Abstract
We investigated the effects of pre-maturational (pre-IVM) culture on the developmental competence of small-sized bovine oocytes (110 and < 115 µm). Oocytes were cultured with 3-isobutyl-1-methylxanthine (IBMX) for 0, 5, or 10 h and subjected to in vitro maturation, fertilization, and culture. The cleavage rate (73%) of small-sized oocytes with 5 h pre-IVM was higher than those with 0 and 10 h pre-IVM (61 and 62%, respectively). The blastocyst rate (16%) of embryos derived from small-sized oocytes with 5 h pre-IVM was higher than those with 0 and 10 h pre-IVM (9 and 8%, respectively). In addition, small-sized oocytes with 5 h pre-IVM had a higher mean cell number in blastocysts (134.1 ± 34.8) than those with 0 and 10 h pre-IVM (100.2 ± 17.2 and 107.8 ± 23.7, respectively). In conclusion, the pre-IVM of small-sized oocytes with IBMX for 5 h improved the developmental competence of bovine oocytes, as well as the quality of blastocysts.
Keywords: IBMX, Oocytes, Pre-IVM duration
Bovine oocytes obtained from slaughterhouse-derived ovaries and from ovum pick-up (OPU) from live cows are widely used for in vitro embryo production (IVEP). Previous studies have focused on establishing a suitable system to efficiently employ these oocytes [1,2,3,4]. However, the oocytes aspirated from these ovaries vary in size, and development to the blastocyst stage is known to increase with larger follicular and oocyte diameters [5]. It was reported that the percentage of oocytes of 115 to < 120 µm in size that achieve metaphase II (MII) and develop to blastocyst stage was higher than that of oocytes sized 110 to < 115 µm [6]. Furthermore, there was a positive correlation between oocyte diameter and follicular size [7]. When OPU is performed, follicles more than 2 mm in diameter are generally aspirated, but the number of harvested oocytes is limited [8]. It was also reported that the mean diameter of oocytes collected from follicles of 2–3 mm in diameter was 112.9 µm [7], thus, the improvement of small-sized oocytes (110 to < 115 µm) should be attempted for the effective IVEP.
Previous studies have demonstrated improvement of the developmental competence of oocytes by inhibiting germinal vesicle breakdown (GVBD) and holding oocytes at the germinal vesicle (GV) stage before in vitro maturation (IVM), because oocytes require time to acquire full developmental competence during meiotic arrest [9]. We speculated that oocytes collected by OPU, especially small-sized oocytes, need time prior to IVM to acquire developmental competence, and this time is different than that for large-sized oocytes (≥ 115 µm). In our previous study [10], we showed that culture with 3-isobutyl-1-methylxanthine (IBMX) for 20 h before IVM culture (pre-IVM) had a positive effect on the developmental competence of oocytes derived from 12 days of in vitro growth (IVG) culture; however, pre-IVM treatment for 20 h did not improve the rate of development to the blastocyst stage in oocytes derived from 14 days of IVG culture. In addition, we found that the low developmental rate of 14-day IVG oocytes was associated with reduced integrity of the cell membranes of cumulus cells [10]. We also showed that several small-sized oocytes (< 115 µm) derived from antral follicles (2–8 mm) started to degenerate and that some of the cumulus cell process endings lost gap junctions with the oocytes [11], because the cow is a mono-ovulator and most of the oocytes used for IVEP are destined to degenerate. Therefore, the effect of pre-IVM treatment with IBMX on the developmental competence of small-sized oocytes derived from antral follicles (2–8 mm) is unclear. In the present study, we collected bovine oocytes from slaughterhouse-derived ovaries and divided them into small-sized (110 to < 115 µm) and large-sized (≥ 115 µm) oocytes and cultured them for 0, 5, or 10 h with IBMX before IVM culture. The oocytes were then submitted to IVM, in vitro fertilization (IVF), and in vitro culture (IVC) and their development to the blastocyst stage was examined.
Immediately before pre-IVM culture, all small- and large-sized oocytes were at GV (19/19 and 22/22, respectively). Among the small-sized oocytes, the proportions of oocytes that reached metaphase I (MI) and overall meiotic resumption when treated with IBMX for 5 h were lower than those treated for 10 h pre-IVM (P < 0.05) (Table 1). However, the proportion of oocytes at GV stage was higher (P < 0.05) when the oocytes were treated for 5 h than when they were treated for 10 h pre-IVM. Similarly, in large-sized oocytes, the percentage of MI oocytes and the overall meiotic resumption were higher (P < 0.05) in oocytes treated for 10 h than in those treated for 5 h pre-IVM, whereas lower percentages at the GV stage were observed in oocytes treated for 10 h than in those treated for 5 h pre-IVM (P < 0.05). After IVM culture, the overall meiotic resumption was similar between the small and the large-sized oocytes, regardless of the duration of pre-IVM treatment (Table 2). In addition, the percentage of metaphase II (MII) oocytes was similar among oocytes of the same size category that were treated for 0, 5, and 10 h pre-IVM. However, the percentage of MII oocytes among those treated for 0 and 10 h pre-IVM was higher among large-sized than among small-sized oocytes (P < 0.05), while the percentage of MII oocytes among those treated for 5 h pre-IVM tended to be higher among large-sized than among small-sized oocytes (P = 0.06). On the other hand, the rate of MI oocytes was higher among small-sized oocytes than among large-sized oocytes, regardless of the duration of pre-IVM treatment (P < 0.05).
Table 1. Effects of pre-IVM durations on the nuclear status of oocytes before IVM.
| Oocyte diameter (µm) |
Pre-IVM (h) | No. of oocytes (replicates) |
No. of oocytes in each meiotic stage (%) |
GVBD-MII | ||||
|---|---|---|---|---|---|---|---|---|
| GV | GVBD | MI | MII | Deg. | ||||
| 110 to < 115 | 5 | 101 (5) | 93 (92.1) a) | 0 (0) | 6 (5.9) a) | 0 (0) | 2 (0.2) a) | 6 (5.9) a) |
| 10 | 117 (5) | 49 (41.9) b) | 1 (0.9) | 63 (53.8) b) | 0 (0) | 4 (3.4) b) | 64 (54.7) b) | |
| ≥ 115 | 5 | 54 (4) | 47 (87.0) a) | 1 (1.9) | 5 (9.5) a) | 0 (0) | 1 (1.8) | 6 (11.1) a) |
| 10 | 64 (3) | 35 (54.7) b) | 0 (0) | 28 (43.8) b) | 0 (0) | 1 (1.6) | 28 (43.8) b) | |
GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; MII, metaphase II. a, b) Within a column, values without a common superscript differed significantly (P < 0.05).
Table 2. Effects of pre-IVM durations on the nuclear status of oocytes after IVM.
| Oocyte diameter (µm) |
Pre-IVM (h) | No. of oocytes (replicates) |
No. of oocytes in each meiotic stage (%) |
GVBD-MII | ||||
|---|---|---|---|---|---|---|---|---|
| GV | GVBD | MI | MII | Deg. | ||||
| 110 to < 115 | 0 | 49 (3) | 5 (10.2) | 6 (12.2) | 9 (18.4) * | 26 (53.1) | 2 (4.1) | 40 (81.6) |
| 5 | 40 (3) | 1 (2.5) | 0 (0) | 11 (27.5) * | 27 (67.5) | 1 (2.5) | 38 (95) | |
| 10 | 43 (3) | 0 (0) | 0 (0) | 13 (30.2) * | 28 (65.1) | 2 (4.6) | 41 (95.3) | |
| ≥ 115 | 0 | 44 (3) | 1 (2.3) | 3 (6.8) | 2 (4.5) | 37 (84.1) * | 1 (2.3) | 42 (95.5) |
| 5 | 36 (3) | 1 (2.8) | 1 (2.8) | 3 (8.4) | 31 (86.1) | 0 (0) | 35 (97.2) | |
| 10 | 34 (3) | 0 (0) | 0 (0) | 2 (5.9) | 32 (94.1) * | 0 (0) | 34 (100) | |
GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; MII, metaphase II. *Asterisks indicate the difference between different sized-oocytes with the same duration of pre-IVM treatment (P < 0.05).
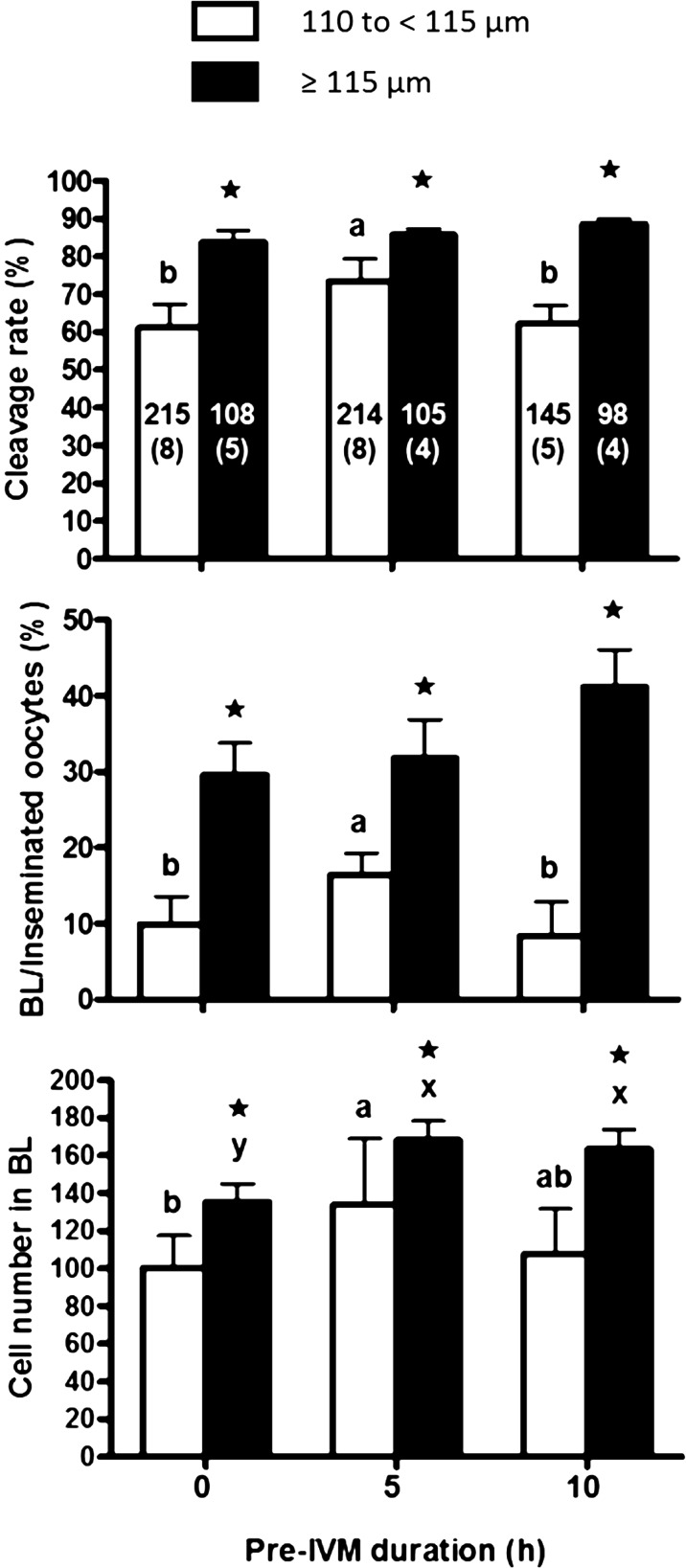
As shown in Fig. 1, the major effects of the size of oocytes and the duration of pre-IVM treatment were evident through cleavage and blastocyst rates (P < 0.05), but not through the cell numbers of blastocysts (P > 0.05). Namely, the cleavage rate (73%) of embryos derived from the small-sized oocytes treated for 5 h pre-IVM was higher (P < 0.05) than that of those treated for 0 and 10 h pre-IVM (61 and 62%, respectively) (Fig. 1). However, the cleavage rates were lower in small-sized oocytes than in large-sized oocytes, regardless of the duration of pre-IVM treatment (P < 0.05). This difference may be caused by the lower maturation rate in small-sized oocytes than in large-sized oocytes. Based on inseminated oocytes, the blastocyst rate (16%) of embryos derived from small-sized oocytes subjected to 5 h pre-IVM treatment was higher (P < 0.05) than that of those subjected to 0 and 10 h pre-IVM treatment (9 and 8%, respectively), but was lower (P < 0.05) than that of large-sized oocytes (31%). In addition, blastocysts derived from small-sized oocytes treated for 5 h pre-IVM had a higher mean cell number (134.1 ± 34.8) than those derived from oocytes treated for 0 and 10 h pre-IVM (100.2 ± 17.2 and 107.8 ± 23.7, respectively). A previous mouse and bovine study using cumulus-oocyte complex (COC) [12] demonstrated that IBMX treatment for 1–2 h pre-IVM increased COC cAMP levels 100-fold and improved embryo cleavage, blastocyst rates, and embryo quality. Additionally, the pre-IVM treatment had a positive influence on the developmental competence of oocytes in pigs by improving cytoplasmic maturation [13]. In the present study, we also added low concentration of FSH during pre-IVM treatment, expecting to increase cAMP levels in oocytes. Sugimura et al. [14] reported that bovine oocytes treated with IBMX for 2 h followed by 22 h IVM with FSH significantly enhanced the ability of oocytes to develop to blastocysts. They suggested that pre-treatment with IBMX enhanced the effectiveness of FSH at improving oocyte developmental competence [14]. In the present study, we cultured bovine oocytes with simultaneous addition of IBMX and FSH for 5 h, and we did not observe a significant increase in blastocyst rate among large-sized oocytes. However, the synergistic effect of IBMX and FSH on developmental competence was observable in small-sized oocytes after 5 h of pre-IVM treatment. These results may suggest that the proper duration of pre-IVM treatment is different for large- and small-sized oocytes. We should thus determine the optimal pre-IVM treatment duration for oocytes of different sizes or oocytes derived from follicles of different sizes (estrous cycle), as well as the dynamics of cAMP concentrations in oocytes during pre-IVM treatment with IBMX and FSH, in a future study.
Fig. 1.
Cleavage rates, blastocyst rates, and blastocyst cell numbers for small-sized (110 to < 115 µm in diameter) and large-sized oocytes (≥ 115 µm in diameter) after 0, 5, and 10 h of pre-IVM treatment with IBMX. The number in the bar is the number of oocytes, and the number of replicates is in parentheses. * Asterisk indicates a significant difference between experimental groups (P < 0.05). a, b Different letters indicate a significant difference among small-sized oocytes (P < 0.05). x, y Different letters indicate a significant difference among large-sized oocytes (P < 0.05).
Large-sized oocytes were previously reported to show higher developmental competence than small-sized oocytes, and bovine oocytes derived from larger follicles exhibited stronger mitochondrial activity and a higher proportion of blastocyst development than those from smaller follicles [15]. Our results demonstrated that the pre-IVM treatment of small-sized oocytes improved their developmental competence. The intracellular secondary messenger cAMP plays an important role in the regulation of mitochondrial activity in mammalian cells [16,17,18]. IBMX prevents the deprivation of cAMP in COCs, which significantly increases cAMP levels and further enhances meiosis progression in oocytes, similar to what occurs during in vivo oocyte maturation [19]. Furthermore, mitochondrial activity of bovine oocytes increases during follicular development, and stronger mitochondrial activity is accompanied by greater developmental competence of immature oocytes [15]. We showed that the mitochondrial activity of in vitro grown oocytes of 105.9–122.7 mm in diameter increased during pre-IVM treatment and was accompanied by the acquisition of developmental competence [20]. In the present study, cAMP concentrations in oocytes may have been increased by the addition of IBMX and a low concentration of FSH to the pre-IVM medium, and mitochondrial activity before the IVM culture may also have increased; it is possible that these phenomena improved the developmental competence of small-sized oocytes treated for 5 h pre-IVM. The extension of the pre-IVM treatment to 10 h may have resulted in the aging of oocytes, subsequently inducing oocyte or cumulus cell degradation and reducing not only mitochondrial activity before IVM culture, but also the developmental competence of oocytes.
In the case of oocytes with a diameter of ≥ 115 µm, 5- and 10-h pre-IVM treatment did not have any detrimental effects (aging) on the cleavage and blastocyst rates; however, the cell number in blastocysts significantly increased after the 5- and 10-h pre-IVM treatment (P < 0.05) compared to untreated oocytes. These results indicate that embryo quality may be improved by pre-IVM treatment due to improved cytoplasmic maturation.
In conclusion, the results of our study show that pre-IVM treatment with FSH and IBMX for 5 h improved the developmental competence of bovine oocytes of a diameter between 110 and < 115 µm as well as of those of a diameter of ≥ 115 µm. These results suggest that the approach to enhance the developmental competence of oocytes using the pre-IVM treatment for 5 h, regardless of oocyte diameter, will ultimately be useful in the management of IVEP in bovines. In the present study, we showed the benefits of pre-IVM treatment for only 5 and 10 h pre-IVM; therefore, the appropriate duration of pre-IVM treatment should be determined in a future study.
Methods
Chemicals
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated.
Collection of cumulus oocyte complexes (COCs)
Bovine ovaries were obtained from a local abattoir. They were transported to the laboratory within 6 h of collection in plastic bags at 20°C. After three washes in sterile physiological saline, follicles (2–8 mm in diameter) were aspirated using an 18-gauge needle attached to a 10-ml syringe containing TALP working medium, supplemented with 10% fetal bovine serum (FBS, Invitrogen, Grand Island, NY, USA). Aspirated follicular fluid was pooled in 50-ml conical tubes and allowed to settle. COCs were searched for under a stereomicroscope and washed three times in TALP working medium; those with more than three layers of cumulus cells and a uniform cytoplasm were selected for further processing. The diameters of oocytes (excluding the zona pellucida) were measured using an ocular scale attached to the stereomicroscope and then divided into COCs having oocytes with diameters between 110 and < 115 µm (small-sized) and those having oocytes with a diameter of ≥ 115 µm (large-sized).
Pre-IVM treatment and IVM of COCs
COCs were submitted to IVM with or without pre-IVM treatment, as described previously [21]. Briefly, COCs were incubated in droplets of pre-IVM medium (approximately 10 COCs/50 µl), which was modified from IVM medium containing 0.5 mM IBMX, and a lower FSH concentration (2 × 10−6 units/ml, from the porcine pituitary), and covered with paraffin for 0, 5, or 10 h. The IBMX stock solution was diluted to 500 mM in dimethyl sulfoxide, and 10 µl of the stock solution was mixed with 10 ml pre-IVM medium, yielding a final concentration of IBMX of 0.5 mM. The maturation medium consisted of HEPES-buffered TCM199 supplemented with 0.2 mM sodium pyruvate, 0.02 units/ml FSH, 1 µg/m1 estradiol-17β, 10% FBS, and 50 µg/ml gentamicin sulfate, covered with paraffin at 39°C for 22 h in a 5% CO2 atmosphere. The total culture periods were 22, 27, or 32 h in the 0, 5, and 10 h pre-IVM groups, respectively.
Evaluation of the oocyte nuclear status after pre-IVM treatment
After pre-IVM and/or IVM, oocytes were denuded from cumulus cells by vortexing and stained with 1% aceto-orcein. The nuclear status was classified as germinal vesicles (GV), germinal vesicle breakdown (GVBD), metaphase I (MI), or metaphase II (MII) by observation under a phase-contrast microscope [3].
In vitro fertilization (IVF) and subsequent culture (IVC)
IVF using frozen semen was performed according to a previously described procedure [22] with slight modifications. Briefly, motile sperm (5 × 106 sperm/ml) separated in a Percoll gradient (45% and 90%) were incubated with COCs in a 100-µl droplet (approximately 10 COCs per droplet) of modified Brackett and Oliphant isotonic medium [23] containing 3 mg/ml fatty acid-free BSA and 2.5 mM theophylline [24] at 39°C for 18 h in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2. IVC of inseminated oocytes (presumptive zygotes) was performed as previously described [25]. Briefly, after an incubation with sperm, presumptive zygotes were freed from cumulus cells by vortexing and washing three times in culture medium. Cumulus-free zygotes were cultured at 39°C for 6 days in 30-µl droplets of culture medium in a 5% CO2, 5% O2, and 90% N2 atmosphere. The culture medium consisted of modified synthetic oviduct fluid containing 1 mM glutamine, 12 essential amino acids for basal medium Eagle, seven non-essential amino acids for minimum essential medium, 10 µg/ml insulin, 5 mM glycine, 5 mM taurine, 1 mM glucose, and 3 mg/ml fatty acid-free BSA. Cleavage and blastocyst rates were assessed after 2 days (approximately 30 h) and 6 days (approximately 150 h) of IVC, respectively. The total cell number in blastocysts obtained after 6 days of IVC was counted using an air-drying method [24].
Statistical analysis
All statistical analyses were performed using JMP software version 11.0.0 (SAS Institute, Cary, NC, USA). The oocyte nuclear status was analyzed by the chi-square test. Interactions among the size of collected COCs, duration of the pre-IVM culture, and developmental competence were compared by two-way ANOVA followed by Turkey-Kramer’s HSD as a post-hoc test. Differences of P < 0.05 were regarded as significant.
Conflict of interests
Authors declare that they have no competing interests.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number JP16K08043 to M. Nagano.
References
- 1.Boelhauve M, Sinowatz F, Wolf E, Paula-Lopes FF. Maturation of bovine oocytes in the presence of leptin improves development and reduces apoptosis of in vitro-produced blastocysts. Biol Reprod 2005; 73: 737–744. [DOI] [PubMed] [Google Scholar]
- 2.Diógenes MN, Guimarães ALS, Leme LO, Maurício MF, Dode MAN. Effect of prematuration and maturation with fibroblast growth factor 10 (FGF10) on in vitro development of bovine oocytes. Theriogenology 2017; 102: 190–198. [DOI] [PubMed] [Google Scholar]
- 3.Nagano M, Katagiri S, Takahashi Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote 2006; 14: 299–304. [DOI] [PubMed] [Google Scholar]
- 4.Sagirkaya H, Misirlioglu M, Kaya A, First NL, Parrish JJ, Memili E. Developmental potential of bovine oocytes cultured in different maturation and culture conditions. Anim Reprod Sci 2007; 101: 225–240. [DOI] [PubMed] [Google Scholar]
- 5.Arlotto T, Schwartz JL, First NL, Leibfried-Rutledge ML. Aspects of follicle and oocyte stage that affect in vitro maturation and development of bovine oocytes. Theriogenology 1996; 45: 943–956. [DOI] [PubMed] [Google Scholar]
- 6.Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997; 48: 769–774. [DOI] [PubMed] [Google Scholar]
- 7.Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev 1995; 42: 437–442. [DOI] [PubMed] [Google Scholar]
- 8.Merton JS, de Roos APW, Mullaart E, de Ruigh L, Kaal L, Vos PLAM, Dieleman SJ. Factors affecting oocyte quality and quantity in commercial application of embryo technologies in the cattle breeding industry. Theriogenology 2003; 59: 651–674. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto S, Minami N, Takakura R, Imai H. Bovine immature oocytes acquire developmental competence during meiotic arrest in vitro. Biol Reprod 2002; 66: 1696–1701. [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Nagano M, Kang SS, Yanagawa Y, Takahashi Y. Effects of in vitro growth culture duration and prematuration culture on maturational and developmental competences of bovine oocytes derived from early antral follicles. Theriogenology 2013; 80: 793–799. [DOI] [PubMed] [Google Scholar]
- 11.Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 2006; 14: 53–61. [DOI] [PubMed] [Google Scholar]
- 12.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod 2010; 25: 2999–3011. [DOI] [PubMed] [Google Scholar]
- 13.Park B, Lee H, Lee Y, Elahi F, Lee J, Lee ST, Park CK, Hyun SH, Lee E. Cilostamide and forskolin treatment during pre-IVM improves preimplantation development of cloned embryos by influencing meiotic progression and gap junction communication in pigs. Theriogenology 2016; 86: 757–765. [DOI] [PubMed] [Google Scholar]
- 14.Sugimura S, Yamanouchi T, Palmerini MG, Hashiyada Y, Imai K, Gilchrist RB. Effect of pre-in vitro maturation with cAMP modulators on the acquisition of oocyte developmental competence in cattle. J Reprod Dev 2018; 64: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machatkova M, Jeseta M, Hulinska P, Knitlova D, Nemcova L, Kanka J. Characteristics of bovine oocytes with different meiotic competence in terms of their mitochondrial status and expression of nuclear-encoded factors. Reprod Domest Anim 2012; 47: 806–814. [DOI] [PubMed] [Google Scholar]
- 16.Eppig JJ. The participation of cyclic adenosine monophosphate (cAMP) in the regulation of meiotic maturation of oocytes in the laboratory mouse. J Reprod Fertil Suppl 1989; 38: 3–8. [PubMed] [Google Scholar]
- 17.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007; 67: 6–15. [DOI] [PubMed] [Google Scholar]
- 18.Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology (Bethesda) 2013; 28: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng HT, Ren Z, Guzman L, Wang X, Sutton-McDowall ML, Ritter LJ, De Vos M, Smitz J, Thompson JG, Gilchrist RB. Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence. Hum Reprod 2013; 28: 1536–1545. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Kang SS, Nagai K, Yanagawa Y, Takahashi Y, Nagano M. Mitochondrial activity during pre-maturational culture in in vitro-grown bovine oocytes is related to maturational and developmental competences. Reprod Fertil Dev 2016; 28: 349–356. [DOI] [PubMed] [Google Scholar]
- 21.Nagano M, Kang SS, Koyama K, Huang W, Yanagawa Y, Takahashi Y. In vitro maturation system for individual culture of bovine oocytes using micro-volume multi-well plate. Jpn J Vet Res 2013; 61: 149–154. [PubMed] [Google Scholar]
- 22.Takahashi Y, Kanagawa H. Effect of oxygen concentration in the gas atmosphere during in vitro insemination of bovine oocytes on the subsequent embryonic development in vitro. J Vet Med Sci 1998; 60: 365–367. [DOI] [PubMed] [Google Scholar]
- 23.Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod 1975; 12: 260–274. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992; 37: 963–978. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, Kanagawa H. Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium. J Vet Med Sci 1998; 60: 433–437. [DOI] [PubMed] [Google Scholar]