Abstract
Heat stress (HS) negatively affects reproduction in cattle; however, its effect on endocrine function in bovine endometrial cells remains unclear. In this study, we examined the effects of HS on the production of prostaglandin (PG) E2 and PGF2α in the cultured bovine endometrial epithelial and stromal cells separately. To evaluate the effect of HS on endocrine function, the cells were cultured at 38.5°C (control) or 40.5°C (HS). After treatment, PGE2 and PGF2α levels were measured via enzyme immunoassay (EIA) and mRNA expressions of enzymes involved in PG synthesis were examined via quantitative reverse transcription polymerase chain reaction (RT-PCR). HS did not influence the production of PGE2 or PGF2α in the epithelial cells; however, HS significantly enhanced the production of both PGE2 and PGF2α in the stromal cells (P < 0.05). In addition, HS significantly increased phospholipase A2 (PLA2), cyclooxygenase 2 (COX2), prostaglandin F synthase (PGFS), prostaglandin E synthase (PGES), and carbonyl reductase 1 (CBR1) mRNA expression in the stromal cells (P < 0.05). The overall results suggest that HS induces mRNA expression of enzymes involved in PG synthesis, resulting in the upregulation of PGE2 and PGF2α production in the stromal cells, but not in the epithelial cells. The HS-induced increase of PGE2 and PGF2α secretion in bovine endometrial stromal cells may disrupt the normal estrous cycle and cause infertility in cows during summer.
Keywords: Bovine, Endometrial cell, Heat stress, Prostaglandin
In recent years, global warming has received increasing attention. Particularly in the livestock industry, the effect of heat stress (HS) due to global warming is a special concern. In cows, HS influences physiological properties, such as milk production, a decrease in feed intake, and rapid shallow breathing [1]. In animal reproduction, HS decreases estradiol-17β secretion from the dominant follicle, resulting a in suppression of overt estrous behavior [2]. In addition, HS increases uterine temperature, leading to reduced blood flow to the uterus [3], and impedes sperm and embryo survival [4]. These effects of HS on reproduction lead to a decrease in the bovine conception rate during summer and cause economic losses in livestock management [5]. Therefore, an improvement in the conception rate of cattle during summer is required.
The uterine endometrium secretes prostaglandins (PGs), which are involved in implantation, parturition, luteolysis and pregnancy recognition in mammals [6]. The production of PGs is mainly controlled by phospholipase A2 (PLA2) and cyclooxygenase 2 (COX2). Arachidonic acid is liberated from phospholipids in the plasma membrane by PLA2. Then, COX2 converts it to PGH2, which is a precursor of all PGs including PGF2α and PGE2. In addition, PGH2 is converted to PGF2α and PGE2 by prostaglandin F synthase (PGFS) and prostaglandin E synthase (PGES), respectively. PGE2 is then converted to PGF2α by carbonyl reductase 1 (CBR1) [7]. In cows, PGE2 is known as a luteotrophic factor, since it induces the secretion of progesterone (P4) from the corpus luteum (CL) by increasing intracellular cyclic AMP [8, 9]. Contrarily, PGF2α secreted by the endometrium induces luteolysis in cows [10]. The endometrium consists of two types of cells, epithelial cells and stromal cells both of which produce PGE2 and PGF2α. Epithelial cells principally secrete PGF2α, while stromal cells mainly secrete PGE2 [11]. During bovine luteolysis, tumor necrosis factor (TNF) α induces PGF2α secretion from the stromal cells, and subsequently induces the production of oxytocin (OT) from the CL. Then OT further enhances PGF2α secretion from the epithelial cells [12]. This positive feedback mechanism between PGF2α secreted from the endometrium and OT secreted from the CL induces luteolysis. Thus, the endocrine function of the endometrial epithelial and stromal cells plays central roles in the regulation of the estrous cycle in cows. The endocrine functions of the bovine endometrium are influenced by HS. Exposing endometrial tissues to HS (43°C, 18 h) was shown to increase PGF2α secretion [13]. However, the specific effect of HS on the epithelial and stromal cells of bovine endometrium remains unclear. In this study, to suggest possible solutions to bovine reproductive disorders caused by HS, we examined the in vitro effect of HS on the endocrine functions of the endometrial epithelial and stromal cells, individually.
Materials and Methods
Collection of endometrial tissues
Healthy cow uteri without visible conceptus were obtained from a local slaughterhouse (Okayama Meat Center), within 10–20 min of exsanguination of the animals; the organs were submerged in ice-cold physiological saline and immediately transported to the laboratory. The stages of the estrous cycle were confirmed by macroscopic observation of the ovaries and uteri, as described previously [14, 15]. After trimming of the uteri ipsilateral to the CL, intercaruncular endometrial tissues were immediately frozen and stored at –80°C until mRNA extraction and protein analysis.
Isolation and culture of endometrial cells
Uteri at the early-luteal and follicular stages (Day 2–3 and Day 19–21) were used for the isolation and culture of endometrial cells. Epithelial and stromal cells from bovine endometrium were enzymatically separated and cultured according to procedures described previously [16, 17]. The collected epithelial and stromal cells were separately resuspended in culture medium (DMEM/Ham’s F-12, 1:1 (v/v); Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) bovine serum (Invitrogen), 20 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO, USA) and 2 μg/ml amphotericin B (Sigma-Aldrich). These epithelial and stromal cells were seeded with 750 µl, 1 ml, and 20 ml, having a density of 1 × 105 viable cells/ml, in 4-well cluster dishes (Thermo Fisher Scientific, Yokohama, Japan), 24-well cluster dishes (Greiner Bio-One, Frickenhausen, Germany) and 75 cm2 culture flasks, respectively, and were cultured at 38.5°C in a humidified atmosphere of 5% CO2 in air, until the cells reached confluence.
Enzyme immunoassay (EIA)
The concentrations of PGE2 and PGF2α in the culture medium were determined via EIA, as described previously [18, 19]. The PGE2 standard curve ranged from 0.039 to 10 ng/ml, and the median effective dose (ED50) of the assay was 0.625 ng/ml. The intra- and inter-assay coefficients of variation, on average, were 7.83% and 13.02%, respectively. The PGF2α standard curve ranged from 0.016 to 4 ng/ml and the ED50 of the assay was 0.25 ng/ml. The intra- and inter-assay coefficients of variation, on average, were 6.18% and 12.11%, respectively.
Total RNA extraction and quantitative reverse transcription - polymerase chain reaction (RT-PCR)
Total RNA was extracted from the endometrial tissues and cultured cells using TRIsure (Bioline, London, UK), according to the manufacturer’s directions. One microgram of the respective total RNA was reverse transcribed using a ThermoScript RT-PCR System (no. 11146-016; Invitrogen) and 10% of the reaction mixture was used in each PCR reaction using specific primers for bovine heat shock protein (HSP) 70, HSP90, PLA2, COX2, PGFS, cytosolic PGES (cPGES), CBR1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of primers were listed in Table 1. Quantifications of mRNA expressions were determined by SsoAdvanced SYBR Green Supermix (no. 170-8880; Bio-Rad Laboratories, Berkeley, CA, USA), as described previously [20]. Serial dilutions of arbitrary cDNA were used as standards to analyze the relative level of the expressions of each mRNA. The mRNA expression of GAPDH was used as an internal control. For quantification of the mRNA expression levels, PCR was performed under the following conditions: 95°C for 15 min followed by 45 cycles of 95°C for 10 sec, 60°C for 10 sec, and 72°C for 15 sec. Use of the MyiQ Single-Colour Real-Time PCR Detection system at elevated temperatures resulted in reliable and sensitive quantification of the RT-PCR products with high linearity (Pearson’s product moment correlation coefficient, r > 0.99).
Table 1. Sequences of primers used for real-time RT-PCR analysis.
| Genes | Forward and reverse primers | Accession no. |
|---|---|---|
| PLA2 | 5'-AGGTGCACAACTTCATGCTG-3' | BC134610 |
| 5'-GGCATCCAATTCGTCTTCAT-3' | ||
| COX2 | 5'-TGTGAAAGGGAGGAAAGAGC-3' | AF004944 |
| 5'-GGCAAAGAATGCAAACATCA-3' | ||
| PGFS | 5'-GCAGGAGAAAGTGGTGAAGC-3' | S54973 |
| 5'-GCCAGTGGATGAGGTAGAGG-3' | ||
| cPGES | 5'-AGGACGCTCAGAGACATGGA-3' | NM_174443 |
| 5'-TTCGGTCCGAGGAAAGAGTA-3' | ||
| CBR1 | 5'-AAAACCGCAAGGCAGAGTGGTG-3' | NM_001034513.1 |
| 5'-CTCCATATGCGGTATCGGGCCA-3' | ||
| GAPDH | 5'-CACCCTCAAGATTGTCAGCA-3' | BC102589 |
| 5'-GGTCATAAGTCCCTCCACGA-3' | ||
Western blotting
The protein expression levels of HSP70 and HSP90 in the bovine endometrial tissues and cultured endometrial cells were determined via western blotting. Protein concentrations in the lysates were determined by bovine serum albumin (BSA) as a standard [21]. The proteins were then solubilized in sodium dodecyl sulfate (SDS) gel-loading buffer (50 mM Tris-HCL, 2% SDS 31607-94, Nakarai Tesque, Tokyo, Japan), 10% glycerol, and 1% B-mercaptoethanol (pH 6.8; 137-06862; Wako Pure Chemical Industries, Osaka, Japan) and heated at 95°C for 10 min. Samples (25 μg protein/lane) were analyzed on a 10% (v/v) SDS-polyacrylamide gel electrophoresis (PAGE) gel and then transferred to a polyvinylidene (PVDF) membrane (RPN303F; GE Healthcare, Little Chalfront, Buckinghamshire, UK). The membrane was washed in TBS-T (0.1% Tween 20 in tris-buffered saline (TBS), pH 7.5) and incubated in blocking buffer (5% nonfat dry milk in TBS-T) for 1 h at 25°C. After washing, the membrane was cut into two pieces, and each piece was incubated separately with specific primary antibodies to HSP70 (anti-HSP70-IgG-rabbit; Assay designs, 1:2000 dilution) or HSP90 (anti-HSP90-IgG-mouse; Abcam, Cambridge, UK, 1:2000 dilution) and β-actin (anti-β-actin-IgG-mouse; Sigma-Aldrich, 1:40000 dilution) in TBS-T for 24 h at 4°C. After incubation, the membrane pieces were incubated again with secondary antibodies (anti-rabbit, horseradish peroxidase (HRP)-linked whole antibody produced in donkey; GE Healthcare, 1:5000 dilution for HSP70, and anti-mouse, HRP-linked whole antibody produced in sheep; GE Healthcare, 1:5000 dilution for HSP90 and 1:4000 dilution for β-actin, respectively) in TBS-T 1 h at room temperature. The signal was detected using an ECL Western Blotting Detection System (GE Healthcare), and the intensity of the immunological reaction mixture was estimated by measuring the optical density in the defined area by computerized densitometry using Image J (National Institutes of Health, Bethesda, MD, USA).
Experimental design
Experiment 1: Comparisons of mRNA and protein expressions of HSP70 and HSP90 between summer and winter in bovine endometrial tissues
In the present study, in order to examine whether bovine endometrium is influenced by HS in the summer, we examined the expressions of HSP70 and HSP90, both of which are generally used as HS makers in the bovine endometrium, oviduct, and embryos [13, 22, 23]. For the measurement of the expressions of HSP70 and HSP90 mRNA and protein, endometrial tissues (days 8–12 after ovulation) were collected from five cows in winter (December-April, 2013–2014) and six cows in summer (July-August, 2014 and August, 2015). Average temperatures in Okayama during the sampling periods were 6.7°C in winter and 27.3°C in summer, respectively. The temperature data were obtained from the Japan Meteorological Agency.
Experiment 2: Effects of various incubation temperatures on HSP70 and HSP90 mRNA and protein expression in the cultured bovine endometrial epithelial and stromal cells
Endometrial epithelial and stromal cells that reached confluence in the 4-well cluster dishes and 75 cm2 culture flasks were used for this experiment. The cells were incubated with phenol red-free Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 supplemented with 0.1% (w/v) BSA, 5 ng/ml sodium selenite (Sigma-Aldrich), 0.5 mM ascorbic acid (Wako Pure Chemical Industries), 5 mg/ml transferrin (Sigma-Aldrich), 2 mg/ml insulin (Sigma-Aldrich), and 20 mg/ml gentamicin. Control samples were incubated at 38.5°C for 34 h. HS treatment samples were incubated at 39.5°C or 40.5°C for 10 h and allowed to recover from stress at 38.5°C for 14 h. After recovery, the cells were incubated again at 39.5°C or 40.5°C for 10 h. We used this duration for HS treatment in order to reproduce in vivo HS conditions, based on our observation that daytime lasts 10 h and nighttime lasts 14 h in summer. After incubation, the cells were collected to analyze the expressions of HSP70 and HSP90 mRNAs and proteins, respectively. Cells to be used for HSP70 and HSP90 mRNAs analysis were immediately frozen and stored with TRIsure at –80°C until total RNA extraction. Cells to be used for HSP70 and HSP90 proteins analysis were immediately frozen and stored with lysis buffer at –30°C until protein analysis.
Experiment 3: Effect of elevated temperature on the production of PGE2 and PGF2α in the cultured bovine endometrial epithelial and stromal cells
Endometrial epithelial and stromal cells that reached confluence in the 24-well cluster dishes were used for this experiment. The cells were incubated as described above in experiment 2. After incubation, the medium was collected in a 1.5 ml tube containing a 1% stabilizer solution (0.3 M EDTA and 1% (w/v) acetylsalicylic acid, pH 7.3) and immediately frozen and stored at –30°C until EIA. The concentrations of PGE2 and PGF2α in the medium were measured via EIA. The cells were collected for measuring the amount of DNA via DNA assay [24].
Experiment 4: Effect of elevated temperature on the expressions of PG synthetase mRNA in the cultured bovine endometrial epithelial and stromal cells
Endometrial epithelial and stromal cells that reached confluence in the 4-well cluster dishes were used for this experiment. The cells were incubated as described above in experiment 2. After incubation, the cells were collected to measure the expressions of PLA2, COX2, PGFS, cPGES and CBR1 mRNAs via quantitative RT-PCR.
Statistical analysis
All experimental data are shown as the mean ± SEM. The statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). The statistical significance of differences in experiments 1, 3, and 4 were assessed via Student’s t-test, while the statistical significance of differences in experiment 2 was assessed via ANOVA followed by Dunnett’s multiple comparisons tests. Statistical level of significance was set at 5% (P < 0.05).
Results
Experiment 1: Comparisons of mRNA and protein expressions of HSP70 and HSP90 between summer and winter in bovine endometrial tissues
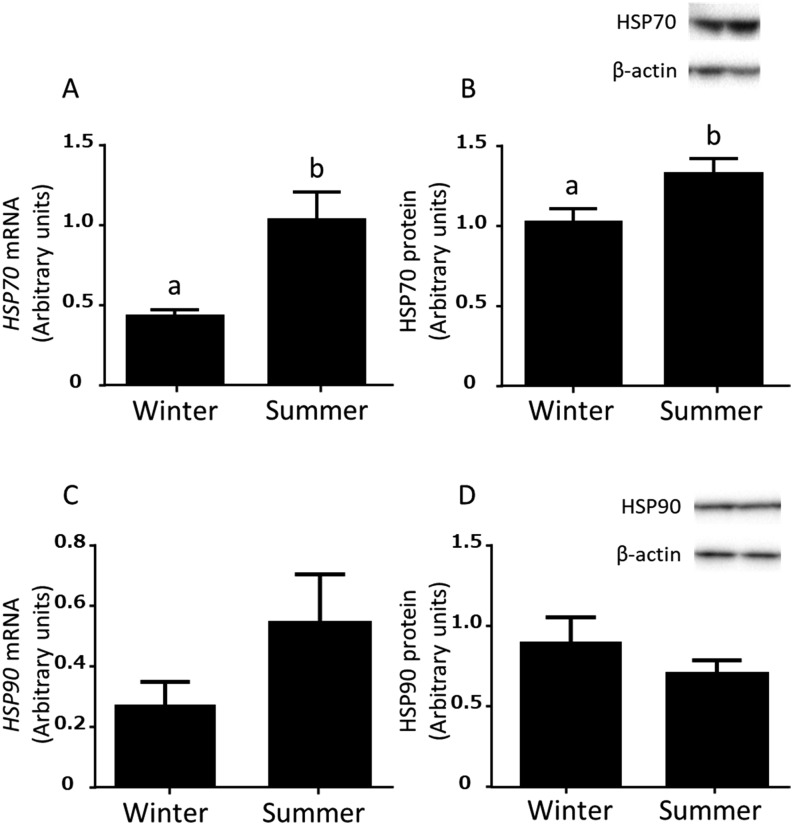
The expressions of HSP70 mRNA and protein in the bovine endometrial tissues were higher (P < 0.05) in summer than in winter (Fig. 1A and B). However, the expressions of HSP90 mRNA and protein in bovine endometrial tissues were not different between summer and winter (Fig. 1C and D).
Fig. 1.
Expressions of HSP70 and HSP90 mRNA (A and C) and protein (B and D) in the bovine endometrium in the mid luteal stages in winter (December 2013 – April 2014; n = 5 uteri) and summer (July – August 2014 and August 2015; n = 6 uteri). Different superscripts indicate significant difference (P < 0.05), as determined by Student’s t-test.
Experiment 2: Effects of various incubation temperatures on HSP70 and HSP90 mRNA and protein expressions in the cultured bovine endometrial epithelial and stromal cells
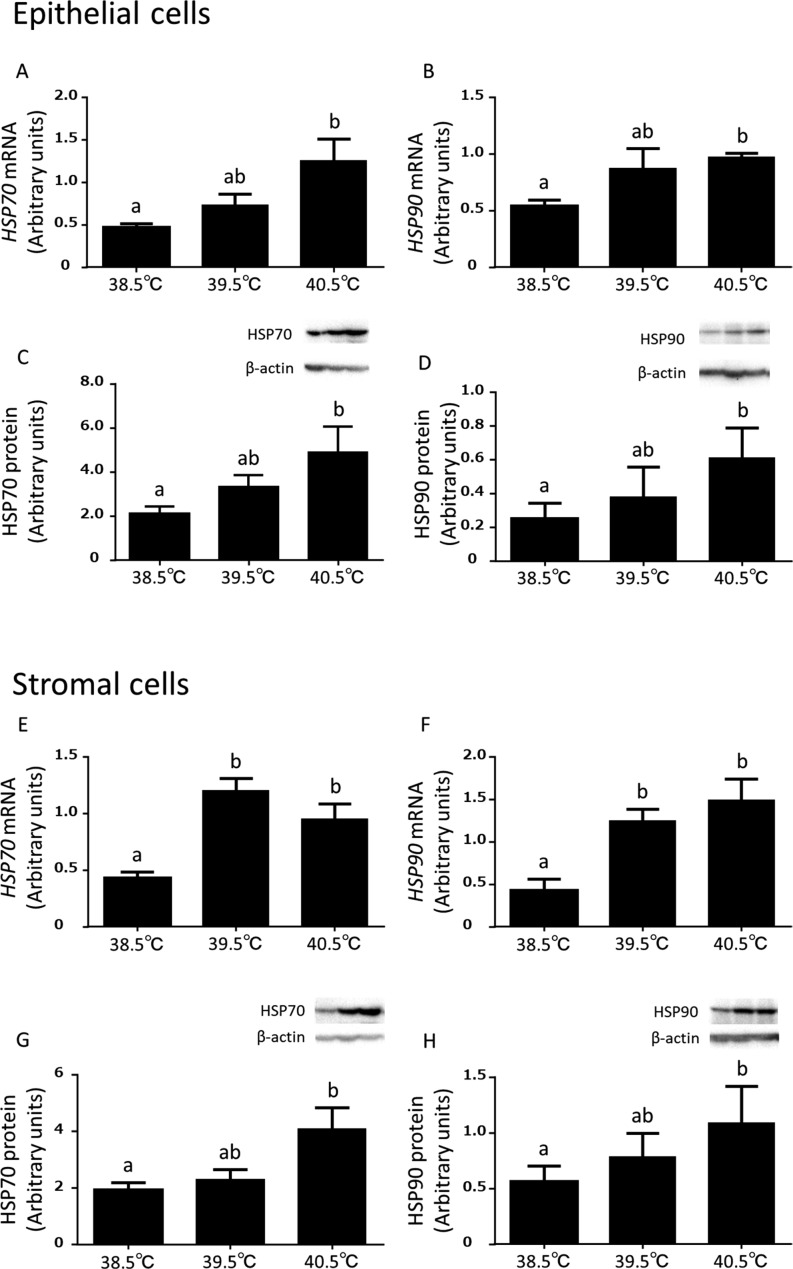
The expressions of HSP70 and HSP90 mRNA were higher (P < 0.05) at 40.5°C than at 38.5°C in the epithelial cells (Fig. 2A and B). In the stromal cells, the expression of HSP70 and HSP90 mRNA were higher (P < 0.05) at 39.5°C and 40.5°C than at 38.5°C (Fig. 2E and F). The expressions of HSP70 and HSP90 protein were greater (P < 0.05) at 40.5°C than 38.5°C in the epithelial cells (Fig. 2C and D) and stromal cells (Fig. 2G and H).
Fig. 2.
Effects of elevated incubation temperature (38.5°C, 39.5°C, and 40.5°C) on HSP70 and HSP90 mRNA and protein expressions in the cultured bovine endometrial epithelial (A, B, C and D) and stromal (E, F, G and H) cells (mean ± S.E.M, n = 8–13 uteri). Different superscripts indicate significant differences (P < 0.05), as determined by ANOVA followed by Dunnett’s multiple comparisons test.
Experiment 3: Effect of elevated temperature on the production of PGE2 and PGF2α in the cultured bovine endometrial epithelial and stromal cells
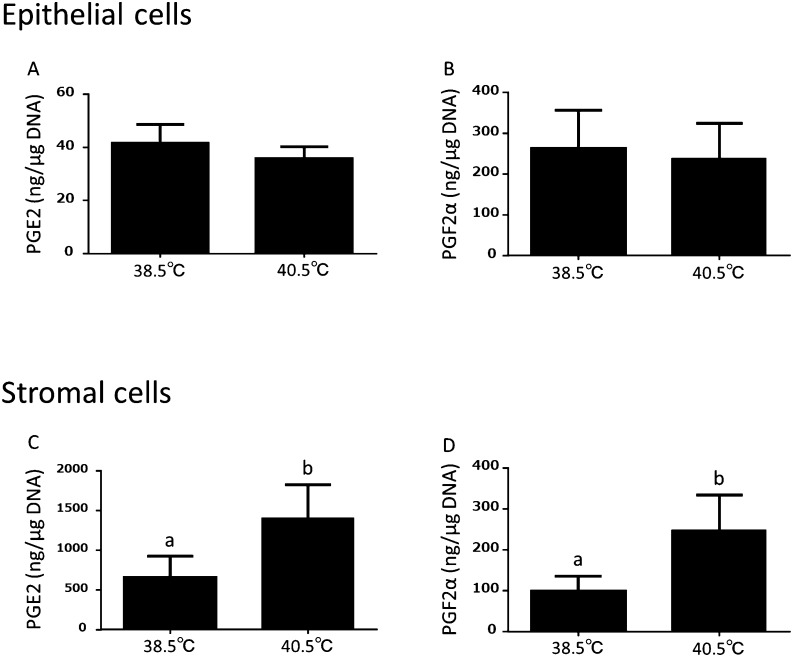
As indicated in Fig 3, in the epithelial cells, the production of PGE2 and PGF2α were not changed by elevated temperatures (Fig. 3A and B). In contrast, the production of PGE2 and PGF2α were significantly (P < 0.05) increased at 40.5°C than at 38.5°C in the stromal cells (Fig. 3C and D).
Fig. 3.
Effect of elevated temperature on the production of PGE2 and PGF2α in the cultured bovine endometrial epithelial (A, B) and stromal (C, D) cells (mean ± S.E.M., n = 4–6 uteri). Different superscripts indicate significant differences (P < 0.05), as determined by Student’s t-test.
Experiment 4: Effect of elevated temperature on the expressions of PG synthetase mRNA in the cultured bovine endometrial epithelial and stromal cells
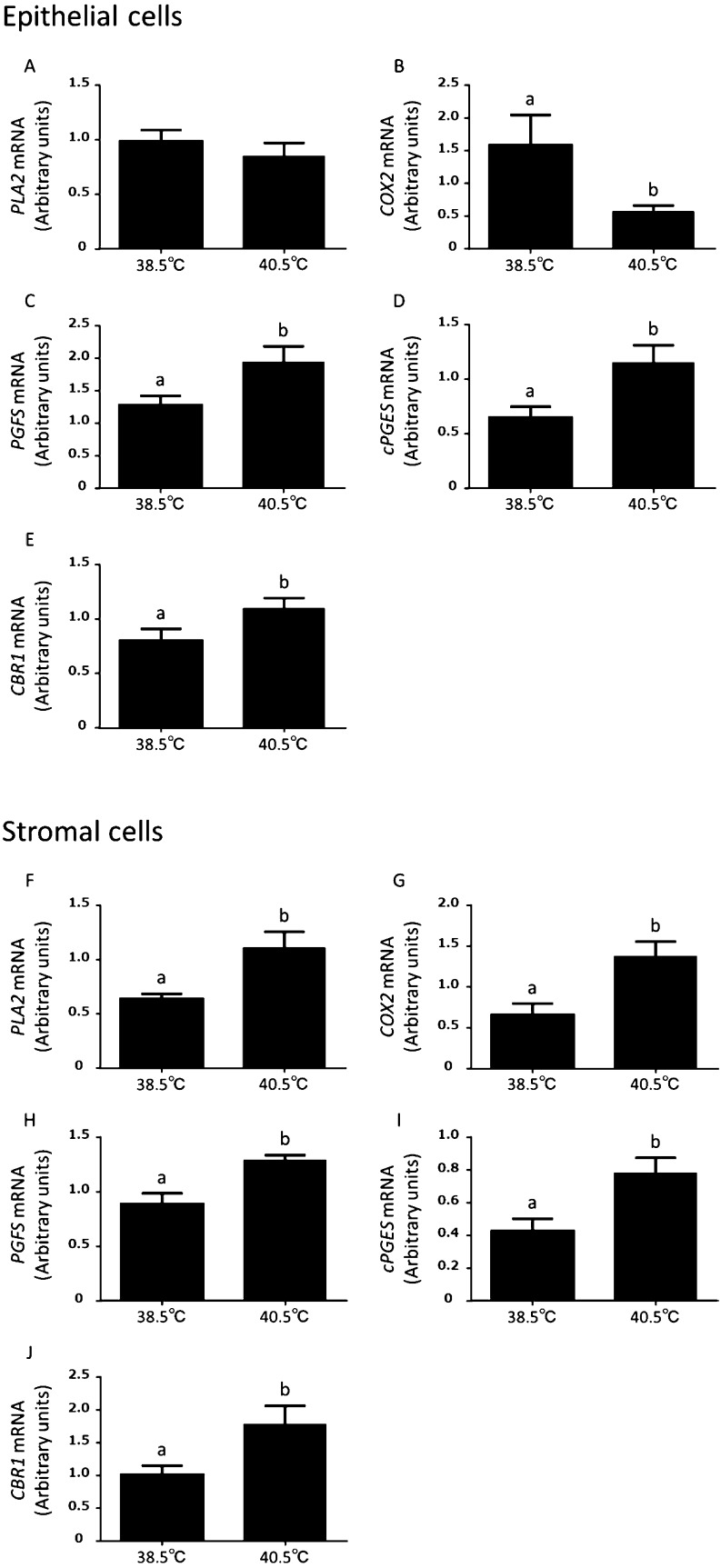
As indicated in Fig 4, in the epithelial cells, the expression of PLA2 mRNA was not changed by an elevated temperature (Fig. 4A), but the expression of COX2 mRNA was lower (P < 0.05) at 40.5°C than at 38.5°C (Fig. 4B). In contrast, the expressions of PGFS, cPGES and CBR1 mRNA were significantly (P < 0.05) increased at 40.5°C than at 38.5°C in the endometrial epithelial cells (Fig. 4C, D, and E). In addition, the expressions of PLA2, COX2, PGFS, cPGES, and CBR1 mRNA were greater (P < 0.05) at 40.5°C than 38.5°C in the stromal cells (Fig. 4F, G, H, I, and J).
Fig. 4.
Effect of elevated temperature on the expressions of PLA2, COX2, PGFS, cPGES, and CBR1 mRNA in the cultured bovine endometrial epithelial (A, B, C, D and E) and stromal (F, G, H, I, and J) cells (mean ± S.E.M., n = 6–13 uteri). Different superscripts indicate significant differences (P < 0.05), as determined by Student’s t-test.
Discussion
In cows, HS influences reproductive performance [5]. For example, it disrupts estrous cyclicity, reduces estrous behavior [25], suppresses follicular development [2], and induces early embryo mortality [26]. In this study, we revealed that the endocrine function of bovine endometrial cells was influenced by HS. Especially, in the endometrial stromal cells HS enhanced the gene expressions of enzymes involved in PG synthesis, resulting in an increase in PG production.
Bovine body temperature is approximately 38.5°C but increases under high ambient temperature [27], and HS influences the functions of various organs [2]. In a previous study, the expressions of HS markers HSP70 and HSP90 were increased in bovine endometrial explants cultured in vitro under HS conditions [13]. However, since it has not yet been determined whether bovine uteri are influenced by HS in in vivo, we examined the expressions of HSP70 and HSP90 in the bovine endometrium collected in summer and winter. The mRNA and protein expressions of HSP90 were not different in the uterine endometrial tissues between summer and winter (Fig. 1C and D), while those of HSP70 were higher in summer than in winter (Fig. 1A and B). Min et al. reported that HS increased both HSP70 and HSP90 in bovine serum; however HSP90 in serum was decreased after 8 weeks nevertheless HSP70 in serum was still high in HS exposed cows, suggesting that HSP70 was more sensitive and appropriate as a HS marker [28]. Therefore, present results suggest that bovine endometrium in summer might experience HS conditions. Subsequently, to mimic HS conditions in in vitro experiments, we cultured bovine endometrial cells at various temperatures and examined the expression of HS markers. The results showed that mRNA and protein expressions of HSP70 and HSP90 in the cultured bovine endometrial epithelial and stromal cells were increased at 40.5°C (Fig. 2A–H), which is equivalent to the vaginal temperature of cows under high temperature conditions [27]. Therefore, endometrial cells were cultured at 40.5°C to mimic the HS conditions in the subsequent experiments.
We examined whether HS affected endocrine function in the cultured bovine endometrial cells. In the endometrial stromal cells, HS increased PGF2α and PGE2 production (Fig. 3C and D). In a study of bovine endometrium, Skarzynski et al. reported that upregulation of mRNA expression of the enzymes associated with PG synthesis induced PG production [17]. In this study as well, HS increased mRNA expressions of PLA2, COX2, PGFS, cPGES, and CBR1 in the stromal cells (Fig. 4F-J). Therefore, the increase of PG production induced by HS may be caused by the upregulation of mRNA expression of enzymes associated with PG synthesis. Indeed, the effects of HS on enzymes associated with PG synthesis have previously been reported. Under HS conditions, heat shock factor 1, a transcription factor for HSP gene expression, is activated. Heat shock factor 1 binds directly to the heat shock element (HSE) of the COX2 promoter and regulates its expression in human umbilical vein endothelial cells [29]. The HSE sequence (GAA-CTC-GAA) [29] is found in the promoter region of bovine COX2 (1415-1427, Accession No; AY260143, National Center for Biotechnology Information). In addition, HSP90 regulates the phosphorylation of PLA2 under the stimulation of okadaic acid, resulting in the release of arachidonic acid in murine macrophages [30] and increases in cPGES activity in rat fibroblast cells [31]. In this study, as HS induced not only the mRNA expressions of PLA2 and COX2 but also the mRNA and protein level of HSPs in the endometrial stromal cells, enzymes for PG synthesis in bovine endometrium might also be regulated by the mechanisms described above.
Unlike in endometrial stromal cells, HS did not influence PGF2α and PGE2 production in the epithelial cells (Fig. 4C–E). The mRNA expression of COX2 was decreased by HS (Fig. 4B); however, the mRNA expressions of PGFS, cPGES and CBR1 were significantly induced by HS (Fig. 4C–E). These results may be one of the reasons for the minor effect of HS on the production of PGs in epithelial cells. It remains unclear why endometrial epithelial and stromal cells are affected differently by HS. The differences of reactivity between these two types of cells against various stimuli have been reported previously. When Trueperella pyogenes contaminate bovine uterus after parturition, it induces cytolysis of stromal cells but not of epithelial cells, because stromal cells contain more cholesterol than epithelial cells [32]. As such, the difference in responses against external stimuli between the epithelial and stromal cells may have resulted in the observations made in the present study. Moreover, in this study, even though both the epithelial and stromal cells upregulated HSP70 and HSP90 expressions under HS conditions, only the stromal cells had increased production of PGs. Factors other than HSP may increase the production of PGs under HS conditions. Therefore, it is necessary to study how bovine endometrial epithelial and stromal cells sense HS.
There are two contradictory reports regarding the effect of HS on the estrous cycle of cows. Heat stress increased PGF2α production in bovine endometrial tissues and might have shortened the estrous cycle, following early regression of the CL [13]. In contrast, HS extended the estrous cycle in a previous in vivo experiment [33]. In cows, the estrous cycle is regulated by endocrine functions, such as the production of PGs, LH pulse [11], and the plasma concentrations of P4 and estradiol [34]. Wolfenson et al. and Younas et al. suggested that a smaller number of luteal cells or lower plasma cholesterol availability under HS condition caused a decrease in the plasma concentration of P4 [35, 36]. In contrast, Wilson et al. reported that the serum concentration of P4 was higher in HS conditions and that HS delayed the regression of the CL [37]. In this study, we observed that the productions of both PGF2α (a luteolytic factor) and PGE2 (a luteotrophic factor) were increased by HS in the bovine endometrial stromal cells. It remains unclear whether HS extends or shortens the lifespan of the CL. Further in vivo studies are required to further explore this question.
The present results show that HS affected the bovine endometrial epithelial and stromal cells, particularly the secretion of PGs from the stromal cells. Disturbance of endocrine functions may be one of the reasons of HS-induced reproductive disorders in cows.
Acknowledgments
This work was supported by the Grants-in-Aid for Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development (REP-1002) from the Ministry of Agriculture, Forestry, and Fisheries of Japan, and JSPS KAKENHI Grant Number 17K19322.
We greatly appreciate Okayama meat center (Okayama, Japan) for providing the bovine uteri.
References
- 1.Hansen PJ. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci 2004; 82−83: 349–360. [DOI] [PubMed] [Google Scholar]
- 2.De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cowa review. Theriogenology 2003; 60: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 3.Roman-Ponce H, Thatcher WW, Caton D, Barron DH, Wilcox CJ. Thermal stress effects on uterine blood flow in dairy cows. J Anim Sci 1978; 46: 175–180. [DOI] [PubMed] [Google Scholar]
- 4.De Rensis F, Garcia-Ispierto I, López-Gatius F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015; 84: 659–666. [DOI] [PubMed] [Google Scholar]
- 5.Cavestany D, el-Wishy AB, Foote RH. Effect of season and high environmental temperature on fertility of Holstein cattle. J Dairy Sci 1985; 68: 1471–1478. [DOI] [PubMed] [Google Scholar]
- 6.Poyser NL. The control of prostaglandin production by the endometrium in relation to luteolysis and menstruation. Prostaglandins Leukot Essent Fatty Acids 1995; 53: 147–195. [DOI] [PubMed] [Google Scholar]
- 7.Okuda K, Miyamoto Y, Skarzynski DJ. Regulation of endometrial prostaglandin F(2α) synthesis during luteolysis and early pregnancy in cattle. Domest Anim Endocrinol 2002; 23: 255–264. [DOI] [PubMed] [Google Scholar]
- 8.Fitz TA, Mayan MH, Sawyer HR, Niswender GD. Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol Reprod 1982; 27: 703–711. [DOI] [PubMed] [Google Scholar]
- 9.Marsh J. The effect of prostaglandins on the adenyl cyclase of the bovine corpus luteum. Ann N Y Acad Sci 1971; 180: 416–425. [DOI] [PubMed] [Google Scholar]
- 10.McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev 1999; 79: 263–323. [DOI] [PubMed] [Google Scholar]
- 11.Fortier MA, Guilbault LA, Grasso F. Specific properties of epithelial and stromal cells from the endometrium of cows. J Reprod Fertil 1988; 83: 239–248. [DOI] [PubMed] [Google Scholar]
- 12.Asselin E, Bazer FW, Fortier MA. Recombinant ovine and bovine interferons tau regulate prostaglandin production and oxytocin response in cultured bovine endometrial cells. Biol Reprod 1997; 56: 402–408. [DOI] [PubMed] [Google Scholar]
- 13.Putney DJ, Malayer JR, Gross TS, Thatcher WW, Hansen PJ, Drost M. Heat stress-induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium. Biol Reprod 1988; 39: 717–728. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto Y, Skarzynski DJ, Okuda K. Is tumor necrosis factor alpha a trigger for the initiation of endometrial prostaglandin F(2alpha) release at luteolysis in cattle? Biol Reprod 2000; 62: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Kito S, Sumi N, Sato K. A study of the central cavity in the bovine corpus luteum. Vet Rec 1988; 123: 180–183. [DOI] [PubMed] [Google Scholar]
- 16.Murakami S, Shibaya M, Takeuchi K, Skarzynski DJ, Okuda K. A passage and storage system for isolated bovine endometrial epithelial and stromal cells. J Reprod Dev 2003; 49: 531–538. [DOI] [PubMed] [Google Scholar]
- 17.Skarzynski DJ, Miyamoto Y, Okuda K. Production of prostaglandin F2α by cultured bovine endometrial cells in response to tumor necrosis factor alpha: cell type specificity and intracellular mechanisms. Biol Reprod 2000; 62: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 18.Tanikawa M, Acosta TJ, Fukui T, Murakami S, Korzekwa A, Skarzynski DJ, Piotrowska KK, Park CK, Okuda K. Regulation of prostaglandin synthesis by interleukin-1alpha in bovine endometrium during the estrous cycle. Prostaglandins Other Lipid Mediat 2005; 78: 279–290. [DOI] [PubMed] [Google Scholar]
- 19.Uenoyama Y, Hattori S, Miyake M, Okuda K. Up-regulation of oxytocin receptors in porcine endometrium by adenosine 3,5-monophosphate. Biol Reprod 1997; 57: 723–728. [DOI] [PubMed] [Google Scholar]
- 20.Sakumoto R, Komatsu T, Kasuya E, Saito T, Okuda K. Expression of mRNAs for interleukin-4, interleukin-6 and their receptors in porcine corpus luteum during the estrous cycle. Domest Anim Endocrinol 2006; 31: 246–257. [DOI] [PubMed] [Google Scholar]
- 21.Osnes T, Sandstad O, Skar V, Osnes M, Kierulf P. Total protein in common duct bile measured by acetonitrile precipitation and a micro bicinchoninic acid (BCA) method. Scand J Clin Lab Invest 1993; 53: 757–763. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Wakamiya K, Kohka M, Yamamoto Y, Okuda K. Summer heat stress affects prostaglandin synthesis in the bovine oviduct. Reproduction 2013; 146: 103–110. [DOI] [PubMed] [Google Scholar]
- 23.Mori M, Hayashi T, Isozaki Y, Takenouchi N, Sakatani M. Heat shock decreases the embryonic quality of frozen-thawed bovine blastocysts produced in vitro. J Reprod Dev 2015; 61: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 1980; 102: 344–352. [DOI] [PubMed] [Google Scholar]
- 25.Monty DE, Jr, Wolff LK. Summer heat stress and reduced fertility in Holstein- Friesian cows in Arizona. Am J Vet Res 1974; 35: 1495–1500. [PubMed] [Google Scholar]
- 26.Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev 2004; 67: 77–82. [DOI] [PubMed] [Google Scholar]
- 27.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J Reprod Dev 2011; 57: 450–456. [DOI] [PubMed] [Google Scholar]
- 28.Min L, Cheng JB, Shi BL, Yang HJ, Zheng N, Wang JQ. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J Zhejiang Univ Sci B 2015; 16: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A, Coccia M, Trotta E, Angelini M, Santoro MG. Regulation of cyclooxygenase-2 expression by heat: a novel aspect of heat shock factor 1 function in human cells. PLoS ONE 2012; 7: e31304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker DE, Gijón MA, Spencer DM, Qiu ZH, Gelb MH, Leslie CC. Regulation of cytosolic phospholipase A2alpha by hsp90 and a p54 kinase in okadaic acid-stimulated macrophages. J Leukoc Biol 2008; 84: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanioka T, Nakatani Y, Kobayashi T, Tsujimoto M, Oh-ishi S, Murakami M, Kudo I. Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem Biophys Res Commun 2003; 303: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 32.Amos MR, Healey GD, Goldstone RJ, Mahan SM, Düvel A, Schuberth HJ, Sandra O, Zieger P, Dieuzy-Labaye I, Smith DG, Sheldon IM. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol Reprod 2014; 90: 54. [DOI] [PubMed] [Google Scholar]
- 33.Sakatani M, Balboula AZ, Yamanaka K, Takahashi M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim Sci J 2012; 83: 394–402. [DOI] [PubMed] [Google Scholar]
- 34.Badinga L, Thatcher WW, Diaz T, Drost M, Wolfenson D. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology 1993; 39: 797–810. [DOI] [PubMed] [Google Scholar]
- 35.Wolfenson D, Bartol FF, Badinga L, Barros CM, Marple DN, Cummins K, Wolfe D, Lucy MC, Spencer TE, Thatcher WW. Secretion of PGF2alpha and oxytocin during hyperthermia in cyclic and pregnant heifers. Theriogenology 1993; 39: 1129–1141. [DOI] [PubMed] [Google Scholar]
- 36.Younas M, Fuquay JW, Smith AE, Moore AB. Estrous and endocrine responses of lactating Holsteins to forced ventilation during summer. J Dairy Sci 1993; 76: 430–436. [DOI] [PubMed] [Google Scholar]
- 37.Wilson SJ, Marion RS, Spain JN, Spiers DE, Keisler DH, Lucy MC. Effects of controlled heat stress on ovarian function of dairy cattle. 1. Lactating cows. J Dairy Sci 1998; 81: 2124–2131. [DOI] [PubMed] [Google Scholar]