Highlights
-
•
Reactive oxygen species (ROS) are produced in abundance by photosynthesis.
-
•
ROS and antioxidants function in redox signal transduction that is important in chloroplast to nucleus communication.
-
•
Some chloroplasts have specialized signaling functions that regulate epigenetic as well as genetic programming.
-
•
Photoinhibition and slowly reversible decreases in photosynthetic capacity are not necessarily the result of light-induced damage to PSII reaction centers.
Keywords: Ascorbate, Antioxidants, Acclimation, Chloroplast-to-nucleus retrograde signaling, Epigenetics, Glutathione, High Light, Hydrogen peroxide, Mehler reaction, MSH1 pathway, NPQ, Peroxiredoxins, Photodamage, Photoinhibition, Photosystem II, Photosynthesis, Post translational modifications, Reactive oxygen species, Redox signaling, Singlet Oxygen
Abstract
Reduction-oxidation (redox) reactions, in which electrons move from a donor to an acceptor, are the functional heart of photosynthesis. It is not surprising therefore that reactive oxygen species (ROS) are generated in abundance by photosynthesis, providing a plethora of redox signals as well as functioning as essential regulators of energy and metabolic fluxes. Chloroplasts are equipped with an elaborate and multifaceted protective network that allows photosynthesis to function with high productivity even in resource-limited natural environments. This includes numerous antioxidants with overlapping functions that provide enormous flexibility in redox control. ROS are an integral part of the repertoire of chloroplast signals that are transferred to the nucleus to convey essential information concerning redox pressure within the electron transport chain. Current evidence suggests that there is specificity in the gene-expression profiles triggered by the different ROS signals, so that singlet oxygen triggers programs related to over excitation of photosystem (PS) II while superoxide and hydrogen peroxide promote the expression of other suites of genes that may serve to alleviate electron pressure on the reducing side of PSI. Not all chloroplasts are equal in their signaling functions, with some sub-populations appearing to have better contacts/access to the nucleus than others to promote genetic and epigenetic responses. While the concept that light-induced increases in ROS result in damage to PSII and photoinhibition is embedded in the photosynthesis literature, there is little consensus concerning the extent to which such oxidative damage happens in nature. Slowly reversible decreases in photosynthetic capacity are not necessarily the result of light-induced damage to PSII reaction centers.
1. Introduction
Electron flow is the basis of life on earth, whether it is driven by light in photosynthesis or through chemical redox couples in respiration. The incorporation of photosynthesis into eukaryotic cells during evolution not only provided enormous benefits in terms of energy metabolism, but also significantly extended the cellular capacity for reduction/oxidation (redox) regulation and signaling. The incorporation of the photosynthetic electron transport chain appears to have been accompanied by a large expansion of protein cysteine thiols used to sense and regulate cellular redox state (Woehle et al., 2017). The operation of photosynthesis also resulted in a large increase in the production of reactive oxygen species (ROS), such as superoxide, hydrogen peroxide and singlet oxygen. The explosion of oxidative signals arising from photosynthesis, together with the significant expansion of the redox-sensitive proteome, not only provided cells with mechanisms to monitor photosynthetic electron flow and thus prevent over-reduction or over-oxidation, but also produced redox regulatory networks that enable plants to sense and respond to fluctuating environmental conditions (Martin and Sies, 2017). While the concept that cells need to manage ROS production and removal to avoid excessive and irreversible oxidation is embed in the animal and plant literature, the consensus of opinion is now shifting towards a recognition of the positive roles of ROS as essential pro-life signals (Noctor and Foyer, 2016; Foyer et al., 2017). Within the complex redox landscape of photosynthetic cells, ROS production is essential for redox sensing, signaling and regulation. The best characterized signaling mechanisms involve redox changes in cysteines either on low-molecular-weight thiols, particularly glutathione, or on proteins thiol groups with oxidation states of the sulphur moieties ranging from thiols (–RSH) and disulfides (–RSSR–) to trisulfides and possibly even tetrasulfides (Noctor et al., 2011). In photosynthesis, this thiol-disulphide exchange system is used to co-ordinate the rates of NADP and ATP generation to the rates of their utilization in photosynthetic carbon assimilation through the mediation of thioredoxins (TRXs). This extensive network of interactive redox regulation occurs together with other post-translational modifications (PTM), particularly protein phosphorylation/dephosphorylation reactions that also serve to co-ordinate processes occurring in the thylakoids and stroma. Exposure to oxidative stress influences two other poorly characterized plant PTMs, protein succinylation and acetylation (Zhou et al., 2017). This analysis of H2O2-triggered interactions between the rice leaf lysine succinylome and acetylome revealed specific effects on photosynthetic proteins, but the functional significance of these PTMs remains to be established (Zhou et al., 2017). Moreover, the chloroplast thylakoid lumen also contains redo-regulated proteins. Within the lumen, proteins such as the immunophilin, FKBP13, polyphenol oxidase and violaxanthin de-epoxidase, are regulated by oxidative activation facilitated by the oxygen produced by PSII (Buchanan & Luan, 2005).
Light-driven redox changes in the photosynthetic electron transport system drive energy conversion that is harnessed in the forms of reducing power (reduced ferredoxin and NADPH) and ATP. The rate of carbon assimilation is matched to prevailing electron fluxes through the reduction of TRXs. Reduced TRXs then reduce disulphide bridges on target proteins within the Calvin cycle (Buchanan and Balmer, 2005; Nikkanen and Rintamäki, 2014). Chloroplasts house a large number of different TRX forms (Meyer et al., 2012). These TRXs can be reduced by two pathways that show some overlap with regard to their target proteins (Nikkanen and Rintamäki, 2014; Nikkanen et al., 2017). The ferredoxin-dependent TRX system is driven by reduced ferredoxin produced by PSI. Reduced TRX is generated through the mediation of ferredoxin-thioredoxin reductases. The pathway is responsible for the light-induced activation of enzymes of photosynthetic carbon assimilation (Michelet et al., 2013; Geigenberger and Fernie, 2014), including fructose-1,6-bisphosphatase (FBPase) and sedoheptulose-1,7-bisphosphatase (SBPase), phosphoribulokinase (PRK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In addition, this pathway regulates the thylakoid chloroplast ATP synthase (Hisabori et al., 2013), the malate-oxaloacetate shuttle (Scheibe and Dietz, 2012), the ADP-glucose pyrophosphorylase step of starch synthesis (Thormählen et al., 2013) and the Mg-protoporphyrin methyltransferase step in chlorophyll biosynthesis (Richter et al., 2013). In addition to the ferredoxin-dependent TRX system, there is an NADPH-dependent thioredoxin reductase (NTRC) pathway, which can also activate the carbon assimilation enzymes either directly or through interaction with the ferredoxin-TRX system. The operation of the NTRC pathway may be crucial in activating the photosynthetic enzymes under low light intensities (Nikkanen et al., 2017).
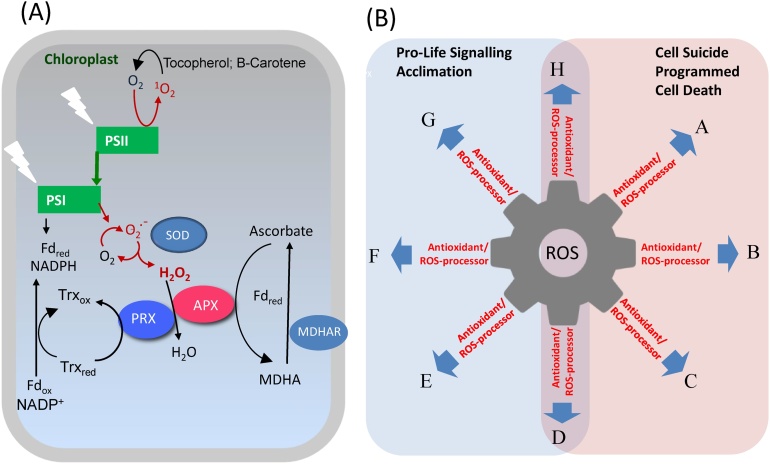
The ferredoxin-dependent TRX and NTRC systems function together with 2-cysteine peroxiredoxins (2-Cys PRX) as a two-component hydrogen peroxide-detoxifying system under stress conditions (Perez-Ruiz et al., 2006; Bernal-Bayard et al., 2014). 2-Cys PRXs act together with the ascorbate-glutathione cycle to limit the accumulation of hydrogen peroxide produced by photosynthesis (Foyer and Shigeoka, 2011). It is also worthy of note that TRXz is required for the correct assembly of the plastid PEP polymerase and the developmental transition from nuclear-encoded RNA polymerase-driven transcription to PEP-dependent transcription of plastid genes, such as psbA that encodes the D1 protein of PSII (Dietz and Pfannschmidt, 2011). Moreover, TRXx, TRXy and CDSP32 are important in chloroplast responses to oxidative stress (Collin et al., 2003, Collin et al., 2004; Broin et al., 2002). ROS accumulation within chloroplasts is controlled by a complex antioxidant-scavenging system (Foyer and Shigeoka, 2011) that includes TRXs and 2-Cys PRXs as well as antioxidant enzymes, such as superoxide dismutase (SOD) and ascorbate peroxidase (APX), and low molecular weight antioxidants, such as ascorbate (Fig. 1A) that also play roles in transmitting oxidative signals as well as controlling ROS accumulation (Foyer et al., 2017; Noctor et al., 2017).
Fig. 1.
A. ROS formation and metabolism in chloroplasts. The chloroplast produces singlet oxygen at Photosystem (PS) II, whereas reduction of molecular oxygen to superoxide occurs predominantly at PSI. Hydrogen peroxide is produced from superoxide in a reaction catalyzed by superoxide dismutase (SOD). Hydrogen peroxide can be reduced to water by the action of ascorbate peroxidases (APX) or 2-Cys peroxiredoxins (PRX). Oxidation of ascorbate generates monodehydroascorbate (MDHA), which can be reduced back to ascorbate either by reduced ferredoxin or monodehydroascorbate (reductase (MDHAR). Make consistent between figure and legend] Oxidized PRX are reduced again by the action of thioredoxins (TRX). B. Current model for ROS-antioxidant interplay in cell signaling showing that antioxidants act as ROS-processing and –signaling mediators, allowing different options (for signal transduction. Letters indicate different possible pathways that are not mutually exclusive. The model indicates that loss of any one of these antioxidant components would drive processing and signaling through the other pathways..
For many years, the dominant concept in redox signaling has been that cells balance ROS on one side and antioxidants on the other (Noctor et al., 2017). According to this concept, oxidative signaling shifts this balance such that ROS accumulate, either through an increase in their production or a decrease in antioxidant capacity. This concept suggests that, when ROS are low, cell signaling entrains acclimation and improved stress tolerance and that, when ROS are in excess of antioxidant capacity, the resulting enhanced oxidation causes damage and entrains programmed cell death. While this concept continues to be useful, recent work suggests that it needs to be updated, notably to take account of the complexity and specificity of plant antioxidative systems as well as their roles in signaling (Fig. 1B). It has, therefore, been suggested that “ROS processing systems” would be a more accurate term than “antioxidative systems” to describe cellular components that are most likely to interact with ROS and, in doing so, transmit oxidative signals (Noctor et al., 2017). The direction of signal flow will depend on factors such as the proximity of the ROS target, its relative susceptibility to oxidation and its abundance in relation to other targets. In this way, oxidative signals may have a high level of specificity in a ROS-determined wheel of cell fortune or fate. Within this context, redox signals arising from the chloroplasts will readily interact with components of the phytohormone signaling network to regulate plant growth and defense pathways in response to a changing environment (Bartoli et al., 2013).
2. ROS formation in chloroplasts
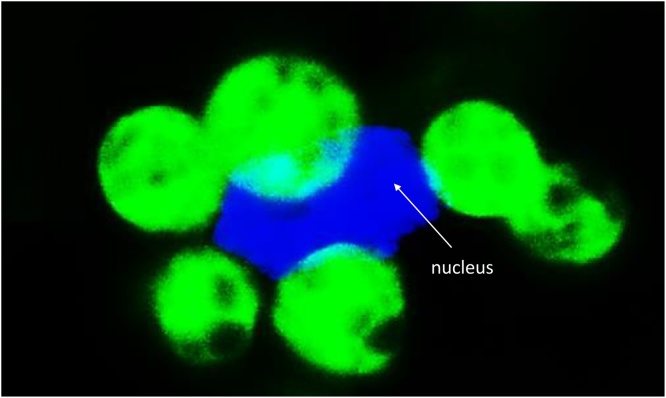
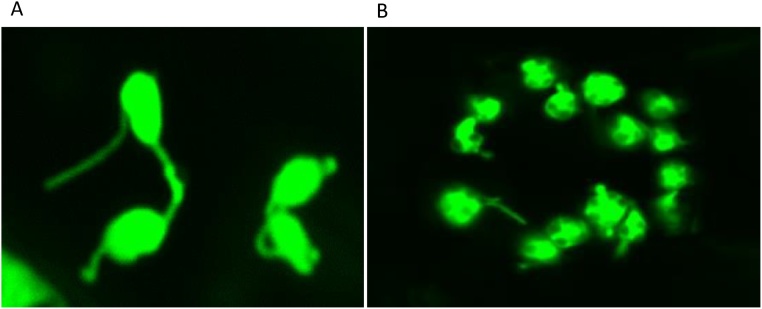
Oxygen is an important electron acceptor in photosynthesis. The reduction of molecular oxygen by PSI in the Mehler reaction is a coupled process leading to the formation of ATP without NADPH. Oxygen reduction provides an alternative electron sink and generates superoxide anion radicals that are converted to H2O2 by the action of the thylakoid copper/zinc SODs. H2O2 can then be reduced to water by chloroplast APXs and the PRXs (Awad et al., 2015). In the overall process, known as the water-water cycle, two electrons are used to produce H2O2 and two are required to metabolize H2O2 to water, allowing the dissipation of excess excitation energy and electrons (Foyer et al., 1991; Osmond and Grace, 1995; Asada, 2000). While this process may only make a minor contribution to thylakoid acidification and the control of PSII activity because it is saturated at relatively low irradiances (Heber, 2002; Driever and Baker, 2011), it is undoubtedly an important source of oxidative signals that may be directly or indirectly be transferred to the nucleus (Fey et al., 2005; Noctor and Foyer, 2016; Exposito-Rodriguez et al., 2007). One possibility is that H2O2 generated by photosynthesis may be directly transferred to the nucleus from attached chloroplasts (Fig. 2; Exposito-Rodriguez et al., 2007). Thus, the Mehler reaction might itself function as a signaling mechanism, with H2O2 production acting as a measure of electron transport activity (Exposito-Rodriguez et al., 2007). Another mechanism of direct transfer of H2O2 to the nucleus is via stromules (Fig. 3; Caplan et al., 2015).
Fig. 2.
The close association of some chloroplasts with the nucleus (stained blue with DAPI) in Arabidopsis thaliana cotyledons, in which chloroplasts are labelled with green fluorescent protein. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Chloroplast stromules in the mesophyll cells (A) and stomatal guard cells (B) of Arabidopsis thaliana cotyledons. Chloroplasts are labelled with green fluorescent protein. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
It is now well-established that ROS are key components of chloroplast‐nucleus retrograde signaling pathways, working together with a complex array of putative retrograde signaling molecules including metabolites and mobile transcription factors, calcium and links to pathways such as the unfolded protein response. Of these, the 3′-phosphoadenosine 5′-phosphate (PAP) phosphatase called SAL1 is perhaps the best characterized; SAL1 is thought to act as a sensor of oxidative stress in the chloroplasts because it undergoes a conformational change in response to redox changes. Chloroplast oxidation results in deactivation of SAL1 and accumulation of its substrate PAP, which acts as plastid signal instigating chloroplast-to-nucleus retrograde signaling pathways (Chan et al., 2016). It should be noted that redox changes in other cellular compartments may influence the susceptibility of photosynthesis to high-light-induced inhibition and also modulate chloroplast-to-nucleus signaling and the transcriptional responses of leaves to high light (Karpinska et al., 2017a, Karpinska et al., 2017b). Superoxide and H2O2 are relatively weak oxidants. Superoxide has received little attention with regard to chloroplast processes because it has no reactivity to most biological molecules and is rapidly converted to H2O2 by the action of thylakoid and stromal SODs. Superoxide interacts with nitric oxide (NO) and it is also reduced to H2O2 by ascorbic acid, which is abundant in chloroplasts. In the mitochondria, superoxide is able to inactivate the tricarboxylic acid cycle enzyme aconitase, which is a sensitive target in the matrix, but it has not been shown to cause inhibition of Calvin cycle enzymes that, in contrast, are very susceptible to oxidation and inactivation by H2O2. Hence, plastid H2O2 levels have to be very low and in the micromolar range. It is worthy of note that H2O2 and superoxide have divergent effects on cell survival and programmed cell suicide pathways in animals, where H2O2 promotes and superoxide inhibits cell death programs and apoptotic signaling (Pervaiz and Clement, 2007). It may be that superoxide also promotes cell survival in plants, particularly in light of observations of spatiotemporal patterns superoxide and H2O2 in root meristems (Foyer et al., 2018). The short lifetimes of superoxide and singlet oxygen make them unlikely candidates to diffuse over any distances within the cell. In contrast, H2O2 is more stable and is thus the most likely ROS form to act as a mobile signal (Foyer and Noctor, 2016).
Chloroplast ROS are essential signals underpinning acclimation responses through retrograde signaling to the nucleus, and they are also components of whole-plant systemic signaling pathways. The small plastids of the vascular parenchyma and also bundle-sheath plastids fulfil signaling functions that regulate mesophyll plastid development (Lundquist et al., 2014; Petrillo et al., 2014). Phytochrome B-mediated auxin pathways transmit signals perceived by apical leaves to increase the speed of induction of photosynthesis in distal leaves (Guo et al., 2016). This pathway, which transmits information concerning high light perceived at the apex, results in stimulation of H2O2 production in systemic leaves that are distant from the apical leaves [?] leading to increased cyclic electron flow around PSI during photosynthetic induction (Guo et al., 2016). Moreover, altering the ascorbate/DHA ratio of the apoplast/cell wall compartment by manipulating ascorbate oxidase activities was sufficient to alter the ability of photosynthesis to acclimate to high light (Karpinska et al., 2017a). Tobacco leaves with low ascorbate oxidase activities maintained higher photosynthesis rates under high light than wild type controls or plants with high ascorbate oxidase activities (Karpinska et al., 2017a). Taken together, such findings demonstrate that photosynthesis is not only responsive to ROS produced directly in the chloroplasts, but also to oxidants generated in other parts of the cell or via phytohormone-mediated pathways to allow acclimation to local and systemic cues (Bartoli et al., 2013).
In contrast to H2O2, singlet oxygen (1O2), is a powerful oxidant that reacts rapidly with macromolecules in the vicinity of where it is generated, resulting in oxidation that is often referred to as “damage” (Apel and Hirt, 2004; Watabe et al., 2007). Much of the oxidative inactivation, caused by over-excitation of the photosynthetic electron transport chain is caused by 1O2 (Telfer et al., 1999; Telfer, 2014; Krieger-Liszkay et al., 2008). The vulnerability of PSII to oxidative damage/inactivation means that each reaction center has to be rebuilt several times an hour, even under optimal irradiances. Oxidative modifications to the D1 and D2 proteins increase reaction center turnover (Malnoë et al., 2014). It is possible that the breakdown products of D1 protein might fulfil signaling roles, but this has not as yet been demonstrated in higher plants. The subunits of the oxygen-evolving complex are also prone to ROS- mediated damage/modification (Henmi et al., 2004). Any damaged/inactivated subunits are removed by luminal proteases that degrade damaged/inactivated components during the repair cycle, in which newly synthesized subunits are shuttled from the stroma lamellae, where they are inserted into the thylakoids, to the granal thylakoids. Singlet oxygen can also impair de novo synthesis of D1 by targeting the protein elongation factor G (CpEF-G) (Nishiyama et al., 2006). D1 is co-translationally inserted into PSII during repair via the cpSECY translocase (Nilsson and van Wijk, 2002). However, 1O2 would have to diffuse over a considerable distance if it were to target the elongation factors, and there are many other susceptible molecules in the thylakoid system, particularly poly-unsaturated fatty acids (PUFA) and other membrane lipids (Przybyla et al., 2008). These peroxidation products are generated in a singlet oxygen-dependent fashion in plants exposed to high light and other stresses (Triantaphylidès et al., 2008). Lipid peroxidation, accompanied by the generation of oxylipins, is often taken to be a signature of photo-oxidative damage (Girotti, 2001; Mueller et al., 2006; Przybyla et al., 2008). Moreover, excessive lipid peroxidation, leading to the apparent bursting of the chloroplast membranes, is often considered by the basis for the “accidental cell death” phenomena described in mutants such as flu and ch1 (Laloi and Havaux, 2015). However, chloroplasts contain many 1O2 scavengers, including β-carotene ascorbate, tocopherol and glutathione that prevent such negative outcomes. Moreover, photo-protective mechanisms are deployed in order to protect the photosynthetic antenna complexes from over-excitation, when the absorption of light intensity exceeds the capacity of photochemistry to use the absorbed energy.
Superoxide and hydrogen peroxide are largely generated at PSI on the stromal side of non-appressed thylakoid membranes. Singlet oxygen is generated by PSII in the grana core and also by the PSII repair process occurring on non-appressed margins of the grana (Wang et al., 2016). Accumulating evidence suggests that hydrogen peroxide and singlet oxygen function as separate redox signals that are transduced by different pathways to trigger the expression of specific suites of nuclear genes. Two distinct 1O2 −triggered chloroplast-to-nucleus signaling pathways have been described to date: firstly, 1O2 may modify gene expression through interaction with β-carotene and the generation of carotenoid breakdown products (β-CC), some of which are volatile (Ramel et al., 2012); secondly, the nuclear-encoded chloroplast protein EXECUTER (EX)1 plays an essential role together with EX2 in the transmission of 1O2 signals (Apel and Hirt, 2004; Lee et al., 2007, op den Camp et al., 2003; Wagner et al., 2004, Wang et al., 2016). The β-CC-induced and EX1-dependent pathways have only a small number of common 1O2 −responsive genes, suggesting that the two pathways are separate (Dogra et al., 2017). Moreover, β-CC-mediated signaling is independent of EX1/EX2 (Ramel et al., 2013; Shumbe et al., 2016). The EX1 and EX2 proteins are required for the local and systemic signaling pathways leading to gene expression changes that underpin acclimation to stress caused by high light (Carmody et al., 2016). The EX1 proteins are mostly localized in the non-appressed margins of the grana, suggesting that EX1 transmits signals from 1O2 produced in the grana margins (Wang et al., 2016), where the repair of damaged/inactivated PSII reaction centers (RCII) takes place through disassembly and degradation of damaged/inactivated proteins by the membrane-bound FtsH protease. EX1 is associated with RCII proteins, including D1 and D2, and FtsH2 proteases, as well as with protein-elongation factors and chlorophyll biosynthetic enzymes (Wang et al., 2016). In contrast, β-carotene is likely to be more important in quenching 1O2 produced in the grana core (Ramel et al., 2012, Ramel et al., 2013).
Perception of 1O2 signals induces cell suicide programs in seedlings and leads to growth inhibition in mature plants (Wagner et al., 2004). Phytohormones such as salicylic acid and jasmonate act as important promoters or inhibitors of cell death in the fluorescent (flu) and chlorina 1 (ch1) mutants, acting as key players in the decision between acclimation and cell death as they do in H2O2–mediated induction of programmed cell death observed in catalase (cat)2 mutants (Mhamdi et al., 2010, Mhamdi et al., 2012). The FLU protein acts as a negative feedback regulator in the Mg-branch of the tetrapyrrole biosynthetic pathway in the dark. The flu mutants accumulate the chloroplast precursor protochlorophyllide (Pchlide) in the dark because conversion of Pchlide to Chlorophyllide is light-dependent. Pchlide molecules are potent photosensitizers that generate 1O2 in abundance upon illumination (Meskauskiene et al., 2001).
3. Photodamage and photo-protection
Photosynthesis converts light energy into chemical energy through an interacting series of biophysical and biochemical reactions that provide the essential driving force for reductive metabolism in plants. The dynamic regulation of the photosynthetic machinery allows continuous adjustments to changes in the availability of light and carbon dioxide. Plants cannot easily turn off photosystem chemistry and this flexibility is thus crucial, not least because PSII is extremely sensitive to light-induced oxidative inactivation (Aro et al., 1993; Yokthongwattana and Melis, 2006). PSI is also susceptible to light-induced inhibition, particularly at low temperatures or under high light, and this has consequences for photosynthetic carbon fixation and cell signaling (Gollan et al., 2017). Photosynthetic regulation is designed to protect the system, while maintaining the balance between energy-producing and energy-consuming processes. Optimizing the efficiency of photosynthesis is a priority when light is limiting, allowing effective use of solar power and ensuring that metabolism is not limited by energy supply. Since light is a potentially dangerous substrate, light use must be carefully managed.
Electron transport and formation of ATP and NADPH proceed at much slower rates than light harvesting and energy transfer to RCII by antennae (Ruban et al., 2012). As an immediate consequence, the amount of energy absorbed recurrently overcomes the metabolic energy demands. This leads to accumulation of excess energy in the thylakoid membrane that can potentially be harmful to PSII, leading to the permanent closure of the PSII reaction centers and photoinhibition, which can be defined as a sustained depression of Fv/Fm ratio (Aro et al., 1993). The probability of light-induced inactivation of RCII increases when QA is reduced, i.e., when electron transport away from PSII is limited. However, the PSII reaction centers are protected by mechanisms that diminish the rate of excitation of PSII by harmlessly dissipating excess energy in the PSII antenna complexes as heat. This protection is quantified by processes that can be assessed from non-photochemical quenching of chlorophyll fluorescence (NPQ). Several processes that contribute to NPQ can be distinguished through their recovery kinetics. These are the state transition (qT), photoinhibition (qI), heat dissipation (qE) and zeaxanthin-dependent (qZ) quenching (Baker, 2008; Jahns and Holzwarth, 2012; Ruban et al., 2012). Although these NPQ components are kinetically distinct, they have overlapping quenching mechanisms and functions (Logan et al., 2014; Demmig-Adams et al., 2014). The pH-dependent NPQ component that is typically rapidly reversible in the dark can be maintained in darkness under conditions that maintain trans-thylakoid pH in darkness. Similarly, the zeaxanthin-correlated NPQ component can be reversed in minutes in some circumstances but can be maintained for days or weeks in darkness under photoinhibitory conditions. The qT component is associated with redistributing energy absorption between the two photosystems, which is achieved by the partial detachment and migration of LHCII between PSII and PSI (Bellafiore et al., 2005). The qI component is a slowly reversible process and the consequence of photoinhibitory events reflecting inactivation of the PS II reaction centres, where closed PS II reaction centres protect the open PS II reaction centres (Matsubara and Chow 2004). The major and fastest component of NPQ, qE, is believed to act in RCII photoprotection, and is related to the dissipation of excess energy as heat (Baker, 2008; Ruban et al., 2012). More recently described and with a formation/relaxation lifetime in the order of 10–15 min, qZ is believed to be correlated with the synthesis and epoxidation of zeaxanthin, and is also related to photoprotection (Nilkens et al., 2010; Jahns and Holzwarth, 2012). The site of qE formation is believed to be the light-harvesting antenna of PSII, triggered by ΔpH and enhanced by violaxanthin de-epoxidation to zeaxanthin (Horton and Ruban, 1992). The protein PsbS was discovered to play a crucial role in sensing lumen acidification and transducing the signal to the antenna. Although NPQ is the most commonly encountered form of PSII regulation, other mechanisms may also be important (Demmig-Adams and Adams, 2006). However, such assessments are complicated because multiple pathways contribute to photoprotection and these operate over different timescales. For example, the pH-dependent NPQ component that is typically rapidly reversible in the dark can be maintained in darkness if a trans-thylakoid pH persists (Gilmore and Björkman, 1995). Similarly, the zeaxanthin-correlated NPQ component can be reversed in minutes in some circumstances and can be maintained for days or weeks in darkness under photoinhibitory conditions (Demmig-Adams et al., 2012). Thus, it is not trivial to measure or quantify the different NPQ components accurately and this has led to a lack of consensus concerning the molecular mechanisms involved (Aro et al., 1993; Jahns and Holzwarth, 2012; Ruban et al., 2012; Tyystjärvi, 2013). A new method of NPQ measurement was proposed by Ruban and Murchie (2012) that involves determination of fluorescence responses to a gradually increasing actinic light routine. The yield of chlorophyll fluorescence can then be tracked to provide a measure of the onset of photo-inactivation (Ruban and Murchie, 2012). While sustained NPQ formation and zeaxanthin retention have been typically associated with the degradation of D1 degradation and other PSII proteins in overwintering conifers and other evergreens (Demmig-Adams et al., 2014), photoinhibitory damage may not be a common phenomenon in nature (Foyer et al., 2017). Within this context, photoinhibition may be consequence, rather than a cause, of limited productivity (Adams et al., 2013). Stress-induced sustained decreases in Fv/Fm ratios are more likely to be related to PSII downregulation via sustained components of protective NPQ and hence should not be used as a measure of damage resulting from photoinhibition (Foyer et al., 2017). Moreover, recent evidence shows that leaves adjust photosynthetic efficiency in response to changing irradiance relatively slowly. For example, wheat leaves were shown to take 15 min to regain maximum photosynthetic efficiency following transfer from shade to sun conditions, the major limitation being the activation of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Taylor and Long, 2017). Moreover, decreasing the time required for NPQ relaxation was found to increase the efficiency of CO2 assimilation in tobacco leaves such that productivity was increased by up to 20% (Kromdijk et al., 2016). The observed enhancement of biomass production may not only be due to increased energy availability as a result of an improved efficiency of photon capture, but also to an increase in the production of ROS signals that stimulate cell expansion and growth (Demmig-Adams et al., 2018). An association between increased growth, decreased thermal dissipation and enhanced ROS production is consistent with findings of Esteban et al. (2009).
4. Chloroplast antioxidants
Chloroplasts are armed with an elaborate arsenal of antioxidants that often have overlapping or interacting functions. The function of chloroplast antioxidants is not to totally eliminate superoxide, H2O2 and 1O2, but rather to achieve an appropriate balance between production and removal that is compatible with the operation of photosynthesis and yet allows effective transmission of these redox signals to the nucleus. Carotenoids such as lutein and zeaxanthin are effective thylakoid antioxidants located in close proximity to chlorophylls in the light-harvesting complexes. These pigments are able to quench the triplet state of chlorophyll (3Chl*) and 1O2, resulting in a higher-energy triplet state (Car*), which can decay to the ground state via different reaction mechanisms such as intersystem crossing, triplet-triplet annihilation and ground state quenching (Burke et al., 2000). The PSII reaction center contains two β-carotene molecules (Ferreira et al., 2004), which are positioned too far from the reaction centre special pair, P680* to allow direct quenching. However, when 1O2 is generated by P680*, the distance to the β-carotene is close enough to allow oxidative modification of β-carotene and signaling (Ramel et al., 2012). Tocopherols are also important membrane 1O2 quenchers, which function as general protectors of thylakoid membranes against lipid peroxidation (Piller et al., 2014). These membrane-bound antioxidants are oxidized either in a one-electron-transfer reaction to a tocopheryl-radical or are oxidized 1O2 to a hydroperoxide. Both reactions can be reversed by ascorbate, which can reduce both the radical and hydroperoxide (Krieger-Liszkay et al., 2008). The α − tocopherols in the thylakoid membranes are also able to terminate chain reactions of PUFA free radicals generated by lipid oxidation. Lipid alkoxyl and peroxyl radicals are reduced to alcohols or hydroperoxides, and chromanoxyl radicals, which are all less efficient in propagation of lipid peroxidation. The chromanoxyl radical may be reduced by ascorbate, thereby regenerating α − tocopherol molecule. While Arabidopsis mutants lacking tocopherol cyclase (vte1) showed little increase in stress sensitivity compared to the wild type, crossing vte1 with the npq1 mutant that is deficient in violaxanthin deepoxidase, the enzyme responsible for the conversion of violaxanthin to zeaxanthin in the thylakoid membrane, resulted in oxidation of lipids and pigments, as well as photoinhibition (Havaux et al., 2005). In addition to its functions in NPQ, zeaxanthin can function directly as an antioxidant.
Chloroplasts contain a number of pathways that limit H2O2 accumulation, including the ascorbate-glutathione cycle (Foyer and Shigeoka, 2009). In its basic form, this pathway consists of a network of reactions involving APX and enzymes that thereafter serve to regenerate ascorbate. APX produces monodehydroascorbate radicals (MDHA), which can then be reduced back to ascorbate by ferredoxin or by NAD(P)H in a reaction catalysed by MDHA reductase (MDHAR; Fig. 1A). MDHA that escapes these reactions can spontaneously disproportionate to ascorbate and dehydroascorbate (DHA). DHA must then be recycled to ascorbate in order to avoid depletion of ascorbate. In chloroplasts, reduced glutathione (GSH) can reduce DHA non-enzymatically or via the enzyme DHA reductase (DHAR), in a reaction in which GSH is oxidized to glutathione disulfide (GSSG). However, an analysis of Arabidopsis dhar mutants, including DHAR3 that has a chloroplastic location, showed that DHAR activity can be decreased to negligible levels without marked effects on the leaf ascorbate pool (Rahantaniaina et al., 2017). Intriguingly, the dhar1 dhar2 double mutants showed decreased GSH oxidation in a cat2 background with inhibition of cat2-triggered induction of the salicylic acid and cell death pathways. However, these effects were reversed in the cat2 dhar1 dhar2 dhar3 mutant, suggesting the presence of complex redox signaling pathways that remain to be elucidated (Rahantaniaina et al., 2017). Recycling of GSSG to GSH is catalysed by glutathione reductase (GR) using NADPH produced by the thylakoid electron transport chain.
Analysis of Arabidopsis mutants lacking both stromal (sAPX) and thylakoid (tAPX) forms of APX, however, revealed that the chloroplasts do not depended on the ascorbate-glutathione cycle alone to remove H2O2 (Giacomelli et al., 2007; Kangasjärvi et al., 2008; Maruta et al., 2010). In contrast, mutants lacking the thylakoid 2-Cys PRXs (2cpa 2cpb) had impaired photosynthetic efficiency and showed signs of oxidation suggesting that the chloroplast 2-Cys PRXs are essential and perhaps more important than tAPX in preventing H2O2 accumulation. However, 2-Cys PRXs are also involved in the reduction of reactive lipid peroxides, thus providing additional protection to the chloroplasts. Interestingly, while the 2cpa 2cpb tapx triple mutants showed oxidative stress markers including expression of H2O2-responsive marker genes, leaf H2O2 levels were similar to those measured in the 2cpa 2cpb (Awad et al., 2015). These findings suggest that APXs work together with the 2-Cys PRXs and perhaps other systems to remove H2O2 produced by the photosynthetic electron transport chain and keep stromal H2O2 levels low (Awad et al., 2015).
While the mechanisms by which ascorbate is transported into chloroplasts remain to be fully characterized, a member of the phosphate transporter 4 family (AtPHT4;4) was shown to transport ascorbate across the envelope membrane (Miyaji et al., 2015) and at least one member of the nucleobase ascorbate transporter (NAT) family may be localized on the thylakoid membrane. Knockout mutants lacking AtPHT4;4 were compromised in thermal energy dissipation (Miyaji et al., 2015). In the thylakoid membrane, ascorbate can act as an alternative electron donor to PSII, and it is also required for the conversion of violaxanthin to zeaxanthin in the xanthophyll cycle (Müller-Moulé et al., 2003; Jahns et al., 2009). Violaxanthin deepoxidase (VDE) is a luminal enzyme that is active only in its completely oxidized form that has six disulfide bonds. It is, therefore, possible that this enzyme is regulated by dithiol/disulfide exchange reactions (Simionato et al., 2015). The VDE reaction has a low affinity for ascorbate. Moreover, the affinity of the latter enzyme for ascorbate is strongly pH-dependent (Bratt et al., 1995; Jahns et al., 2009). Since chloroplasts contain about 10 mM ascorbate (Zechmann et al., 2011) and VDE requires very high levels of ascorbate for saturation e.g. 100 mM at pH 6.0 (Bratt et al., 1995; Jahns et al., 2009), it is highly unlikely that these levels are achieved within the thylakoid lumen. The granal thylakoid lumen significantly expands in the light, allowing better movement of plastocyanin and alleviating restrictions imposed on protein diffusion and the transport of electrons both to and from the cytochrome b6f complex in this compartment in the dark (Kirchhoffa et al., 2011). Nevertheless, measured levels of ascorbate within the thylakoid lumen are much lower than those measured in the stroma (Zechmann et al., 2011; Heyneke et al., 2013). Hence, VDE activity must always be limited by ascorbate availability in the wild type even under optimal conditions. The Arabidopsis vtc2-1 mutants, which have low ascorbate, showed higher levels of photoinhibition and photooxidation than the wild type under high light (Müller-Moulé et al., 2003, Müller-Moulé et al., 2014). However, other antioxidants such as glutathione are increased to compensate for low ascorbate and maintain antioxidant capacity (Pastori et al., 2003; Pavet et al., 2005).
Adaptation to biotic and abiotic threats in the environment is thought to have a long-lasting impact on plant processes that is passed to successive generations through persistent epigenetic mechanisms that provide a pre-emptive advantage. While a growing body of evidence suggests that changes in the reduction/oxidation (redox) status of stress signaling molecules and the level of DNA methylation play a major role in the transgenerational embedding of stress tolerance, little information is available concerning the specific pathways and mechanisms involved. The plant’s environmental sense and response mechanism contains a proteome distinct but overlapping with that of the photosynthetic chloroplast, with several stress proteins, including MSH1, appearing uniquely localized to the sensory plastid.
5. Chloroplast pathways contribute to epigenetic stress memory in plants
Chloroplasts are important sensors of environmental change, producing redox and other signals that can be transferred directly, or indirectly, to the nucleus to influence gene expression leading to acclimation to prevailing environmental conditions. Redox signals arising in chloroplasts also participate in epigenetic controls (Dietzel et al., 2015). Recent evidence suggests the presence of specific sub-sets of chloroplasts that specialize in sensory/signaling functions and participate in epigenetic reprogramming (Virdi et al., 2016). This sensory role is highlighted by analysis of the functions of MUTS HOMOLOG1 (MSH)1, a nucleoid protein that binds DNA and localizes to plastid and mitochondrial nucleoids (Xu et al., 2012; Virdi et al., 2016). The MSH1 protein, which is unique to plants, is targeted to both the mitochondria and chloroplasts and fulfils important functions in the induction of epigenetic stress memories in plants. MSH1-associated genetic reprogramming is a chloroplast-driven process accompanied by mitochondrial genomic and epigenetic responses that are intimately associated with organellar redox changes and stress responses (Virdi et al., 2016). Consequently, MSH1-containing organelles are considered to function as sensory plastids (Virdi et al., 2016).
Adaptation to the environment has both short term and long-lasting impacts on plant processes. In some cases, such stress memories are passed to successive generations through persistent epigenetic mechanisms that provide a pre-emptive advantage in a changing climate. Crucially, cellular redox status is a regulator of the level of DNA methylation, which plays a major role in the transgenerational embedding of stress tolerance memories in animals and plants. Reprogramming of the methylome i.e. the set of nucleic acid methylation modifications in the genome, predisposes the next generation for enhanced stress responses. The redox state of the sensory chloroplast population may also play a pivotal role in the regulation of such epigenetic controls in plants. The specialized sensory chloroplasts that house the MSH1 pathway have been found to be localized in the epidermal and vascular parenchyma, as well as in reproductive cells. The sensory chloroplasts, which are about 30% of the size of photosynthetic chloroplasts and have decreased granal stacking, appear to be otherwise very similar to the more general chloroplast population, whose main function is photosynthesis, but they contain additional stress proteins, including MSH1, which associates with the thylakoid membrane and plastoglobuli (Virdi et al., 2016). Silencing MSH1 produces a strong plant-stress response with differential expression of genes associated with abiotic and biotic stress pathways, together with genome-wide methylome re-patterning (Virdi et al., 2015). Subsequent segregation of the MSH1-RNAi transgene produced a trans-generational stress memory in 10-25% of progeny, with reduced growth, pale leaves, delayed maturity transition, delayed flowering, enhanced stress tolerance and altered circadian rhythms (Virdi et al., 2015). The msh1 memory is stable and heritable indefinitely.
The sensory function of MSH1 is clearly associated with the redox functions of the chloroplasts. It is possible that the MSH1 protein is able to move from the chloroplast to the nucleus, as has been suggested for another plastid single-stranded DNA-binding protein, WHIRLY1 (Grabowski et al., 2008). The partitioning of WHIRLY1 between chloroplasts and nuclei was shown to depend on phosphorylation by Calcineurin BLike‐Interacting Protein Kinase14 (CIPK14), providing a potential link between protein phosphorylation, Ca2+ signalling and WHIRLY1 functions (Ren et al., 2017). It is likely that these and other important multi-targeted and multifunctional organellar nucleoid proteins function in plastid-to-nucleus communication during developmental and stress responses, and perhaps passing directly from the chloroplasts to the nuclei (Caplan et al., 2015).
6. Conclusions and perspectives
The driving force for photosynthesis, sunlight, is an almost limitless supply. Hence, energy conservation is not a key issue for photosynthetic regulation. A sophisticated network of mechanisms that dissipate energy are essential to the operation of light harvesting linked to electron transport in photosynthesis, allowing effective protection against excess irradiance. The flexibility of photosynthesis has become optimized during evolution allowing photosynthesis to operate over a wide range of irradiances and environmental variables. The photosynthetic processes have evolved to be highly plastic and flexible, allowing other processes such as ROS production to occur at the expense of efficiency. Multiple layers of overlapping protective systems serve to prevent damage to the photosynthetic machinery, via combined action of preemptive energy dissipation and ROS removal through antioxidants. Give the requirement to perceive fluctuations in factors that influence the effective operation of photosynthesis in order to regulate these protective mechanisms, it is not surprising that the chloroplast became a major sensor of environmental and metabolic change with the ability to transmit retrograde signals to the nucleus in order to make appropriate adjustments in gene expression. Direct regulation of plastome gene expression is achieved via activation of protein kinases through the reduction state of photosynthetic electron transport chain components, notably plastoquinone and the cytochrome b6f complex. This regulation extends as far as regulation of nuclear alternative splicing to adjust plant responses to varying light conditions (Petrillo et al., 2014). This involves regulation of protein kinases located in the thylakoid membrane and in the stroma that, together with thioredoxin z, control the function of the plastid polymerase complex (Allen, 2015). While it is less clear how the redox state of the photosynthetic electron transport chain influences the expression of nuclear genes, ROS-related signals are established components of chloroplast-to-nuclear signaling. The importance of such communication pathways may have led to further specialization of certain sub-sets of chloroplasts to fulfil a crucial role in the epigenetic preservation of stress memories.
Despite compelling evidence that ROS are pro-life signals with multi-faceted roles in plant growth and development, the concept that oxidative damage is a major cause of light-induced loss of cellular functions remains prominent in the photosynthesis literature. This may be in part due to the heavy reliance on measured decreases in Fv/Fm ratios as a measure of photoinhibition, without consideration that such changes may be related to sustained PSII downregulation via protective systems. Hence, caution should be exercised in the use of Fv/Fm ratios to measure of photo-damage (Foyer et al., 2017).
Chloroplasts are equipped with an abundance of antioxidants that clearly have overlapping functions as ROS targets. Some of these antioxidants, such as glutathione and PRX, have well characterized roles in signal transduction. While others, such as ascorbate, may simply serve to attenuate the ROS signal, the complexity of the chloroplast oxidant and antioxidant system affords an extensive repertoire of discrete and probably specific redox signals that provides enormous flexibility in the control of gene expression. This communication with the nucleus not only conveys essential information on redox pressure within the electron transport chain to triggers appropriate short-term genetic responses, but also allows strategic provision of future defenses through epigenetic modifications.
Acknowledgements
CHF thanks the Biotechnology and Biological Sciences Research Council (BBSRC) UK (BB/M009130/1) for financial support. I thank Barbara Karpinska for the photos in Figs. 2 and 3.
References
- Adams W.W., III, Muller O., Cohu C.M., Demmig-Adams B. May photoinhibition in plants be a consequence, rather than a cause, of limited productivity? Photosynth. Res. 2013;117:31–44. doi: 10.1007/s11120-013-9849-7. [DOI] [PubMed] [Google Scholar]
- Allen J.F. Why chloroplasts and mitochondria retain their own genomes and genetic systems: colocation for redox regulation of gene expression. Proc. Natl. Acad. Sci. U. S. A. 2015;112:10231–10238. doi: 10.1073/pnas.1500012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Aro E.M., Virgin I., Andersson B. Photoinhibition of PS II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asada K. The water–water cycle as alternative photon and electron sinks. Philosophical Transactions of the. R. Soc. Lond. B. 2000;355:1419–1431. doi: 10.1098/rstb.2000.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad J., Stotz H.U., Fekete A., Krischke M., Engert C., Havaux M., Berger S., Mueller M.J. 2-Cysteine peroxiredoxins and thylakoid ascorbate peroxidase create a water-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions. Plant Physiol. 2015;167:1592–1603. doi: 10.1104/pp.114.255356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N.R. Chlorophyll fluorescence: a probe of photosynthesis 610 in vivo. Ann. Rev. Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Bartoli C.G., Casalongue C., Simontacchi M., Marquez-Garcia B., Foyer C.H. Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ. Exp. Bot. 2013;94:73–88. [Google Scholar]
- Bellafiore S., Barneche F., Peltier G., Rochaix J.-D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature. 2005;433:892–895. doi: 10.1038/nature03286. [DOI] [PubMed] [Google Scholar]
- Bernal-Bayard P., Ojeda V., Hervas M., Cejudo F.J., Navarro J.A., Velazquez-Campoy A., Perez-Ruiz J.M. Molecular recognition in the interaction of chloroplast 2-cys peroxiredoxin with NADPH-thioredoxin reductase C (NTRC) and thioredoxin. FEBS Lett. 2014;588:4342–4347. doi: 10.1016/j.febslet.2014.09.044. [DOI] [PubMed] [Google Scholar]
- Bratt C.E., Arvidsson P.O., Carisson M., Akerlund H.E. Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth. Res. 1995;45:169–175. doi: 10.1007/BF00032588. [DOI] [PubMed] [Google Scholar]
- Broin M., Cuine S., Eymery F., Rey P. The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. Plant Cell. 2002;14:1417–1432. doi: 10.1105/tpc.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B.B., Balmer Y. Redox regulation: A broadening horizon. Ann. Rev. Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- Buchanan B.B., Luan S. Redox regulation in the chloroplast thylakoid lumen: a new frontier in photosynthesis research. J. Exp. Bot. 2005;56:1439–1447. doi: 10.1093/jxb/eri158. [DOI] [PubMed] [Google Scholar]
- Burke M., Land E.J., McGarvey D.J., Truscott T.G. Carotenoid triplet state lifetimes. J. Photochem. Photobiol. B. 2000;59:132–138. doi: 10.1016/s1011-1344(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Caplan J.L., Kumar A.S., Park E., Padmanabhan M.S., Hoban K., Modla S., Czymmek K., Dinesh-Kumar S.P. Chloroplast stromules function during innate immunity. Dev. Cell. 2015;34:45–57. doi: 10.1016/j.devcel.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody M., Crisp P.A., d'Alessandro S., Ganguly D., Gordon M., Havaux M., Albrecht-Borth V., Pogson B.J. Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation. Plant Physiol. 2016;171:1734–1749. doi: 10.1104/pp.16.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.X., Mabbitt P.D., Phua S.Y., Mueller J.W., Nisar N., Gigolashvili T., Stroeher E., Grass J., Arlt W., Estavillo G.M., Jackson C.J., Pogson B.J. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc. Natl. Acad. Sci. U. S. A. 2016;113:4567–4576. doi: 10.1073/pnas.1604936113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin V., Issakidis-Bourguet E., Marchand C., Hirasawa M., Lancelin J., Knaff D., Miginiac-Maslow M. The arabidopsis plastidial thioredoxins – new functions and new insights into specificity. J. Biol. Chem. 2003;278:23747–23752. doi: 10.1074/jbc.M302077200. [DOI] [PubMed] [Google Scholar]
- Collin V., Lamkemeyer P., Miginiac-Maslow M., Hirasawa M., Knaff D.B., Dietz K.J., Issakidis-Bourguet E. Characterization of plastidial thioredoxins from arabidopsis belonging to the new y-type. Plant Physiol. 2004;136:4088–4095. doi: 10.1104/pp.104.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams W.W., III Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 2006;172:11–21. doi: 10.1111/j.1469-8137.2006.01835.x. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Cohu C.M., Muller O., Adams W.W., III Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth. Res. 2012;113:75–88. doi: 10.1007/s11120-012-9761-6. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Koh S.-C., Cohu C.M., Muller O., Adams W.W., III . Non-photochemical quenching in contrasting plant species and environments. In: Demmig-Adams B., Garab G., Adams W.W., Govindjee, editors. Vol. 40. Springer; Dordrecht: 2014. pp. 531–551. (Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration). [Google Scholar]
- Demmig-Adams B., Stewart J.J., Baker C.R., Adams W.W., III Optimization of photosynthetic productivity in contrasting environments by regulons controlling plant form and function. Int. J. Mol. Sci. 2018;19:872. doi: 10.3390/ijms19030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K.J., Pfannschmidt T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol. 2011;155:1477–1485. doi: 10.1104/pp.110.170043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel L., Glaßer C., Liebers M., Hiekel S., Courtois F., Czarnecki O., Schlicke H., Zubo Y., Borner T., Mayer K. Identification of early nuclear target genes of plastidial redox signals that trigger the long-term response of Arabidopsis to light quality shifts. Mol. Plant. 2015;8 doi: 10.1016/j.molp.2015.03.004. 1237–125. [DOI] [PubMed] [Google Scholar]
- Dogra V., Duan J., Lee K.P., Lv S., Liu R., Kim C. FtsH2-dependent proteolysis of EXECUTER1 is essential in mediating singlet oxygen-triggered retrograde signaling in Arabidopsis thaliana. Front. Plant Sci. 2017;8:1145. doi: 10.3389/fpls.2017.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever S.M., Baker N.R. The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 2011;34:837–846. doi: 10.1111/j.1365-3040.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- Esteban R., Olano J.M., Castresana J., Fernández-Marín B., Hernández A., Becerril J.M., García-Plazaola J.I. Distribution and evolutionary trends of photoprotective isoprenoids (xanthophylls and tocopherols) within the plant kingdom. Physiol. Plant. 2009;135:379–389. doi: 10.1111/j.1399-3054.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- Exposito-Rodriguez M., Philippe Laissue P., Yvon-Durocher G., Smirnoff N., Mullineaux P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2007:8. doi: 10.1038/s41467-017-00074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira K.N., Iverson T.M., Maghlaoui K., Barber J., Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- Fey V., Wagner R., Brauetigam K., Wirtz M., Hell R., Dietzmann A., Leister D., Oelmueller R., Pfannschmidt T. Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J. Biol. Chem. 2005;280:5318–5328. doi: 10.1074/jbc.M406358200. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Lelandais M., Edwards E.A., Mullineaux P.M. The role of ascorbate in plants, interactions with photosynthesis, and regulatory significance. In: Pell E., Steffen K., editors. Vol. 6. 1991. pp. 131–144. (Active oxygen/oxidative stress and plant metabolism, Current Topics in Plant Physiology). American Society of Plant Physiologists. [Google Scholar]
- Foyer C.H., Ruban A.V., Noctor G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017;474:877–883. doi: 10.1042/BCJ20160814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Wilson M., Wright M. Redox regulation of cell proliferation: bioinformatics and redox proteomics approaches to identify redox-sensitive cell cycle regulators. Free Radic. Biol. Med. 2018 doi: 10.1016/j.freeradbiomed.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P., Fernie A.R. Metabolic control of redox and redox control of metabolism in plants. Antioxid. Redox Signal. 2014;21:1389–1421. doi: 10.1089/ars.2014.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli L., Masi A., Ripoll D.R., Lee M.J., van Wijk K.J. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol. Biol. 2007;65:627–644. doi: 10.1007/s11103-007-9227-y. [DOI] [PubMed] [Google Scholar]
- Gilmore A.M., Björkman O. Temperature-sensitive coupling and uncoupling of ATPase-mediated, nonradiative energy-dissipation – similarities between chloroplasts and leaves. Planta. 1995;197:646–654. [Google Scholar]
- Girotti A.W. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- Gollan P.J., Lima-Melo Y., Tiwari A., Tikkanen M., Aro E.-M. Interaction between photosynthetic electron transport and chloroplast sinks triggers protection and signalling important for plant productivity. Philosophical Transactions of the. R. Soc. Lond. B. 2017;372:20160390. doi: 10.1098/rstb.2016.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski E., Miao Y., Mulisch M., Krupinska K. Singlestranded DNA-binding protein in barley leaves is located in plastids and the nucleus of the same cell. Plant Physiol. 2008;147:1800–1804. doi: 10.1104/pp.108.122796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Li H., Xiang X., Ahammed G.J., Wang M., Onac E., Zhou J., Xia X., Shi K., Yu J., Foyer C.H., Zhou Y. Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome B, auxin and hydrogen peroxide in tomato. Plant Physiol. 2016;172:1259–1272. doi: 10.1104/pp.16.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M., Eymery F., Porfirova S., Rey P., Dormann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17:3451–3469. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosynth. Res. 2002;73:223–231. doi: 10.1023/A:1020459416987. [DOI] [PubMed] [Google Scholar]
- Henmi T., Miyao M., Yamamoto Y. Release and reactive-oxygen-mediated damage of the oxygen-evolving complex subunits of PSII during photoinhibition. Plant Cell Physiol. 2004;45:243–250. doi: 10.1093/pcp/pch027. [DOI] [PubMed] [Google Scholar]
- Heyneke E., Luschin-Ebengreuth N., Krajcer I., Wolkinger V., Müller M., Zechmann B. Dynamic compartment specific changes in glutathione and ascorbate levels in Arabidopsis plants exposed to different light intensities. BMC Plant Biol. 2013;13:104. doi: 10.1186/1471-2229-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisabori T., Sunamura E., Kim Y., Konno H. The chloroplast ATP synthase features 695 the characteristic redox regulation machinery. Antioxid. Redox Signal. 2013;19:1846–1854. doi: 10.1089/ars.2012.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns P., Holzwarth A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochimica et Biophysica Acta. 2012;1817:182–193. doi: 10.1016/j.bbabio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Jahns P., Latoeski D., Strzalka K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochimica et Biophysica Acta. 2009;1787:3–14. doi: 10.1016/j.bbabio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Kangasjärvi S., Lepistö A., Hännikäinen K., Piippo M., Luomala E.M., Aro E.M., Rintamäki E. Diverse roles for chloroplast stromal and thylakoid bound ascorbate peroxidases in plant stress responses. Biochem. J. 2008;412:275–285. doi: 10.1042/BJ20080030. [DOI] [PubMed] [Google Scholar]
- Karpinska B., Zhang K., Rasool B., Pastok D., Morris J., Verrall S., Hedley P., Hancock R., Foyer C.H. The redox state of the apoplast influences the acclimation of photosynthesis and leaf metabolism to changing irradiance. Plant Cell Environ. 2017;41:1083–1097. doi: 10.1111/pce.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinska B., Alomrani S.O., Foyer C.H. Inhibitor-induced oxidation of the nucleus and cytosol in Arabidopsis thaliana: Implications for organelle to nucleus retrograde signalling. Philos. Trans. R. Soc. Lond. B. 2017;372:20160392. doi: 10.1098/rstb.2016.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoffa H., Halla C., Wooda M., Herbstováa M., Tsabarib O., Nevob R., Charuvib D., Shimonic E., Reich Z. Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20248–20253. doi: 10.1073/pnas.1104141109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A., Fufezan C., Trebst A. Singlet oxygen production in PS II and related protection mechanism. Photosynth. Res. 2008;98:551–564. doi: 10.1007/s11120-008-9349-3. [DOI] [PubMed] [Google Scholar]
- Kromdijk J., Glowacka K., Leonelli L., Gabilly S.T., Iwai M., Niyogi K.K., Long S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science. 2016;354:857–861. doi: 10.1126/science.aai8878. [DOI] [PubMed] [Google Scholar]
- Laloi C., Havaux M. Key players of singlet oxygen-induced cell death in plants. Front. Plant Sci. 2015;6:39. doi: 10.3389/fpls.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Kim C., Landgraf F., Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan B.A., Demmig-Adams B., Adams 3rd W.W., Bilger W. Context, quantification, and measurement guide for non-photochemical quenching of chlorophyll fluorescence. In: Demmig-Adams B., Garab G., Adams 3rd W.W., Govindjee, editors. Vol. 40. Springer; Dordrecht: 2014. pp. 187–201. (Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration). [Google Scholar]
- Lundquist P.K., Rosar C., Brautigam A., Weber A.P. Plastid signals and the bundle sheath: mesophyll development in reticulate mutants. Mol. Plant. 2014;7:14–29. doi: 10.1093/mp/sst133. [DOI] [PubMed] [Google Scholar]
- Müller-Moulé P., Conklin P.L., Niyogi K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2003;128:970–977. doi: 10.1104/pp.010924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P., Golan T., Niyogi K.K. Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photo-oxidative stress. Plant Physiol. 2014;133:748–760. doi: 10.1104/pp.103.032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoë A., Wang F., Girard-Bascou J., Wollman F.-A., de Vitry C. Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions. Plant Cell. 2014;26:373–390. doi: 10.1105/tpc.113.120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W.F., Sies H. Genomic redox footprints. Nat. Plants. 2017;3:17071. doi: 10.1038/nplants.2017.71. [DOI] [PubMed] [Google Scholar]
- Maruta T., Tanouchi A., Tamoi M., Yabuta Y., Yoshimura K., Ishikawa T., Shigeoka S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200. doi: 10.1093/pcp/pcp177. [DOI] [PubMed] [Google Scholar]
- Matsubara S., Chow W. Populations of photoinhibited photosystem II reaction centers characterized by chlorophyll a fluorescence lifetime in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18234–18239. doi: 10.1073/pnas.0403857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proceed. Natl. Acad. Sci. U. S. A. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Belin C., Delorme-Hinoux V., Reichheld J., Riondet C. Thioredoxin and glutaredoxin systems in plants: Molecular mechanisms crosstalks, and functional significance. Antioxid. Redox Signal. 2012;17:1124–1160. doi: 10.1089/ars.2011.4327. [DOI] [PubMed] [Google Scholar]
- Mhamdi A., Hager J., Chaouch S., Queval G., Han Y., Taconnat Y., Saindrenan P., Issakidis-Bourguet E., Gouia H., Renou J.P., Noctor G. Arabidopsis GLUTATHIONE REDUCTASE 1 is essential for the metabolism of intracellular H2O2 and to enable appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010;153:1144–1160. doi: 10.1104/pp.110.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A., Noctor G., Baker A. Plant catalases: peroxisomal redox guardians. Arch. Biochem. Biophys. 2012;525:181–194. doi: 10.1016/j.abb.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Michelet L., Zaffagnini M., Morisse S., Sparla F., Perez-Perez M.E., Francia F., Lemaire S.D. Redox regulation of the calvin-benson cycle: Something old, something new. Front. Plant Sci. 2013;4:470. doi: 10.3389/fpls.2013.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T., Kuromori T., Takeuchi Y., Yamaji N., Yokosho K., Shimazawa A., Sugimoto E., Omote H., Ma J.F., Shinozaki K., Moriyama Y. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat. Commun. 2015;6:5928. doi: 10.1038/ncomms6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M.J., Mene-Saffrane L., Grun C., Karg K., Farmer E.E. Oxylipin analysis methods. Plant J. 2006;45:472–489. doi: 10.1111/j.1365-313X.2005.02614.x. [DOI] [PubMed] [Google Scholar]
- Nikkanen L., Rintamäki E. Thioredoxin-dependent regulatory networks in chloroplasts under fluctuating light conditions. Philos. Trans. R. Soc. B. 2014;369:20130224. doi: 10.1098/rstb.2013.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkanen L., Toivola J., Diaz M.D., Rintamäki E.E. Chloroplast thioredoxin systems: prospects for improving photosynthesis. Signal. Philos. Trans. R. Soc. Lond. B. 2017;372:20160474. doi: 10.1098/rstb.2016.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M., Kress E., Lambrev P., Miloslavina Y., Muller M., Holzwarth A.R., Jahns P. Identification of a slowly inducible zeaxanthin-dependent component of non- photochemical quenching of chlorophyll fluorescence generated under steady-745 state conditions in Arabidopsis. Biochimica et Biophysica Acta. 2010;1797:466–475. doi: 10.1016/j.bbabio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Nilsson R., van Wijk K.J. Transient interaction of cpSRP54 with elongating nascent chains of the chloroplast-encoded D1 protein; ‘cpSRP54 caught in the act'. FEBS Lett. 2002;524:127–133. doi: 10.1016/s0014-5793(02)03016-8. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Allakhverdiev S.I., Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochimica et Biophysica Acta. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Noctor G., Foyer C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016;171:1581–1592. doi: 10.1104/pp.16.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G., Queval G., Mhamdi A., Chaouch S., Foyer C.H. Glutathione. In: Millar H., editor. Vol. 9. American Society of Plant Biologists Rockville; MD: 2011. pp. 1–32. (The Arabidopsis Book). [Google Scholar]
- Noctor G., Riechheld J.-P., Foyer C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2017 doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Osmond C.B., Grace C.E. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J. Exp. Bot. 1995;46:1351–1362. [Google Scholar]
- Pastori G.M., Kiddle G., Antoniw J., Bernard S., Veljovic-Jovanovic S., Verrier P.J., Noctor G., Foyer C.H. Leaf vitamin C contents modulate plant defense transcripts and regulate genes controlling development through hormone signaling. Plant Cell. 2003;15:939–951. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V., Olmos E., Kiddle G., Mowla S., Kumar S., Antoniw J., Alvarez M.E., Foyer C.H. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis thaliana. Plant Physiol. 2005;139:1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ruiz J.M., Spinola M.C., Kirchsteiger K., Moreno J., Sahrawy M., Cejudo F.J. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell. 2006;18:2356–2368. doi: 10.1105/tpc.106.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S., Clement M.V. Superoxide anion: oncogenic reactive oxygen species? Int. J. Biochem. Cell Biol. 2007;39:1297–1304. doi: 10.1016/j.biocel.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Petrillo E., Godoy Herz M.A., Fuchs A., Reifer D., Fuller J., Yanovsky M.J., Simpson C., Brown J.W.S., Barta A., Kalyna M., Kornblihtt A.R. A chloroplast retrograde signal regulates nuclear alternative splicing. Science. 2014;344:427–430. doi: 10.1126/science.1250322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller E.L., Glauser G., Kessler F., Besagni C. Role of plastoglobules in metabolite repair in the tocopherol redox cycle. Front. Plant Sci. 2014;5:298. doi: 10.3389/fpls.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla D., Gobel C., Imboden A., Hamberg M., Feussner I., Apel K. Enzymatic, but not nonenzymatic, 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J. 2008;54:236–248. doi: 10.1111/j.1365-313X.2008.03409.x. [DOI] [PubMed] [Google Scholar]
- Rahantaniaina M.-S., Li S., Chatel-Innocenti G., Tuzet A., Issakidis-Bourguet E., Mhamdi A., Noctor G. Chloroplastic and cytosolic dehydroascorbate 1 reductases co-operate in oxidative stress-driven activation of the salicylic acid pathway. Plant Physiol. 2017;174:956–971. doi: 10.1104/pp.17.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Ginies C., Soubigou-Taconnat L., Triantaphylides C., Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F., Ksas B., Akkari E., Mialoundama A.S., Monnet F., Krieger-Liszkay A., Ravanat J.L., Mueller M.J., Bouvier F., Havaux M. Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell. 2013;25:1445–1462. doi: 10.1105/tpc.113.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Li Y., Jiang Y., Wu B., Miao Y. Phosphorylation of WHIRLY1 by CIPK14 shifts its localization and dual functions in Arabidopsis. Mol. Plant. 2017;10:749–763. doi: 10.1016/j.molp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Richter A.S., Peter E., Rothbart M., Schlicke H., Toivola J., Rintamäki E., Grimm B. Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiol. 2013;162:63–73. doi: 10.1104/pp.113.217141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban A.V., Murchie E.H. Assessing the photoprotective effectiveness of nonphotochemical chlorophyll fluorescence quenching: a new approach. Biochimica et Biophysica Acta. 2012;1817:977–982. doi: 10.1016/j.bbabio.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Ruban A.V., Johnson M.P., Duffy C.D.P. The photoprotective molecular switch in the photosystem II antenna. Biochimica et Biophysica Acta. 2012;1817:167–181. doi: 10.1016/j.bbabio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Scheibe R., Dietz K.J. Reduction-oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant Cell Environ. 2012;35:202–216. doi: 10.1111/j.1365-3040.2011.02319.x. [DOI] [PubMed] [Google Scholar]
- Shumbe L., Chevalier A., Legeret B., Taconnat L., Monnet F., Havaux M. Singlet oxygen-induced cell death in Arabidopsis under high-light stress is controlled by OXI1 kinase. Plant Physiol. 2016;170:1757–1771. doi: 10.1104/pp.15.01546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionato D., Basso S., Zaffagnini M., Lana T., Marzotto F., Trost P., Morosinotto T. Protein redox regulation in the thylakoid lumen: The importance of disulfide bonds for violaxanthin de-epoxidase. FEBS Lett. 2015;589:919–923. doi: 10.1016/j.febslet.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Taylor S.H., Long S.P. Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity, Philosophical Transactions of the. R. Soc. Lond. B. 2017:372. doi: 10.1098/rstb.2016.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A., Oldham T.C., Phillips D., Barber J. Singlet oxygen formation detected by near-infrared emission from isolated PS II reaction centres: Direct correlation between P680 triplet decay and luminescence rise kinetics and its consequences for photoinhibition. J. Photochem. Photobiol. B: Biol. 1999;48:89–96. [Google Scholar]
- Telfer A. Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of beta-carotene. Plant Cell Physiol. 2014;55:1216–1223. doi: 10.1093/pcp/pcu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormählen I., Ruber J., Von Roepenack-Lahaye E., Ehrlich S., Massot V., Huemmer C., Issakidis-Bourguet E., Geigenberger P. Inactivation of thioredoxin f1 leads to decreased light activation of ADP834 glucose pyrophosphorylase and altered diurnal starch turnover in leaves of arabidopsis plants. Plant Cell Environ. 2013;36:16–29. doi: 10.1111/j.1365-3040.2012.02549.x. [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C., Krischke M., Hoeberichts F.A., Ksas B., Gresser G., Havaux M., Van Breusegem F., Mueller M.J. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008;148:960–968. doi: 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E. Photoinhibition of photosystem II. Intent. Rev. Cell Mol. Biol. 2013;300:243–303. doi: 10.1016/B978-0-12-405210-9.00007-2. [DOI] [PubMed] [Google Scholar]
- Virdi K.S., Laurie J.D., Xu Y.-Z., Yu J., Shao M.-R., Sanchez R., Kundariya H., Wamboldt Y., Wang D., Riethoven J.-J., Wamboldt Y., Arrieta-Montiel M.P., Mackenzie S.A. Arabidopsis MSH1 mutation alters the epigenome to produce heritable changes in plant growth. Nat. Commun. 2015;6:6386. doi: 10.1038/ncomms7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdi K.S., Wamboldt Y., Kundariya H., Laurie J.D., Keren I., Kumar K.R.S., Block A., Basset G., Luebker S., Elowsky C., Day P.M., Roose J.L., Bricker T.M., Elthon T., Mackenzie S.A. MSH1 is a plant organellar DNA binding and thylakoid protein under precise spatial regulation to alter development. Mol. Plant. 2016;9:245–260. doi: 10.1016/j.molp.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Wagner D., Przybyla D., Op den Camp R., Kim C., Landgraf F., Lee K.P., Wursch M., Laloi C., Nater M., Hideg E., Apel K. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- Wang L., Kim C., Xu X., Piskurewicz U., Dogra V., Singh S., Mahler H., Apel K. Singlet oxygen- and EXECUTER1-mediated signaling is initiated in grana margins and depends on the protease FtsH2. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E3792–3800. doi: 10.1073/pnas.1603562113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe N., Ishida Y., Ochiai A., Tokuoka Y., Kawashima N. Oxidation decomposition of unsaturated fatty acids by singlet oxygen in phospholipid bilayer membranes. J. Oleo Sci. 2007;56:73–80. doi: 10.5650/jos.56.73. [DOI] [PubMed] [Google Scholar]
- Woehle C., Dagan T., Landan G., Vardi A., Rosenwasser S. Expansion of the redox-sensitive proteome coincides with the plastid endosymbiosis. Nat. Plants. 2017;3:17066. doi: 10.1038/nplants.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.Z., Santamaria Rde L., Virdi K.S., Arrieta-Montiel M.P., Razvi F., Li S., Ren G., Yu B., Alexander D., Guo L. The chloroplast triggers developmental reprogramming when MUTS HOMOLOG1 is suppressed in plants. Plant Physiol. 2012;159:710–720. doi: 10.1104/pp.112.196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokthongwattana K., Melis A. Photoinhibition and recovery in oxygenic photosynthesis: Mechanism of a PS II damage and repair. In: Demmig-Adams B., Adams W.W., Matoo A., editors. Advances in Photosynthesis and Respiration, Photoprotection, Photoinhibition, Gene Regulation, and Environment. Springer; Dordrecht: 2006. pp. 175–191. [Google Scholar]
- Zechmann B., Stumpe M., Mauch F. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta. 2011;233:1–12. doi: 10.1007/s00425-010-1275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Finkemeier I., Guan W., Tossounian M., Wei B., Young D., Jingjing J., Messens J., Yang X., Zhu J., Wilson M., Shen W.-B., Xie Y., Foyer C.H. Oxidative stress-triggered interactions between the succinyl- and acetyl-proteomes of rice leaves. Plant. Cell Environ. 2017;41:1139–1153. doi: 10.1111/pce.13100. [DOI] [PubMed] [Google Scholar]
- op den Camp R.G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Gobel C., Feussner I., Nater M., Apel K. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]