Abstract
A chylothorax may be due to either direct trauma or occlusion of the thoracic duct. Treatments include antegrade or retrograde glue and coil embolization as well as thoracic duct stent graft placement. This report describes a patient with chylothorax secondary to venous outflow occlusion. Left upper extremity venography demonstrated multifocal left brachiocephalic and axillary vein occlusions with retrograde filling of an engorged and disrupted thoracic duct. Retrograde thoracic duct lymphangiography with embolization and left upper extremity venous reconstruction were performed with complete resolution of chylothorax.
Keywords: Chylothorax, Central venous occlusion, Retrograde, Thoracic duct, Embolization, Venous reconstruction
Chylothorax is a rare condition typically caused by traumatic or spontaneous disruption of the thoracic duct.1, 2, 3, 4, 5, 6, 7 Patients with thoracic duct injury commonly suffer from respiratory compromise due to chylothorax, metabolic abnormalities, malnutrition, dehydration, and immune compromise secondary to fat, protein, and fluid losses.1, 2, 3, 4, 5, 6, 7 Chylous leaks <1 L/d may be managed conservatively with diet modification and somatostatin analogues, whereas high-output leaks >1 L/d may be treated with thoracic duct embolization or stent graft placement.1, 2, 4, 8, 9
Various methods of thoracic duct access, including antegrade and retrograde access, with subsequent coil and n-butyl cyanoacrylate glue embolization, sodium tetradecyl sulfate sclerosis, and stent graft placement have been described.1, 3, 4, 6, 7, 8, 9, 10, 11 This case describes a patient with an unusual chylothorax secondary to venous outflow obstruction of the brachiocephalic and axillary veins treated with retrograde opacification and embolization of the thoracic duct with concomitant venous reconstruction (Fig 1). Institutional Review Board approval was not required for preparation of this report. The patient's consent was obtained for publication of this article.
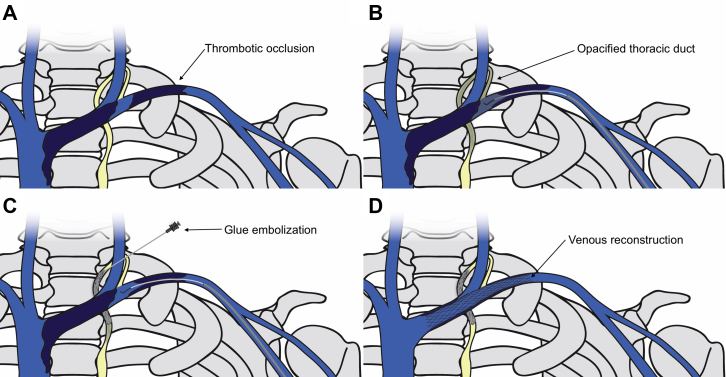
Fig 1.
Schematic illustration of transcervical retrograde thoracic duct glue embolization and venous reconstruction. A, Thrombotic occlusion of the left axillary and brachiocephalic veins. B, Opacification of the proximal thoracic duct through pressurized venography. C, Transcervical access and glue embolization of the thoracic duct. D, Venous recanalization and stenting of the left axillary and distal brachiocephalic veins.
Case report
A 62-year-old morbidly obese (body mass index of 46.9 kg/m2) woman with ovarian cancer but no surgical or traumatic history presented with a large right pleural effusion and left arm swelling. Thoracentesis yielded 1.5 L of milky white fluid with a triglyceride level of 1960 mg/dL. A 10F right chest tube was placed.
An antegrade approach of thoracic duct embolization was initially attempted. Under ultrasound guidance, 25-gauge spinal needles (Becton Dickinson, Franklin Lakes, NJ) were inserted into the bilateral inguinal lymph nodes. Ethiodized oil (Ethiodol; Guerbet, Villepinte, France) was infused by hand at 0.5 mL/min under continuous fluoroscopic visualization. Multiple attempts were made to cannulate the cisterna chyli using a 21-gauge, 20-cm Chiba needle (Cook Medical, Bloomington, Ind); however, they were ultimately unsuccessful because of the patient's large body habitus (distance from the skin to the cisterna chyli was 33 cm).
Percutaneous transcervical retrograde thoracic duct cannulation was attempted but was unsuccessful because of the lack of thoracic duct opacification. In addition, attempts were made to puncture the thoracic duct under ultrasound guidance in the neck; however, they were also unsuccessful because of poor visualization, given the patient's large body habitus. Under ultrasound guidance, left brachial vein access was obtained, and left upper extremity venography demonstrated occlusion of the left axillary vein with multiple chest wall venous collaterals. The procedure was aborted.
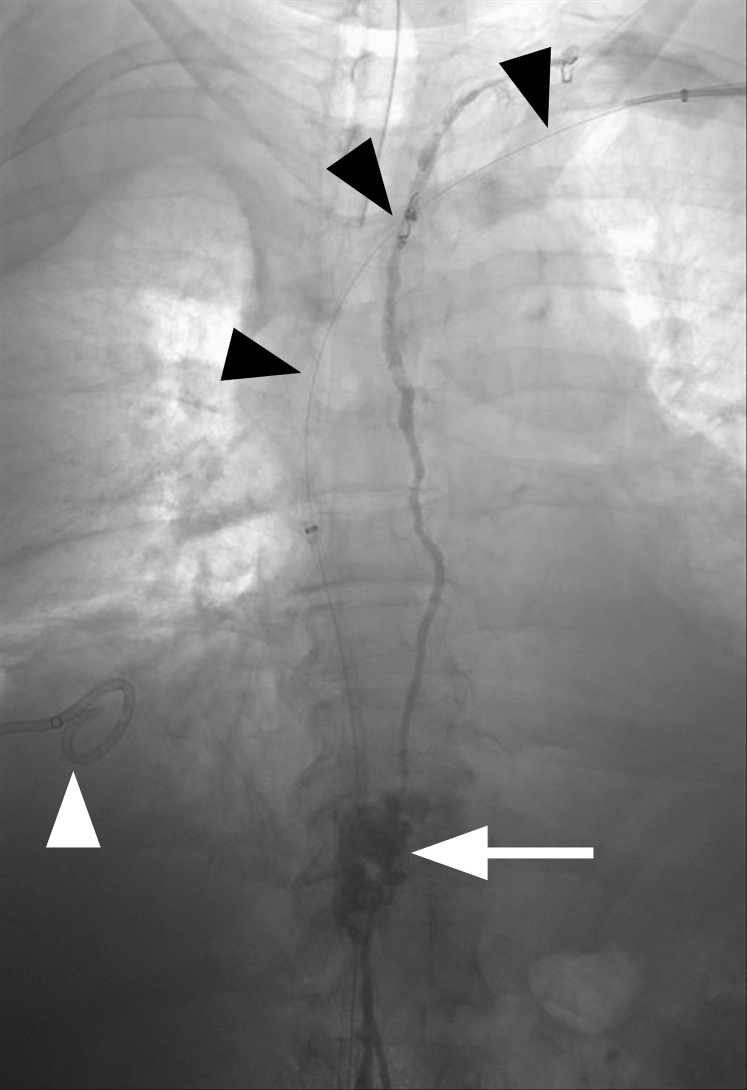
Given the left upper extremity venous occlusions, it was surmised that the right chylothorax may be due to venous outflow obstruction resulting in thoracic duct outlet obstruction. Seven days later, left upper extremity recanalization and reconstruction were performed. The patient was placed under general anesthesia. Under ultrasound guidance, left brachial and right greater saphenous vein access was obtained. Venography of the left upper extremity and superior vena cava demonstrated occlusions of the axillary and brachiocephalic veins (Fig 2).
Fig 2.
Frontal digital subtraction venography of the left arm showing complete occlusion of the left axillary and brachiocephalic veins (arrowhead). Numerous collateral veins are seen in the upper chest that reconstitute the superior vena cava (arrow).
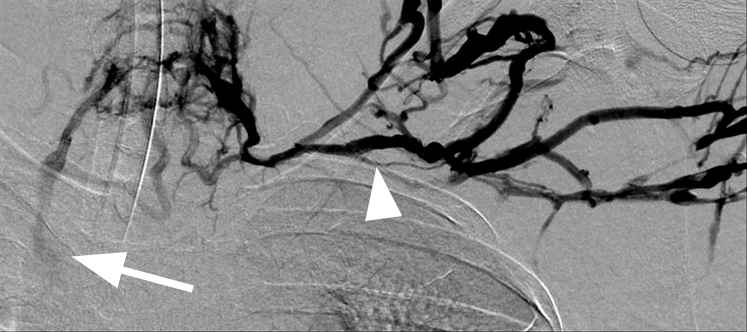
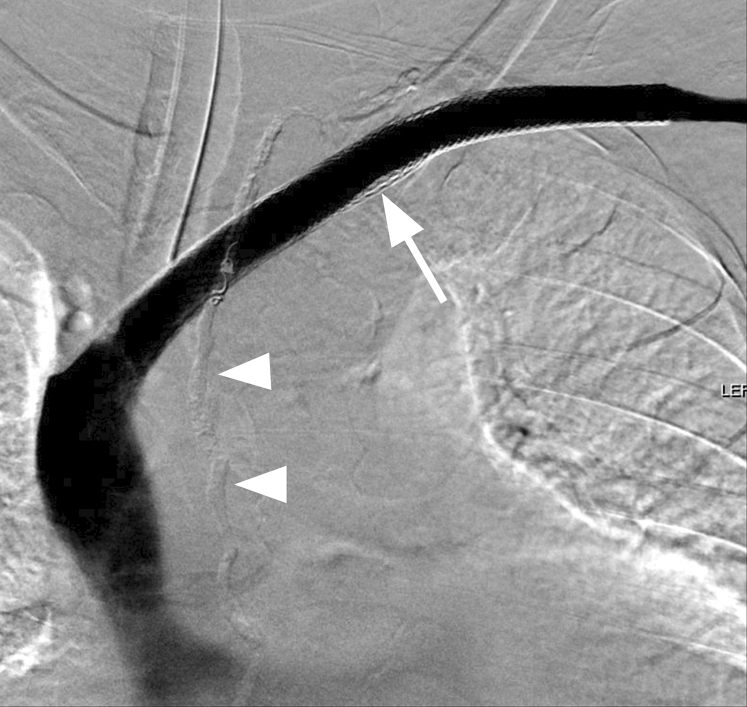
From the left arm, a straight stiff Glidewire (Terumo Interventional Systems, Tokyo, Japan) and angled Glidecath (Terumo Interventional Systems) were advanced into the subclavian vein. Injection of contrast material (Isovue 300; Bracco Diagnostics, Monroe Township, NJ) demonstrated a dilated subclavian vein, axillary and brachiocephalic vein occlusions, and retrograde reflux of contrast material into a disrupted caudal thoracic duct (Fig 3). A straight stiff Glidewire and angled Glidecath were advanced from the right groin into the left subclavian vein and snared from the left arm using a 15-mm Amplatz GooseNeck snare (Covidien, Plymouth, Minn) to obtain through-and-through access.
Fig 3.
After successful crossing of the left axillary and brachiocephalic vein occlusions, pressurized digital subtraction venography within the excluded subclavian vein shows retrograde filling of the thoracic duct (arrow). Tandem occlusions are noted in the axillary and brachiocephalic veins (white arrowheads). The left internal jugular vein is occluded. Contrast material is seen refluxing into a straight-appearing segment of a left lymphatic duct (black arrowhead) and the main thoracic duct.
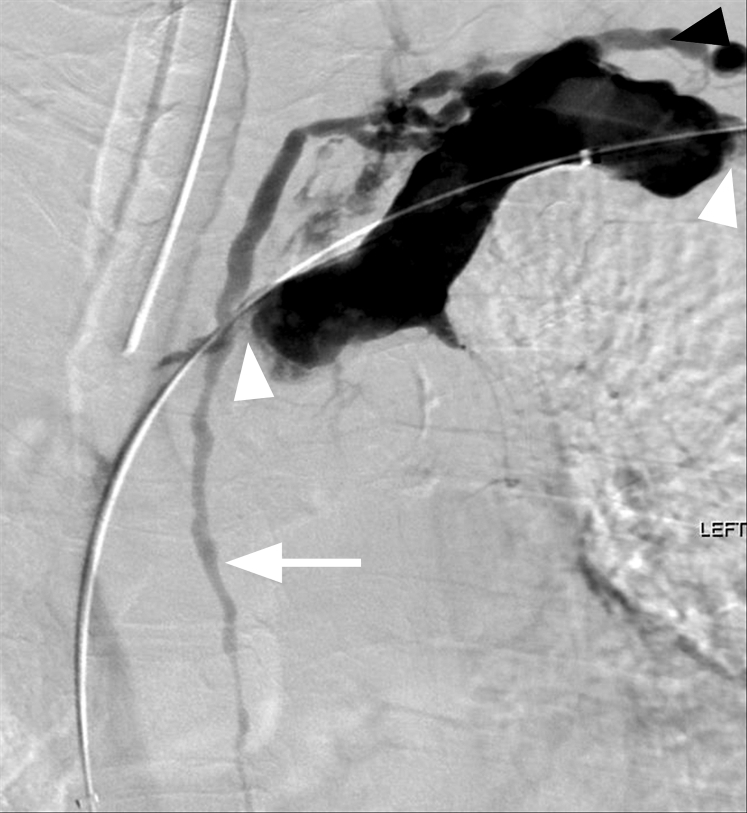
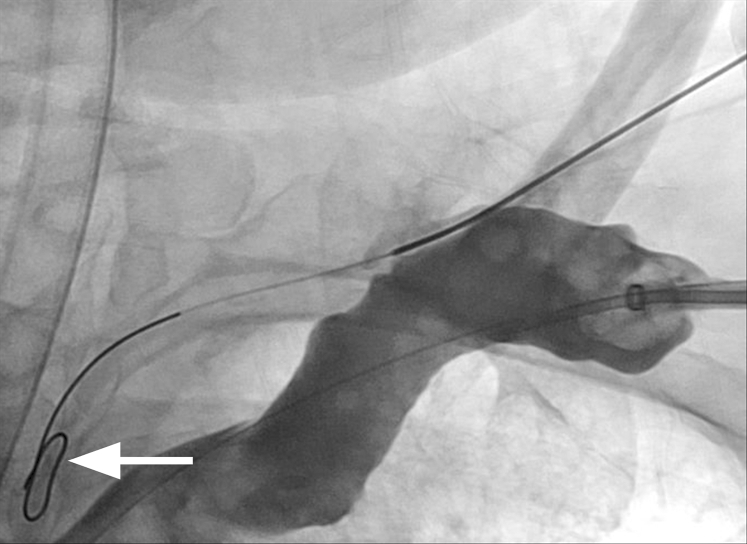
Multiple attempts were made to access the thoracic duct through a transvenous retrograde approach but were ultimately unsuccessful. A transcervical retrograde thoracic duct cannulation was performed. While pressurized contrast material was simultaneously being injected from the left arm sheath to opacify the thoracic duct through retrograde reflux of contrast material, a horizontal lymphatic channel leading to the thoracic duct was accessed in a percutaneous transcervical fashion using a 21-gauge × 10-cm Chiba needle under fluoroscopic guidance (Fig 4). Once access was obtained, an 0.018-inch Nitrex wire (Covidien) and a 2.4F Progreat microcatheter (Terumo Interventional Systems) were advanced into the thoracic duct (Fig 5). Lymphangiography demonstrated a leak at the level of the diaphragm (Fig 5). Embolization of the thoracic duct above and below the leak was performed with 2:1 ethiodized oil (Guerbet) and n-butyl cyanoacrylate (TruFill; DePuy Synthes, New Brunswick, NJ) and 3-mm Tornado coils (Cook Medical; Fig 6).
Fig 4.
After multiple unsuccessful transvenous attempts to cannulate the terminal thoracic duct (arrowheads) with various reverse curve catheters, a 21-gauge Chiba needle (arrow) was used to access a lymphatic channel under fluoroscopic guidance.
Fig 5.
Once access was obtained, an 0.018-inch Nitrex wire was navigated into the main thoracic duct (arrow).
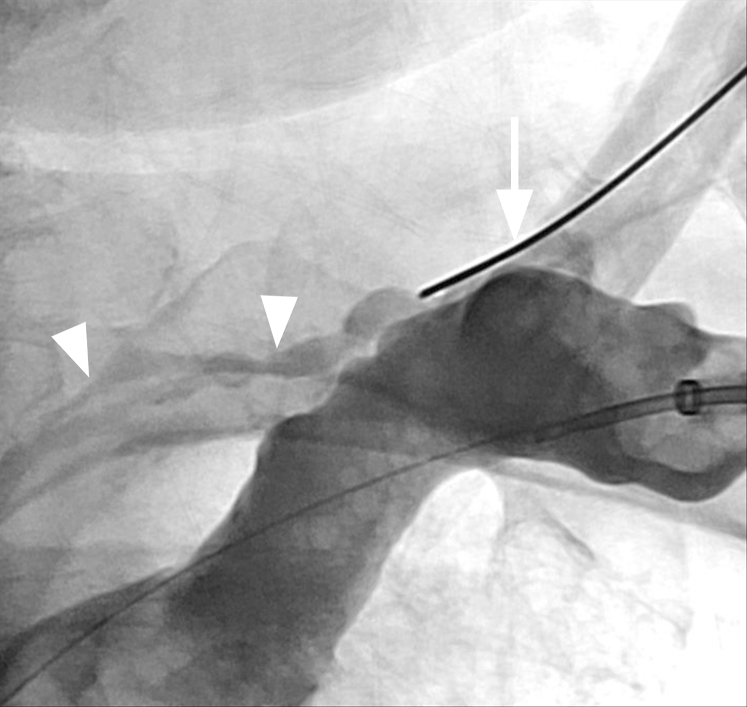
Fig 6.
Frontal fluoroscopic image showing n-butyl cyanoacrylate filling the thoracic duct. The area of disruption within the thoracic duct, likely from central venous occlusion, is seen with glue filling this segment (arrow). A wire is identified crossing the central venous occlusions (black arrowheads). A pigtail catheter is visualized in the right pleural space (white arrowhead).
Left upper extremity recanalization and reconstruction were then performed. The venous recanalization track was initially dilated with a 4-mm Mustang balloon (Boston Scientific, Marlborough, Mass). Integrity of the track was confirmed by injections of contrast material and intravascular ultrasound. Additional balloon angioplasty was performed with 8-mm and 12-mm balloons. Venous reconstruction of the left upper extremity was then performed using two overlapping 16- × 60-mm and one 10- × 90-mm Wallstents (Boston Scientific). Completion venography demonstrated brisk inline flow from the left brachial vein to the superior vena cava (Fig 7).
Fig 7.
Frontal left upper extremity venogram showing successful placement of Wallstents in the brachiocephalic and subclavian veins (arrow) with free flow of contrast material from the left arm to the superior vena cava. Stents were extended into the left axillary vein to ensure adequate inflow. Glue is seen within the thoracic duct (arrowheads).
Chest tube output ranged from 1402 to 3970 mL/d 5 days before the procedure and decreased to 65 mL 1 day after the procedure. The chest tube was removed 2 days after the procedure. Repeated chest radiography demonstrated resolution of the chylothorax. The patient remains well, without chylothorax, 136 days after the procedure.
Discussion
Thoracic duct injuries are typically iatrogenic as a result of esophagectomy or lung resection.5 This report, however, offers an alternative, unusual cause of chylothorax, namely, venous outflow obstruction. This was confirmed by pressured venography, which demonstrated brisk retrograde reflux of contrast material into the thoracic duct and identification of the leak. Traditional antegrade lymphangiography has been described; however, this failed in this patient because of the large body habitus.10 Whereas retrograde ultrasound-guided direct thoracic duct access has been described, this report suggests that perhaps pressurized transvenous balloon-occluded venography may offer an additional method of thoracic duct opacification and percutaneous targeting.12 This method may be particularly useful in cases of failed antegrade access due to body habitus or an inability to identify the thoracic duct in the neck under ultrasound guidance. Given the patient's left arm swelling and the presence of tandem venous occlusions, the left subclavian and brachiocephalic veins were also stented.
Although additional studies are needed to clarify the findings of this report, venous outflow obstruction may be a cause of thoracic duct disruption that may be treated with transcervical retrograde thoracic duct embolization or venous recanalization and reconstruction.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Maldonado F., Cartin-Ceba R., Hawkins F.J., Ryu J.H. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci. 2010;339:314–318. doi: 10.1097/MAJ.0b013e3181cdcd6c. [DOI] [PubMed] [Google Scholar]
- 2.Chen E., Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol. 2011;28:63–74. doi: 10.1055/s-0031-1273941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pamarthi V., Stecker M.S., Schenker M.P., Baum R.A., Killoran T.P., Suzuki Han A. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol. 2014;25:1398–1404. doi: 10.1016/j.jvir.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Johnson O.W., Chick J.F., Chauhan N.R., Fairchild A.H., Fan C.M., Stecker M.S. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26:2482–2493. doi: 10.1007/s00330-015-4112-6. [DOI] [PubMed] [Google Scholar]
- 5.Doerr C.H., Allen M.S., Nichols F.C., Ryu J.H. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80:867–870. doi: 10.4065/80.7.867. [DOI] [PubMed] [Google Scholar]
- 6.Toliyat M., Singh K., Sibley R.C., Chamarthy M., Kalva S.P., Pillai A.K. Interventional radiology in the management of thoracic duct injuries: anatomy, techniques and results. Clin Imaging. 2017;42:183–192. doi: 10.1016/j.clinimag.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Cope C., Kaiser L.R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13:1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 8.Binkert C.A., Yucel E.K., Davison B.D., Sugarbaker D.J., Baum R.A. Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol. 2005;16:1257–1262. doi: 10.1097/01.rvi.0000167869.36093.43. [DOI] [PubMed] [Google Scholar]
- 9.Chick J.F., Reddy S.N., Murrey D.A., Castle J.C., Gemmete J.J., Saad W.E. Single-session endolymphatic thoracic duct stent-graft placement for recurrent idiopathic chylothorax. J Vasc Interv Radiol. 2017;28:1063–1067. doi: 10.1016/j.jvir.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Nadolski G.J., Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23:613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 11.Deso S., Ludwig B., Kabutey N.K., Kim D., Guermazi A. Lymphangiography in the diagnosis and localization of various chyle leaks. Cardiovasc Intervent Radiol. 2012;35:117–126. doi: 10.1007/s00270-010-0066-x. [DOI] [PubMed] [Google Scholar]
- 12.Guevara C.J., Rialon K.L., Ramaswamy R.S., Kim S.K., Darcy M.D. US-guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: feasibility and efficacy. J Vasc Interv Radiol. 2016;27:1890–1896. doi: 10.1016/j.jvir.2016.06.030. [DOI] [PubMed] [Google Scholar]