WCK 5222 is a combination of cefepime and the novel β-lactam enhancer zidebactam being developed for the treatment of serious Gram-negative bacterial infections. The objective of this study was to compare plasma (total), epithelial lining fluid (ELF), and alveolar macrophage (AM) concentrations of cefepime and zidebactam in healthy adult subjects.
KEYWORDS: WCK 5222, alveolar macrophages, cefepime, epithelial lining fluid, pharmacokinetics, zidebactam
ABSTRACT
WCK 5222 is a combination of cefepime and the novel β-lactam enhancer zidebactam being developed for the treatment of serious Gram-negative bacterial infections. The objective of this study was to compare plasma (total), epithelial lining fluid (ELF), and alveolar macrophage (AM) concentrations of cefepime and zidebactam in healthy adult subjects. The WCK 5222 dosing regimen was 2 g cefepime/1 g zidebactam administered as a 1-h intravenous infusion every 8 h for a total of 7 doses. Subjects were assigned to one bronchoalveolar lavage (BAL) sampling time at 0.5, 1.25, 3, 6, 8, or 10 h after the seventh dose. Noncompartmental pharmacokinetic parameters were determined from serial plasma concentrations collected over 8-hour and 10-hour intervals following the first and seventh doses, respectively. Penetration ratios were calculated from the area under the plasma concentration-time curve from 0 to 8 h (AUC0–8) for plasma, ELF, and AM using mean and median concentrations at each BAL sampling time. The plasma maximum concentration of drug (Cmax) and AUC values of cefepime and zidebactam increased by 8% to 9% after the seventh versus the first dose of WCK 5222. The respective AUC0–8 values based on mean concentrations of cefepime and zidebactam in ELF were 127.9 and 52.0 mg · h/liter, and 87.9 and 13.2 mg · h/liter in AM. The ELF to total plasma penetration ratios of cefepime and zidebactam based on mean AUC0–8 values were 0.39 and 0.38, respectively. The AM to total plasma ratios were 0.27 and 0.10, respectively. The observed plasma, ELF, and AM concentrations of cefepime and zidebactam support studies of WCK 5222 for treatment of pneumonia caused by susceptible pathogens.
INTRODUCTION
WCK 5222 is a combination of cefepime and zidebactam that is currently undergoing a clinical development program for the treatment of multidrug-resistant infections caused by Gram-negative bacteria (1–4). Zidebactam (WCK 5107) is a novel non-β-lactam bicyclo-acyl hydrazide that has a dual mechanism of action, including both β-lactamase and penicillin-binding protein 2 (PBP2) inhibition (2–6). Zidebactam is considered a broad-spectrum inhibitor of Ambler class A, C, and D β-lactamases; however, it is not an inhibitor of metallo-β-lactamases (MBLs). Zidebactam can also selectively bind to Gram-negative PBP2 with high affinity. Cefepime has high affinity for Gram-negative PBP3 and modest affinity for PBP1a/1b and PBP2. Zidebactam acts as a “β-lactam enhancer” with antibacterial activity through complementary PBP binding even when resistance mechanisms impact the availability of cefepime (5, 6). Several recent reports have demonstrated the in vitro antimicrobial activity of WCK 5222 against multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter spp. (2–4). WCK 5222 has also shown in vitro activity against strains of colistin-nonsusceptible mcr-1- and MBL-producing Gram-negative pathogens (2–6). The U.S. Food and Drug Administration has granted a fast-track clinical trial and approval process (QIDP status) for WCK 5222.
Zidebactam and WCK 5222 have completed several phase 1 studies evaluating the safety, tolerability, and pharmacokinetics of single and multiple ascending intravenous doses in healthy adult subjects (ClinicalTrials.gov registration no. NCT02674347 and NCT02707107). In brief, the pharmacokinetics of cefepime and zidebactam were linear and demonstrated dose-proportional increases in plasma exposure parameters, including maximum concentration of drug (Cmax) and area under the plasma concentration-time curve (AUC) (7–10). The two agents had similar pharmacokinetic parameters, including an average total body clearance (CL) between 4 and 8 liters/h and apparent volume of distribution (V) between 15 and 18 liters. The plasma protein binding rates of cefepime and zidebactam were approximately 20% and 15%, respectively. The mean terminal elimination half-lives (t1/2) of cefepime and zidebactam were approximately 2.0 and 1.8 h, respectively. Both cefepime and zidebactam were primarily (≥80%) excreted as unchanged drug in the urine.
Bronchoalveolar lavage (BAL) has become a reliable method for measuring antibiotic concentrations in the lungs of healthy subjects or patients undergoing diagnostic bronchoscopy (11, 12). Epithelial lining fluid (ELF) and alveolar macrophages (AM) are considered the relevant intrapulmonary infection sites of extracellular and intracellular pathogens, respectively, in acute bacterial pneumonia (11–14). The primary objective of this study was to compare plasma and intrapulmonary concentrations of cefepime and zidebactam and to determine the tolerability and safety of intravenous administration of WCK 5222 in healthy adult male and female subjects.
RESULTS
Subjects.
Forty-six adult male and female subjects signed informed consent and were screened for participation in the study. Ten subjects did not meet the inclusion and exclusion criteria. A total of 36 subjects were included in the study, having received at least one dose of WCK 5222, and are included in the safety assessment (Table 1). One subject (assigned to the 1.25-hour BAL sampling time) was withdrawn from the study due to an adverse event.
TABLE 1.
Characteristics of 36 healthy adult subjects enrolled in the studya
| Sampling time (h)b | Sex of patientsc | Age (yr) | Height (cm) | Weight (kg) | eCLCRd (ml/min) | Total cell count in BAL fluid (cells/mm3) | Macrophages (%) |
|---|---|---|---|---|---|---|---|
| 0.5 | 5 M, 1 F | 37 ± 7 | 180 ± 12 | 88.9 ± 15.2 | 133 ± 26 | 92 ± 27 | 84 ± 9 |
| 1.25 | 3 M, 3 F | 43 ± 11 | 172 ± 6 | 73.7 ± 11.5 | 96 ± 9 | 110 ± 38e | 80 ± 16c |
| 3 | 6 M | 43 ± 7 | 175 ± 7 | 79.8 ± 7.7 | 106 ± 21 | 132 ± 52 | 89 ± 7 |
| 6 | 5 M, 1 F | 37 ± 10 | 176 ± 7 | 78.3 ± 10.6 | 122 ± 20 | 65 ± 41 | 78 ± 12 |
| 8 | 5 M, 1 F | 45 ± 6 | 172 ± 9 | 77.1 ± 13.4 | 116 ± 38 | 118 ± 79 | 76 ± 21 |
| 10 | 5 M, 1 F | 38 ± 9 | 172 ± 7 | 75.0 ± 12.7 | 110 ± 21 | 131 ± 62 | 82 ± 8 |
Data are expressed as means ± SD except for the data on sex.
Six subjects per sampling period.
M, male; F, female.
eCLCR, estimated creatinine clearance based on the Cockcroft-Gault equation (40).
Five subjects had BAL samples to determine total cell and macrophage counts.
Three subjects (8.3%) experienced a total of three treatment-emergent adverse events (TEAEs) over the course of the study. Two subjects experienced headaches, which were mild and were considered not related to study drug administration. One subject had a hypersensitivity reaction, which was moderate in severity and considered certainly related to WCK 5222 administration. This TEAE occurred with the first dose of WCK 5222, and the subject was withdrawn from further study activities. No clinically significant changes were observed in clinical laboratory test parameters, vital signs, electrocardiogram (ECG) results, and physical examination findings.
Thirty-five subjects completed all study procedures and were included in the pharmacokinetic analyses. The most notable difference in demographic characteristics was gender, with equal numbers (n = 3) of male and female subjects enrolled to the 1.25-hour BAL sampling time, whereas five or six male subjects were included at each of the other sampling times (Table 1).
Pharmacokinetics.
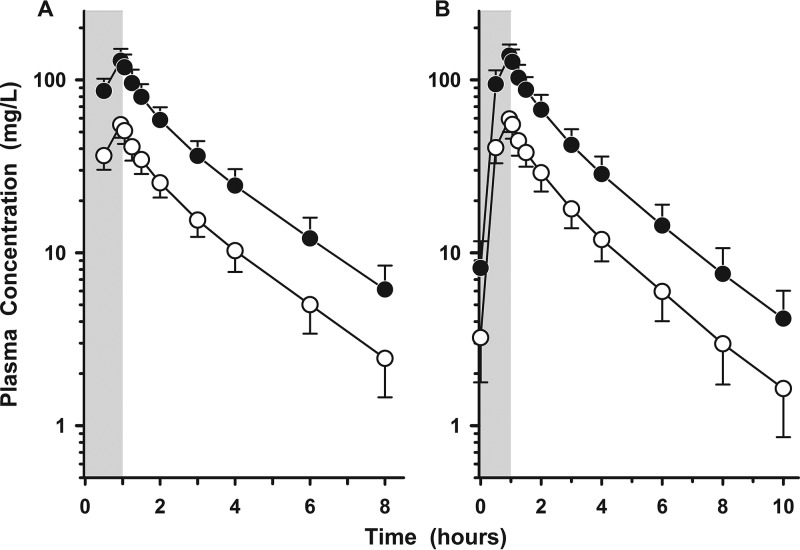
Figure 1A and B display the plasma concentration-time profiles of cefepime and zidebactam after the first and seventh intravenous doses of WCK 5222, respectively. Plasma trough concentrations (Cmin) of cefepime (i.e., means ± standard deviations [SD] of 6.13 ± 2.30, 7.48 ± 2.99, 8.87 ± 3.23, 7.49 ± 3.06, and 7.35 ± 3.20 mg/liter before the second, third, fourth, fifth, and sixth doses, respectively) and zidebactam (i.e., means ± SD of 2.45 ± 0.99, 2.97 ± 1.25, 3.54 ± 1.33, 3.01 ± 1.28, and 3.01 ± 1.35 mg/liter before the second, third, fourth, fifth, and sixth doses, respectively) marginally increased with repeated dosing. Table 2 lists the mean (±SD) pharmacokinetic parameters of cefepime and zidebactam in plasma after the first and seventh intravenous doses of WCK 5222. The average increases in Cmax and AUC of cefepime and zidebactam from the first and seventh doses were approximately 9% and 8%, respectively.
FIG 1.
Mean (±SD) plasma concentration-versus-time profile of cefepime (filled circles) and zidebactam (open circles) with the first (A) and seventh (B) doses of WCK 5222 (2 g cefepime/1 g zidebactam) administered as a 1-h intravenous infusion every 8 h. The shaded regions represent the 1-h infusion period. The y axes are in log scale.
TABLE 2.
Pharmacokinetic parameters of cefepime and zidebactam in plasma following the first and seventh intravenous dosesa
| Drug and dose no. | Cmax (mg/liter) | AUCb (mg · h/liter) | t1/2 (h) | Vss (liters) | CL (liters/h) |
|---|---|---|---|---|---|
| Cefepime | |||||
| First | 129.3 ± 22.2 | 305.7 ± 60.7 | 1.8 ± 0.2 | 15.4 ± 2.4 | 6.79 ± 1.32 |
| Seventh | 139.5 ± 21.4 | 327.0 ± 63.7 | 2.0 ± 0.2 | 15.4 ± 2.9 | 6.36 ± 1.35 |
| Zidebactam | |||||
| First | 55.4 ± 8.6 | 129.4 ± 24.1 | 1.8 ± 0.2 | 17.6 ± 2.7 | 8.00 ± 1.52 |
| Seventh | 60.0 ± 9.0 | 139.5 ± 25.6 | 1.9 ± 0.3 | 17.4 ± 3.2 | 7.44 ± 1.54 |
The data are from 35 subjects per parameter estimate and are expressed as means ± SD.
The AUC for the first dose was the AUC0–∞, and the AUC for the seventh dose was the AUC0–8.
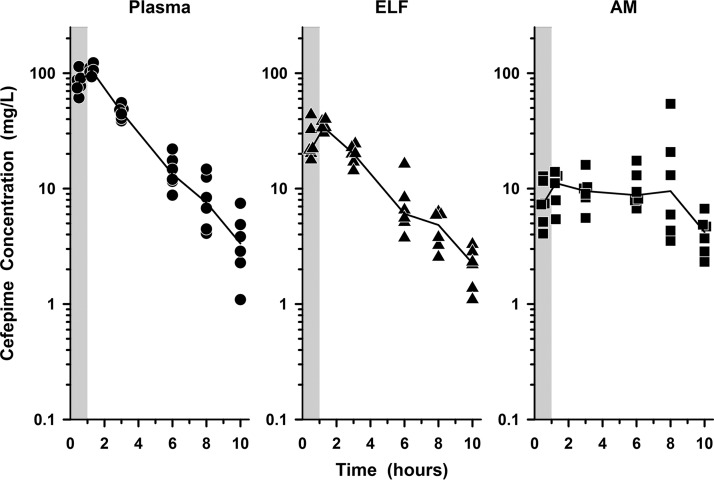
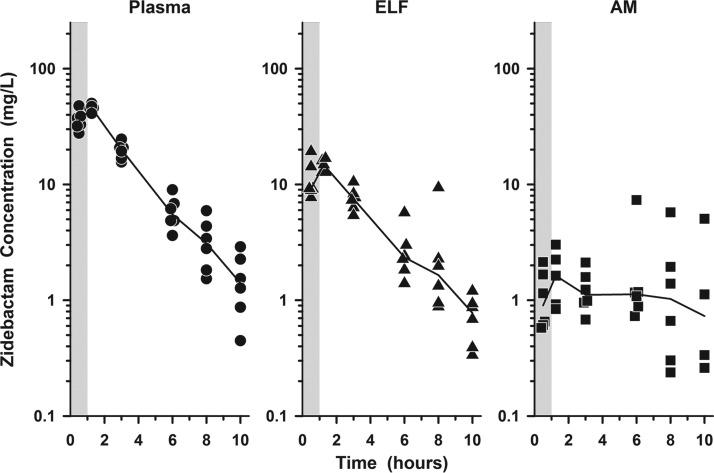
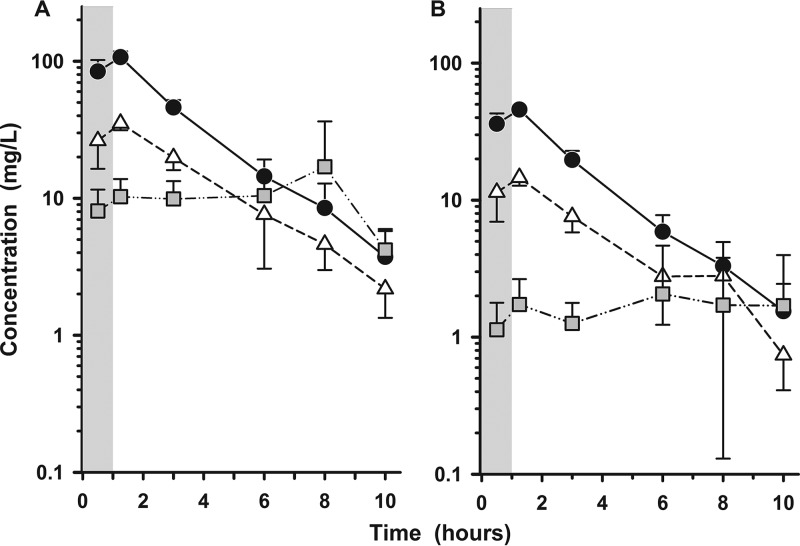
The individual concentrations of cefepime and zidebactam in plasma, ELF, and AM at the BAL sampling times are illustrated in Fig. 2 and 3, respectively. The mean (±SD) concentrations of cefepime and zidebactam after the seventh dose in plasma, ELF, and AM for the six bronchopulmonary sampling time points are reported in Tables 3 and 4, respectively. The mean (±SD) concentrations of cefepime and zidebactam in plasma, ELF, and AM at the bronchopulmonary sampling times are illustrated in Fig. 4A and B, respectively.
FIG 2.
Individual concentrations of cefepime in plasma (circles), ELF (triangles), and AM (squares) at 0.5, 1.25, 3, 6, 8, and 10 h after the seventh dose of WCK 5222 (2 g cefepime/1 g zidebactam) administered as a 1-h intravenous infusion every 8 h. The lines represent the median concentrations. The shaded regions represent the 1-h infusion period. The y axes are in log scale.
FIG 3.
Individual concentrations of zidebactam in plasma (circles), ELF (triangles), and AM (squares) at 0.5, 1.25, 3, 6, 8, and 10 h after the seventh dose of WCK 5222 (2 g cefepime/1 g zidebactam) administered as a 1-h intravenous infusion every 8 h. The lines represent the median concentrations. The shaded regions represent the 1-h infusion period. The y axes are in log scale.
TABLE 3.
Cefepime concentrations and ratios in plasma (total), ELF, and AM at various times of bronchoscopy and BALa
| Sampling time (h) | Cefepime concn (mg/liter) in: |
Ratio of cefepime |
|||
|---|---|---|---|---|---|
| Plasma (total) | ELF | AM | ELF to plasma (total) | AM to plasma (total) | |
| 0.5b | 84.06 ± 17.94 | 26.31 ± 9.89 | 8.07 ± 3.49 | 0.31 ± 0.07 | 0.09 ± 0.03 |
| 1.25c | 107.04 ± 11.03 | 35.24 ± 3.89 | 10.28 ± 3.55 | 0.33 ± 0.06 | 0.10 ± 0.04 |
| 3b | 45.97 ± 6.16 | 19.77 ± 3.71 | 9.90 ± 3.46 | 0.43 ± 0.08 | 0.22 ± 0.07 |
| 6b | 14.43 ± 4.76 | 7.62 ± 4.56 | 10.45 ± 4.06 | 0.52 ± 0.18 | 0.76 ± 0.27 |
| 8b | 8.50 ± 4.34 | 4.62 ± 1.63 | 16.99 ± 19.41 | 0.60 ± 0.18 | 1.83 ± 1.44 |
| 10b | 3.73 ± 2.24 | 2.18 ± 0.84 | 4.19 ± 1.58 | 0.74 ± 0.42 | 1.35 ± 0.61 |
The data are expressed as means ± SD.
Six subjects per sampling period.
Five subjects per sampling period.
TABLE 4.
Zidebactam concentrations and ratios in plasma (total), ELF, and AM at various times of bronchoscopy and BALa
| Sampling time (h) | Zidebactam concn (mg/liter) in: |
Ratio of zidebactam |
|||
|---|---|---|---|---|---|
| Plasma (total) | ELF | AM | ELF to plasma (total) | AM to plasma (total) | |
| 0.5b | 36.03 ± 6.94 | 11.41 ± 4.46 | 1.13 ± 0.65 | 0.31 ± 0.07 | 0.03 ± 0.01 |
| 1.25c | 45.80 ± 3.29 | 14.61 ± 1.87 | 1.73 ± 0.92 | 0.32 ± 0.05 | 0.04 ± 0.02 |
| 3b | 19.65 ± 3.22 | 7.59 ± 1.77 | 1.26 ± 0.52 | 0.39 ± 0.09 | 0.06 ± 0.03 |
| 6b | 5.89 ± 1.89 | 2.77 ± 1.54 | 2.06 ± 2.59 | 0.46 ± 0.15 | 0.34 ± 0.37 |
| 8b | 3.30 ± 1.65 | 2.80 ± 3.27 | 1.71 ± 2.08 | 0.95 ± 1.19 | 0.48 ± 0.47 |
| 10b | 1.55 ± 0.90 | 0.74 ± 0.33 | 1.70 ± 2.28d | 0.55 ± 0.21 | 1.15 ± 1.18d |
The data are expressed as means ± SD.
Six subjects per sampling period.
Five subjects per sampling period.
Four samples (2 of 6 samples were below the quantification limits).
FIG 4.
Mean (±SD) plasma (circles), ELF (triangles), and AM (squares) concentrations of cefepime (A) and zidebactam (B) at the BAL sampling times after the seventh dose of WCK 5222 (2 g cefepime/1 g zidebactam) administered as a 1-h intravenous infusion every 8 h. The shaded regions represent the 1-h infusion period. The y axes are in log scale.
The mean ratios of ELF and AM to simultaneous total plasma concentrations of cefepime during the 10-hour period after the seventh dose ranged from 0.31 to 0.74 and 0.09 to 1.83, respectively (Table 3). The AUC0–8 values based on mean and median ELF concentrations of cefepime were 127.9 and 119.7 mg · h/liter, respectively. The ratio of ELF to total plasma cefepime concentrations based on the mean and median AUC0–8 values were 0.39 and 0.38, respectively. The AUC0–8 values based on mean and median AM concentrations of cefepime were 87.9 and 74.9 mg · h/liter, respectively. The ratio of AM to total plasma cefepime concentrations based on the mean and median AUC0–8 values were 0.27 and 0.24, respectively.
The respective mean ratios of ELF or AM to simultaneous total plasma concentrations of zidebactam at the BAL sampling times ranged from 0.31 to 0.95 and 0.03 to 1.15 (Table 4).
The AUC0–8 values based on mean and median ELF concentrations of zidebactam were 52.0 and 47.8 mg · h/liter, respectively. The ratio of ELF to total plasma zidebactam concentrations based on the mean and median AUC0–8 values were 0.38 and 0.35, respectively. The AUC0–8 values based on mean and median AM concentrations of zidebactam were 13.2 and 9.3 mg · h/liter, respectively. The ratio of AM to total plasma zidebactam concentrations based on the mean and median AUC0–8 values were 0.10 and 0.07, respectively.
DISCUSSION
The plasma concentration-time profiles of cefepime and zidebactam in this study were similar to those observed in previous pharmacokinetic studies in healthy subjects (7–10). The observed values for Cmax and AUC of cefepime (Table 2) in this study were within the respective geometric mean ranges of 130 to 160 mg/liter and 341 to 479 mg · h/liter seen in recent studies (8, 15), as well as arithmetic mean values of 129 to 137 mg/liter and 238 to 263 mg · h/liter reported from early phase 1 studies of cefepime (9, 10). The observed t1/2 of cefepime ranged between 1.8 and 2.3 h and were similar to those from other studies. The largest observed differences occurred with CL, where a larger range of values (4.33 to 8.58 liters/h) were reported in other phase 1 studies (8–10, 15). A similar trend was observed for the pharmacokinetic parameters of zidebactam, where the mean Cmax, AUC, and t1/2 values (Table 2) were within the ranges for geometric mean values (57.2 to 66.3 mg/liter, 144 to 172 mg · h/liter, and 1.7 to 2.1 h, respectively) reported in recent pharmacokinetic studies (7, 8, 16). In line with previous reports, we observed minimal accumulation in plasma concentrations for either drug (e.g., Cmax and AUC values increased by 8% to 9%) between the first and subsequent doses when the dosing interval was every 8 h (7–10). The most likely explanations for minor differences in parameter values between studies include the length of intravenous infusion (e.g., 30 versus 60 min), blood-sampling schemes, analytical assays, and subject characteristics (e.g., age, weight, and gender).
The mean plasma Cmax and AUC values for cefepime were approximately 2.33 times higher than for zidebactam. This is explained, in part, by the difference in the doses of each agent administered (e.g., 2 g of cefepime versus 1 g of zidebactam). In addition, the apparent volume of distribution and CL values were slightly higher for zidebactam than for cefepime (Table 2). Plasma protein binding levels are similar for both agents. The mean plasma protein binding of cefepime is approximately 20% and is concentration independent (17). The mean plasma protein binding of zidebactam in humans is <15% over a concentration range of 10 to 100 mg/liter (data not shown). Protein binding analyses were not conducted as part of this study. The measured concentrations in ELF and AM were assumed to represent unbound concentrations, since only unbound plasma fractions are considered to penetrate the lung compartments. Since both agents have low plasma protein binding, the reported penetration ratios in this study are based on total plasma concentrations.
The time courses and patterns of simultaneous concentrations in plasma and ELF were similar for both cefepime and zidebactam. However, the magnitude of concentrations in ELF differs from the systemic exposure in plasma. The ELF exposures (based on the AUC0–8) for cefepime and zidebactam were approximately 35% to 39% of the total plasma concentrations. The penetration ratios for cefepime are similar to those reported in animal studies and are lower than those observed with continuous infusions in critically ill patients (18, 19). The ratios of ELF to total plasma concentrations of cefepime and zidebactam gradually increased (e.g., from approximately 0.31 to 0.95) throughout the 10-hour BAL sampling times after the seventh dose (Tables 3 and 4). The changing ratios throughout the BAL sampling times illustrate why AUC determination of plasma and site compartments is the preferred method for determining penetration ratios of intermittent dosing (e.g., every 8 h) (20).
Penetration into ELF has been considered an important characteristic of antibiotics for the treatment of serious hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) (11, 12, 21, 22). WCK 5222, as a combination of cefepime and zidebactam, has demonstrated potent in vitro and in vivo activity against both MDR and XDR extracellular pathogens commonly associated with HABP/VABP (2–4). The MIC90 values of WCK 5222 for 1:1 and 2:1 ratios of cefepime-zidebactam against carbapenem-resistant Enterobacteriaceae (n = 153), colistin-nonsusceptible Klebsiella spp. (n = 54), and Pseudomonas aeruginosa (n = 1,291) were 4.0 and 8.0 mg/liter, respectively (2, 3). Rapid bactericidal activity due to the novel β-lactam enhancer effects of zidebactam contribute to these lower MIC values of WCK 5222 compared to cefepime or zidebactam tested alone (2–6).
The pharmacodynamic parameter target value for cefepime (e.g., percent time of unbound concentrations during the dosing interval above the MIC value [%fT>MIC]) is lower when administered with zidebactam than when cefepime is given alone (23). When administered as WCK 5222, the %fT>MIC values of cefepime needed for >2 log10 kill of P. aeruginosa (WCK 5222 MIC = 1 mg/liter) and Acinetobacter baumannii (WCK 5222 MIC = 16 mg/liter) in the murine neutropenic infection model of the lung were 16.1 and 12.0, respectively (23). In comparison, cefepime alone required %fT>MIC target values of 46.8 and 40, respectively. A threshold concentration range between 1 and 8 mg/liter for zidebactam has also been identified for the efficacy of WCK 5222 against carbapenemase-producing isolates of Klebsiella pneumoniae and P. aeruginosa, MBL-expressing P. aeruginosa, and carbapenem-resistant A. baumannii (24–26). The target values for %fT>MIC of cefepime and threshold concentrations of zidebactam are likely to be achieved in both plasma and ELF based on the observed concentration-time profiles in our healthy subjects. Greater variability in plasma and ELF concentrations of β-lactam agents has been observed in critically ill patients (compared with healthy adult subjects), and alterations in pharmacokinetic parameters and/or intrapulmonary penetration should also be considered in designing dosage regimens of WCK 5222 for the treatment of severe nosocomial pneumonia (11, 27, 28).
The ELF exposure of zidebactam at the intended therapeutic dose of 1 g every 8 h was found to be higher than those observed for the β-lactam inhibitors relebactam and avibactam. The mean AUC in the ELF after multiple intravenous doses of relebactam 250 mg every 6 h and avibactam 500 mg every 8 h were reported as 12.2 and 13.7 mg · h/liter, respectively, compared to 52 mg · h/liter of zidebactam (29, 30). The favorable pharmacokinetic profiles in ELF and plasma, along with the rapid bactericidal action through its novel mechanism of action, provide zidebactam with advantageous features for the treatment of nosocomial pneumonia.
Concentrations of cefepime and zidebactam were detected in alveolar macrophages. The concentrations tended to remain constant over the 10 sampling periods at approximately 10 mg/liter for cefepime and between 1 and 2 mg/liter for zidebactam. Thus, intrapulmonary penetration of cefepime and zidebactam based on AUC0–8 values of mean AM and total plasma concentrations were 27% and 10%, respectively. Although β-lactam antibiotics are considered to have poor cellular uptake, measured AM concentrations have been observed in older single-dose studies of the oral β-lactams amoxicillin and cefuroxime (31, 32). Previous studies have observed AM concentrations of the β-lactamase inhibitors clavulanate, vaborbactam (RPX7009), and relebactam at magnitudes similar to that of zidebactam in this study (29, 31, 33). Potential explanations for the observed AM concentrations may be the higher dose of cefepime (2 g every 8 h), administration of seven doses of WCK 5222, and the lower quantification level of the liquid chromatography-tandem mass spectrometry (LC–MS-MS) assay. The clinical significance of these findings has not been determined.
In summary, intravenous administration of WCK 5222 (2 g cefepime and 1 g zidebactam) every 8 h for seven doses was observed to be safe and well tolerated. The concentrations of cefepime in plasma and ELF were approximately 2.33 times higher than the zidebactam concentrations in both matrices. The intrapulmonary penetrations of cefepime and zidebactam into ELF were nearly identical at approximately 38% of the total plasma concentrations. Concentrations of cefepime and zidebactam were detected in AM over the 10-hour sampling period and tended to remain at constant values of approximately 10 mg/liter and 1 to 2 mg/liter, respectively. The AM penetrations of cefepime and zidebactam based on AM and total plasma mean AUC0–8 values were 27% and 10%, respectively. Overall, the data obtained in this study support further consideration of WCK 5222 for treatment of severe nosocomial pneumonia caused by susceptible extracellular pathogens.
MATERIALS AND METHODS
Study design and subjects.
This was a phase 1, multiple-dose, open-label pharmacokinetic study conducted in healthy adult male and female subjects at Pulmonary Associates, PA (Phoenix, AZ, USA). The protocol was reviewed and approved by the Institutional Review Board at Quorum Review (Seattle, WA, USA). The study protocol and procedures were completely explained to each subject, and all participants provided written informed consent before any study procedures were initiated. The study was performed in accordance with the principles outlined in good clinical practice guidelines.
Nonsmoking, healthy subjects between 18 and 55 years old of either gender were eligible for study entry. The subjects were required to have a body mass index and total body weight between 18.5 and 30 kg/m2 and 55 and 100 kg, respectively. Subject eligibility was confirmed based on medical history, physical examination, vital signs, 12-lead ECGs, pulmonary function test, and screening laboratory tests (hematology, blood chemistry, and urinalysis). Male subjects engaging in sexual activity were required to use two highly effective methods of birth control from the time of the screening visit until 90 days following the last dose of WCK 5222. Male subjects were also not allowed to donate sperm during this time period. Female subjects of childbearing potential were required to use two highly effective methods of birth control (as defined in the protocol) from the screening visit until 30 days following the last dose of WCK 5222. Subjects had to be willing to refrain from consumption of alcohol, caffeine, or methylxanthine-containing beverages or food, Seville orange, and grapefruit or grapefruit juice from 48 h before entry into the clinical research center until the time of discharge.
Exclusion criteria included a history or presence of clinically significant medical disorders, surgeries, clinically significant infection (within 30 days before the screening visit), and a history of Clostridium difficile-induced diarrhea or infections within 1 year before the screening visit. Subjects with a known hypersensitivity or idiosyncratic reaction to β-lactam agents or a history of allergic or other serious adverse reactions to lidocaine or benzodiazepines were excluded. Female subjects could not be pregnant or lactating. Subjects were excluded if a clinically significant pulmonary or any other disease would increase the risk of a standardized bronchoscopy and BAL. Subjects must not have had a >500-ml blood loss or blood/plasma donation within 60 days before the screening visit. Subjects with a history of seizures, head injury, or meningitis; a bleeding disorder; and evidence of difficulty in donating blood were excluded. Laboratory test findings that excluded subjects included a positive result for human immunodeficiency virus, hepatitis B virus surface antigen, or anti-hepatitis C virus antibody at screening; a white blood cell count of <3,000/mm3; an absolute neutrophil count of <1,200/mm3; hemoglobin of <11 g/dl; a platelet count of <120,000/mm3; estimated creatinine clearance of <60 ml/min (Cockroft-Gault equation [40]) at screening or confinement; and a positive alcohol breath test or urine drug screen test at screening or confinement. The subjects must not have had a baseline QT interval using Fridericia's correction method (QTcF) of >450 ms for males or >470 ms for females or a history of prolonged QT syndrome. Subjects were not allowed to use tobacco or other smoking materials within 6 months of the screening visit or to have a history or presence of alcohol or drug abuse within the 2 years before screening. The subjects could not have received a prescription drug (with the exception of hormonal contraceptives or hormone replacement therapy) within 14 days before the first dose of WCK 5222. Unless prior approval was granted by the investigators and sponsor, the use of acetaminophen, multivitamins, or vitamin C and all other nonprescription medications (including health supplements and herbal remedies) was prohibited within 3, 7, and 14 days before the first dose of WCK 5222, respectively. Subjects were excluded if they received an investigational drug or device or participated in another research study within 30 days or 5 half-lives of the investigational agents (whichever was longer) before the screening visit.
The subjects received a total of seven intravenous doses of WCK 5222 (2 g cefepime/1 g zidebactam) administered every 8 h as an intravenous infusion over 1 h. Blood for determining plasma cefepime and zidebactam concentrations was collected within 15 min prior to and 0.5, 0.95, 1.05, 1.25, 1.5, 2, 3, 4, 6, and 8 h after the start of the first dose of WCK 5222. The 8-hour blood sample was collected before administration of the second dose of WCK 5222. A second serial blood collection for determining plasma cefepime and zidebactam concentrations was performed within 15 min prior to and 0.5, 0.95, 1.05, 1.25, 1.5, 2, 3, 4, 6, 8, and 10 h after the start of the seventh dose of WCK 5222. In addition, blood samples for determining drug concentrations were collected within 15 min before administration of the third, fourth, fifth, and sixth doses of WCK 5222.
The subjects were assigned to one of six bronchoscopy sampling times at either 0.5, 1.25, 3, 6, 8, or 10 h following the seventh dose of WCK 5222. Each subject underwent a standardized bronchoscopy with BAL, and samples were collected and processed to determine the cell count and differential in BAL fluid, urea concentrations in BAL fluid, and concentrations of cefepime and zidebactam in BAL fluid and cell pellets. A blood sample was collected at the time of bronchoscopy to determine plasma urea and drug concentrations. Details regarding bronchoscopy and BAL procedures, methods for sample handling, and storage conditions have been previously described (34–36).
Determination of cefepime and zidebactam concentrations.
Plasma, BAL fluid, and cell pellet concentrations of cefepime and zidebactam were determined by a high-performance LC–MS-MS method at Keystone Bioanalytical (North Wales, PA) (report numbers 170506.00 and 170615.00). Eighteen analytical runs were used to measure drug concentrations in 947 plasma samples between 16 May 2017 and 22 June 2017. Ninety-four samples (9.93%) were reassayed to assess the incurred sample assay reproducibility (ISR) of previously analyzed samples.
An LC–MS-MS procedure was developed and validated for the quantification of cefepime and zidebactam in sodium heparin human plasma. In brief, the LC–MS-MS system consisted of an Applied Biosystems Sciex 5500 QTRAP tandem mass spectrometer, a Shimadzu SIL-30AC autosampler, two Shimadzu LC-20AD pumps, and Unison UK-C18 3-μm, 50- by 3-mm analytical columns. Cefepime, zidebactam, and the internal standards (cefepime-d3 and avibactam were used as the internal standards for cefepime and zidebactam, respectively) were isolated using protein precipitation (0.1% formic acid in methanol was used as a solvent). After vortex mixing and centrifugation, 14 μl of the supernatant was transferred to clean plastic injection vials, which contained 1 ml of 10 mM ammonium acetate in water. After vortex mixing, 250 μl of the reconstituted samples was transferred to autosampler injection vials. A total injection volume between 5 and 15 μl was used for LC–MS-MS analysis. Quantification of drug concentrations was performed using Analyst software (version 1.5.2; Sciex), and sample concentrations were calculated from the curve parameters with the Watson LIMS software (version 7.4.1; Thermoscientific).
Calibration curves in human plasma were linear (r2 > 0.997) over the concentration ranges of 0.5 to 200 μg/ml for cefepime and 0.25 to 100 μg/ml for zidebactam. The respective precision (i.e., the percent coefficient of variation) and accuracy (i.e., the percent bias) from analyses of three quality control (QC) plasma samples were 7.97% and 1.20% at 1.5 μg/ml, 3.64% and −3.41% at 30 μg/ml, and 2.56% and 0.03% at 150 μg/ml for cefepime and 4.53% and −2.93% at 0.75 μg/ml, 2.19% and 0.37% at 15 μg/ml, and 5.02% and −0.98% at 75 μg/ml for zidebactam. The lower limits of quantification (LLOQ) in plasma were 0.5 μg/ml and 0.25 μg/ml for cefepime and zidebactam, respectively. Ninety-three of 94 (98%) samples for each drug were within a 20.0% difference between the original and ISR concentration values for cefepime or zidebactam.
A total of 35 BAL samples and 35 cell pellet suspensions were assayed for cefepime and zidebactam in one analytical run of each matrix between 30 June 2017 and 2 July 2017. Human BAL fluid was used as a blank control matrix for lining fluid sample analysis. Human BAL fluid and sodium heparin plasma were used for control matrices for macrophage sample analysis. Cell pellets were resuspended with nanopure water and carried through three freeze-thaw cycles. The standard curves were linear for both drugs over the concentration range of 2.0 to 1,000 ng/ml for both BAL fluid (r2 ≥ 0.997) and cell suspensions (r2 ≥ 0.996). The respective precision and accuracy for QC samples in BAL fluid for cefepime were 1.71% and 7.30% at 6 ng/ml, 3.40% and −6.31% at 80 ng/ml, and 0.89% and −5.28% at 750 ng/ml. The respective precision and accuracy for cefepime QC samples in cell suspensions were 2.50% and −0.80% at 6 ng/ml, 2.38% and −5.08% at 80 ng/ml, and 1.20% and 5.55% at 750 ng/ml. For zidebactam, the respective precision and accuracy for QC samples in BAL fluid were 6.94% and −1.37% at 6 ng/ml, 3.27% and −5.73% at 80 ng/ml, and 3.07% and −9.88% at 750 ng/ml. The respective precision and accuracy for QC samples in cell suspensions of zidebactam were 1.37% and −1.42% at 6 ng/ml, 1.88% and −3.31% at 80 ng/ml, and 1.46% and 3.92% at 750 ng/ml. The LLOQ for cefepime and zidebactam in human BAL fluid and cell suspensions was 2 ng/ml. A total of 20 samples (57%) for both the BAL fluid and cell pellet of each drug were selected for ISR testing, and the calculated assay variability values were within 20% for all cefepime values and 19 of 20 zidebactam values.
Determination of urea concentrations.
Concentrations of urea in plasma and BAL fluid supernatants were determined by an LC–MS-MS method at Keystone Bioanalytical (North Wales, PA) (report numbers 170606 and 170610, respectively). In brief, the LC–MS-MS system consisted of an Applied Biosystems/MDS Sciex API 4000 tandem mass spectrometer, a Shimadzu autosampler, an LC-20AD pump, and Phenomenex Partisil 5-μm, 100- by 4.6-mm analytical columns. Chromatograms were integrated using the Analyst software package (version 1.4.2; Sciex), and further data processing, including the calculation of concentration data, was performed with the Watson software package (version 7.4.1; Thermoscientific). Sodium chloride 0.9% solution was used for calibration standards, and blank human BAL fluid was used for QC sample preparation. The samples with urea and the internal standard ([13C, 15N2]urea) were diluted and vortexed, and the mixture was transferred to plastic injection vials. A 10-μl sample was injected into the LC–MS-MS system for analysis.
A total of 35 human plasma and BAL samples were assayed for urea concentrations between 14 June 2017 and 22 June 2017. The assay for urea in human plasma was linear (r2 > 0.998) over a range of concentrations from 100 to 3,000 μg/ml. The precision and accuracy for urea QC samples in phosphate-buffered saline (PBS) at 300 μg/ml were 1.37% and 2.97%, respectively. The respective precision and accuracy for urea QC samples in plasma were 4.77% and −6.09% at 115.1 μg/ml, 1.80% and 0.77% at 300 μg/ml, 1.62% and −2.98% at 1,000 μg/ml, and 1.95% and −6.61% at 2,250 μg/ml. The LLOQ for urea in human plasma was 100 μg/ml. The assay for urea in BAL fluid was linear (r2 > 0.998) for the range of concentrations from 0.2 to 10 μg/ml. The respective precision and accuracy for urea QC samples in human BAL fluid were 3.16% and 0.33% at 0.6 μg/ml, 1.96% and −4.83% at 3.0 μg/ml, and 7.35% and −2.80% at 7.5 μg/ml. The LLOQ of urea in human BAL fluid was 0.2 μg/ml. A total of 20 samples (57%) of both plasma and BAL fluid were selected for ISR testing, and the calculated assay variability values were within 20%.
Calculation of concentrations of cefepime and zidebactam in ELF and AM.
Previously described urea dilution methods were used to determine the apparent volume of ELF in BAL fluid (37). The estimated drug concentration (DRUGELF) in ELF was calculated as follows: DRUGELF = DRUGBAL × (ureaplasma/ureaBAL), where DRUGBAL is the measured cefepime or zidebactam in BAL fluid and ureaplasma and ureaBAL are the measured concentrations of urea in plasma and BAL fluid, respectively. The estimated drug concentration (DRUGAM) in AM was calculated as follows: DRUGAM = DRUGS/VAM, where DRUGS and VAM are the measured concentration of cefepime or zidebactam and the volume of alveolar cells in the cell suspension, respectively. The absolute cell numbers and differential cell count percentage were determined from the BAL fluid. A mean macrophage volume of 2.42 μl/106 cells was used for the calculations of VAM (38, 39).
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic parameters for cefepime and zidebactam were calculated using Phoenix WinNonlin software (version 7.0; Pharsight Corp., Cary, NC). Actual sampling times relative to WCK 5222 dosing were used to determine pharmacokinetic parameter values. Maximum (Cmax) and minimum (Cmin) plasma concentrations were directly obtained from the observed plasma concentration-time profiles following the first and seventh intravenous doses of WCK 5222. The 8-hour plasma concentration represented the Cmin after a specific dose. The AUC after the first and seventh doses was calculated with the linear-log trapezoidal method. The AUC for the first dose was extrapolated to infinity (AUC0–∞), and the AUC for the seventh dose was determined for the 8-hour dosing interval (AUC0–8). The elimination rate constant (λZ) was determined by nonlinear least-squares regression, and the t1/2 was calculated by dividing λZ into the natural logarithm of 2. The apparent volume of distribution at steady-state (Vss) and CL were calculated using standard noncompartmental equations for intravenous infusions.
Mean and median plasma, ELF, and AM concentration values at the BAL sampling times were used to estimate the AUC0–8 by the linear-log trapezoidal method. The cefepime and zidebactam concentrations at the 8-hour sampling time after the seventh dose also served as the time zero values for determining the AUC0–8 of each matrix. The intrapulmonary penetration ratios of cefepime and zidebactam were estimated from the ratio of the AUC0–8 for ELF or AM to the AUC0–8 for total plasma concentrations.
Safety and laboratory assessments.
All enrolled subjects who had received at least one dose of WCK 5222 were included in the safety analyses. Safety and tolerability were assessed by adverse event monitoring, physical examination findings, vital sign measurements, standard 12-lead ECG readings, and clinical laboratory test results (serum chemistry, hematology, coagulation, and urinalysis). The observed and reported adverse events of each subject were assessed by investigators during the entire study duration (i.e., from the time of signing informed consent until completion of the follow-up visit). The Common Terminology Criteria for Adverse Events (CTCAE) (U.S. National Cancer Institute) was used as a reference for grading the severity of all adverse events (i.e., mild, moderate, severe, life-threatening, or fatal). Causality assessment of adverse events was categorized as certain, probable/likely, possible, unlikely, not related, and unknown.
ACKNOWLEDGMENTS
This study was supported by Wockhardt Bio Ag.
Regarding conflicts of interest, K. A. Rodvold and M. H. Gotfried have been consultants to Wockhardt Bio Ag; M. H. Gotfried has received research support from Wockhardt Bio Ag; and R. Chugh, M. Gupta, A. Patel, R. Chavan, R. Yeole, H. D. Friedland, and A. Bhatia are current employees of Wockhardt Bio Ag.
REFERENCES
- 1.Bush K, Page MGP. 2017. What we may expect from novel antibacterial agents in the pipeline with respect to resistance and pharmacodynamics principles. J Pharmacokinet Pharmacodyn 44:113–132. doi: 10.1007/s10928-017-9506-4. [DOI] [PubMed] [Google Scholar]
- 2.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 4.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 56:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 5.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonoma RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonoma RA, Oliver A. 2017. Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinebacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61:e01238-17. doi: 10.1128/AAC.01238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia A, Gupta M, Chugh R. 2016. Zidebactam phase 1 single ascending dose and crossover study in healthy subjects, abstr 2236. IDWeek, 26 to 30 October 2016, New Orleans, LA, U S A. [Google Scholar]
- 8.Chugh R, Lakdavala F, Friedland HD, Bhatia A. 2017. Safety and pharmacokinetics of multiple ascending doses of WCK 5107 (zidebactam) and WCK 5222 (cefepime and zidebactam), abstr P1301. In: Abstracts of the 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Vienna, Austria. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 9.Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, Martin RR. 1990. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother 34:1118–1122. doi: 10.1128/AAC.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, Movahhed H, Tenney J, Martin RR. 1992. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother 36:552–557. doi: 10.1128/AAC.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodvold KA, Hope WW, Boyd SE. 2017. Considerations for effect site pharmacokinetics to estimate drug exposure concentrations of antibiotics in the lung. Curr Opin Pharmacol 36:114–123. doi: 10.1016/j.coph.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin DR, Honeybourne D, Wise R. 1992. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother 36:1176–1180. doi: 10.1128/AAC.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nix DE. 1998. Intrapulmonary concentrations of antimicrobial agents. Infect Dis Clin North Am 12:631–646. doi: 10.1016/S0891-5520(05)70202-6. [DOI] [PubMed] [Google Scholar]
- 15.Muller A, Bhagwat S, Patel M, Mouton JW. 2016. Development of a population pharmacokinetic model of cefepime, session 308. ASM Microbe, 16 to 20 June 2016, Boston, MA, USA. [Google Scholar]
- 16.Muller A, Mouton J. 2017. Population pharmacokinetics of zidebactam (WCK 5107), a novel beta-lactam enhancer antibiotic, abstr P1299. Abstr 27th Eur Congr Clin Microbiol Infect Dis (ECCMID), Vienna, Austria. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 17.Barbhaiya RH, Forgue ST, Shyu WC, Papp EA, Pittman KA. 1987. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother 31:55–59. doi: 10.1128/AAC.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayat S, Louchahi K, Verdiere B, Anglade D, Rahoui A, Sorin PM, Tod M, Petitjean O, Fraisse F, Grimbert FA. 2004. Comparison of 99mTc-DTPA and urea for measuring cefepime concentrations in epithelial lining fluid. Eur Respir J 24:150–156. doi: 10.1183/09031936.04.00106803. [DOI] [PubMed] [Google Scholar]
- 19.Boselli E, Breilh D, Duflo F, Saux MC, Debon R, Chassard D, Allaouchiche B. 2003. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit Care Med 31:2102–2106. doi: 10.1097/01.CCM.0000069734.38738.C8. [DOI] [PubMed] [Google Scholar]
- 20.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. 2008. Tissue concentrations: do we ever learn? J Antimicrob Chemother 61:235–237. doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]
- 21.Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin Infect Dis 51(Suppl 1):S103–S110. doi: 10.1086/653057. [DOI] [PubMed] [Google Scholar]
- 22.Ambrose PG. 2017. Antibacterial drug development program successes and failures: a pharmacometric explanation. Curr Opin Pharmacol 36:1–7. doi: 10.1016/j.coph.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222 [cefepime (FEP) plus WCK 5107 (zidebactam, ZID)]: in vivo demonstration of ZID-mediated β-lactam enhancer effect leading to lowering of FEP %fT>MIC against P. aeruginosa (PA) and A. baumannii (AB), abstr 284. ASM Microbe, 1 to 5 June 2017, New Orleans, LA, USA. [Google Scholar]
- 24.Takalkar SS, Chavan RP, Patel AM, Umarkar KV, Satav JS, Udaykar AP, Kulkarni AM, Shaikh JU, Bhagwat SS, Patel MV. 2015. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: lung PK/PD studies for carbapenamase-expressing K. pneumoniae (KP) and P. aeruginosa (PA), abstr Sunday-443. ASM Microbe, 16 to 20 June 2016, Boston, MA, USA. [Google Scholar]
- 25.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: unravelling sub-MIC pharmacodynamics (PD) effects employing in vivo dose fractionation studies and translating into MIC based PK/PD targets for MBL-expressing P. aeruginosa (PA), abstr 283. ASM Microbe, 1 to 5 June 2017, New Orleans, LA, USA. [Google Scholar]
- 26.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: unravelling sub-MIC PD effects employing in vivo dose fractionation studies and translating into MIC based PK/PD targets for carbapenem-resistant A. baumannii (AB), abstr 282. ASM Microbe, 1 to 5 June 2017, New Orleans, LA, USA. [Google Scholar]
- 27.Goncalves-Pereira J, Povoa P. 2011. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care 15:R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felton TW, Ogungbenro K, Boselli E, Hope WW, Rodvold KA. 2018. Comparison of piperacillin exposure in the lungs of critically ill patients and healthy volunteers. J Antimicrob Chemother 73:1340–1347. doi: 10.1093/jac/dkx541. [DOI] [PubMed] [Google Scholar]
- 29.Rizk ML, Rhee EG, Jumes PA, Gotfried MH, Zhao T, Mangin E, Bi S, Chavez-Eng CM, Zhang Z, Butterton JR. 2018. Intrapulmonary pharmacokinetics of relebactam, a novel β-lactamase inhibitor, dosed in combination with imipenem-cilastatin in healthy subjects. Antimicrob Agents Chemother 62:e01411-17. doi: 10.1128/AAC.01411-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolau DP, Siew L, Armstrong J, Li J, Edeki T, Learoyd M, Das S. 2015. Phase 1 study assessing the steady-state concentrations of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother 70:2862–2869. doi: 10.1093/jac/dkv170. [DOI] [PubMed] [Google Scholar]
- 31.Cook PJ, Andrews JM, Woodcook J, Wise R, Honeybourne D. 1994. Concentrations of amoxycillin and clavulanate in lung compartments in adults without pulmonary infection. Thorax 49:1134–1138. doi: 10.1136/thx.49.11.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin DR, Andrews JM, Wise R, Honeybourne D. 1992. Bronchoalveolar distribution of cefuroxime axetil and in-vitro efficacy of observed concentrations against respiratory tract pathogens. J Antimicrob Chemother 30:377–385. doi: 10.1093/jac/30.3.377. [DOI] [PubMed] [Google Scholar]
- 33.Wenzler E, Gotfried MH, Loutit JS, Durso S, Griffith DC, Dudley MN, Rodvold KA. 2015. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother 59:7232–7239. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodvold KA, Gotfried MH, Danziger LH, Servi RJ. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob Agents Chemother 41:1399–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodvold KA, Danziger LH, Gotfried MH. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother 47:2450–2457. doi: 10.1128/AAC.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotfried MH, Danziger LH, Rodvold KA. 2003. Steady-state plasma and bronchopulmonary characteristics of clarithromycin extended-release tablets in normal healthy adults. J Antimicrob Chemother 52:450–456. doi: 10.1093/jac/dkg355. [DOI] [PubMed] [Google Scholar]
- 37.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker for dilution. J Appl Physiol 60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin DR, Honeybourne D, Wise R. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother 36:1171–1175. doi: 10.1128/AAC.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D. 1990. Azithromycin concentrations at the site of pulmonary infections. Eur Respir J 3:886–890. [PubMed] [Google Scholar]
- 40.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]