The pharmacokinetics (PK), safety, and tolerability of two repeated dosing regimens of oral fosfomycin tromethamine were evaluated in 18 healthy adult subjects. Subjects received 3 g every other day (QOD) for 3 doses and then every day (QD) for 7 doses, or vice versa, in a phase I, randomized, open-label, two-period-crossover study.
KEYWORDS: fosfomycin, pharmacokinetics, safety, tolerability, antimicrobial safety
ABSTRACT
The pharmacokinetics (PK), safety, and tolerability of two repeated dosing regimens of oral fosfomycin tromethamine were evaluated in 18 healthy adult subjects. Subjects received 3 g every other day (QOD) for 3 doses and then every day (QD) for 7 doses, or vice versa, in a phase I, randomized, open-label, two-period-crossover study. Serial blood (n = 11) and urine (n = 4 collection intervals) samples were collected before and up to 24 h after dosing on days 1 and 5, along with predose concentrations on days 3 and 7. PK parameters were similar between days 1 and 5 within and between dosing regimens. The mean (± standard deviation [SD]) PK parameters for fosfomycin in plasma on day 5 during the respective QOD and QD dosing regimens were as follows: maximum concentration of drug in serum (Cmax) = 24.4 ± 6.2 versus 23.8 ± 5.6 μg/ml, time to Cmax (Tmax) = 2.2 ± 0.7 versus 2.0 ± 0.4 h, apparent volume of distribution (V/F) = 141 ± 67.9 versus 147 ± 67.6 liters, apparent clearance (CL/F) = 21.4 ± 8.0 versus 20.4 ± 5.3 liters/h, renal clearance (CLR) = 7.5 ± 4.1 versus 7.3 ± 3.5 liters/h, area under the concentration-time curve from 0 to 24 h (AUC0–24) = 151.6 ± 35.6 versus 156.6 ± 42.5 μg · h/ml, and elimination half-life (t1/2) = 4.5 ± 1.1 versus 5.0 ± 1.7 h. Urine concentrations peaked at approximately 600 μg/ml through the 0- to 8-h urine collection intervals but displayed significant interindividual variability. Roughly 35 to 40% of the 3-g dose was excreted in the urine by 24 h postdose. No new safety concerns were identified during this study. The proportion of diarrhea-free days during the study was significantly lower with the QD regimen than with the QOD regimen (61% versus 77%; P < 0.0001). Further studies to establish the clinical benefit/risk ratio for repeated dosing regimens of oral fosfomycin tromethamine are warranted. (This trial is registered at ClinicalTrials.gov under registration no. NCT02570074.)
INTRODUCTION
Oral fosfomycin tromethamine is currently approved by the U.S. Food and Drug Administration (FDA) as a one-time 3-g dose for women for the treatment of uncomplicated urinary tract infections due to susceptible strains of Escherichia coli and Enterococcus faecalis (1) and is approved in Europe for adults for the treatment of acute uncomplicated lower urinary tract infections caused by susceptible strains of Enterobacteriaceae (2). It is also a recommended first-line treatment for acute uncomplicated cystitis in women by the international clinical practice guidelines published by the Infectious Diseases Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases (ESCMID) (3). Despite these recommendations, fosfomycin's broad in vitro activity against clinically significant multidrug-resistant (MDR) pathogens, lack of cross-resistance and cross-allergy sensitivity, and minimal propensity for collateral damage have made it an attractive option for expanded use in the era of increasing bacterial resistance (4). This expanded use includes more aggressive, off-label dosing schemes ranging from 3 g every other day (QOD) for 5 to 10 days to 3 g daily (QD) for weeks to months (5–8). Despite the frequent use of these off-label dosing regimens in clinical practice, they are not supported by modern robust pharmacokinetic (PK) or safety data. The vast majority of data regarding the PK of fosfomycin were generated in the 1970s and 1980s, prior to advanced sampling and bioanalytical techniques and the recognized need to supplement microbiological assays with glucose-6-phosphate (G6P) (9). Many previous studies also used the calcium salt of fosfomycin, which is known to have significantly decreased bioavailability compared to that of the tromethamine salt (10–13). As such, there is an urgent need to establish reliable PK and safety data to inform the clinical use and future research investigations of oral fosfomycin tromethamine. Given the dearth of effective treatment options in the current landscape of MDR bacteria, it is crucial to fully understand the PK of antibacterial agents in order to assess the PK/pharmacodynamic (PK/PD) parameters associated with efficacy.
The purpose of this study was to determine the PK, safety, and tolerability of two dosing regimens of oral fosfomycin tromethamine in a randomized, two-period-crossover study in healthy subjects.
(This work was presented in part at the 2017 IDWeek meeting in San Diego, CA, USA.)
RESULTS
A total of 19 healthy adult subjects were enrolled in the study. Of the 19 subjects enrolled, 18 received both dosing regimens, while 1 subject completed only the QOD regimen due to scheduling conflicts. This subject was included in safety analyses but excluded from PK analyses. The baseline demographics of the pharmacokinetically evaluable subjects are presented in Table 1. Overall the subjects were young, and the majority were white (non-Hispanic or -Latino), with an equal distribution of males and females. No significant difference between the mean (± standard deviation [SD]) Cockroft-Gault estimated creatinine clearance (eCLCR) and measured 24-h creatinine clearance (mCLCR) was observed (110 ± 19.9 ml/min versus 109 ± 30 ml/min; P = 0.892).
TABLE 1.
Characteristics of healthy adult subjects receiving oral fosfomycin tromethamine
| Treatment (n) | No. (%) of male subjects | No. (%) of white subjects | Mean ± SD |
||||
|---|---|---|---|---|---|---|---|
| Age (yr) | Ht (cm) | Wt (kg) | Body mass index (kg/m2) | eCLCRa (ml/min) | |||
| Oral fosfomycin (18) | 9 (50) | 13 (72) | 28 ± 7 | 173.2 ± 9.9 | 75.4 ± 11.5 | 24.9 ± 2.5 | 110 ± 19.9 |
eCLCR, Cockroft-Gault estimated creatinine clearance.
Pharmacokinetics of QOD fosfomycin dosing.
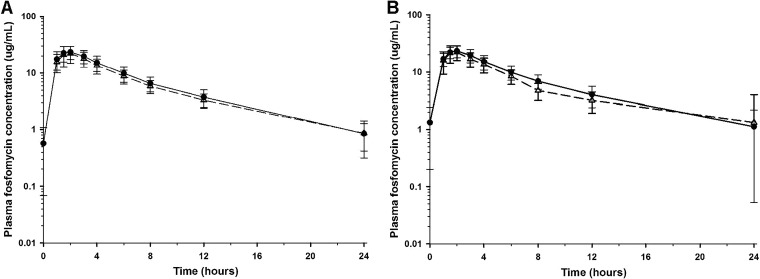
Mean (± SD) plasma concentrations of fosfomycin on study day 1, after a single 3-g dose, are displayed in Fig. 1A. The mean (± SD) plasma PK parameters of fosfomycin on study day 1 are summarized in Table 2. After oral administration of 3 g, all 18 subjects had quantifiable plasma concentrations within 1 h after ingestion and at 24 h postdose. Eight subjects (44%) also had measurable plasma fosfomycin concentrations at 48 h postdose. Mean (± SD) plasma concentrations of fosfomycin on study day 5, after three 3-g doses, are displayed in Fig. 1B. The mean (± SD) plasma PK parameters of fosfomycin on study day 5 are summarized in Table 2. Again, all subjects had quantifiable concentrations 1 h after ingestion and at 24 h postdose. Five subjects (28%) had measurable plasma concentrations at 48 h postdose. Table 3 compares the analysis of variance (ANOVA)-generated means and 95% confidence intervals (CI) for the primary PK parameters between study days and dosing regimens. Plasma PK parameters were similar between study days 1 and 5 after QOD dosing. Mean (± SD) predose plasma concentrations on days 3 and 7 were 0.2 ± 0.3 and 0.1 ± 0.2 μg/ml, respectively.
FIG 1.
Mean (± SD) concentration-versus-time profiles of fosfomycin in plasma after oral administration of 3 g oral fosfomycin tromethamine on study day 1 (A) and study day 5 (B). The QOD dosing regimen data are illustrated by open triangles and a dashed line, and the QD regimen data are illustrated by filled circles and a solid line. The y axis is on a log scale.
TABLE 2.
Pharmacokinetic parameters of fosfomycin in plasma on study days 1 and 5 after two oral dosing regimens
| Study day and dosing regimena (n) | Mean ± SD |
|||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUCb (μg · h/ml) | V/F (liters) | CL/F (liters/h) | t1/2 (h) | |
| Day 1 | ||||||
| QOD (18) | 23.8 ± 7.5 | 2.0 ± 0.5 | 148.8 ± 35.4 | 172 ± 70.5 | 21.6 ± 6.8 | 5.6 ± 1.5 |
| QD (18) | 23.5 ± 6.6 | 2.1 ± 0.6 | 149.8 ± 67.3 | 138.6 ± 57.4 | 22.2 ± 5.9 | 4.4 ± 1.3 |
| Day 5 | ||||||
| QOD (18) | 24.4 ± 6.2 | 2.2 ± 0.7 | 151.6 ± 35.6 | 141 ± 67.9 | 21.4 ± 8.0 | 4.5 ± 1.1 |
| QD (18) | 23.8 ± 5.6 | 2.0 ± 0.4 | 156.6 ± 42.5 | 147 ± 67.6 | 20.4 ± 5.3 | 5.0 ± 1.7 |
QOD, every other day; QD, every day.
AUC0–∞ on study day 1 and AUC0–24 on study day 5.
TABLE 3.
Comparison of primary pharmacokinetic parameters of fosfomycin between dosing regimens and study days after two oral dosing regimensa
| Study day and dosing regimen (n) | Geometric mean (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | V/F (liters) | CL/Fb (liters/h) | t1/2 (h) | AUCc (μg · h/ml) | Cum Ae (mg) | CLR (liters/h) | |
| Day 1 | |||||||
| QOD (18) | 24 (20–28.8) | 172.3 (142.5–208.3) | 21.6 (20–23.2) | 5.6 (4.8–6.5) | 149.5 (130.6–171.2) | 1,063 (745.5–1,515.9) | 7.2 (5.5–9.3) |
| QD (18) | 23.6 (20.3–27.4) | 144.1 (119.8–173.3) | 22 (20.4–23.5) | 5.4 (4–7.3) | 148.64 (125.9–175.5) | 1,082.4 (832.5–1,407.3) | 7.9 (6.3–10) |
| Day 5 | |||||||
| QOD (18) | 24.7 (20.9–29.1) | 140.2 (114–172.5) | 21.4 (19.8–23) | 4.5 (4–5.2) | 152.8 (132.1–176.7) | 1,209.5 (820.4–1,783.3) | 7.7 (5.6–10.6) |
| QD (18) | 23.8 (20.9–27.2) | 146.7 (118.1–182.2) | 20.4 (18.9–22) | 5 (4.2–6) | 156.9 (137.4–179.1) | 1,159.3 (888.8–1,512.1) | 7.3 (6–8.9) |
QOD, every other day; QD, every day; CI, confidence interval.
CL/F data are arithmetic means.
AUC0–∞ on study day 1 and AUC0–24 on study day 5.
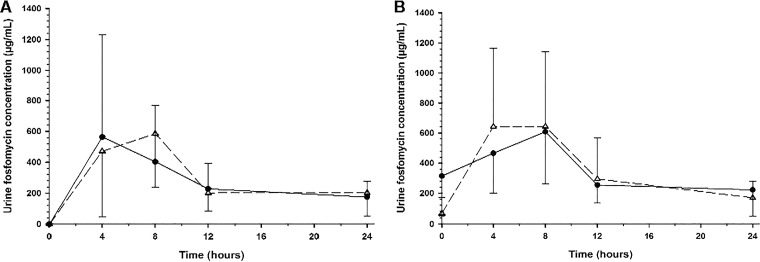
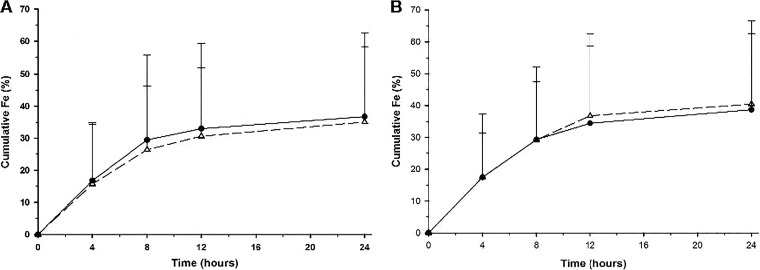
Mean (± SD) urine concentrations of fosfomycin on study day 1, after a single 3-g dose, are displayed in Fig. 2A and Table 4. Peak urinary concentrations of fosfomycin occurred through the first 8 h of urine collection (Fig. 2A), and 15 subjects (83%) had measurable urine concentrations at 48 h postdose. Approximately 35% of the 3-g dose was excreted by 24 h postdose (Fig. 3A), and the mean renal clearance (7.1 ± 3.6 liters/h) approximated that for normal glomerular filtration (Table 4). Mean (± SD) urine concentrations of fosfomycin on study day 5, after three 3-g doses, are displayed in Fig. 2B and Table 4. Urine concentrations peaked through the 8-h collection interval, and 11 subjects (61%) had measurable urine concentrations at 48 h postdose. Approximately 40% of the 3-g dose was excreted by 24 h postdose (Fig. 3B), and the mean renal clearance (7.5 ± 4.1 liters/h) approximated that for normal glomerular filtration (Table 4). Urinary excretion levels were similar between study days 1 and 5 (Table 3) after QOD dosing. Mean (± SD) predose urine concentrations on days 3 and 7 were 45.2 ± 84.2 and 20.3 ± 32.9 μg/ml, respectively (Table 4).
FIG 2.
Mean (± SD) concentration-versus-time profiles of fosfomycin in urine after oral administration of 3 g oral fosfomycin tromethamine on study day 1 (A) and study day 5 (B). The QOD dosing regimen data are illustrated by open triangles and a dashed line, and the QD regimen data are illustrated by filled circles and a solid line.
TABLE 4.
Urine concentrations, cumulative amounts excreted, and renal clearance of fosfomycina
| Dosing regimen (n) | Mean ± SD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Study day 1 |
Study day 3 |
Study day 5 |
Study day 7 |
|||||
| Avg urine concn over 24 h (μg/ml) | Cum Ae (mg) | CLR (liters/h) | Predose concn (μg/ml) | Avg urine concn over 24 h (μg/ml) | Cum Ae (mg) | CLR (liters/h) | Predose concn (μg/ml) | |
| QOD (18) | 361.7 ± 254.2 | 1,047.1 ± 710.5 | 7.1 ± 3.6 | 45.2 ± 84.2 | 434.6 ± 343.4 | 1,177.2 ± 790.8 | 7.5 ± 4.1 | 20.3 ± 32.9 |
| QD (18) | 342.4 ± 324.7 | 1,102.3 ± 772.7 | 8.1 ± 5.6 | 312.7 ± 263.3 | 387.9 ± 224.8 | 1,161.6 ± 718.1 | 7.3 ± 3.5 | 231.0 ± 227.6 |
QOD, every other day; QD, every day; Cum Ae, cumulative amount of fosfomycin excreted into urine over a 24-h collection period; CLR, renal clearance of fosfomycin.
FIG 3.
Mean (± SD) cumulative fractions (percentages) of the fosfomycin dose excreted in urine over time following oral administration of 3 g oral fosfomycin tromethamine on study day 1 (A) and study day 5 (B). The QOD dosing regimen data are illustrated by open triangles and a dashed line, and the QD regimen data are illustrated by filled circles and a solid line.
Pharmacokinetics of QD fosfomycin dosing.
Mean (± SD) plasma concentrations of fosfomycin on study day 1, after a single 3-g dose, are displayed in Fig. 1A. The mean (± SD) plasma PK parameters of fosfomycin on study day 1 are summarized in Table 2. After oral administration of 3 g, all 18 subjects had quantifiable plasma concentrations within 1 h after ingestion and at 24 h postdose. Mean (± SD) plasma concentrations of fosfomycin on study day 5, after five 3-g doses, are displayed in Fig. 1B. The mean (± SD) plasma PK parameters of fosfomycin on study day 5 are summarized in Table 2. Again, all subjects had quantifiable concentrations 1 h after ingestion and at 24 h postdose. Table 3 compares the ANOVA-generated means and 95% confidence intervals for the primary PK parameters between study days and dosing regimens. Plasma PK parameters were similar between study days 1 and 5 after QD dosing. Mean (± SD) predose plasma concentrations on days 3 and 7 were 1.3 ± 1.1 and 1.3 ± 1.1 μg/ml, respectively.
Mean (± SD) urine concentrations of fosfomycin on study day 1, after a single 3-g dose, are displayed in Fig. 2A and Table 4. Peak urinary concentrations of fosfomycin occurred through the first 8 h of urine collection, approximately 37% of the 3-g dose was excreted by 24 h postdose (Fig. 3A), and the mean renal clearance (8.1 ± 5.6 liters/h) approximated that for normal glomerular filtration (Table 4). Mean (± SD) urine concentrations of fosfomycin on study day 5, after five 3-g doses, are displayed in Fig. 2B and Table 4. Urine concentrations peaked through the 8-h collection interval, approximately 39% of the 3-g dose was excreted by 24 h postdose (Fig. 3B), and the mean renal clearance (7.3 ± 3.5 liters/h) approximated that for normal glomerular filtration (Table 4). Urinary excretion levels were similar between study days 1 and 5 (Table 3) after QD dosing. Mean (± SD) predose urine concentrations on days 3 and 7 were 312.7 ± 263.3 and 231.0 ± 227.6 μg/ml, respectively (Table 4).
Safety and tolerability.
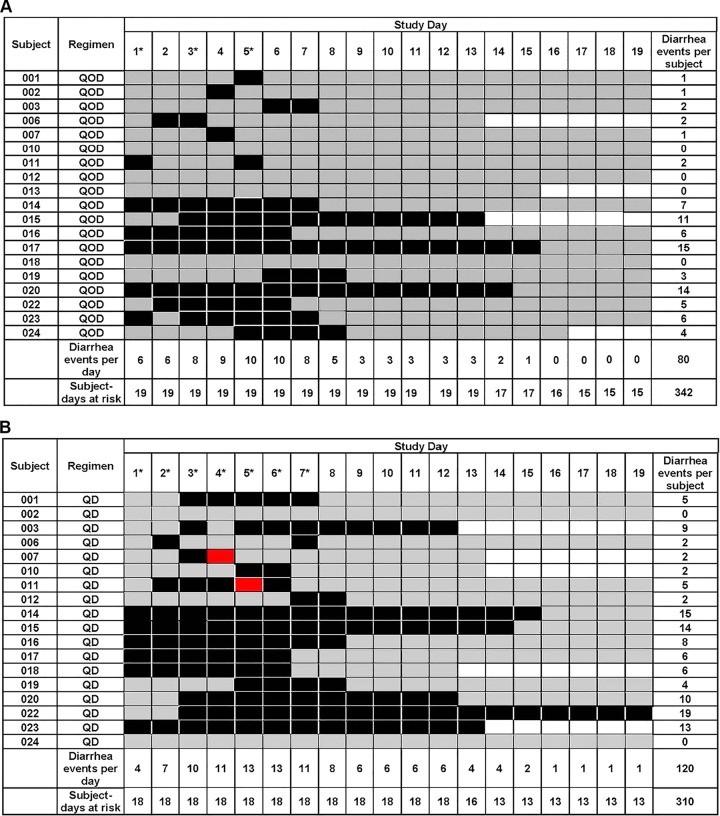
Treatment-emergent adverse events (TEAEs) were reported by 17 (89%) subjects during days 1 to 9 of the QOD regimen. The majority of TEAEs (13 subjects) were gastrointestinal disorders which were considered related to study drug, followed by skin and subcutaneous tissue disorders (3 subjects) which were considered not related to study drug. There was one grade 3 adverse event, namely, a laboratory abnormality of increased aspartate aminotransferase (AST) that was asymptomatic, resolved without intervention or sequelae, and was considered possibly related to study drug. During days 1 to 9 of the QD regimen, 16 (89%) subjects reported a TEAE. The majority of these were gastrointestinal disorders (14 subjects) and were considered related to study drug, followed by nervous system disorders (3 subjects) which were not considered related to study drug. There were two grade 2 adverse events of diarrhea (related to study drug) and one of skin and subcutaneous tissue disorder (not related). There was one grade 3 serious adverse event of Clostridium difficile-associated diarrhea that occurred approximately 14 days after the last dose of study drug in the QD regimen and was considered related to study drug administration. Overall, grade 1 diarrhea (an increase of up to 3 stools per day above baseline) represented 57% (52/91 events) of all reported TEAEs for both dosing regimens. Figure 4 displays the number of days of diarrhea experienced by each subject during days 1 to 19 for the QOD and QD regimens. The proportion of diarrhea-free days was significantly lower with the QD regimen than with the QOD regimen (190/310 days [0.61] versus 262/342 days [0.77]; P < 0.0001). No subjects were withdrawn from the study due to TEAEs, and all TEAEs were considered resolved by the end of the study follow-up period.
FIG 4.
Days of diarrhea per subject and study day during the QOD regimen (A) and the QD regimen (B). Cases of CTCAE grade 1 diarrhea are shaded in black, and cases of grade 2 diarrhea are shown in red. Gray shaded days indicate that no diarrhea events occurred during these at-risk days. Unshaded days represent days beyond a participant's follow-up period. Asterisks represent days of study drug administration.
DISCUSSION
This phase I, randomized, open-label, two-period-crossover, multiple-dose study evaluated the PK and safety of fosfomycin in plasma and urine after repeated oral administration in healthy subjects. The plasma PK of fosfomycin were comparable between days 1 and 5 of each regimen. The plasma PK between the QOD and QD dosing regimens were also similar, and daily dosing did not lead to increased systemic exposure at steady state (Table 3). Urine concentrations of fosfomycin were also comparable between study days 1 and 5 and between the QOD and QD regimens, although predose urine concentrations on days 3 and 7 were significantly higher with the QD dosing regimen. No new safety concerns were identified during this study. Diarrhea was the most commonly reported adverse event, consistent with other studies of healthy subjects and of the clinical use of repeated doses of fosfomycin in patients (1, 5, 7, 8). The incidence of diarrhea in this study was higher than that reported in a recently completed phase I study of fosfomycin in healthy subjects (14); however, only a single oral dose of fosfomycin tromethamine was administered in that study. Figure 4 shows that the number of subjects experiencing diarrhea in the current study increased on or after day 3, once they had received multiple repeated doses of fosfomycin.
The plasma PK parameters observed in this study compare well to those in previous studies despite differences in study populations, doses, bioanalytical methods, and sampling schemes. Borsa et al. examined the PK of oral fosfomycin tromethamine in young and elderly adults (10). Thirteen healthy subjects were administered a single oral dose of 25 mg/kg of body weight (∼2 g) of fosfomycin tromethamine under fasted conditions. The mean (± SD) maximum concentration of drug in serum (Cmax) and time to Cmax (Tmax) in young subjects (26 to 33 years of age; n = 5) were 18.48 ± 10.27 μg/ml and 1.61 ± 0.23 h, respectively. The mean (± SD) volume of distribution (V), elimination half-life (t1/2), and area under the concentration-time curve from 0 h to infinity (AUC0–∞) were 2.42 ± 1.68 liters/kg, 5.37 ± 2.56 h, and 102.85 ± 42.1 μg · h/ml, respectively. Total body and renal clearance values were determined to be 33.6 ± 14.5 liters/h and 18.6 ± 2.6 liters/h, respectively, and 57.7 ± 30.2% of the administered dose was eliminated renally by 24 h. Other studies administering 3 g of oral fosfomycin tromethamine have demonstrated Cmax values ranging from 22 to 32 μg/ml, Tmax values of 2 to 2.5 h, t1/2 values of 2.4 to 7.3 h, and AUC values of 145 to 228 μg · h/ml (11–13).
The urine concentrations of fosfomycin achieved after oral dosing of fosfomycin tromethamine have varied considerably throughout the published literature. Older PK studies of adults demonstrated mean peak urinary concentrations ranging from 1,053 to 4,415 μg/ml within 4 h of administration of a single dose of 3 g of fosfomycin tromethamine (15, 16). In a 1987 study of 10 healthy subjects administered a single oral dose of 50 mg/kg (∼4 g) of fosfomycin tromethamine, serum and urine concentrations were measured at 2, 4, 6, 8, and 24 h postdose via a Proteus mirabilis ATCC 2100 bioassay (17). Urine concentrations at 2 h reached 2,000 to 2,500 μg/ml and were maintained between 1,200 and 2,750 μg/ml at 8 h postdose. A similar 1987 study of 5 healthy subjects administered the same oral dose demonstrated concentrations of fosfomycin in the urine above 1,000 μg/ml at 12 h postdose (18). Conversely, more recent studies describe urine fosfomycin concentrations similar to those observed in the current study. A 1996 PK study included in the Monurol prescribing information reports a peak urine concentration from 6 to 8 h of 537.7 ± 251.8 μg/ml and a 24-h concentration of 163.5 ± 99.3 μg/ml after a single 3-g oral dose (1). The aforementioned phase I PK study included 28 subjects administered a single 3-g dose of oral fosfomycin tromethamine and demonstrated peak urine concentrations of 1,049 ± 867.8 μg/ml during the 0- to 4-h collection interval and 947.5 ± 791.9 μg/ml during the 4- to 8-h collection interval (14). By 12 h, the average urine concentration was below 300 μg/ml. Finally, a study of 40 healthy adult women given a single 3-g oral dose of fosfomycin tromethamine demonstrated a peak urine concentration of 1,982 ± 1,257.4 μg/ml, although this study included only subjects with an eCLCR of ≥90 ml/min and the urine collection times were not standardized (19). Notably, all studies evaluating urine concentrations of fosfomycin after oral administration of fosfomycin tromethamine have demonstrated significant levels of inter- and intrasubject variability. This variability is likely due in large part to the timing of the fosfomycin dose in relation to bladder emptying and the subjects' urinary output. This variability creates uncertainty in the estimation of PK/PD indices and the ability to predict treatment efficacy and may contribute to the treatment failure rate of up to 30% observed in randomized controlled trials of fosfomycin (20).
An understanding of the PK/PD index that links antimicrobial exposure with efficacy is an important step in designing regimens that optimize safety and efficacy. A recent neutropenic murine thigh infection model demonstrated the AUC/MIC ratio to be the PK/PD index most closely associated with the efficacy of fosfomycin, with average net stasis and 1-log kill ratios for Enterobacteriaceae of 23 and 83, respectively (21). If these data are applied to the mean plasma AUC values observed in our study, a 3-g oral dose of fosfomycin at steady state would be expected to achieve net stasis against Enterobacteriaceae isolates with MICs of ≤4 μg/ml and a 1-log kill against those with MICs of ≤1 μg/ml. If the same indices are applied to the urine exposures observed in this study (urine AUC0–24 of approximately 4,800 μg · h/ml), stasis and a 1-log kill could be achieved at MICs of up to 128 μg/ml and 32 μg/ml, respectively, corresponding well to the Clinical and Laboratory Standards Institute (CLSI) (22) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) (23) susceptibility breakpoints for oral fosfomycin tromethamine of ≤64 μg/ml and ≤32 μg/ml, respectively. Importantly, given the lower urine concentrations observed in this and other recent PK studies of fosfomycin tromethamine, PK/PD studies utilizing peak urine concentrations of up to 4,000 μg/ml, based on older PK analyses, may need to be reevaluated (24). Ideally, these PK/PD targets should be validated for humans and correlated with clinical outcomes.
This study is not without limitations. Subjects were not confined to the study unit for the entire 24-h urine collection period and therefore were instructed to collect their urine at home during the 12- to 24-h collection period; the reliability of this procedure could not be confirmed directly. Additionally, enteral fluid intake was encouraged throughout the course of the study but was not standardized across subjects. We did not formally assess the effect of diarrhea on the systemic exposure of fosfomycin. Finally, the homogenous subject population included in this study does not allow for exploration of the influence of covariates on PK parameters.
In summary, the results of this study provide important information on the time course and magnitudes of plasma and urine concentrations of fosfomycin following single and repeated oral doses of fosfomycin tromethamine. There was no observed increase in systemic exposure on day 5 after repeated doses of fosfomycin given either QOD or QD compared to that after a single dose. Additionally, day 5 systemic exposure was not significantly increased after 5 daily doses of fosfomycin compared to that after 3 doses given QOD. Plasma predose concentrations were marginally higher after daily dosing, while urine predose concentrations were significantly higher but highly variable. The lower urine concentrations observed in this and other modern PK studies than those in previous studies may necessitate reevaluation of achievable PK/PD indices and revision of the susceptibility breakpoints. Daily dosing of fosfomycin tromethamine was associated with a significant increase in the number of days of diarrhea experienced by healthy subjects in this study compared to that with QOD dosing. Further clinical studies to evaluate the efficacy and safety of repeated dosing regimens of oral fosfomycin tromethamine in patients with urogenital infections are warranted in order to establish an appropriate benefit/risk ratio.
MATERIALS AND METHODS
Study design and subjects.
The present study (ClinicalTrials.gov registration number NCT02570074) was a phase I, randomized, open-label, two-period-crossover, multiple-dose study of oral fosfomycin tromethamine (Monurol; Forest Pharmaceuticals, Inc., St. Louis, MO) in healthy adult subjects. This study was approved by the University of Illinois at Chicago (UIC) Office for the Protection of Research Subjects Institutional Review Board and conducted in accordance with good clinical practices at the UIC Clinical Research Center. Written informed consent was obtained from each subject prior to the conduct of any study-related procedures.
Inclusion criteria included healthy, nonsmoking male or female subjects between 18 and 55 years of age inclusive, with no clinically significant findings on medical history, physical examination, vital signs, 12-lead electrocardiogram, or clinical laboratory evaluation. Subjects of childbearing potential were required to use protocol-defined acceptable methods of birth control. Eligible body weight was ≥50 kg with a body mass index of ≥18.5 and <30 kg/m2. Exclusion criteria included an intolerance or hypersensitivity history to phosphonic acid derivative antibiotics, history of any significant cardiac, neurological, thyroid, muscular, or immune disorder, Cockroft-Gault estimated creatinine clearance (eCLCR) of <60 ml/min (25), or history of alcohol abuse in the previous 6 months. Subjects could not have had prescription and nonprescription drugs (including vitamins and herbal or dietary supplements) within 7 days prior to day 1 or have donated blood within a 56-day period.
Subjects were enrolled in study drug administration sequences in parallel so that each subject received both regimens in a randomized, crossover fashion. Randomization was stratified by gender. The two dosing regimens were 3 g every other day (QOD) for 3 doses followed by 3 g every day (QD) for 7 doses and vice versa. Fosfomycin was delivered as a powder sachet mixed in 3 to 4 oz. water under fasted conditions. Each administration sequence was separated by a 5- to 14-day washout period.
Pharmacokinetic samples.
On study days 1 and 5, blood samples for measurement of fosfomycin concentrations were collected before dosing and at 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h postdose. Urine samples for measurement of fosfomycin concentrations and measured 24-h creatinine clearance (mCLCR) were collected before and 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after ingestion. Predose blood and urine samples were also collected on days 3 and 7. Blood was collected and centrifuged, and plasma was separated for bioanalytical analysis, frozen within 60 min of collection, and stored at −70°C until shipment. Urine samples were collected and stored at ≤4°C during collection intervals. After completion of the collection interval, aliquots of urine were extracted, frozen, and stored at −70°C until shipment.
Bioanalytical procedures for determination of fosfomycin concentrations.
Concentrations of fosfomycin in plasma and urine samples were measured by Keystone Bioanalytical, Inc. (North Wales, PA), via validated liquid chromatography-tandem mass spectrometry (LC-MS/MS). The validation procedure included short- and long-term assessments of room temperature, refrigerated, frozen, and freeze-thaw stability of fosfomycin in extracted and unextracted samples. The accuracy of the method was determined by comparing the mean measured concentrations with theoretical concentrations of each analyte in the quality control (QC) samples. The lower and upper limits of quantitation for fosfomycin in human plasma samples were 0.1 and 80 μg/ml, respectively. Eight hundred fourteen unique plasma samples were analyzed in 11 analytical runs which met the acceptance criteria for standard curve and QC samples. All 11 batches met prespecified acceptance criteria, with a coefficient of variation (%CV) of ≤15% and relative error (%RE) within 15%. The lower and upper limits of quantitation for fosfomycin in human urine samples were 2 and 1,000 μg/ml, respectively. A total of 444 unique urine samples were analyzed in 8 analytical runs which met the acceptance criteria for standard curve and QC samples. All 8 batches met prespecified acceptance criteria, with a %CV of ≤15% and a %RE within 15%.
Pharmacokinetic analysis.
Noncompartmental analyses (Phoenix WinNonlin, version 7; Pharsight Corporation, Cary, NC) were used to generate PK parameters for each subject for fosfomycin in plasma. Reported parameters following oral administration of fosfomycin tromethamine included peak plasma concentration (Cmax), time to maximum concentration (Tmax), apparent volume of distribution (V/F), apparent clearance (CL/F), and elimination half-life (t1/2). The area under the plasma concentration-time curve (AUC) was calculated by use of the linear-up log-down trapezoidal method. Reported parameters for fosfomycin in human urine following oral administration included the amount of drug excreted during the urine collection interval (Ae), cumulative amount excreted from time zero (Cum Ae), fraction of the dose excreted during the collection interval (fe), cumulative fraction of the dose excreted from time zero (Cum fe), and renal clearance (CLR).
Laboratory and safety assessment.
Safety was monitored by clinical laboratory tests, physical examination, 12-lead electrocardiogram, vital signs, and monitoring of adverse events. Safety evaluations were conducted at screening and during each visit to the study center. A follow-up safety call was made to each subject 60 days after the last dose of study drug. The investigators assessed subjects for the occurrence of adverse events throughout the study, along with their severity, as assessed via the common terminology criteria for adverse events (CTCAE) (26), and their relationship with the study drug. A safety monitoring committee of independent evaluators was also appointed to monitor subject safety.
Statistical analysis.
The primary objectives of the study were (i) to assess the safety and tolerability and (ii) to estimate the Cmax, AUC, V/F, CL/F, t1/2, Ae, and CLR of two oral dosing regimens of fosfomycin tromethamine. The primary safety/tolerability objective was addressed by reporting numbers and percentages of subjects exhibiting adverse events by regimen, grade, and MedDRA preferred term. Primary adverse event analyses were restricted to study days 1 to 9 to allow for comparison between dosing regimens. Secondary adverse event analyses covered all study days up to and including the 60-day follow-up. Additionally, the cumulative proportion of diarrhea-free days observed from the first dose of study drug until the start of the second dosing regimen or the end of the washout period (whichever occurred first) was calculated in a post hoc analysis and compared between regimens via the χ2 test. The primary PK objective was addressed by reporting summary statistics (number of observations, mean, standard deviation, coefficient of variation, minimum, maximum, median, and quartiles) for PK parameters by regimen and study day. For each PK parameter (Cmax, AUC, V/F, CL/F, t1/2, Ae, and CLR), a mixed-effects ANOVA model was fit by using log-transformed PK parameters as the outcome (except for CL/F, which was analyzed on a linear scale) and including fixed effects for dosing regimen, study day, a dosing regimen-by-study-day interaction term, and a random effect for subject ID within the dosing regimen sequence. ANOVA-generated means and 95% confidence intervals are reported.
Assuming a coefficient of variation of 35% for the fosfomycin Cmax in plasma, consistent with previous reports (10), it was determined that with complete data from 18 participants, the regimen-specific Cmax could be estimated with a precision of ±4 μg/ml, and if the true difference in Cmax between regimens was ≥30%, there would be 87% power to declare such a difference statistically significant. The effect size was based on plasma Cmax in order to provide a conservative estimate of adequate sample size, as time-weighted parameters, such as AUC, are more precise. This sample size was not expected to provide as much precision for binary events (occurrence of specific toxicities or discontinuation), as an observed event rate of 5% would have a 95% CI of 0.1% to 27.3%.
ACKNOWLEDGMENTS
We thank the study participants and acknowledge the contributions of the staff at the UIC Clinical Research Center and Investigational Drug Service for their support of this work.
This study was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number 1UM1AI104681-01. The Center for Clinical and Translational Science at the University of Illinois at Chicago is supported by National Center for Advancing Translational Sciences NIH grant UL1TR002003.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
All authors report no relevant conflicts.
REFERENCES
- 1.Anonymous. July 2007. Monurol (fosfomycin tromethamine) package insert. Forest Pharmaceuticals, Inc, St Louis, MO: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf Accessed 11 May 2015. [Google Scholar]
- 2.Anonymous. 2015. Fosfomycin trometamol package insert. Mercury Pharmaceuticals Limited, London, United Kingdom: https://www.medicines.org.uk/emc/product/7219/smpc Accessed 30 May 2018. [Google Scholar]
- 3.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao LD, Zheng B, Chen S, Yang Y, Zhang K, Guo HF, Yang B, Niu YJ, Wang Y, Shi BK, Yang WM, Zhao XK, Gao XF, Chen M. 2013. Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open 3:e004157. doi: 10.1136/bmjopen-2013-004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayson ML, Macesic N, Trevillyan J, Ellis AG, Zeglinski PT, Hewitt NH, Gardiner BJ, Frauman AG. 2015. Fosfomycin for treatment of prostatitis: new tricks for old dogs. Clin Infect Dis 61:1141–1143. doi: 10.1093/cid/civ436. [DOI] [PubMed] [Google Scholar]
- 7.Moroni M. 1987. Monuril in lower uncomplicated urinary tract infections in adults. Eur Urol 13(Suppl 1):S101–S104. doi: 10.1159/000472872. [DOI] [PubMed] [Google Scholar]
- 8.Pullukcu H, Tasbakan M, Sipahi OR, Yamazhan T, Aydemir S, Ulusoy S. 2007. Fosfomycin in the treatment of extended spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 29:62–65. doi: 10.1016/j.ijantimicag.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Dette GA, Knothe H, Schonenbach B, Plage G. 1983. Comparative study of fosfomycin activity in Mueller-Hinton media and in tissues. J Antimicrob Chemother 11:517–524. doi: 10.1093/jac/11.6.517. [DOI] [PubMed] [Google Scholar]
- 10.Borsa F, Leroy A, Fillastre JP, Godin M, Moulin B. 1988. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob Agents Chemother 32:938–941. doi: 10.1128/AAC.32.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergan TTS, Albini E. 1993. Pharmacokinetic profile of fosfomycin trometamol. Chemotherapy (Basel) 29:297–301. doi: 10.1159/000239140. [DOI] [PubMed] [Google Scholar]
- 12.Bergan T. 1990. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 18(Suppl 2):S65–S69. doi: 10.1007/BF01643430. [DOI] [PubMed] [Google Scholar]
- 13.Segre G, Bianchi E, Cataldi A, Zannini G. 1987. Pharmacokinetic profile of fosfomycin trometamol (Monuril). Eur Urol 13(Suppl 1):56–63. doi: 10.1159/000472864. [DOI] [PubMed] [Google Scholar]
- 14.Wenzler E, Ellis-Grosse EJ, Rodvold KA. 2017. Pharmacokinetics, safety, and tolerability of single-dose intravenous (ZTI-01) and oral fosfomycin in healthy volunteers. Antimicrob Agents Chemother 61:e00775-. doi: 10.1128/AAC.00775-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SS, Balfour JA, Bryson HM. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637–656. [DOI] [PubMed] [Google Scholar]
- 16.Bergan T, Mastropaolo G, Di Mario F, Naccarato R. 1988. Pharmacokinetics of fosfomycin and influence of cimetidine and metodopramide on the bioavailability of fosfomycin trometamol, p 157–166. In Neu HC, Williams JD (ed), New trends in urinary tract infections. Karger, Basel, Switzerland. [Google Scholar]
- 17.Bergogne-Berezin E, Muller-Serieys C, Joly-Guillou ML, Dronne N. 1987. Trometamol-fosfomycin (Monuril) bioavailability and food-drug interaction. Eur Urol 13(Suppl 1):S64–S68. [DOI] [PubMed] [Google Scholar]
- 18.Segre G, Bianchi E, Cataldi A, Zannini G. 1987. Pharmacokinetic profile of fosfomycin trometamol (Monuril). Eur Urol 13(Suppl 1):S56–S63. doi: 10.1159/000472864. [DOI] [PubMed] [Google Scholar]
- 19.Wijma RA, Koch BCP, van Gelder T, Mouton JW. 2018. High interindividual variability in urinary fosfomycin concentrations in healthy female volunteers. Clin Microbiol Infect 24:528–532. doi: 10.1016/j.cmi.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. 2010. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 65:1862–1877. doi: 10.1093/jac/dkq237. [DOI] [PubMed] [Google Scholar]
- 21.Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, Andes DR. 2017. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (fosfomycin for injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e00476-17. doi: 10.1128/AAC.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CLSI. 2017. Performance standards for antimicrobial susceptibility testing: approved 27th ed Document M100-S27. CLSI, Wayne, PA. [Google Scholar]
- 23.European Committee on Antimicrobial Susceptibility Testing. 2015. Breakpoint tables for interpretations of MICs and zone diameters, version 6. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf Accessed 23 December 2017.
- 24.Zhanel GG, Parkinson K, Higgins S, Denisuik A, Adam H, Pitout J, Noreddin A, Karlowsky JA. 2017. Pharmacodynamic activity of fosfomycin simulating urinary concentrations achieved after a single 3-g oral dose versus Escherichia coli using an in vitro model. Diagn Microbiol Infect Dis 88:271–275. doi: 10.1016/j.diagmicrobio.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous. 2015. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE), version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 1 March 2018.