Abstract
A systematic review was performed to estimate the duration of protection of Hepatitis-B vaccine after primary vaccination during infancy. The number of seropositive participants with anti-HBs antibody titer ≥ 10 mIU/ml and seronegative participants who had anti-HBs antibody titer ≤ 10 mIU/ml after booster dose was the main outcome criteria to find out the protection time of Hepatitis-B vaccine. Twelve studies were selected for systematic review. Overall, results from the meta-analysis have revealed that the risk of Anti-HBs Titer ≤ 10 mIU/ml reduced by 50%. Upon performing the sub-group analysis it was revealed that the overall risk of having Anti-HBs Titre ≤ 10 mIU/ml was reduced up to 62% among the subjects age 21–30 years (0.38 [0.34, 0.44]; I2 = 0.0%, p = 0.938). Furthermore, it was observed that the risk of having titre level less than 10 mIU/ml for plasma derived vaccines were to be 56% [0.44, CI 0.33–0.57, I2 90.9%, p = <0.001]. Vaccination in early infancy does not ensure protection against Hepatitis-B infection. There is a strong correlation between the duration of protection and time elapsed after primary immunization during infancy.
Introduction
Hepatitis-B is a serious health problem worldwide. According to World Health Organization (WHO) in year 2016 about 240 million people were infected with Hepatitis-B1, and about 686,000 death were reported2,3. Countries with the highest disease burden are China, Indonesia, Nigeria, parts of Africa and Asia4,5. Norway and United Kingdom are observed to be the countries with the prevalence of hepatitis-B, as low as 0.01%, while the highest prevalence is observed in Sudan where it is up to 22.70%6.
In South Asia the prevalence rate of chronic Hepatitis-B is 2–5%1. A decrease in hepatitis-B prevalence has been seen in countries where routine immunization plan have been implemented7.
Vaccination with Hepatitis-B has been considered as a very important tool for protection against HBV infection8. The protective response to Hepatitis-B vaccine is quantified by measuring anti-HBS level in 6–8 weeks after vaccination, for successful immunization the anti-HBS level should be greater than 10 mIU/ml. According to WHO the Hepatitis-B vaccination should produce the protective level of antibodies in ≥95% of the individuals after completion of the recommended vaccination schedule9. However, in some cases primary and secondary vaccination failure led to occurrence of hepatitis B infection among the individual. When the Infections occur in short time after the vaccination, it is termed as primary vaccination failures. However, in the case when there is loss of seroprotective response that is termed as secondary vaccination failure due to loss of immunity10, which is due to decline in the immunological memory which wane over time11. The individual whose anti-HBS level falls below 10 mIU/ml is not protected anymore. However the individual is not under the threat from hepatic disease because of immune memory related to Hepatitis-B surface antigen (HBsAg). The specific memory after Hepatitis-B vaccination is due to an anamnestic anti-HBs response after booster dose of vaccine. The booster dose lead to spontaneous rise in anti-HBs level in the population who have completed their initial vaccination series12,13. According to European consensus group on Hepatitis-B immunity the duration of protection among fully vaccinated children is 15 years12.
The protection period of Hepatitis-B vaccine (either derived from plasma or recombinant vaccine) is not well understood8,14–16. According to WHO global immunization coverage data sheet 2014 highest coverage for Hepatitis-B vaccine is seen in western pacific where it is estimated to be 92%,while lowest is 10% in African region.
Study Question and Aim
The duration of protection of Hepatitis-B vaccine is still variable and a very limited data is available on this topic especially in developing countries. Most regions/countries have different vaccination schedule, this results in failure to develop the policies and procedures to control the spread of Hepatitis-B in the world, particularly in under developed countries. Currently available vaccines are highly safe and effective but the available data shows that antibody titer declines with time17,18. Secondary vaccination failure is one of the main concern for the most of the policy makers in order to ensure the decline in mortality and morbidity of.
Therefore, this systematic review aims at assessing the duration of protection of Hepatitis-B vaccine after primary vaccination during infancy/childhood as well as the need for booster dose in case the antibody titer is below immunoprotection level. Findings in this regard will assist the policy makers to develop guidelines for booster dose.
Method
A systematic review of the scientific literature was performed. All studies published from 1st Jan 2000 till 31st December 2016 was assessed for potential inclusion in this systematic review.
Search Strategy
The syntax used for literature survey is Immune Memory OR immunopersistence AND Hepatitis-B AND Vaccine OR vaccination OR Immunization AND infants OR newborn OR birth OR cohort. The relevant studies were identified through Pubmed, Medline, Embase, Google Scholar and Cochrane Library.
Population, intervention, comparator and outcomes
Population = Seronegative children/adults
Intervention = Hepatitis-B vaccine
Comparator = None
Outcome = The outcomes studied in this systematic review include the percentage of the children/adults with antibody titer ≥10 mIU/ml and percentage of seronegative children/adults who have seroprotective antibody titer ≥10 mIU/ml in response to single booster dose. In addition, continuous data i.e. antibody titer, standard deviation and number patients before and after the booster dose will also be extracted to estimate the differences in the titer before and after vaccination.
Inclusion Exclusion Criteria
All observational studies reporting the antibody titer after primary vaccination with Hepatitis-B vaccine during infancy with or without the information regarding the administration of booster dose were included for further assessment.
We excluded all case studies/reports, letter to the editors, review papers, personal opinions or any other type of study with inconsistent data or not reporting original data. Similarly the studies were excluded if primary vaccination was done six months after birth. All studies with follow up duration less than two years were also excluded. Studies were excluded if the booster dose was administered between the primary vaccination series and follow up study.
Data extraction
The titles, abstracts and the contents of the study were reviewed according to our inclusion and exclusion criteria. Any question/concern regarding the selection of articles was settled through consensus. The data collected was Name of investigator, Country of study, Year of study, Study population, No. of patients with Hepatitis-B antibody titer ≥ 10 mIU/ml, No. of patients with Hepatitis-B antibody titer ≥ 10 mIU/ml after booster dose, Vaccine type, Dosage of vaccine and Dosage schedule. Two investigators independently evaluated the identified research studies and extracted the relevant outcomes of the studies. The outcomes of the studies were extracted by using the data extraction table especially designed for this purpose (Table 1). Any confusion regarding the results of selected study was resolved through mutual consensus.
Table 1.
Anti-HBs Titer ≥ 10 mIU/ml before and after Booster Dose.
| Authors | Country of Study | Study Design | Year of Study | Age at Follow up (Years) | Study Population | Patients with anti-HBS ≥ 10 mIU/ml | Anti-HBS antibody Titer After Boosting | Vaccine Type | Dosage of Vaccine | Vaccination Schedule 1 s 2nd 3rd | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruce et al. | Alaska | Prospective Cohort | 2011 | 30 | 243 | 51% | 88% | Plasma Derived | Standard | Vaccinated at 06 months age. | ||
| Wu et al. | China | Randomized placebo controlled cohort | 2010 | 23 | 126 | 48.1% | 84% | Plasma Derived | Standard | 00 | 01 | 06 |
| Poovorawan et al. | Thailand | Cohort Study | 2009 | 20 | 109 | 60.5% | 83.9% | Recombinant | Standard | 00 | 01/02 | 12 |
| Al-Ghamdi et al. | Saudi Arabia | Cross Sectional Cohort Study | 2012 | 20 | 238 | 58.8% | Not Specified | Standard | ||||
| Su et al. | Taiwan | Cross Sectional Study | 2012 | 18 | 1734 | 35.9% | 95% | Plasma Derived | Standard | 01 | 02 | 12 |
| Al Faleh et al. | Saudi Arabia | Cross Sectional Study | 2008 | 18 | 1355 | 38.0% | Not Specified | Recombinant | Standard | 00 | 01 | 06 |
| Spada et al. | Italy | Follow up Study | 2010 | 17 | 571 | 72.9% | Not Specified | Standard | ||||
| Middleman et al. | U.S.A | Cross Sectional Study | 2014 | 16–19 | 420 | 24.0% | 92% | Recombinant | Standard | 00 | 01 | 12 |
| Avidoca et al. | Slovakia | Longitudinal Study | 2010 | 10–11 | 31 | 48.4% | 96% | Standard | 03 | 05 | 12 | |
| Gold et al. | Israel | Cross Sectional Study | 2000 | 08 | 122 | 77.1% | Not Specified | Recombinant | Standard | 00 | 01 | 06 |
| Tsega et al. | Ethiopia | Longitudinal Study | 2010 | 05 | 314 | 89% | Not Specified | Recombinant | Standard | 00 | 01 | 06 |
| Amini et al. | Iran | Longitudinal Study | 1999 | 02 | 542 | 97% (Children) 82% (Adults) | Not Specified | Recombinant | Standard | 00 | 01 | 06 |
Data analysis
Meta-analysis was carried out by using STATA version 14.3®. Risk ratios were estimated using random effect model. The binary data was analyzed to estimate the risk of having Hepatitis-B antibody titer ≥ 10 mIU/ml after booster dose. Subgroup analysis was performed to address heterogeneity. Age and type of vaccines were used as the grouping variables. Tau2 was used to interpret the heterogeneity at a confidence interval of 95%. In addition, quality of studies was also performed using New Castel Ottawa scale for observational studies.
Results
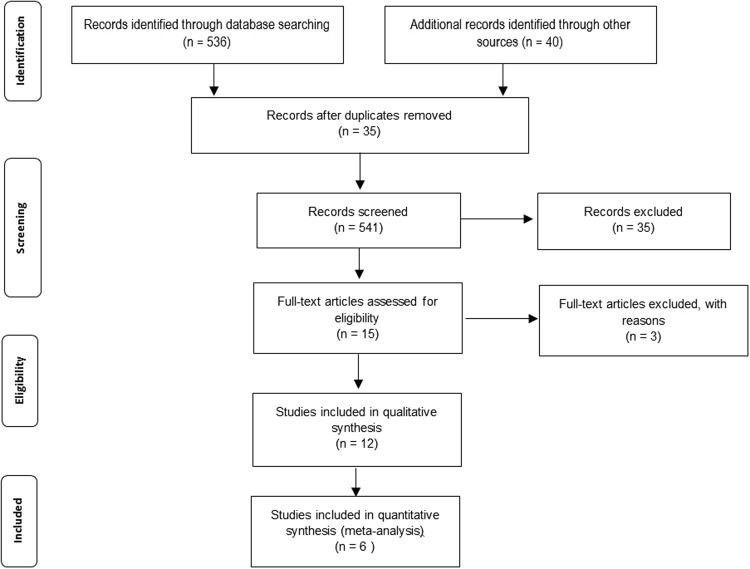
A total of N = 576 studies were identified for the initial assessment and potential inclusion in this systematic review. Upon applying the inclusion criteria n = 12 studies were eligible for the qualitative synthesis. Upon screening the extracted data, n = 6 studies were selected for the meta-analysis. Details illustration of the screening the selected articles are shown in the Fig. 1.
Figure 1.
PRISMA 2009 Flow Diagram.
Persistence of seroprotective Anti-HBs Titer
A total of N = 5805 patients were presented across the twelve studies8,19–29. Only six studies have reported the anti HBS titer after booster dose of hepatitis-B vaccine, with a follow up of two years8,19,20,22,25,26. A single booster dose of vaccine was administered only to those participants who had antibody titer ≤ 10 mIU/ml at the time of follow up, antibody titer was again determined one month after challenge dose. However Su et al. administered two subsequent booster doses of Hepatitis-B vaccine. Second booster dose was administered six months after the first dose, if the participant had ant-HBV titer ≤ 10 mIU/ml after the first challenge dose. Anti-HBV antibody titer was again determined one month after the second challenge dose22. An increase in anti HBS titer ≥ 10 mIU/ml after booster dose ranging from 23.4% to 68.0% of participants than the anti HBS titer before booster dose. Details are given in Table 1. Among the eligible studies one was randomized placebo controlled study19, one study was Phase-4 Open follow up and Challenge Study26, three were Cohort Studies8,20,21. Three were follow up studies24,28,29 four were cross sectional studies22,23,25,27.
All participants of the study were fully immunized during infancy with either recombinant or plasma derived hepatitis-B vaccine. Bruce et al. in a long term prospective cohort study conducted in Alaska, data was collected after thirty years of the primary vaccination with plasma derived vaccine on 243 participants. Of whom 51% of the participants has anti-HBs titer above seroprotection level (titer ≥ 10 mIU/ml)8. In a randomized placebo controlled trial cohort study conducted in China on 126 persons immunized with plasma derived vaccine during infancy it was observed that 48.1% of subjects were seroprotective with an antibody titer ≥ 10 mIU/ml after 23 years19. In a cohort study conducted in Thailand by Poovorawan et al. reported data of 109 participants with recombinant Hepatitis-B vaccine, of whom 60.5% of participants were maintained with the seroprotective level of Anti-HBs anti-bodies20. Al-Ghamidi et al. reported data of 238 participants from a cross sectional study conducted in Saudi Arabia, it was observed that 58.8% participants were seroprotected after twenty years after primary vaccination with recombinant hepatitis-B vaccine21. During an eighteen years cross sectional study in Taiwan on 1734 participants, only 35.9% of participants were seroprotected22. In eighteen years cross sectional cohort study in Saudi Arabia by Al-Faleh et al. on 1355 participants, only 38% of the participant had seroprotective anti-HBs tier ≥ 10 mIU/ml23. Spada et al. conducted a 17 years follow up study on 571 participants in Italy and noticed that 72.9% of participants were seroprotected24. In a recent cross sectional study conducted by Middleman et al. in USA among 420 Participants, of whom only 24% of the participants were seroprotected, 16–19 years after primary vaccination with Recombinant Hepatitis-B vaccine during infancy25. In an eleven years phase-4 open follow up and challenge study on 31 participants in Slovakia by Avidoca et al., the seroprotection level was to be 48.4%26. Gold et al. in a cross sectional study in Israel found that 77.1% of the participants were seropotected27. Tsega et al. in a follow up study carried out five years after primary vaccination with recombinant Hepatitis-B vaccine in Ethiopia revealed that 89% of participants were still seroprotected28. In a two years follow up study conducted by Amini et al. in Iran on 542 participants establish that 97% of the participants had anti-HBs titer ≥ 10 mIU/ml after primary immunization with recombinant Hepatitis-B vaccine during infancy29.
Response to Booster Dose
The antibody titer ≤ 10 mIU/ml does not always means the loss of immunity because the vaccine protection persists beyond the time during which antibody titer is above seroprotective level. The rise in anti-HBs titer above seroprotection level after the booster dose means that anamnestic response to vaccine still persists30. For example, results Bruce et al. revealed that Anti-HBs antibody titer above seroprotection level raised from 51% to 88% after booster dose which shows that immune memory still persists even though the Anti-HBs level is below seroprotection level8. Similarly in the randomized placebo controlled trial cohort study carried out by Qian Wu et al. the number of seroprotected individuals rise from 48.1% to 84% after booster dose19. Poovorawan et al. in a cohort study in Thailand observed that number of seroprotected individuals rose from 60.5% to 83.9% after a single booster dose20. Su et al. in across sectional study in Taiwan observed a rise in Anti-HBs level above seroprotection level from 35.9% to 95% after booster dose which shows the persistence of immune memory22. Middleman et al. in a cross sectional study conducted in USA observed an increase in the number of seroprotected individuals from 24% to 92% after single booster dose25. Avidoca et al. in a phase-4 open follow up and challenge study in Slovakia observed that the number of seroprotected individuals rose from 48.4% to 96% showing the persistence of immune memory Eleven years after primary vaccination during infancy26.
Quality of studies
All the studies included in this systematic review were observational studies. New castle Ottawa scale was used to estimate the quality. Overall most studies were of good quality with a NOS score ranging from 7 to 8. Details are shown in Table 2.
Table 2.
Quality assessment using New Castel Ottawa Scale.
| Authors/reference | Selection | Comparability | Outcomes | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representative of Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcomes not Present at Baseline | Assessment of Outcomes | Sufficient Follow up Duration | Adequate Follow up | |||
| Bruce et al. | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Wu et al. | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Poovorawan et al. | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Al-Ghamdi et al. | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Su et al. | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Al Faleh et al. | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Spada et al. | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Middleman et al. | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Avidoca et al. | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Gold et al. | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Tsega et al. | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Amini et al. | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
Meta-analysis for the post booster titer comparison
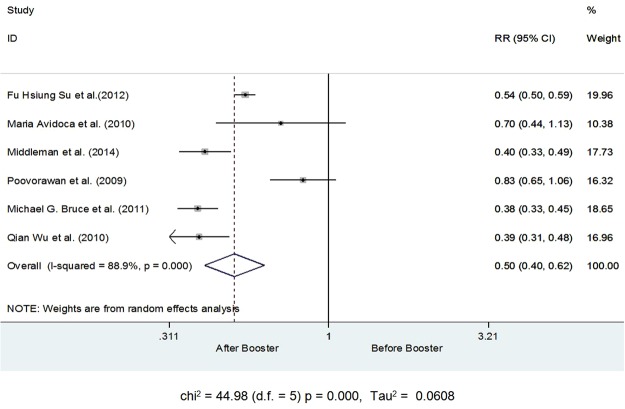
Quantitative data from six studies were considered suitable for the Meta-analysis. Number of subjects with Anti-HBs Titer ≥ 10 mIU/ml before and after the booster dose was used to estimate the overall effect of the booster dose. Upon initial analysis it was revealed that after receiving the booster dose the risk of having Anti-HBs Titer ≤ 10 mIU/ml reduced by 50% [RR 0.50, CI 0.40–0.63, I2 88.9%, Tau2 0.0608, p = <0.001] after receiving the booster dose. The decline in the risk of having Anti-HBs Titer ≤ 10 mIU/ml was variable across all studies, ranging from 17% to 62%. The decline in risk was highest for the study by Bruce et al. (2011) and Qian Wu et al. (2010) details are shown in Fig. 2.
Figure 2.
Number of subjects with Anti-HBs Titer ≥ 10 mIU/ml before and after the booster dose.
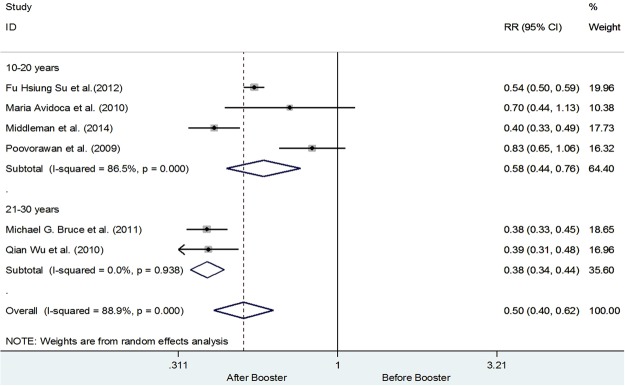
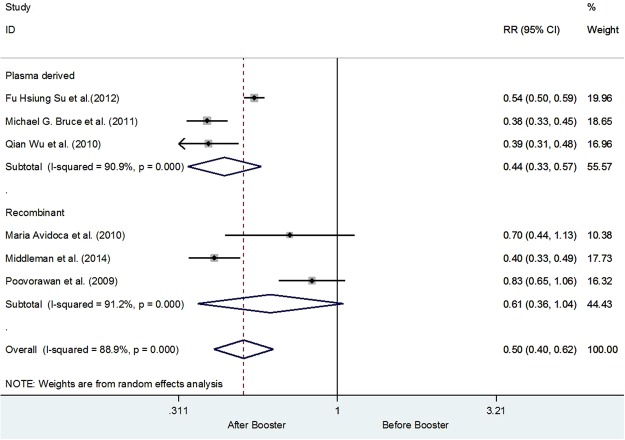
Keeping in view the heterogeneity subgroups analysis was performed using age group 10–20 years and 21–30 years respectively. Four studies have recruited subjects from the age of 10–20 years upon receiving the booster dose20,22,25,26. While only two studies were from the age group 21–30 years8,19. Upon performing the sub-group analysis it was revealed that the overall risk of having Anti-HBs Titer ≤ 10 mIU/ml was reduced up to 42% among the subjects age 10–20 years (0.0.58 [0.44, 0.76]). However, heterogeneity was still 86.5%, while for the age 21–30 years were observed to have 62% decline in the risk of having Anti-HBs Titer ≤ 10 mIU/ml (0.38 [0.34, 0.44]; I2 = 0.0%, p = 0.938), In addition, all the studies from the subgroup of 10–20 years of age have adapted a 12 month vaccination schedule in comparison to the 21–30 years of age group who adapted a 6 month vaccination schedule. Details are shown in Fig. 3. Furthermore, it was also observed that the risk of having titer level less than 10 mIU/ml was 39% (RR 0.61 CI 95% 0.36–1.04) less in the recombinant vaccines than the plasma derived vaccines. It is observed that the risk of having titer level less than 10 mIU/ml for plasma derived vaccines were to be 56% [0.44, CI 0.33–0.57, I2 90.9%, p = <0.001]. Details are shown in the Fig. 4.
Figure 3.
Number of subjects with Anti-HBs Titer ≥ 10 mIU/ml before and after the booster dose using age as the grouping variable.
Figure 4.
Anti-HBs Titer ≥ 10 mIU/ml before and after the booster dose using types of vaccines as a grouping variable.
Discussion
The current systematic review is perhaps the first to explore the dosing frequency impact on the anti-HBs antibody titer. Anti-HBs antibody titer ≥ 10 mIU/ml and response to booster dose are considered as protection markers against Hepatitis-B infection. Overall, results from the meta-analysis have revealed that the risk of Anti-HBs Titer ≤ 10 mIU/ml reduced by 50%. In addition it was observed that likelihood of the having Anti-HBs Titer ≤ 10 mIU/ml reduced by 42% among the subjects age 10–20 years (0.58 [0.44, 0.76]. Furthermore, with the use of plasma derived vaccines the risk of having Anti-HBs Titer ≤ 10 mIU/ml reduced by 56%. In the studies referred above the protective antibody concentration decreases with time since primary vaccination and booster dose is required to keep antibody titer above seroprotective level. A decrease in anamnestic response has been observed after twenty years of primary vaccination. The response to booster dose decreases with age. This decrease in vaccine protection level is accelerated by vaccination with lower vaccine dose than currently recommended dose at the time of primary vaccination. Therefore without the administration of booster dose the duration of protection in fully vaccinated individuals remains under question.
Data studied for review reveals that after two years of primary vaccination more than 95% of individuals were seroprotected with anti-HBs level ≥ 10 mIU/ml. The level of protection continue to decrease with passage of time as after five years of primary vaccination only 80–90% individuals were found to be seroprotected with anti-HBs level more than 10 mIU/ml. This protection level drops further to 70% ten years after primary vaccination with Hepatitis-B vaccine. Booster dose presents viral challenge in previously immunized individuals which result in spontaneous rise in anti-HBs antibody level due to immunological memory. This rise in antibody level suggests that there is no need for booster dose administration. From the data it is evident that no booster dose is required until 10 years after primary vaccination with Hepatitis-B vaccine.
Limited data is available on duration of protection in adults after the primary vaccination during infancy. Several studies in high risk population show the persistence of immunological memory for as much as 12 years after primary vaccination. Studies on duration of action of Hepatitis-B vaccine is important for healthcare authorities in order to plan for the immunization programs and to make booster dose policy. Lower dose of vaccine and the gap between two consecutive doses seem to be contributing factors in the duration of protection of Hepatitis-B vaccine. The individuals who received the primary vaccination during infancy are better protected. The differences between recombinant vaccine and plasma derived vaccine are also under debate in many countries which require further investigations. No connection is found between the endemic status of the region and duration of protection. The persistence of vaccine immunity requires further studies because large scale seroepidemiological studies in adults are still not been conducted, who are born after the implementation of Hepatitis-B vaccination program. The studies in different ethnic populations are required to determine that same observations are replicable in other ethnic populations as well.
Limitations
A higher heterogeneity among the studies is one of the main issues that should be kept in view while interpreting the results of the meta-analysis. The heterogeneity among the studies was not statistical and can be due to the clinical and demographics related factors among the participants from the included studies. Secondly we did not investigate the data on duration of protection of Hepatitis-B vaccine in children born with Hepatitis B antigen carrier mothers. The effects of under dose of vaccine and gap between the last dose and the preceding dose during primary vaccination should also be studied for the duration of protection of Hepatitis-B vaccine. Finally the entire analysis was performed using published data, and due to variability of data reporting among the studies further analysis to estimate the dose response relationship was not possible to estimate.
Conclusion
Results have revealed that risk of having Anti-HBs Titer ≤ 10 mIU/ml reduced by 50% after receiving the booster dose. Use of plasma derived vaccines and participant age of 21–30 years were identified to be the other two factors resulting in higher Anti-HBs titer.
Recommendations
The protection of an individual remains under question without the administration of booster dose 12 years after primary vaccination during infancy. After this period a booster dose should be administered to check the persistence of immune memory. If the anti-HBS tier remains less than 10 mIU/ml even after the administration of booster dose, revaccination should be done.
Author Contributions
S.M., T.M.K. conceived the idea; K.U.S., S.M. performed the search and data extraction; S.M. and K.U.S. devised the method and analyzed the data; TMK verified the data and analysis. S.M. and T.M.K. wrote the initial draft; K.U.S., S.M. and T.M.K. finalized the draft for submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kifayat Ullah Shah, Email: kushah@qau.edu.pk.
Tahir Mehmood Khan, Email: tahir.khan@uvas.edu.pk.
References
- 1.World Health Organization fact sheet published in July, 2016, http://www.who.int/mediacentre/factsheets/fs204/en/.
- 2.MacLachlan JH, Locarnini S, Cowie BC. Estimating the global prevalence of hepatitis B. The Lancet. 2015;386:1515–1517. doi: 10.1016/S0140-6736(15)61116-3. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, et al. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein ST, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. International journal of epidemiology. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 5.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiologic reviews. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 6.Ott J, Stevens G, Groeger J, Wiersma S. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 7.Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization’s Western Pacific Region: targets, strategies, status. Vaccine. 2013;31:J85–J92. doi: 10.1016/j.vaccine.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 8.Bruce, M. G. et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. Journal of Infectious Diseases, jiv748 (2016). [DOI] [PubMed]
- 9.Ahmed W, et al. Evaluation of Antibody Response of Hepatitis B Vaccine Provided by the Chief Minister Program of Sindh Province. Pakistan Journal of Medical Research. 2015;54:57. [Google Scholar]
- 10.Schönberger K, et al. Determinants of long-term protection after hepatitis B vaccination in infancy: a meta-analysis. The Pediatric infectious disease journal. 2013;32:307–313. doi: 10.1097/INF.0b013e31827bd1b0. [DOI] [PubMed] [Google Scholar]
- 11.Chaves SS, et al. Improved anamnestic response among adolescents boosted with a higher dose of the hepatitis B vaccine. Vaccine. 2010;28:2860–2864. doi: 10.1016/j.vaccine.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Kane M, Banatvala J, Da Villa G, Esteban R. Are booster immunisations needed for lifelong hepatitis B immunity? The Lancet. 2000;355:561. doi: 10.1016/S0140-6736(99)07239-6. [DOI] [PubMed] [Google Scholar]
- 13.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clinical Infectious Diseases. 2011;53:68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 14.Keck JW, et al. Hepatitis B virus antibody levels 7 to 9 years after booster vaccination in Alaska native persons. Clinical and Vaccine Immunology. 2014;21:1339–1342. doi: 10.1128/CVI.00263-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dentinger CM, et al. Persistence of antibody to hepatitis B and protection from disease among Alaska natives immunized at birth. The Pediatric infectious disease journal. 2005;24:786–792. doi: 10.1097/01.inf.0000176617.63457.9f. [DOI] [PubMed] [Google Scholar]
- 16.Hammitt LL, et al. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine. 2007;25:6958–6964. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 17.Watson B, West DJ, Chilkatowsky A, Piercy S, Ioli VA. Persistence of immunologic memory for 13 years in recipients of a recombinant hepatitis B vaccine. Vaccine. 2001;19:3164–3168. doi: 10.1016/S0264-410X(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 18.McMahon BJ, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Annals of internal medicine. 2005;142:333–341. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, et al. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine. 2011;29:2302–2307. doi: 10.1016/j.vaccine.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Poovorawan Y, et al. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28:730–736. doi: 10.1016/j.vaccine.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 21.Al Ghamdi SS, Fallatah HI, Fetyani DM, Al‐Mughales JA, Gelaidan AT. Long‐term efficacy of the hepatitis B Vaccine in a high‐risk group. Journal of Medical virology. 2013;85:1518–1522. doi: 10.1002/jmv.23658. [DOI] [PubMed] [Google Scholar]
- 22.Su F-H, et al. Significance and anamnestic response in isolated hepatitis B core antibody-positive individuals 18 years after neonatal hepatitis B virus vaccination in Taiwan. Vaccine. 2012;30:4034–4039. doi: 10.1016/j.vaccine.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 23.AlFaleh F, et al. Long-term protection of hepatitis B vaccine 18 years after vaccination. Journal of infection. 2008;57:404–409. doi: 10.1016/j.jinf.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Spada E, et al. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17‐year follow‐up study. Clinical Microbiology and Infection. 2014;20:O680–O686. doi: 10.1111/1469-0691.12591. [DOI] [PubMed] [Google Scholar]
- 25.Middleman AB, et al. Duration of protection after infant hepatitis B vaccination series. Pediatrics. 2014;133:e1500–e1507. doi: 10.1542/peds.2013-2940. [DOI] [PubMed] [Google Scholar]
- 26.Avdicova M, Crasta PD, Hardt K, Kovac M. Lasting immune memory against hepatitis B following challenge 10–11 years after primary vaccination with either three doses of hexavalent DTPa-HBV-IPV/Hib or monovalent hepatitis B vaccine at 3, 5 and 11–12 months of age. Vaccine. 2015;33:2727–2733. doi: 10.1016/j.vaccine.2014.06.070. [DOI] [PubMed] [Google Scholar]
- 27.Gold Y, Somech R, Mandel D, Peled Y, Reif S. Decreased immune response to hepatitis B eight years after routine vaccination in Israel. Acta Paediatrica. 2003;92:1158–1162. doi: 10.1111/j.1651-2227.2003.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsega E, et al. Antibody levels in Ethiopian children five years after vaccination with two different doses of hepatitis B vaccine: Is there a need for booster vaccine? Canadian Journal of Gastroenterology and Hepatology. 1998;12:57–60. doi: 10.1155/1998/213028. [DOI] [PubMed] [Google Scholar]
- 29.Amini S, Andalibi S, Mahmoodi M. Anti-HBs response and its protective effect in children and adults receiving hepatitis B recombinant vaccine in Tehran. Iranian Journal of Medical Sciences. 2015;27:101–105. [Google Scholar]
- 30.Lao TT. Immune persistence after hepatitis B vaccination in infancy–Fact or fancy? Human vaccines & immunotherapeutics. 2016;12:1172–1176. doi: 10.1080/21645515.2015.1130195. [DOI] [PMC free article] [PubMed] [Google Scholar]