Summary
Objective
Multicomponent lifestyle modification interventions designed for gestational and early postnatal periods may be key to preventing obesity in children. The primary objective of the study was to determine if infant growth outcomes differed between treatment arms of an 18‐month, maternal, infant and early childhood home visiting project.
Methods
Pregnant women at least 18 years of age, less than 19 weeks pregnant and residing in a lower Mississippi Delta county were recruited between March 2013 and December 2014. Postnatal data were collected from 24 experimental and 30 control participants between September 2013 and May 2016. Infant growth outcomes were modelled as time‐to‐event data using Kaplan–Meier survival curves with log‐rank tests to determine if survival curves differed between treatment arms.
Results
Retention rates for the experimental and control arms were 88% (21/24) and 83% (25/30), respectively. Approximately three‐fourths of infants in both treatment arms were classified as overweight and experienced rapid weight gain during the first 12 months of life. No differences between median times neither to classification as overweight (3–4 months) nor to experiencing rapid weight gain (6–7 months) were observed between treatment arms.
Conclusions
As compared with a standard educational (control) curriculum, an educational curriculum enhanced with diet and physical activity components was not effective at improving infant growth outcomes.
Keywords: African American, home visiting, infant overweight, rapid infant weight gain
Introduction
In the USA, 8% of infants and toddlers are affected by overweight, and 17% of children are affected by obesity with rates higher in racial/ethnic minority children 1. Further, 7 of the 10 states with the highest rates of children with overweight/obesity are in the South with Mississippi ranking third (37%) nationwide 2. The prenatal through early childhood periods are likely key to the development and thus prevention of obesity and its consequences in children 3. During the first year of life, modifiable risk factors for childhood overweight include maternal pre‐pregnancy overweight, high infant birth weight and rapid weight gain, formula feeding, and early introduction of solid foods 4. Thus, lifestyle interventions designed for both the gestational period (i.e. promotion of appropriate gestational weight gain) and the early postnatal period (i.e. promotion of breastfeeding and solid food introduction after 6 months of age) may have a positive impact on childhood overweight/obesity.
The Delta Healthy Sprouts Project was designed to compare the impact of two maternal, infant and early childhood home visiting curricula on weight status, dietary intake, physical activity and other health behaviours of women and their infants residing in the rural Lower Mississippi Delta region of the USA. This region was chosen because it is characterized by high rates of infants with low birth weight, preterm infants, child poverty and childhood overweight/obesity 5. Infant diet, activity and sleep outcomes are being reported elsewhere. The primary objective of the paper was to determine if infant growth outcomes differed between treatment arms. Secondary objectives were to explore associations among infant characteristics and growth outcomes. It was hypothesized that infants born to participants in the experimental arm of the intervention would have improved growth outcomes (i.e. experience less overweight and/or rapid weight gain) as compared with infants born to participants in the control arm of the intervention.
Methods
Design
Delta Healthy Sprouts, an 18‐month, randomized, two‐arm, parallel, controlled trial, was designed to evaluate the impact of the Parents as Teachers® (PAT, control) curriculum as compared with a nutrition and physical activity enhanced PAT curriculum (PATE, experimental) on the primary outcomes of maternal gestational weight gain and postpartum weight control and childhood obesity prevention. Participants were randomly assigned to one of the two treatment arms (PAT [n = 43] or PATE [n = 39]) using a random generator function in SAS® (version 9.4, SAS Institute Inc., Cary, NC, USA) and equal allocation blocks of 25. Participants were followed for 18 months via monthly home visits, starting at approximately gestational month (GM) 4 through postnatal month (PM) 12. Participants provided written informed consent. The project was approved by the Institutional Review Board of Delta State University (protocol number 12‐024) and is registered at http://clinicaltrials.gov (NCT01746394).
Participants and setting
Recruitment occurred via distribution of flyers and brochures, and study staff on site at health clinics and medical facilities serving pregnant women and at health fairs. Referrals also came from health clinic/department staff, Special Supplemental Program for Women, Infants and Children (WIC) nutritionists, social service agencies and enrolled study participants. Inclusion criteria included at least 18 years of age; less than 19 weeks pregnant with first, second or third child; singleton pregnancy; and resident of Washington, Bolivar or Humphreys County in Mississippi. Baseline data were collected from 82 pregnant women between March 2013 and December 2014.
Target enrolment was 75 women in each of the two treatment arms. This sample size was based on the following assumptions: 20% attrition rate, 15% of infants in the control arm classified as obese in their first year of life and a 12% difference between treatment arms for percentage of infants classified as obese during their first year of life. Power and sample size calculations for gestational weight gain within the Institute of Medicine recommendations and 12‐month postnatal maternal weight loss also were performed 5. Recruitment was stopped prior to reaching these numbers due to unexpected difficulties recruiting pregnant women meeting study criteria and fiscal reasons. Data collection was completed in May 2016.
Because infant growth outcomes were the primary focus of the paper, analyses were conducted only for the postnatal cohort (participants who completed the gestational period and had at least one visit in the postnatal period; n = 54). Figure 1 illustrates the flow of Delta Healthy Sprouts participants through all study phases.
Figure 1.
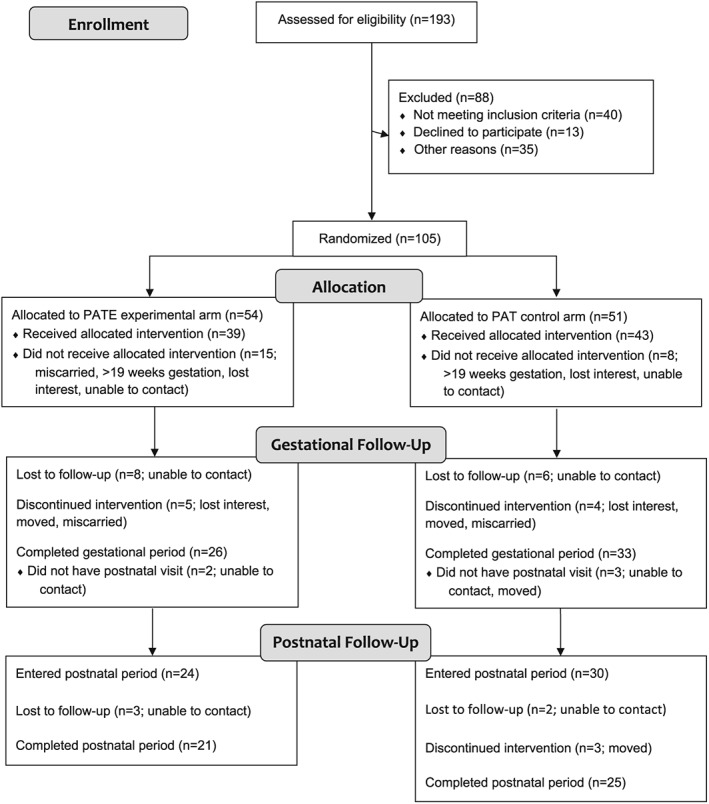
Flow diagram of recruitment, assignment, enrollment and completion of gestational and postnatal periods for Delta Healthy Sprouts participants in the two treatment arms. PAT, Parents as Teachers control treatment; PATE, Parents as Teachers Enhanced experimental treatment.
Intervention
The PAT control arm followed the nationally recognized, evidence‐based Parents as Teachers® curriculum, which included one‐on‐one home visits, optional monthly group meetings, developmental screenings and a resource network for families. The program seeks to increase parental knowledge of child development, improve parenting practices, provide early detection of developmental delays, prevent child abuse and increase school readiness 6. Using the PAT model, Parent Educators provided mothers with evidence‐based information and activities during monthly home visits. Materials were responsive to parental information requests and tailored to the child's age.
The PATE experimental arm built upon the PAT curriculum by adding culturally tailored, maternal weight management and early childhood obesity prevention components. The PATE curriculum was guided by theoretical underpinnings of the social cognitive theory 7 (e.g. maternal modelling of positive health behaviour) and the transtheoretical model of behaviour change 8 (e.g. information to positively affect attitudes and decisional balance and encouragement for engagement in healthy behaviours to improve self‐efficacy). Foundational elements from the Diabetes Prevention Program, including a culturally sensitive, individualized educational curriculum taught on a one‐to‐one basis, also were incorporated 9. Additional elements included anticipatory guidance and parenting support 10. Anticipatory guidance involves providing practical, developmentally appropriate, child health information to parents in anticipation of significant physical, emotional and psychological milestones 11. Parenting support emphasizes children's psychological and behavioural goals, logical and natural consequences, mutual respect and encouragement techniques 12.
Intervention components of the PATE arm included appropriate weight gain during pregnancy and weight management after pregnancy, nutrition and physical activity in the gestational and postnatal periods, and parental modelling of healthful nutrition and physical activity behaviours. Monthly lesson topics included breastfeeding (GM 8 and GM 9 visits), mixed and formula feeding (GM 9 visit), infant feeding cues (GM 9 and PM 1 visits), tummy and confinement time for infants (PM 1 visit), appropriate introduction of solid foods (PM 3 and PM 4 visits), family play time and decreased TV time (PM 6 visit), toddler feeding (PM 8 visit) and healthy, toddler friendly meals and managing toddler food rejection and demands (PM 10 visit). Postnatal lessons also included maternal weight loss charts, infant growth charts, hands‐on activities, instructional DVDs, and goal setting and barrier reduction for both diet and physical activity.
At each monthly postnatal visit, the infants' growth was plotted on World Health Organization sex‐specific reference curves for length‐for‐age and weight‐for‐age percentiles. Subsequently, Parent Educators interpreted and discussed the infant's growth with the mother. If the infant's weight‐for‐age or length‐for‐age were between the 5th and 95th percentiles, the mother was told that her infant was growing at a healthy rate. If the infant's weight‐for‐age or length‐for‐age were less than the 5th percentile, the mother was told that her infant was growing at a low rate and recommended she consult her infant's pediatrician to monitor the infant's growth. If the infant's weight‐for‐age or length‐for‐age were greater than the 95th percentile, the mother was told that her infant was growing at a high rate and strategies to slow her infant's growth rate were recommended. These recommendations included using non‐food soothing strategies, allowing self‐regulation of food intake, waiting to introduce juice or sugar‐sweetened beverages until after 12 months of age and providing more tummy time and floor play.
Parent Educators were African American, college educated women residing in the target communities who completed the onsite PAT foundational training program. Parent Educators were trained to deliver the nutrition and physical activity lessons and to enroll and collect data from participants, including dietary intake, by PhD level senior research staff members who were certified master trainers in the Nutrition Data System for Research software (versions 2012, 2013, 2014, University of Minnesota Nutrition Coordinating Center, Minneapolis, MN, USA). The PAT and PATE lessons were approximately 60–90 and 90–120 min in length, respectively. All participants received incentives (e.g. gift card, diapers, and baby bottles, books and toys) at every visit. A comprehensive description of the Delta Healthy Sprouts Project, including additional details regarding study methodology, lesson plan outlines and Parent Educator training, has been published elsewhere 5.
Measures
Anthropometric measures obtained on the infants included length and weight, which were measured in duplicate if the two measures agreed or in triplicate if the two measures did not agree. For analytic purposes, the measures were averaged. Length was measured using an infantometer (model seca 416, seca, Birmingham, UK). Weight was measured using a digital scale (model SR241, SR Instruments, Tonawanda, NY, USA) with the infant dressed in a diaper only and held in the mother's arms (mother's weight zeroed out). Infant length and weight were measured at each postnatal visit. Infant weight‐for‐length and weight‐for‐age percentiles and z‐scores were computed based on World Health Organization reference growth curves for sex and age 13.
Anthropometric measures obtained on the mothers at the baseline (GM 4) visit included height, which was measured in duplicate using a portable stadiometer (model 217, seca) and weight, which was measured using a digital scale. Both measures were performed without shoes or heavy clothing. Pre‐pregnancy body weight was self‐reported. Body mass index was calculated as weight (kg) divided by height (m) squared where height was averaged if the two measurements differed. Weight also was measured at each of the 17 subsequent (5 gestational and 12 postnatal) visits.
Multiple pass 24‐h dietary recall data for the infants were collected from the mothers at each postnatal visit using Nutrition Data System for Research software. Adherence to the American Academy of Pediatrics infant feeding recommendation pertaining to no solid foods before 6 months of age was determined based on these recalls. This recommendation was chosen because it has been associated with infant weight outcomes in a systematic review with an associated meta‐analysis 4.
Breastfeeding initiation and duration and infant sleep duration were collected from the participants via electronic surveys. Breastfeeding initiation was assessed at the PM 1 visit by recording the participant's response to the question ‘Are you currently breastfeeding?’ If the response was ‘yes’ or ‘no – stopped’, then breastfeeding was considered initiated. Breastfeeding duration was not included in the current analysis because so few participants were currently breastfeeding at the PM 1 visit 14. Infant sleep duration (time spent sleeping in a 24‐h period including naps) was collected at each postnatal visit and included six responses ranging from <8 to ≥16 h in 2‐h time intervals. These six responses were used to classify infants' sleep as aligned or not aligned with the National Sleep Foundation's age‐specific recommendations at each time point. The sleep duration recommendations are 14 to 17 h for newborns (0 to 3 months) corresponding to survey responses 5 and 6 (14 to more than 16 h); 12 to 15 h for infants (4 to 11 months of age), corresponding to survey responses 4 and 5 (12 to less than 16 h); and 11 to 14 h for toddlers (1 to 2 years of age) corresponding to survey responses 3 through 5 (10 to less than 16 h) 15. Breastfeeding initiation and infant sleep duration were included in these analyses because they have been associated with child weight outcomes 4, 16, 17.
Participants provided information regarding socio‐demographic characteristics, Supplemental Nutrition Assistance Program (SNAP) and WIC participation, health history and current health conditions at baseline. At the first postnatal visit, participants provided information regarding birth outcomes. Details regarding other measures and questionnaire data that were collected but are not relevant to the present paper have been published elsewhere 5. All measures and questionnaires were collected or administered by Parent Educators using laptop computers loaded with relevant software (Snap Surveys, version 11.20, Snap Surveys Ltd, Portsmouth, NH, USA).
Statistical analyses
Statistical analyses were performed using SAS. Results were considered significant at p ≤ 0.05. Chi square tests of association or Fisher's exact tests (categorical measures) and two sample t‐tests or Wilcoxon rank scores with exact p‐values (continuous measures) were used to assess differences between PAT and PATE participants' baseline and postnatal characteristics and measures.
Infant growth outcomes were modelled as time‐to‐event data (survival analysis) because conventional methods (e.g. logistic or linear regression) ignore information about the timing of events, cannot handle censored observations and often cannot properly account for unusual distributions of time‐to‐event data. Infant overweight was defined as >97.7th percentile of weight‐for‐length (2 standard deviations > median) 18. Rapid infant weight gain (RIWG) was defined as an increase, from birth, in weight‐for‐age z‐score above 0.67 standard deviation 19. The infant's age (in months) at the time his or her growth first met these criteria was used as the time to the event of interest (overweight or RIWG). Observations were considered censored if the participant dropped from the study prior to the PM 12 visit or the infant did not experience the event at study end.
Kaplan–Meier survival curves using the product‐limit method were used to estimate median survival times. Log‐rank tests were used to determine if survival curves differed between treatment arms or between values of specific household and infant characteristics. Survival analysis represents the most appropriate statistical methods available to handle time‐to‐event data, especially when censoring is involved 20. Treatment, SNAP participation, infant gender, breastfeeding initiation, introduction of solid foods before 6 months of age and alignment with age‐specific sleep duration recommendations were modelled as strata in separate models. Covariate adjustments were not performed in the model testing for treatment arm differences because of small sample sizes. Only one infant's sleep was 100% aligned with recommendations. Hence, a cut‐off value of 80% (≥80% vs. <80% of responses in alignment) was used because it provided a sufficient division for analytic purposes. SNAP participation and infant gender were modelled because these characteristics differed between treatment arms. Median survival times and 95% confidence limits were computed using a log–log transformation.
Results
Postnatal retention rates for PAT and PATE treatment arms were 83% (25/30) and 88% (21/24) (p = 0.668). Mean number of postnatal visits was 10.2 and 9.9 (p = 0.717) for PAT and PATE participants.
Table 1 displays the comparisons between treatment arms for maternal socio‐demographic and anthropometric characteristics of the postnatal cohort. Significant differences between PAT and PATE participant characteristics at baseline were not found with the exception of percentages receiving SNAP benefits (food and nutrition assistance for low‐income individuals and families). More PAT participants (87%) received SNAP benefits as compared with PATE participants (63%). The majority of PAT and PATE participants were African American, single and receiving WIC. Additionally, mean post‐pregnancy body mass index was in the obese category for both treatment arms.
Table 1.
Baseline socio‐demographic and anthropometric characteristics of Delta Healthy Sprouts participants by treatment arm
| PAT (N = 30) | PATE (N = 24) | ||||
|---|---|---|---|---|---|
| Characteristic | n | % | n | % | p |
| Race | >0.999 | ||||
| African American | 29 | 96.7 | 23 | 95.8 | |
| White | 1 | 3.3 | 1 | 4.2 | |
| Relationship status | 0.682 | ||||
| Singlea | 26 | 86.7 | 22 | 91.7 | |
| Married | 4 | 13.3 | 2 | 8.3 | |
| Educational level | 0.462 | ||||
| ≤High school graduate | 12 | 40.0 | 12 | 50.0 | |
| ≥Some college/technical | 18 | 60.0 | 12 | 50.0 | |
| Employment status | 0.608 | ||||
| Full time/part‐time | 10 | 33.3 | 11 | 45.8 | |
| Unemployed (looking) | 12 | 40.0 | 7 | 29.2 | |
| Homemaker/student | 8 | 26.7 | 6 | 25.0 | |
| Smoker in household | 7 | 23.3 | 9 | 37.5 | 0.257 |
| Smoking statusb | 0.620 | ||||
| Current | 1 | 3.3 | 1 | 4.2 | |
| Stopped before pregnancy | 1 | 3.3 | 0 | 0.0 | |
| Stopped after became pregnant | 1 | 3.3 | 0 | 0.0 | |
| Never | 27 | 90.0 | 23 | 95.8 | |
| Medicaid health insurance | 30 | 100.0 | 24 | 100.0 | 0.703 |
| Receiving SNAP | 26 | 86.7 | 15 | 62.5 | 0.039 |
| Receiving WIC | 28 | 93.3 | 20 | 83.3 | 0.389 |
| Mean | SD | Mean | SD | p | |
| Age (years) | 24.1 | 4.76 | 23.0 | 4.96 | 0.380 |
| Household size | 3.6 | 1.61 | 4.2 | 1.52 | 0.221 |
| Pre‐pregnancy BMIc | 28.6 | 8.18 | 29.2 | 7.72 | 0.762 |
| Post‐pregnancy BMId | 30.4 | 7.73 | 31.6 | 7.77 | 0.577 |
BMI, body mass index; PAT, Parents as Teachers control treatment; PATE, Parents as Teachers Enhanced experimental treatment; SNAP, Supplemental Nutrition Assistance Program; WIC, Special Supplement Program for Women, Infants and Children.
Includes one participant who indicated she is divorced.
Comparison: never vs. all other responses.
Based on measured height and self‐reported weight.
Based on weight measured at first postnatal visit.
Table 2 displays the comparisons between treatment arms for infant socio‐demographic, behavioural and birth characteristics. Significant differences between PAT and PATE infant characteristics at birth were not found with the exception of gender. More PATE infants were female (58%) as compared with PAT infants (30%). The majority of PAT and PATE infants were fed formula within the first 24 h of birth, and half or less were ever breastfed. Mean weight‐for‐length birth percentiles were 56.6 and 46.1 for PAT and PATE infants.
Table 2.
Socio‐demographic, behavioural and birth characteristics of Delta Healthy Sprouts participants' infants by treatment arm
| PAT (N = 30) | PATE (N = 24) | ||||
|---|---|---|---|---|---|
| Characteristic | n | % | n | % | p |
| Female gender | 9 | 30.0 | 14 | 58.3 | 0.036 |
| Non‐Hispanic ethnicity | 29 | 96.7 | 24 | 100.0 | >0.999 |
| Race | >0.999 | ||||
| African American | 29 | 96.7 | 23 | 95.8 | |
| White | 1 | 3.3 | 1 | 4.2 | |
| Fed formula within 24 h of birth | 27 | 90.0 | 21 | 87.5 | >0.999 |
| Premature (<37 weeks gestation)a | 5 | 16.7 | 2 | 8.3 | 0.443 |
| Ever breastfed | 9 | 30.0 | 12 | 50.0 | 0.134 |
| Fed solid foods before 6 months | 14 | 46.7 | 7 | 29.2 | 0.190 |
| Met sleep recommendationsb | 6 | 20.0 | 5 | 20.8 | >0.999 |
| Mean | SD | Mean | SD | p | |
| Weeks gestationa | 38.6 | 1.50 | 38.8 | 1.93 | 0.580 |
| Birth weight (g) | 3242.2 | 475.57 | 2994.4 | 646.84 | |
| Birth length (cm) | 49.3 | 2.80 | 47.7 | 2.90 | |
| Birth weight‐for‐length percentilec | 56.6 | 36.06 | 46.1 | 39.10 | 0.322 |
PAT, Parents as Teachers control treatment; PATE, Parents as Teachers Enhanced experimental treatment.
Based on conception date (from online pregnancy calculator and using self‐reported due date).
Infant's sleep duration aligned with expert recommended amounts for at least 80% of visits.
Based on World Health Organization age‐specific and sex‐specific growth curves for children.
Table 3 displays the results of the time‐to‐event analysis for infant growth. Approximately three‐fourths of infants in both treatment arms were classified as overweight and experienced RIWG during the first 12 months of life. Median times to overweight and RIWG were 3 and 7 months, respectively, for PAT infants and 4 and 6 months, respectively, for PATE infants. Treatment differences were not apparent for either of the infant growth outcomes. None of the household or infant characteristics tested were significantly associated with time to overweight. However, both SNAP participation and alignment with expert recommendations for sleep duration were significantly associated with time to RIWG. The majority, 71% and 85%, respectively, of infants who did and did not live in households receiving SNAP benefits experienced RIWG and median event times were 8 and 6 months, respectively. Fifty‐five per cent and 80%, respectively, of infants whose sleep duration was and was not aligned with expert recommendations experienced RIWG and median event times were 11 and 6 months, respectively.
Table 3.
Time‐to‐event analysis for growth outcomes of Delta Healthy Sprouts participants' infants by treatment arm and SNAP participation
| Yes | No | Droppeda | Survival time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Group | N | n | % | n | % | n | % | Median | 95% CL | p b | |
| Overweightc | PAT | 30 | 22 | 73.3 | 6 | 20.0 | 2 | 6.7 | 2.9 | 1.35 | 6.37 | 0.820 |
| PATE | 24 | 18 | 75.0 | 4 | 16.7 | 2 | 8.3 | 3.6 | 1.25 | 10.71 | ||
| Rapid infant weight gaind | PAT | 30 | 22 | 73.3 | 3 | 10.0 | 5 | 16.7 | 7.0 | 3.94 | 8.84 | 0.683 |
| PATE | 24 | 18 | 75.0 | 3 | 12.5 | 3 | 12.5 | 6.0 | 3.58 | 10.20 | ||
| Rapid infant weight gaind | SNAP Yes | 41 | 29 | 70.7 | 6 | 14.6 | 6 | 14.6 | 8.3 | 4.86 | 10.25 | 0.024 |
| SNAP No | 13 | 11 | 84.6 | 0 | 0.0 | 2 | 15.4 | 5.7 | 2.69 | 7.10 | ||
| Rapid infant weight gaind | Sleepe Yes | 11 | 6 | 54.5 | 4 | 36.4 | 1 | 9.1 | 10.8 | 2.69 | NE | 0.048 |
| Sleepe No | 43 | 34 | 79.1 | 2 | 4.7 | 7 | 16.3 | 5.9 | 3.94 | 8.31 | ||
CL, confidence limits; NE, not estimable; PAT, Parents as Teachers control treatment; PATE, Parents as Teachers Enhanced experimental treatment; SNAP, Supplemental Nutrition Assistance Program.
Participants dropped from the study (censored event).
p‐Value for log‐rank test of equality of survival curves.
Defined as exceeding 97.7th percentile for weight for height based on World Health Organization growth curves.
Defined as exceeding 0.67 increase in weight for age z‐score based on World Health Organization growth curves.
Defined as infant's sleep duration aligned with expert recommended amounts for at least 80% of the visits.
Discussion
To the authors' knowledge, this is the first paper to present infant weight outcomes as time‐to‐event outcomes rather than percentages representing one specific point in time. Study results indicate that treatment differences were not observed between PAT and PATE infants for overweight classification and RIWG during the first 12 months of life. Arguably more concerning than this study's lack of effect is that 74% of infants experienced overweight within their first year of life, five times the amount expected 21, and overweight was occurring prior to 4 months of age. Clearly, implementation of effective lifestyle interventions in this region of the country is paramount to improving the health of its residents, particularly during childhood.
In contrast to the current study's findings, infants in the Intervention Nurses Start Infants Growing on Healthy Trajectories responsive parenting group were less likely to be overweight at 1 year of age as compared with control infants (5.5% vs. 12.7%) 22. Likewise, a significantly smaller percentage of Nurse‐Family Partnership infants was classified as high weight‐for‐length as compared with WIC infants (15.5% vs. 17.5%) at 24 months of age 23. Similar to the current study, no differences in overweight were observed between infants whose mothers participated in a prenatal nutrition intervention and control infants (17% vs. 15%) 24. Again, the comparison of infant overweight percentages between the current study and these other programs, particularly Nurse‐Family Partnership and WIC, which serve pregnant women and their children at high risk for adverse health outcomes, is alarming.
The relationship between obesity and short sleep duration in children has been previously reported in the literature 25. A meta‐analysis of prospective cohort studies found evidence that sleep duration may be inversely and longitudinally associated with risk of overweight/obesity in children 16. Additionally, significant inverse associations between infant nighttime sleep and physical growth, even after controlling for potential confounders (i.e. sex, infant birth weight, breastfeeding, sleep position and parental education), were found in infants 6 months of age 17. However, this is the first study to suggest an association between RIWG, a risk factor for later childhood and adult obesity 3, and insufficient sleep in infancy. This finding provides further evidence supporting the importance of adequate amounts of sleep for infants and the inclusion of infant sleep recommendations in future interventions for preventing the onset of RIWG in populations at risk for childhood obesity.
The lack of effect on infant growth outcomes apparent in the Delta Healthy Sprouts trial may be attributed to several factors including prevalence of socioeconomic disadvantage in this region, lack of intervention effect on maternal weight outcomes and cultural beliefs or traditions. Given participants' basic needs, such as stable housing and/or safe living conditions, food security, dependable transportation, and enduring telephone service, discussions concerning weight, diet, exercise and infant growth may have been somewhat underwhelming or overwhelming for participants. Additionally, significant differences between treatment arms were not found for either gestational weight gain or maternal weight loss in the postnatal period 26, 27. The lack of effect may be at least partly attributed to the tendency of African American women to evaluate their attractiveness independently of perceived weight 28, and this behaviour may extend to weight perceptions of their children. Finally, cultural traditions in the South such as family consumption of ‘soul foods’ can increase the risk of obesity in African American children 29.
The current study had a number of strengths including its longitudinal design, use of time‐to‐event analysis for infant growth and the population studied. However, several limitations bear mentioning. With the exception of the anthropometric measures, information was self‐reported, and data collection was not blinded. Additionally, the sample size was small, which may have limited the ability to detect meaningful associations with infant growth outcomes. Difficulties with recruitment included low rates of referral by prenatal healthcare providers and most local WIC and health department nutritionists, relocation of a healthcare clinic to a smaller building that could not accommodate onsite recruitment by research staff, and competition for this population of pregnant women by other programs operating in the same area. Difficulties with retention were mainly attributed to the gestational period during which 100% turnover of the Parent Educators resulted in the largest loss of participants. It is possible that the length of the lessons also may have adversely affected retention.
In conclusion, as compared with the standard PAT curriculum, the diet and physical activity enhanced PATE curriculum was not effective at improving growth outcomes of infants born to this cohort of rural, Southern, primarily African American women. Given the majority of infants experienced adverse weight outcomes in the first year of life, concerted efforts are needed to break the intergenerational cycle of obesity present in this region of the nation.
Conflicts of Interest Statement
All authors declare they have no conflicts of interest to report.
Acknowledgements
J. T. contributed to the study design and data acquisition, analysis and interpretation and drafted the paper. M. G. contributed to data acquisition, analysis and interpretation. L. T.‐H. contributed to the study design and data analysis and interpretation. A. L. contributed to data acquisition and interpretation. All authors participated in the revising of this paper and have read and approved the final paper. The authors thank Debra Johnson and Donna Ransome for their research support, including reviewing an early version of this paper. This work was supported by the United States Department of Agriculture, Agricultural Research Service [Project 6401‐5300‐003‐00D] and in‐kind support from the Delta Health Alliance. The views expressed are solely those of the authors and do not reflect the official policy or position of the US government.
Thomson, J. L. , Goodman, M. H. , Tussing‐Humphreys, L. M. , and Landry, A. S. (2018) Infant growth outcomes from birth to 12 months of age: findings from the Delta Healthy Sprouts randomized comparative impact trial. Obesity Science & Practice, 4: 299–307. 10.1002/osp4.272.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA‐J Am Med Assoc 2014; 311: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trust for America's Health, Robert Wood Johnson Foundation . The state of childhood obesity. https://stateofobesity.org/childhood. Accessed January 9, 2018.
- 3. Dixon B, Peña M‐M, Taveras EM. Lifecourse approach to racial/ethnic disparities in childhood obesity. Adv Nutr 2012; 3: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta‐analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child 2012; 97: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomson JL, Tussing‐Humphreys LM, Goodman MH. Delta Healthy Sprouts: a randomized comparative effectiveness trial to promote maternal weight control and reduce childhood obesity in the Mississippi Delta. Contemp Clin Trials 2014; 38. [DOI] [PubMed] [Google Scholar]
- 6. Parents as Teachers National Center Inc . Parents as Teachers. http://www.parentsasteachers.org. Accessed April 12, 2017.
- 7. Bandura A. Human agency in social cognitive theory. Annu Rev Psychol 1989; 44: 1175–1184. [DOI] [PubMed] [Google Scholar]
- 8. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot 1997; 12: 38–48. [DOI] [PubMed] [Google Scholar]
- 9. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell KJ, Lioret S, McNaughton SA, et al. A parent‐focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics 2013; 131: 652–660. [DOI] [PubMed] [Google Scholar]
- 11. Nowak AJ, Casamassimo PS. Using anticipatory guidance to provide early dental intervention. J Am Dent Assoc 1995; 126: 1156–1163. [DOI] [PubMed] [Google Scholar]
- 12. Mullis F. Active parenting: an evaluation of two Adlerian parent education programs. J Individ Psychol 1999; 55: 359–364. [Google Scholar]
- 13. National Center for Health Statistics . WHO growth charts. https://www.cdc.gov/growthcharts/who_charts.htm. Accessed May 12, 2017.
- 14. Thomson JL, Tussing‐Humphreys LM, Goodman MH, Landry AS, Olender SE. Low rate of initiation and short duration of breastfeeding in a maternal and infant home visiting project targeting rural, Southern, African American women. Int Breastfeed J 2017; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Heal 2015; 1: 40–43. [DOI] [PubMed] [Google Scholar]
- 16. Ruan H, Xun P, Cai W, He K, Tang Q. Habitual sleep duration and risk of childhood obesity: systematic review and dose‐response meta‐analysis of prospective cohort studies. Sci Rep 2015; 5: 16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tikotzky L, De Marcas G, Har‐Toov J, Dollberg S, Bar‐Haim Y, Sadeh A. Sleep and physical growth in infants during the first 6 months. J Sleep Res 2010; 19: 103–110. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization Department of Nutrition for Health and Development . WHO child growth standards: methods and development.; 2006.
- 19. Ong K, Loos R. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 2006; 95: 904–908. [DOI] [PubMed] [Google Scholar]
- 20. Hosmer D, Lemehow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley & Sons: New York, NY, 1999. [Google Scholar]
- 21. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. J Am Med Assoc 2012; 307: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savage JS, Birch LL, Marini M, Anzman‐Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatr 2016; 170: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aldrich H, Gance‐Cleveland B. Comparing weight‐for‐length status of young children in two infant feeding programs. Matern Child Health J 2016; 20: 2518–2526. [DOI] [PubMed] [Google Scholar]
- 24. Gregory EF, Goldshore MA, Henderson JL, Weatherford RD, Showell NN. Infant growth following maternal participation in a gestational weight management intervention. Child Obes 2016; 12: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Felső R, Lohner S, Hollódy K, Erhardt É, Molnár D. Relationship between sleep duration and childhood obesity: systematic review including the potential underlying mechanisms. Nutr Metab Cardiovasc Dis 2017; 27: 751–761. [DOI] [PubMed] [Google Scholar]
- 26. Thomson JL, Tussing‐Humphreys LM, Goodman MH, Olender SE. Gestational weight gain: results from the Delta Healthy Sprouts comparative impact trial. J Pregnancy 2016: 5703607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tussing‐Humphreys LM, Thomson JL, Hemphill NO, Goodman MH, Landry AS. Maternal weight in the postpartum: results from the Delta healthy sprouts trial. Matern Heal Neonatol Perinatol 2017; 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chithambo TP, Huey SJ. Black/white differences in perceived weight and attractiveness among overweight women. J Obes 2013; 2013: 320326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yancey AK, Kumanyika SK. Bridging the gap: understanding the structure of social inequities in childhood obesity. Am J Prev Med 2007; 33: S172–S174. [DOI] [PubMed] [Google Scholar]


