Data are needed from outpatient settings to better inform antimicrobial stewardship. In this study, a random sample of outpatient antibiotic prescriptions by primary care providers (PCPs) at our health care system was reviewed and compared to consensus guidelines.
KEYWORDS: antimicrobial stewardship, inappropriate prescribing, outpatient, prescribing patterns, primary care
ABSTRACT
Data are needed from outpatient settings to better inform antimicrobial stewardship. In this study, a random sample of outpatient antibiotic prescriptions by primary care providers (PCPs) at our health care system was reviewed and compared to consensus guidelines. Over 12 months, 3,880 acute antibiotic prescriptions were written by 76 PCPs caring for 40,734 patients (median panel, 600 patients; range, 33 to 1,547). PCPs ordered a median of 84 antibiotic prescriptions per 1,000 patients per year. Azithromycin (25.8%), amoxicillin-clavulanate (13.3%), doxycycline (12.4%), amoxicillin (11%), fluoroquinolones (11%), and trimethoprim-sulfamethoxazole (10.6%) were prescribed most commonly. Medical records corresponding to 300 prescriptions from 59 PCPs were analyzed in depth. The most common indications for these prescriptions were acute respiratory tract infection (28.3%), urinary tract infection (23%), skin and soft tissue infection (15.7%), and chronic obstructive pulmonary disease (COPD) exacerbation (6.3%). In 5.7% of cases, no reason for the prescription was listed. No antibiotic was indicated in 49.7% of cases. In 12.3% of cases, an antibiotic was indicated, but the prescribed agent was guideline discordant. In another 14% of cases, a guideline-concordant antibiotic was given for a guideline-discordant duration. Therefore, 76% of reviewed prescriptions were inappropriate. Ciprofloxacin and azithromycin were most likely to be prescribed inappropriately. A non-face-to-face encounter prompted 34% of prescriptions. The condition for which an antibiotic was prescribed was not listed in primary or secondary diagnosis codes in 54.5% of clinic visits. In conclusion, there is an enormous opportunity to reduce inappropriate outpatient antibiotic prescriptions.
INTRODUCTION
The overuse of antibiotics leads to drug resistance, excess adverse events, complications such as Clostridium difficile infections, and increased costs. Most antibiotics are prescribed in outpatient settings (1, 2). There has been increasing attention to outpatient antimicrobial stewardship. The White House's National Action Plan for Combating Antibiotic Resistant Bacteria set a goal of a 50% reduction in inappropriate outpatient antibiotic use by 2020 (https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf). The U.S. Centers for Disease Control (CDC) recently recommended core elements of outpatient antibiotic stewardship (3). However, more data are needed to establish baseline outpatient antibiotic usage, identify target levels of prescribing and performance benchmarks, and better inform stewardship efforts (4).
A national study estimated that approximately 30% of antibiotic prescribing at ambulatory care visits was not indicated according to professional society guidelines (5). This figure likely underestimates inappropriate usage (6), since the study did not account for the selection of an improper agent or duration of therapy. Indeed, data suggest unnecessarily broad-spectrum antibiotic use is common in ambulatory care settings (7). Recent studies demonstrated that physicians prescribed first-line antibiotics for only 37% of adults who were treated for sinusitis and pharyngitis (8), and fluoroquinolones were most commonly used to treat uncomplicated cystitis, despite not being first-line agents (9, 10).
To our knowledge, there has not been a study of appropriate overall antibiotic prescribing in a primary care setting that considers agent selection and duration, in addition to whether an antibiotic was necessary. We sought to establish a baseline for overall inappropriate antibiotic prescribing in our Veterans Affairs (VA) Healthcare System primary care clinics. Our objectives were to determine the most common antibiotics prescribed, the indications for which antibiotics were prescribed, and the rates of inappropriate usage (defined as failure to comply with consensus guidelines with regard to indication, agent selection, and duration).
RESULTS
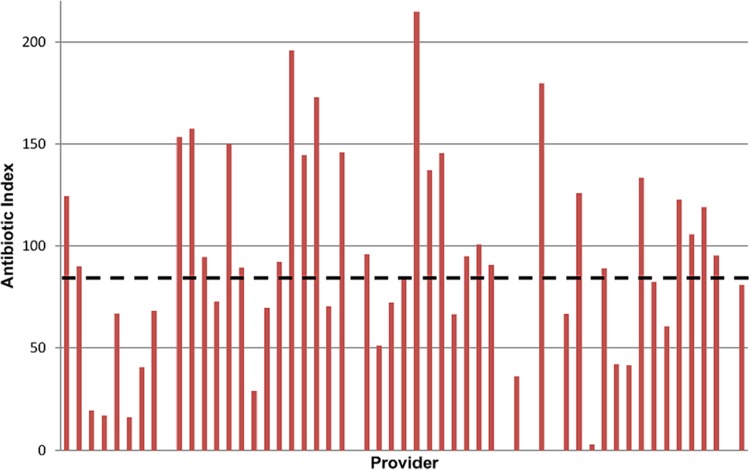
Over 12 months, 3,880 acute antibiotic prescriptions were written by 76 primary care providers (PCPs) caring for 40,734 patients (median panel, 600 patients; range, 33 to 1,547). The median antibiotic index was 84 antibiotic prescriptions per 1,000 patients per year, with wide variation between providers (Fig. 1). The most commonly prescribed antibiotics were azithromycin (25.8%), amoxicillin-clavulanate (13.3%), doxycycline (12.4%), amoxicillin (11%), trimethoprim-sulfamethoxazole (10.6%), and cephalexin (7.9%). Fluoroquinolones accounted for 11% of prescriptions (ciprofloxacin, 7.6%; moxifloxacin, 2%; levofloxacin, 1.4%). Other agents represented 8% of prescriptions.
FIG 1.
Distribution of antibiotic indices for providers with panel sizes >100 patients. Considerable variation was observed. The median antibiotic index was 84 (dashed line).
Of the 59 PCPs (39 women, 20 men) who had prescriptions reviewed, 41 were physicians (36 medical doctors [MDs], 5 doctors of osteopathic medicine [DOs], 30 doctors of internal medicine, and 11 from family practice) and 18 were advanced practice providers ([APPs]12 nurse practitioners, 6 physicians' assistants). A total of 316 prescriptions were reviewed. After exclusions (chronic antibiotic prescription, n = 12; patient seen as an inpatient, n = 3; medication prescribed but patient subsequently told not to take it, n = 1), 300 were available for analysis. The conditions for which antibiotics were prescribed were acute respiratory tract infection ([ARTI] 28.3%), urinary tract infection ([UTI] 23%), skin and soft tissue infection ([SSTI] 15.7%), chronic obstructive pulmonary disease (COPD) exacerbation (6.3%), dental (4.7%), community acquired pneumonia (4%), and Lyme disease (3.7%). Other conditions comprised the remaining 8.7% of prescriptions. In 5.7% of cases, no reason for a prescription was stated anywhere in the chart. The respective mean and median duration of antibiotic prescriptions were 6.7 and 5 days for ARTI, 8.9 and 7 days for UTI, 8.5 and 7 days for SSTI, and 5.3 and 5 days for COPD exacerbation.
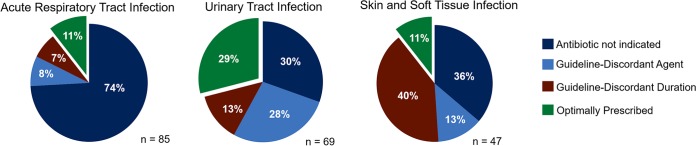
On the basis of consensus guidelines, antibiotics were not indicated in 49.7% (149/300) of cases in which they were prescribed. In 12.3% (37/300) of cases, an antibiotic was indicated, but the prescribed agent was guideline discordant. In another 14% (42/300) of cases, a guideline-concordant antibiotic was given for a guideline-discordant duration (too long in 95% [40/42]). Therefore, 76% (228/300) of reviewed prescriptions were inappropriate. The appropriateness of antibiotic prescriptions for the three most common conditions is shown in Fig. 2. Ciprofloxacin and azithromycin were the antibiotics that were most likely to be prescribed inappropriately (Table 1). The prevalence of unnecessary antibiotic prescribing was not different between physicians and APPs (48.5% [98/202] versus 52% [51/98], respectively, not significant [NS]), MD and DO physicians (48.3% [83/172] versus 50% [15/30], respectively, NS), or men and women providers (46.6% [48/103] versus 51.3% [101/197], respectively, NS). Family practice physicians were more likely to write unnecessary antibiotic prescriptions than internal medicine physicians (58.5% [38/65] versus 43.8% [60/137], respectively, P = 0.07). When also considering agent selection and duration, the prevalence of inappropriate antibiotic prescribing was higher for APPs than for physicians (82.7% [81/98] versus 72.8% [147/202], respectively, P = 0.06), though no significant differences were observed between MD and DO physicians (73.3% [126/172] versus 70% [21/30], respectively, NS), men and women providers (72.8% [75/103] versus 77.7% [153/197], respectively, P = 0.39), or family practice and internal medicine physicians (78.5% [51/65] versus 70.1% [96/137], respectively, P = 0.24).
FIG 2.
Appropriateness of antibiotic prescribing for the most common outpatient indications. The data highlight that there are vast opportunities for interventions to improve practice and that optimal interventions are likely to differ by indication.
TABLE 1.
Inappropriate prescriptions, by antibiotica
| Antibiotic | No. (%) of prescriptions |
Most common reasons for inappropriate prescriptions (n) | |
|---|---|---|---|
| Reviewed | Inappropriate | ||
| Amoxicillin-clavulanate | 43 | 30 (69.8) | No indication: ARTI (12), dental (3), otitis externa (3); guideline-discordant agent: UTI (4), SSTI (3) |
| Amoxicillin | 31 | 19 (61.3) | No indication: ARTI (7), unknown (4), endocarditis prophylaxis (3) |
| Azithromycin | 74 | 58 (78.4) | No indication: ARTI (33), COPD exacerbation (9), unknown (5); guideline-discordant agent: ARTI (7) |
| Cephalexin | 26 | 12 (46.2) | No indication: SSTI (9) |
| Ciprofloxacin | 25 | 21 (84.0) | No indication: UTI (9); guideline-discordant agent: UTI (10) |
| Doxycycline | 33 | 14 (42.4) | No indication: ARTI (8) |
| Nitrofurantoin | 7 | 0 (0) | |
| TMP-SMX | 28 | 13 (46.4) | No indication: ARTI (7), UTI (7) |
For this analysis, an antibiotic was considered inappropriate if there was no indication for its usage or if the agent chosen was guideline discordant.
No positive C. difficile tests were observed within 90 days among reviewed cases. Related return visits within 30 days were more common following unnecessary antibiotic prescriptions than for indicated antibiotic prescriptions (21.5% [32/149] versus 13.9% [21/151], respectively, P = 0.10) and for inappropriate antibiotic prescriptions than for those optimally prescribed (19.7% [45/228] versus 11.1% [8/72], respectively, P = 0.11). An unnecessary antibiotic prescription was significantly more likely to be given at a routine versus an acute visit (61% [58/95] versus 44.7% [46/103], respectively, P = 0.02); however, prescriptions were equally likely to be inappropriate at either type of encounter (81.1% [77/95] versus 80.6% [83/103], respectively, NS). The condition for which an antibiotic was prescribed was not listed in primary or secondary diagnosis codes in 54.5% of clinic visits. A non-face-to-face encounter prompted 34% (102/300) of prescriptions.
DISCUSSION
To our knowledge, this is the first study to describe a comprehensive estimate of unnecessary and guideline-discordant antibiotic prescribing in a primary care setting. The study was unique in utilizing all antibiotic prescriptions as a starting point, focusing on PCPs, and using chart review for determinations of appropriateness (rather than assuming appropriateness based on coding diagnoses). It also provides data from a VA setting, which were not captured in the recent national ambulatory care study (5).
We observed substantial antibiotic overuse and a lack of adherence with guidelines for front-line agents and durations of treatment. Approximately half of prescriptions were for conditions for which no antibiotic was indicated. There are several reasons our unnecessary usage may have been greater than the ∼30% rate estimated for ambulatory care providers (5). First, our methodology of chart review may have enabled us to assess in greater detail the rationale (or lack of rationale) for prescribing. Second, our focus was exclusively on the primary care setting, whereas national estimates were inclusive of emergency department visits and outpatient specialties as well. Lastly, our findings suggest that national data may underestimate inappropriate prescribing for at least some conditions. For example, the national study assumed that all antibiotics given for UTIs were appropriate, whereas we found 30% were inappropriately prescribed for asymptomatic bacteriuria.
Over a quarter of prescriptions in our clinics were for conditions for which an antibiotic was indicated, but the agent chosen or treatment duration was guideline discordant. These observations were not surprising, as previous data have shown that broad-spectrum antibiotic use is common in ambulatory care settings (7), as are guideline-discordant prescriptions for ARTIs (8, 11–13), UTIs (9, 14), and SSTIs (15). Our experience highlights vast opportunities for improvements in antibiotic utilization, since as few as 11% of agents were optimally prescribed for common conditions such as ARTIs and SSTIs.
Indeed, our study has important implications for designing outpatient antimicrobial stewardship interventions. Condition-based interventions focusing on ARTIs, UTIs, or SSTIs may be high yield, but the focus of the intervention will vary by condition. For ARTIs, the biggest impact is likely to stem from avoiding prescriptions against conditions for which antibiotics are not indicated. For UTIs, the greatest benefit may be obtained through education on not treating asymptomatic bacteriuria and by focusing on prescribing guideline-concordant agents or those deemed appropriate on the basis of local susceptibility patterns. For SSTIs, there is a particular opportunity to reduce treatment durations. Drug-based interventions directed against azithromycin or ciprofloxacin also may be high yield, since the vast majority of prescriptions for these agents were inappropriate. As suggested elsewhere (16), interventions focusing on family practice providers may be useful in our clinics. Other literature has highlighted increased antibiotic prescribing among APPs (17, 18). While we did not find differences in unnecessary prescriptions between APPs and physicians, we did find more inappropriate antibiotic prescribing among APPs if antibiotic selection and duration were considered. Lastly, we noted considerable variation in the antibiotic indices between providers (Fig. 1). We hypothesize that a relationship exists between the antibiotic index (overall prescribing) and inappropriate prescribing that may explain some of this variation. However, such a relationship was not fully evaluated in the present study and requires further analysis. The data here highlight the need for programs to understand prescribing patterns at their own centers.
Finally, our experience indicates that interventions based on diagnosis codes may miss many stewardship opportunities. Indeed, only 30% (90/300) of prescriptions reviewed in this study would have been identified by diagnosis code. The rate is low for two reasons. First, PCPs often failed to enter an infection-related diagnosis code for office visits at which an antibiotic was prescribed, even if a diagnosis was mentioned in the clinic note. Second, approximately one-third of antibiotic prescriptions were generated by non-face-to-face encounters, which typically lack an associated code. Our rate of such “phantom” prescribing was greater than the ∼15% rate estimated in a study of Medicare patients with recent myocardial infarction (19), suggesting that it may be fruitful for stewardship programs to address such practices. To ascertain a true estimate of antibiotic utilization, centers may find greater benefit in reviewing lists of all antibiotics prescribed, as was done here, rather than focusing exclusively on infection-associated diagnosis codes. A shortcoming of our approach is that it limits the ability to review appropriate nonprescription of antibiotics (e.g., withholding antibiotics in a patient with the common cold). Thus, an argument could be made for incorporating both techniques into a comprehensive outpatient stewardship program.
Other limitations of this study included its retrospective design and the inclusion of a single VA health care system. Therefore, the results may not be generalizable to other regions or to community primary care settings; coding practices, in particular, may differ. Also, the dose of antibiotic was not considered in the determination of appropriateness. Had dosing been evaluated, the rates of inappropriate use likely would have been higher. Decisions regarding the necessity of antibiotic prescribing assume accurate documentation. As such, it is possible that some prescriptions that we classified as unnecessary were indicated, but incompletely documented. Similarly, with regard to guideline concordance, there could be individual patient factors unknown to reviewers that might justify a provider's decision to deviate from the guidelines. Thus, it is possible that some guideline-discordant prescriptions in our sample were justified but classified as inappropriate. Finally, we reviewed a subset of randomly chosen prescriptions. While it is possible that results may have differed if more prescriptions were assessed, our findings are plausible and broadly in keeping with data from other studies.
Conclusions.
Our data suggest that prior studies of inappropriate outpatient antibiotic use may have underestimated the extent of the problem. Through a detailed review of medical records for PCPs, we identified substantial room for improvement in antibiotic prescribing, especially when agent selection and duration were also considered. Our findings raise important considerations for the design of outpatient stewardship programs, targeted to specific types of infection, antibiotic agents, and types of providers. Moreover, the data support interventions directed toward prescriptions generated by non-face-to-face encounters and those without a corresponding diagnosis code. We are following up this baseline investigation with an intervention to improve outpatient antibiotic prescribing, in which initial education sessions are followed by email-based peer comparison of overall antibiotic prescribing rates.
MATERIALS AND METHODS
Data from 1 September 2015 to 31 August 2016 were collected retrospectively, as part of an initiative to improve outpatient antibiotic prescribing. The data collection form is included as Spreadsheet S1 in the supplemental material. There were no active outpatient stewardship efforts under way during this time period. As the data presented here were gathered as part of quality improvement activities, VA Pittsburgh Institutional Review Board (IRB) approval was waived. All outpatient antibiotic prescriptions written by VA Pittsburgh Healthcare System primary care providers (PCPs) from our seven clinics were catalogued. Prescriptions for >28 days were excluded to focus on acute infections.
Charts were reviewed from PCPs who were expected to be present for a subsequent intervention phase. Among these PCPs, a subset of prescriptions (a 5% random sample with a minimum of 5 prescriptions per provider, or all prescriptions if <5 were ordered by a provider) were assessed for compliance with consensus guidelines. Each case was evaluated by one of the authors, who determined the indication for the antibiotic prescribed on the basis of the documentation in the patient's chart. It was determined if the prescribed antibiotic was indicated for the condition documented and if the prescription was guideline concordant for the agent and duration of therapy. Guidelines for acute respiratory tract infections (defined as acute uncomplicated bronchitis, pharyngitis, rhinosinusitis, and the common cold) were taken from the American College of Physicians and the CDC (20). For other conditions, guidelines were from the Infectious Diseases Society of America (IDSA) (21–27). If IDSA guidelines were not available for a given condition, other national or international guidelines were used (28–34, https://www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html, http://ginasthma.org/wp-content/uploads/2016/04/wms-GINA-2016-main-report-final.pdf, http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/, http://www.sdcep.org.uk/wp-content/uploads/2016/03/SDCEP-Drug-Prescribing-for-Dentistry-3rd-edition.pdf). If consensus guidelines were not available, an antibiotic was considered appropriate if deemed to be so by at least one of two infectious diseases physicians (N. R. Shively, B. K. Decker) upon independent review. If an antibiotic was prescribed for an unstated condition, the prescription was considered inappropriate. To ensure reliability of the overall estimates of appropriateness reached, a random sample of 10% (n = 30) of cases were reviewed by two reviewers (N. R. Shively, B. K. Decker), who independently determined that antibiotic prescriptions were not indicated and indicated in 18 and 12 cases, respectively.
Other data collected included the type of encounter (clinic visit versus non-face-to-face (e.g., telephone call, patient email, walk-in to front desk only), type of clinic visit (acute versus routine), primary and secondary diagnoses at the time of the visit, as indicated by diagnosis code, prescriber characteristics, such as gender, physician versus advanced practice provider ([APP] nurse practitioner or physician assistant), physician degree (MD versus DO), and physician specialty (internal medicine versus family practice), related return visits within 30 days, and whether there was a positive C. difficile test within 90 days of the antibiotic prescription. Antibiotic index was defined as the number of antibiotic prescriptions per 1,000 patients per year. Statistical analysis was performed using Fisher's exact test, and a P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Janelle Altman and Jeff Wagner for their assistance with data acquisition and Lloyd Clarke and Ryan Shields for their assistance with data analysis and statistical advice. We have no financial conflicts to disclose.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00337-18.
REFERENCES
- 1.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Danziger LH. 2013. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother 68:715–718. doi: 10.1093/jac/dks445. [DOI] [PubMed] [Google Scholar]
- 2.Schumock GT, Li EC, Suda KJ, Matusiak LM, Hunkler RJ, Vermeulen LC, Hoffman JM. 2014. National trends in prescription drug expenditures and projections for 2014. Am J Health Syst Pharm 71:482–499. doi: 10.2146/ajhp130767. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. 2016. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 65:1–12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]
- 4.Metlay JP. 2015. Editorial commentary: setting national targets for antibiotic use. Clin Infect Dis 60:1317–1318. doi: 10.1093/cid/civ081. [DOI] [PubMed] [Google Scholar]
- 5.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, Lynfield R, Margolis DJ, May LS, Merenstein D, Metlay JP, Newland JG, Piccirillo JF, Roberts RM, Sanchez GV, Suda KJ, Thomas A, Woo TM, Zetts RM, Hicks LA. 2016. Prevalence of inappropriate antibiotic prescriptions among U.S. ambulatory care visits, 2010–2011. JAMA 315:1864. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 6.Tamma PD, Cosgrove SE. 2016. Addressing the appropriateness of outpatient antibiotic prescribing in the United States: an important first step. JAMA 315:1839–1841. doi: 10.1001/jama.2016.4286. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. 2014. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 8.Hersh AL, Fleming-Dutra KE, Shapiro DJ, Hyun DY, Hicks LA, Outpatient Antibiotic Use Target-Setting Workgroup . 2016. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med 176:1870. doi: 10.1001/jamainternmed.2016.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigoryan L, Zoorob R, Wang H, Trautner BW. 2015. Low concordance with guidelines for treatment of acute cystitis in primary care. Open Forum Infect Dis 2:ofv159. doi: 10.1093/ofid/ofv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. 2016. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002–2011. Open Forum Infect Dis 3:ofw159. doi: 10.1093/ofid/ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett ML, Linder JA. 2014. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA Intern Med 174:138–140. doi: 10.1001/jamainternmed.2013.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong SY, Jordan MR, Wanke C. 2011. Antimicrobial prescribing in the USA for adult acute pharyngitis in relation to treatment guidelines. J Eval Clin Pract 17:1176–1183. doi: 10.1111/j.1365-2753.2010.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett K, Geide T, Rehman D, Garrett A, Thompson J. 2015. Assessment of empiric antibiotic prescribing patterns for acute sinusitis in a Veterans Affairs (VA) ambulatory care system. Open forum Infect Dis 2:165. doi: 10.1093/ofid/ofv133.43. [DOI] [Google Scholar]
- 14.Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. 2016. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002–2011. Open Forum Infect Dis 3:ofw159. doi: 10.1093/ofid/ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley HJ, Knepper BC, Price CS, Mehler PS, Burman WJ, Jenkins TC. 2013. Avoidable antibiotic exposure for uncomplicated skin and soft tissue infections in the ambulatory care setting. Am J Med 126:1099–1106. doi: 10.1016/j.amjmed.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlam TF, Morgan JR, Wetzler LM, Christiansen CL, Drainoni M-L. 2015. Antibiotics for respiratory tract infections: a comparison of prescribing in an outpatient setting. Infect Control Hosp Epidemiol 36:153–159. doi: 10.1017/ice.2014.21. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez GV, Hersh AL, Shapiro DJ, Cawley JF, Hicks LA. 2016. Outpatient antibiotic prescribing among United States nurse practitioners and physician assistants. Open Forum Infect Dis 3:ofw168. doi: 10.1093/ofid/ofw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt ML, Spencer MD, Davidson LE. 2018. Patient, provider, and practice characteristics associated with inappropriate antimicrobial prescribing in ambulatory practices. Infect Control Hosp Epidemiol 39:307–315. doi: 10.1017/ice.2017.263. [DOI] [PubMed] [Google Scholar]
- 19.Riedle BN, Polgreen LA, Cavanaugh JE, Schroeder MC, Polgreen PM. 2017. Phantom prescribing: examining the frequency of antimicrobial prescriptions without a patient visit. Infect Control Hosp Epidemiol 38:273–280. doi: 10.1017/ice.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris AM, Hicks LA, Qaseem A. 2016. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American college of physicians and the centers for disease control and prevention. Ann Intern Med 164:425–434. doi: 10.7326/M15-1840. [DOI] [PubMed] [Google Scholar]
- 21.Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJC, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM, Infectious Diseases Society of America . 2012. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 54:e72–e112. doi: 10.1093/cid/cis370. [DOI] [PubMed] [Google Scholar]
- 22.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC, Infectious Diseases Society of America . 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 23.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM, Infectious Diseases Society of America American Society of Nephrology American Geriatric Society . 2005. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 40:643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 24.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 25.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 26.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America American Thoracic Society . 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44 Suppl 2:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare Epidemiology of America Infectious Diseases Society of America . 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 28.National Asthma Education and Prevention Program. 2007. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 120:S94–S138. doi: 10.1016/j.jaci.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT. 2007. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 30.Workowski KA, Bolan GA, Centers for Disease Control and Prevention . 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. doi: 10.15585/mmwr.rr6404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sollecito TP, Abt E, Lockhart PB, Truelove E, Paumier TM, Tracy SL, Tampi M, Beltrán-Aguilar ED, Frantsve-Hawley J. 2015. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitioners–a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 146:11.e8–16.e8. doi: 10.1016/j.adaj.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld RM, Schwartz SR, Ron Cannon C, Roland PS, Simon GR, Ashok Kumar K, Huang WW, Haskell HW, Robertson PJ. 2014. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg 150:1–24. doi: 10.1177/0194599813514365. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD. 2015. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg 152:S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen M, Qaseem A. 2016. Hematuria as a marker of occult urinary tract cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 164:488. doi: 10.7326/M15-1496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.