ABSTRACT
Infections caused by biofilm-producing methicillin-resistant Staphylococcus aureus (MRSA) bacteria are challenging due to increasing antibiotic resistance. Synergistic activities of lipopeptides and lipoglycopeptides with β-lactams have been demonstrated for MRSA, but little is known about biofilm-embedded organisms. Our objective was to evaluate two telavancin (TLV) dosage regimens (7.5 mg/kg of body weight and 10 mg/kg every 24 h [q24h]) alone and in combination with ceftaroline (CPT) (600 mg every 8 h [q8h]) or rifampin (RIF) (450 mg every 12 h [q12h]) against two biofilm-producing MRSA strains (494 and N315). Pharmacokinetic/pharmacodynamic CDC biofilm reactor models with polyurethane coupons were used to evaluate the efficacies of the antibiotic combinations over 72 h. Overall, there were no significant differences observed between the two TLV dosing regimens either alone or in combination with RIF or CPT against these strains. Both TLV dosing regimens and CPT alone demonstrated killing but did not reach bactericidal reduction at 72 h. However, both TLV regimens in combination with RIF demonstrated enhanced activity against both strains, with a rapid decrease in CFU/ml at 4 h that was bactericidal and maintained over the 72-h experiment (−Δ3.75 log10 CFU/ml from baseline; P < 0.0001). Of interest, no enhanced activity was observed for TLV combined with CPT. No development of resistance was observed in any of the combination models. However, resistance to RIF developed as early as 24 h, with MIC values exceeding 32 mg/liter. Our results show that TLV plus RIF displayed therapeutic improvement against biofilm-producing MRSA. These results suggest that TLV at 7.5 and 10 mg/kg q24h are equally effective in eradicating biofilm-associated MRSA strains in vitro.
KEYWORDS: MRSA, antibiotic combinations, biofilms, telavancin
INTRODUCTION
Staphylococcus aureus is the leading cause of hospital-associated infections in the United States. Moreover, it is the second most common cause of health care-associated pneumonia and bloodstream infections, and methicillin-resistant S. aureus (MRSA) strains comprise up to 50% of isolates (1, 2). The prevalence of multidrug-resistant isolates of S. aureus, especially MRSA, remains persistently high, and it is recognized as a major cause of nosocomial infections, including infections of prosthetic material (3). Often, S. aureus produces biofilms, which encapsulate microorganisms and render many antimicrobials ineffective due to compromised penetration, diffusion, and the stationary-growth phase of microorganisms when present in this matrix (4, 5). These infections involving bacterial biofilms are difficult to treat and are associated with significant morbidity and cost (6, 7).
Telavancin (TLV), a new lipoglycopeptide antibiotic, was approved in 2009 for the treatment of complicated skin and skin structure infections (cSSSI) caused by susceptible Gram-positive bacteria (8). TLV is a vancomycin derivative with the same mechanism of action inhibiting cell wall synthesis. In addition, TLV also binds to lipid II in the cell membrane and causes depolarization of the cells and disruption of cell barrier function (9, 10). TLV demonstrates potent in vitro activity against various Gram-positive bacteria, including MRSA more than vancomycin (11, 12). Traditionally, vancomycin has been the mainstay of treatment for serious MRSA infections (13). However, treatment failure with vancomycin in MRSA biofilm-associated infections has been reported, highlighting the need for the development of effective novel antibiotic combinations that could be used in situations where vancomycin treatment is not successful (14, 15). Telavancin may be an intriguing option for biofilm infections, because it has been shown to have activity against bacteria under conditions of high inocula or in stationary phase due to its ability to disrupt bacterial membrane function (16, 17).
Thus far, there are limited numbers of in vitro studies published regarding the activity of telavancin against biofilm-producing MRSA strains (18, 19), and initial findings suggest a potential role for telavancin in treating infections involving indwelling medical devices. In a study performed by LaPlante and Mermel, telavancin was active against bacteria embedded in biofilm, with minimal biofilm eradication concentrations (MBECs) of 0.25 μg/ml and 1 μg/ml for two MRSA clinical isolates, an 8- to 16-fold increase in susceptibility over vancomycin (17). We have previously demonstrated that telavancin compares favorably to vancomycin against various multidrug-resistant S. aureus strains (20). Often, vancomycin-based antibiotic combinations are used to combat difficult-to-treat infections with MRSA. One of our objectives in this study was to evaluate combination options. Of these, rifampin (RIF) is one of the most frequently used adjunctive agent for biofilm-associated medical device infections, and it appears to increase efficacy (21). In time-kill studies including 40 MRSA strains with different resistance phenotypes, synergy was observed in 28 strains (70%) when rifampin was added to telavancin (22). Ceftaroline (CPT), a broad-spectrum cephalosporin with activity against MRSA, has demonstrated potent bactericidal activity against biofilm-producing MRSA strains in an in vitro study performed at our laboratory (23).
These data suggest that telavancin combined with either rifampin or ceftaroline may have greater synergistic activity than vancomycin-based combinations. Therefore, the objective of present study was to evaluate the activity of telavancin alone and in combination with either ceftaroline or rifampin against biofilm-producing MRSA strains in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model of bacterial biofilms.
RESULTS
Susceptibility testing.
The susceptibilities of the MRSA strains 494 and N315 to telavancin, ceftaroline, and rifampin, as well as the telavancin MIC in the presence of an adjunctive antimicrobial agent at 0.5× the MIC, are displayed in Table 1. Both strains were susceptible to TLV, CPT, and RIF, with MIC values of 0.0625 (for 494) 0.0156 (for N315), 1, and 0.0078 μg/ml, respectively. Biofilm MICs (MBIC) were 3 to 5 dilutions higher than the reported MICs for telavancin, 1 dilution higher for ceftaroline, and similar for rifampin.
TABLE 1.
MIC and MBIC values of each antimicrobial agent evaluated against the two MRSA strains
| Antimicrobial agent(s) | MRSA 494 |
MRSA N315 |
||
|---|---|---|---|---|
| MIC (μg/ml) | MBIC (μg/ml) | MIC (μg/ml) | MBIC (μg/ml) | |
| Telavancin | 0.0625 | 0.5 | 0.0156 | 0.5 |
| Ceftaroline | 1 | 2 | 0.5 | 1 |
| Rifampin | 0.0078 | 0.0078 | 0.0078 | 0.0078 |
| Telavancin + ceftaroline | 0.0156 | 0.5 | 0.0156 | 0.5 |
| Telavancin + rifampin | 0.0625 | 0.25 | 0.0156 | 0.125 |
In vitro PK/PD model.
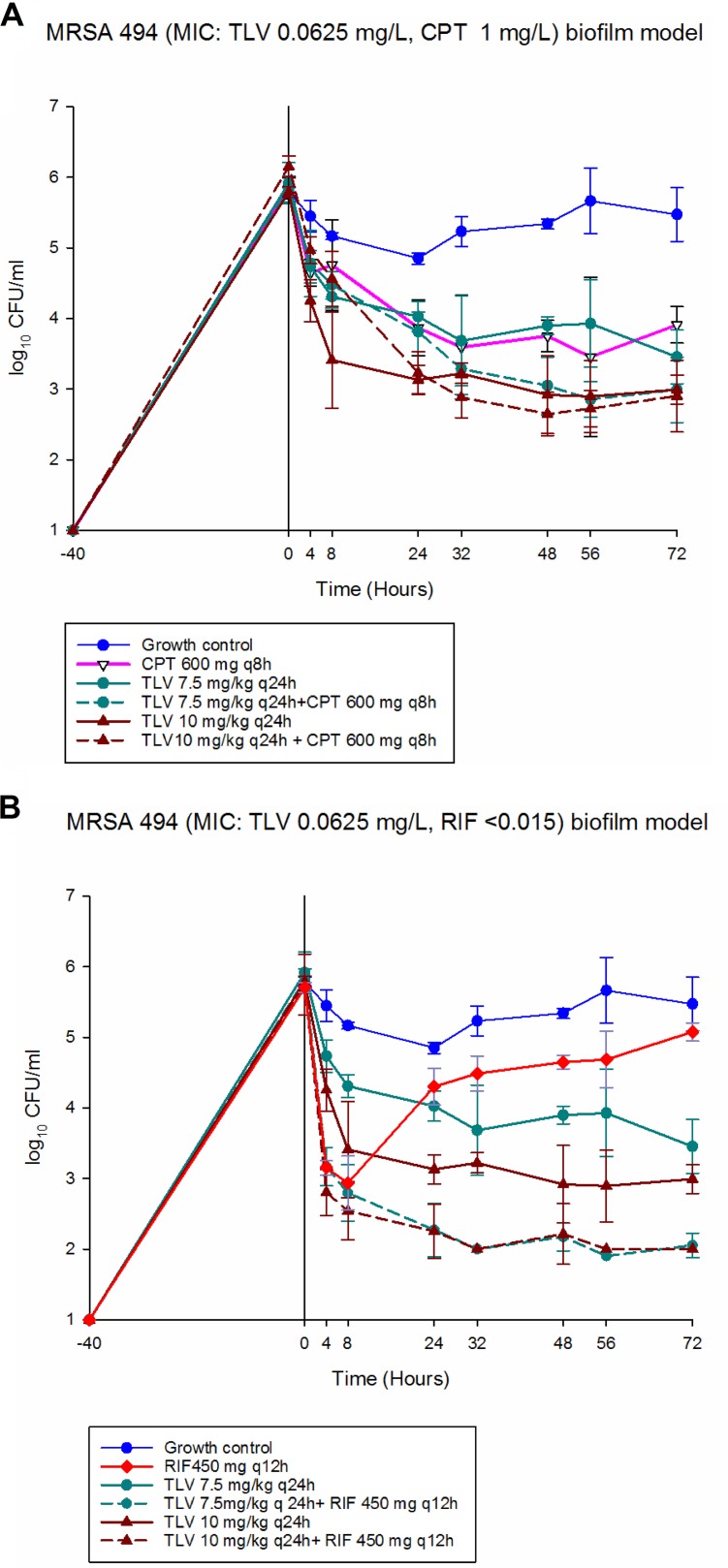
Overall, no significant differences between the two TLV dosing regimens (TLV 7.5 or 10 mg/kg of body weight every 24 h [q24h]) either alone or in combination with RIF or CPT were observed against MRSA 494 and N315 at 72 h (P > 0.05) There was no antagonism observed with TLV combined with RIF or CPT (Fig. 1 and 2). The changes in bacterial burden for the evaluated regimens against MRSA strain 494 are shown in Fig. 1. Both TLV dosing regimens alone demonstrated killing but did not reach bactericidal reduction (−Δ2.46 and 2.8 log10 CFU/ml from baseline for TLV 7.5 and TLV 10, respectively) at 72 h. The CPT alone regimen showed bacteriostatic killing (−Δ1.49 log10 CFU/ml from baseline) at 72 h. Both dosing regimens of TLV in combination with RIF showed improved activity against the strain, with a rapid decrease in CFU per milliliter at 4 h that was bactericidal and maintained over the 72-h experiment (−Δ3.75 log10 CFU/ml from baseline) (P < 0.0001). However, the addition of CPT to both dosing regimens of TLV provided no benefit and resulted in kill similar to that with TLV monotherapy (P = 0.1676 and 0.8865 for TLV 7.5 and TLV 10, respectively). The rifampin monotherapy model demonstrated activity against this strain with a rapid decrease in CFU per milliliter at 4 h, but regrowth was observed as early as 24 h.
FIG 1.
In vitro PK/PD biofilm model results for MRSA 494. (A) CPT 600 mg q6h. (B) RIF 450 mg q12h.
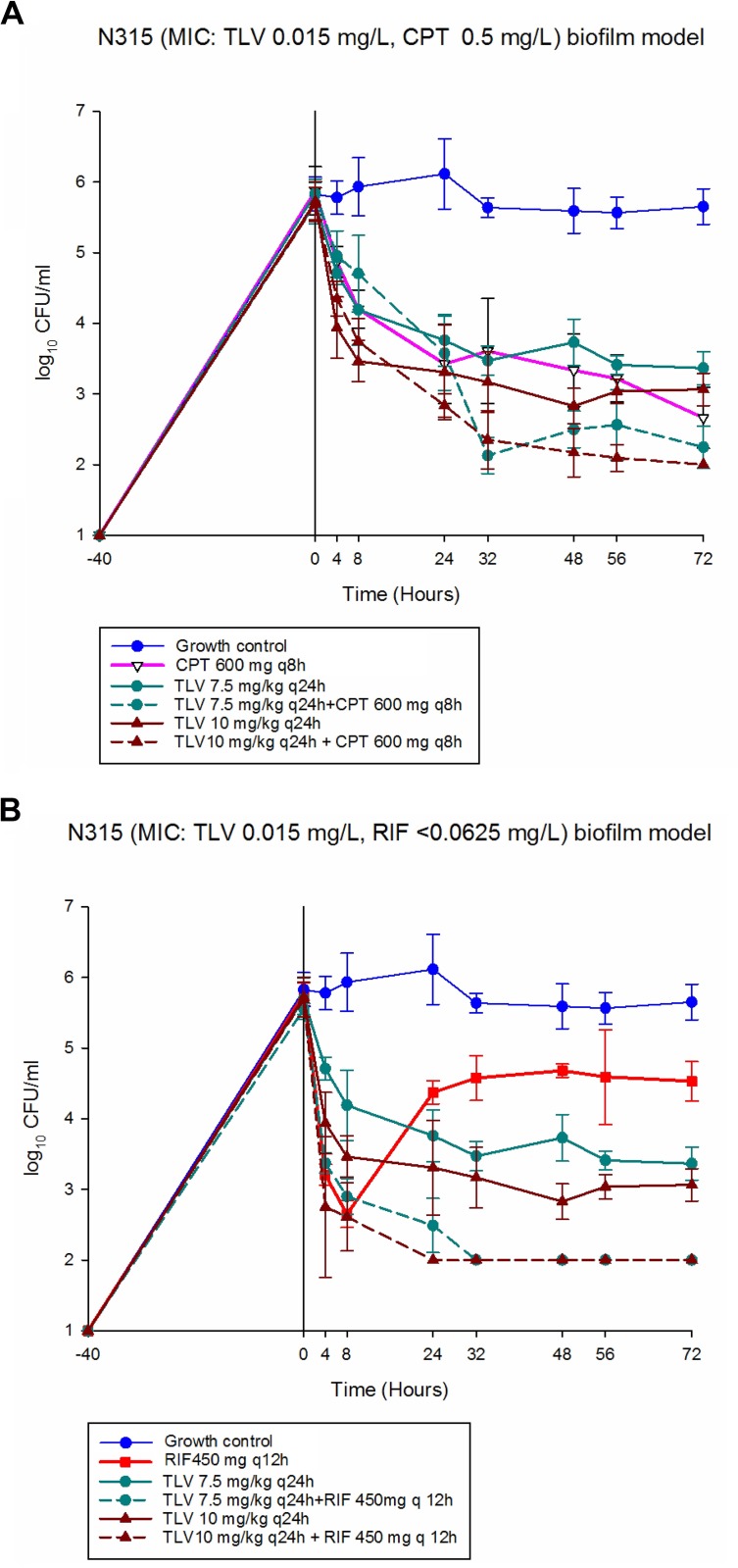
FIG 2.
In vitro PK/PD biofilm model results for MRSA N315. (A) CPT 600 mg q6h. (B) RIF 450 mg q12h.
The pharmacodynamic responses to the evaluated regimens against MRSA strain N315 are shown in Fig. 2. The CPT-only regimen demonstrated bactericidal activity (−Δ3.21 log10 CFU/ml from baseline), whereas both TLV dosing regimens showed bacteriostatic killing (−Δ2.33 and 2.66 log10 CFU/ml from baseline for TLV 7.5 and TLV 10, respectively) at 72 h. Both dosing regimens of TLV in combination with RIF were improved exhibited bactericidal activity against this strain, with a rapid decrease in CFU per milliliter at 4 h and maintained over the 72-h experiment (−Δ3.55 and −Δ3.69 log10 CFU/ml from baseline for TLV 7.5 and TLV 10, respectively; P < 0.0001). However, there was no improved activity for either dosing regimen of TLV combined with CPT that resulted in bactericidal killing similar to that with CPT monotherapy (P > 0.05). The RIF-only model showed similar activity against MRSA 494, with a rapid decrease in CFU per milliliter at 4 h; then, regrowth with an increase in the rifampin MIC (see changes in susceptibility) was observed at 24 h.
Pharmacokinetics.
The observed PK parameters are summarized in Table 2. Overall, the measured PK concentrations were nearly similar to the target values.
TABLE 2.
Pharmacokinetic parameters of antimicrobials achieved in the PK/PD model
| Antimicrobial agent (dosage) | Cmax (μg/ml) (targeted value) | t1/2 (h) (targeted value) | AUC0–24 (μg · h/ml)a |
|---|---|---|---|
| Telavancin (7.5 mg/kg/day) | 84.75 ± 1.34 (84.75) | 7.17 ± 0.71 (8.1) | 819.86 ± 85.75 |
| Telavancin (10 mg/kg/day) | 99.30 ± 3.82 (108) | 8.32 ± 0.20 (8.1) | 1,136.26 ± 39.10 |
| Ceftaroline (600 mg q8h) | 21.25 ± 0.45 (21.3) | 2.71 ± 0.04 (3) | 83.75 ± 1.7 |
| Rifampin (450 mg q12h) | 10.58 ± 0.05 (10.54) | 2.98 ± 2.84 (2.66) | 43.62 ± 1.35 |
AUC0–24, AUC from 0 to 24 h.
SEM.
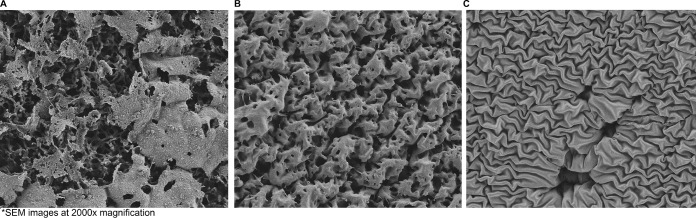
Antibiotic clearance of established biofilm in the model was visualized by scanning electron microscopy (SEM) of the coupons. Overall, visual biofilm development and embedded bacteria occur on the coupons in the model prior to the initiation of antibiotic exposure (Fig. 3A). While TLV has activity against biofilm-embedded MRSA in the model, SEM confirms that biofilm still is present on the material at the end of TLV 7.5-mg/kg q24h treatment regimen (Fig. 2B). The combination of TLV 7.5 mg/kg/day and RIF was most effective in the model, and this is confirmed by the complete clearance of bacteria and biofilm on SEM showing the underlying polyurethane coupon after 72 h of treatment (Fig. 2C).
FIG 3.
SEM images of coupon surfaces to assess the presence and structure of the matrix of a MRSA 494 biofilm. (A) Before drug exposure, showing vast biofilms. (B) After 72 h of TLV exposure, showing decreased biofilms. (C) After 72 h of TLV+RIF exposure, showing no biofilms. SEM images are shown at ×2,000 magnification.
Changes in susceptibility.
No increase in MIC (no visible growth on antibiotic resistance plates) to either telavancin or ceftaroline was detected for any strain at any of the time points during these experiments. Resistance, however, was detected for rifampin monotherapy runs as soon as 24 h in both strains, with MICs of >32 μg/ml within 72 h of experimentation.
DISCUSSION
Medical device infections are associated with over 250,000 catheter-associated bloodstream infections (CLABSI), with 25% mortality annually (24). Biofilm-associated MRSA infections pose significant challenges due to increased organism resistance and decreased antimicrobial penetration (25). The Infectious Diseases Society of America has recognized several pathogens for which novel therapies are needed, including S. aureus (26). Alternative therapeutic approaches, such as combination therapies, have been demonstrated to be synergistic for MRSA, but little is known about their activity against biofilm-embedded MRSA. The potential benefits of combination antimicrobial chemotherapy over monotherapy include decreased resistance development, synergistic antibacterial activity, and a potential broadened antibacterial spectrum (27). Treatment of MRSA infections is challenging due to limited therapeutic options and increasing resistance to glyco- and lipopeptides, especially in cases of biofilm-associated infections. Therefore, it is important to explore the potential of antibiotics used alone and in combination against organisms that are frequently associated with biofilms. In the present study, we report the in vitro activities of antimicrobials currently available, used alone or in combination, against two strains of biofilm-producing MRSA.
In this study, both TLV and CPT demonstrated activities against biofilm-embedded MRSA strains. In the case of CPT, it showed a tendency for bactericidal killing. In agreement with previous studies performed at our laboratory, CPT has demonstrated potent bactericidal activity against biofilm-producing MRSA strains in a CDC biofilm model (28) and biofilm time-kill analysis (23). In addition, we recently demonstrated in an in vitro one-compartment PK/PD model that TLV was rapidly bactericidal at 4 h and maintained its activity over 96 h against MRSA (29). Likewise, Rolston et al. reported in a time-kill study that TLV exhibited activity against clinical MRSA isolates (30).
In the present study, TLV at 7.5 mg or 10 mg q24h plus RIF had equal activity and demonstrated the greatest reduction in biofilm-embedded MRSA compared to any regimen alone or combination with CPT. These results are in good agreement with the data of Lin et al., who reported that when rifampin was added to telavancin, synergetic activity was observed in 70% of the strains by time-kill analysis (22). Indeed, telavancin inhibits bacterial cell wall synthesis by interfering with the polymerization and cross-linking of peptidoglycan, as well as by increasing the permeability of the cell membrane. Telavancin might enhance the entry of rifampin, which specifically inhibits bacterial RNA polymerase and the production of RNA (9, 31).
The relatively high rate of emergence of resistance to rifampin is consistent with previous data (1–3). The mechanism of resistance is not well understood; however, rpoB gene mutations and efflux mechanisms are thought to contribute (4). Emergence of resistance to rifampin has previously been observed when used as monotherapy in a catheter-lock model (5). However, for our study, we had some limitations that should be noted, including the low variety of tested organisms (two strains of S. aureus) and the short length of therapy (72 h), which might preclude the emergence of resistance. Likewise, we used only one type of material (polyurethane) commonly found in medical devices. While our results might simulate catheter-related biofilm-embedded organisms, different results may be obtained with other types of materials used in prostheses, such as titanium, Teflon, or steel. In addition, the conditions under which the biofilm is formed in vitro versus those in vivo may be different on the basis of the contributions of extracellular proteins, such as fibrin, or the differences that may exist under nutrition-rich or -poor conditions (32).
In conclusion, we found that combination therapy with telavancin plus rifampin was bactericidal and improved the killing of biofilm-embedded staphylococci compared to that with monotherapy against both strains tested. Additionally, no significant differences were observed using the two different treatment regimens of TLV either alone or in combination. Moreover, telavancin and ceftaroline retained susceptibility despite biofilm formation and displayed activity against both strains, including reaching bactericidal activity for ceftaroline in one strain at 72 h. While no improved activity was observed for both dosing regimens of TLV combined with CPT, these results suggest that the TLV 7.5 and 10 mg/kg q24h are equally effective in eradicating biofilm-associated MRSA bacteria in vitro. The lower dose of TLV may have clinical implications as it relates to improved safety. The addition of RIF to TLV significantly improved the activity against biofilm-embedded S. aureus. This combination may be a consideration for biofilm-associated prosthetic infections. Further experiments with TLV 7.5 and 10 mg/kg q24h in combination with RIF against S. aureus are warranted.
MATERIALS AND METHODS
Bacterial strains and culture media.
Two biofilm-producing MRSA strains, 494 and N315, were selected from the Anti-Infective Research Laboratory collection and were evaluated in this study. Both strains were susceptible to telavancin. Tryptic soy broth supplemented with 1% glucose (gSTSB) was used for the 24-h incubation phase, and 10% gSTSB was used for the 16-h conditioning phase, allowing for biofilm formation. After the conditioning phase, Mueller-Hinton broth (Difco, Detroit, MI) was used for all in vitro experiments and was supplemented with albumin to physiologic conditions (3.5 g/dl) to account for protein binding. All media contained 0.002% Tween 80 (Sigma Chemical Company, St. Louis, MO) to protect telavancin from adsorptive loss, in accordance with recent CLSI guidelines (33). Samples were plated for colony enumeration on tryptic soy agar (TSA; Difco). Brain heart infusion agar (BHIA; Difco) was used for resistance plating.
Antimicrobial agents.
Ceftaroline (Allergan, Parsippany, NJ) and telavancin powder (Theravance Biopharma, San Francisco, CA) were provided by the manufacturers. Telavancin stock solution was prepared using dimethyl sulfoxide (DMSO) as the solvent, and 0.002% Tween 80 was added, as per the CLSI guidelines (33). Rifampin was purchased commercially (Sigma Chemical Company, St. Louis, MO). All antimicrobials were prepared and stored in accordance with CLSI guidelines.
Susceptibility testing.
Susceptibility testing of all antimicrobials was performed in duplicate by broth microdilution at an inoculum of ∼1 × 106 CFU/ml, according to the CLSI guidelines (33). A telavancin stock solution was prepared using DMSO as the solvent and diluent. Tween 80 (0.002%) was added to the media. Biofilm MIC (MBIC) testing was carried out using the pin-lid method, as previously described (34). Following a determination of the MIC and MBIC values for each isolate, telavancin MICs and MBICs were determined again in the presence of rifampin or ceftaroline at 0.5% the MIC and MBIC to determine the potential for synergy, evidenced by the telavancin MIC-lowering effect of rifampin and ceftaroline (35).
In vitro PK/PD model.
Strains were inoculated into TSA plates incubated at 37°C for 24 h and then suspended in normal saline to reach a concentration equivalent to a 0.5 McFarland standard. The in vitro model consisted of a previously described CDC biofilm reactor (CBR) model (BioSurface Technologies, Bozeman, MT) that was set up with polyurethane coupons inserted into eight rods, with flow rates simulating human PK, to evaluate the in vitro activities of antimicrobials (28, 36). Briefly, a 40-h biofilm conditioning phase was performed prior to an evaluation of the antimicrobials and consisted of 24 h of incubation at 37°C of inoculated 1% gSTSB, followed by 16 h of continuous flow with a 1/10 concentration of gSTSB carried out with peristaltic pumps (Masterflex; Cole-Parmer Instrument Co., Chicago, IL, USA). After the completion of the conditioning and continuous-flow phases, Mueller-Hinton broth supplemented with albumin was utilized as the medium for the model experiments. Boluses of antimicrobials were injected into the reactor after the biofilm conditioning phase was completed. Each CBR model allowed for 8 rods with 2 polyurethane coupons each. The CBR was placed in a 37°C walk-in incubator throughout the procedure. Fresh medium was continuously supplied and removed from the model along with the drug via a peristaltic pump set to simulate the half-life of the drugs. Supplemental telavancin was added at an appropriate rate to ceftaroline or rifampin combination models to compensate for the higher flow rate required to simulate ceftaroline and rifampin clearance. The regimens evaluated were telavancin 7.5 mg/kg q24h (peak drug concentrations [Cmax], 87.5 mg/liter; t1/2, 8.1 h; protein binding, 90%), telavancin 10 mg/kg q24h (Cmax, 108 mg/liter; t1/2, 8.1 h; protein binding, 90%) (37), ceftaroline 600 mg q8h (Cmax, 21.3 mg/liter; t1/2, 2.66 h; protein binding, 20%) (38), rifampin 450 mg q12h (Cmax, 10.54 mg/liter; t1/2, 3 h; protein binding, 80%) (39), telavancin 7.5 mg/kg q24h plus ceftaroline 600 mg q8h, telavancin 7.5 mg/kg q24h plus rifampin 450 mg q12h, telavancin 10 mg/kg q24h plus ceftaroline 600 mg q8h, telavancin 10 mg/kg q24h plus rifampin 450 mg q12h, and a growth control. All model experiments were completed in duplicate to ensure reproducibility.
Pharmacodynamic analysis.
One rod from each model was aseptically removed at 0, 4, 8, 24, 32, 48, 56, and 72 h. Each coupon was washed twice in sterile normal saline to remove excess planktonic cells. Biofilm bacteria were recovered by 3 alternating 60-s cycles of vortexing and sonication at 20 Hz (Bransonic 12 Branson Ultrasonic Corporation) and a final 60 s of vortexing. Recovered biofilm cells were serially diluted in normal saline, and colony counts were determined by spiral plating appropriate dilutions using an automatic spiral plater (WASP; DW Scientific, West Yorkshire, England) on TSA to enumerate the CFU per milliliter and avoid antibiotic carryover. Plates were incubated at 37°C for 24 h, and then colonies were counted using a laser colony counter (Scan 1200; Interscience, France). The biofilm-embedded cell concentration (mean and standard deviation in CFU per milliliter) was computed for each coupon (28, 40). The limit of detection of these methods of colony count determination was 2 log10 CFU/ml. The total reduction in log10 CFU/ml over 72 h was determined by plotting time-kill curves based on the number of viable organisms over the time period. Bactericidal (99.9% kill) and bacteriostatic effects were defined as a ≥3-log10 CFU/ml reduction and a <3-log10 CFU/ml reduction in the colony count compared to the starting inoculum baseline, respectively. Enhancement and improvement of activity by the addition of a drug were defined as a ≥2-log10 CFU/ml increase and a 1- to 2-log10 CFU/ml increase in kill compared to the more active single agent of the combination, respectively (36). Combinations that resulted in a >1-log10 CFU/ml increase in bacterial growth in comparison to the lesser-active single agent were considered to represent antagonism (28).
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained through the injection port of each model at 0, 4, 8, 24, 32, 48, 56, and 72 h for verification of target antimicrobial concentrations. All samples were stored at −80°C until ready for analysis. Telavancin concentrations were measured by a validated liquid chromatography-mass spectrometry (LC/MS) assay. Rifampin and ceftaroline concentrations were determined by bioassay using Escherichia coli strain ATCC 25922 for ceftaroline PK and Kocuria rhizophila (formerly Micrococcus luteus) strain ATCC 9394 for rifampin PK (28). In brief, 0.25-in. disks were placed on preswabbed agar plates with organisms were spotted with 10 μl of the standards or samples. Each standard was tested in duplicate by placing the disk on antibiotic medium agar no. 11 plates, which were inoculated with a 0.5 McFarland suspension of the test organism. Plates were incubated at 37°C for 18 to 24 h, at which time the zone sizes were measured using an automatic colony counter (Scan 1200; Interscience, Woburn, MA). A standard curve was created using inhibition zone size versus known concentrations, and the inhibition zone size at each sample time point was plotted against this curve to obtain sample concentrations. The half-life (t1/2), area under the curve (AUC), and total peak concentration (Cmax) were determined by the trapezoidal method using PKAnalyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
SEM.
Coupons from rods recovered at 0 and 72 h were evaluated for the presence and structure of biofilm by SEM. After removal, coupons were rinsed in normal saline to remove nonadherent cells and immersed in a fixative solution containing 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer. Coupons were then dehydrated in a graded ethanol series and carbon coated at 30 A for 3 min utilizing a SeeVac Conductavac IV sputter coater (SeeVac, Inc., Pittsburgh, PA, USA). The coupons were imaged using a Hitachi S570 SEM at ×2,000 magnification and evaluated visually for the presence and characteristics of biofilm. All experiments had 3 to 5 coupon SEM images taken. In addition, uniform representation of the high-magnification image was ensured, with a broader view of the coupon at 10-fold lower magnification than the publication quality images provided.
Emergence of resistance.
The development of resistance was determined at 72 h. Samples of 100 μl were plated on BHI containing 3× the MIC of the respective antibiotic to assess the development of resistance. Plates were then examined for growth after 48 h of incubation at 37°C. MIC testing by broth microdilution following CLSI guidelines was performed on any isolate observed to grow on drug-containing agar plates used for resistance screening during model experiments. Similarly, biofilm MIC was performed to evaluate any changes in biofilm MIC (MBIC).
Statistical analysis.
Changes in log10 CFU per milliliter were evaluated for each regimen by analysis of variance with Tukey's post hoc test at 24, 32, 48, and 72 h. A P value of ≤0.05 was considered significant. All statistical analyses will be performed using SPSS Statistical Software (release 22.0; SPSS, Inc., Chicago, IL).
REFERENCES
- 1.Loffler CA, Macdougall C. 2007. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther 5:961–981. doi: 10.1586/14787210.5.6.961. [DOI] [PubMed] [Google Scholar]
- 2.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities . 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 3.Boyce JM, Cookson B, Christiansen K, Hori S, Vuopio-Varkila J, Kocagoz S, Oztop AY, Vandenbroucke-Grauls CM, Harbarth S, Pittet D. 2005. Meticillin-resistant Staphylococcus aureus. Lancet Infect Dis 5:653–663. doi: 10.1016/S1473-3099(05)70243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 6.Barberán J. 2006. Management of infections of osteoarticular prosthesis. Clin Microbiol Infect 12(Suppl 3):S93–S101. doi: 10.1111/j.1469-0691.2006.01400.x. [DOI] [PubMed] [Google Scholar]
- 7.Trampuz A, Widmer AF. 2006. Infections associated with orthopedic implants. Curr Opin Infect Dis 19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 8.Hughes B. 2010. 2009 FDA drug approvals. Nat Rev Drug Discov 9:89–92. doi: 10.1038/nrd3101. [DOI] [PubMed] [Google Scholar]
- 9.Saravolatz LD, Stein GE, Johnson LB. 2009. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 49:1908–1914. doi: 10.1086/648438. [DOI] [PubMed] [Google Scholar]
- 10.Attwood RJ, LaPlante KL. 2007. Telavancin: a novel lipoglycopeptide antimicrobial agent. Am J Health Syst Pharm 64:2335–2348. doi: 10.2146/ajhp070080. [DOI] [PubMed] [Google Scholar]
- 11.Draghi DC, Benton BM, Krause KM, Thornsberry C, Pillar C, Sahm DF. 2008. Comparative surveillance study of telavancin activity against recently collected Gram-positive clinical isolates from across the United States. Antimicrob Agents Chemother 52:2383–2388. doi: 10.1128/AAC.01641-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King A, Phillips I, Kaniga K. 2004. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration-dependent anti-infective with multiple mechanisms of action against Gram-positive bacteria. J Antimicrob Chemother 53:797–803. doi: 10.1093/jac/dkh156. [DOI] [PubMed] [Google Scholar]
- 13.Holland TL, Fowler VG Jr. 2011. Vancomycin minimum inhibitory concentration and outcome in patients with Staphylococcus aureus bacteremia: pearl or pellet? J Infect Dis 204:329–331. doi: 10.1093/infdis/jir275. [DOI] [PubMed] [Google Scholar]
- 14.Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. 2011. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 66:2386–2392. doi: 10.1093/jac/dkr301. [DOI] [PubMed] [Google Scholar]
- 15.Dombrowski JC, Winston LG. 2008. Clinical failures of appropriately-treated methicillin-resistant Staphylococcus aureus infections. J Infect 57:110–115. doi: 10.1016/j.jinf.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard SN, Szeto YG, Zolotarev M, Grigoryan IV. 2011. Comparative in vitro activity of telavancin, vancomycin and linezolid against heterogeneously vancomycin-intermediate Staphylococcus aureus (hVISA). Int J Antimicrob Agents 37:558–561. doi: 10.1016/j.ijantimicag.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 17.LaPlante KL, Mermel LA. 2009. In vitro activities of telavancin and vancomycin against biofilm-producing Staphylococcus aureus, S. epidermidis, and Enterococcus faecalis strains. Antimicrob Agents Chemother 53:3166–3169. doi: 10.1128/AAC.01642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K, Gemmell CG, Lang S. 2013. Telavancin shows superior activity to vancomycin with multidrug-resistant Staphylococcus aureus in a range of in vitro biofilm models. Eur J Clin Microbiol Infect Dis 32:1327–1332. doi: 10.1007/s10096-013-1883-z. [DOI] [PubMed] [Google Scholar]
- 19.Kirker KR, Fisher ST, James GA. 2015. Potency and penetration of telavancin in staphylococcal biofilms. Int J Antimicrob Agents 46:451–455. doi: 10.1016/j.ijantimicag.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Steed ME, Vidaillac C, Rybak MJ. 2012. Evaluation of telavancin activity versus daptomycin and vancomycin against daptomycin-nonsusceptible Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 56:955–959. doi: 10.1128/AAC.05849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. 2008. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 168:805–819. doi: 10.1001/archinte.168.8.805. [DOI] [PubMed] [Google Scholar]
- 22.Lin G, Pankuch GA, Ednie LM, Appelbaum PC. 2010. Antistaphylococcal activities of telavancin tested alone and in combination by time-kill assay. Antimicrob Agents Chemother 54:2201–2205. doi: 10.1128/AAC.01143-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber KE, Werth BJ, McRoberts JP, Rybak MJ. 2014. A novel approach utilizing biofilm time-kill curves to assess the bactericidal activity of ceftaroline combinations against biofilm-producing methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 58:2989–2992. doi: 10.1128/AAC.02764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. 2014. Catheter-related bloodstream infections. Int J Crit Illn Inj Sci 4:162–167. doi: 10.4103/2229-5151.134184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Ray P, Das A, Sharma M. 2010. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 26.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 27.Eliopoulos GM, Eliopoulos CT. 1988. Antibiotic combinations: should they be tested? Clin Microbiol Rev 1:139–156. doi: 10.1128/CMR.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber KE, Smith JR, Ireland CE, Boles BR, Rose WE, Rybak MJ. 2015. Evaluation of ceftaroline alone and in combination against biofilm-producing methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin and vancomycin in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 59:4497–4503. doi: 10.1128/AAC.00386-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yim J, Smith JR, Barber KE, Hallesy JA, Rybak MJ. 2016. Evaluation of pharmacodynamic interactions between telavancin and aztreonam or piperacillin/tazobactam against Pseudomonas aeruginosa, Escherichia coli and methicillin-resistant Staphylococcus aureus. Infect Dis Ther 5:367–377. doi: 10.1007/s40121-016-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolston KV, Wang W, Nesher L, Smith JR, Rybak MJ, Prince RA. 2017. Time-kill determination of the bactericidal activity of telavancin and vancomycin against clinical methicillin-resistant Staphylococcus aureus isolates from cancer patients. Diagn Microbiol Infect Dis 87:338–342. doi: 10.1016/j.diagmicrobio.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Wehrli W. 1983. Rifampin: mechanisms of action and resistance. Rev Infect Dis 5(Suppl 3):S407–S411. doi: 10.1093/clinids/5.Supplement_3.S407. [DOI] [PubMed] [Google Scholar]
- 32.Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E. 2015. An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device-related infections. J Infect Dis 212:1883–1893. doi: 10.1093/infdis/jiv319. [DOI] [PubMed] [Google Scholar]
- 33.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 34.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yim J, Smith JR, Singh NB, Rice S, Stamper K, Garcia de la Maria C, Bayer AS, Mishra NN, Miro JM, Tran TT, Arias CA, Sullam P, Rybak MJ. 2017. Evaluation of daptomycin combinations with cephalosporins or gentamicin against Streptococcus mitis group strains in an in vitro model of simulated endocardial vegetations (SEVs). J Antimicrob Chemother 72:2290–2296. doi: 10.1093/jac/dkx130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parra-Ruiz J, Vidaillac C, Rose WE, Rybak MJ. 2010. Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob Agents Chemother 54:4329–4334. doi: 10.1128/AAC.00455-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong SL, Barriere SL, Kitt MM, Goldberg MR. 2008. Multiple-dose pharmacokinetics of intravenous telavancin in healthy male and female subjects. J Antimicrob Chemother 62:780–783. doi: 10.1093/jac/dkn273. [DOI] [PubMed] [Google Scholar]
- 38.Werth BJ, Barber KE, Ireland CE, Rybak MJ. 2014. Evaluation of ceftaroline, vancomycin, daptomycin, or ceftaroline plus daptomycin against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob Agents Chemother 58:3177–3181. doi: 10.1128/AAC.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peloquin CA, Namdar R, Singleton MD, Nix DE. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12–18. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 40.Hall Snyder AD, Vidaillac C, Rose W, McRoberts JP, Rybak MJ. 2014. Evaluation of high-dose daptomycin versus vancomycin alone or combined with clarithromycin or rifampin against Staphylococcus aureus and S. epidermidis in a novel in vitro PK/PD model of bacterial biofilm. Infect Dis Ther 4:51–65. doi: 10.1007/s40121-014-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]