Abstract
Multiresistant microorganism infection often can produce a life-threatening situation. We report two cases in which fecal microbiota transplantation used for the treatment of recurrent Clostridium difficile infection were effective in eradicating colonization by carbapenemase-producing Enterobacteriaceae. The presented cases illustrate the potential benefit of fecal microbiota transplantation in resolution of asymptomatic carrier states of multiresistant microorganisms, suggesting the need for further investigations with a view to their applicability in this area.
Keywords: Multiresistant microorganisms, Carbapenemase-producing enterobacteriaceae, Fecal microbiota transplantation
Introduction
Multiresistant microorganism (MRM) infection can evolve into life-threatening situations. Since colonization constitutes a risk factor for the development of infection by these agents, the attempt to resolve the carrier state can be an extremely useful intervention. We report two cases in which Fecal Microbiota Transplantation (FMT) used for the treatment of recurrent Clostridium difficile (CD) infection was effective in eradicating colonization by carbapenemase-producing Enterobacteriaceae (CPE).
Cases
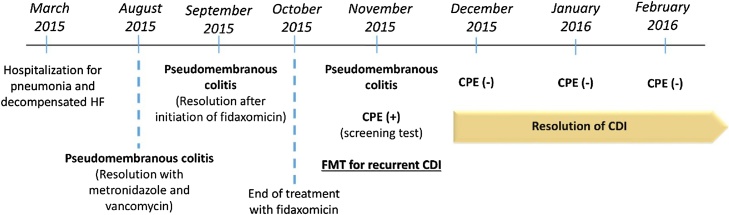
Patient 1 (Fig. 1) was a 66-year-old woman with medical history of chronic heart failure, diabetes mellitus type 2, obesity, dyslipidemia and subclinical hypothyroidism who was hospitalized for pneumonia and decompensation of her heart disease and was treated with amoxicillin/clavulanic acid. Six months later, she was readmitted for pseudomembranous colitis that resolved after therapy with metronidazole and vancomycin. About a month later, she was again admitted to hospital for septic shock with pseudomembranous colitis. In addition to the support measures, the patient was treated with fidaxomicin resulting in clinical improvement. One month after the end of treatment, she presented with a new recurrence. At this time, positivity was detected in a carrier screening test for CPE that was carried out according to the institutional protocol and performed through rectal swabs. In view of the recurrence of CD infection, the patient was considered a candidate for FMT, which occurred 9 days after being positive in the CPE screening test. After transplantation, the patient remained asymptomatic from the point of view of CD infection and negative results from the CPE screening tests were also observed in 3 consecutive samples spaced in about 1 month from each other.
Fig. 1.
Chronological clinical evolution of patient 1.
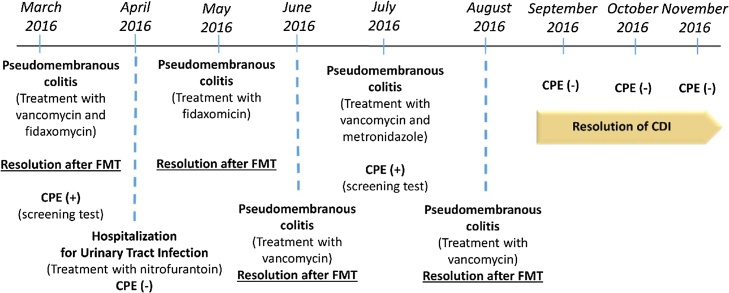
Patient 2 (Fig. 2) was a 70-year-old woman with medical history of arterial hypertension, dyslipidemia and a past frontal subarachnoid hemorrhage. She had 6 episodes of hospitalization in a period of 6 months, 5 of them due to pseudomembranous colitis and one (the second) due to an urinary tract infection. She was received a total of 4 FMT, presenting with recurrence of CD infection after the first 3 which were temporally related to the use of antimicrobial for the treatment of intercurrent urinary tract infections. In the first hospitalization episode she presented a positive screening test for CPE. Shortly after the first transplant she presented negative for the screening test, however, the CPE screening test also returned to positive on the third recurrence of CD infection. After the last transplantation, both the resolution of pseudomembranous colitis and negativity for CPE carrier screening test were verified (also in 3 consecutive samples spaced in about 1 month from each other).
Fig. 2.
Chronological clinical evolution of patient 2.
Discussion
Nowadays it is practically global knowledge that infections by MRM are a major health care problem [1]. They are often serious and life-threatening situations, especially given the low effectiveness of antimicrobial therapy in eliminating these microorganisms and the lack of therapeutic alternatives. This scenario translates into prolonged hospitalizations, significant expenses and exhaustion of the patient, family members and health professionals. Mortality from multidrug resistant gram negative bacterial infections has been revealed in some studies as high as 55% [2].
It has been demonstrated that the asymptomatic carrier state with dominant colonization by intestinal microorganisms such as extended-spectrum β lactamases (ESBL)–producing Enterobacteriaceae, vancomycin-resistant Enterococci (VRE) and CPE can proceed the colonization of other sites of the organism and, consequently, the occurrence of infection [3,4]. Therefore, the attempt to solve asymptomatic carrier status appears as a potentially beneficial intervention for the reduction of infections by this type of microorganisms. Even so, decolonization strategies are scarce and the use of selective antimicrobial therapy for these cases has not presented long-lasting results, while increasing the risk of developing resistance [5].
The use of FMT has already proven its efficacy in the treatment of recurrent or refractory CD infections [[6], [7], [8]]. In these patients, where the intestinal flora has been altered in some way (for example by antibacterial therapy), it is believed that the restoration of the healthy fecal microbiome provided by the donor, by multiple mechanisms of competition and by altering the characteristics of the environment, is capable to prevent CD replication, culminating in its intestinal elimination. It is thought that the mechanism of elimination of MRM with FMT may consist of these same mechanisms of competition [[9], [10], [11]].
Isolated cases have been described of patients who underwent FMT for treatment of CD infections and in whom negative carrier status and even resolution of MRM infections have been achieved. More recently, a small study reported 7 cases in which FMT was used to decolonize ESBL–producing Enterobacteriaceae, VRE or methicillin-resistant Staphylococcus aureus [12]. However, the FMT is not yet formally indicated or approved for the isolated purpose of eradicating MRM. The cases presented here, in which the FMT was used with the initial intention of treating CD infection, also presented as an additional effect the persistent decolonization by CPE, emphasizing the potential of this technique as a way to control MRM burden and associated morbidity and mortality. On the other hand, the fact that patient 2 was again positive for the CPE screening test after antibacterial for recurrent urinary tract infections supports the theory that the FMT mechanism of action for MRM eradication requires a healthy intestinal flora, with relapses being associated with new cycles of antimicrobial therapy.
Conclusion
The presented cases emphasize the potential benefit of FMT in resolution of asymptomatic carrier states of multiresistant microorganisms, stressing the need for further investigations with a view to their applicability in this area.
Authors’ contributions
Cátia Dias: Conceptualization; Investigation; Project administration; Writing original draft; Review and editing, Sara Pipa: Writing original draft; Review and editing, Filipa Duarte – Ribeiro: Writing original draft; Review and editing, Margarida Mota: Conceptualization; Investigation; Project administration; Writing original draft; Review and editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Cátia Dias, Email: catia.dias@chvng.min-saude.pt.
Sara Pipa, Email: sara.pipa@chvng.min-saude.pt.
Filipa Duarte-Ribeiro, Email: filipa.duarte.ribeiro@chvng.min-saude.pt.
Margarida Mota, Email: mmota@chvng.min-saude.pt.
References
- 1.Marston H.D., Dixon D.M., Knisely J.M., Palmore T.N., Fauci A.S. Antimicrobial resistance. JAMA. 2016;316:1193. doi: 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- 2.Vardakas K.Z., Rafailidis P.I., Konstantelias A.A., Falagas M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66 doi: 10.1016/j.jinf.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Taur Y., Xavier J.B., Lipuma L., Ubeda C., Goldberg J., Gobourne A. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ubeda C., Taur Y., Jenq R.R., Equinda M.J., Son T., Samstein M. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oostdijk E.A.N., de Smet A.G.A., Kesecioglu J., Bonten M.J.M. Decontamination of cephalosporin-resistant Enterobacteriaceae during selective digestive tract decontamination in intensive care units. J Antimicrob Chemother. 2012;67 doi: 10.1093/jac/dks187. 2250e3225. [DOI] [PubMed] [Google Scholar]
- 6.Gough E., Shaikh H., Manges A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 7.Kassam Z., Lee C.H., Yuan Y., Hunt R.H. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and metaanalysis. Am J Gastroenterol. 2013;108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 8.van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E.G., de Vos V.M. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 9.Nancy F., Crum-Cianflone, Sullivan E., Ballon-Landa G. Fecal microbiota transplantation and successful resolution of multidrug-resistant-organism colonization. J Clin Microbiol. 2015;53:1968–1989. doi: 10.1128/JCM.00820-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamada N., Chen G.Y., Inohara N., Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buffie C.G., Pamer E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davido B., Batista R., Michelon H., Lepainteur M., Bouchand F., Lepeule R. Is faeacal microbiota transplantation an option to eradicate highly drug-resistent enteric bactéria carriage? J Hosp Infect. 2017;95:433–437. doi: 10.1016/j.jhin.2017.02.001. [DOI] [PubMed] [Google Scholar]