Abstract
Speckle-type poz protein (SPOP) is an E3-ubiquitin ligase adaptor for turnover of a diverse number of proteins involved in key cellular processes such as chromatin remodeling, transcriptional regulation, and cell signaling. Genomic analysis revealed that SPOP somatic mutations are found in a subset of endometrial cancers, suggesting that these mutations act as oncogenic drivers of this gynecologic malignancy. These studies also raise the question as to the role of wild-type SPOP in normal uterine function. To address this question, we generated a mouse model (Spopd/d) in which SPOP is ablated in uterine cells that express the PGR. Fertility studies demonstrated that SPOP is required for embryo implantation and for endometrial decidualization. Molecular analysis revealed that expression levels of the PGR at the protein and transcript level are significantly reduced in the Spopd/d uterus. While this result was unexpected, this finding explains in part the dysfunctional phenotype of the Spopd/d uterus. Moderate increased levels of the ESR1, GATA2, and SRC2 were detected in the Spopd/d uterus, suggesting that SPOP is required to maintain the proteome for normal uterine function. With age, the Spopd/d endometrium exhibits large glandular cysts with foci of epithelial proliferation, further supporting a role for SPOP in maintaining a healthy uterus. Collectively, studies on the Spopd/d mouse support an important role for SPOP in normal uterine function and suggest that this mouse model may prove useful to study the role of SPOP-loss-of-function mutations in the etiopathogenesis of endometrial cancer.
Keywords: mouse, speckle-type poz protein, progesterone receptor, estrogen receptor, embryo implantation, decidualization
Summary Sentence
SPOP is required for embryo implantation, endometrial decidualization, and uterine health in the mouse
Introduction
Efforts to improve diagnosis and treatment of uterine dysfunction are stymied by an incomplete understanding of the key endometrial molecular signals that are required for early establishment of the maternofetal interface. Recent whole exome sequencing has revealed that speckle-type poz (pox virus and zinc finger) protein (SPOP; also known as PCIF1 [1]) is frequently mutated in human endometrial cancers [2–5]. This observation suggests that SPOP mutations may act as oncogenic drivers of this gynecological malignancy and raises the question: Is wild-type SPOP required for normal uterine biology? Acting as a 42 kDa adaptor protein for the cullin3 (CUL3)-based E3 ubiquitin ligase complex, SPOP is composed of a N-terminal MATH domain, which selectively recruits substrates for ubiquitination and subsequent proteasomal degradation (reviewed in [6, 7]). To date, all human endometrial cancer-associated SPOP mutations have been mapped to the MATH substrate-recognition cleft [7]; therefore, such mutations are predicted to block normal substrate recruitment for proteasomal turnover. Toward the C-terminus, the bric-a-brac/tramtrack/broad (BTB) domain of SPOP is required for interaction with the CUL3 scaffold protein [6, 7], which then targets substrates for ubiquitin-mediated degradation by the 26S proteasome.
Underscoring its evolutionary conserved and pleiotropic role in proteome homeostasis, SPOP is associated with the ubiquitination and degradation of a rapidly expanding list of diverse substrates involved in a broad spectrum of physiological processes. Some of these substrates include Daxx, the death-associated protein; Puckered (Puc), a signaling phosphatase; MacroH2A, a core histone variant; Gli (glioma associated oncogene), a transcriptional regulator; CHOP (C/EBP homologous protein), a transcription factor; CDC20 (cell division cycle 20), a cell cycle regulator; ERG (ETS-related gene), an ETS transcription factor family member; AR (androgen receptor), a nuclear transcription factor; coactivators: steroid receptor coactivator-3 (SRC-3) and tripartite motif containing 24 (TRIM 24), and more recently c-MYC [8–20]. Although wild-type SPOP is considered a tumor suppressor in a number of human cancers (reviewed in [7]), high expression levels of wild-type SPOP have been shown to act as a tumor promoter in other target tissues such as the kidney [21–24]. These paradoxical findings may be explained in part by the cell-type specific make-up of a given SPOP substrate population, which may constitute either a majority of tumor suppressors or tumor promoters depending on cellular context [7].
Although there is compelling evidence that mutations of SPOP may drive endometrial cancer, the in vivo role of wild-type SPOP in normal uterine function is not known. To address this issue, we recently applied conditional genetic technology in the mouse to selectively abrogate SPOP expression in uterine cells that express the PGR. Our data strongly support an important role for SPOP in normal uterine function in the mouse by regulating the homeostasis of key signaling cues required for embryo implantation and endometrial decidualization.
Materials and methods
Generation of a Spop conditional knockout mouse
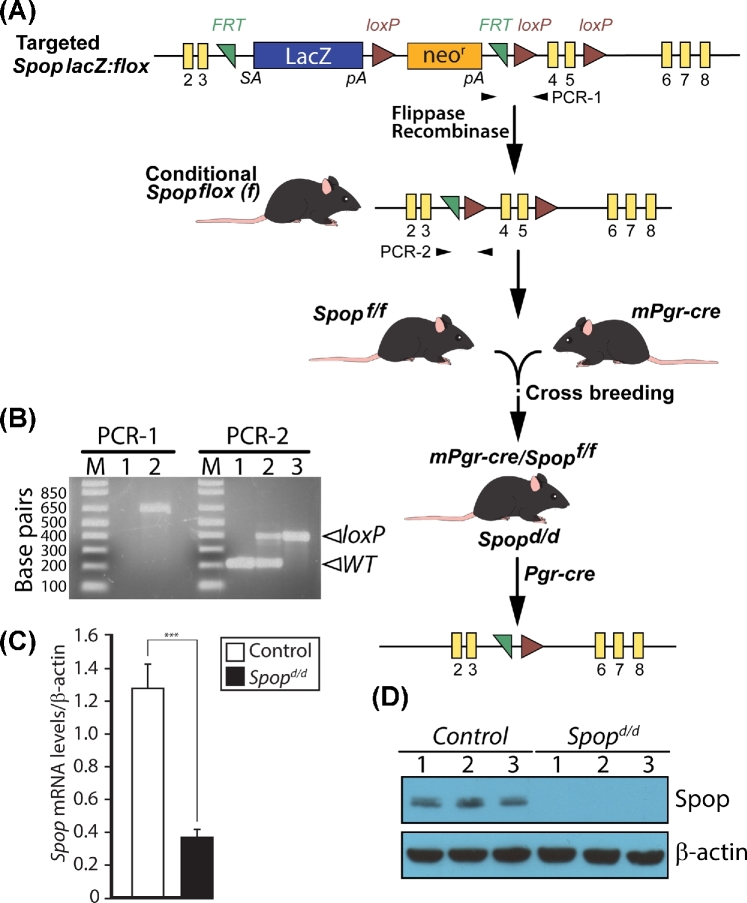
As described previously [13, 25], C57BL/6 mice carrying the Spoptm1a(KOMP)Wtsi allele were obtained from the international knockout mouse phenotyping (KOMP) consortium (www.knockoutmouse.org). Termed a knockout first allele, the engineered Spoptm1a(KOMP)Wtsi allele consists of a targeted insertion into the third intron of the murine Spop gene. In the 5΄ to 3΄ direction, the insertion cassette is composed of an FRT site followed by the LacZ gene and a loxP site. The first loxP site is followed by a neomycin resistance (neor) gene under the control of the human beta-actin promoter; the antibiotic resistance gene also contains a strong SV40 polyA termination signal. A second FRT site and loxP site follow the neor gene, while a third loxP site is inserted downstream of exon 5 of the Spop gene. A “conditional ready” Spop allele, in which exons 4 and 5 are flanked by loxP sites (floxed), was generated by crossing Spoptm1a(KOMP)Wtsi mice with C57BL6 mice carrying the flip recombinase gene (encoding flippase) targeted to the ROSA locus [26]. Progeny carrying a Spop conditional allele (Spopf/f) was crossed with our Pgrcre mice (Pgr < tm2(cre)Lyd > (C57BL6)) to selectively abrogate SPOP expression in cells expressing the PGR [27]. The PCR primer sequences used to genotype the Spoptm1a(KOMP)Wtsi allele are as follows: forward-primer 5΄-GGGATCTCATGCTGGAGTTCTTC-3΄; and reverse-primer 5΄-GAGCGTTCACATCCCTTACATCTC-3΄, which generates a 659-bp amplicon (PCR-1; Figure 1A and B). The PCR primer sequences to genotype the Spopf/f allele are as follows: forward-primer 5΄-GCAGAAGCAGGCAGATCTTT-3΄ and reverse-primer 5΄-GCCCTTAGTTTTTCATGATGG-3΄, which generates a 179-bp and 397-bp amplicon (PCR-2; Figure 1A and B) for the wild-type and floxed Spop allele respectively. For brevity, resultant Pgrcre:Spopf/f bigenic mice are termed Spopd/d. For studies described herein, Pgrcre monogenics served as controls for Spopd/d mice. When possible, control and Spopd/d mice were euthanized at the same stage of their cycles for histological and molecular studies described herein.
Figure 1.
Generation of the Spopd/d mouse. (A) Obtained from KOMP [13], mice carrying the Spop lacZ: flox allele were crossed with ROSA flippase mice to generate mice harboring the conditional Spop floxed allele (Spopf/f) in which exons 4 and 5 are flanked by loxP sites. The Spopf/f and mPgr-cre mouse (both C57BL6) were crossed to generate the bigenic Spopd/d mouse. Driven by the Pgr promoter, the cre recombinase excises exons 4 and 5 of the Spop gene in the Spopd/d mouse; exons 4 and 5 encode the essential MATH domain of SPOP [13]. (B) Typical PCR results are shown for genotyping mice carrying the targeted Spop lacZ: flox allele and the Spopf/f allele using the PCR-1 and PCR-2 reaction primers respectively. For the PCR-1 reaction, lanes 1 and 2 represent genotype results using tail tip genomic DNA from control mice and mice heterozygous for the Spop lacZ: flox allele, respectively; the positive PCR amplicon is 659 bp. For the PCR-2 reaction, lanes 1, 2, and 3 denote control, Spopf/+ (heterozygous for the Spop floxed allele) and Spopf/f (homozygous for Spop floxed allele) respectively; PCR-positive amplicon is 179 bp for WT allele and 397 bp for Spop floxed allele. Primer sequences for PCR-1 and -2 are listed in Materials and methods section. (C) Real-time PCR analysis clearly demonstrates a significant reduction in Spop transcript levels in the uterus of Spopd/d mice (n = 9) as compared to controls (n = 6). (D) Western analysis of protein isolated from control and Spopd/d uteri confirms that SPOP protein is not detected in the Spopd/d uterus; each lane represents a protein sample pooled from four individual adult mice per genotype (β-actin serves as a loading control).
Animal husbandry and housing was undertaken in an AAALAC accredited vivarium at Baylor College of Medicine. In temperature controlled mouse rooms (22 ± 2°C) on a 6 am–7 pm light cycle, mice were fed irradiated Tekland global soy protein-free extruded rodent diet (Harlan Laboratories, Inc., Indianapolis, IN) and fresh water ad libitum. Mouse experiments were conducted in strict accordance with the guidelines described in the Guide for the Care and Use of Laboratory Animals (“The Guide” (Eighth Edition 2011)) published by the National Research Council of the National Academies, Washington, D.C. (www.nap.edu). Animal protocols used in these studies were prospectively approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Fertility trials, timed pregnancies, and superovulation
For breeding trials, sexually mature control and Spopd/d females were housed with proven stud/breeder C57BL/6 males. Over a 6-month breeding period, the date of pup delivery, the number of litters, and the number of pups per litter were recorded for each female. For timed pregnancy studies, females were housed with fertility-proven C57BL/6 males overnight. The detection of a copulatory (or vaginal) plug the following morning was designated as day 1 of pregnancy (1 dpc); pregnant females were individually housed. To locate incipient implantation sites in uterine horns of 5-dpc pregnant dams, Chicago sky blue dye (1%; 100 μl PBS/mouse) was injected into one of the two lateral tail veins before mice were euthanized 2 min later.
To induce superovulation, 21-day old female mice were intraperitoneally (IP) injected with pregnant mare serum gonadotropin (PMSG; Sigma-Aldrich, St. Louis, MO (5 international units (IU)/100 μl of sterile 0.9% saline)). Forty-eight hours later, mice received an IP injection of human chorionic gonadotropin (hCG; Sigma-Aldrich (5 IU/100 μl of sterile 0.9% saline)). Sixteen hours post-hCG injection, oocytes were collected from oviducts and counted using a dissecting microscope as previously described [28]. Ovaries were fixed in 4% paraformaldehyde (PFA) for histological analysis. To assess ovarian cyclicity, the estrous cycle was monitored over 3–4 weeks by examining the cytology of daily vaginal lavages on slides stained with 10% crystal violet (Sigma-Aldrich) [29].
To measure circulating serum levels of E2 and P4, blood was collected from the orbital sinus of anesthetized 5-dpc Spopd/d and control mice. After blood clotting at room temperature for 90 min in BD microtainer tubes (Fisher Scientific Inc.), serum was isolated through the microtainer tubes’ serum separator; serum was stored at –80°C until hormone measurement. Serum levels of E2 and P4 were measured by the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia (Charlottesville, VA).
Artificial decidual response assay and hormone treatments
Elicitation of an artificial decidual response has been described [28, 30]. Briefly, mice were ovariectomized at 6 weeks of age and rested for 2 weeks before receiving three daily subcutaneous (sc) injections of E2 (100 ng). Following 2 days of rest, mice were administered three daily sc injections of E2 plus P4 (E2 (6.7 ng) and P4 (1 mg)). Six hours following the third E2P4 injection, sesame oil (50 μl) was instilled into the lumen of the left uterine horn (stimulated); the right horn did not receive oil (unstimulated). After intraluminal instillation of the deciduogenic stimulus, mice received daily sc injections of E2P4 for 5 days, and then weighed before euthanasia. Trimmed of mesometrial membrane and vasculature, extracted stimulated and unstimulated uterine horns from each mouse were weighed for wet-weight measurements before further analysis.
To assay the ability of P4 to suppress E2 induced uterine epithelial proliferation in the ovariectomized mouse, an established protocol with modifications was used [28]. Briefly, mice were ovariectomized at 6 weeks of age, rested for 2 weeks before injection with (1) 100 μl of hormone vehicle (sesame oil; termed untreated); (2) E2 (100 ng/100 μl) for 18 h; or (3) E2 (100 ng/100 μl) and P4 (1 mg/100 μl) for 24 h before euthanasia. Tissues were immediately removed for histological examination.
Immunohistochemical analyses
Tissues, fixed in 4% PFA overnight, were processed and embedded in paraffin as previously reported [28]. Tissue sections from serially sectioned tissue blocks were placed on Superfrost plus glass slides (Fisher Scientific, Pittsburgh, PA). Prior to immunohistochemical analysis, sections were deparaffinized, rehydrated, and processed through an antigen unmasking step. Following a 1-h blocking step at room temperature, tissue sections were incubated with one of the appropriate antibodies listed in Supplementary Table S1 overnight at 4°C. After primary antibody incubation, sections were incubated with a goat anti-rabbit IgG secondary antibody (Vector laboratories Inc.) for 1 h at room temperature followed by incubation with the R.T.U Vectastain® Universal ABC reagent (Vector laboratories Inc.) for 30 min at room temperature. Immunopositivity was visualized in situ through incubation with 3, 3΄-diaminobenzidine (DAB, Vector laboratories Inc.); slides were counterstained with hematoxylin for contrast. Finally, sections were dehydrated before coverslips were mounted using slowfade mounting medium (Fisher Scientific Inc.). For 5΄-bromo-2΄-deoxyuridine (BrdU) immunohistochemical analysis, mice were first IP injected with BrdU (10 mg/ml; Amersham Biosciences Corporation, Piscataway, NJ (0.1 ml/10 g body weight)) 2 h before euthanasia. Following tissue processing as described above, tissue sections were incubated with a biotinylated anti-BrdU antibody (BrdU In-Situ Detection Kit (BD Pharmingen Inc., San Jose, CA; 1:10 dilution)) overnight at room temperature. Sections were then incubated with the Vectastain ABC reagent at room temperature for 1 h and the immunoreaction was visualized using the DAB peroxidase substrate kit and subsequently processed as described above.
Raw images of immunostained tissue sections were digitally captured using a color chilled AxioCam MRc5 digital camera interfaced with a Carl Zeiss AxioImager A1 upright microscope (Zeiss, Jena, Germany). Postprocessing, collation, and annotation of images were performed with Photoshop and Illustrator (version 6) software programs (Adobe Systems Inc., San Jose, CA).
Molecular analyses
For studies using quantitative real-time PCR, total RNA was extracted from homogenized tissues using TRIzol® reagent (ThermoFischer Scientific Inc., Waltham, MA) and further purified using the RNeasy® Plus Mini Kit (Qiagen). Reverse transcription of total RNA into cDNA was performed using the Superscript IV VILO Master Mix (ThermoFisher Scientific Inc.) before real-time PCR amplification. Details on TaqMan ® gene expression assays used in these studies are provided in Supplementary Table S2; the 18S ribosomal RNA TaqMan® assay was used as an internal control. To evaluate Spop transcript levels by real-time PCR, the SensiFAST SYBR® Hi-ROX One-Step kit (Bioline Inc., #BIO-82005) was used. The sequences of the PCR primers in the 5΄-3΄reaction are as follows: Spop forward primer: GGAGGAAATGGGTGAAGTCAT; Spop reverse primer: GGGTTTACTCGCAAACACCA; β-actin forward primer: GTGGTACGACCAGAGGCATAC; and β-actin reverse primer: AAGGCCAACCGTGAAAAGAT.
Conditions for western immunoblotting have been described previously [31]; the primary antibodies used in these studies are listed in Supplementary Table S1. The SuperSignal West Pico Chemiluminescent Substrate kit (ThermoFisher Scientific) was used to detect the chemiluminescent signal. To facilitate the re-probing of western blots with different antibodies when required, immunoblots were stripped of primary and secondary antibodies using Restore Western Blot Stripping buffer ((#21059) Thermo Fisher Scientific Inc.).
Statistical analyses
When applicable, two-tailed Student t tests along with one-way ANOVA with Tukey post hoc multiple range tests were performed using the GraphPad Prism and Instat tools (GraphPad Software Inc., La Jolla, CA). A P-value < 0.05 was considered statistically significant; asterisks in figures denote the level of significance: *P < 0.05; **P < 0.01; and ***P < 0.001.
Results
The Spopd/d female mouse is infertile
Because whole body abrogation of SPOP expression in the mouse results in early neonatal death [1, 13, 25], we used a recently engineered mouse (the Spopf/f mouse) in which critical exons (encoding the MATH domain [7]) of the Spop gene are flanked (or floxed) by loxP sites to allow for tissue selective ablation of SPOP with a cre driver of choice (Figure 1A and B). The Spopf/f mouse was recently used by our colleague, Dr Nicholas Mitsiades at Baylor College of Medicine, to successfully abrogate SPOP in the prostate epithelium using the probasin-cre driver mouse [13]. Crossing the Spopf/f mouse with our Pgr-cre mouse [27] to generate the Spopd/d bigenic (Figure 1A and B), we similarly demonstrated that uterine Spop transcript levels are significantly reduced in the Spopd/d uterus (Figure 1C); importantly, western analysis did not detect SPOP protein in the Spopd/d uterus (Figure 1D). Unfortunately, we and others [13] have not been able to find a commercial SPOP antibody suitable for immunohistochemistry on adult murine tissues. Irrespective, numerous studies have shown that the Pgr-cre driver can successfully ablate gene expression in PGR-positive cells of the murine uterus (reviewed in [32]).
Long-term breeding studies revealed that Spopd/d female mice mated with stud males exhibited a comparable vaginal plugging latency to control females. Unlike control females (N = 12; 60 litters; 395 pups; average number of pups per litter = 6.58); however, none of the plugged Spopd/d females (N = 11) became pregnant over the 6-month breeding period. To examine whether impairment of ovarian function underpinned the Spopd/d infertility phenotype, prepubescent Spopd/d and control females were superovulated by administrating an established gonadotropin hormone sequential treatment regimen [28]. Both oocyte counts and ovarian histological evaluation clearly showed that ovarian activity in the Spopd/d female is comparable to that of control females (Supplementary Figure S1). This observation is further supported by the finding that Spopd/d females display a normal iterative estrous cycle profile by cytological evaluation of vaginal lavages taken daily (Supplementary Figure S1).
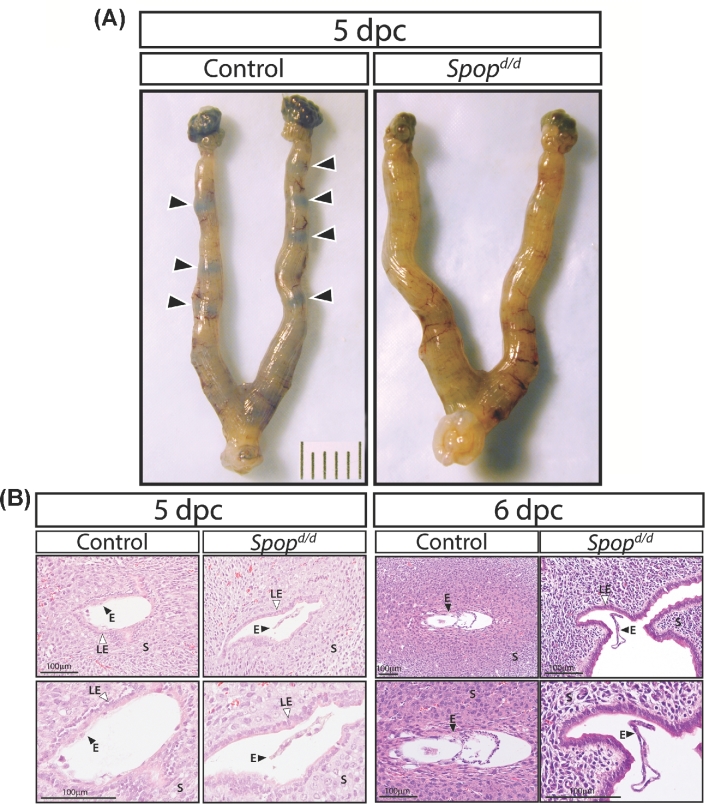
Because ovarian activity in the Spopd/d mouse is normal, we next tested whether Spopd/d uterine function is compromised during the implantation period. Timed pregnancy studies revealed that Chicago sky blue tail vein injection did not detect nascent implantation sites in the uterine horns of the 5-dpc Spopd/d mouse as compared with similarly treated timed-pregnant controls (Figure 2A). Examination of serial H&E stained uterine sections showed typical tight embryo attachment to the luminal epithelial compartment of the 5-dpc control uterus; however, embryo attachment was not observed in the 5-dpc or 6-dpc Spopd/d uterus (Figure 2B). In all cases, embryos were found floating in the luminal space of the Spopd/d uterus with no evidence of local luminal cavity closure around the embryo (Figure 2B). At 5 dpc, there was no significant difference in the serum levels of P4 and E2 in Spopd/d and control mice (P4 levels (ng/ml): Spopd/d = 25.0 ± 2.5; control = 22.9 ± 2.0; and E2 levels (pg/ml): Spopd/d = 4.0 ± 0.6; control = 3.6 ± 0.6; n = 6 mice per group)). These results show that ovarian luteal function is normal in the Spopd/d mouse during this period and concur with ovarian data presented in Supplementary Figure S1.
Figure 2.
Embryo attachment and implantation fails in Spopd/d mice. (A) At the gross level, the location of implantation sites are clearly observed as blue bands (indicated by arrowheads) in uteri of control 5 dpc mice (n = 5) following tail vein injection with 1% Chicago sky blue dye. However, blue bands are not observed in the uterus of similarly treated Spopd/d mice (n = 6). (B) Serial sections stained with hematoxylin and eosin (H&E) of uterine tissue from control and Spopd/d mice at 5 dpc clearly show tight embryo attachment (E; black arrowhead) to the intact luminal epithelium (LE; white arrowhead) of the control mouse (n = 5); however, embryo attachment does not occur in Spopd/d mice (n = 6). Similarly, continued advancement of embryo implantation following breakdown of the luminal epithelium is seen in control 6 dpc mice (n = 5) but not in Spopd/d 6 dpc mice (n = 5); instead, the latter show floating embryos in the uterine lumen. Note: bottom panels are high power magnifications of images shown in the upper panels.
The Spopd/d infertility phenotype is intrinsic to the uterus
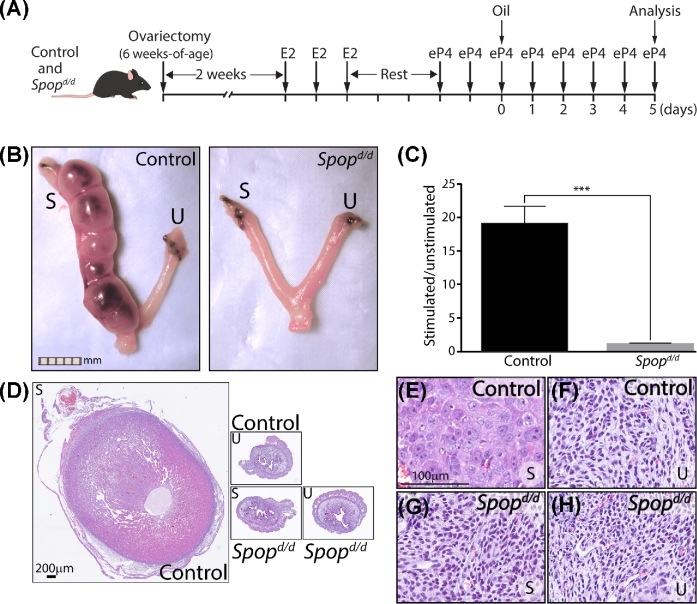
To confirm that the Spopd/d female infertility phenotype originates in the uterus, we used a standard artificial decidual response assay [30] on steroid hormone treated ovariectomized Spopd/d and control siblings (Figure 3). Using an E2 and P4 administration regimen shown in Figure 3A, we found that the Spopd/d uterine horn does not develop into a typical deciduoma following intraluminal instillation of sesame oil, which was the deciduogenic stimulus used in these experiments (Figure 3B–H). This result strongly supports a critical role for SPOP in steroid hormone-dependent endometrial stromal decidualization, a cellular process that is essential for the early establishment of pregnancy.
Figure 3.
Uterine decidualization is impaired in the Spopd/d mouse. (A) Established protocol to induce an artificial decidual response in the uterus of ovariectomized control and Spopd/d mice (n = 6 per genotype). (B) Gross morphology of control and Spopd/d uterus following the protocol described in (A) above; stimulated and unstimulated horns are denoted by “S” and “U”, respectively (scale bar applies to both panels). (C) Histogram shows the average of wet-weight ratios (±standard deviation) of stimulated uterine horns over contralateral unstimulated horns for control and Spopd/d mice; ***P-value ≤ 0.001. (D) Low power magnification images of H&E stained transverse sections of stimulated (S) control uterine horn; unstimulated (U) control horn; stimulated (S) Spopd/d horn; and unstimulated Spopd/d horn. Panels (E–H) are corresponding higher magnification images of regions within images shown in (D); scale bar in (E) also applies to (F–H).
The expression level of PGR is markedly reduced in the murine Spopd/d uterus
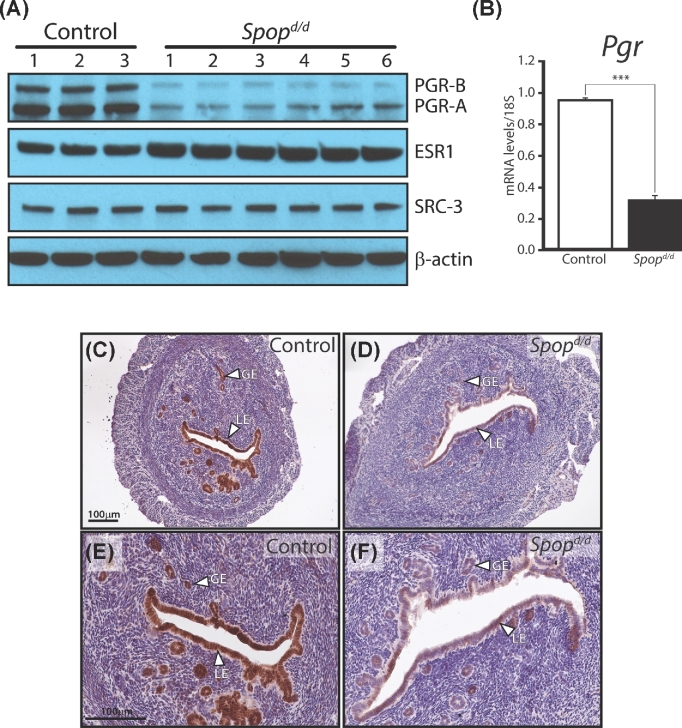
Of the key uterine molecular signaling cues examined by western analysis, our first line of molecular investigations revealed that the expression level of both isoforms of PGR protein is markedly decreased in Spopd/d uteri as compared to control uteri of virgin mice (Figure 4A). This finding was unexpected as one study using cultured human breast cancer cells showed that PGR protein is targeted for turnover by SPOP [33], suggesting absence of SPOP would lead to an increase (rather than a decrease) in the levels of uterine PGR. However, real-time PCR analysis demonstrated that Pgr transcript levels are also reduced by SPOP silencing (Figure 4B), indicating that the observed reduction in uterine PGR expression levels is most likely an indirect molecular consequence of SPOP depletion.
Figure 4.
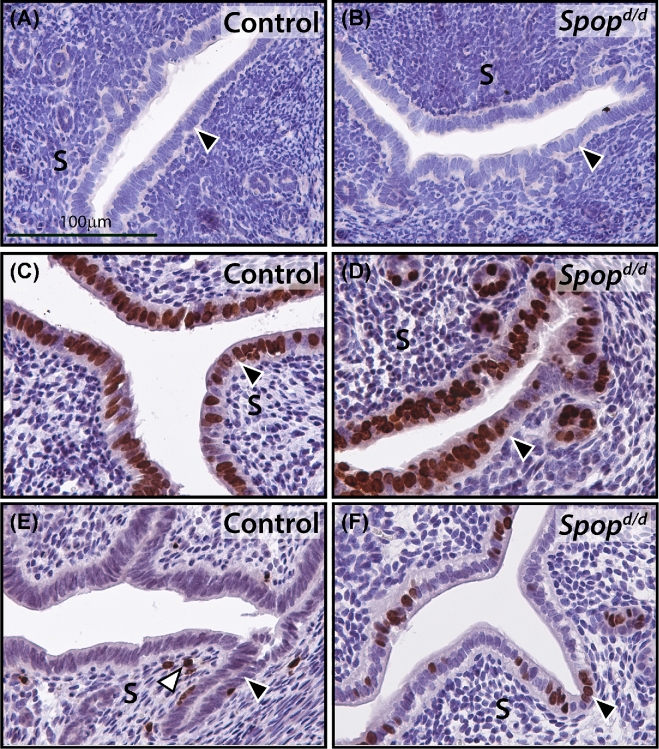
Protein levels of the PGR are significantly reduced in the Spopd/d uterus. (A) Western immunoblot analysis shows that the A and B isoforms of PGR are significantly reduced in the Spopd/d uterus as compared to controls. Expression levels of ESR1 are moderately increased in the Spopd/d uterus compared to controls. The expression levels for SRC-3 are not significantly changed between control and Spopd/d uteri; β-actin serves as the loading control. Note: each lane on the gel represents pooled protein samples from three individual mice. (B) Real-time PCR analysis shows that Pgr transcript levels are also significantly reduced in the Spopd/d uterus (n = 4) as compared to controls (n = 4). (C) Immunohistochemical detection of PGR expression in the uterus of an ovariectomized control mouse. Note strong immunoreactivity for PGR in the luminal epithelium (LE) and glandular epithelium (GE). (D) Immunohistochemical detection of PGR expression in the uterus of an ovariectomized Spopd/d mouse; note the significantly low levels of PGR expression in the luminal and glandular epithelial compartments. (E) and (F) are higher magnification images of (C) and (D) respectively; scale bar in (C) and (E) applies to (B) and (F), respectively.
Although the expression levels of ESR1 are moderately increased in the Spopd/d uterus (Figure 4A), the induction of established ESR1 molecular targets is not significantly different between the Spopd/d and control uterus (Supplementary Figure S2). The expression levels of steroid receptor coactivator 3 (SRC-3) are unchanged between genotypes (Figure 4A). Apart from their importance in murine uterine function [34, 35], ESR1 and SRC-3 have been shown to be targets of SPOP in vitro [8, 36] as well as substrates for other ubiquitin-mediated mechanisms of protein turnover [34]. We also detected a moderate increase in protein levels of GATA2 and SRC-2 in the Spopd/d uterus as compared to the control uterus (Supplementary Figure S3); importantly, these proteins exert important roles in endometrial responsiveness to P4 [29, 37, 38].
The significant depletion of uterine PGR in the Spopd/d mouse was also confirmed by follow-up immunohistochemical detection for PGR expression in uterine sections derived from untreated ovariectomized Spopd/d and control mice (Figure 4C–F). It is known that PGR protein expression is markedly robust and consistent in the epithelial compartment of the uterus of the ovariectomized wild-type mouse [38], making for an ideal model to compare the relative expression levels of this nuclear receptor in control and mutant mice by immunohistochemistry [38]. Importantly, as a consequence of the reduction of uterine PGR levels, the transcriptional induction of a number of PGR molecular targets is significantly attenuated in the Spopd/d uterus (Figure 5), a subset of these targets are critical for embryo implantation [39–41] and endometrial stromal cell decidualization [31, 42].
Figure 5.
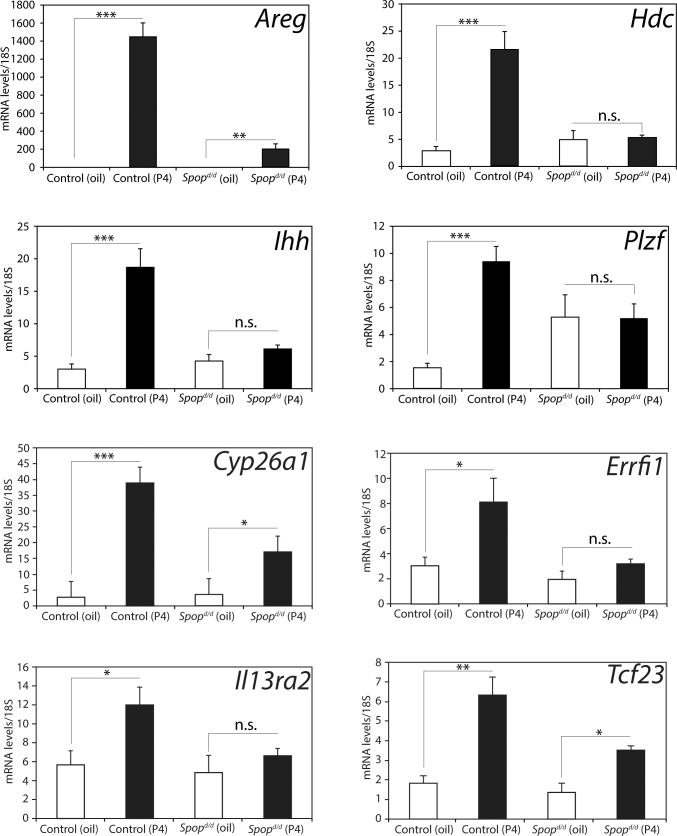
Absence of SPOP in the murine uterine results in a significant attenuation in the induction of established transcriptional responses to acute progesterone exposure. Quantitative real-time PCR analysis of the transcriptional induction of established P4 gene targets in the uterus from ovariectomized control and Spopd/d mice treated with vehicle (sesame oil (white column)) and 1 mg P4 (1 mg/100 μl (black column)) for 6 h (n = 5 mice per treatment/genotype). Note the marked decrease in transcriptional induction of Areg; Hdc; Ihh; Plzf; Cyp26a1; Errfi1; IL13ra2; and Tcf23 in the Spopd/d uterus in response to P4 administration. P values ≤0.05, ≤0.01 and ≤0.001 are indicated by *, **, and *** respectively; n.s. denotes nonspecific (P > 0.05).
Reduction in PGR levels in the Spopd/d uterus also results in a significant attenuation in the ability of P4 to suppress E2-induced uterine epithelial proliferation (Figure 6). This result is significant as endometrial P4 resistance is linked to a number of endometrial pathologies, including endometriosis and endometrial cancer [43].
Figure 6.
Progesterone suppression of estrogen-induced epithelial proliferation is significantly attenuated in the Spopd/d endometrium. (A) Uterine tissue from ovariectomized untreated control mice that were stained for BrdU incorporation. Luminal epithelium is indicated by arrowhead. (B) Uterine tissue from ovariectomized untreated Spopd/d mice that were similarly stained for BrdU incorporation. (C) Uterine tissue from ovariectomized control mice treated with E2 (100 ng for 18 h) that were stained for BrdU incorporation. Note the expected increase in the number of luminal epithelial cells that score positive for BrdU incorporation (arrowhead). (D) Uterine tissue from ovariectomized Spopd/d mice treated with E2 (100 ng for 18 h) that were stained for BrdU incorporation. Note a similar increase in the number of luminal epithelial cells scoring positive for BrdU incorporation. (E) Uterine tissue from ovariectomized control mice treated with E2 for 18 h and then P4 (1 mg) for 8 h that were stained for BrdU incorporation. Note the expected marked reduction in the number of luminal epithelial cells scoring positive for BrdU incorporation (black arrowhead). White arrowhead indicates a stromal cell positive for BrdU incorporation. (F) Uterine tissue from ovariectomized Spopd/d mice treated with E2 for 18 h and then P4 (1 mg) for 8 h that were stained for BrdU incorporation. Note: a significant number of luminal epithelial cells remain positive for BrdU incorporation (arrowhead). Scale bar in (A) applies to (B–F); data representative of n = 5 mice per genotype/treatment group.
Cystic endometrial glands develop in the Spopd/d uterus with age
By 10 months of age, the untreated Spopd/d uterus exhibits overt dilated cystic glandular structures that are not observed in age-matched control mice (Figure 7A–E). Throughout the Spopd/d uterine horn, numerous cystoid structures—irregular in size and shape—crowd the endometrial stroma in cribriform patterns that extend to the circular smooth muscle layer of the myometrium (Figure 7A–E). However, the dilated Spopd/d uterine glands do not show epithelial atypia, pseudostratification, or excessive secretory (or eosin positive) material. Interestingly, immunohistochemical evaluation for FOXA2 expression (a marker for normal glandular epithelial cells [44]) is significantly reduced in epithelial cells that comprise the larger cysts, which suggests that these cells have lost their normal glandular epithelial identity (Figure 8A–E). Although the Spopd/d glandular epithelium scores significantly higher in the number of proliferating cells as compared to controls, many of these proliferating cells are distributed in focal areas rather than evenly distributed (Figure 8F and G); this cellular distribution pattern has been reported for similar uterine pathologies with dilated cystic uterine glands [45]. Therefore, the Spopd/d uterine histopathology that emerges with age may arise from these foci of hyperproliferation.
Figure 7.
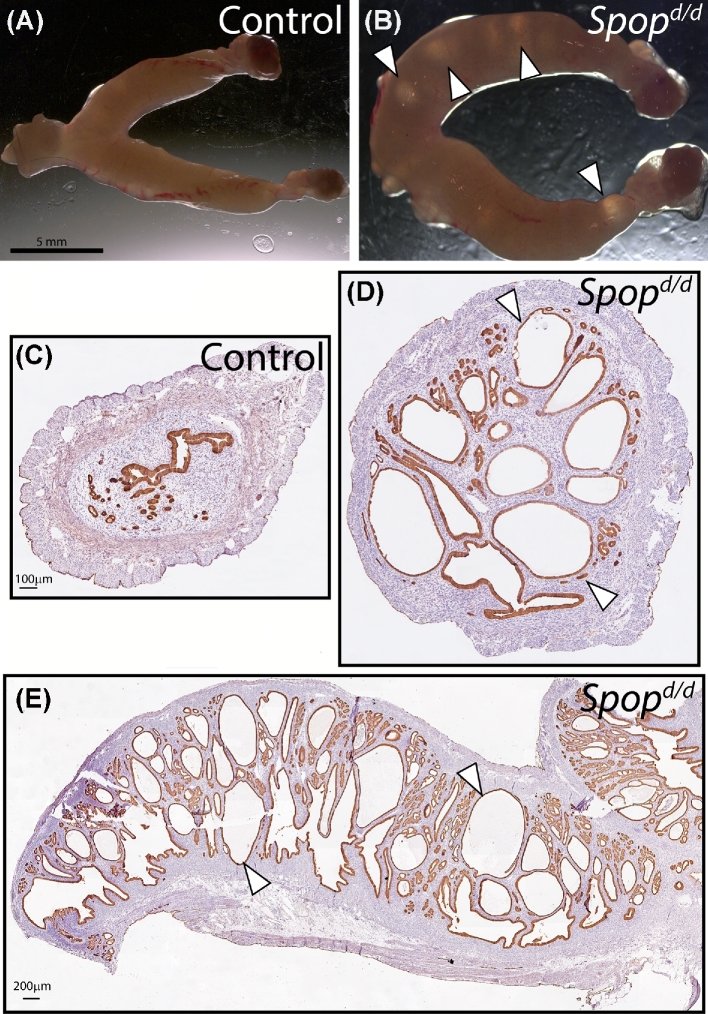
The Spopd/d uterus exhibits large cystic endometrial glands with age. (A) Light microscopy using a dissecting scope of control uterine tissue at the gross level (n = 5 mice). (B) Light microscopy of Spopd/d uterus shows conspicuously large cysts (arrowheads); n = 5 mice. Scale bar in (A) applies to (B). (C) Transverse section of control uterus stained for cytokeratin 8, an epithelial marker. (D) Transverse section of Spopd/d uterus similarly stained for cytokeratin 8; note the numerous large glandular epithelial cystic structures (arrowhead). Scale bar in (C) applies to (D). (E) Longitudinal section of the Spopd/d uterus stained for cytokeratin 8. Note that cystic glands of variable size and shape appear throughout the uterine horn (arrowhead).
Figure 8.
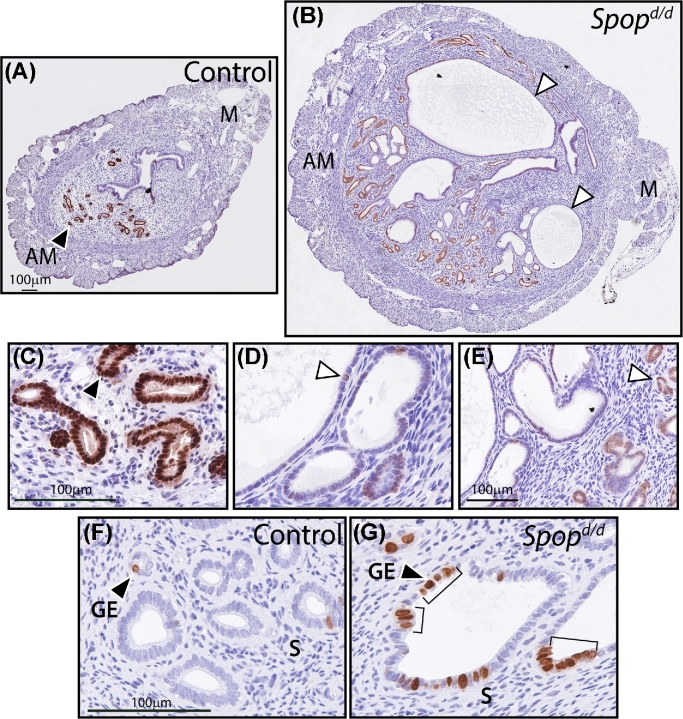
Epithelial cells in Spopd/d endometrial cysts express low levels of FOXA2 and exhibit increased proliferative capacity. (A) Transverse section of uterine horn from a control mouse stained for FOXA2 expression. Note the expected strong immunopositivity for FOXA2 expression in the glandular epithelia located in the antimesometrial (AM) pole (arrowhead); M indicates mesometrial pole. (B) Transverse section of uterine horn from Spopd/d mouse similarly stained for FOXA2 expression. Note the significant decreased expression of FOXA2 in the larger cystoid structures (arrowheads). Scale bar in (A) applies to (B). (C) Higher magnification of region shown in (A) above. (D) and (E) are higher magnifications of regions shown in (B) above. Scale bar in (C) applies to (D). (F) Control glandular epithelia (GE) stained for BrdU incorporation. Note the sparse distribution of BrdU-positive GE cells (arrowhead). (G) Immunostaining for BrdU incorporation in Spopd/d GE cells. Note the foci (brackets) of BrdU-positive cells in the larger cystic glands; S indicates stroma. Scale bar in (F) applies to (G).
Discussion
Although SPOP was discovered over two decades ago [46], conditional SPOP knockout mouse models have only been generated and characterized within the last two years [13, 25]. In the case of the murine prostate, SPOP ablation in the epithelium results in hyperplasia and dysplasia that develops into prostatic intraepithelial neoplasia (PIN) in the dorsolateral and ventral prostate by 38 weeks of age [13]. Using a similar approach that allows for the targeted ablation of SPOP in cells which express PGR, we demonstrate the importance of uterine SPOP for embryo implantation and endometrial decidualization. By revealing a critical role for SPOP in hormone-dependent endometrial decidualization, we provide essential in vivo support for recent in vitro studies [47] that report a pivotal involvement for murine SPOP in endometrial stromal cell decidualization in culture.
Intriguingly, our molecular analysis demonstrates that the expression levels of both isoforms of the PGR protein are significantly attenuated with uterine SPOP depletion, providing one mechanistic explanation for the uterine dysfunction displayed by the Spopd/d mice. The marked reduction in PGR protein levels following SPOP ablation was unexpected since SPOP has been shown by in vitro studies to turnover PGR [33] as well as other nuclear receptors, such as ESR1 and the AR [9, 36, 48, 49]. Moreover, a recent in vivo study—using a similar engineered mouse approach—showed that the expression levels of AR—a close relative of PGR—are markedly increased as expected in the prostate epithelium following conditional SPOP ablation in the mouse [13]. However, the observation that Pgr transcript levels are also reduced in the Spopd/d uterus argues for an indirect regulation of PGR protein levels by SPOP. Instead, these findings suggest other regulatory factors of PGR protein stability and/or transcription are substrates for uterine SPOP. Accordingly, the striking reduction in uterine PGR expression levels results in adverse molecular repercussions in terms of a significant attenuated induction of key downstream transcriptional programs required for endometrial implantation processes and decidualization in the mouse. Furthermore, the P4 resistance phenotype of the Spopd/d uterus may suggest a cellular mechanism to explain the functional defects of this tissue, when SPOP function is derailed. The latter proposal will be a major focus for future investigations.
Further molecular analysis disclosed that SPOP ablation results in a moderate increase in the expression levels of uterine ESR1, GATA2, and SRC-2, suggesting that perturbation of SPOP action most likely derails the normal homeostatic levels of many proteins in the proteome required for uterine function. Although the changes in expression levels are moderate for a given protein, we speculate that the collective alterations in levels of many proteins are expected to exert significant adverse effects on uterine function. Interestingly, we have recently shown that GATA2 is required for maintaining uterine PGR expression levels [38]. The observation that GATA2 is moderately increased in the Spopd/d uterus may represent a molecular mechanism to increase PGR expression in response to the significant decrease in PGR expression levels as result of SPOP ablation. In the case of SRC-2, we previously demonstrated that increasing the levels of this coregulator results in severe uterine dysfunction in the mouse [29], resulting in a striking subfertility phenotype. The uterine defects resulting from increased SRC-2 levels include a marked impairment in uterine decidualization and an enhanced proliferative response to E2 exposure leading to endometrial glandular cysts and epithelial hyperplasia [29].
In view of the strengthening link between SPOP somatic mutations and a subset of human endometrial cancers [2–5], the question arose as to whether the Spopd/d uterus would exhibit histopathological signatures consistent with the early stages of endometrial tumorigenesis. Although the Spopd/d conditional knockout allele in the mouse does not directly model the human SPOP point mutations found in human endometrial cancers, both SPOP mutation types result in loss-of-function phenotypes. In keeping with recent in vivo prostate studies in the mouse [13], we found that the aging Spopd/d endometrium develops conspicuously dilated glandular epithelial cysts with foci of hyperproliferative epithelial cells. Interestingly, these aberrant histomorphic features emerge at approximately the same time (∼38–40 weeks) that PIN lesions are observed in the prostate of mice in which the Spop allele is conditionally abrogated [13]. Whether the histopathology of the Spopd/d endometrium predisposes this tissue to neoplasia with time or accelerates the progression of oncogene-dependent endometrial cancer constitute important questions for future investigation.
Supplementary Material
Acknowledgments
The authors thank Jie Li, Yan Ying, and Rong Zhao for their essential technical contributions. We would also like to thank Dr Nicholas Mitsiades, Baylor College of Medicine, for providing his findings from studies on the conditional knockout of Spop in the murine prostate prior to publication. We also acknowledge the services of The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) grant source: P50-HD28934.
Footnotes
Grant Support: This research was supported in part by a Cancer Prevention Research Institute of Texas pre-doctoral fellowship grant (CPRIT: RP101499 (to MMS)); National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) grant (U01: HD-076596 (to DML)); a NIH/NCI R01: CA-211861 to BH; a NIH/NICHD K99 HD080742 to RK; and a NIH/NICHD: R01: HD-042311 grant (to JPL).
Supplementary data
Supplementary data are available at BIOLRE online.
Supplementary Table S1. List of antibodies used in the describe experiments.
Supplementary Table S2. List of TaqMan assays used in the described experiments.
Supplementary Figure S1. Ovarian function is normal in the Spopd/d mouse. (A) Control (n = 8) and Spopd/d (n = 6) mice ovulated similar numbers of oocytes following administration of an established superovulation hormone regimen. (B–E) Histological analysis shows both control (B, D) and Spopd/d (C, E) ovaries exhibit corpora lutea (CL) following ovulation. (F) Typical estrous cyclicity profiles for both control (n = 10) and Spopd/d (n = 10) mice supports normal ovarian activity including steroidogenesis in Spopd/d female. (G) Representative examples of crystal violet stained vaginal cytology from control and Spopd/d mice at defined stages of the estrous cycle.
Supplementary Figure S2. Expression of estrogen molecular targets is not significantly altered in the Spopd/d uterus.
Supplementary Figure S3. Western analysis of GATA2, SRC-1, and SRC-2 protein expression in the control and Spopd/d uterus.
References
- 1. Claiborn KC, Sachdeva MM, Cannon CE, Groff DN, Singer JD, Stoffers DA. Pcif1 modulates Pdx1 protein stability and pancreatic beta cell function and survival in mice. J Clin Invest 2010; 120(10):3713–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, Mohanty AS, Cheng DT, Berger MF, Soslow RA, Weigelt B. The genetic landscape of endometrial clear cell carcinomas. J Pathol 2017; 243(2):230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones S, Stransky N, McCord CL, Cerami E, Lagowski J, Kelly D, Angiuoli SV, Sausen M, Kann L, Shukla M, Makar R, Wood LD et al. . Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nat Commun 2014; 5:5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Gallo M, Bell DW. The emerging genomic landscape of endometrial cancer. Clin Chem 2014; 60:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Gallo M, O’Hara AJ, Rudd ML, Urick ME, Hansen NF, O’Neil NJ, Price JC, Zhang S, England BM, Godwin AK, Sgroi DC. Program NIHISCCS et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 2012; 44:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen HY, Chen RH. Cullin 3 ubiquitin ligases in cancer biology: functions and therapeutic implications. Front Oncol 2016; 6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mani RS. The emerging role of speckle-type POZ protein (SPOP) in cancer development. Drug Discov Today 2014; 19:1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, Blattner M, Lonard DM et al. . Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci USA 2013; 110:6997–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geng C, Rajapakshe K, Shah SS, Shou J, Eedunuri VK, Foley C, Fiskus W, Rajendran M, Chew SA, Zimmermann M, Bond R, He B et al. . Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res 2014; 74:5631–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An J, Ren S, Murphy SJ, Dalangood S, Chang C, Pang X, Cui Y, Wang L, Pan Y, Zhang X, Zhu Y, Wang C et al. . Truncated ERG oncoproteins from TMPRSS2-ERG fusions are resistant to SPOP-mediated proteasome degradation. Mol Cell 2015; 59:904–916. [DOI] [PubMed] [Google Scholar]
- 11. An J, Wang C, Deng Y, Yu L, Huang H. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell Rep 2014; 6:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan W, Dai X, Lunardi A, Li Z, Inuzuka H, Liu P, Varmeh S, Zhang J, Cheng L, Sun Y, Asara JM, Beck AH et al. . SPOP promotes ubiquitination and degradation of the erg oncoprotein to suppress prostate cancer progression. Mol Cell 2015; 59:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geng C, Kaochar S, Li M, Rajapakshe K, Fiskus W, Dong J, Foley C, Dong B, Zhang L, Kwon OJ, Shah SS, Bolaki M et al. . SPOP regulates prostate epithelial cell proliferation and promotes ubiquitination and turnover of c-MYC oncoprotein. Oncogene 2017; 36:4767–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groner AC, Cato L, de Tribolet-Hardy J, Bernasocchi T, Janouskova H, Melchers D, Houtman R, Cato ACB, Tschopp P, Gu L, Corsinotti A, Zhong Q et al. . TRIM24 is an oncogenic transcriptional activator in prostate cancer. Cancer Cell 2016; 29:846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, Baek SH, Chiba T, Tanaka K, Bang OS, Joe CO, Chung CH. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem 2006; 281:12664–12672. [DOI] [PubMed] [Google Scholar]
- 16. Li C, Ao J, Fu J, Lee DF, Xu J, Lonard D, O’Malley BW. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene 2011; 30:4350–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu F, Dai X, Gan W, Wan L, Li M, Mitsiades N, Wei W, Ding Q, Zhang J. Prostate cancer-associated mutation in SPOP impairs its ability to target Cdc20 for poly-ubiquitination and degradation. Cancer Lett 2017; 385:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang P, Gao K, Tang Y, Jin X, An J, Yu H, Wang H, Zhang Y, Wang D, Huang H, Yu L, Wang C. Destruction of DDIT3/CHOP protein by wild-type SPOP but not prostate cancer-associated mutants. Human Mutat 2014; 35:1142–1151. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Q, Shi Q, Chen Y, Yue T, Li S, Wang B, Jiang J. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci USA 2009; 106:21191–21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu H, Ren S, Bitler BG, Aird KM, Tu Z, Skordalakes E, Zhu Y, Yan J, Sun Y, Zhang R. SPOP E3 ubiquitin ligase adaptor promotes cellular senescence by degrading the SENP7 deSUMOylase. Cell Rep 2015; 13:1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo ZQ, Zheng T, Chen B, Luo C, Ouyang S, Gong S, Li J, Mao LL, Lian F, Yang Y, Huang Y, Li L et al. . Small-molecule targeting of E3 ligase adaptor SPOP in kidney cancer. Cancer Cell 2016; 30:474–484. [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Sun G, Sun X. RNA interference-mediated silencing of speckle-type POZ protein promotes apoptosis of renal cell cancer cells. Onco Targets Ther 2016; 9:2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stone L. Kidney cancer: on target - inhibiting SPOP in ccRCC. Nat Rev Urol 2016; 13:630. [DOI] [PubMed] [Google Scholar]
- 24. Zhao W, Zhou J, Deng Z, Gao Y, Cheng Y. SPOP promotes tumor progression via activation of beta-catenin/TCF4 complex in clear cell renal cell carcinoma. Int J Oncol 2016; 49:1001–1008. [DOI] [PubMed] [Google Scholar]
- 25. Cai H, Liu A. Spop promotes skeletal development and homeostasis by positively regulating Ihh signaling. Proc Natl Acad Sci USA 2016; 113:14751–14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 2000; 28:106–110. [PubMed] [Google Scholar]
- 27. Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41:58–66. [DOI] [PubMed] [Google Scholar]
- 28. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9:2266–2278. [DOI] [PubMed] [Google Scholar]
- 29. Szwarc MM, Kommagani R, Jeong JW, Wu SP, Tsai SY, Tsai MJ, O’Malley BW, DeMayo FJ, Lydon JP. Perturbing the cellular levels of steroid receptor coactivator-2 impairs murine endometrial function. PLoS ONE 2014; 9:e98664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, DeMayo FJ, Lydon JP, O’Malley BW. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet 2013; 9:e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kommagani R, Szwarc MM, Vasquez YM, Peavey MC, Mazur EC, Gibbons WE, Lanz RB, DeMayo FJ, Lydon JP. The promyelocytic leukemia zinc finger transcription factor is critical for human endometrial stromal cell decidualization. PLoS Genet 2016; 12:e1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu SP, DeMayo FJ. Progesterone receptor signaling in uterine myometrial physiology and preterm birth. Curr Top Dev Biol 2017; 125:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao K, Jin X, Tang Y, Ma J, Peng J, Yu L, Zhang P, Wang C. Tumor suppressor SPOP mediates the proteasomal degradation of progesterone receptors (PRs) in breast cancer cells. Am J Cancer Res 2015; 5:3210–3220. [PMC free article] [PubMed] [Google Scholar]
- 34. Kawagoe J, Li Q, Mussi P, Liao L, Lydon JP, DeMayo FJ, Xu J. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell 2012; 23:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 1993; 90:11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang P, Gao K, Jin X, Ma J, Peng J, Wumaier R, Tang Y, Zhang Y, An J, Yan Q, Dong Y, Huang H et al. . Endometrial cancer-associated mutants of SPOP are defective in regulating estrogen receptor-alpha protein turnover. Cell Death Dis 2015; 6:e1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 1998; 279:1922–1925. [DOI] [PubMed] [Google Scholar]
- 38. Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, Jeong JW, Lydon JP et al. . A Gata2 -dependent transcription network regulates uterine progesterone responsiveness and endometrial function. Cell Rep 2016; 17:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han BC, Xia HF, Sun J, Yang Y, Peng JP. Retinoic acid-metabolizing enzyme cytochrome P450 26a1 (cyp26a1) is essential for implantation: functional study of its role in early pregnancy. J Cell Physiol 2010; 223:471–479. [DOI] [PubMed] [Google Scholar]
- 40. Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol Reprod 2010; 82:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 2006; 38:1204–1209. [DOI] [PubMed] [Google Scholar]
- 42. Kommagani R, Szwarc MM, Kovanci E, Creighton CJ, O’Malley BW, Demayo FJ, Lydon JP. A murine uterine transcriptome, responsive to steroid receptor coactivator-2, reveals transcription factor 23 as essential for decidualization of human endometrial stromal cells. Biol Reprod 2014; 90:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013; 34:130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ, Spencer TE. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci USA 2017; 114:E1018–E1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gonzalez G, Mehra S, Wang Y, Akiyama H, Behringer RR. Sox9 overexpression in uterine epithelia induces endometrial gland hyperplasia. Differentiation 2016; 92:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagai Y, Kojima T, Muro Y, Hachiya T, Nishizawa Y, Wakabayashi T, Hagiwara M. Identification of a novel nuclear speckle-type protein, SPOP. FEBS Lett 1997; 418:23–26. [DOI] [PubMed] [Google Scholar]
- 47. Liu N, Liu X, Yu Q, Chen X, Ding Y, He J, Gao R, Wang Y, Liu X. SPOP regulates endometrial stromal cell decidualization in mice. Reprod Sci 2016; 23:1565–1574. [DOI] [PubMed] [Google Scholar]
- 48. Lai J, Batra J. Speckle-type POZ protein mutations interrupt tumor suppressor function of speckle-type POZ protein in prostate cancer by affecting androgen receptor degradation. Asian J Androl 2014; 16:659–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Byun B, Jung Y. Repression of transcriptional activity of estrogen receptor alpha by a Cullin3/SPOP ubiquitin E3 ligase complex. Mol Cell 2008; 25:289–293. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.