Abstract
Background/Objectives
Serum S100A8/S100A9 and S100A12 levels have been shown to be elevated in giant cell arteritis (GCA). This study aimed to determine if levels of serum S100 proteins perform as markers in a comparable fashion to standard markers of disease activity in large-vessel vasculitis.
Methods
Serum samples were obtained from the Vasculitis Clinical Research Consortium (VCRC) Longitudinal Prospective Cohort Study of GCA and Takayasu’s arteritis (TAK). A mixed effects model compared S100 proteins during active and inactive disease states. Receiver operating characteristic curves compared models using S100 proteins to models using ESR and CRP.
Results
There were 106 samples (50 during active disease) from patients with GCA and 32 samples (16 during active disease) from patients with TAK. In GCA, S100A8/S100A9 and S100A12 were significantly elevated during active disease (1445.6 ng/mL vs. 1095.7 ng/mL, p = 0.003;163.2 ng/mL vs. 116.6ng/mL, p=0.016, respectively). There were weak correlations between levels of S100 proteins and ESR or CRP. A model including S100A8/S100A9, S100A12, ESR, and CRP was a better indicator of disease activity compared to ESR and CRP together. In TAK, there were no significant differences between active and inactive disease for either the S100 proteins or ESR/CRP.
Conclusions
Serum levels of S100A8/S100A9 and S100A12 are elevated during active disease and perform comparably to ESR and CRP as measures of disease activity in giant cell arteritis.
INTRODUCTION
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are widely used biomarkers in giant cell arteritis (GCA) and Takayasu’s arteritis (TAK), however, both can be normal in smoldering disease1–3 and other biomarkers have proven unhelpful4. A search for improved biomarkers is warranted. Calgranulins, including S100A8 (calgranulin A; myeloid-related protein 8), S100A9 (calgranulin B; myeloid-related protein 14) and S100A12 (calgranulin C; myeloid-related protein 6), are calcium binding proteins expressed during myeloid differentiation. S100 proteins have been shown to be useful biomarkers in various rheumatic diseases including: oligoarticular and polyarticular juvenile rheumatoid arthritis (JRA), ulcerative colitis, systemic lupus erythematosus, reactive arthritis, and rheumatoid arthritis5–10. In GCA S100A8/S100A9 complexes can be identified in the adventitia and media of affected arteries, whereas S100A12 expression is found around the vasa vasorum of the adventitia11. Serum levels of S100 proteins are also elevated in GCA compared to healthy controls11. Moreover, gene expression profiles of temporal arteries from patients with GCA, revealed a significant increase in the gene expression of S100A8, S100A9 and S100A12 as compared to control temporal arteries12. In addition, S100 positive cells are prominent in the inflammatory infiltrate of the aortic wall of patients with TAK 13. The aim of this study is to assess the association of peripheral S100 proteins with disease activity in two forms of large vessel vasculitis, GCA and TAK.
METHODS
Serum samples were obtained from a longitudinal prospective cohort including GCA and TAK patients from multiple centers located in the United States and Canada. This study is in compliance with the Declaration of Helsinki and has been approved by the local institutional review board. All patients met either the modified American College of Rheumatology (ACR) criteria for GCA or the modified ACR criteria for TAK14,15. Active disease was defined as a Birmingham Vasculitis Activity Score version 1 score greater than 0 and a physician’s global assessment greater than 0. The serum concentrations of S100A8/S100A9 heterodimers and S100A12 were measured by ELISA11. Comparisons were made to S100A8/S100A9 levels in healthy, historical controls (n=35) measured in the same laboratory using a two tailed student’s T-test11.
A mixed effects model was used to compare average levels of S100A8/S100A9 or S100A12 in active and inactive disease states. The qualities of predictions of disease activity from these models using either the traditional measures (ESR and CRP) or the two new measurements (S100A8/S100A9 and S100A12) were assessed using receiver operating characteristic (ROC) curves with confidence intervals using DeLong approach. A significance level of 5% was used for all testing.
RESULTS
Giant Cell Arteritis
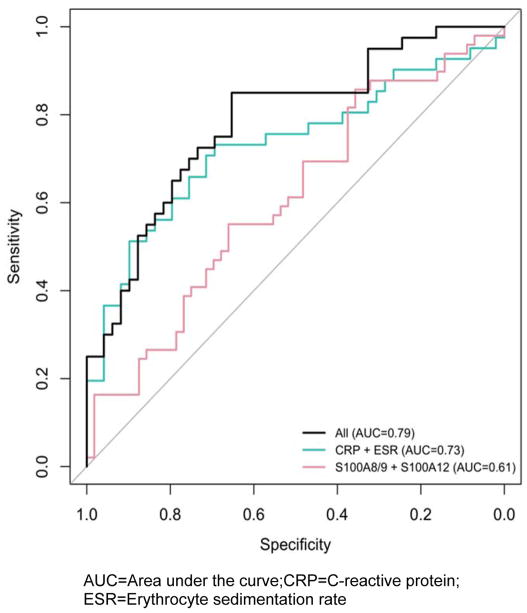
Serum samples obtained from 59 patients with GCA (Table 1). The majority of patients were female (74.6%) and Caucasian (98.3%). Forty-two patients (71%) had positive temporal artery biopsies diagnostic for GCA. Forty-six (78%) patients had samples drawn during both active and inactive disease. Serum S100A8/S100A9 and S100A12 levels were measured on 50 samples during active disease and 56 samples during inactive disease. The mean serum levels of S100A8/S100A9 and S100A12 were both significantly higher during active disease compared with inactive disease; S100A8/S100A9: 1445.6 ng/mL [95% CI 1189.2,1757.1] vs. 1095.7 ng/mL [95% CI 910.7, 1318.3], p = 0.003; S100A12: 163.2 ng/mL [95% CI 124.3,214.2] vs. 116.6ng/mL [95% CI 90.3,150.6], p=0.016. Mean S100A8/S100A9 and S100A12 levels (including both active and inactive disease) were significantly higher compared to healthy controls; S100A8/S100A9: 305ng/mL [95% CI 246.2–363.8]; S100A12: 40ng/mL [95% CI 20.4–59.6]. An AUC of 0.61 was obtained using the combination of S100A8/S100A9 and S100A12 which was not statistically different for a model using the combination of ESR and CRP (AUC 0.73, p=0.13). A model utilizing all variables increased the AUC to 0.79 (Figure 1).
Table 1.
Baseline Patient Characteristics
| Factor | Giant Cell Arteritis | Takayasu’s Arteritis | ||
|---|---|---|---|---|
| N | Statistic | N | Statistic | |
| 59 | 16 | |||
| Positive TABa | 42 | 71.2% | ||
| Sexa | ||||
| Female | 44 | 74.6% | 14 | 87.5% |
| Male | 15 | 25.4% | 2 | 12.5% |
| Racea | ||||
| Asian | 1 | 1.7% | 1 | 6.3% |
| White | 58 | 98.3% | 15 | 93.8% |
| Age at enrollment (years)b | 59 | 70.65±13.28 | 35.4±12.8 | |
| Age at visit (by sample, years)de | ||||
| Active | 50 | 70.9(67.5,74.4) | ||
| Inactive | 56 | 71.5(68.1,74.9) | ||
| Observation times | 59 | 16 | ||
| Active and Inactive | 46 | 78.0% | 14 | 87.5% |
| Both Active | 1 | 1.7% | ||
| Just Active | 2 | 3.4% | 1 | 6.3% |
| Just Inactive | 10 | 16.9% | 1 | 6.3% |
| Medicationsa | Active | Inactive | ||
| Prednisone | 84.0% | 92.7% | 75.0% | 68.8% |
| Duration (months)c | 2 | 3 | 15 | 3.5 |
| Aspirin | 58.0% | 66.4% | 68.8% | 68.8% |
| Azathioprine | 0.0% | 0.0% | 12.5% | 18.8% |
| Cyclophosphamide | 2.0% | 0.0% | 0% | 0% |
| Infliximab | 0.0% | 0.0% | 12.5% | 18.8% |
| Methotrexate | 14.0% | 23.6% | 37.5% | 50.0% |
| Mycophenolate | 0.0% | 0.0% | 6.3% | 0.0% |
Percentage;
Mean±SD;
Median;
p<0.001, TAB = temporal artery biopsy;
95% Confidence intervals
Figure 1.
ROC curves for tested biomarkers as measures of disease activity in patients with giant cell arteritis
AUC=Area under the curve; CRP=C-reactive protein;
ESR=Erythrocyte sedimentation rate
Takayasu’s Arteritis
Serum samples were obtained from 16 patients with TAK (Table 1). The majority of patients were female (87.5%) and Caucasian (93.8%). The mean age when the serum was collected was 35 years old (range 19–65). There was a non-statistically significant increase in S100A8/S100A9 levels during active disease compared to levels during inactive disease: 1028 ng/mL [95%CI 640, 1652] vs 843ng/mL [95%CI 536, 1327], p=0.38. There were no differences in the S100A12 levels between active and inactive disease (86.8 ng/mL [95%CI 50.2, 150.6] vs 85.1 ng/mL [47.9, 151.1], p=0.93). Mean S100A8/S100A9 and S100A12 levels (including both active and inactive disease) were significantly higher compared to healthy controls. None of the biological markers (CRP, ESR, S100A8/S100A9 nor S100A12) significantly correlated with disease activity in logistic regression models for each of these measurements.
When comparing GCA to TAK, there were no significant differences between the means (including both active and inactive disease) for either S100A8/S100A9 (p=0.1130) or S100A12 (p=0.0734).
DISCUSSION
This is the first study to investigate the role of S100 proteins as a marker of disease activity in primary large vessel vasculitis. In GCA, both S100A8/S100A9 and S100A12 were significantly higher during active disease compared to inactive disease and healthy controls. The S100 proteins better predict disease activity when combined with traditional biomarkers (ESR and CRP) compared to using traditional biomarkers alone. While the S100 proteins did not outperform traditional biomarkers, we suspect that the ability of traditional biomarkers to predict disease activity was overestimated since physicians rely on both ESR and CRP for disease assessment. In TAK, this study is the first to demonstrate that both S100A8/S100A9 and S100A12 are higher in patients compared to healthy controls with a trend toward S100A8/S100A9 being higher during active disease. This may have some future implication in the pathogenesis of large vessel vasculitis and future studies should explore these pathway.
There are several strengths to this study including this being a large registry of patients from multiple centers, the prospective nature of the assessment, all S100 protein measurements done in the same laboratory and 71% of GCA patients were biopsy proven. The primary limitation is the inability to determine to what degree the traditional biomarkers influenced the physicians’ assessment of disease activity.
There is a critical need to develop better methods of assessing disease activity in both GCA and TAK. Our group has demonstrated that gene expression of S100A8, S100A9 and S100A12 are increased in the GCA temporal arteries12, even in arteries that did not show inflammation. This suggests S100 proteins may be elevated early on in disease flares, possibly rising earlier than traditional biomarkers. Future studies are warranted to determine the longitudinal change in serum S100 proteins in GCA during active and inactive disease.
Acknowledgments
Funding for this research was provided in part by the Vasculitis Foundation. The Vasculitis Clinical Research Consortium has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Research Resources, the Office of Rare Diseases Research, and the National Center for Advancing Translational Science (U54AR057319, U54RR019497). A portion of the work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational research #UL1TR000001(formerly #UL1RR033179). The contents are solely the responsibility of the author and do not necessarily represent the official views of the NIH or NCATS.
Contributor Information
Jason Springer, University of Kansas Medical Center Kansas City, KS USA.
Paul Monach, Boston University Boston, MA USA.
David Cuthbertson, University of South Florida Tampa, FL USA.
Simon Carette, Mount Sinai Hospital University of Toronto Toronto, Ontario Canada.
Nader A Khalidi, McMaster University Hamilton, Ontario Canada.
Carol A McAlear, University of Pennsylvania Philadelphia, PA USA.
Christian Pagnoux, Mount Sinai Hospital University of Toronto Toronto, Ontario Canada.
Philip Seo, John Hopkins Medical Center Baltimore, MD USA.
Kenneth J Warrington, Mayo Clinic Rochester, MN USA.
Steven R Ytterberg, Mayo Clinic Rochester, MN USA.
Gary Hoffman, Cleveland Clinic Cleveland, OH USA.
Carol Langford, Cleveland Clinic Cleveland, OH USA.
Thomas Hamilton, Cleveland Clinic Cleveland, OH USA.
Dirk Foell, University Hospital Muenster Muenster, Germany.
Thomas Vogl, University of Muenster Muenster, Germany.
Dirk Holzinger, University of Muenster Muenster, Germany.
Peter A Merkel, University of Pennsylvania Philadelphia, PA USA.
Johannes Roth, University of Munster Muenster, Germany.
Rula A Hajj-Ali, Cleveland Clinic Cleveland, OH USA.
References
- 1.Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Annals of internal medicine. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Dagna L, Salvo F, Tiraboschi M, et al. Pentraxin-3 as a marker of disease activity in Takayasu arteritis. Annals of internal medicine. 2011;155:425–33. doi: 10.7326/0003-4819-155-7-201110040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ostberg G. Morphological changes in the large arteries in polymyalgia arteritica. Acta medica Scandinavica Supplementum. 1972;533:135–59. [PubMed] [Google Scholar]
- 4.Visvanathan S, Rahman MU, Hoffman GS, et al. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis--a prospective longitudinal study. Rheumatology. 2011;50:2061–70. doi: 10.1093/rheumatology/ker163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frosch M, Strey A, Vogl T, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis and rheumatism. 2000;43:628–37. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Schulze zur Wiesch A, Foell D, Frosch M, Vogl T, Sorg C, Roth J. Myeloid related proteins MRP8/MRP14 may predict disease flares in juvenile idiopathic arthritis. Clinical and experimental rheumatology. 2004;22:368–73. [PubMed] [Google Scholar]
- 7.Lugering N, Stoll R, Schmid KW, et al. The myeloic related protein MRP8/14 (27E10 antigen)--usefulness as a potential marker for disease activity in ulcerative colitis and putative biological function. European journal of clinical investigation. 1995;25:659–64. doi: 10.1111/j.1365-2362.1995.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 8.Soyfoo MS, Roth J, Vogl T, Pochet R, Decaux G. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. The Journal of rheumatology. 2009;36:2190–4. doi: 10.3899/jrheum.081302. [DOI] [PubMed] [Google Scholar]
- 9.Foell D, Kane D, Bresnihan B, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology. 2003;42:1383–9. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
- 10.Chen YS, Yan W, Geczy CL, Brown MA, Thomas R. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis research & therapy. 2009;11:R39. doi: 10.1186/ar2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foell D, Hernandez-Rodriguez J, Sanchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. The Journal of pathology. 2004;204:311–6. doi: 10.1002/path.1660. [DOI] [PubMed] [Google Scholar]
- 12.Hajj-Ali RH, Thomas, Lee Michael, Li Jianbo, Hernandez-Rodriguez Jose, Cid Maria, García-Martínez Ana, Brown Earl, Prayson Prayson, Hoffman Gary. Distinct patterns of gene expression in temporal artery biopsies associated with pathogenesis of giant cell arteritis. Arthritis and rheumatism. 2006;54(Supplemental):S756. [Google Scholar]
- 13.Inder SJ, Bobryshev YV, Cherian SM, et al. Immunophenotypic analysis of the aortic wall in Takayasu’s arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immuno-inflammatory reactions. Cardiovascular surgery (London, England) 2000;8:141–8. doi: 10.1016/s0967-2109(99)00100-3. [DOI] [PubMed] [Google Scholar]
- 14.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis and rheumatism. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 15.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis and rheumatism. 1990;33:1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]