ABSTRACT
Regimens containing topical polymyxin appear highly effective at preventing ventilator-associated pneumonia (VAP) overall and, more so, VAP caused by Gram-negative bacteria. However, Stoutenbeek's postulates that VAP incidences within studies of topical antibiotics depend on the context of whether the component (control and intervention) groups of each study were concurrent versus nonconcurrent remain untested. The literature was searched for concurrent control (CC) versus nonconcurrent control (NCC) designed studies of respiratory tract applications of topical polymyxin to mechanically ventilated (MV) patients that reported incidences of Pseudomonas-associated ventilator-associated pneumonia (PsVAP). Studies of various interventions other than topical polymyxin (nonpolymyxin studies) served to provide additional points of reference. The PsVAP incidences within the component groups of all studies were benchmarked against groups from observational studies. This was undertaken by meta-regression using generalized estimating equation methods. Dot plots, caterpillar plots, and funnel plots enable visual benchmarking. The PsVAP benchmark (and 95% confidence interval [CI]) derived from 102 observational groups is 4.6% (4.0 to 5.3%). In contrast, the mean PsVAP within NCC polymyxin intervention groups (1.6%; CI, 1.0 to 4.5%) is lower than that of all other component group categories. The mean PsVAP within CC polymyxin control groups (9.9%; CI, 7.6 to 12.8%) is higher than that of all other component group categories. The PsVAP incidences of control and intervention groups of studies of respiratory tract applications of polymyxin are dependent on whether the groups were within a concurrent versus nonconcurrent study. Stoutenbeek's concurrency postulates are validated.
KEYWORDS: ventilator-associated pneumonia, Pseudomonas, antibiotic prophylaxis, study design, intensive care, mechanical ventilation, selective digestive decontamination, polymyxin
INTRODUCTION
Polymyxin is a common component within selective oral-pharyngeal decontamination and selective digestive decontamination (SOD/SDD) regimens (1–6). The evidence in support of SOD/SDD in protection against ventilator-associated pneumonia (VAP) and other intensive care unit (ICU)-acquired infections appears compelling. Numerous studies and systematic reviews report apparent reductions in VAP incidence of >50% (1–6) in comparison to other methods of VAP prevention, which achieve reductions of generally <50% (7–18). Moreover, SOD/SDD appears most protective against Gram-negative colonization and infection (4).
However, the optimal study design for the evaluation of decontamination interventions in the ICU context is unclear. Stoutenbeek postulated that it would be difficult to interpret the infection incidences in, on the one hand, the intervention group (postulate 1) and, on the other hand, the control group (postulate 2) of a concurrent control (CC) study, as the influence of contextual effects may be impossible to exclude within the traditional CC trial format. Hence, the original ICU study of SOD/SDD (19) and at least 20 of the more than 60 studies that followed were undertaken with a nonconcurrent control (NCC) design. Several had either a hybrid design with both NCC and CC groups or crossover designs.
The objective here is to test these postulates. To this end, several obstacles need to be resolved. First, Pseudomonas aeruginosa-associated VAP (PsVAP) incidence provides a more specific endpoint than overall VAP incidence. Pseudomonas aeruginosa, against which polymyxin is a key antibiotic, accounts for approximately 20% of VAP isolates (20, 21), and resistance, even in the context of prophylactic colistin use, is rare (22).
Second, an externally derived reference PsVAP incidence is required with which to benchmark the PsVAP incidences within the component (control and intervention) groups within the published studies of SOD/SDD. To this end, a category of observational studies serves to derive external benchmarks for PsVAP (and VAP) incidence. Additionally, a composite category of studies of interventions other than topical polymyxin (nonpolymyxin studies) provides additional points of reference.
Third, the impact of a range of other contextual (group level) factors that could influence the PsVAP incidence needs to be modeled.
RESULTS
Characteristics of the studies.
Of the 191 studies identified by the search (Fig. 1), 119 were sourced from 20 systematic reviews and 72 were sourced from elsewhere (Table 1; see Tables S1 to S4 in the supplemental material). Only a minority of NCC studies of topical polymyxin had been cited within a systematic review. The majority of studies were published between 1980 and 2010, and a minority originated from trauma ICUs. Bronchoscopic methods for VAP sampling and diagnosis were used more commonly among observational studies than topical polymyxin studies. The nonpolymyxin studies were drawn from studies of gastric acid-based, airway-based, and topical antiseptic- or antibiotic-based interventions for the prevention of ICU-acquired infections among mechanically ventilated (MV) patients.
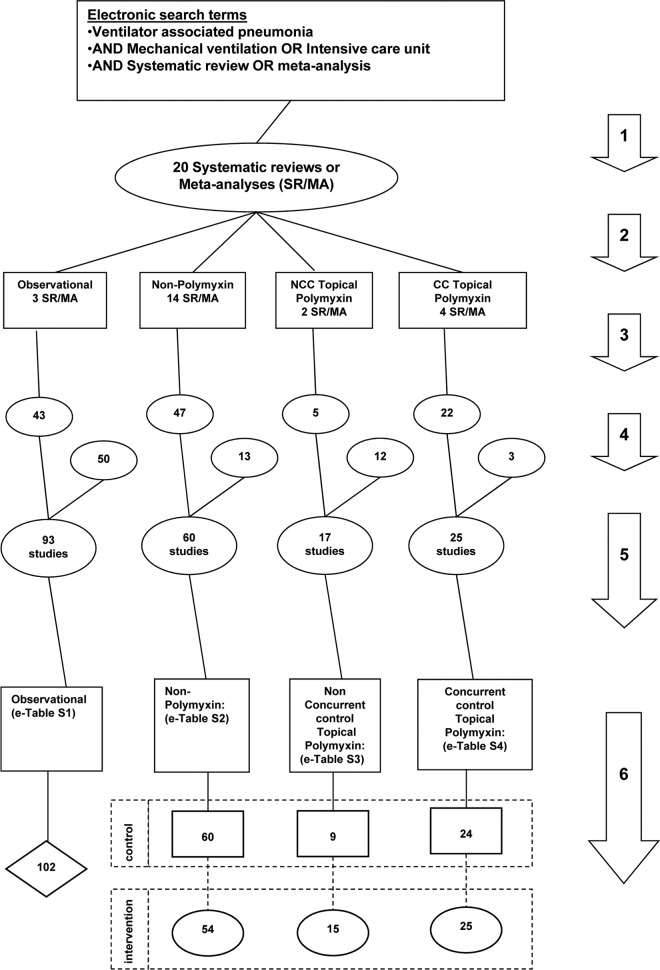
FIG 1.
Search method, screening criteria, and resulting classification of eligible studies and subsequent derivation of component groups. The arrows numbered 1 to 6 indicate the following steps. (1) An electronic search for systematic reviews containing potentially eligible studies was performed using the search terms “ventilator-associated pneumonia,” “mechanical ventilation,” and “intensive care unit,” each combined with either “meta-analysis” or “systematic review,” up to December 2015. (2) Studies were streamed into one of four categories: studies in which there was no intervention (observational studies), studies with topical polymyxin with or without a CC design, and studies of nonpolymyxin methods of VAP prevention. The studies of nonpolymyxin methods of VAP prevention encompass a broad range of methods. (3) The studies were screened against inclusion and exclusion criteria. (4) A hand search was undertaken for additional studies. (5) Eligible studies were then collated, and any duplicate studies were removed. (6) The component groups, being control (rectangles), intervention (ovals), and observation (diamond) groups, were extracted from each study. Note that the total numbers do not tally, as some systematic reviews provided studies in more than one category and some studies provided groups in more than one category.
TABLE 1.
Characteristics of studiesa
| Characteristic | Result for: |
|||
|---|---|---|---|---|
| Observational studies (no intervention) | Studies of VAP prevention |
|||
| Nonpolymyxin studies | Topical polymyxin studies |
|||
| NCC | CC | |||
| Study characteristics | ||||
| Source | Table S1 | Table S2 | Table S3 | Table S4 |
| Total no. of studies | 93 | 60 | 17 | 25 |
| No. of studies with origin from systematic reviewb | 43 | 47 | 5 | 22 |
| No. of studies originating from North American ICUsc | 25 | 15 | 2 | 3 |
| No. of studies with MV for >48 h for <90%d | 6 | 1 | 1 | 4 |
| No. of studies originating from trauma ICUse | 21 | 13 | 2 | 8 |
| No. of studies with bronchoscopic samplingf | 57 | 25 | 6 | 6 |
| Study publication yr (range) | 1986–2015 | 1987–2016 | 1975–2017 | 1987–2007 |
| Overall VAP prevention effect sizeg | NA | 0.74; 0.67–0.82 | 0.40; 0.31–0.52 | 0.35; 0.29–0.43 |
| Group characteristics | ||||
| No. of patients per study group (median; IQR)h | 290; 135–660 | 76; 60–149 | 84; 60–228 | 42; 31–74 |
| VAP incidence per 100 patients (mean %; 95% CI) (no. of groups) | ||||
| Cohort | 20; 18–22 (102) | NA | NA | NA |
| Control | NA | 21; 19–24 (60) | 18; 11–30 (9) | 33; 26–41 (24) |
| Intervention | NA | 15; 13–18 (54) | 10; 6–15 (15) | 14; 11–18 (25) |
| PsVAP incidence per 100 patients (mean %; 95% CI) (no. of groups) | ||||
| Cohort | 4.6i; 4.0–5.3 (102) | |||
| Control | 4.8j; 4.0–5.8 (60) | 6.4k,l; 5.1–7.9 (9) | 9.9l,m,n; 7.6–12.8 (24) | |
| Intervention | 3.5o; 2.8–4.3 (54) | 1.6k,p,q; 1.0–4.5 (15) | 3.8m,n,p; 2.9–5.0 (25) | |
Note that several studies had more than one control and/or intervention group. Hence, the number of groups does not equal the number of studies. Abbreviations: NCC, nonconcurrent control; CC, concurrent control; NA, not applicable.
Studies that were sourced from 20 systematic reviews (references available in supplemental material).
Studies originating from an ICU in Canada or the United States.
Studies for which fewer than 90% of patients were reported to receive >48 h of MV.
A trauma ICU was arbitrarily defined as an ICU with >50% of admissions for trauma.
Bronchoscopic instead of tracheal sampling for VAP diagnosis.
See Fig. S1 and S2 in the supplemental material.
Data are the median and interquartile range (IQR).
This value is the PsVAP benchmark as derived in Fig. S3.
See Fig. S4.
See Fig. S6.
The incidences of PsVAP within control groups from CC and NCC design studies of topical polymyxin were statistically different (P = 0.049).
The incidence of PsVAP within CC control groups of studies of topical polymyxin in which observer blinding was achieved by using a topical placebo was 10.9% (7.0 to 16.0%; n = 11) compared to 8.9% (6.2 to 12.7%; n = 14) in those not using a topical placebo.
See Fig. S7.
See Fig. S5.
The incidences of PsVAP within intervention groups from CC and NCC design studies of topical polymyxin were statistically different (P = 0.017).
A total of 289 component groups were derived from these 191 studies. Fifteen studies, including three having a hybrid design, had more than one observational, control, or intervention group. The majority of groups from studies of topical polymyxin methods had fewer than 50 patients per group versus more than 75 patients in the majority of all remaining groups. There were 16 different topical polymyxin-containing regimens within the topical polymyxin-based intervention groups.
Effect sizes: overall VAP incidence.
The study-specific effect sizes of the topical polymyxin and nonpolymyxin interventions against overall VAP incidence are presented as forest plots (Fig. S1 and S2 [see additional file S1]), and the summary odds ratios are presented in Table 1.
Benchmarking: visual.
The PsVAP incidences in all groups are displayed in caterpillar plots (Fig. S3 to S7), funnel plots (Fig. S9 to S15), and scatter plots (Fig. 2). There was significant disparity in the summary incidences of both VAP (Fig. S8) and PsVAP (Fig. 2) among the component groups versus the respective benchmarks (Table 1). For VAP and PsVAP, the incidences among the control groups of CC studies of topical polymyxin were each higher by >50% than the respective benchmarks, whereas the incidences for control groups of studies of nonpolymyxin methods and the control groups of NCC design studies of topical polymyxin were more similar to the corresponding benchmarks. Conversely, for both VAP and PsVAP, the incidences for the intervention groups of NCC studies of topical polymyxin were >50% less than the respective benchmarks, whereas the incidences for intervention groups of studies of nonpolymyxin methods and the intervention groups of CC designed studies of topical polymyxin were more similar to the corresponding benchmarks (Table 1 and Fig. 2; Fig. S3 to S7). Among the studies of topical polymyxin, the difference in PsVAP incidence between control groups of NCC design studies and CC design studies and likewise the difference in PsVAP incidence between intervention groups of NCC design studies and CC design studies were each significantly different (P < 0.05).
FIG 2.
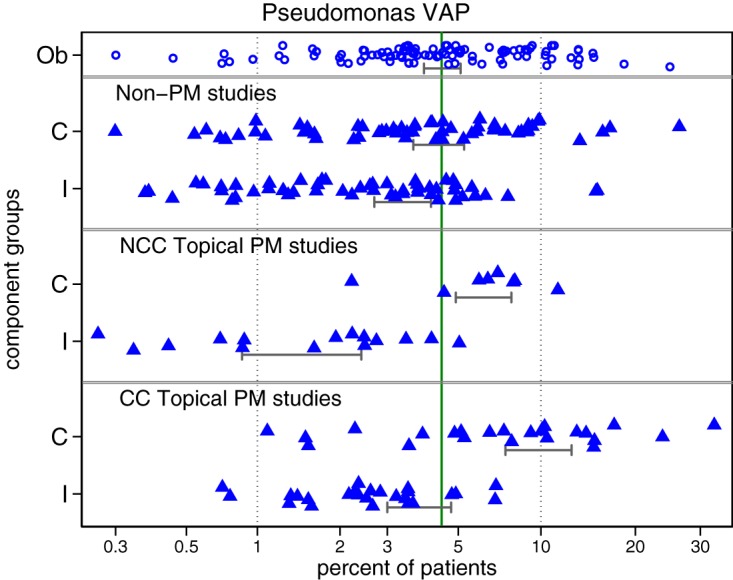
Incidence of PsVAP versus the benchmark. Results for the component (Ob, observational [open symbols]; C, control, and I, intervention [closed triangles]) groups of studies of either topical polymyxin- or nonpolymyxin-based methods of VAP prevention are shown (95% CI of summary incidence). The PsVAP benchmark is the summary mean (central unbroken vertical line) derived from the observational studies. Note that the x axis is a logit scale and groups with a zero count have a continuity correction (N + 0.5) to enable them to appear in the plot. These data are displayed in more detail as caterpillar plots (see Fig. S3 to S7 in the supplemental material).
In the PsVAP caterpillar plots (Fig. S3 to Fig. S7) and the VAP (Fig. S8) and PsVAP (Fig. 2) dot plots, there is no impression that the summary VAP or PsVAP incidences for any category of component group was driven by a minority of outlier studies that may have been subject to either outbreaks or to unusually large prevention effects. Of the 41 zero-event groups, 10 were among the 25 intervention groups of the CC design studies compared to 2 among the 15 intervention groups of the NCC design studies of topical polymyxin. This uneven distribution is apparent as an asymmetry in the funnel plots (Fig. S10, S11, S14, and S15) of study groups, with an excess of high standard error (low-precision) groups with PsVAP <5%. These groups mostly represent zero-event groups whose analytic weights were each <1% and, in aggregate, <10% toward the derivation of each group summary (data not shown).
Among the observational groups, there was a slight downward trend in PsVAP incidence versus year of publication (Fig. 3).
FIG 3.
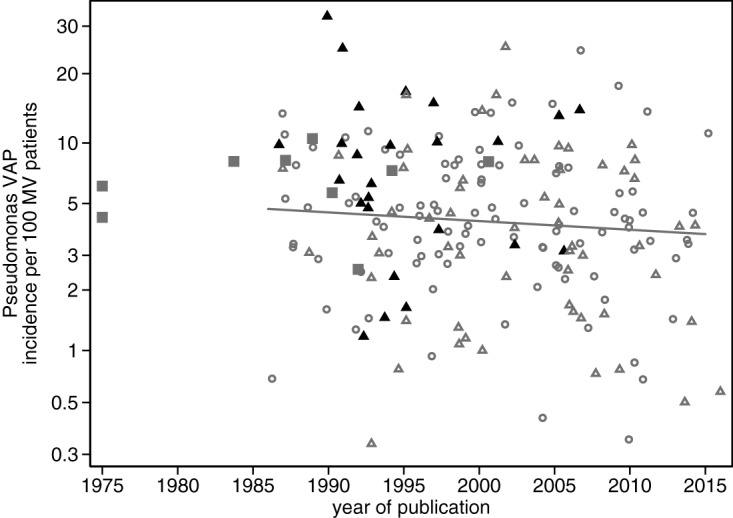
Pseudomonas VAP incidence per 100 patients versus year of publication. Symbols: open gray circles, observational groups; open triangles, control group from nonpolymyxin studies; solid gray squares, control group from NCC polymyxin studies; solid black triangles, control group from CC polymyxin studies. Note that the y axis is a logit scale and that groups with a zero count have a continuity correction (N + 0.5) to enable them to appear in the plot. The regression line is derived from only the observational groups.
Benchmarking: meta-regression.
In models adjusting for all the group level factors as detailed in Table 2, origin from a North American ICU was negatively associated with both VAP and PsVAP, group origin from a trauma ICU was positively associated with both VAP and PsVAP, and year of publication was negatively associated with PsVAP (Fig. 3).
TABLE 2.
Regression models (GEE methods)a
| Factor | VAP |
PsVAP |
||||
|---|---|---|---|---|---|---|
| Coefficientb | 95% CI | P | Coefficientb | 95% CI | P | |
| Groups from observational studies (reference group) | −1.3 | −1.8 to −0.8 | <0.001 | −3.1 | −3.7 to −2.4 | <0.001 |
| Control groups | ||||||
| Nonpolymyxin studies | +0.20 | −0.03 to +0.43 | 0.08 | +0.12 | −0.18 to +0.43 | 0.43 |
| NCC topical polymyxin studies | +0.15 | −0.31 to +0.61 | 0.52 | +0.49 | −0.09 to +1.07 | 0.1 |
| CC topical polymyxin studies | +0.46 | +0.03 to +0.88 | 0.036 | +0.56 | +0.18 to +0.94 | 0.004 |
| Intervention groups | ||||||
| Nonpolymyxin studies | −0.16 | −0.44 to +0.11 | 0.24 | −0.15 | −0.48 to +0.18 | 0.36 |
| NCC topical polymyxin studies | −0.57 | −1.26 to −0.19 | 0.008 | −1.35 | −1.96 to −0.73 | 0.001 |
| CC topical polymyxin studies | −0.41 | −1.03 to −0.21 | 0.003 | −0.54 | −0.93 to −0.15 | 0.006 |
| Trauma ICUc | +0.80 | +0.58 to +1.01 | 0.001 | +0.43 | +0.14 to +0.72 | 0.004 |
| Mode of diagnosisd | −0.16 | −0.45 to +0.12 | 0.26 | −0.21 | −0.55 to +0.13 | 0.23 |
| MV of <90%e | −0.57 | −0.92 to −0.22 | 0.002 | −0.50 | −1.05 to +0.05 | 0.075 |
| North American studyf | −0.41 | −0.73 to −0.08 | 0.013 | −0.59 | −1.03 to −0.16 | 0.007 |
| Yr of publicationg | −0.01 | −0.03 to +0.005 | 0.16 | −0.02 | −0.05 to −0.006 | 0.01 |
Abbreviations: GEE, generalized estimating equation methods; ICU, intensive care unit; MV, mechanical ventilation; NCC, nonconcurrent control; CC, concurrent control. The regression models derived using random-effects methods are presented in Table S5 in the supplemental material.
In our interpretation, for each model, the reference group is the observational study (benchmark) group, and the coefficient for the reference group equals the difference in logits from 0 (a logit equal to 0 equates to a proportion of 50%; a logit equal to −1.4 equates to a proportion of 20%; a logit equal to −2.9 equates to a proportion of 5%), and the other coefficients represent the difference in logits for groups positive for that factor versus the reference group.
A trauma ICU is arbitrarily defined as an ICU for which >50% of admissions were for trauma.
Diagnosis of VAP using bronchoscopic rather than trachea-based sampling.
Less than 90% of the group receiving prolonged MV.
Originating from an ICU in the United States or Canada.
Year of study publication, with the coefficient representing the increment for each year post-1985.
The strongest negative factor in each of the PsVAP and VAP models was that associated with membership of an NCC topical polymyxin intervention group. In contrast, for each of the PsVAP and VAP models, the strength of the association between membership of a CC topical polymyxin study intervention group was equal in magnitude, although contrary in direction, to the strength of the association with membership of a CC control group. Repeating this analysis limited to studies obtained from systematic reviews revealed similar findings (data not shown).
Repeating this analysis using the DerSimonian and Laird random-effects method as an alternative method of meta-regression analysis revealed similar findings (Table S5).
DISCUSSION
This analysis of a broad range of studies has been undertaken in an attempt to test the postulated contextual effect of concurrency of control and intervention groups within studies of topical polymyxin on the incidence of PsVAP. This analysis is informed by data from other studies in this patient group; PsVAP and VAP benchmarks are derived from studies without an intervention, and studies of nonpolymyxin interventions provide additional points of reference. The number of studies with PsVAP data included here is larger than that contained in a previous review (4), which catalogued 54 randomized controlled trials of SOD/SDD from which data on Gram-negative bacterial pneumonia was available from only 14.
Of note, the summary effect sizes seen here of the polymyxin and nonpolymyxin interventions are similar to the summary effects seen in the respective systematic reviews from which these studies were largely drawn (2–18). Interestingly, the magnitudes of the summary effect sizes for topical polymyxin appear similar for CC and NCC designed studies, and each, in contrast, exceed the effect size derived from the studies of nonpolymyxin interventions.
Moreover, the PsVAP benchmark, being 4.6% (95% confidence interval [CI], 4.0 to 5.3), is comparable to an incidence estimate reported in the literature. Among a multinational survey of ICUs, the PsVAP incidence among MV patients was 4.8% in Europe and 3.4% in the United States (23).
There are three hybrid design studies with PsVAP data available (24–26). These three studies were each relatively small. The PsVAP incidence within these studies fit the pattern of PsVAP results among the broader panel of studies of topical polymyxin (Fig. S16).
The summary PsVAP and VAP incidences derived from the control groups of the CC studies of topical polymyxin methods are higher than the respective benchmarks, than the same incidences derived from the NCC polymyxin studies, and also than the same incidences derived from the control groups of nonpolymyxin-based methods. Surprisingly, the PsVAP and VAP incidences derived from the intervention groups of the CC polymyxin-based methods are in each case more similar to the respective benchmarks.
In contrast, the summary PsVAP and VAP incidences derived from the intervention groups of the NCC studies of topical polymyxin methods are lower than the respective benchmarks, than the same incidences derived from the CC polymyxin studies, and also than the same incidences derived from the intervention groups of nonpolymyxin-based methods.
These findings remain robust to three sensitivity tests. First, the use of a topical placebo to achieve observer blinding is a key marker of a high-quality study, especially where the endpoint is somewhat subjective, as is the case for VAP. However, approximately half of the studies of topical polymyxin used a topical placebo to achieve observer blinding (27). The PsVAP incidences derived from CC studies that were or were not observer blinded were similar.
Second, the low PsVAP incidence within intervention groups of NCC studies of topical polymyxin remains apparent after exclusion of two early U.S. studies. Finally, a meta-regression model was undertaken. Several candidate group level predictors of PsVAP incidence were used to base meta-regression models in an effort to account for the disparate observations between the CC and NCC studies of topical polymyxin. However, membership of an intervention group from an NCC design topical polymyxin study retained the strongest association with PsVAP incidence (Table 2). In contrast, membership of a control group from a CC design topical polymyxin study remained positively associated with PsVAP incidence (Table 2).
There are four key limitations to this analysis, the first being that the studies have been published over a period of more than 3 decades. There was considerable heterogeneity in the interventions, populations, VAP isolate reporting practices, prevalence of antibiotic resistance, and study designs among the studies here, together with a slight downward trend in PsVAP over this time. Moreover, the inclusion criteria for both the nonpolymyxin and the topical polymyxin interventions here have been intentionally broadly specified. Of note, many of the topical polymyxin interventions under study were within a regimen that often also included other SOD/SDD components, such as topical antifungal and aminoglycoside antimicrobials and protocolized parenteral antibiotics.
Second, the analysis is not intended to be a systematic review to estimate the effect size of topical polymyxin in preventing PsVAP. There is no rating for study quality here. Generally, NCC design studies are rated as too low in quality to warrant inclusion within a systematic review of effect size. The summary effect sizes here are derived merely for internal reference within this review.
The third limitation is that there are too few NCC nonpolymyxin studies with which to compare the effect of membership of component group of such a study versus that of an NCC topical polymyxin study.
Finally, the analysis here is inherently observational. Only a limited number of key group level factors were entered into the regression models, and there was no ability to adjust for the underlying patient level risk within the analysis. Moreover, the analysis here has merely identified a high incidence of PsVAP among the control groups of CC design topical polymyxin studies that remains without an explanation. It remains possible that a high PsVAP or even VAP incidence, whether due to an apparent or an inapparent outbreak (28), served as a prompt to undertake and publish a CC study of a topical polymyxin regimen within the ICU. This was not explicitly stated in any of the studies analyzed here. However, there are studies that have reported the use of regimens containing topical polymyxin in relation to the control (29–31) and emergence (32–34) of outbreaks of Gram-negative infections. These outbreak studies (29–34) were excluded from the analysis here.
The importance of using methods that include zero-event groups together with estimates of their precision in the analysis has previously been considered and discussed within an analysis of candidemia (35). Moreover, without these zero-event groups, the summary PsVAP estimates for CC studies of topical polymyxin would have been higher and the discrepancy with the benchmark would have been greater than that obtained here with the zero-event groups included. The funnel plots indicate a disproportionate number of small (imprecise) zero-event groups among the CC studies here. The explanation for this is unclear, but this would be consistent with underreporting of PsVAP.
The disparity in the incidence of PsVAP among studies of topical polymyxin versus the respective benchmarks recapitulates similar observations for various endpoints among studies of SOD/SDD versus externally derived benchmarks from populations of MV patients. For example, with respect to Staphylococcus aureus as a VAP isolate (27), Staphylococcus aureus bacteremia (36), Candida as a respiratory tract isolate (37), and candidemia incidence (35), in each case, the endpoint is higher among control groups of SOD/SDD studies than the respective benchmarks.
The basis for the difference in PsVAP incidence between control and intervention groups of NCC versus CC studies of topical polymyxin remains to be explained.
With respect to postulate 1, it is plausible that cross colonization from the concurrent colocated control group patients nullified the decolonization in the intervention patients receiving SDD. As predicted by Stoutenbeek et al. (19), “… having heavily contaminated controls next to decontaminated patients might adversely affect the potential beneficial results.”
With respect to postulate 2, the PsVAP incidence in control group patients is highest in the presence of concurrent colocated intervention patients receiving SDD. These findings here are paradoxical and contrary to the prediction of Stoutenbeek et al. (19) that “…. a reduction in the number of contagious patients by applying SDD in half of them, might reduce the acquisition, colonization and infection incidence in the not SDD treated control group.”
There are five considerations to explain this paradox. First, patients receiving prolonged MV are a major reservoir of Pseudomonas in the ICU (20, 21). Second, the MV patient population, to which SOD/SDD has been targeted in these studies, may have ICU lengths of stay that are above average. Third, the mechanism of allocation of an intervention, or not, to patients within a CC study is on a patient-by-patient basis and typically involves random assignment. As a consequence of this allocation, adjacent to intervention group MV patients within a CC design study will be a predominance of untreated MV patients. In contrast, in an NCC design study, this allocation is typically to all eligible patients within the unit, and there may be no adjacent untreated MV patients.
Fourth, is a topical placebo used within a randomized double-blind placebo-controlled evaluation of topical antibiotics as prophylaxis without effect within the ICU context? Such studies require careful consideration of any potential perfidious effect of a topical placebo (27) contributing to herd peril (38) within this context.
Finally, the Pseudomonas decolonization achieved with SOD/SDD is incomplete (4, 22). Decolonization fails using even an intensified SDD regimen in a minority of patients (39). These patients may continue to serve as reservoirs within the unit, and the potential exists for rebound on the discontinuation of SOD/SDD (40). One study examined the epidemiology of Pseudomonas pyocine and resistogram types within an ICU by undertaking a CC designed study of SDD and found that the patients receiving SDD served as a reservoir for persistent Pseudomonas strains in the unit that accounted for the majority of control group Pseudomonas infections (41). This persistence within the SDD-treated patients was not a consequence of polymyxin resistance. Studies within Dutch ICUs confirm that the prevalence of resistance to antibiotics, including colistin among Gram-negative bacteria colonizing the respiratory tract, does not increase over a period of several years of SDD use (22). However, on the sudden discontinuation of SDD, a rebound in the prevalence of antibiotic resistance in the ICU can occur (39). A similar rebound was observed in a two-ICU crossover study undertaken without a washout period (42). There were no PsVAP (and no Gram-negative bronchopneumonia) events in 99 SDD-exposed intervention group patients versus 13 PsVAP (among 31 Gram-negative bronchopneumonia) events in 61 NCC group patients in the post-SDD period (42).
Two NCC polymyxin studies undertaken in the 1970s are of particular interest (43, 44). These American studies, with >1,500 “seriously ill” ICU patients overall, represent the largest studies of polymyxin preventative therapy undertaken outside Europe. Despite the findings of substantial reductions in Pseudomonas pneumonia, both studies urged caution with this preventative therapy. One observed a higher pneumonia mortality despite the reduction in Pseudomonas pneumonia and raised concern that “continuous use of polymyxin B aerosols appears to be a dangerous form of therapy” (43). The other study was placebo blinded and conducted over 11 alternating placebo and polymyxin cycles without washout periods. This study was “terminated … in the middle of the 11th cycle, a placebo cycle, because of the sudden increase in colonization and infection with P. aeruginosa” (44). With the rebound in this last cycle, the incidence of Pseudomonas pneumonia reached 12%, having been 0.8% in the previous five polymyxin cycles.
Conclusion.
The PsVAP incidences of control and intervention groups of studies of respiratory tract applications of polymyxin are dependent on whether the groups were within a CC versus a NCC study. These discrepancies highlight the challenges to study design in the evaluation of topical antibiotics as prophylaxis within the ICU context. These findings, which would not be apparent within any study examined in isolation nor within a systematic review as conventionally undertaken, validate the Stoutenbeek hypotheses.
MATERIALS AND METHODS
Being an analysis of published work, ethics committee review of this study was not required.
Study selection and derivation of groups.
The literature search and analytic approach used here (Fig. 1) are as described previously (35, 38). The six steps used (Fig. 1, numbered arrows) were as follows.
In step 1, an electronic search of PubMed, the Cochrane database, and Google Scholar for systematic reviews containing potentially eligible studies was undertaken using the following search terms: “ventilator-associated pneumonia,” “mechanical ventilation,” and “intensive care unit,” each combined with either “meta-analysis” or “systematic review” up to December 2015.
In step 2, systematic reviews of studies of patient populations requiring prolonged (>24-h) ICU admission for which the VAP incidence was analyzed were then streamed into one of four categories: systematic reviews of studies in which there was no intervention (observational studies), systematic reviews of NCC or CC design studies with topical polymyxin in any formulation (2–7), and systematic reviews of studies of nonpolymyxin interventions (nonpolymyxin studies). The studies of nonpolymyxin methods of VAP prevention encompass a broad range of methods delivered either via the gastric route (8–10), the airway route (11–16), or the oral care route (17, 18).
In step 3, the studies were screened against the following eligibility criteria. Criteria for inclusion were prevention studies with or without concurrent controls for which incidence data for both PsVAP and VAP were extractable as an incidence proportion. The denominator for this incidence proportion is the number of MV patients with an ICU stay of at least 24 h. Criteria for exclusion were studies limited to patients with the acute respiratory distress syndrome, studies with less than 50% of patients receiving MV, and studies of topical antibiotics in the context of an ICU outbreak (29–34). Due to the significant worldwide variation in PsVAP (23), and the finding that there were no studies of topical polymyxin conducted outside Europe and North America, studies undertaken from Asia and Central and South America were excluded from this analysis.
In step 4, a hand search was undertaken for additional studies not identified within systematic reviews.
In step 5, all eligible studies were then collated, and any duplicate studies were removed and streamed into groups of patients from studies without a VAP prevention method (observational groups) or component groups of the studies of polymyxin with CC or NCC design and nonpolymyxin interventions. Groups within multiarm studies that received antibiotic prophylaxis with regimens other than topical polymyxin were excluded. The working definition for an NCC design study used here was that the control and study patients were not colocated in the same ICU at the same time. Some NCC design studies fit this description because the control and intervention group patients, while concurrent, were located in separate ICUs.
In step 6, the component groups were extracted from each study as observational, control, or intervention groups.
Data analysis.
The PsVAP incidence is the number of patients with VAP and with any Pseudomonas isolate per 100 MV patients together with the incidence proportion of VAP overall. Those groups for which the proportion of admissions for trauma was >50% were arbitrarily designated as originating from trauma ICUs. Other parameters extracted were whether the mode of VAP diagnosis required bronchoscopic sampling and whether the study originated from either the United States or Canada (North America).
The analytic approach is as undertaken previously (27, 28, 36, 37, 45, 46). Two methods of meta-regression were used. The main method was generalized estimating equation regression models using an exchangeable correlation matrix together with empirical robust variance estimates. A second method based on a DerSimonian and Laird random-effects meta-regression analysis was undertaken for comparison. The full details are provided in the supplemental material.
Data availability.
The data sets analyzed during the current study are provided in the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Australian Government Department of Health and Ageing through the Rural Clinical Training and Support (RCTS) program.
J.C.H. declares that he has no competing interests.
As the sole author, J.C.H. produced the design of the study, performed the statistical analysis, and wrote the manuscript. J.C.H. read and approved the final manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00291-18.
REFERENCES
- 1.de Smet AMGA, Kluytmans JAJW, Cooper BS, Mascini EM, Benus RFJ, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ. 2009. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 2.Liberati A, D'Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. 2009. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 4:CD000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pileggi C, Bianco A, Flotta D, Nobile CG, Pavia M. 2011. Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care 15:R155. doi: 10.1186/cc10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestri L, Van Saene HK, Casarin A, Berlot G, Gullo A. 2008. Impact of selective decontamination of the digestive tract on carriage and infection due to Gram-negative and Gram-positive bacteria: a systematic review of randomised controlled trials. Anaesth Intensive Care 36:324–338. [DOI] [PubMed] [Google Scholar]
- 5.Vandenbroucke-Grauls CM, Vandenbroucke JP. 1991. Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet 338:859–862. doi: 10.1016/0140-6736(91)91510-2. [DOI] [PubMed] [Google Scholar]
- 6.Hurley JC. 1995. Prophylaxis with enteral antibiotics in ventilated patients: selective decontamination or selective cross-infection? Antimicrob Agents Chemother 39:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan EY, Ruest A, Meade MO, Cook DJ. 2007. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 334:889–900. doi: 10.1136/bmj.39136.528160.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Cao Y, Liao C, Wu L, Gao F. 2010. Effect of histamine-2-receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: a meta-analysis of 10 randomized controlled trials. Crit Care 14:R194. doi: 10.1186/cc9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhazzani W, Almasoud A, Jaeschke R, Lo BW, Sindi A, Altayyar S, Fox-Robichaud A. 2013. Small bowel feeding and risk of pneumonia in adult critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care 17:R127. doi: 10.1186/cc12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrof EO, Dhaliwal R, Manzanares W, Johnstone J, Cook D, Heyland DK. 2012. Probiotics in the critically ill: a systematic review of the randomized trial evidence. Crit Care Med 40:3290–3302. doi: 10.1097/CCM.0b013e318260cc33. [DOI] [PubMed] [Google Scholar]
- 11.Subirana M, Solà I, Benito S. 2007. Closed tracheal suction systems versus open tracheal suction systems for mechanically ventilated adult patients. Cochrane Database Syst Rev 4:CD004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. 2011. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 39:1985–1991. doi: 10.1097/CCM.0b013e318218a4d9. [DOI] [PubMed] [Google Scholar]
- 13.Siempos II, Vardakas KZ, Kopterides P, Falagas ME. 2007. Impact of passive humidification on clinical outcomes of mechanically ventilated patients: a meta-analysis of randomized controlled trials. Crit Care Med 35:2843–2851. doi: 10.1097/00003246-200712000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Delaney A, Gray H, Laupland KB, Zuege DJ. 2006. Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care 10:R70. doi: 10.1186/cc4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, Gattinoni L. 2010. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 36:585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Liu Y. 2010. Effect of ventilator circuit changes on ventilator-associated pneumonia: a systematic review and meta-analysis. Respir Care 55:467–474. [PubMed] [Google Scholar]
- 17.Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. 2011. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis 11:845–854. doi: 10.1016/S1473-3099(11)70127-X. [DOI] [PubMed] [Google Scholar]
- 18.Alhazzani W, Smith O, Muscedere J, Medd J, Cook D. 2013. Toothbrushing for critically ill mechanically ventilated patients: a systematic review and meta-analysis of randomized trials evaluating ventilator-associated pneumonia. Crit Care Med 41:646–655. doi: 10.1097/CCM.0b013e3182742d45. [DOI] [PubMed] [Google Scholar]
- 19.Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. 1984. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 10:185–192. doi: 10.1007/BF00259435. [DOI] [PubMed] [Google Scholar]
- 20.Agodi A, Barchitta M, Cipresso R, Giaquinta L, Romeo MA, Denaro C. 2007. Pseudomonas aeruginosa carriage, colonization, and infection in ICU patients. Intensive Care Med 33:1155–1161. doi: 10.1007/s00134-007-0671-6. [DOI] [PubMed] [Google Scholar]
- 21.Wunderink RG, Mendoza DL. 2007. Epidemiology of Pseudomonas aeruginosa in the intensive care unit, p 218–225. In Rello J, Kollef M, Díaz E, Rodríguez A (ed), Infectious diseases in critical care. Springer, Berlin, Germany. [Google Scholar]
- 22.Oostdijk EA, Smits L, de Smet AM, Leverstein-van Hall MA, Kesecioglu J, Bonten MJ. 2013. Colistin resistance in gram-negative bacteria during prophylactic topical colistin use in intensive care units. Intensive Care Med 39:653–660. doi: 10.1007/s00134-012-2761-3. [DOI] [PubMed] [Google Scholar]
- 23.Kollef MH, Chastre J, Fagon JY, François B, Niederman MS, Rello J, Torres A, Vincent JL, Wunderink RG, Go KW, Rehm C. 2014. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med 42:2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 24.Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, Beysens AJ, de Leeuw PW, Stobberingh EE. 2001. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 164:382–388. doi: 10.1164/ajrccm.164.3.2005003. [DOI] [PubMed] [Google Scholar]
- 25.Bonten MJ, Gaillard CA, Johanson WG Jr, van Tiel FH, Smeets HG, van der Geest S, Stobberingh EE. 1994. Colonization in patients receiving and not receiving topical antimicrobial prophylaxis. Am J Respir Crit Care Med 150:1332–1340. doi: 10.1164/ajrccm.150.5.7952561. [DOI] [PubMed] [Google Scholar]
- 26.Winter R, Humphreys H, Pick A, MacGowan AP, Willatts SM, Speller DC. 1992. A controlled trial of selective decontamination of the digestive tract in intensive care and its effect on nosocomial infection. J Antimicrob Chemother 30:73–87. doi: 10.1093/jac/30.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Hurley JC. 2013. The perfidious effect of topical placebo: calibration of Staphylococcus aureus ventilator-associated pneumonia incidence within selective digestive decontamination studies versus the broader evidence base. Antimicrob Agents Chemother 57:4524–4531. doi: 10.1128/AAC.00424-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley JC. 2005. Inapparent outbreaks of ventilator-associated pneumonia: an ecological analysis of prevention and cohort studies. Infect Control Hosp Epidemiol 26:374–390. doi: 10.1086/502555. [DOI] [PubMed] [Google Scholar]
- 29.Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, Meakins JL, Soussy CJ, Lemaire F. 1989. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli: study of an outbreak in an intensive care unit. Ann Intern Med 110:873–881. doi: 10.7326/0003-4819-110-11-873. [DOI] [PubMed] [Google Scholar]
- 30.Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, Eskira S, Yousef B, Smolykov R, Codish S, Borer A. 2012. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol 33:14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 31.Agusti C, Pujol M, Argerich MJ, Ayats J, Badia M, Dominguez MA, Corbella X, Ariza J. 2002. Short-term effect of the application of selective decontamination of the digestive tract on different body site reservoir ICU patients colonized by multi-resistant Acinetobacter baumannii. J Antimicrob Chemother 49:205–208. doi: 10.1093/jac/49.1.205. [DOI] [PubMed] [Google Scholar]
- 32.Brown RB, Phillips D, Barker MJ, Pleczarka R, Sands M, Tares D. 1989. Outbreak of nosocomial Flavobacterium meningosepticum respiratory infections associated with use of aerosolized polymyxin B. Am J Infect Control 17:121–125. doi: 10.1016/0196-6553(89)90197-1. [DOI] [PubMed] [Google Scholar]
- 33.Halaby T, al Naiemi N, Kluytmans J, van der Palen J, Vandenbroucke-Grauls CM. 2013. Emergence of colistin resistance in Enterobacteriaceae after the introduction of selective digestive tract decontamination in an intensive care unit. Antimicrob Agents Chemother 57:3224–3229. doi: 10.1128/AAC.02634-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lübbert C, Faucheux S, Becker-Rux D, Laudi S, Dürrbeck A, Busch T, Gastmeier P, Eckmanns T, Rodloff AC, Kaisers UX. 2013. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: a single-centre experience. Int J Antimicrob Agents 42:565–570. doi: 10.1016/j.ijantimicag.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Hurley JC. 2015. ICU-acquired candidemia within selective digestive decontamination studies: a meta-analysis. Intensive Care Med 41:1877–1885. doi: 10.1007/s00134-015-4004-x. [DOI] [PubMed] [Google Scholar]
- 36.Hurley JC. 2018. Unusually high incidences of Staphylococcus aureus infection within studies of ventilator associated pneumonia prevention using topical antibiotics: benchmarking the evidence base. Microorganisms 6:E2. doi: 10.3390/microorganisms6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurley JC. 2016. Impact of selective digestive decontamination on respiratory tract Candida among patients with suspected ventilator-associated pneumonia. A meta-analysis. Eur J Clin Microbiol Infect Dis 35:1121–1135. doi: 10.1007/s10096-016-2643-7. [DOI] [PubMed] [Google Scholar]
- 38.Hurley JC. 2014. Ventilator-associated pneumonia prevention methods using topical antibiotics: herd protection or herd peril? Chest 146:890–898. doi: 10.1378/chest.13-2926. [DOI] [PubMed] [Google Scholar]
- 39.Oostdijk EA, de Smet AM, Blok HE, Thieme Groen ES, van Asselt GJ, Benus RF, Bernards SA, Frénay IH, Jansz AR, de Jongh BM, Kaan JA. 2010. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med 181:452–457. doi: 10.1164/rccm.200908-1210OC. [DOI] [PubMed] [Google Scholar]
- 40.Frencken JF, Wittekamp BH, Plantinga NL, Spitoni C, van de Groep K, Cremer OL, Bonten MJ. 2018. Associations between enteral colonization with Gram-negative bacteria and intensive care unit-acquired infections and colonization of the respiratory tract. Clin Infect Dis 66:497–503. doi: 10.1093/cid/cix824. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong PJ, Barr JG, Webb CH, Blair PH, Rowlands BJ. 1992. Epidemiology of Pseudomonas aeruginosa in an intensive care unit using selective decontamination of the digestive tract. J Hosp Infect 20:199–208. doi: 10.1016/0195-6701(92)90088-4. [DOI] [PubMed] [Google Scholar]
- 42.Hartenauer U, Thulig B, Diemer W, Lawin P, Fegeler W, Kehrel R, Ritzerfeld W. 1991. Effect of selective flora suppression on colonization, infection, and mortality in critically ill patients: a one-year, prospective consecutive study. Crit Care Med 19:463–473. doi: 10.1097/00003246-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Feeley TW, Du Moulin GC, Hedley-Whyte J, Bushnell LS, Gilbert JP, Feingold DS. 1975. Aerosol polymyxin and pneumonia in seriously ill patients. N Engl J Med 293:471–475. doi: 10.1056/NEJM197509042931003. [DOI] [PubMed] [Google Scholar]
- 44.Klick JM, Du Moulin GC, Hedley-Whyte J, Teres D, Bushnell LS, Feingold DS. 1975. Prevention of gram-negative bacillary pneumonia using polymyxin aerosol as prophylaxis. II. Effect on the incidence of pneumonia in seriously ill patients. J Clin Invest 55:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurley JC. 2014. Topical antibiotics as a major contextual hazard toward bacteremia within selective digestive decontamination studies: a meta-analysis. BMC Infect Dis 14:714. doi: 10.1186/s12879-014-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurley JC. 2011. Paradoxical ventilator associated pneumonia incidences among selective digestive decontamination studies versus other studies of mechanically ventilated patients. Benchmarking the evidence base. Crit Care 15:R7. doi: 10.1186/cc9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analyzed during the current study are provided in the supplemental material.