ABSTRACT
Hepatotoxicity induced by antituberculosis drugs is a serious adverse reaction with significant morbidity and even, rarely, mortality. This form of toxicity potentially impacts the treatment outcome of tuberculosis in some patients. Covering only first-line antituberculosis drugs, this review addresses whether and how oxidative stress and, more broadly, disturbance in redox homeostasis alongside mitochondrial dysfunction may contribute to the hepatotoxicity induced by them. Risk factors for such toxicity that have been identified, in addition to genetic factors, principally include old age, malnutrition, alcoholism, chronic hepatitis C and chronic hepatitis B infection, HIV infection, and preexisting liver disease. Importantly, these comorbid conditions are associated with oxidative stress and drugs related to antioxidants, especially those for management of mitochondrial dysfunction. Thus, the shared pathogenetic mechanism(s) for liver injury might be in operation due to disease-drug interaction. Our current ability to predict, prevent, or treat hepatotoxicity (other than removing potentially hepatotoxic drugs) remains limited. More translational research to unravel the pathogenesis, inclusive of the underlying molecular bases, regarding antituberculosis drug-induced hepatotoxicity is needed, and so is clinical research pertaining to the advances in therapy, with antioxidants and beyond. The role of pharmacogenetics in the clinical management of drug-induced hepatotoxicity also likely merits further evaluation.
KEYWORDS: drugs, hepatotoxicity, tuberculosis
INTRODUCTION
Inappropriate oxidative stress (and nitrosative stress) generally results from an imbalance in the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), alongside their reduced elimination and/or the suboptimal response of antioxidative defense (1, 2) (Table 1). Oxidative stress is exogenous or endogenous in origin. Exogenous oxidative stress can be induced by drugs, and endogenous oxidative stress is inherently associated with some conditions and diseases (1). Inappropriate oxidative stress is harmful to the host and may result in organ injury (1, 2). Covering only first-line antituberculosis drugs, this review addresses whether and how oxidative stress may contribute to antituberculosis drug-induced hepatotoxicity, which has been a longstanding concern in the treatment of tuberculosis (3, 4).
TABLE 1.
Some reactive oxygen species, reactive nitrogen species, and components of antioxidative defense
| Category | Substance |
|---|---|
| Reactive oxygen species | Hydroxyl ion (OH−) |
| Hydroxyl radical (·OH) | |
| Peroxide (·O22−) | |
| Hydrogen peroxide (H2O2) | |
| Superoxide anion (·O2−) | |
| Singlet oxygen (1O2) | |
| Reactive nitrogen species | Nitric oxide (NO) |
| Nitrogen dioxide (NO2) | |
| Peroxynitrite (ONOO−) | |
| Nitrosoperoxycarbonate (ONOOCO2−) | |
| Antioxidative defense | Nitric oxide (NO) |
| Glutathione transferase (GST) | |
| Glutathione peroxidase (GTPx) | |
| Catalase (CAT) | |
| Superoxide dismutase (SOD) | |
| Nuclear factor-erythroid 2 related factor (Nrf2) | |
| Beta-carotene | |
| Ascorbic acid (vitamin C) | |
| Alpha-tocopherol (vitamin E) | |
| Selenium | |
| Zinc |
OXIDATIVE STRESS AND DRUG-INDUCED HEPATOTOXICITY
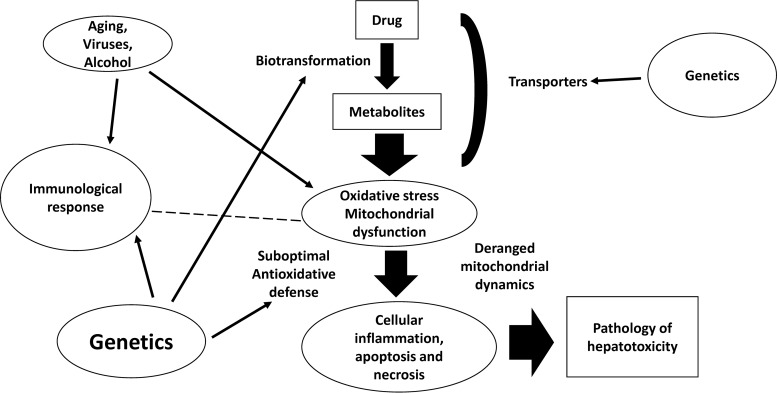
The liver is an important organ with substantial vulnerability to the deleterious effects of oxidative stress (1, 2). Mitochondria, microsomes, and peroxisomes of hepatocytes are associated with the production of ROS, which impact the regulation of signaling pathways, including peroxisome proliferator-activated receptor alpha (PPARα) governing fatty acid oxidation, and mitogen-activated protein kinase (MAPK) and related stress-sensitive kinases associated with proapoptosis. Furthermore, in Kupffer cells, oxidative stress might induce the elaboration of cytokines, such as tumor necrosis factor alpha, that contribute to the progression of tissue inflammation and cell apoptosis. In hepatic stellate cells, oxidative stress-mediated lipid peroxidation can lead to increased collagen synthesis. Complex cross talk between oxidative stress (nitrosative stress) and immune responses has been suggested to play a critical role in the pathogenesis of liver injury (Fig. 1). In humans and other mammalian species, a sophisticated antioxidative system to preserve redox homeostasis is found in the liver (1). However, when perturbation of the homeostasis is to such a degree that it culminates in overwhelming oxidative stress that challenges the liver of the host, jeopardy of the organ status ensues due to damage to intracellular targets, notably lipids, proteins, and DNA, and there is an adverse impact on key signaling pathways for the maintenance of optimal biological functions of the liver involved, and even other organs (2). Some examples of drug-induced hepatotoxicity associated with oxidative stress are as follows. It has been shown that acetaminophen-induced hepatotoxicity is related to its metabolic derivative, N-acetyl-p-benzoquinone imine, which depletes glutathione (GSH) from cellular storage and promotes protein adducts in mitochondria. Mitochondrial dysfunction and oxidative stress thus ensue, with subsequent activation of c-jun N-terminal kinase (JNK) and ultimate induction of mitochondrial membrane permeability transition (MPT). Apoptosis-inducing factor and endonuclease G are then released, leading to nuclear DNA fragmentation and programmed necrosis (5, 6). Also, in diclofenac-induced hepatotoxicity, mitochondrial dysfunction and oxidative stress are now viewed as likely underlying mechanisms based on some molecular evidence (7).
FIG 1.
A simplified representation of the pathogenesis of drug-induced hepatotoxicity. Oxidative stress and mitochondrial dysfunction are important mechanisms contributing to drug-induced hepatotoxicity. Genetic polymorphisms associated with drug metabolism, oxidative stress, and immune response interact in an intricate way, with conditions/diseases associated with oxidative stress per se, leading to cellular inflammation, apoptosis, and necrosis, which manifest as histopathological changes of hepatotoxicity. The dashed line indicates possible interaction between immunological response and oxidative stress.
OXIDATIVE STRESS AND ANTITUBERCULOSIS DRUG-INDUCED HEPATOTOXICITY
Hepatotoxicity induced by antituberculosis drugs might result in significant morbidity and, rarely, even mortality (8–10). Such toxicity also hampers patient adherence to therapy and could negatively impact the treatment outcome of patients. Isoniazid, pyrazinamide, and rifampin, the three key first-line antituberculosis drugs, have potential hepatotoxicity. Both dose-dependent and hypersensitivity/idiosyncrasy mechanisms are at play, with either one often predominating, as reported, though with some disparity (8, 10). For example, for isoniazid, the idiosyncratic mechanism is generally favored, and for pyrazinamide, the dose-dependent mechanism is usually preferred.
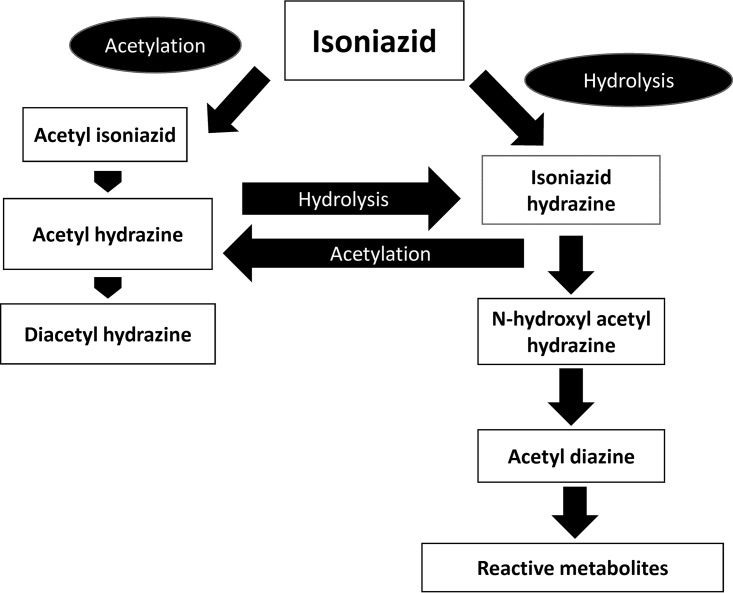
The association of oxidative stress with isoniazid-induced hepatotoxicity appears to be reasonably well understood (8–10). As shown in Fig. 2, isoniazid is metabolized by N-acetyltransferase 2 (NAT-2) to acetylhydrazine and diacetylhydrazine. Diacetylhydrazine is nontoxic and is readily eliminated from the body of the host. However, the acetylhydrazine metabolite of isoniazid can be further hydrolyzed to the hepatotoxic isoniazid hydrazine, and so can isoniazid itself. These hydrolysis pathways are more activated in subjects with a slow-acetylator phenotype (with any two of the several alleles of the NAT2 gene). A meta-analysis demonstrates that slow acetylators have an increased risk of antituberculosis drug-induced liver injury (11). Another study involving a mixed-ethnicity patient group has also demonstrated the association after controlling for possible confounding effects by ethnicity (12). Isoniazid hydrazine can be further metabolized by cytochrome P450 (CYP) 2E1 isoenzymes to reactive derivatives/metabolites with greater toxicity (Fig. 2). Subjects with CYP2E1 c1/c1 alleles and higher enzymatic activity have been shown to be more prone to isoniazid-associated hepatotoxicity (13, 14). GSH, an important antioxidant, protects against oxidative damage by furnishing a thiol (-SH) group for conjugation with the reactive metabolites. Deficiency in glutathione S-transferase (GST) activity because of homozygous null mutations at GSTM1 and GSTT1 loci can influence susceptibility to isoniazid-induced hepatotoxicity. In a case-control study, the frequency of GSTM1 null mutations in subjects with hepatic dysfunction induced by an antituberculosis drug was found to be twice as common (15). In another study, the occurrence of hepatic dysfunction in patients with tuberculosis was 2.6 times more likely in those with GSTT1 null mutations (16). Earlier animal experiments have shown reduced levels of GST and other antioxidative enzymes after the administration of isoniazid hydrazine (9). In a recent study using isolated liver mitochondria from rats, oxidative stress and mitochondrial dysfunction induced by isoniazid have been well shown (17). Another study in mice has established the role of micro-RNA-122 in oxidative stress-related liver injury by isoniazid (18). It has been suggested that isoniazid toxicity might be mediated through an interaction with electron transport chain, lipid peroxidation, mitochondrial membrane potential change, and cytochrome c extrusion, resulting in detrimental cell signaling (17). As there have been some data regarding a better correlation of higher severity of isoniazid-induced hepatotoxicity with RNS than with ROS, peroxynitrite (ONOO−) generation and mitochondrial dysfunction probably have significant contributions to such toxicity (19, 20).
FIG 2.
Metabolism of isoniazid in the liver. Isoniazid is metabolized by acetylation to acetylhydrazine and diacetylhydrazine. Acetylhydrazine can be hydrolyzed further to become isoniazid hydrazine, which also results from the hydrolysis of isoniazid itself. Isoniazid hydrazine is toxic to hepatocytes, and so are the more toxic reactive metabolites further derived from it through the activities of cytochrome P450 enzymes.
Rifampin alone has low potential for hepatotoxicity (6), but it exhibits additive or even synergistic hepatotoxicity when used with isoniazid concomitantly in treating tuberculosis (21). Pregnane X receptor (PXR), a member of the nuclear receptor superfamily of ligand-dependent transcription factors, can be activated by rifampin, resulting in the upregulation of phase I and phase II drug-metabolizing enzymes, including CYPs and GSTs, as well as drug/substrate transporters, such as ATP binding cassette transporters (ABCB) (10, 22). The induction of hydrolases, and perhaps other enzymes, by rifampin has been suggested to increase the generation of reactive metabolites from isoniazid that are hepatotoxic, thus helping to explain the interactive toxicity of isoniazid and rifampin (23). Another suggested mechanism contributing to such interactive toxicity is PXR-mediated effects on heme biosynthesis leading to an accumulation of hepatotoxic protoporphyrin IX (24). In a study involving patients who received both antiretroviral agents and antituberculosis drugs, a significant association was found between drug-induced hepatic dysfunction and an NAT-2 slow-acetylator genotype, as well as the ABCB1 3435TT genotype (25). In another study, the PXR TT genotype was found to be associated with an increased risk of antituberculosis drug-induced liver injury (26).
Pyrazinamide is increasingly recognized as an antituberculosis drug that can result in significant hepatotoxicity in clinical settings. In a case-control study, the adjusted odds ratio of hepatotoxicity for continuation-phase regimens incorporating pyrazinamide, isoniazid, and/or rifampin, relative to standard regimens, was shown to be about three (27). In a rat model, changes in the activities of major antioxidant enzymes and nonenzymatic antioxidants, principally including superoxide dismutase (SOD), antioxidant capacity, GSH, and malondialdehyde, were found to be significantly associated with pyrazinamide-induced injury, alluding to the role of oxidative stress in the pathogenesis of the drug-induced hepatotoxicity (28). In a subsequent experiment using the same model, the expression of PPARα, alongside the target genes downstream, was shown to be downregulated in the face of pyrazinamide-induced hepatotoxicity, with a negative correlation of the expression level with the severity of liver injury (29). The liver injury was ameliorated by fenofibrate, a PPARα agonist. These data further support the role of oxidative stress in pyrazinamide-induced hepatotoxicity.
RISK FACTORS OF ANTITUBERCULOSIS DRUG-INDUCED HEPATOTOXICITY AND THEIR ASSOCIATION WITH OXIDATIVE STRESS
Aside from the slow-acetylator status and the genetic polymorphisms pertaining to CYP2E1 and GST mentioned, predisposing one to hepatotoxicity induced by antituberculosis drugs, the other clinical conditions that increase the risk of such drug-associated toxicity mainly include old age, malnutrition, alcoholism, chronic viral hepatitis B and C infections, and HIV infections, as well as preexisting liver diseases (8–10). It is imperative to briefly review these comorbid conditions, in the context of oxidative stress (or more broadly redox imbalance), to enable a better understanding of the pertinent drug-disease interaction.
Aging and oxidative stress.
Oxidative stress and beyond is now strongly believed to contribute to the biophysiology of aging and the pathogenesis of a number of degenerative diseases involving principally the cardiovascular, neurological, and dermatological systems (30, 31). In old age, immunological senescence often develops, and there is likely an interaction between immunological dysfunction and oxidative stress (and redox homeostasis and mitochondrial dysfunction) in elderly subjects (32). The association between tuberculosis and old age has been reported in many countries and geographical regions, largely related to a high prevalence of latent tuberculosis infection, with increased risk of progression/reactivation to disease, especially in the presence of comorbid conditions, such as smoking, alcoholism, and diabetes mellitus (33, 34). A possible underlying mechanism is the propensity for the formation of dormant/semidormant Mycobacterium tuberculosis persisters in the face of oxidative stress (34). As discussed, oxidative stress plays an important role in the pathogenesis of hepatotoxicity induced by antituberculosis drugs. Recent clinical evidence also points to an increased risk of antituberculosis drug-induced adverse reactions (inclusive of hepatotoxicity) in older patients (35), especially in the presence of diabetes mellitus (36, 37). In older people with type 2 diabetes mellitus, complications of the metabolic disease resulting in organ dysfunction often have oxidative stress likely contributing to their pathogenesis (34, 37). The results from animal experiments have helped substantiate the hypothesis. For example, alloxan-induced diabetes has been shown to cause morphological and ultrastructural changes in the rat liver that resemble the natural history of chronic fatty liver disease in humans (38). Other experiments have similarly demonstrated cardiovasculopathy, neuropathy, and nephropathy associated with oxidative stress in the animal models (39–41). Thus, due to the shared pathogenetic basis for organ injury in diabetic patients who receive antituberculosis therapy concomitantly, it is biologically plausible that the diabetic complications can aggravate the aftermath of antituberculosis drug-induced toxicities (37). Indeed, more research regarding the metabolic control of diabetes mellitus and the risk of toxicities induced by antituberculosis therapy appears to be warranted.
Malnutrition and oxidative stress.
In malnutrition, oxidative stress is heightened, and impoverished nutritional status is conspicuously associated with immune dysfunction (42, 43). Hepatic steatosis has also been found in the malnourished animals and humans, with a likely link to oxidative stress and mitochondrial dysfunction (44, 45). It is conceivable that malnutrition would especially merit attention in the elderly population with tuberculosis. Relevant knowledge hopefully can further accumulate.
Alcoholic liver disease and oxidative stress.
The main pathway of conversion of ethanol to acetaldehyde, together with the reduction of NAD to NADH, using the alcohol dehydrogenase system takes place in the liver (2). Acetaldehyde is further oxidized to acetate by aldehyde dehydrogenase. The other important pathway, also in the liver, involves the inducible microsomal ethanol-oxidizing system, which entails CYP2E1, an NADPH-requiring enzyme (2). During ethanol metabolism in these two systems, NADH or NADP+ is produced, together with an increase in ROS generation, and thus oxidative stress. DNA damage, lipid peroxidation, and formation of protein adducts then result, along with mitochondrial dysfunction (2). Pathologically, steatosis, fibrosis, cirrhosis, and even malignant changes are manifested in the hepatocytes of patients with clinical liver disease. Studies of enzymatic and nonenzymatic systems in addressing the pro-oxidant and antioxidant status in animal models and subjects with chronic alcoholism have been performed, however, with somewhat disparate results (2). As in diabetes mellitus, the shared pathogenetic basis of oxidative stress for organ injury highlights the possible mechanism underlying disease-drug interaction when patients with alcoholic liver disease receive antituberculosis treatment.
Chronic viral hepatitis and oxidative stress.
The relative risk of developing antituberculosis drug-induced hepatotoxicity among patients with chronic viral hepatitis is about 3- to 5-fold that of the general population (8, 10). Studies have also shown that the severity of hepatotoxicity was related to the viral load at the time of initiating antituberculosis therapy (46, 47). In the face of chronic viral hepatitis, the host immune responses are largely responsible for the generation of ROS and RNS, as well as mitochondrial dysfunction. It has also been shown that hepatitis C infection is associated with a greater production of ROS than other hepatitis viruses (48, 49). The level of ROS was found to correlate with the likelihood of developing chronic hepatic disease, namely, chronic hepatitis, cirrhosis, and hepatocellular carcinoma. ROS has also been found to affect viral genome translation and induce viral genome heterogeneity. A number of core viral proteins of hepatitis C virus are associated with oxidative stress. Hepatitis C virus also affects enzymes, some of which pertain to antioxidant pathways. The availability of much information notwithstanding, huge gaps in the knowledge still exist regarding ROS scenarios in hepatitis C infection. Interestingly, steatosis is one conspicuous histopathological feature in chronic liver disease due to hepatitis C infection. It appears that both host and virus factors contribute to the development of this liver pathology, which is probably caused by ROS/RNS-mediated disturbance in lipid metabolism. Furthermore, there appears a link between chronic hepatitis C infection and insulin resistance and diabetes mellitus (50, 51). Taken together, the information underscores extremely important awareness regarding the enhanced predisposition to hepatotoxicity incurred by chronic hepatitis C disease and antituberculosis therapy. In chronic hepatitis B infection, there is also some evidence for the occurrence of oxidative stress (52). As in chronic hepatitis C infection, sometimes the studies regarding antioxidant status have yielded rather conflicting results in patients with chronic hepatitis B infection (49, 52). This notwithstanding, great vigilance has to be exercised regarding the impact of an ROS-based pathogenetic mechanism in causing antituberculosis drug-associated hepatotoxicity in patients with chronic hepatitis B infection.
HIV infection and oxidative stress.
The relative risk of antituberculosis drug-induced hepatotoxicity in HIV-infected subjects is about 4, but it rises to 14 when concomitant hepatitis C infection is present (53). In HIV-associated tuberculosis, the increased risk of hepatotoxicity could result from the shared mechanisms of liver injury due to antituberculosis drugs and host factors, as related to oxidative stress and beyond (54). To complicate the scenario further, there have been reports regarding the perturbation of redox homeostasis during antiretroviral therapy with nucleoside and nonnucleoside reverse transcriptase inhibitors, as well as viral protease inhibitors (55, 56). Nevirapine is the most hepatotoxic nonnucleoside reverse transcriptase inhibitor, and the majority of nucleoside reverse transcriptase inhibitors, such as didanosine and stavudine, are also potentially hepatotoxic (9). Highly active antiretroviral therapy, with inclusion of HIV protease inhibitors, can result in hepatotoxicity in 18 to 27% of recipients in some studies (9, 10).
PHARMACOGENOMICS OF DRUG-INDUCED LIVER INJURY
In parallel with the improvement of technologies in genome analysis, novel concepts in the mechanisms of drug-induced hepatotoxicity have underlined nonspecific downstream events following drug-specific upstream injury and the complex interactions between environmental and genetic risk factors. Attention to genes beyond the context of drug disposition and metabolism appears imperative, especially in relation to human leukocyte antigen (HLA) system variability, immune response, and oxidative stress (57, 58). In recent years, the pharmacogenomics knowledge base has been accumulating (57–60). Table 2 depicts some important genes, apart from NAT2, CYP2E1, and GST, which may be associated with isoniazid-induced hepatotoxicity. For truly idiosyncratic drug-induced liver injury, it might be difficult to estimate the population-attributable risk and clinically relevant absolute risk regarding these genetic polymorphisms. However, regarding some populations with risk factors for antituberculosis drug-induced hepatotoxicity, further fathoming appears justified. In this connection, it also appears that more research is warranted regarding the possible interaction between genetic polymorphisms associated with drug metabolism and oxidative stress, in the generation of drug-induced liver injury. As an example, genetic variation of SOD2 has recently been found to be associated with alcoholic cirrhosis (61), and genetic polymorphisms of SOD2 and cytochrome CYP 2E1 have been found to be associated with nonalcoholic steatohepatitis (62).
TABLE 2.
Examples of genes putatively associated with isoniazid-induced hepatotoxicitya
| Gene | Genetic polymorphisms | Possible results |
|---|---|---|
| NAT2 | NAT2 *2, *5, *6, *7 | Risk of hepatotoxicity |
| CYP2E1 | CYP2E1*1A,*5,*6 | Risk of hepatotoxicity |
| GSTb | GSTM1, GSTT1 null homozygous | Risk of hepatotoxicity |
| UGT1A | TA insertion in gene promoter | Risk of hyperbilirubinemia and hepatotoxicity |
| HLA | HLA-DQ | Risk of hepatotoxicity |
| NOS | NOS2A (inducible isoform) | Risk of hepatotoxicity |
| BACHb | BACH1 CC genotype at rs11080344 | Risk of hepatotoxicity |
| MAFKb | Homozygous mutant genotype at rs4720833 | Risk of hepatotoxicity |
| MnSODb | Homozygous/heterozygous mutant C allele (T/C or C/C) | Risk of hepatotoxicity |
| ABCB11c | T→C V444A | Risk of hyperbilirubinemia and hepatotoxicity? |
| ABCB4c | Various transversions | |
| ABCC2c | Various transversions | |
| SLCO1B1c | T→C V174A | Risk of hepatotoxicity? |
ABCB4, ATP binding cassette superfamily B member 4; ABCB11, ATP binding cassette superfamily B member 11; ABCC2, ATP binding cassette superfamily C member 2; BACH, BTB and CNC homolog basic leucine zipper transcription factor; HLA, human leukocyte antigen; MAFK, Maf basic leucine zipper protein; MnSOD, manganese superoxide dismutase; NOS, nitric oxide synthase; SLCO1B1, solute carrier organic anion transporter family member 1B1; UGT, UDP glucuronosyltransferase.
Associated with antioxidative defense.
May contribute to interactive hepatotoxicity of rifampin and isoniazid.
EPILOGUE
Oxidative stress, nitrosative stress, and overall redox imbalance likely contribute to antituberculosis drug-induced hepatotoxicity, especially in demographic subpopulation(s) or patient groups with specific comorbidities (34–36). Our current ability to predict, prevent, or treat hepatotoxicity remains limited. The need for clinical vigilance and programmatic management of tuberculosis cannot be overemphasized. This is imperative largely for preventing the development of hepatotoxicity or rendering proactive management when hepatotoxicity is diagnosed at the earliest opportunity to forestall its worsening in severity. One frequent measure is drug regimen modification (8–10). Attention to nutritional status is of particular importance in elderly people with both latent tuberculosis infection and tuberculosis. Optimal metabolic control in diabetes-associated tuberculosis can have a favorable impact on the outcomes of both diseases, including antituberculosis drug-induced toxicities (34).
The use of antioxidants may have an adjunctive role, but the benefit of such therapy requires further delineation (2, 10), perhaps implying the complex nature of perturbation in redox homeostasis, as well as the great likelihood of concomitant mitochondrial dysfunction (63–65). At present, for overall drug-induced hepatotoxicity, animal experiments are abundant, but there are only limited clinical studies (2). Coadministration of N-acetylcysteine was found to be protective against liver injury in animals treated with hepatotoxic doses of isoniazid and rifampin (66). In an open-label trial involving patients >60 years old, N-acetylcysteine appeared to protect against liver chemistry abnormalities during antituberculosis treatment (67). The methodology of this trial, however, precluded unequivocal conclusions to guide the management of antituberculosis drug-induced hepatotoxicity. In many animal experiments, herbal plants were investigated for hepatoprotection. The use of herbal products, albeit occasionally encouraging, requires proper evaluation in well-designed clinical trials (2, 68). An endeavor that systematically reviewed the ingredients and evaluation in studies involving drugs and plants for hepatoprotection during antituberculosis therapy has not yielded sufficient evidence to support advocating such an approach (69).
In chronic viral hepatitis, the use of antioxidants has not been shown to be definitely beneficial for the liver disease per se and indeed might prove to be the contrary in some patients. It has been suggested that patients with chronic hepatitis B infection might harvest therapeutic results with antioxidants, only if appropriate selection of candidates is undergone; thus, more pertinent research is mandatory (52). On the other hand, since the severity of hepatotoxicity has been shown to be related to the viral loads of chronic hepatitis B and hepatitis C infection at the time of commencement of antituberculosis therapy, antiviral therapy would likely have greater value. The possibility of a reduction of risk of antituberculosis drug-induced hepatotoxicity in these patients with antiviral therapy also appears promising from the results of preliminary studies, and evaluations in controlled clinical trials are ongoing (70, 71). Whether the beneficial effect of such viral load modulation is linked with a change in oxidative stress merits further research.
In the coming decade, the pathogenesis of hepatotoxicity during antituberculosis therapy should be further unraveled, using better experimental models and human tissue samples (10). More robust clinical studies should also be performed, including those for evaluating the role of pharmacogenetics in the clinical management of antituberculosis drug-induced hepatotoxicity (58, 72, 73).
REFERENCES
- 1.Cichoż-Lach H, Michalak A. 2014. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong C-W, Feng Y. 2015. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR, ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee . 2006. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 4.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O'Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. 2016. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 63:e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H, McGill MR, Ramachandran A. 2012. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandran A, Jaeschke H. 2017. Mechanisms of acetaminophen hepatotoxicity and their translation to the human pathophysiology. J Clin Transl Res 3(Suppl 1):157–169. doi: 10.18053/jctres.03.2017S1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deavall DG, Martin EA, Horner JM, Roberts R. 2012. Drug-induced oxidative stress and toxicity. J Toxicol 2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yew WW, Leung CC. 2006. Antituberculosis drugs and hepatotoxicity. Respirology 11:699–707. doi: 10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 9.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WCM, van der Ven AJAM, Dekhuijzen R. 2008. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramappa V, Aithal GP. 2013. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol 3:37–49. doi: 10.1016/j.jceh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y, Yi J, Zhou C, Shen X. 2012. Pharmacogenetic study of drug-metabolising enzyme polymorphisms on the risk of anti-tuberculosis drug-induced liver injury: a meta-analysis. PLoS One 7:e47769. doi: 10.1371/journal.pone.0047769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng CS, Hasnat A, Al Maruf A, Ahmed MU, Pirmohamed M, Day CP, Aithal GP, Daly AK. 2014. N-Acetyltransferase 2 (NAT2) genotype as a risk factor for development of drug-induced liver injury relating to antituberculosis drug treatment in a mixed-ethnicity patient group. Eur J Clin Pharmacol 70:1079–1086. doi: 10.1007/s00228-014-1703-0. [DOI] [PubMed] [Google Scholar]
- 13.Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD. 2003. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 37:924–930. doi: 10.1053/jhep.2003.50144. [DOI] [PubMed] [Google Scholar]
- 14.Bose PD, Sarma MP, Medhi S, Das BC, Husain SA, Kar P. 2011. Role of polymorphic N-acetyl transferase2 and cytochrome P4502E1 gene in antituberculosis treatment-induced hepatitis. J Gastroenterol Hepatol 26:312–318. doi: 10.1111/j.1440-1746.2010.06355.x. [DOI] [PubMed] [Google Scholar]
- 15.Roy B, Chowdhury A, Kundu S, Santra A, Dey B, Chakraborty M, Majumder PP. 2001. Increased risk of antituberculosis drug-induced hepatotoxicity in individuals with glutathione S-transferase M1 “null” mutation. J Gastroenterol Hepatol 16:1033–1037. doi: 10.1046/j.1440-1746.2001.02585.x. [DOI] [PubMed] [Google Scholar]
- 16.Leiro V, Fernández-Villar A, Valverde D, Constenla L, Vázquez R, Piñeiro L, González-Quintela A. 2008. Influence of glutathione S-transferase M1 and T1 homozygous null mutations on the risk of antituberculosis drug-induced hepatotoxicity in a Caucasian population. Liver Int 28:835–839. doi: 10.1111/j.1478-3231.2008.01700.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahadpour M, Eskandari MR, Mashayekhi V, Haj Mohammad Ebrahim Tehrani K, Jafarian I, Naserzadeh P, Hosseini M-J. 2016. Mitochondrial oxidative stress and dysfunction induced by isoniazid: study on isolated rat liver and brain mitochondria. Drug Chem Toxicol 39:224–232. doi: 10.3109/01480545.2015.1092039. [DOI] [PubMed] [Google Scholar]
- 18.Song L, Zhang ZR, Zhang JL, Zhu XB, He L, Shi Z, Gao L, Li Y, Hu B, Feng FM. 2015. MicroRNA-122 is involved in oxidative stress in isoniazid-induced liver injury in mice. Genet Mol Res 14:13258–13265. doi: 10.4238/2015.October.26.22. [DOI] [PubMed] [Google Scholar]
- 19.Stine JG, Chalasani N. 2015. Chronic liver injury induced by drugs: a systematic review. Liver Int 35:2343–2353. doi: 10.1111/liv.12958. [DOI] [PubMed] [Google Scholar]
- 20.Shuhendler AJ, Pu K, Cui L, Uetrecht JP, Rao J. 2014. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol 32:373–380. doi: 10.1038/nbt.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steele MA, Burk RF, DesPrez RM. 1991. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest 99:465–471. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y-M, Chai SC, Brewer CT, Chen T. 2014. Pregnane X receptor and drug-induced liver injury. Expert Opin Drug Metab Toxicol 10:1521–1532. doi: 10.1517/17425255.2014.963555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askgaard DS, Wilcke T, Døssing M. 1995. Hepatotoxicity caused by the combined action of isoniazid and rifampicin. Thorax 50:213–214. doi: 10.1136/thx.50.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Lu J, Cheng J, Wang L, Matsubara T, Csanaky IL, Klaassen CD, Gonzalez FJ, Ma X. 2013. Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat Med 19:418–420. doi: 10.1038/nm.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yimer G, Ueda N, Habtewold A, Amogne W, Suda A, Riedel K-D, Burhenne J, Aderaye G, Lindquist L, Makonnen E, Aklillu E. 2011. Pharmacogenetic & pharmacokinetic biomarker for efavirenz based ARV and rifampicin based anti-TB drug induced liver injury in TB-HIV infected patients. PLoS One 6:e27810. doi: 10.1371/journal.pone.0027810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zazuli Z, Barliana MI, Mulyani UA, Perwitasari DA, Ng H, Abdulah R. 2015. Polymorphism of PXR gene associated with the increased risk of drug-induced liver injury in Indonesian pulmonary tuberculosis patients. J Clin Pharm Ther 40:680–684. doi: 10.1111/jcpt.12325. [DOI] [PubMed] [Google Scholar]
- 27.Chang KC, Leung CC, Yew WW, Lau TY, Tam CM. 2008. Hepatotoxicity of pyrazinamide: cohort and case-control analyses. Am J Respir Crit Care Med 177:1391–1396. doi: 10.1164/rccm.200802-355OC. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Jiang Z, Su Y, Chen M, Li F, Liu L, Sun L, Wang Y, Zhang S, Zhang L. 2013. Gene expression profiling reveals potential key pathways involved in pyrazinamide-mediated hepatotoxicity in Wistar rats. J Appl Toxicol 33:807–819. doi: 10.1002/jat.2736. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Guo H, Hassan HM, Ding P-P, Su Y, Song Y, Wang T, Sun L, Zhang L, Jiang Z. 2016. Pyrazinamide induced hepatic injury in rats through inhibiting the PPARα pathway. J Appl Toxicol 36:1579–1590. doi: 10.1002/jat.3319. [DOI] [PubMed] [Google Scholar]
- 30.Breitenbach M, Ralser M, Perrone GG, Iglseder B, Rinnerthaler M, Dawes IW. 2013. Oxidative stress and neurodegeneration: the yeast model system. Front Biosci (Landmark Ed) 18:1174–1193. doi: 10.2741/4171. [DOI] [PubMed] [Google Scholar]
- 31.El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. 2012. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 3:132. doi: 10.3389/fphys.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon AK, Hollander GA, McMichael A. 2015. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung CC, Li T, Lam TH, Yew WW, Law WS, Tam CM, Chan WM, Chan CK, Ho KS, Chang KC. 2004. Smoking and tuberculosis among the elderly in Hong Kong. Am J Respir Crit Care Med 170:1027–1033. doi: 10.1164/rccm.200404-512OC. [DOI] [PubMed] [Google Scholar]
- 34.Yew WW, Leung CC, Zhang Y. 2017. Oxidative stress and TB outcomes in patients with diabetes mellitus? J Antimicrob Chemother 72:1552–1555. doi: 10.1093/jac/dkx046. [DOI] [PubMed] [Google Scholar]
- 35.Leung CC, Yew WW, Chan CK, Chau CH, Tam CM, Lam CW, Tam WO, Lau KS, Liu WT. 2002. Tuberculosis in older people: a retrospective and comparative study from Hong Kong. J Am Geriatr Soc 50:1219–1226. doi: 10.1046/j.1532-5415.2002.50308.x. [DOI] [PubMed] [Google Scholar]
- 36.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J, Drug Induced Liver Injury Network (DILIN) . 2008. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yew WW, Chan DP, Leung CC, Zhang Y, Wang R, Ng P, Lee SM. 2017. Can toxicities induced by antituberculosis drugs be better managed in diabetic patients? Eur Respir J 50:1700409. doi: 10.1183/13993003.00409-2017. [DOI] [PubMed] [Google Scholar]
- 38.Lucchesi AN, Cassettari LL, Spadella CT. 2015. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans. J Diabetes Res 2015:494578. doi: 10.1155/2015/494578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiorentino TV, Prioletta A, Zuo P, Folli F. 2013. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des 19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 40.Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. 2016. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res 2016:3425617. doi: 10.1155/2016/3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins GC, Coughlan MT. 2014. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol 171:1917–1942. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandas A, Congiu M, Balestrieri C, Mereu A, Iorio E. 2008. Nutritional status and oxidative stress in an elderly Sardinian population. Med J Nutrition Metab 1:99–107. doi: 10.1007/s12349-008-0016-1. [DOI] [Google Scholar]
- 43.Bourke CD, Berkley JA, Prendergast AJ. 2016. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 37:386–398. doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Zutphen T, Ciapaite J, Bloks VW, Ackereley C, Gerding A, Jurdzinski A, de Moraes RA, Zhang L, Wolters JC, Bischoff R, Wanders RJ, Houten SM, Bronte-Tinkew D, Shatseva T, Lewis GF, Groen AK, Reijngoud D-J, Bakker BM, Jonker JW, Kim PK, Bandsma RHJ. 2016. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J Hepatol 65:1198–1208. doi: 10.1016/j.jhep.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 45.Tsai JH, Ferrell LD, Tan V, Yeh MM, Sarkar M, Gill RM. 2017. Aggressive non-alcoholic steatohepatitis following rapid weight loss and/or malnutrition. Mod Pathol 30:834–842. doi: 10.1038/modpathol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JY, Liu CH, Hu FC, Chang HC, Liu JL, Chen JM, Yu CJ, Lee LN, Kao JH, Yang PC. 2011. Risk factors of hepatitis during anti-tuberculous treatment and implications of hepatitis virus load. J Infect 62:448–455. doi: 10.1016/j.jinf.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, Albanese AP, Pitchenik AE. 1998. Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med 157:1871–1876. doi: 10.1164/ajrccm.157.6.9711039. [DOI] [PubMed] [Google Scholar]
- 48.Paracha UZ, Fatima K, Alqahtani M, Chaudhary A, Abuzenadah A, Damanhouri G, Qadri I. 2013. Oxidative stress and hepatitis C virus. Virol J 10:251. doi: 10.1186/1743-422X-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medvedev R, Ploen D, Hildt E. 2016. HCV and oxidative stress: implications for HCV life cycle and HCV-associated pathogenesis. Oxid Med Cell Longev 2016:9012580. doi: 10.1155/2016/9012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. 2011. Hepatitis C virus, oxidative stress and steatosis: current status and perspectives. Curr Mol Med 11:373–390. doi: 10.2174/156652411795976592. [DOI] [PubMed] [Google Scholar]
- 51.Mihm S. 2010. Hepatitis C virus, diabetes and steatosis: clinical evidence in favor of a linkage and role of genotypes. Dig Dis 28:280–284. doi: 10.1159/000282103. [DOI] [PubMed] [Google Scholar]
- 52.Alavian SM, Showraki A. 2016. Hepatitis B and its relationship with oxidative stress. Hepat Mon 16:e37973. doi: 10.5812/hepatmon.37973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dworkin MS, Adams MR, Cohn DL, Davidson AJ, Buskin S, Horwitch C, Morse A, Sackoff J, Thompson M, Wotring L, McCombs SB, Jones JL. 2005. Factors that complicate the treatment of tuberculosis in HIV-infected patients. J Acquir Immune Defic Syndr 39:464–470. doi: 10.1097/01.qai.0000152400.36723.85. [DOI] [PubMed] [Google Scholar]
- 54.Ivanov AV, Valuev-Elliston VT, Ivanova ON, Kochetkov SN, Starodubova ES, Bartosch B, Isaguliants MG. 2016. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev 2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagiah S, Phulukdaree A, Chuturgoon A. 2015. Mitochondrial and oxidative stress response in HepG2 cells following acute and prolonged exposure to antiretroviral drugs. J Cell Biochem 116:1939–1946. doi: 10.1002/jcb.25149. [DOI] [PubMed] [Google Scholar]
- 56.Gil L, Martínez G, González I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tápanes R, Pérez J, León OS. 2003. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res 47:217–224. doi: 10.1016/S1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 57.Russmann S, Jetter A, Kullak-Ublick GA. 2010. Pharmacogenetics of drug-induced liver injury. Hepatology 52:748–761. doi: 10.1002/hep.23720. [DOI] [PubMed] [Google Scholar]
- 58.Urban TJ, Daly AK, Aithal GP. 2014. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis 34:123–133. doi: 10.1055/s-0034-1375954. [DOI] [PubMed] [Google Scholar]
- 59.Richardson M, Kirkham J, Dwan K, Sloan D, Davies G, Jorgensen A. 2017. Influence of genetic variants on toxicity to anti-tubercular agents: a systematic review and meta-analysis (protocol). Syst Rev 6:142. doi: 10.1186/s13643-017-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perwitasari DA, Atthobari J, Wilffert B. 2015. Pharmacogenetics of isoniazid-induced hepatotoxicity. Drug Metab Rev 47:222–228. doi: 10.3109/03602532.2014.984070. [DOI] [PubMed] [Google Scholar]
- 61.Huang Y-S, Wang LY, Chang C-H, Perng C-L, Lin H-C. 2016. Superoxide dismutase 2 genetic variation as a susceptibility risk factor for alcoholic cirrhosis. Alcohol Alcohol 51:633–637. doi: 10.1093/alcalc/agw004. [DOI] [PubMed] [Google Scholar]
- 62.Huang YS, Chang CH, Lin TL, Perng CL. 2014. Genetic variations of superoxide dismutase 2 and cytochrome P450 2E1 in non-alcoholic steatohepatitis. Liver Int 34:931–936. doi: 10.1111/liv.12533. [DOI] [PubMed] [Google Scholar]
- 63.Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS, Collins JJ. 2013. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci Transl Med 5:192ra85. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willems PHGM, Rossignol R, Dieteren CEJ, Murphy MP, Koopman WJH. 2015. Redox homeostasis and mitochondrial dynamics. Cell Metab 22:207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Valero T. 2014. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 66.Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC, Nain CK, Singh K. 2000. Isoniazid- and rifampicin-induced oxidative hepatic injury–protection by N-acetylcysteine. Hum Exp Toxicol 19:517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- 67.Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, Velayati AA. 2010. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol 22:1235–1238. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]
- 68.Adhvaryu MR, Reddy N, Vakharia BC. 2008. Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol 14:4753–4762. doi: 10.3748/wjg.14.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q, Garner P, Wang Y, Huang B, Smith H. 2008. Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: systematic review of ingredients and evaluation studies. BMC Public Health 8:365. doi: 10.1186/1471-2458-8-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu WC, Lai ST, Chiu MC, Chau TN, Ng TK, Tam CM. 2006. Lamivudine enabled isoniazid and rifampicin treatment in pulmonary tuberculosis and hepatitis B co-infection. Int J Tuberc Lung Dis 10:824–825. [PubMed] [Google Scholar]
- 71.Dhiman RK, Saraswat VA, Rajekar H, Reddy C, Chawla YK. 2012. A guide to the management of tuberculosis in patients with chronic liver disease. J Clin Exp Hepatol 2:260–270. doi: 10.1016/j.jceh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kinzig-Schippers M, Tomalik-Scharte D, Jetter A, Scheidel B, Jakob V, Rodamer M, Cascorbi I, Doroshyenko O, Sörgel F, Fuhr U. 2005. Should we use N-acetyltransferase type 2 genotyping to personalize isoniazid doses? Antimicrob Agents Chemother 49:1733–1738. doi: 10.1128/AAC.49.5.1733-1738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azuma J, Ohno M, Kubota R, Yokota S, Nagai T, Tsuyuguchi K, Okuda Y, Takashima T, Kamimura S, Fujio Y, Kawase I, Pharmacogenetics-Based Tuberculosis Therapy Research Group . 2013. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol 69:1091–1101. doi: 10.1007/s00228-012-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]