M = 1.45, SD = 0.60) and delayed recognition (M = 2.1, SD = 0.71) on the 5-item scale. Further, sleepiness levels at immediate recall (r = −0.072, p = 0.764) and delayed recognition (r = 0.286, p = 0.222) were not associated with memory performance across the nap bout, nor across the wake bout (immediate: r = 0.005, p = 0.983; delayed: r = 0.025, p = 0.915). Similarly, sleepiness at immediate recall was not associated with performance change across the 24-hr period in either the nap (r = −0.026, p = 0.913) or the wake condition (r = 0.047, p = 0.847).
Memory Performance
Contrary to the findings of Kinzler and Shutts9, across conditions there were no significant differences between immediate recognition accuracy of negative and positive stimuli (F(1,48) = 0.029, p = 0.865). There was, however, a main effect of condition on immediate recognition accuracy (F(1,48) = 5.079, p = 0.029, ηp2 = 0.096) such that the baseline memory performance was greater prior to the wake condition (M = 72.4%, SD = 19.5%) compared to the nap condition (M = 63.7%, SD = 24.6%). For this reason, we focus on change in performance (as opposed to comparison of post-nap/post-wake performance; for absolute recognition memory, see Table 1).
Table 1.
Absolute memory recognition accuracy scores for each session.
| Session | Condition | Emotion | n | Mean | SD |
|---|---|---|---|---|---|
| Immediate Recall | Nap | Negative | 49 | 62.92 | 31.525 |
| Wake | Negative | 49 | 72.51 | 24.714 | |
| Nap | Positive | 49 | 64.39 | 29.132 | |
| Wake | Positive | 49 | 72.20 | 27.331 | |
| Delayed Recall | Nap | Negative | 48 | 60.04 | 32.673 |
| Wake | Negative | 49 | 67.71 | 28.031 | |
| Nap | Positive | 49 | 66.43 | 29.404 | |
| Wake | Positive | 49 | 67.10 | 26.747 | |
| 24-Hr Recall | Nap | Negative | 47 | 69.94 | 31.439 |
| Wake | Negative | 46 | 60.17 | 36.823 | |
| Nap | Positive | 47 | 63.51 | 37.420 | |
| Wake | Positive | 46 | 59.09 | 34.979 |
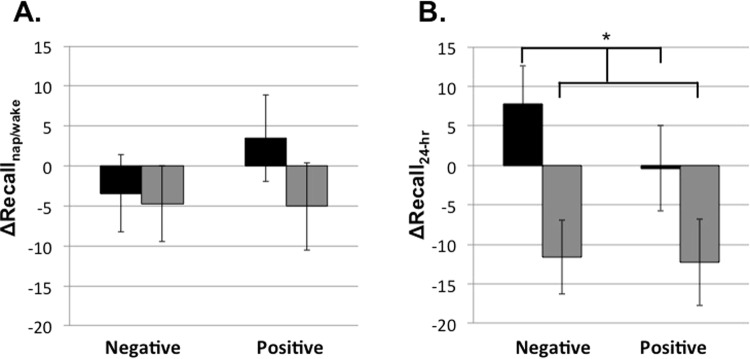
Counter to our prediction, performance changes over the nap did not differ from those over wake (ΔRecallnap/wake; F(1,47) = 0.678, p = 0.414; Fig. 2A). In addition, there was no main effect of emotion (F(1,47) = 0.285, p = 0.596), nor a significant interaction between emotion and condition (F(1,47) = 0.733, p = 0.396). However, when change in memory accuracy was assessed 24-hrs after learning (ΔRecall24-hr), memory recall was greater in the nap compared to the wake condition (main effect condition: F(1,43) = 4.523, p = 0.039; ηp2 = 0.095; Fig. 2B). The nap led to protected memory (one-sample t-test; t(46) = 0.758, p = 0.452; 95% CI: [−6.180, 13.650]), whereas wake resulted in significant forgetting (one-sample t-test; t(45) = −2.819, p = 0.007; 95% CI: [−20.590, −3.430]). Positive and negative stimuli were similarly benefited by the nap (main effect emotion: F(1,43) = 0.234, p = 0.631; emotion x condition: F(1,43) = 0.286, p = 0.595).
Figure 2.
Nap-dependent benefits on emotional memory (change in % correct across the delay) were not observed initially (A) when comparing the nap condition (black bar) to the wake condition (grey bar). Effects were only observed following overnight sleep (B), reflecting a delayed benefit of the nap. Error bars represent SEM.
Given that changes in memory were observed across the 24-hr period, we considered whether overnight sleep alone was sufficient for these effects. Performance changes across the overnight sleep bout (ΔRecallovernight) were not significantly different across conditions (F(1,43) = 0.952, p = 0.335) nor emotion categories (F(1,43) = 1.387, p = 0.245; emotion x condition: F(1,43) = 0.606, p = 0.441).
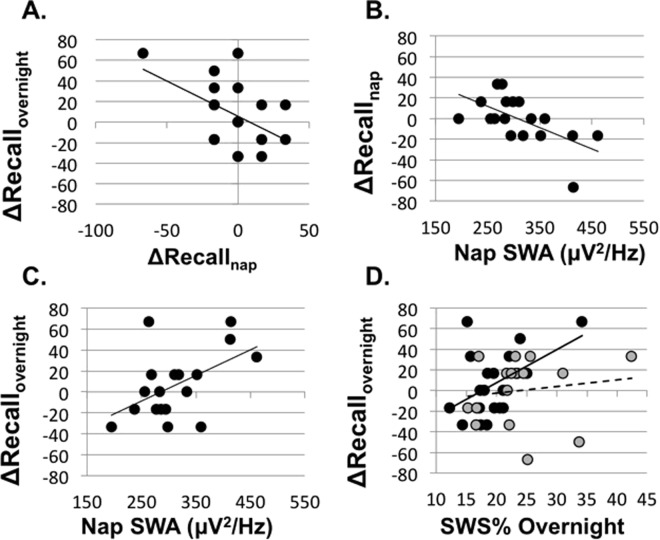
These results suggest a delayed benefit of the nap on emotional memory. We found a similar delayed benefit for procedural learning12. In that study, ΔRecallnap predicted ΔRecallovernight, suggesting an interaction of multiple sleep bouts on memory processing. Notably, here too with emotional memories, we found a significant association between ΔRecallnap and ΔRecallovernight such that declines in accuracy across the nap were associated with improvements across overnight sleep (B = −0.488, p = 0.029; 95% CI: [−1.294, −0.079]; Fig. 3A).
Figure 3.
(A) Performance decrements across the nap predicted performance improvements overnight (p = 0.029). All data points are within 1.5*IQR for both ΔRecallovernight and ΔRecallnap. (B) SWA in the nap predicted performance decay across the nap (p = 0.008). (C) Nap SWA also predicts the performance improvements across nocturnal sleep (p = 0.028). (D) Nocturnal SWS is associated with overnight performance improvements for the nap condition (black/solid line; p = 0.029), but not the wake condition (grey/dashed line; p = 0.606).
Nap habituality was also examined with respect to the nap benefit in post hoc exporatory analyses. Nap habituality was obtained through caregiver report of nap frequency. We did not receive caregiver reports for 9 of the children. Habitually napping children (n = 18) were defined as those who napped 5 or more days per week, whereas nonhabitually napping children (n = 9) were defined as children who napped fewer than 2 days per week (as in13). There were no baseline differences (no differences at immediate recall) across conditions for either habitually napping children (F(1,17) = 2.236, p = 0.153) or nonhabitually napping children (F(1,8) = 1.138, p = 0.391).
For habitually napping children, performance changes over the nap did not differ from those over wake (ΔRecallnap/wake; F(1,17) = 0.009, p = 0.925). There was also no main effect of emotion (F(1,17) = 0.208, p = 0.654), nor an emotion by condition interaction (F(1,17) = 0.028, p = 0.870) across the nap/wake period. However, there was a significant main effect of condition on performance changes across overnight sleep (ΔRecallovernight; F(1,13) = 4.57, p = 0.05, ηp2 = 0.260). When habitually napping children had napped during the prior day, they showed protected memory (one-sample t-test; t(15) = 1.420, p = 0.176; 95% CI: [−5.50, 27.44]) across the overnight sleep bout, whereas significant forgetting was observed for the wake condition (one-sample t-test; t(15) = −2.501, p = 0.024; 95% CI: [−30.85, −2.46]). Positive and negative stimuli were similarly benefited across overnight sleep (main effect emotion: F(1,13) = 0.521, p = 0.483; emotion x condition: F(1,13) = 0.271, p = 0.611). Similarly, for ΔRecall24-hr, there was a significant main effect of condition for habitually napping children (F(1,13) = 7.581, p = 0.016, ηp2 = 0.368). Again, the nap was associated with protected memory across the delay (one-sample t-test; t(15) = 1.398, p = 0.182; 95% CI: [−6.28, 30.21]), whereas the wake condition demonstrated significant forgetting (one-sample t-test; t(15) = −3.314, p = 0.005; 95% CI: [−35.17, −7.64]). There was no main effect of emotion (F(1,13) = 0.223, p = 0.645), nor an emotion x condition interaction (F(1,13) = 0.290, p = 0.599). These results suggest that napping is critical for the retention of memory for habitually napping children. In addition, for habitually napping children, napping in conjunction with overnight sleep equally protects memory for both positively and negatively valenced stimuli.
For nonhabitually napping children, there were no effects of condition (F(1,8) = 0.559, p = 0.476), nor emotion (main effect emotion: F(1,8) = 0.207, p = 0.661; emotion x condition: F(1,8) = 0.087, p = 0.776) on ΔRecallnap/wake. Across the overnight sleep bout (ΔRecallovernight) however, despite no main effect of condition (F(1,8) = 0.105, p = 0.755), or emotion (F(1,8) = 1.07, p = 0.331), there was a significant emotion x condition interaction (F(1,8) = 5.274, p = 0.05, ηp2 = 0.397). Similar trends were observed for ΔRecall24-hr but the interaction was not significant (main effect condition: F(1,8) = 0.379, p = 0.555; main effect emotion: F(1,8) = 0.943, p = 0.360; emotion x condition: F(1,8) = 4.248, p = 0.073).
For both ΔRecallovernight (one-sample t-test; t(8) = 2.405, p = 0.043; 95% CI: [1.07, 5.12]) and for ΔRecall24-hr (one-sample t-test; t(8) = 2.302, p = 0.05; 95% CI: [−0.4, 44.49]), napping during the day lead to significantly improved memory for negative stimuli, but not for positive stimuli (one-sample t-tests - ΔRecallovernight: t(8) = −0.898, p = 0.395; ΔRecall24-hr: t(8) = −1.004, p = 0.345). Staying awake during naptime for the nonhabitually napping children did not impact memory for either negative (one-sample t-tests - ΔRecallovernight: t(8) = −0.241, p = 0.815; ΔRecall24-hr: t(8) = −0.26, p = 0.801) or positive stimuli (one-sample t-tests - ΔRecallovernight: t(8) = 0.546, p = 0.600; ΔRecall24-hr: t(8) = −0.007, p = 0.995). These results indicate that while napping is not necessary for nonhabitually napping children to retain memories learned earlier in the day, napping may still help to prioritize and improve memory for salient emotional information.
Sleep Physiology
Due to issues with lost electrodes (n = 3) or computer malfunction (n = 2), 1 nap record and 4 wake night records were unable to be scored for sleep stages. However, we were able to determine sleep latency on all 4, and REM latency on 2 of the unscorable wake night records, as the recording issues did not occur until later in the night.
Consistent with our prior work6, the naps were primarily comprised of SWS and non-REM stage 2 sleep (nREM2; Table 2). Only 6 of the 20 children with PSG reached REM in their nap (M% REM = 2.56%, SD = 4.51%). For those 6 children who reached REM sleep during their naps, REM comprised only 8.11% (SD = 4.38) of the nap.
Table 2.
Sleep characteristics from polysomnography (Mean (SD)).
| Nap | Nap Overnight | Wake Overnight | p* | |
|---|---|---|---|---|
| TST (min) | 70.88 (24.37) | 522.68 (71.34) | 549.51 (74.76) | 0.006 |
| Sleep Latency (min) | 15.03 (10.04) | 44.70 (31.35) | 26.73 (21.00) | 0.017 |
| WASO (min) | 11.35 (16.32) | 37.08 (40.14) | 36.39 (28.22) | 0.550 |
| Sleep Efficiency (%) | 73.06 (17.78) | 86.54 (7.94) | 90.03 (6.41) | 0.033 |
| nREM1(%) | 9.39 (4.49) | 9.39 (2.51) | 8.42 (2.19) | 0.004 |
| nREM2 (%) | 36.85 (13.52) | 53.22 (4.78) | 50.31 (9.05) | 0.322 |
| SWS (%) | 51.20 (15.36) | 19.73 (4.74) | 23.89 (7.00) | 0.003 |
| REM (%) | 2.56 (4.51) | 17.67 (3.60) | 17.41 (3.96) | 0.624 |
| SWA C3 (μV2/Hz) | 312.07 (67.52) | 244.42 (76.96) | 243.39 (65.20) | 0.970 |
Note TST = total sleep time; WASO = wake after sleep onset; nREM = non-rapid eye movement sleep, SWS = slow wave sleep; REM = rapid eye movement, SWA = slow wave activity.
*p values correspond to paired samples t-tests comparing overnight bouts across conditions.
We compared overnight sleep from the nap and wake conditions. When children napped, it took them longer to fall asleep at night (t(19 = 2.612, p = 0.017; 95% CI: [−3.569, 32.381]), they had reduced overnight sleep efficiency (t(15) = −2.35, p = 0.033; 95% CI: [−9.231, −0.456]), and reduced nocturnal sleep time (t(15) = −3.173, p = 0.006; 95% CI: [−65.781, −12.919]) compared to the night following the wake condition. Notably, when considering the total daily sleep time (nap + overnight sleep), children had more sleep when they napped (M = 579.88, SD = 76.18) compared to the wake condition (overnight sleep only; M = 549.51, SD = 74.75; t(15) = 2.25, p = 0.04; 95% CI: [1.637, 59.113]).
There were no differences in the percent of the night spent in nREM2 (t(15) = 1.03, p = 0.322) or REM sleep (t(15) = 0.501, p = 0.624) across conditions. However, a significantly larger percent of the night was spent in nREM1 (t(15) = 3.37, p = 0.004; 95% CI: [0.554, 2.458]) and a smaller percent of the night was spent in SWS (t(15) = −3.62, p = 0.003; 95% CI: [−6.226, −1.611]) in the nap compared to the wake condition. There were no significant differences in overnight SWA across conditions, in either the frontal (t(16) = 0.021, p = 0.983; 95% CI: [−58.232, 59.422]) or central (t(16) = 0.0908, p = 0.923; 95% CI: [−53.308, 58.485]) cortical leads.
The presence of REM in the nap did not affect overnight REM quantity (t(17) = −1.539, p = 0.142) or latency (t(17) = −1.456, p = 0.164); further, the percentage of the nap spent in REM sleep did not predict the percentage of the night spent in REM sleep (B = 0.317, p = 0.499). The percentage of the nap spent in SWS also did not predict the percentage of the night spent in SWS (B = 0.165, p = 0.186). Interestingly, nap SWA positively predicted overnight SWS percentage, although this was not significant (B = 0.442, p = 0.066).
Relationship between Sleep Physiology and Memory Performance
We first considered whether REM sleep contributed to the delayed performance enhancements. The percentage of the nap spent in REM sleep did not predict ΔRecallnap (B = 0.083, p = 0.736). The percentage of the night spent in REM sleep also did not predict ΔRecallovernight (B = −0.350, p = 0.131). Interestingly, the percentage of REM sleep across all sleep bouts did significantly but negatively predict ΔRecall24-hr (B = −0.458, p = 0.049; 95% CI: [−0.001, 0.000]). Greater REM percentage across all sleep bouts was associated with greater memory decline across the 24-hour period.
Given the emerging research supporting a role of SWS and SWA in emotional memory consolidation, we next examined the contribution of these sleep traits on the change in memory performance across sleep bouts. The percentage of the nap spent in SWS sleep did not predict ΔRecallnap (B = −0.347, p = 0.146); however, there was a significant inverse relationship between ΔRecallnap and nap SWA (B = −0.601, p = 0.008; 95% CI: [−2.982, −0.517]; Fig. 3B) such that greater nap SWA was associated with more memory decay. While this result could indicate poorer consolidation due to greater sleep need or greater sleep inertia of the child, nap SWA was not significantly correlated with sleepiness at either immediate (r = −0.014, p = 0.955) nor delayed recall (r = −0.056, p = 0.825). Further, the relationship between SWA and memory decay was driven by reduced memory for positive stimuli (B = −0.599, p = 0.009; 95% CI: [−2.302, −0.391]) rather than negative stimuli (B = −0.422, p = 0.081). The percentage of the night spent in SWS sleep did positively predict ΔRecallovernight (B = 0.488, p = 0.029; 95% CI: [0.008, 0.140]; Fig. 3D). This effect was driven by memory for negative stimuli (B = 0.556, p = 0.011; 95% CI: [1.713, 11.495]) as compared to positive stimuli (B = 0.178, p = 0.452; Fig. 3D). Overnight SWA did not predict ΔRecallovernight (B = 0.216, p = 0.360). The percentage of SWS sleep across all sleep bouts did not significantly predict ΔRecall24-hr (B = −0.005, p = 0.983), nor was there a significant relationship between ΔRecall24hr and the average SWA across both the nap and the overnight sleep bouts (B = −0.081, p = 0.936).
In light of the reduced accuracy across the nap predicting improvements in accuracy overnight (Fig. 3A), and the associations between nap SWA and overnight SWS with recognition memory, we investigated possible interactions between these two sleep bouts. Supporting an interaction across sleep bouts, SWA in the nap negatively predicted ΔRecallnap (B = −0.601, p = 0.008; 95% CI: [−2.982, −0.517]; Fig. 3B) and positively predicted ΔRecallovernight (B = 0.518, p = 0.028; 95% CI: [0.000, 0.005]; Fig. 3C). Importantly, ΔRecallovernight was also positively predicted by overnight SWS in the nap condition as previously stated; however, this relationship was not observed in the wake condition (B = 0.140, p = 0.606; Fig. 3D). In other words, overnight SWS predicted improved memory performance when a nap rich in SWA had occurred earlier in the day.
Lastly, to further explore the possibility of an interaction between distributed sleep bouts, we assessed whether nap SWA had a mediatory role on memory performance changes. Given that ΔRecallnap significantly predicted ΔRecallovernight, and both significantly predicted by nap SWA, the preliminary conditions for a mediation analysis were met. When nap SWA was controlled for, the standardized regression coefficient for the relationship between ΔRecallnap and ΔRecallovernight was reduced from B = −0.488, (p = 0.029) to B = −0.364 (p = 0.182). These results support that the relationship between memory performance change across both the nap and overnight sleep bouts was fully mediated by nap SWA (Fig. 4).
Figure 4.
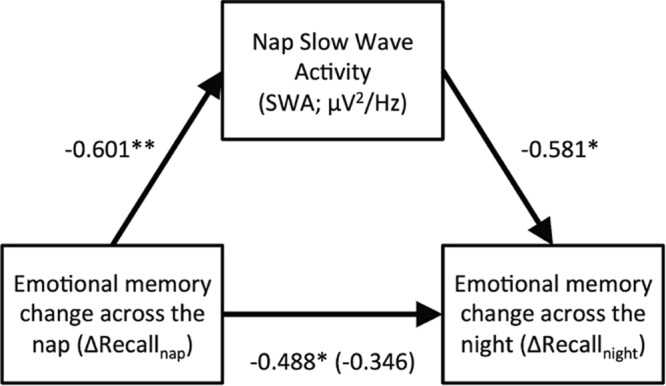
Mediation model of the effect of nap SWA on emotional memory consolidation across both nap and overnight sleep bouts. **p < 0.01, *p < 0.05. Numbers represent the regression coefficient (β). Data indicates full mediation as β becomes non-significant when controlling for the mediator (in parentheses).
Discussion
Individually, the nap and overnight sleep bouts were not sufficient to induce changes in memory. A significant benefit of napping was observed only when changes across the entire 24-hour period were considered. This supports an interplay between the nap and subsequent overnight sleep in the consolidation of memories in young children. A day of continuous wake led to significant forgetting, particularly for the habitually napping children, which could not be reversed with overnight sleep. The results suggest that the nap led to stabilization and protection of new memories from forgetting even though these effects were not apparent directly following the nap/wake manipulation. In addition, for children who no longer nap regularly, a mid-day nap in conjunction with overnight sleep may still be beneficial for emotional memory organization and prioritization.
The results of this study are consistent with those in procedural memory consolidation in preschool-aged children13. Here also, both a nap and subsequent overnight sleep were necessary to observe performance benefits. In that study, preschool children learned a serial reaction time task in the morning. Memory for this procedural task was probed following a nap, and following an equivalent bout of daytime wake (within-subjects design), as well as following the subsequent overnight sleep in both conditions. Memory performance was not significantly different directly following the nap/wake period; however, following overnight sleep, performance was significantly improved when the children had napped the previous day.
A similar delayed benefit of sleep has been demonstrated in juvenile songbirds14. Vocal replication of a model song deteriorated when a period of sleep directly followed learning, but this deterioration predicted improvement when assessed long-term. This indicates an active but delayed role of sleep in learning, which may be particularly evident in juvenile populations. The authors of this study concluded that changes across sleep could represent synaptic15 or cellular16 remodeling, possibly through neuronal replay during sleep. The remodeling across the initial sleep bout presented as performance deterioration but was critical to promote long-term consolidation15.
Likewise, SWA in naps in early childhood may yield an initially destabilized memory that is most labile to plastic processes in overnight SWS. While high SWA during the nap was associated with short-term memory decay, it was advantageous to long-term memory consolidation, potentially via the enhancement of overnight SWS. Slow wave activity has often been linked with synaptic remodeling and cortical plasticity17. The synchrony of neuronal activation during SWS produces regular and periodic neurotransmitter release as well as changes in intracellular calcium concentrations that facilitate plasticity within neuronal populations18. Studies have shown that SWA increases or decreases in cortical regions that are more or less active respectively during previous waking in rats12 as well as in humans19. Moreover, greater SWA may reflect improved efficiency within the hippocampal-neocortical loop20, thereby enhancing memory transfer to long-term storage.
Napping in early childhood may begin the process of synaptic remodeling of the learned memories, which may be sufficient to yield short-term benefits to mood and emotion regulation10,21. However, those memories require additional processing over successive sleep periods to promote observable differences in memory performance. Greater plasticity over the nap, as indicated by greater SWA, may allow nocturnal SWS to be more effective at consolidating and preserving the memories learned earlier in the day. In contrast, with the lack of processing and remodeling directly following learning, information is forgotten across the 24-hour period in the wake condition. Newly formed memories may be more susceptible to daytime waking interference22 without plastic changes across the nap. This could therefore diminish the likelihood of localized SWA acting to induce plastic changes to stabilize and transfer information into long-term storage during nocturnal sleep.
The results of this study replicate previous findings6 that naps in young children contain little-to-no REM sleep. The preschool nap was beneficial to both positive and negative valenced memories despite the lack of REM sleep. In the current study, emotional stimuli were positively associated with nocturnal SWS. These results are consistent with recent reports of SWS being particularly important for emotional memory consolidation in both children23 and adults7,8,20. Thus, the relative effect of SWS and REM sleep on emotional memory in children is an area that warrants future research.
It is important to note a few limitations to this study. First, the circadian timing of encoding for the laboratory- and classroom-based participants was slightly different (~1.5–2 hours later for the laboratory participants). A second limitation was that there were differences in immediate recognition accuracy across conditions. Change scores were used to assess performance with respect to initial encoding, however the baseline difference could still impact condition effects.
Another limitation was the lack of a neutral stimulus comparison. As such, all stimuli presented to the participants were emotional in valence – either positive or negative. While this design was chosen due to its age-appropriateness and to replicate a previous study using a preschool-aged population18, it does hamper direct comparisons to much of the adult emotional memory literature. Further, lacking the neutral stimulus comparison prohibits us from directly assessing the preferential processing of emotional, relative to non-emotional, items as previously reported in young and older adults24. Nevertheless, we feel that these results indicate a need for further research into emotional memory consolidation across a nap and subsequent overnight sleep in preschool-aged chidren.
Finally, we failed to replicate the original findings of Kinzler and Shutts18 in which memory for negative stimuli was greater than memory for positive stimuli immediately after encoding. While on average the Kinzler and Shutts study demonstrated a negativity bias in emotional recognition memory, they did however report large individual differences in emotional bias (differences in accuracy of recognizing positive or negative stimuli). Of their participants, ~44.7% had a negativity bias in memory, ~42.1% had no emotion bias, and ~13.1% had a positivity bias. In our study, fewer participants showed a lack of emotional bias (28.6%) at immediate recognition, and instead participants were equally as likely to have positive (36.7%) or negative (34.7%) emotional recognition memory bias stimuli. Therefore, the lack of main effect of emotion in our data could reflect a different make up of the participant population, or a generational change in emotional processing of faces presented on a screen.
This study demonstrates that napping is beneficial to memory processing. Given the importance of socio-emotional learning in preschool25–27, naps (averaging 70 mins) may support the curricular goals of early childhood education. As such, napping remains an important part of the daily preschool schedule and sufficient time for sleep should be protected.
Methods
Participants
Sixty-four children (43 female; M = 51.55 months, SD = 7.23, range = 34–64 months) participated in the study. Children were required to have normal or corrected-to-normal vision and have no diagnosis (present or past) of sleep or neurological disorders, or learning or developmental disabilities as reported by parents.
Task
The task was modified from a short-term emotional memory task used by Kinzler and Shutts1,7,24 in preschool children. Stimuli were emotionally neutral faces taken from the Radboud Faces Database28 and the Max Planck Institute’s FACES collection29. An equal number of male and female face stimuli as well as mean and nice statements were presented at encoding. To prevent ceiling effects, the task difficulty was adjusted by age (as in6). Older children (>42 months) encoded 18 faces by viewing them for 6 secs each, whereas younger children (<42 months) encoded 12 faces by viewing images for 8 secs each. During the presentation of each image, a statement was read aloud describing the individual as either “mean” or “nice” (e.g., “Jordan is always nice. Today he brought in a book to share with the class.” or “Lena is always nice. Today she helped us pour milk into our cups at lunch time.”). Children were not required to make any responses during encoding. The experimenter visually monitored children to ensure they attended to each stimulus as the statement was read.
In each of three memory assessments (immediate, delayed, and 24-hr recognition), children were given a two-item forced choice recognition task. Children were asked to select the familiar (encoded) face when paired with a novel distractor face, matched for gender. Children who encoded 18 faces were given 6 trials in each of the 3 recognition phases; children who encoded 12 faces were given 4 trials in each recognition phase. Recognition for each encoded image was probed in only one recognition phase. Both the gender and the emotional valence of the targets were balanced across recognition phases.
Questionnaires
Children self-rated sleepiness using the visual sleepiness scale (VSS30) and rated mood using a visual mood scale (VMS31. Experimenters also rated the child’s sleepiness and mood on the same scales. Although these measures are not validated for use in this particular age group, the VSS is reliable (compared to Karolinska Sleepiness Scale in adults, α = 0.7230; and validated for use in children over 6 yrs32. The facial version of the VMS (using faces scaled from happy to sad) has also been shown to be reliable in 5–6 year old children33.
Primary caregivers completed the Child Sleep Habits Questionnaire (CSHQ), providing a measure of the child’s sleep habits and sleep health. This assessment is reliable (α = 0.88) and validated for detecting disordered sleep in preschool-aged children (night wakings, parasomnias34,35. Consistent with previous work13, habitually napping children (n = 18) were defined as children who napped 5 or more days per week, and nonhabitually napping children (n = 9) were defined as children who napped fewer than 2 days per week.
Procedures
All procedures were approved by the University of Massachusetts Institutional Review Board. Accordingly, procedures were carried out in accordance with the approved protocol. Parents or legal guardians provided written informed consent and child assent was obtained before the testing began. Children participated in two conditions (within-subjects design), a nap and a wake condition, separated by at least 1 week. The order of conditions was counterbalanced across participants. Children were either tested in a preschool classroom (n = 44) or in the sleep laboratory (n = 20) so that sleep physiology could be recorded.
Children tested in the preschool classroom completed the encoding phase and subsequent immediate recognition phase between approximately 10:00 and 11:00 am (Fig. 1). Children then went about their typical classroom routine (e.g., outside playtime, lunch). During the classroom nap opportunity (typically 1:00–3:00 pm), children were either wake or nap promoted. In the wake promotion condition, children were required to remain on their cot/mat in the darkened classroom, but were kept awake as needed with quiet activities (e.g., books, puzzles). In the nap promotion condition, back rubbing and soothing were used to encourage children to sleep as necessary. After the classroom nap opportunity and a short recovery time to overcome sleep inertia (~3:30 pm), the delayed recognition phase took place. Children and experimenters then completed the VSS. The following morning, experimenters returned to the classroom for the 24-hr recognition phase, after which both children and experimenters again completed the VSS. Approximately one week later, the alternate condition (wake or nap promotion) took place with all procedures identical to the first week.
Procedures for children tested in the sleep laboratory were identical to the classroom design with the following exceptions: (1) all children, regardless of age, were given the 18-item image set in order to have more range in performance measures to compare with sleep physiology; (2) to reduce time in the laboratory, encoding and immediate recognition took place between 11:30 am and 12:00 pm; (3) due to the encoding and recognition taking place later, children had only ~1 hour between immediate recall and napping, as opposed to the ~2 hours in the classroom; (4) children and experimenters completed the VMS in addition to the VSS, and these were assessed at the immediate, delayed, and 24-hr recognition phases. Additionally, children who came to the sleep lab were equipped with a PSG montage prior to sleep intervals (Fig. 1). In the nap condition, after PSG application, children were given a 2-hr nap opportunity (~1:00–3:00 pm) in which sleep was promoted. Polysomnography was also applied following immediate recognition in the wake condition to ensure wakefulness. Following the 2-hr nap/wake opportunity, delayed recognition was assessed (~3:30 pm). After delayed recognition, children and parents left the laboratory to complete the rest of their daily routine. In both conditions, parents brought their child back to the laboratory in the evening, approximately 1 hr prior to the child’s typical bedtime, for the PSG montage to be re-applied. In the laboratory, typical bedtime for most children was ~8:00 pm, but ranged from ~7:30–10:00 pm. Children were allowed to sleep as long as they could overnight. Children awoke naturally without influence from the researchers. Most children awoke around 7:00am, although wake times also ranged from ~4:45am–8:00am. In the morning, following removal of the PSG montage and morning routines (e.g., teeth-brushing, changing clothes; approximately 30 mins after waking), 24-hr recognition was tested. It should be noted that the 24-hr recall for the laboratory study was not tested exactly 24-hrs after encoding, given that laboratory encoding occurred ~1.5–2 hours later than encoding classroom encoding.
Polysomnography
A 32-electrode electroencephalography (EEG) cap (Easy Cap; Brain Products GmbH, Germany), customized with 2 electromyography (EMG) and 2 electrooculography (EOG) electrodes, was used for physiological recordings. The EEG cap included 25 cortical EEG leads (F3, F4, Fz, F7, F8, FCz, FC1, FC2, FC5, FC6, Cz, C3, C4, CP1, CP2, CP5, CP6, Pz, P3, P4, P7, P8, POz, O1, and O2) referenced to electrodes placed on the mastoids (A1 and A2). For two children, the EasyCap was not available and an AURA PSG ambulatory system (Grass Products, Natus Technologies) with a 14-electrode montage including 7 EEG leads, 2 EMG leads, and 2 EOG leads, was used. EEG leads included F3, F4, Cz, C3, C4, O1, and O2 all referenced to electrodes placed on the mastoids (A1 and A2). A ground electrode was affixed to the forehead. EMG electrodes were placed on the chin and referenced to each other.
Data Analyses
To assess changes in memory over naps and equivalent intervals of wake, baseline recognition accuracy (percent correct) was subtracted from delayed recognition accuracy (ΔRecallnap/wake = delayed recognition − immediate recognition accuracy; Fig. 1). ΔRecallnap was also used for associations with nap physiology and memory performance change across this delay. ΔRecallovernight (24-hr recognition − delayed recognition accuracy) was used to assess the change in memory across the overnight sleep period. ΔRecallovernight was also used to correlate overnight sleep physiology with performance change across the overnight delay. Finally, ΔRecall 24-hr (ΔRecall 24-hr = 24-hr recognition − immediate recognition accuracy) was calculated to measure the change in performance across the entire 24-hr period.
Effects were assessed with 2 × 2 repeated-measures ANOVAs comparing the difference scores with condition (Nap vs. Wake) and emotion (Mean vs. Nice) as within-subjects factors. To assess the relationship between ΔRecallnap and ΔRecallovernight and between behavior and sleep physiology, linear regressions were used. Paired-samples t-tests were used to examine differences in sleep physiology between the two experimental nights, as well as to assess differences in sleepiness and emotionality.
Lastly, mediation was assessed with the Baron and Kenny method36. This method was employed to test the hypothesis that memory performance change across both sleep bouts was mediated by physiological sleep measures. The Baron and Kenny method defines a mediator as a variable that is independently associated with both the predictor and outcome variables, but one that also explains the majority of the variance in the association. As such, the regression coefficient between the predictor and outcome variables must reduce when the mediating variable is controlled for, and for full mediation, the relationship must become non-significant.
PSG recordings were coded for sleep stages using the American Academy of Sleep Medicine criteria37, developed for healthy young adults and suitable for young children6,38. Additionally, spectral analyses of PSG were run using BrainVision Analyzer 2 software (Ver 2.4; Brain Products GmbH, Germany), with interest in SWA defined with a range of 0.5–4 Hz. Specifically, SWS staged segments were extracted and then analyzed using the periodogram method for spectral analysis39. Data were divided into 4-second epochs for artifact rejection on individual channels, and a fast-Fourier transform (FFT) was applied using a Hanning window with 10% overlap and utilizing covariance.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 HL111695; PI: RMCS) and an Honors Research Grant from Commonwealth Honors College (to JK).
Author Contributions
L.K. and R.S. developed the hypothesis and approach. L.K. and J.K. collected and analyzed the data. L.K., J.K. and R.S. wrote the manuscript. L.K. prepared the figures.
Data availability
The data from the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.S. Denham, Social cognition, prosocial behavior, and emotion in preschoolers: Contextual validation. Child Dev. 57 (1986).
- 2.Walker MP. The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennion KA, Payne JD, Kensinger EA. Selective Effects of Sleep on Emotional Memory: What Mechanisms Are Responsible? Transl. Issues Psychol. Sci. 2015;1:79–88. doi: 10.1037/tps0000019. [DOI] [Google Scholar]
- 4.Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiol. Learn. Mem. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurdziel L, Duclos K, Spencer RMC. Sleep spindles in midday naps enhance learning in preschool children. Proc. Natl. Acad. Sci. USA. 2013;110:17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne JD, et al. Napping and the selective consolidation of negative aspects of scenes. Emotion. 2015;15:176–186. doi: 10.1037/a0038683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairney SA, Durrant SJ, Hulleman J, Lewis PA. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep. 2014;37:701–707. doi: 10.5665/sleep.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinzler KD, Shutts K. Memory for “mean” over “nice”: the influence of threat on children’s face memory. Cognition. 2008;107:775–783. doi: 10.1016/j.cognition.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremone, A., Kurdziel, L. B. F., Fraticelli-Torres, A., McDermott, J. M. & Spencer, R. M. C. Napping reduces emotional attention bias during early childhood. Dev. Sci. 10.1111/desc.12411 (2016). [DOI] [PMC free article] [PubMed]
- 11.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9:29–34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- 12.Vyazovskiy V, Borbély AA, Tobler I. Fast track: Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J. Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 13.Desrochers, P. C., Kurdziel, L. B. F. & Spencer, R. M. C. Delayed benefit of naps on motor learning in preschool children. Exp. Brain Res. 234, 10.1007/s00221-015-4506-3 (2016). [DOI] [PMC free article] [PubMed]
- 14.Derégnaucourt S, Mitra PP, Fehér O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- 15.Bottjer SW, Johnson F. Circuits, hormones, and learning: Vocal behavior in songbirds. J. Neurobiol. 1997;33:602–618. doi: 10.1002/(SICI)1097-4695(19971105)33:5<602::AID-NEU8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Nordeen KW, Nordeen EJ. Projection neurons within a vocal motor pathway are born during song learning in zebra finches. Nature. 1988;334:149–151. doi: 10.1038/334149a0. [DOI] [PubMed] [Google Scholar]
- 17.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog. Neurobiol. 2003;69:71–101. doi: 10.1016/S0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 19.Huber R, Felice Ghilardi M, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 20.Benedict C, Scheller J, Rose-John S, Born J, Marshall L. Enhancing influence of intranasal interleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J. 2009;23:3629–3636. doi: 10.1096/fj.08-122853. [DOI] [PubMed] [Google Scholar]
- 21.Berger RH, Miller AL, Seifer R, Cares SR, LeBourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J. Sleep Res. 2012;21:235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 23.Prehn-Kristensen A, et al. Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. PLoS One. 2013;8:e65098. doi: 10.1371/journal.pone.0065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, B. J., Schultz, K. S., Adams, S., Baran, B. & Spencer, R. M. C. Emotional bias of sleep-dependent processing shifts from negative to positive with aging. Neurobiol. Aging. 45, 10.1016/j.neurobiolaging.2016.05.019 (2016). [DOI] [PMC free article] [PubMed]
- 25.Ramey CT, Ramey SL. Early learning and school readiness: Can early intervention make a difference? Merrill. Palmer. Q. 2004;50:471–491. doi: 10.1353/mpq.2004.0034. [DOI] [Google Scholar]
- 26.Brown JA, Jimerson SR, Dowdy E, Gonzalez V, Stewart K. Assessing the effects of school-wide Second Step implementation in a predominately English language learner, low SES, Latino sample. Psychol. Sch. 2012;49:864–875. doi: 10.1002/pits.21639. [DOI] [Google Scholar]
- 27.Powell, D. & Dunlap, G. Evidence-based social-emotional curricula and intervention packages for children 0-5 years and their families (Roadmap to effective intervention practices) (2009).
- 28.Langner O, et al. Presentation and validation of the Radboud FacesDatabase. Cogn. Emot. 2010;24:1377–1388. doi: 10.1080/02699930903485076. [DOI] [Google Scholar]
- 29.Ebner NC, Riediger M, Lindenberger U. FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav. Res. Methods. 2010;42:351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado CC, Bentley AJ, Mitchell D. A pictorial sleepiness scale based on cartoon faces. Sleep. 2004;27:541–8. doi: 10.1093/sleep/27.3.541. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol. Med. 1973;3:479–86. doi: 10.1017/S0033291700054283. [DOI] [PubMed] [Google Scholar]
- 32.Kollins SH, et al. Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2011;21:111–20. doi: 10.1089/cap.2010.0064. [DOI] [PubMed] [Google Scholar]
- 33.Cremeens J, Eiser C, Blades M. Brief report: Assessing the impact of rating scale type, types of items, and age on the measurement of school-age children’s self-reported quality of life. J. Pediatr. Psychol. 2007;32:132–138. doi: 10.1093/jpepsy/jsj119. [DOI] [PubMed] [Google Scholar]
- 34.Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J. Dev. Behav. Pediatr. 2008;29:82–88. doi: 10.1097/DBP.0b013e318163c39a. [DOI] [PubMed] [Google Scholar]
- 35.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. doi: 10.1093/sleep/23.8.1d. [DOI] [PubMed] [Google Scholar]
- 36.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 37.Silber MH, et al. The visual scoring of sleep in adults. J. Clin. sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2007;3:121–131. [PubMed] [Google Scholar]
- 38.Meltzer LJ, Walsh CM, Traylor J, Westin AML. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–166. doi: 10.5665/sleep.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch PD. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967;15:70–73. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from the current study are available from the corresponding author on reasonable request.